Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants often present in aquatic systems as complex mixtures. Embryonic fish are sensitive to the developmental toxicity of some PAHs, but the exact mechanisms involved in this toxicity are still unknown. This study explored the role of the aryl hydrocarbon receptor (AHR) in the oxidative stress response of zebrafish to the embryotoxicity of select PAHs. Embryos were exposed to two PAHs, benzo[k]fluoranthene (BkF; a strong AHR agonist) and fluoranthene (FL; a cytochrome P4501A (CYP1A) inhibitor), alone and in combination. CYP1A, CYP1B1, CYP1C1, and redox-responsive genes glutathione s-transferase pi 2 (GSTp2), glutathione peroxidase 1 (GPx1), the glutamate-cysteine ligase catalytic subunit (GCLc), MnSOD and CuZnSOD mRNA expression was examined. CYP1 activity was measured via an in vivo ethoxyresorufin-O-deethlyase (EROD) activity assay, and the area of the pericardium was measured as an index of cardiotoxicity. BkF or FL alone caused no deformities whereas BkF + FL resulted in extreme pericardial effusion. BkF induced CYP activity above controls and co-exposure with FL inhibited this activity. BkF induced expression of all three CYPs, GSTp2, and GCLc. BkF + FL caused greater than additive induction of the three CYPs, GSTp2, GPx1, and GCLc but had no effect on MnSOD or CuZnSOD. AHR2 knockdown protected against the cardiac deformities caused by BkF + FL and significantly inhibited the induction of the CYPs, GSTp2, GPx1 and GCLc after BkF + FL compared to non-injected controls. These results further show the protective role of AHR2 knockdown against cardiotoxic PAHs and the role of AHR2 as a mediator of redox-responsive gene induction.
Keywords: zebrafish, polycyclic aromatic hydrocarbons (PAHs), oxidative stress, morpholino, aryl hydrocarbon receptor (AHR)
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are widespread environmental contaminants that are produced predominantly by anthropogenic activities via the incomplete combustion of petroleum products and are also components of crude oil. PAH levels have been increasing in the environment over the last two decades in association with increased urban sprawl and increased use of automobiles (Lima et al., 2003; Van Metre and Mahler, 2005). PAHs also enter into the environment during oil spills such as the Cosco Busan oil spill in the San Francisco Bay in November 2007 and the more recent Deepwater Horizon oil spill in the Gulf of Mexico in the spring and summer of 2010, and PAHs from oil spills may persist in aquatic ecosystems for decades (Short et al., 2004; Diercks et al., 2010; Lemkau et al., 2010). As PAHs have increased in aquatic ecosystems, their effects on fish have begun to receive greater attention. Some PAHs, as well as coplanar polychlorinated biphenyls (PCBs) and dioxins, elicit a range of developmental toxicities, including cardiac deformities, in various fish species (Incardona et al., 2004; Wassenberg and Di Giulio, 2004; Scott and Hodson, 2008). The developmental cardiac toxicity caused by some PAHs, coplanar PCBs, and dioxins is mediated through the aryl hydrocarbon receptor (AHR) (Schmidt and Bradfield, 1996). Upon ligand-activated binding by an AHR agonist, the AHR dissociates from chaperone proteins in the cytoplasm and is translocated into the nucleus. Once in the nucleus, the AHR dimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT) and binds to xenobiotic response elements (XREs), causing the upregulation of numerous phase I and II metabolic enzymes, including the cytochrome P450 1 (CYP1) gene family, glutathione s-transferases (GSTs), and NADP(H):oxidoreductase (Nebert et al., 2000; Denison and Nagy, 2003).
Zebrafish (Danio rerio) have three characterized AHRs (Tanguay et al., 1999; Karchner et al., 2005), and knockdown of the AHR2 protects against the developmental toxicity of 2,3,7,8-tetrachlorodibenzodioxin (TCDD) (Teraoka et al., 2003), certain low molecular weight PAHs (Incardona et al., 2006), and the model PAHs β-naphthoflavone (BNF) and α-naphthoflavone (ANF) (Billiard et al., 2006). AHR2 knockdown has also been shown to protect against the cardiac toxicity of BNF, 3,3′,4,4′,5-pentachlorobiphenyl (PCB-126), and the PAH benzo[k]fluoranthene (BkF) in killifish, Fundulus heteroclitus (Clark et al., 2010). While exposure of fish to certain individual AHR agonists can induce cardiac deformities, co-exposure of an AHR agonist and a CYP inhibitor results in severe, synergistic toxicity (Wassenberg and Di Giulio, 2004; Billiard et al., 2006). However, the exact molecular mechanisms and cell signaling pathways responsible for PAH-induced developmental toxicity in fish remain unclear.
One possible mechanism by which PAHs elicit toxicity is through the induction of oxidative stress. The formation of reactive oxygen species (ROS) is essential for normal cell function; however, ROS production in excess of levels that cellular enzymes are able to neutralize can lead to oxidative stress and cellular damage (Di Giulio and Meyer, 2008). PAHs can lead to the overproduction of ROS in multiple ways. PAHs may be enzymatically, auto- or photo-metabolized into reactive o-quinone metabolites that produce ROS via redox cycling (Penning et al., 1996; Li et al., 2003). Furthermore, PAH metabolites may also act to increase uncoupling of the mitochondrial electron transport chain and uncoupling of CYP redox reactions (Winston and Di Giulio, 1991; Livingstone, 2001).
Various studies indicate that the toxicity caused by some PAHs, coplanar PCBs, and dioxins may involve oxidative stress. TCDD has been shown to induce H2O2 production in mice mitochondria, an effect that was dependent upon TCDD binding to the AHR (Senft et al., 2002). In scup (Stenotomus chrysops), PCB-126 has been shown to induce ROS production in liver microsomes and cause increased levels of catalase, glutathione peroxidase, glutathione reductase, and superoxide dismutase activities in the liver (Schlezinger and Stegeman, 2001). In addition, killifish from a creosote-contaminated Superfund site have been shown to have elevated hepatic total glutathione levels (Bacanskas et al., 2004), and their F1 progeny were resistant to the acute toxicity of the model pro-oxidant tert-butyl hydroperoxide (Meyer et al., 2003). The F1 progeny also exhibited higher manganese superoxide dismutase (MnSOD) protein levels, glutathione concentrations, and total oxy-radical scavenging capacity as compared to reference site F1 fish. Furthermore, BNF + ANF co-exposure in zebrafish has been shown to cause an upregulation of various redox-responsive genes (Timme-Laragy et al., 2009).
In this study, we examined the cardiac deformities and phase I and redox-responsive gene expression changes caused by two environmentally relevant PAHs, BkF and fluoranthene (FL), individually and in combination. BkF is a strong AHR agonist (Billiard et al., 2002), and FL is a CYP1 enzyme inhibitor (Willett et al., 2001). We then examined the affect of AHR2 knockdown on cardiac deformities and gene expression induced by PAH exposure.
Materials and Methods
Fish Care
Adult Ekkwill zebrafish (Danio rerio; Ekkwill Waterlife Resources, Ruskin, FL, USA) were maintained at 28°C in a recirculating AHAB system (Aquatic Habitats, Apopka, FL, USA) under a 14:10 h light:dark cycle. Adults were fed brine shrimp and a mix of Ziegler’s Adult Zebrafish Complete Diet (Aquatic Habitats) and Cyclop-eeze (Argent Chemical Laboratories, WA, USA). Embryos were collected after natural spawning of adult zebrafish and were maintained in 30% Danieau (Nasevicius and Ekker, 2000) under the same temperature and photoperiod as adults. Adult care and reproductive techniques were non-invasive and approved by the Duke University Institutional Animal Care & Use Committee (A279–08–10).
Chemicals and Exposure
Benzo[k]fluoranthene (BkF) and fluoranthene (FL) standards were purchased from AccuStandard (Hamden, CT, USA). Dimethyl sulfoxide (DMSO), 7-ethoxyresorufin (7-ER), and tricaine methanesulfonate (MS-222) were purchased from Sigma-Aldrich (St. Louis, MO). BkF, FL, and 7-ER were dissolved in DMSO, protected from light, and kept at −20°C until use.
At 24 hours post fertilization (hpf), embryos exhibiting normal development were dosed in 7.5 mL 30% Danieau in 20-mL glass scintillation vials, with five embryos per vial and three vials per treatment. Embryos were dosed with DMSO, 50 μg/L BkF, μ150 g/L FL, or a co-exposure of 50 μg/L BkF + μ150 g/L FL. Final DMSO concentration was < 0.03%. For experiments examining CYP1 activity via the 7-ethoxyresorufin-O-deethylase (EROD) assay, 7-ER was added at a final concentration of 21 g/L at the time of PAH dosing. Deformity assessment and EROD assay were performed at 96 hpf. For gene expression experiments, six vials per treatment with five embryos per vial were dosed with either DMSO, BkF, FL, or BkF + FL at 24 hpf. At 48 hpf, embryos were dechorionated, pooled in groups of 10 (two vials), and fixed in RNAlater® (Applied Biosystems, Foster City, CA, USA). Samples were stored at −80°C until RNA extraction.
Morpholino Injection
A previously designed morpholino (Teraoka et al., 2003) shown to block the translation of zebrafish aryl hydrocarbon receptor 2 (AHR2-mo) was purchased from Gene Tools, LLC (Philomath, OR, USA). The sequence of the AHR2-mo is 5′-TGTACCGATACCCGCCGACATGGTT-3′. Gene Tools’ standard control morpholino (5′-CCTCTTACCTCAGTTACAATTTATA-3′) was used as a morpholino injection control. The morpholinos were fluorescein-tagged at the 3′ end to monitor injection success and were diluted to 100 μM working stocks in 30% Danieau.
The morpholinos (approximately 3 nL) were injected by hand into the yolk of zebrafish embryos at the 1–4 cell stage using a microinjection system consisting of a Nikon SMZ-1500 zoom stereomicroscope (Nikon Instruments Inc., Lewisville, TX, USA) and an MDI PM 1000 Cell Microinjector (MicroData Instrument Inc., S. Plainfield, NJ, USA). Embryos exhibiting normal development and strong, uniform incorporation of the morpholinos were used for experiments.
Deformity Assessment
At 96 hpf, hatched zebrafish embryos were screened for cardiac deformities via measurement of pericardial effusion (Billiard et al., 2006). Fish were rinsed with 30% Danieau and anesthetized with MS-222. Fish were then placed in a left lateral orientation in 3% methylcellulose on depression slides and were imaged under 50x magnification (Zeiss Axioskop, Thornwood, NY, USA). The two-dimensional image of the pericardial area was manually traced and then quantified using IPLab software (Scanalytics Inc., Fairfax, VA, USA). Deformity values are expressed as a percentage of the two-dimensional pericardial area of non-injected (NI) control embryos.
In vivo EROD assay
At the same time as deformity assessment (96 hpf), CYP1 activity was measured via a modified in vivo EROD assay (Nacci et al., 1998; Billiard et al., 2006; Matson et al., 2008b). The CYP1 enzymes metabolize 7-ER into a fluorescent product, resorufin, which accumulates in the gastrointestinal tract in zebrafish (Billiard et al., 2006). Fluorescence was measured under 50× magnification using a rhodamine red filter set (Zeiss Axioskop) and quantified by IPLab software (Scanalytics Inc.). EROD values are expressed as a percentage of the mean fluorescence of NI control embryos.
RNA Extraction and Reverse Transcription
Samples were homogenized with a sterile hand-held homogenizer for 30 s, and RNA was extracted according to the RNA-Bee protocol (Tel-Test Inc., Friendswood, TX, USA). RNA quantity was analyzed spectrophotometrically using a NanoDrop ND-100 (NanoDrop Technologies, Wilmington, DE, USA). cDNA was synthesized using the Omniscript Reverse Transcriptase kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer’s instructions with 500 ng RNA, random hexamers, and RNaseOut (Invitrogen, Carlsbad, CA, USA). The reverse transcription reaction was performed in a thermocycler for 1 hour at 37°C, and the resulting cDNA was diluted to a concentration of 2 ng/μL.
QPCR
The expression of the following genes was examined: CYP1A, CYP1B1, CYP1C1, pi class of glutathione s-transferase (GSTp2), the catalytic subunit of glutamate cysteine ligase (GCLc), glutathione peroxidase 1 (GPx1), manganese superoxide dismutase (MnSOD), and copper zinc superoxide dismutase (CuZnSOD). β-actin was used as a housekeeping gene. CYP1A, CYP1B1, and CYP1C1 primers were published previously (Timme-Laragy et al., 2007). GPx1, MnSOD, and CuZnSOD primers were also published previously (Malek et al., 2004). Primers for GCLc and GSTp2 were designed using PrimerQuest software (Integrated DNA Technologies, Inc., www.idtdna.com). β-actin primers were supplied by Kyle Erwin; the forward primer was published previously (Grimes et al., 2008) and the reverse was designed using PrimerQuest software. Primer sequences are listed in Table 1.
Table 1.
Primers and GenBank Accession Numbers for QRT-PCR
Real-time QPCR primers.
| Gene | GenBank ID | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|---|
| β–actin | AF057040 | AAGATCAAGATCATTGCTCC | CCAGACTCATCGTACTCCT |
| CYP1A | NM_131879 | AGGACAACATCAGACACATCACCG | GATAGACAACCGCCCAGGACAGAG |
| CYP1B1 | NM_001013267 | CCACCCGAACTCTGAAACTC | AAACACACCATCAGCGACAG |
| CYP1C1 | NM_001020610 | TGGAGGCTGAGTTTGGACTGAAGA | GAGGAAGAAGAGGATGACGAAGGATG |
| GSTp2 | AB194128 | TCTGGACTCTTTCCCGTCTCTCAA | ATTCACTGTTTGCCGTTGCCGT |
| GCLc | NM_199277 | AAGTGGATGAGGGAGTTTGTTGCC | CTTGTGGAGCAGGTCGTAGTTGAT |
| GPx1 | AW232474 | AGATGTCATTCCTGCACACG | AAGGAGAAGCTTCCTCAGCC |
| MnSOD | AW07696 | CTAGCCCGCTGACATTACATC | TTGCCCACATAGAAATGCAC |
| CuZnSOD | Y12236 | CGCATGTTCCCAGACATCTA | GAGCGGAAGATTGAGGATTG |
Each 25 μL QPCR reaction consisted of 12.5 μL SYBR Green PCR Master Mix (Applied Biosystems), 9.5 μL dH2O, 200 nM forward and reverse primer, and 2 μL cDNA template. The reaction was carried out using an Applied Biosystems 7300 Real-Time PCR System with the following thermal profile: 10 min at 95°C and 40 replicates of 15 s at 95°C, 1 min at 60°C. A dissociation curve was calculated for each sample at the end of each profile. All samples were run in duplicate and technical replicates were averaged prior to analysis.
The ABI PRISM 7300 Sequence Detection System Software, Version 1.1 (Applied Biosystems) was used to carry out data analysis. The average mRNA fold induction of each target gene was calculated by comparing the CT (threshold cycle) of the target gene to that of β-actin according to Livak and Schmittgen (2001).β-actin expression remained constant across treatments. Each experiment consisted of three biological replicates (10 pooled embryos) per treatment and each experiment was replicated at least three times for a final n = 9–12.
Statistical Analysis
Statistical analyses were performed using JMP 8.1.1 (SAS Institute Inc., Cary, NC, USA). For deformity and EROD analysis of non-injected (NI) experiments, data were analyzed via one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. For morpholino experiments, the data were analyzed via two-way ANOVA to determine an overall effect of the morpholino injection and dose followed by least square means (LSMeans) procedures. Tukey’s post-hoc test was used to determine differences between groups. All experiments were replicated at least three times with at least three samples per treatment per experiment, and no differences between experimental replicates were observed for any test. Data are represented as mean ± standard error of the mean (SEM). Values were considered significantly different at p ≤0.05.
Results
Deformities and EROD activity
Individually, 50 μg/L BkF and 150 μg/L FL did not cause cardiac deformities in zebrafish embryos; embryos exposed to BkF had an average pericardial area of 97±2% and those exposed to FL had an average pericardial area of 105±2% of control pericardial area (Fig. 1). However, exposure to BkF + FL caused significant pericardial effusion (226±11%; p < 0.0001) compared to DMSO controls.
Figure 1.
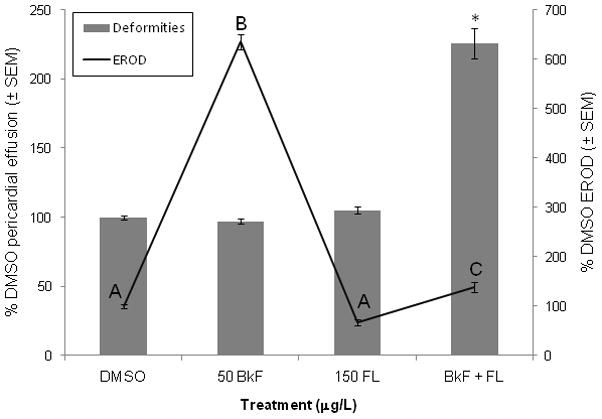
Effect of PAHs on deformities and EROD induction. Embryos were dosed at 24 hpf and scored at 96 hpf. Deformities are represented by bars, and scale is along the left y-axis; values are expressed as percent control (DMSO) pericardial effusion ± SEM (n = 12 per treatment; each n represents the average of five embryos). EROD values are represented by lines, and scale is along the right y-axis; values are expressed as percent DMSO EROD ± SEM (n = 9 per treatment). For EROD comparisons, groups not sharing a common letter are significantly different (p ≤0.05; ANOVA, Tukey adjusted LSMeans). For deformity comparisons, an asterisk (*) represents a significant difference from control (p ≤0.05; ANOVA, Tukey adjusted LSMeans).
Exposure to FL decreased EROD activity to below control levels but the difference was not significant (67±9%; p = 0.0940) (Fig. 1). BkF exposure caused a large and significant induction of EROD response (635±15%; p < 0.0001) compared to controls. Exposure to FL with BkF inhibited the increase in EROD activity caused by BkF alone; the EROD activity of the co-exposure was slightly greater than controls (139±10%; p = 0.0366).
Gene expression
As expected, the three examined CYPs (CYP1A, 1B1, and 1C1) were significantly upregulated by exposure to BkF (Fig. 2). CYP1A mRNA expression was induced 115.2±10.9-fold above control levels, CYP1B1 9.9±0.8-fold, and CYP1C1 15.1±2.6-fold (p < 0.0001, p < 0.004, and p < 0.0001, respectively). FL exposure did not significantly alter CYP1 expression compared to control levels (CYP1A–3.4±0.8-fold; CYP1B1 - 1.5±0.3-fold; and CYP1C1 - 0.8±0.1-fold, all p > 0.05). Exposure to BkF + FL caused a significant (p < 0.0001) and greater than additive induction of CYP1A, CYP1B1, and CYP1C1 of 281.7±29.0-fold, 23.1±3.1-fold, and 65.1±12.1-fold, respectively (Fig. 2).
Figure 2.
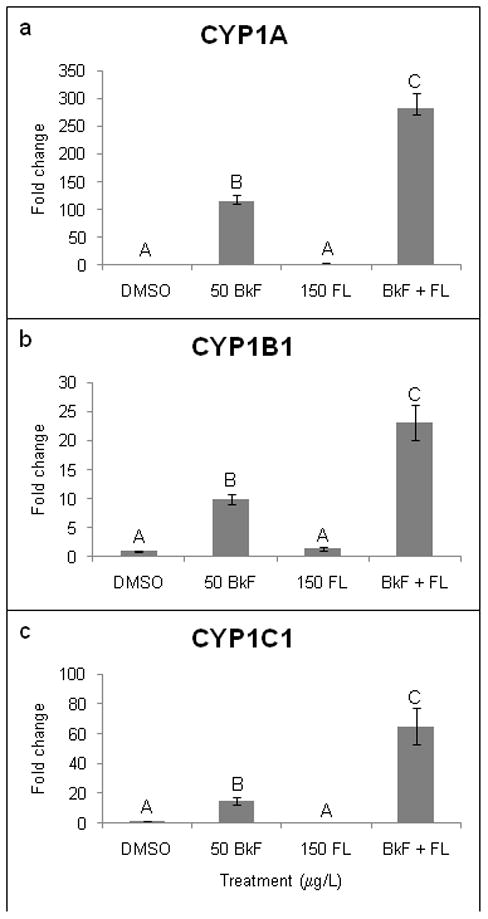
Effect of PAHs on CYP1 mRNA expression. Embryos were dosed at 24 hpf, and expression was measured at 48 hpf. (a) CYP1A; (b) CYP1B1; (c) CYP1C1. Expression is shown as fold induction compared to DMSO controls. n = 9–12 per treatment; each n represents 10 pooled embryos. Groups not sharing a common letter are significantly different (p ≤0.05; ANOVA, Tukey adjusted LSMeans).
Exposure to BkF alone also caused significant induction of certain redox-responsive genes. The greatest induction was observed for GSTp2, which was induced 3.1±0.7-fold (p = 0.037) (Fig. 3). BkF exposure also significantly upregulated GCLc expression (1.9±0.3-fold, p = 0.030). FL did not induce mRNA expression of GSTp2, GPx1, or GCLc. Co-exposure to BkF + FL caused a significant and greater than additive upregulation of GSTp2 (11.0±0.9-fold; p < 0.0001) and GPx1 (3.3±0.2-fold; p < 0.0001). The co-exposure also caused a significant 2.0±0.1-fold induction of GCLc, but this induction was not different than that caused by BkF alone (p < 0.005 vs. control, p = 0.9372 vs. BkF). None of the treatments caused a significant change in MnSOD or CuZnSOD expression (data not shown).
Figure 3.
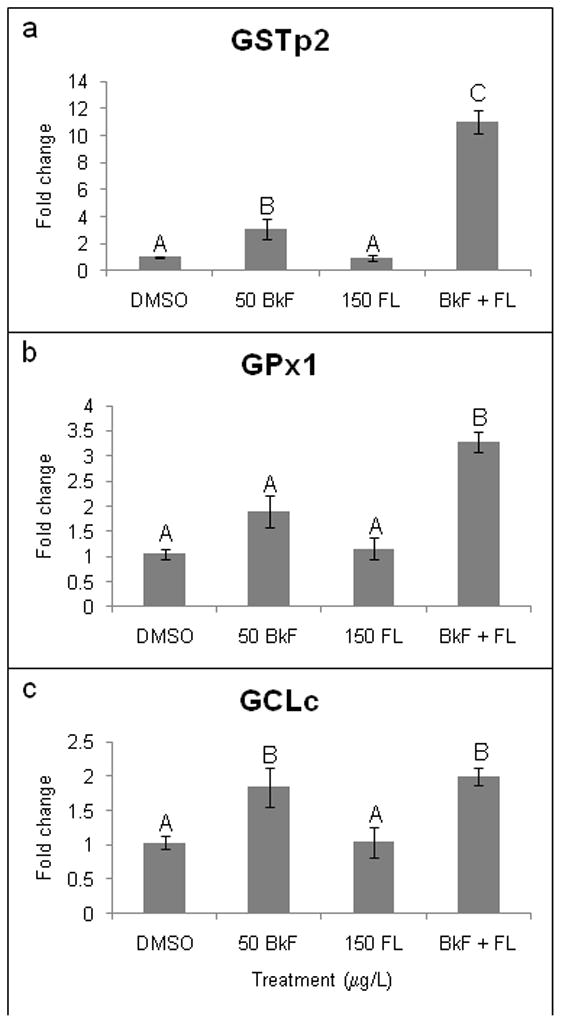
Effect of PAHs on redox-responsive gene expression. Embryos were dosed at 24 hpf, and expression was measured at 48 hpf. (a) GSTp2; (b) GPx1; (c) GCLc. Expression is shown as fold induction compared to DMSO controls. n = 9–12 per treatment; each n represents 10 pooled embryos. Groups not sharing a common letter are significantly different (p ≤0.05; ANOVA, Tukey adjusted LSMeans).
Effect of AHR2 knockdown on deformities and EROD activity
AHR2 knockdown via morpholino injection did not alter the average pericardial area of controls, BkF-exposed, or FL-exposed embryos compared to NI controls (Fig. 4). However, AHR2 knockdown in fish co-exposed to BkF + FL resulted in a mean pericardial effusion of 116±5%; this effusion was significantly (p < 0.0001) less than the extreme pericardial effusion of NI BkF + FL-exposed fish and not statistically different than NI DMSO fish. Injection of the control morpholino did not cause any differences in deformities, EROD, or gene expression as compared to controls (data not shown).
Figure 4.
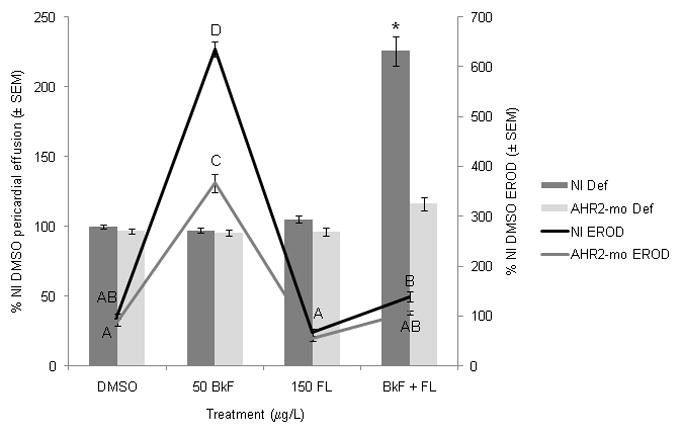
Effect of PAHs on deformities and EROD induction in non-injected (NI) and AHR2-morpholino-injected (AHR2-mo) embryos. Embryos were dosed at 24 hpf and scored at 96 hpf. Deformities are represented by bars, and scale is along the left y-axis; values are expressed as percent NI control (DMSO) pericardial effusion ± SEM (n = 12 per treatment; each n represents average of five embryos). EROD values are represented by lines and scale is along the right y-axis; values are expressed as percent NI control EROD ± SEM (n = 9 per treatment). For EROD comparisons, groups not sharing a common letter are significantly different (p ≤0.05; ANOVA, Tukey adjusted LSMeans). For deformity comparisons, an asterisk (*) represents a significant difference from control (p ≤0.05; ANOVA, Tukey adjusted LSMeans).
AHR2 knockdown caused reduced EROD activity compared to NI fish. In fish exposed to BkF, AHR2 knockdown did not completely prevent EROD activity; the fish still had a significant induction of EROD activity (367±19% of NI control activity; p < 0.0001), but the induction was significantly less than the NI fish exposed to BkF (p < 0.0001 vs. NI BkF activity) (Fig. 4). Fish injected with AHR2-mo and co-exposed to BkF + FL had an average EROD activity of 107±4%, which was not statistically different than NI controls or NI fish co-exposed to BkF + FL.
Effect of AHR2 knockdown on gene expression
AHR2 knockdown reduced the mRNA expression of the three examined CYPs. CYP1A expression in AHR2-mo embryos following exposure to BkF was not different than NI control levels (p = 0.9795) and was significantly less than the induction in NI embryos exposed to BkF (p < 0.0001 vs. NI BkF) (Fig. 5). After exposure to BkF + FL, CYP1A expression in AHR2-mo embryos was still significantly higher (83.7±10.4-fold) than NI controls (p < 0.0002) but was significantly less than the expression in NI embryos exposed to BkF + FL (p < 0.0001 vs. NI BkF + FL). AHR2 knockdown prevented the upregulation of CYP1B1 after exposure to BkF individually (2.3±0.3-fold) and BkF + FL (4.8±0.5-fold) that occurred in NI embryos. These small inductions were significantly reduced compared to NI embryos of the same treatment (p = 0.0026 vs. NI BkF and p < 0.0001 vs. NI BkF + FL, respectively) and were not significantly different than NI control levels. Expression of CYP1C1 in AHR2-mo embryos following BkF exposure (11.4±1.1-fold ) was not induced above NI control levels and was not different than induction in NI embryos exposed to BkF (p = 0.9137 vs. NI control and p = 0.9996 vs. NI BkF). After exposure of AHR2-mo embryos to BkF + FL, CYP1C1 expression (34.4±6.0-fold) was significantly less than expression in NI embryos (p = 0.0016 vs. NI BkF + FL) but was still significantly greater than control levels (p = 0.0006). FL exposure did not significantly alter CYP1A, 1B1, or 1C1 expression in AHR2-mo embryos.
Figure 5.
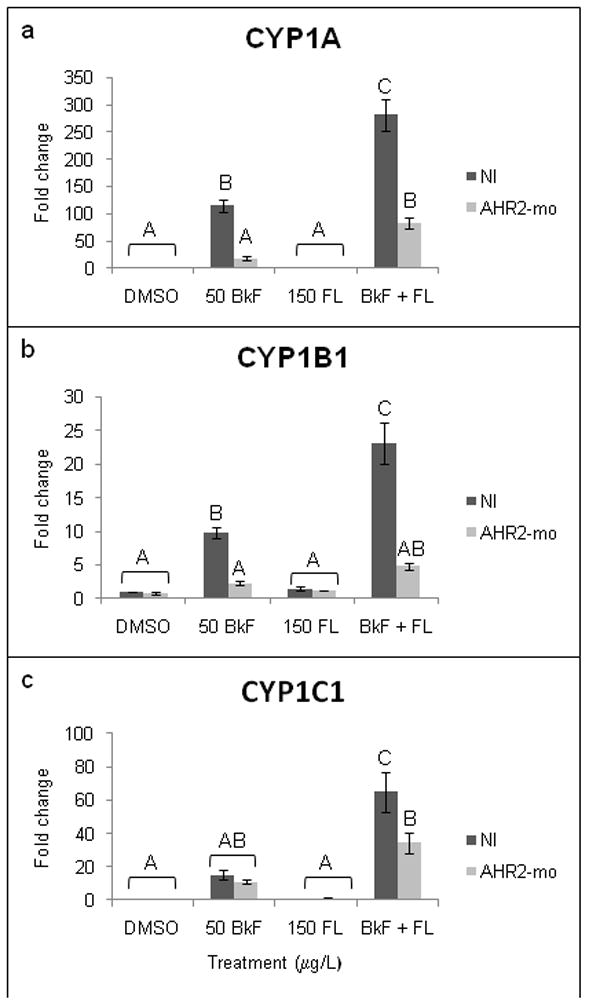
Effect of PAHs on CYP1 mRNA expression in non-injected (NI) and AHR2-morpholino-injected (AHR2-mo) embryos. Embryos were dosed at 24 hpf, and expression was measured at 48 hpf. (a) CYP1A; (b) CYP1B1; (c) CYP1C1. Expression is shown as fold induction compared to NI DMSO controls. n = 9–12 per treatment; each n represents 10 pooled embryos. Groups not sharing a common letter are significantly different (p ≤0.05; ANOVA, Tukey adjusted LSMeans).
AHR2 knockdown also altered the expression of several redox-responsive genes. The significant induction of GSTp2 that occurred in NI embryos after BkF exposure was prevented by AHR2 knockdown (1.0±0.2-fold) (Fig. 6). AHR2 knockdown also prevented the upregulation of GSTp2 in embryos exposed to BkF + FL (2.7±0.3-fold). The upregulation of GCLc that occurred after BkF exposure in NI embryos was prevented by AHR2 knockdown (0.8±0.2-fold). In embryos exposed to BkF + FL, AHR2 knockdown also prevented upregulation of GCLc (1.0±0.1-fold) and GPx1 (1.2±0.2-fold). As in NI embryos, AHR2 knockdown did not alter MnSOD or CuZnSOD expression (data not shown).
Figure 6.
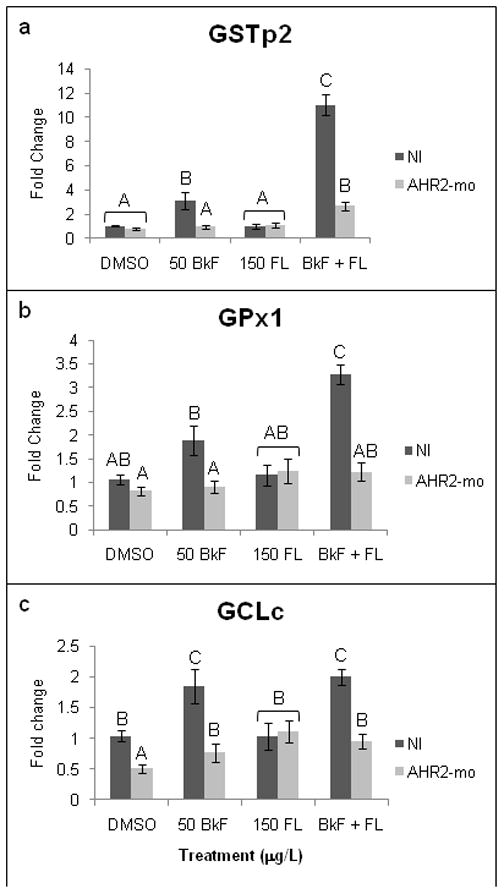
Effect of PAHs on redox-responsive gene expression in non-injected (NI) and AHR2-morpholino-injected (AHR2-mo) embryos. Embryos were dosed at 24 hpf, and expression was measured at 48 hpf. (a) GSTp2; (b) GPx1; (c) GCLc. Expression is shown as fold induction compared to NI DMSO controls. n = 9–12 per treatment; each n represents 10 pooled embryos. Groups not sharing a common letter are significantly different (p ≤0.05; ANOVA, Tukey adjusted LSMeans).
Discussion
Co-exposure to the strong AHR agonist BkF and the CYP1 inhibitor FL resulted in severe pericardial effusion in zebrafish. This pericardial effusion was accompanied by increased expression in phase I and redox-responsive genes. Some PAHs, including benzo[a]pyrene (BaP) and BNF, are considered bifunctional inducers. They induce phase I metabolism via ligand binding to the AHR, which binds to and upregulates genes containing XREs, most notably, CYPs. They can also upregulate genes following their metabolism into electrophilic metabolites, which can induce phase II and redox-responsive genes containing antioxidant response elements (AREs) (Prochaska et al., 1985; Rushmore et al., 1991). It has been shown that gene signaling through AREs is regulated by the transcription factor NF-E2 p45–related factor 2 (Nrf2) (Itoh et al., 1997). It was initially thought that these two mechanisms of induction, AHR-XRE-mediated and Nrf2-ARE-mediated, occurred independently of one another. However, there is evidence for crosstalk between the AHR pathway and Nrf2-mediated oxidative stress pathways: the mouse Nrf2 contains three XREs in its promoter region, and in mouse embryonic fibroblasts, Nrf2 has been shown to bind to a functional ARE in the proximal promoter of the AHR (Miao et al., 2005; Shin et al., 2007). In accordance with AHR binding and phase I metabolic induction, BkF + FL-induced cardiac deformities were indeed preceded by large increases in CYP1A, 1B1, and 1C1 mRNA expression in the present study. Greater than additive induction of these CYPs has also been shown after co-exposure to BNF + ANF; however, ANF alone significantly induces CYP1 expression (Timme-Laragy et al., 2007). The greater than additive induction of the CYPs caused by BkF + FL is unique in that FL by itself does not induce expression of CYP1A, 1B1, or 1C1.
In further support of PAHs as bifunctional inducers, the deformities caused by BkF + FL co-exposure were also preceded by upregulation of GSTp2, GCLc, and GPx1. The greatest induction occurred in GSTp2 expression. GSTp was also the most highly induced after BNF + ANF co-exposure (Timme-Laragy et al., 2009) and BaP + FL co-exposure (unpublished data). GSTs participate in phase II metabolism, exhibit glutathione peroxidase activities via catalyzing the reduction of organic hydroperoxides into alcohols, and are induced in response to pro-oxidants in a variety of organisms (reviewed in Hayes and Pulford, 1995; Hayes et al., 2005). The pi class GSTs are particularly efficient in conjugating PAH metabolites to glutathione (Robertson et al., 1986). It is not currently known whether zebrafish GSTp2 contains XREs or AREs in its promoter region. However, ARE-like sequences are present in the single human and rat GSTp, both known as GSTp1, and mouse GSTp1 (Ikeda et al., 2002; 2004). XREs have also been found in other mammalian GST isoforms (Pimental et al., 1993). Moreover, Suzuki et al. (2005) found that zebrafish GSTp1, which shares 90.4% identity of the coding region sequence with GSTp2, contains a proximal ARE-like sequence 50 bp upstream of the transcription initiation and that Nrf2 activates GSTp1 gene expression through this element in zebrafish embryos. GCLc, which catalyzes the rate-limiting step of glutathione synthesis, and GPx1, which reduces hydrogen peroxide into water and detoxifies lipid peroxides, were also upregulated by BkF alone and BkF + FL co-exposure. As with GSTp2, the presence of XREs and AREs in these two genes in piscine species is not yet known. Previous studies have shown that GCLc induction by BNF occurs via a distal ARE sequence in human HepG2 cells (Mulcahy et al., 1997; Wild et al., 1998), and human intestinal GPx2 and plasma GPx3 contain an ARE in their promoter regions (Bierl et al., 2004; Banning et al., 2005). Because antioxidant defense systems are highly conserved across species, it is plausible that zebrafish redox-responsive genes contain ARE-like sequences and potentially XREs.
Various studies have examined changes in GST and GPx in response to different PAHs in aquatic organisms. However, most studies have examined protein activity and not mRNA expression response and have focused on exposures to a single PAH. In the common goby, Pomatoschistus microps, Vieira et al. (2008) found that GPx activity was induced in response to low doses of BaP (4, 8, and 16 μg/L) and anthracene (4 μg/L), a three-ring PAH. However, GST activity was induced after BaP exposure yet inhibited after anthracene exposure. This difference could be due to the two compounds different affinities for the AHR; BaP is a strong agonist while anthracene is a very weak agonist (Barron et al., 2004). In the same study, exposure to a fuel-oil mixture induced GST activity almost two times higher than the induction caused by BaP. However, because the PAH congeners in the mixture were not examined individually, it is not clear if the PAHs exhibited an additive or synergistic effect on GST activity. Greater than additive induction of GSTp2 was caused by the simple BkF + FL mixture in the present study. Environmentally relevant concentrations of the three-ring PAH phenanthrene were shown to have different effects on GPx activity in different tissues of golden grey mullet (Liza aurata): GPx was inhibited in the gill, unaffected in the kidney, and increased in the liver in a dose-dependent manner (Oliveira et al., 2008), showing that antioxidant defenses within the same organism are variable. In another study, GPx mRNA expression was induced in the liver of adult polar cod, Boreogadus saida, two days after i.p. injection of 6.6, 85, and 378 μg/kg wwt BaP, while GSTp expression was not induced until four days after injection and only after the low and high dose (Nahrgang et al., 2009). The results of these two studies highlight the fine balance in oxidative stress responses. An increase in redox-responsive genes and protein may indicate that an organism will be able to mount a response to a toxicant and overcome its toxicity. However, an organism’s redox-responsive enzymes may become overwhelmed and lead to oxidative stress.
Very few studies have examined GCLc response to PAHs in aquatic species. GCLc mRNA expression was upregulated 1.7-fold in the liver of juvenile largemouth bass (Micropterus salmoides) 48 h after i.p. injection of 66 mg/kg BNF (Hughes and Gallagher, 2004), a response that was concomitant with a 9-fold increase in microsomal CYP activity. In a mammalian study, GST-mu and GPx2 were upregulated 20- and 5-fold, respectively, in rat preneoplastic lesions following BNF exposure (Dewa et al., 2008). This same study showed that BNF also increased microsomal ROS production and lipid peroxidation. In another mammalian study, 1,6 BaP-quinone (BPQ) and 1,3-BPQ induced GCLc expression in MCF-10A human mammary epithelial cells, and it was determined that the gene contained an XRE in its promoter region (Burchiel et al., 2007).
MnSOD and CuZnSOD were not upregulated by BkF or FL individually or by BkF + FL co-exposure in zebrafish embryos in our study. Once again, knowledge of XREs or AREs in fish MnSOD and CuZnSOD is lacking. Human CuZnSOD contains a functional XRE in the 5′ flanking region that can bind and be induced by TCDD (Cho et al., 2001), and an ARE sequence has been identified in the 5′ flanking region of mouse MnSOD (Jones et al., 1995). Though MnSOD and CuZnSOD gene expression were not affected by BkF or FL, other studies have shown that PAHs can induce SOD expression and activity. SOD activity increased significantly after a four-day exposure of gilthead seabream (Sparus aurata) to 1.20 μM fluorene but not after exposure to lower doses (Kopecka-Pilarczyk and Correia, 2009). By comparison, the concentrations of BkF and FL used in this study, 0.20 μM and 0.74 μM, respectively, were significantly lower than the dose at which increased SOD activity was observed. In the liver of polar cod exposed to 85 μg/kg BaP, CuZnSOD mRNA expression was upregulated 15-fold, while MnSOD was also induced but to a much lesser extent, approximately 1.5-fold (Nahrgang et al., 2009). Vieira et al. (2008) found that SOD activity was induced in the common goby after BaP and anthracene exposure. Our laboratory also previously found that MnSOD and CuZnSOD expression were each upregulated approximately three-fold above control levels at 24 h after exposure to BNF + ANF, but not 72 h after exposure in zebrafish (Timme-Laragy et al., 2009), which is further evidence that in addition to variability in response amongst different tissues, antioxidant responses are also variable in time.
AHR2 knockdown protected embryos from the cardiac deformities induced by BkF + FL. AHR2 knockdown also decreased expression of CYP1A, 1B1, and 1C1 compared to NI embryos after BkF + FL exposure. CYP1B1 expression was reduced to control levels in AHR2-mo embryos exposed to BkF and BkF + FL, while CYP1A and 1C1 were reduced compared to NI BkF + FL levels but were still significantly greater than control levels. The inability of the AHR2 morpholino to completely prevent CYP1A and 1C1 expression may be due to the fact that the morpholino only provides a knockdown, not knockout, of AHR2, and thus, AHR2 ligand binding and signaling events may still occur (Eisen and Smith, 2008; Matson et al., 2008a). Furthermore, the CYPs may be partly controlled via AHR-independent pathways. For example, CYP1A1 and CYP1B1 have been shown to be inducible by PAHs in various tissues of AHR null knockout mice (Kerzee and Ramos, 2001; Nakatsuru et al., 2004). Another explanation may be that zebrafish have two other identified AHR isoforms, AHR1A and AHR1B, and CYP1A and 1C1 may be at least partially controlled by one or both of these isoforms. AHR1A is present predominantly in the liver of adult zebrafish and was initially thought to be nonfunctional, as it does not bind TCDD in tissue culture (Andreasen et al., 2002). However, Incardona et al. (2006) showed that embryos injected with AHR1A-mo were protected from the abnormal liver development and pericardial effusion caused by high doses of the four-ringed PAH pyrene. AHR1B was shown to be expressed as early as 24 hpf in zebrafish development and at higher levels than AHR1A. It was shown in vitro to have high affinity binding of TCDD but was not inducible by TCDD (Karchner et al., 2005). The role of zebrafish AHR1A and AHR1B in the toxicity of high molecular weight PAHs, including BkF and mixtures, has not yet been examined.
AHR2 knockdown, which protected against BkF + FL-induced deformities and caused a reduction of CYP1 mRNA expression compared to NI embryos, also prevented the upregulation of GSTp2, GPx1, and GCLc that occurred in NI embryos in response to the BkF + FL co-exposure. The absence of redox-responsive gene expression after BkF + FL co-exposure in AHR2-mo embryos may be due to the lack of metabolism of PAHs in these embryos. When AHR2 is knocked down, there is less receptor present to which the PAHs can bind. This reduction of ligand binding prevents CYP activity, thereby preventing phase I metabolism and the potential formation of redox-cycling quinone metabolites and uncoupling of the electron transport chain and CYP reactions. Furthermore, oxidative stress responses may be a secondary result of the AHR2-mediated developmental toxicity caused by PAHs. Thus, preventing overall PAH toxicity via AHR2 knockdown may prevent oxidative stress. The lack of redox-responsive gene induction in PAH-exposed fish after AHR knockdown provides further evidence of that AHR-XRE-mediated pathways and Nrf2-ARE-mediated pathways are not completely independent of one another.
Various experiments have shown that knockdown or knockout of Nrf2 exacerbates the toxicity of PAHs and ROS (e.g., Zhu et al., 2005; Timme-Laragy et al., 2009). However, few studies have looked at the effects of AHR knockdown or knockout on oxidative stress parameters and none that we know of have done so in fish. siRNA experiments that eliminated the AHR in human esophageal epithelial cells showed that elimination of the AHR prevented the upregulation of various UDP-glucuronosyltransferases (UGTs), another class of detoxifying enzymes that contain both XREs and AREs, by TCDD (Kalthoff et al., 2010). Furthermore, in wild-type Hepa 1c1c7 cells, BNF and BaP increased H2O2 production and lipid peroxidation, but this increase did not occur in cells lacking the AHR, indicating that the AHR is necessary for production of oxidative stress in these cells (Elbekai et al., 2004). In mouse aortic endothelial cells overexpressing CuZnSOD and/or catalase, knockdown of the AHR via siRNA prevented the BaP-induced increase in GSTp1 mRNA expression and GST activity that occurred in cells treated with control siRNA (Wang et al., 2009). These studies provide evidence that the absence of the AHR in cell culture prevents an oxidative stress response consistent with our results with zebrafish.
In summary, co-exposure of zebrafish embryos to two environmentally relevant PAHs, BkF and FL, resulted in pericardial effusion and upregulation of CYP1A, 1B1, 1C1, and the redox-responsive genes GSTp2, GPx1, and GCLc. The co-exposure did not affect the expression of two other redox-responsive genes, MnSOD and CuZnSOD. AHR2 knockdown prevented the cardiac toxicity and upregulation of the various CYPs and redox-responsive genes caused by BkF + FL co-exposure. AHR2 mediates the cardiac toxicity of some PAHs in zebrafish and appears to be involved in the oxidative stress response of zebrafish to PAHs. Additional studies are required to clarify the role of the AHR in the oxidative stress response of zebrafish to the embryotoxicity of select PAHs.
Research Highlights
Co-exposure of the PAHs BkF and FL causes cardiotoxicity in zebrafish.
BkF and FL co-exposure upregulates certain XRE- and ARE-associated genes.
AHR2 knockdown prevents the deformities caused by BkF and FL co-exposure.
AHR2 knockdown prevents upregulation of certain XRE- and ARE-associated genes.
Acknowledgments
We would like to thank Kyle Erwin for use of his β-actin primers. We would also like to thank lab members Dr. Cole Matson and Dr. Bryan Clark for their advice. This work was funded by the NIEHS-supported Duke University Superfund Research Center (P42ES010356) and Duke Integrated Toxicology and Environmental Health Program (T32ES07031). No funding source was involved in the design or implementation of the experiments described herein.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol Pharmacol. 2002;62:234–249. doi: 10.1124/mol.62.2.234. [DOI] [PubMed] [Google Scholar]
- Bacanskas LR, Whitaker J, Di Giulio RT. Oxidative stress in two populations of killifish (Fundulus heteroclitus) with differing contaminant exposure histories. Mar Environ Res. 2004;58:597–601. doi: 10.1016/j.marenvres.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Banning A, Deubel S, Kluth D, Zhou ZW, Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MG, Heintz R, Rice SD. Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res. 2004;58:95–100. doi: 10.1016/j.marenvres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Bierl C, Voetsch B, Jin RC, Handy DE, Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem. 2004;279:26839–26845. doi: 10.1074/jbc.M401907200. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Hahn ME, Franks DG, Peterson RE, Bols NC, Hodson PV. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs) Comp Biochem Physiol B-Biochem Mol Biol. 2002;133:55–68. doi: 10.1016/s1096-4959(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The Role of the Aryl Hydrocarbon Receptor Pathway in Mediating Synergistic Developmental Toxicity of Polycyclic Aromatic Hydrocarbons to Zebrafish. Toxicol Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Thompson TA, Lauer FT, Oprea TI. Activation of dioxin response element (DRE)-associated genes by benzo(a)pyrene 3,6-quinone and benzo(a)pyrene 1,6-uinone in MCF-10A human mammary epithelial cells. Toxicol Appl Pharmacol. 2007;221:203–214. doi: 10.1016/j.taap.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Chang, Rho Transcriptional activation of the human Cu/Zn superoxide dismutase gene by 2,3,7,8-tetrachlorodibenzo-p-dioxin through the xenobiotic-responsive element. Mol Genet and Genom. 2001;266:133–141. doi: 10.1007/s004380100536. [DOI] [PubMed] [Google Scholar]
- Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat Toxicol. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Dewa Y, Nishimura J, Muguruma M, Jin M, Saegusa Y, Okamura T, Tasaki M, Umemura T, Mitsumori K. beta-Naphthoflavone enhances oxidative stress responses and the induction of preneoplastic lesions in a diethylnitrosamine-initiated hepatocarcinogenesis model in partially hepatectomized rats. Toxicology. 2008;244:179–189. doi: 10.1016/j.tox.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Di Giulio RT, Meyer JN. The Toxicology of Fishes. Crc Press-Taylor & Francis Group; 2008. Reactive oxygen species and oxidative stress. [Google Scholar]
- Diercks AR, Highsmith RC, Asper VL, Joung DJ, Zhou ZZ, Guo LD, Shiller AM, Joye SB, Teske AP, Guinasso N, Wade TL, Lohrenz SE. Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon site. Geophys Res Lett. 2010;37:6. [Google Scholar]
- Eisen JS, Smith JC. Controlling morpholino experiments: don’t stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- Elbekai RH, Korashy HM, Wills K, Gharavi N, El-Kadi AOS. Benzo[a]pyrene, 3-methylcholanthrene and beta-naphthoflavone induce oxidative stress in hepatoma hepa 1c1c7 cells by an AHR-dependent pathway. Free Radic Res. 2004;38:1191–1200. doi: 10.1080/10715760400017319. [DOI] [PubMed] [Google Scholar]
- Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol Sci. 2008;106:193–205. doi: 10.1093/toxsci/kfn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-Transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hughes EM, Gallagher EP. Effects of [beta]-naphthoflavone on hepatic biotransformation and glutathione biosynthesis in largemouth bass (Micropterus salmoides) Mar Environ Res. 2004;58:675–679. doi: 10.1016/j.marenvres.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Nishi S, Sakai M. Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem J. 2004;380:515–521. doi: 10.1042/BJ20031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Serria MS, Kakizaki I, Hatayama I, Satoh K, Tsuchida S, Muramatsu M, Nishi S, Sakai M. Activation of mouse Pi-class glutathione S-transferase gene by Nrf2 (NF-E2-related factor 2) and androgen. Biochem J. 2002;364:563–570. doi: 10.1042/BJ20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2 small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jones PL, Kucera G, Gordon H, Boss JM. Cloning and characterization of the murine manganous superoxide dismutase-encoding gene. Gene. 1995;153:155–161. doi: 10.1016/0378-1119(94)00666-g. [DOI] [PubMed] [Google Scholar]
- Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Interaction between Oxidative Stress Sensor Nrf2 and Xenobiotic-activated Aryl Hydrocarbon Receptor in the Regulation of the Human Phase II Detoxifying UDP-glucuronosyltransferase 1A10. J Biol Chem. 2010;285:5993–6002. doi: 10.1074/jbc.M109.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Hahn ME. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: tandem arrangement of ahr1b and ahr2 genes. Biochem J. 2005;392:153–161. doi: 10.1042/BJ20050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerzee JK, Ramos KS. Constitutive and inducible expression of Cyp1a1 and Cyp1b1 in vascular smooth muscle cells - Role of the Ahr bHLH/PAS transcription factor. Circ Res. 2001;89:573–582. doi: 10.1161/hh1901.097083. [DOI] [PubMed] [Google Scholar]
- Kopecka-Pilarczyk J, Correia AD. Biochemical response in gilthead seabream (Sparus aurata) to in vivo exposure to pyrene and fluorene. Journal of Experimental Marine Biology and Ecology. 2009;372:49–57. [Google Scholar]
- Lemkau KL, Peacock EE, Nelson RK, Ventura GT, Kovecses JL, Reddy CM. The M/V Cosco Busan spill: Source identification and short-term fate. Mar Pollut Bull. 2010;60:2123–2129. doi: 10.1016/j.marpolbul.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang MY, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima ALC, Eglinton TI, Reddy CM. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ Sci Technol. 2003;37:53–61. doi: 10.1021/es025895p. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Livingstone DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull. 2001;42:656–666. doi: 10.1016/s0025-326x(01)00060-1. [DOI] [PubMed] [Google Scholar]
- Malek RL, Sajadi H, Abraham J, Grundy MA, Gerhard GS. The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp Biochem Physiol C-Toxicol Pharmacol. 2004;138:363–373. doi: 10.1016/j.cca.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT. Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat Toxicol. 200y;87:289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CW, Timme-Laragy AR, Di Giulio RT. Fluoranthene, but not benzo[a]pyrene, interacts with hypoxia resulting in pericardial effusion and lordosis in developing zebrafish. Chemosphere. 2008b;74:149–154. doi: 10.1016/j.chemosphere.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Smith JD, Winston GW, Di Giulio RT. Antioxidant defenses in killifish (Fundulus heteroclitus) exposed to contaminated sediments and model prooxidants: short-term and heritable responses. Aquat Toxicol. 2003;65:377–395. doi: 10.1016/j.aquatox.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Miao WM, Hu LG, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway - Direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- Nacci D, Coiro L, Kuhn A, Champlin D, Munns W, Specker J, Cooper K. Nondestructive indicator of ethoxyresorufin-O-deethylase activity in embryonic fish. Environ Toxicol Chem. 1998;17:2481–2486. [Google Scholar]
- Nahrgang J, Camus L, Gonzalez P, Goksoyr A, Christiansen JS, Hop H. PAH biomarker responses in polar cod (Boreogadus saida) exposed to benzo(a)pyrene. Aquat Toxicol. 2009;94:309–319. doi: 10.1016/j.aquatox.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Nakatsuru Y, Wakabayashi K, Fujii-Kuriyama Y, Ishikawa T, Kusama K, Ide F. Dibenzo[A,L]pyrene-induced genotoxic and carcinogenic responses are dramatically suppressed in aryl hydrocarbon receptor-deficient mice. Int J Cancer. 2004;112:179–183. doi: 10.1002/ijc.20365. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nature Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Pacheco M, Santos MA. Organ specific antioxidant responses in golden grey mullet (Liza aurata) following a short-term exposure to phenanthrene. Sci Total Environ. 2008;396:70–78. doi: 10.1016/j.scitotenv.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Penning TM, Ohnishi ST, Ohnishi T, Harvey RG. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem Res Toxicol. 1996;9:84–92. doi: 10.1021/tx950055s. [DOI] [PubMed] [Google Scholar]
- Pimental RA, Liang B, Yee GK, Wilhelmsson A, Poellinger L, Paulson KE. Dioxin receptor and C/EBP regulate the function of the glutathione S-transferase Ya gene xenobiotic response element. Mol Cell Biol. 1993;13:4365–4373. doi: 10.1128/mcb.13.7.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska HJ, Delong MJ, Talalay P. On the mechanisms of induction of cancer-protective enzymes - a unifying proposal. Proc Natl Acad Sci U S A. 1985;82:8232–8236. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IGC, Guthenberg C, Mannervik B, Jernstrom B. Differences in stereoselectivity and catalytic efficiency of 3 human glutathione transferases in the conjugation of glutathione with 7-beta,8-alpha-dihydroxy-9-alpha,10-alpha,oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. Cancer Res. 1986;46:2220–2224. [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antiodixant repsonsive element - activation by oxidative stress and identification of the DNA consensus sequence required for functional-activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Schlezinger JJ, Stegeman JJ. Induction and suppression of cytochrome P450 1A by 3,3 ′,4,4 ′,5-pentachlorobiphenyl and its relationship to oxidative stress in the marine fish scup (Stenotomus chrysops) Aquat Toxicol. 2001;52:101–115. doi: 10.1016/s0166-445x(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Scott JA, Hodson PV. Evidence for multiple mechanisms of toxicity in larval rainbow trout (Oncorhynchus mykiss) co-treated with retene and alpha-naphthoflavone. Aquat Toxicol. 2008;88:200–206. doi: 10.1016/j.aquatox.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, Kerzee JK, Uno S, Shertzer HG. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic Biol Med. 2002;33:1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: Influence on adipogenesis. Mol Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short JW, Lindeberg MR, Harris PM, Maselko JM, Pella JJ, Rice SD. Estimate of oil persisting on the beaches of Prince William Sound 12 years after the Exxon Valdez oil spill. Environ Sci Technol. 2004;38:19–25. doi: 10.1021/es0348694. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takagi Y, Osanai H, Li L, Takeuchi M, Katoh Y, Kobayashi M, Yamamoto M. Pi class glutathione S-transferase genes are regulated by Nrf2 through an evolutionarily conserved regulatory element in zebrafish. Biochem J. 2005;388:65–73. doi: 10.1042/BJ20041860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay RL, Abnet CC, Heideman W, Peterson RE. Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochim Biophys Acta-Gene Struct Expression. 1999;1444:35–48. doi: 10.1016/s0167-4781(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem Biophys Res Commun. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat Toxicol. 2007;85:241–250. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Van Tiem LA, Linney EA, Di Giulio RT. Antioxidant Responses and NRF2 in Synergistic Developmental Toxicity of PAHs in Zebrafish. Toxicol Sci. 2009;109:217–227. doi: 10.1093/toxsci/kfp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ Sci Technol. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Vieira LR, Sousa A, Frasco MF, Lima I, Morgado F, Guilhermino L. Acute effects of Benzo[a]pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Sci Total Environ. 2008;395:87–100. doi: 10.1016/j.scitotenv.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Yang H, Ramesh A, Roberts LJ, Zhou LC, Lin XH, Zhao YF, Guo ZM. Overexpression of Cu/Zn-superoxide dismutase and/or catalase accelerates benzo(a) pyrene detoxification by upregulation of the aryl hydrocarbon receptor in mouse endothelial cells. Free Radic Biol Med. 2009;47:1221–1229. doi: 10.1016/j.freeradbiomed.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor Agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild AC, Gipp JJ, Mulcahy RT. Overlapping antioxidant response element and PMA response element sequences mediate basal and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase catalytic subunit gene. Biochem J. 1998;332:373–381. doi: 10.1042/bj3320373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett KL, Wassenberg D, Lienesch L, Reichert W, Di Giulio RT. In vivo and in vitro inhibition of CYP1A-dependent activity in Fundulus heteroclitus by the polynuclear aromatic hydrocarbon fluoranthene. Toxicol Appl Pharmacol. 2001;177:264–271. doi: 10.1006/taap.2001.9296. [DOI] [PubMed] [Google Scholar]
- Winston GW, Di Giulio RT. Prooxidant and antioxidant mechanisms in aquatic orgnaisms. Aquat Toxicol. 1991;19:137–161. [Google Scholar]
- Zhu H, Itoh K, Yamamoto M, Zweier JL, Li YB. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]


