Abstract
Neurogenesis in the adult brain, a process once thought to be essentially absent, has now been demonstrated to occur throughout adult mammalian life within several brain regions. Adult neurogenesis normally occurs only within the subventricular zone (SVZ) bordering the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG). Neurogenic progenitors within these regions produce distinct neuron types, with progenitors in the SGZ giving rise to glutamatergic neurons that populate the DG granule cell layer and those within the SVZ producing neurons destined for the olfactory bulb. In this review, we highlight recent research on transcription factor expression and function during adult hippocampal neurogenesis. In this regard, recent evidence indicates that adult neurogenesis replicates important aspects of progenitor cell development in the embryonic brain. Specifically, work from our laboratory and others indicates that transcription factor cascades active in progenitor cells during neurogenesis in the embryonic cerebral cortex are also activated in adult hippocampal progenitor cells, where they play an important role in determining neuronal fate and regulating progenitor cell proliferation and maintenance. These findings suggest that conserved transcription factor cascades regulate genetic programs that delineate progenitor cell lineages and control progenitor cell proliferation and differentiation.
Introduction
In recent years, numerous studies have confirmed that adult neurogenesis is a robust and continual process that occurs within two specific neurogenic niches of the adult brain (Lie et al., 2004). One of these neurogenic niches, the subgranular zone (SGZ) of the hippocampal dentate gyrus, contains neural progenitor cells (NPCs) that give rise to neurons destined to populate the granule cell layer (GCL) of the dentate gyrus. The subventricular zone (SVZ) adjacent to the lateral ventricles represents the other main neurogenic niche in the adult brain. Within the SVZ, NPCs divide to give rise to neuroblasts that travel through the rostral migratory stream (RMS) and eventually differentiate into olfactory bulb neurons. Additionally, the existence of neurogenesis in otherwise non-neurogenic regions of the adult brain has been demonstrated in models of neurodegenerative diseases and following various brain injuries (Mitchell et al., 2004; Emsley et al., 2005).
The functional significance of adult neurogenesis is an area of ongoing investigation. Several studies suggest that hippocampal neurogenesis in the adult brain may contribute to learning and memory, as well as the regulation of emotional status (Encinas et al., 2006; Clelland et al., 2009; Deng et al., 2009; Deng et al., 2010).
In terms of cellular and molecular mechanisms, recent work suggests that neurogenesis in the adult brain recapitulates many aspects of neurogenesis in the developing brain (Espósito et al., 2005; Nacher et al., 2005; Song et al., 2005; Hevner et al., 2006; Hodge et al., 2008). In accordance with this idea, transcription factors known to influence progenitor cell development in the embryonic brain may likewise play a conserved role in directing proliferation and differentiation in the adult brain. Studies of embryonic development have shown that coordinated transcription factor expression is important for the specification of neuronal identity and neurotransmitter fate (Hevner et al., 2006). In many cases, transcription factor cascades (sequential expression of multiple transcription factors) appear to be important for controlling progenitor proliferation and specifying neuronal identity in the developing brain (Arlotta et al., 2005; Englund et al., 2005; Hevner et al., 2006). This review will summarize current data on the expression and actions of transcription factors during progenitor cell development in the adult brain, focusing specifically on recent work in the hippocampal dentate gyrus.
Neural Progenitor Cells in the Subgranular Zone of the Dentate Gyrus
NPCs in the dentate gyrus are located in the SGZ, which lies at the border of the hilus and the granule cell layer (Fig. 1A, B). Neural stem cells or primary progenitors, referred to as type-1 cells, are multipotent progenitors capable of producing neurons and glia; they can be subdivided into active and quiescent populations (Suh et al., 2007; Ehninger and Kempermann, 2008; Lugert et al., 2010). Quiescent type-1 NPCs typically exhibit radial morphology characterized by a large, triangular soma and a long radial process that traverses the granule cell layer and branches into numerous small processes within the molecular layer of the dentate gyrus (Fig. 1B, C) (Kempermann et al., 2004; Lugert et al., 2010). These quiescent radial type-1 cells are characterized by expression of the glial cell marker GFAP and the neural progenitor marker nestin, divide at a slow rate, and represent a small fraction of the total population of dividing cells in the SGZ (Encinas et al., 2006; Suh et al., 2007; Lugert et al., 2010). A second class of type-1 cell with short, horizontal processes has recently been characterized. These type-1 cells with horizontal morphology express markers similar to radial type-1 cells, but typically divide at a greater frequency and account for a larger proportion of the total population of dividing cells in the adult dentate gyrus (Encinas et al., 2006; Suh et al., 2007; Lugert et al., 2010).
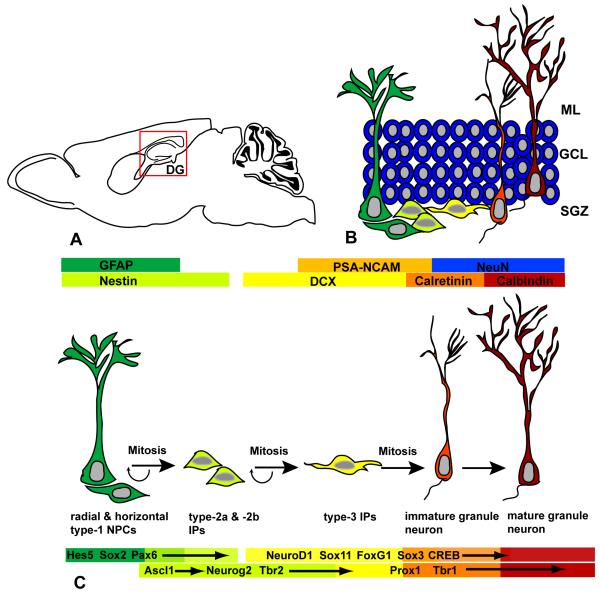
Figure 1.
Schematic diagram of neural progenitor cell development and transcription factor expression in the adult hippocampal dentate gyrus. A) Location of the dentate gyrus neurogenic niche in sagittal view. B) Neural progenitors are localized in the subgranular zone (SGZ) of the dentate gyrus. Within this zone, radial and horizontal type-1 cells (green) give rise to intermediate progenitors (IPs, yellow). IPs in turn divide to produce postmitotic granule neurons (orange), which are typically localized to the inner half of the granule cell layer (GCL). As these newborn neurons mature, the complexity of their dendritic arbors increases with multiple branch points apparent in the molecular layer (ML). C) Schematic diagram illustrating the progression of neural progenitor cell development and corresponding expression patterns of relevant transcription factors in the adult dentate gyrus.
The type-1 cells are thought to produce type 2 and 3 transit amplifying cells, that can be further subdivided into several developmental stages and appear to be responsible for the bulk of neuron production in the adult dentate gyrus (Fig. 1B, C) (Kempermann et al., 2004). Type-2a intermediate progenitors (IPs) express nestin (as determined by nestin-GFP transgene labeling), but do not express microtubule-associated Doublecortin (DCX). DCX expression is generally correlated with commitment of cells to a neuronal lineage and its expression is greatest in cells with a migratory phenotype. A second subclass of IPs, called type-2b cells, is characterized by expression of nestin-GFP and DCX, suggestive of commitment to a neuronal lineage. Both type-2a and –2b progenitors are small cells that exhibit very short tangential processes and tend to form clusters in the SGZ (Kempermann et al., 2004). Type-3 cells are IPs that remain mitotically active, but express further markers of neuronal differentiation such as PSA-NCAM, and do not express nestin. Given that expression of neuronal lineage markers increases with progression from type-2 to type-3 cells, it is likely that these cell types represent continuous steps in progenitor cell development rather than heterogeneous progenitor cell types within the neurogenic niche. However, the exact relationship between these different classes of intermediate stage progenitors remains to be determined.
Newly generated neuroblasts in the SGZ are characterized by transient expression of the calcium binding protein calretinin and upregulation of NeuN, a marker of postmitotic neuronal nuclei (Fig. 1B, C) (Kempermann et al., 2004). Calretinin expression is estimated to continue for approximately two weeks, after which time newborn neurons begin to express calbindin. It is estimated that the time required for new granule neurons to be generated and integrated into the existing hippocampal circuitry is in the range of four to seven weeks (Kempermann et al., 2004; Ehninger and Kempermann, 2008).
Expression and functions of transcription factors in the adult hippocampal SGZ
In order to determine the underlying molecular mechanisms controlling neuron production in the adult hippocampus, recent studies have focused on the roles that transcription factors play in regulating progenitor proliferation and differentiation. In general, the functions of transcription factors in adult hippocampal neurogenesis are not as well understood as the roles of transcription factors during embryonic neurogenesis. However, recent data suggests that transcription factor sequences expressed during the embryonic generation of neocortical glutamatergic neurons are part of a conserved program of neurogenesis responsible for the generation of glutamatergic neurons in the adult dentate gyrus. In addition, analyses of transcription factor expression that have helped to further understanding of the functions of neurogenesis in the adult dentate gyrus will be discussed.
Pax6
During embryonic generation of cortical glutamatergic neurons, Pax6, a paired domain and homeodomain-containing transcription factor, is expressed in radial glia (Götz et al., 1998; Englund et al., 2005). Radial glia act as the primary progenitor cells of the cerebral cortex, and divide to give rise to IPs that, in turn, are responsible for generating most cortical glutamatergic neurons (Hevner et al., 2006; Pontious et al., 2008). Current evidence suggests that Pax6 expression in the SGZ parallels its embryonic pattern of expression (Fig. 1C). Several studies have shown that Pax6 is expressed in cells with morphology typical of radial type-1 cells and have indicated coexpression with the type-1 cell markers GFAP, Glast and nestin (Maekawa et al., 2005; Nacher et al., 2005; Hodge et al., 2008; Roybon et al., 2009). Pax6 expression appears to persist to some extent in type-2 progenitors, as approximately 30% of Pax6-positive cells coexpress DCX, but Pax6 is not present in postmitotic neuroblasts (Nacher et al., 2005; Roybon et al., 2009). Analyses of heterozygous rSey2 Pax6 mutant mice support a role for Pax6 expression in the regulation of type-1 cell development. In these heterozygous Pax6-deficient mice, GFAP-positive cells are reduced in the SGZ, and GFAP-positive cells that persist in this zone exhibit decreased proliferation as evidenced by a reduction in GFAP/BrdU-positive cells (Maekawa et al., 2005).
T-box Transcription Factors
Tbr1 and Tbr2 are T-box transcription factors that are expressed in both the developing cerebral cortex and the adult hippocampus. Analyses in the embryonic cortex first demonstrated the expression of the T-box transcription factor Tbr2 in cortical IPs (Englund et al., 2005). Work from our laboratory and others has since shown that Tbr2 is likewise expressed specifically in IPs in the adult SGZ (Fig. 1B, C) (Hodge et al., 2008; Roybon et al., 2009). Tbr2-positive cells are localized to the SGZ, where they are often found in clusters and exhibit morphology typical of type-2/3 cells (Hodge et al., 2008; Roybon et al., 2009). A subset of Tbr2-positive cells colocalizes with Pax6, which parallels the partially overlapping expression pattern of these transcription factors observed during embryonic development (Englund et al., 2005; Hevner et al., 2006; Hodge et al., 2008; Roybon et al., 2009). Consistent with type-2 progenitor identity, we found that Tbr2-positive cells coexpressed nestin-GFP (Hodge et al., 2008). Additionally, Tbr2 expression likely persists into type-3 cells to some extent as Tbr2-positive cells often expressed low levels of DCX and PSA-NCAM (Hodge et al., 2008; Roybon et al., 2009). Interestingly, Tbr2-positive cells are dramatically increased in the dentate gyrus after voluntary wheel running, consistent with previous data suggesting that proliferation of type-2 cells is regulated by neurogenic stimuli (Hodge et al., 2008). Recent work suggests that Tbr2 is also particularly important for proper development of the dentate gyrus (Arnold et al., 2008). While the exact functions of Tbr2 in adult neurogenesis have not yet been determined, ongoing studies in our laboratory suggest that Tbr2 is required for proper production and maturation of newborn granule neurons in the adult dentate gyrus.
During embryonic cortical development, downregulation of Tbr2 as cells exit the cell cycle and differentiate into postmitotic neuroblasts coincides with upregulation of the T-box transcription factor Tbr1(Hevner et al., 2001; Englund et al., 2005). This expression pattern is recapitulated in the adult SGZ, where Tbr1 expression is particularly strong in newly generated neurons (Fig. 1C) (Hodge et al., 2008). Tbr1 does not colocalize with nestin-GFP or with markers of cell cycle activity, suggesting that expression of this transcription factor is largely confined to postmitotic cells (Hodge et al., 2008; Roybon et al., 2009). The functions of Tbr1 in regulating adult neurogenesis remain to be elucidated.
SRY-related HMG-box (Sox) transcription factors
One of the best studied transcription factors in adult hippocampal neurogenesis, Sox2, is a member of the SoxB1 subgroup (Sox 1-3) that is strongly expressed in both radial and horizontal type-1 cells within the SGZ, and colocalizes with the stem cell markers with GFAP, nestin, and BLBP (Fig. 1C) (Ferri et al., 2004; Komitova and Eriksson, 2004; Suh et al., 2007; Lugert et al., 2010). Expression of Sox2 is apparent in the majority of dividing cells in the SGZ, with some studies estimating that greater than 90% of PCNA-positive cells coexpress Sox2 (Lugert et al., 2010). Sox2 expression persists at least into type-2a cells, where it overlaps with the IP marker Tbr2 (Hodge et al., 2008). Rarely, Sox2-positive cells coexpress markers of neuronal fate commitment, such as PSA-NCAM and DCX (Ferri et al., 2004; Suh et al., 2007), indicating that Sox2 is downregulated as postmitotic neuroblasts are produced. Running has been shown to increase the proliferation of Sox2-positive cells without changing the overall density of this cell population, indicating that Sox2-positive progenitors are responsive to physiological stimuli (Suh et al., 2007). Evidence of a role for Sox2 in regulating neurogenesis in the dentate gyrus comes from studies of Sox2 null mutant, and conditional null mutant mice. Conditional null mutant Sox2 mice in which Sox2 is deleted during embryonic development exhibit almost complete absence of the dentate gyrus by P7 (Favaro et al., 2009). Similarly, conditional deletion of Sox2 in adult hippocampal NSCs results in a decline of nestin/GFAP-positive type-1 cells in the SGZ (Favaro et al., 2009), suggesting that Sox2 is required for NPC maintenance in the dentate gyrus. Sox2 may act to maintain the NPC pool in the dentate gyrus, at least in part, through regulation of Sonic hedgehog (Shh), which has been identified as a direct target of Sox2 (Favaro et al., 2009). Additionally, recent evidence suggests that Sox2 represses expression of NeuroD1, a bHLH transcription factor required for granule neuron differentiation (discussed below), and that this Sox2-dependent repression of NeuroD1 must be removed in order for neurogenesis to progress (Kuwabara et al., 2009). Sox2 expression itself appears to be regulated by Notch/RBPJκ signaling (Ehm et al., 2010). Specifically, Notch signaling has been shown to increase activity of the Sox2 promoter and increase Sox2 expression (Ehm et al., 2010).
Sox3, another member of the SoxB1 subgroup, exhibits a pattern of expression similar to that of DCX. Whereas Sox2 is expressed predominantly in type-1 cells, Sox3 expression is upregulated in intermediate stage progenitors and immature granule neurons (Wang et al., 2006). Sox3-positive cells colabel with acutely administered BrdU, indicating that at least a subset of these cells are actively proliferating. Most Sox3 positive cells coexpress PSA-NCAM and calretinin, markers of immature granule neurons, indicating that Sox3 expression persists to some extent in newly born neurons (Fig. 1C). Sox3-positive cells do not coexpress the mature neuronal marker NeuN, suggesting that this transcription factor is only involved in progenitor proliferation and early stages of neuron maturation (Wang et al., 2006).
Sox11 is a member of the SoxC subgroup (Sox4, Sox11, Sox12) whose expression has been characterized in the adult hippocampus (Haslinger et al., 2009). Within the adult dentate gyrus, Sox11 expression is restricted to DCX-positive type-3 cells and postmitotic neuroblasts (Fig. 1C). While the exact role of Sox11 in adult hippocampal neurogenesis remains unclear, overexpression of Sox11 has been shown to promote neuron generation from NPCs in vitro (Haslinger et al., 2009).
Basic-helix-loop-helix (bHLH) transcription factors
A number of bHLH family members appear to have transcriptional roles in controlling NPC development in the SGZ. Hes5, a read-out of canonical Notch signaling, is expressed in both quiescent and active type-1 cells exhibiting either radial or horizontal morphology (Lugert et al., 2010). Hes5 expression overlaps with that of Sox2 in many hippocampal NPCs (Fig. 1C) (Lugert et al., 2010). Recent work indicates that Hes5-positive cells are responsive to exercise, exhibiting increased proliferation after running (Lugert et al., 2010). Similarly, seizures have been shown to increase the proliferation of Hes5-positive type-1 cells and to increase the density of these cells in the SGZ (Lugert et al., 2010). Ascl1 (also called Mash1), is expressed predominantly in type-2a NPCs in the SGZ. Ascl1 expression can also be detected in some type-1 cells where it colocalizes with Sox2 (Kim et al., 2007). Ascl1 colocalizes with a small percentage of Tbr2-positive cells, but does not coexpress markers of type-3 progenitors or postmitotic neuroblasts (Kim et al., 2007; Hodge et al., 2008; Roybon et al., 2009). Lineage tracing experiments using Ascl1-CreER™ mice suggest that Ascl1-postive NPCs give rise predominantly to granule neurons in the dentate gyrus (Kim et al., 2007). However, retroviral overexpression of Ascl1 has been shown to redirect the fate of newborn cells from granule neurons to oligodendrocytes, which populate the hilus of the dentate gyrus (Jessberger et al., 2008), indicating that the quantitative level of Ascl1 expression may be an important determinant of its in vivo functions in the dentate gyrus.
The transition from type-2a to type 2b progenitors is marked by downregulation of Ascl1 and upregulation of another bHLH transcription factor, Neurogenin-2 (Neurog2) (Roybon et al., 2009). Expression of Neurog2 is initiated in late type-2a progenitors, where colocalization with Pax6 and nestin is apparent (Ozen et al., 2007; Hodge et al., 2008; Roybon et al., 2009). Transition to the type-2b phase is marked by coexpression of Tbr2 (Hodge et al., 2008; Roybon et al., 2009). Neurog2 protein does not typically colocalize with DCX or other markers of neuronal commitment, indicating downregulation of this transcription factor as IPs transition to the type-3 stage (Fig. 1C). Many Neurog2-positive cells colocalize with Ki67 and acutely administered BrdU, confirming expression of Neurog2 in rapidly dividing progenitor populations (Ozen et al., 2007; Roybon et al., 2009). Phenotypes in Neurog2 null mutant mice support a role for Neurog2 in postnatal neurogenesis in the dentate gyrus. These animals exhibit decreased greatly reduced cell proliferation and a failure to form the infrapyramidal blade of the dentate gyrus (Ozen et al., 2007; Roybon et al., 2009). Conversely, retroviral overexpression of Neurog2 produces mostly neuronal progeny, indicating that Neurog2 expression is involved in granule neuron specification in the dentate gyrus (Roybon et al., 2009).
As IPs become committed to a neuronal lineage in the type-2b and type-3 stage of development, expression of the basic-helix-loop-helix (bHLH) transcription factor NeuroD1 (also known as NeuroD and BETA2) is upregulated (Fig. 1C). NeuroD1-positive cells are localized to the SGZ and inner granule cell layer, and studies suggest that at least some of these cells are mitotically active progenitors (Lee et al., 2000; Seki, 2002; Gao et al., 2009). The majority of NeuroD1-positive cells coexpress PSA-NCAM and DCX, suggesting that they are committed to a neuronal fate (Seki, 2002; Hodge et al., 2008; Roybon et al., 2009). NeuroD1 is coexpressed with Tbr2 in many type-3 progenitor cells (Hodge et al., 2008); interestingly, this pattern of expression closely parallels that seen in the embryonic cortex where NeuroD1 and Tbr2 are coexpressed in terminally dividing IPs in the cortical subventricular zone (Englund et al., 2005; Hevner et al., 2006). However, PSA-NCAM-positive cells with well developed dendrites and morphology typical of immature neurons express NeuroD1 weakly or not at all, indicating that NeuroD1 expression is downregulated as new granule neurons mature (Seki, 2002; Seri et al., 2004; Gao et al., 2009). Studies of several mutant mouse models indicate a role for NeuroD1 in controlling neuroblast differentiation in the SGZ. In most mouse strains, NeuroD1 null mutants die several days after birth, limiting their usefulness in studying adult neurogenesis. However, these mice lack a well demarcated dentate gyrus as early as postnatal day (P)0 and P2 (Schwab et al., 2000). Slice cultures harvested from NeuroD1 null mice develop normally in vitro, but fail to form a distinct dentate gyrus (Schwab et al., 2000). When backcrossed onto certain mouse strains, the viability of NeuroD1 null mutants increases, allowing for studies of dentate gyrus development during later postnatal stages (Liu et al., 2000). These studies confirm that NeuroD1 null mice fail to develop an organized dentate gyrus, and exhibit only a disorganized cap of cells with some granule cell features such as expression of calbindin (Liu et al., 2000). Early GFAP-positive type-1 progenitor populations appear to be present in null mice, suggesting that intermediate stage progenitors fail to differentiate properly in the absence of NeuroD1 expression (Del Turco et al., 2004). Intriguingly, a small number of mature granule neurons are present within the disorganized dentate gyrus and exhibit normal afferent and efferent connectivity patterns, suggesting that NeuroD1 is not required for proper target innervation (Del Turco et al., 2004). More recently, studies of conditional NeuroD1 knockout mice have revealed a critical role for this transcription factor in adult neurogenesis (Gao et al., 2009). Conditional deletion of NeuroD1 in the adult dentate gyrus results in loss of newborn granule neurons due to failure of these cells to survive and integrate into existing circuitry (Gao et al., 2009). This loss of newborn granule neurons appears to occur without an effect on progenitor cell numbers in the SGZ (Gao et al., 2009). Recent evidence suggests that upregulation of NeuroD1, which is required for granule neuron differentiation, is mediated by Wnt/β-catenin transcriptional activation of NeuroD1 and removal of Sox2 repression on the NeuroD1 promoter (Kuwabara et al., 2009).
NPAS3, a brain enriched bHLH PAS domain transcription factor, also appears to be involved in regulating progenitor cell development in the adult hippocampus. Studies of NPAS3 null mutant mice suggest that progenitor cell proliferation in the dentate gyrus is disrupted in the absence of expression of this transcription factor (Pieper et al., 2005). Counts of BrdU-positive cells in the NPAS3 null mutant granule cell layer show that proliferation is reduced to 84% of control levels in mutant mice. Correspondingly, the thickness of the granule cell layer is reduced in mutant mice, indicating failure to produce a full complement of new granule cells. NPAS3 expression may be related to the pathophysiology of Schizophrenia as translocation of the Npas3 gene has been described in a family with Schizophrenia present across several generations (Pieper et al., 2005).
Forkhead transcription factors
Expression patterns of FoxG1 (formerly called BF-1), a forkhead transcription factor expressed in the telencephalon during early development, suggest that this transcription factor is also expressed in IPs (Fig. 1C). FoxG1-positive cells are restricted to the SGZ of the dentate gyrus, and are often present in clusters typical of type-2/3 cells (Shen et al., 2006). Analyses of Foxg1 mutant mice indicate reductions in hippocampal volume, dentate gyrus volume, and granule cell number in heterozygous mutants (Shen et al., 2006; Eagleson et al., 2007). BrdU labeling analyses in adult animals reveal reduced progenitor proliferation in the SGZ and decreased production and differentiation of immature granule neurons in mutant mice. Additionally, newly born PSA-NCAM-positive cells do not extend fully formed dendrites into the granule cell layer and calretinin expression is decreased in mutant animals (Shen et al., 2006). Early embryonic development of the dentate gyrus proceeds normally in FoxG1 heterozygous mutants, suggesting that FoxG1 is specifically required for directing progenitor differentiation in the adult SGZ. These mice also offer insight into the function of adult neurogenesis as they exhibit abnormal contextual fear responses and hyperactivity (Shen et al., 2006). Previous studies have shown that normal hippocampal activity is required for appropriate contextual fear conditioning, and the results from FoxG1 mutant mice suggest that functional adult neurogenesis may be required for this process (Shen et al., 2006). Similarly, FoxG1 heterozygous mutant mice do not show a response to antidepressant treatment, which may be due to deficiencies in adult neurogenesis (Kinsler et al., 2010).
Prox1
Prox1, a homeobox transcription factor homologous to the Drosophila melanogaster gene prospero, is upregulated in type-3 progenitor cells and expression of this transcription factor is maintained in all granule neurons in the dentate gyrus (Fig. 1C) (Lavado and Oliver, 2007). The functions of Prox1 in regulating granule cell maturation have only recently been described (Lavado et al., 2010). Conditional Prox1 knockout mice reveal that Prox1 expression is necessary for formation of the dentate gyrus as the infrapyramidal blade fails to form in these mice and the size of the suprapyramidal blade is reduced. Additionally, postnatal deletion of Prox1 expression results in reduced numbers of DCX-positive and Calretinin-positive new granule neurons in the dentate gyrus (Lavado et al., 2010). Interestingly, Prox1 conditional knockout mice also display decreased numbers of IPs and transiently increased numbers of type-1 NPCs (Lavado et al., 2010). This disruption of NPC maintenance in Prox1 conditional knockouts may be due to disregulation of Notch signaling as illustrated by decreased Jagged1 expression in these mice (Lavado et al., 2010).
CREB (Creb1)
The transcription factor cAMP response-element binding protein (Creb1, CREB) is a member of the CREB transcription factor family that acts in conjunction with its transcriptional coactivators p300/CBP/Crebbp as an intracellular effector that has been implicated in regulating neuronal survival and plasticity (Lonze and Ginty, 2002; Nakagawa et al., 2002a, b). CREB is activated via phosphorylation (pCREB) and this activated form is expressed predominantly in immature neurons in the adult dentate gyrus, where it colocalizes with DCX and calretinin (Nakagawa et al., 2002a, b; Jagasia et al., 2009). Pharmacological activation of the CREB signaling cascade has been shown to increase proliferation in adult hippocampal progenitor cells (Nakagawa et al., 2002a). However, inhibition of CREB signaling primarily in immature neuroblasts does not appear to alter progenitor proliferation (Jagasia et al., 2009). Several studies indicate that CREB signaling has a role in regulating morphological development and differentiation of newborn granule neurons (Fujioka et al., 2004; Jagasia et al., 2009). Increased CREB activity has been shown to enhance dendrite length and increase dendritic branching, whereas cell autonomous loss of CREB function has been shown to decrease dendritic branching and expression of DCX and NeuroD1 (Fujioka et al., 2004; Jagasia et al., 2009). Moreover, loss of CREB function reduces the survival of newborn neurons in the adult dentate gyrus (Jagasia et al., 2009). Interestingly, GABA-mediated excitation increases CREB activation, whereas inhibition of GABA-mediated excitation results in loss of pCREB from newborn neurons and recapitulates many of the morphological defects seen with CREB loss of function (Jagasia et al., 2009), suggesting that regulation of CREB is activity dependent. Indeed, forced CREB signaling in cells deficient for NKCC1, a cotransporter required for GABA-mediated depolarization, rescues many of the morphological defects observed after loss of GABA-mediated excitation (Jagasia et al., 2009). Taken together, these results suggest that CREB signaling has a crucial role in regulating neuronal maturation in the adult dentate gyrus.
Summary
The above results suggest that the sequence of transcription factors expressed during development of SGZ progenitor cells precisely recapitulates many aspects of embryonic neocortical neurogenesis, and suggest a role for a diverse array of transcription factors in controlling hippocampal neurogenesis. Specifically, recent studies indicate that sequential expression of Pax6 → Neurog2 → Tbr2 → NeuroD1 → Tbr1 in progenitor and post-mitotic stages of differentiation is important for the production of glutamatergic neurons in the hippocampus and neocortex. In addition, the roles of several other important transcription factors in regulating the balance between NPC maintenance and granule neuron production are currently being delineated. However, the study of transcription factor expression and function specifically in the adult hippocampus is currently in its infancy, and will no doubt be the subject of much future research on adult hippocampal neurogenesis.
Table 1.
Summary of transcription factor expression in the dentate gyrus.
| Transcription Factor |
Expression Pattern |
Type of Study and Stage of Development |
References |
|---|---|---|---|
| Pax6 | Type-1 and type-2a cells |
Expression (postnatal and adult) Loss of function (postnatal) |
Maekawa et al., 2005
Nacher et al., 2005 Hodge et al., 2008 Roybon et al., 2009 |
| Tbr2 | Type-2b and type-3 cells |
Expression (adult) Loss of function (embryonic conditional null mutant) |
Arnold et al., 2008
Hodge et al., 2008 Roybon et al., 2009 |
| Tbr1 | Immature neurons and mature neurons |
Expression (adult) |
Hodge et al., 2008
Roybon et al., 2009 |
| Sox2 | Type-1 and type-2a cells |
Expression (adult) Loss of Function (embryonic null mutant) Loss of function (adult inducible conditional knockout) |
Eriksson, 2004 Ferri et al., 2004 Komitova and Suh et al., 2007 Favaro et al., 2009 Kuwabara et al., 2009 Ehm et al., 2010 Lugert et al., 2010 |
| Sox3 | Type-3 cells and immature neurons |
Expression (adult) | Wang et al., 2006 |
| Sox11 | Type-3 cells and immature neurons |
Expression (adult) Gain of function (in vitro overexpression) |
Haslinger et al., 2011 |
| Hes5 | Type-1 cells | Expression (adult) | Lugert et al., 2010 |
| Ascl1 | Type-1 and type-2a cells |
Expression (adult) Lineage tracing (adult) Gain of function (retroviral overexpression in vivo) |
Kim et al., 2007
Hodge et al., 2008 Roybon et al., 2009 |
| Neurog2 | Type-2a and type- 2b cells |
Expression (postnatal, adult) Loss of function (postnatal) Gain of function (postnatal retroviral overexpression) |
Ozen et al., 2007
Hodge et al., 2008 Roybon et al., 2009 |
| NeuroD1 | Type-2b, type-3, and immature neurons |
Expression (postnatal, adult) Loss of function (embryonic null mutant) Loss of function (adult conditional knockout) |
Lee et al., 2000
Liu et al., 2000 Schwab et al., 2000 Seki, 2002 Del Turco et al., 2004 Seri et al., 2004 Hodge et al., 2008 Gao et al., 2009 Kuwabara et al., 2009 Roybon et al., 2009 |
| NPAS3 | Not determined | Loss of function (embryonic null mutant) |
Pieper et al., 2005 |
| FoxG1 | Type-2 and type-3 cells |
Loss of function (postnatal) |
Shen et al., 2006
Eagleson et al., 2007 |
| Prox1 | Type-3 cells, immature and mature neurons |
Expression (postnatal, adult) Loss of function (postnatal and adult conditional knockout) |
Lavado and Oliver, 2007
Lavado et al., 2010 |
| CREB | Immature neurons | Expression (adult) Loss of function (adult) Gain of function (adult) |
Nakagawa et al., 2002a, b Fujioka et al., 2004 Jagasia et al., 2009 |
Acknowledgements
Research in the Hevner lab was supported by NIH R01 MH080766. R.D.H is the recipient of a postdoctoral training fellowship from the American Heart Association (10POST2610067).
References
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Huang G-J, Cheung AFP, Era T, Nishikawa S-I, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A Functional Role for Adult Hippocampal Neurogenesis in Spatial Pattern Separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Turco D, Gebhardt C, Burbach GJ, Pleasure SJ, Lowenstein DH, Deller T. Laminar organization of the mouse dentate gyrus: insights from BETA2/Neuro D mutant mice. J Comp Neurol. 2004;477:81–95. doi: 10.1002/cne.20239. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-Born Hippocampal Dentate Granule Cells Undergoing Maturation Modulate Learning and Memory in the Brain. Journal of Neuroscience. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Schlueter McFadyen-Ketchum LJ, Ahrens ET, Mills PH, Does MD, Nickols J, Levitt P. Disruption of Foxg1 expression by knock-in of cre recombinase: effects on the development of the mouse telencephalon. Neuroscience. 2007;148:385–99. doi: 10.1016/j.neuroscience.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisen J, Lie DC. RBPJ-Dependent Signaling Is Essential for Long-Term Maintenance of Neural Stem Cells in the Adult Hippocampus. Journal of Neuroscience. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RAM, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri ALM, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis SK. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Ferri ALM, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Duman RS. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci. 2004;24:319–328. doi: 10.1523/JNEUROSCI.1065.03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave K-A, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Haslinger A, Schwarz TJ, Covic M, Chichung Lie D. Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci. 2009;29:2103–2114. doi: 10.1111/j.1460-9568.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RAM, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hodge RD, Kowalczyk T, Wolf S, Encinas J, Rippey C, Enikolopov G, Kempermann G, Hevner RF. Intermediate Progenitors in Adult Hippocampal Neurogenesis: Tbr2 Expression and Coordinate Regulation of Neuronal Output. J Neurosci. 2008;28:3707. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;6 doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsler R, Taylor MM, Flores NM, Leffert JJ, Beech RD. Altered response to antidepressant treatment in FoxG1 heterozygous knockout mice. Synapse. 2010;64:169–171. doi: 10.1002/syn.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Chow LML, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. Plos Biol. 2010;8 doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Oliver G. Prox1 expression patterns in the developing and adult murine brain. Developmental Dynamics. 2007;236:518–524. doi: 10.1002/dvdy.21024. [DOI] [PubMed] [Google Scholar]
- Lee JK, Cho JH, Hwang WS, Lee YD, Reu DS, Suh-Kim H. Expression of neuroD/BETA2 in mitotic and postmitotic neuronal cells during the development of nervous system. Dev Dyn. 2000;217:361–367. doi: 10.1002/(SICI)1097-0177(200004)217:4<361::AID-DVDY3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming G-l, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, Tsai MJ, Lowenstein DH. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci USA. 2000;97:865–870. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Takashima N, Arai Y, Nomura T, Inokuchi K, Yuasa S, Osumi N. Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells. 2005;10:1001–1014. doi: 10.1111/j.1365-2443.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Emsley JG, Magavi SSP, Arlotta P, Macklis JD. Constitutive and induced neurogenesis in the adult mammalian brain: manipulation of endogenous precursors toward CNS repair. Dev Neurosci. 2004;26:101–117. doi: 10.1159/000082131. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Blasco-Ibañez JM, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res. 2005;81:753–761. doi: 10.1002/jnr.20596. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002a;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002b;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen I, Galichet C, Watts C, Parras C, Guillemot F, Raineteau O. Proliferating neuronal progenitors in the postnatal hippocampus transiently express the proneural gene Ngn2. Eur J Neurosci. 2007;25:2591–2603. doi: 10.1111/j.1460-9568.2007.05541.x. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, McKnight SL. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proceedings of the National Academy of Sciences. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of Intermediate Progenitor Cells in Cerebral Cortex Development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Roybon L, Hjalt T, Stott S, Guillemot F, Li J-Y, Brundin P, Reh TA. Neurogenin2 Directs Granule Neuroblast Production and Amplification while NeuroD1 Specifies Neuronal Fate during Hippocampal Neurogenesis. PLoS ONE. 2009;4:e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab MH, Bartholomae A, Heimrich B, Feldmeyer D, Druffel-Augustin S, Goebbels S, Naya FJ, Zhao S, Frotscher M, Tsai MJ, Nave KA. Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. J Neurosci. 2000;20:3714–3724. doi: 10.1523/JNEUROSCI.20-10-03714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J Neurosci Res. 2002;70:327–334. doi: 10.1002/jnr.10387. [DOI] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Shen L, Nam H-S, Song P, Moore H, Anderson SA. FoxG1 haploinsufficiency results in impaired neurogenesis in the postnatal hippocampus and contextual memory deficits. Hippocampus. 2006;16:875–890. doi: 10.1002/hipo.20218. [DOI] [PubMed] [Google Scholar]
- Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J Neurosci. 2005;25:10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-W, Stromberg GP, Whitney JT, Brower NW, Klymkowsky MW, Parent JM. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. The Journal of Comparative Neurology. 2006;497:88–100. doi: 10.1002/cne.20984. [DOI] [PubMed] [Google Scholar]