Abstract
Rationale
Excessive alcohol consumption is less common among aged compared to young adults, with aged adults showing greater sensitivity to many behavioral effects of ethanol.
Objectives
This study compared the discriminative stimulus effects of ethanol in young and middle-aged adult cynomolgus monkeys (Macaca fascicularis) and its γ-amino-butyric acid (GABA)A receptor mediation.
Methods
Two male and two female monkeys trained to discriminate ethanol (1.0 g/kg, i.g.; 60-min pre-treatment interval) from water at 5-6 years of age (Grant et al. 2000) were re-trained in the current study more than a decade later (19.3 ± 1.0 years of age) for a within-subjects comparison. Also, four experimentally-naïve middle-aged (mean ± SEM, 17.0 ± 1.5 years of age) female monkeys were trained to discriminate ethanol for between-subjects comparison with published data from young adult naïve monkeys.
Results
Two of the naïve middle-aged monkeys attained criterion performance, with weak stimulus control and few discrimination tests, despite greater blood-ethanol concentration (BEC) 60 min after 1.0 g/kg ethanol in middle-aged compared to young adult female monkeys (Green et al. 1999). The efficacy of the GABAA receptor positive modulators pentobarbital, midazolam, allopregnanolone, pregnanolone and androsterone to substitute for the discriminative stimulus effects of 1.0 g/kg ethanol was maintained from young adulthood to middle age.
Conclusions
The data suggest that 1.0 g/kg ethanol is a weak discriminative stimulus in naive middle-aged monkeys. Nevertheless, the GABAA receptor mechanisms mediating the discriminative stimulus effects of ethanol, when learned as a young adult, appear stable across one-third of the primate lifespan.
Keywords: ethanol, discrimination, monkey, aging, GABA receptor
Introduction
Aged adults are less likely to engage in heavy drinking and have lower incidence of alcoholism compared to young adults (Grant et al. 2006; Center for Disease Control 2010). However, the prevalence of alcohol abuse is increasing among older adults (Grant et al. 2004), a trend predicted to continue for the next decade with the aging baby-boomer population (Groerer et al. 2003). Further, the negative health consequences of heavy drinking (e.g., impaired psychosocial functioning, depression, sleep disorders, cognitive impairment) occur after lower levels of consumption in aged adult females compared to males (Blow and Barry 2002). Peak blood-ethanol concentration (BEC) and area under the curve (AUC) are greater in older (≥ 60-years) compared to younger (21-40 years) men and women when fasted but not after a meal (Lucey et al. 1999), while alcohol-induced memory impairment appears greater in aged compared to young adult women at the same BEC (Jones and Jones 1980). Research on the neural mechanisms mediating the effects of ethanol has primarily been conducted in young adult subjects, leaving gaps in our understanding of the effects of aging on the neuropharmacology of ethanol.
Key receptors mediating the behavioral effects of ethanol are γ-aminobutyric acid (GABA)A receptors (Grant 1999; Kumar et al. 2009), pentameric Cl- channels with distinct putative binding sites for, and receptor subunit conformations sensitive to, barbiturates (e.g., pentobarbital), benzodiazepines (e.g., midazolam), neuroactive steroids (allopregnanolone, pregnanolone) and possibly ethanol (Mennerick et al. 2004; Thompson et al. 1996; Belelli et al. 2002; Möhler et al. 2002; Akk et al. 2007; Jung and Harris 2006). Aging reportedly has mixed effects on the subunit composition and sensitivity of GABAA receptors. For example, compared to young adults, aged rodents (Mhatre and Ticku 1992; Yu et al. 2006) and humans (Kanaumi et al. 2006) show differential GABAA receptor subunit expression and increased pharmacological sensitivity to GABAA receptor ligands (Albrecht et al. 1999). Expression of GABAA receptor subunit genes also varies with hormonal treatment in rhesus macaques (Noriega et al. 2010). The decline in reproductive hormones during aging in adult females and males (Schumacher et al. 2003) may be associated with altered GABAA receptor expression and correlate with increased sensitivity to the behavioral effects of ethanol. However, in 24- compared to 5-year old rhesus monkeys, there was lower α1 immunolabeling in the hippocampus (Rissman et al., 2006), an outcome that would predict decreased sensitivity to positive GABAA receptor modulators, including ethanol (Helms et al. 2008).
Drug discrimination studies provide valuable information about the receptor-mediated basis of the subjective effects of psychoactive drugs. A limitation of drug discrimination studies is that the receptor mechanisms mediating discriminative stimulus effects are not always identical to those mediating self-reported subjective effects (Kelly et al. 2003), and the relationship between discriminative stimulus and reinforcing effects of drugs is unclear. Nonetheless, in all species studied, positive modulators of GABAA receptors produce ethanol-like discriminative stimulus effects (Grant 1999; Grant et al. 2000). There are only a couple of studies that have addressed aging and sensitivity to the discriminative stimulus effects of ethanol. On the younger end of the developmental spectrum, 3- and 9-month old female mice required a similar number of training sessions to acquire discrimination of 1.0 g/kg ethanol (Groseclose and Middaugh, 1997). Using a within-subjects design, rats trained to discriminate 0.60 g/kg ethanol showed similar sensitivity to ethanol at approximately 12 and 28 months of age (Schechter et al. 1988). In both studies, substitution tests with drugs other than ethanol were not conducted.
The present study investigated acquisition of ethanol discrimination in middle-aged monkeys and the effects of aging on a previously-acquired ethanol discrimination. Specifically, two male and two female monkeys that were trained to discriminate ethanol from vehicle at 5-6 years of age were retested over a decade later. In addition, published data from young (5-6 years of age) adults (Grant et al. 2000, 2008) was compared with data from four naïve middle-aged (13, 17, 18 and 20 years of age) monkeys. Drug-specific changes in efficacy or potency to substitute for the discriminative stimulus effects of ethanol could suggest changes in GABAA receptors with age.
Materials and Methods
Subjects
Adult cynomolgus monkeys (Macaca fascicularis; male, n = 2, 6.2 and 7.2 kg, 18 and 21 years of age; female, n = 6, 2.8 – 5.2 kg, 13, 17, 17, 18, 20 and 21 years of age; Table 1) purchased from World Wide Primates (Miami, FL) were housed in stainless steel quadrant cages (1.6 × 0.8 × 0.8 m) modified for social housing in a humidity- (65%) and temperature- (22°C) controlled room set to a 12-h light-dark cycle (lights on, 0700 h). The monkeys were housed individually (males) or in pairs (females). Monkeys housed individually had auditory and visual or tactile contact with conspecifics. The monkeys' daily diet included nutritionally-complete biscuits or banana-flavored pellets (Bio-Serv, Frenchtown, NJ) and fruit. The monkeys were fed once daily but had unlimited access to water except during training and testing. For the within-subjects study, four monkeys (two male, 25078 and 25079; two female, 25057 and 25070; Table 1) were first trained to discriminate ethanol during 1996-1998 at Wake Forest University School of Medicine (WFUSM) and the last drug discrimination test sessions at WFUSM were conducted April and May 2004. For the between-subjects study, four monkeys (all female 25060, 25075, 25082, 25083) were naïve to ethanol. Three monkeys had ovarian stimulation with follicle-stimulating hormone (15-45 IU) followed by human chorionic gonadotropin (2,000 IU): 25075 had five treatments between August 2002 and August 2004, 25082 had three treatments between September 2002 and December 2003 and 25083 had two treatments between July 2002 and January 2003. The monkeys were transported to Oregon National Primate Research Center and response training for naïve monkeys began in April 2007. Training of the ethanol discrimination for previously-trained monkeys began in July 2007. Monkey 25057 developed uterine and ovarian endometriosis necessitating hysterectomy and bilateral ovariectomy in October 2007 and resumed participating in the ethanol discrimination procedure in December 2007. Discrimination training was stopped in 25075 due to intractable diarrhea. The experiments were conducted in accordance with the guidelines of the Oregon Health & Science University Animal Care and Use Committee and the Guidelines of the Committee on the Care and Use of Laboratory Animal Resources (National Research Council 1996).
Table 1.
Age and menstrual cycle characteristics of female cynomolgus monkeys from the beginning of operant training to completion of the study. The data were obtained over 21 months (except 25075, 15 months). Progesterone values are from cycles < 38 days in duration.
| Monkey | Age | Number of ovulatory cycles/menses | Mean ovulatory cycle duration | Mean (± SEM) peak progesterone (ng/ml) | Minimum- maximum inter-mense interval, median |
|---|---|---|---|---|---|
| 25057 | 21 | 9/9 a | 24.6 ± 0.9 | 11.5 ± 1.3 | 22-29, 24 |
| 25060 | 17 | 18/18 | 30.7 ± 1.2 | 8.7 ± 0.8 | 25-44, 29 |
| 25070 | 17 | 15/17 | 30.3 ± 2.3 | 9.1 ± 0.8 | 24-58, 27 |
| 25075 | 18 | 2/13 | 24.3 ± 1.7 | 3.4 ± 0.5 | 21-56, 29.5 |
| 25082 | 13 | 2/8 | 37 b | – c | 13-115, 68 |
| 25083 | 20 | 19/19 | 33.8 ± 1.0 | 12.3 ± 0.9 | 25-45, 33 |
Ovariectomized after nine cycles.
The second menses for 25082 with confirmed ovulation occurred 115 days after the previous menses.
All menses with luteal phase progesterone > 4 ng/ml occurred within cycles > 37 days.
Apparatus
Training and test sessions were conducted 5-6 days/week in ventilated and sound-attenuating chambers (1.50 × 0.74 × 0.76 m; Med Associates, Inc., St. Albans, VT) that accommodated a primate chair (1.17 × 0.61 × 0.61 m; Plas Labs, Lansing, MI). The front panel of the chamber (0.48 × 0.69 m) contained two retractable levers, a set of three lights (amber, green and red) above each lever, and a centrally-located white light. Two house lights were mounted near the ceiling at the rear of the chamber. Reinforcers (1-g pellets, Bio-Serv) were delivered to a tray on top of the primate chair via vinyl tubing from a feeder set outside of the chamber. A PC- or Macintosh-compatible computer connected to an interface (Med Associates) and programmed with LabView software (National Instruments, Austin, TX) controlled event scheduling and data acquisition.
Procedure
Experimental design
All monkeys (25057, 25060, 25070, 25075, 25082, 25083, 25078, 25079) were trained to discriminate 1.0 g/kg ethanol (20% w/v, 5 ml/kg) from water with a 60-min pre-treatment interval. Female monkeys 25060, 25075, 25082 and 25083 were ethanol-naïve at the beginning of the experiment (Table 1). Both male (25078 and 25079, previously 4995 and 4865, respectively, 1.0 g/kg ethanol discrimination) and two of the female (25057 and 25070, previously 2830 and 5400, 1.0 and 2.0 g/kg ethanol discrimination, respectively) monkeys were previously trained (Grant et al. 2000).
Discrimination training
Before each session, the monkeys were seated in a primate chair. The monkeys were habituated to nasogastric (i.g.) gavage, in which an infant feeding tube (5 French, 1.7 × 381 mm) was passed down one nostril through the esophagus and into the stomach. Monkeys 25057, 25070, 25078, and 25079 began the study with discrimination training sessions. For the other monkeys (25060, 25075, 25082, and 25083), the response requirement for each reinforcer was increased from fixed ratio (FR)-1 to a final schedule of FR-20 to FR-65. Simultaneously, the pre-treatment interval was introduced in 5-min increments over 12 sessions for 25060, 25075, and 25083. The house lights were illuminated after each pre-treatment and until the end of the session. The center light above the lever shut off (< 1 s) after the FR requirement was completed and a banana pellet was delivered. Due to low response rate, the pre-treatment interval was omitted for 25082 during shaping of the operant response beginning on session 35. Once responding was reliable, the pre-treatment interval was incremented in 10- and 15-min intervals over 5 sessions at the final FR for this monkey (25082). The FR was chosen for each monkey to result in approximately 5-min training sessions to capture a consistent ethanol stimulus as the basis of discrimination. Males and females, respectively, received a maximum of 20 and 10 pellets each session. Sessions ended after all the pellets were delivered or 30 min. Response training was complete when response rate was ≥ 1.0 responses/s for four consecutive sessions under the final FR schedule, except for 25082, who was slightly slower, making 0.19-0.74 responses/s for four consecutive sessions. Naïve females completed response training in 36, 32, 122 and 44 sessions, respectively, for monkeys 25060, 25075, 25082 and 25083.
For naïve monkeys, tap water (5-10 ml/kg, i.g.) was administered during the first five consecutive sessions of discrimination training. After each ethanol or water gavage, a banana pellet was given to mask any taste differences between the water and ethanol and the monkey was wheeled into the chamber. After 60 min, the “water-appropriate” lever extended into the chamber and the lights above the lever were illuminated. For subsequent sessions, ethanol (0.50 g/kg and 0.75 g/kg for two sessions each, 1.0 g/kg for five sessions) was administered and 60 min later, only the “ethanol-appropriate” lever was extended and the lights above the lever illuminated. Monkey 25083 failed to respond on the lever after the series of ethanol administrations, so it was repeated after a water training session. Subsequently, 25083 responded at her pre-ethanol rates.
For discrimination training sessions, with which previously-trained monkeys began the study, ethanol or water was administered (i.g.) and after 60 min, all lights above the levers illuminated and both levers extended simultaneously. Completion of the FR on the appropriate lever resulted in delivery of a pellet. Responses on the inappropriate lever reset the response count on the appropriate lever to zero. Ethanol and water training sessions alternated after two consecutive sessions with either condition. However, if ≥ 90% of the responses during the entire session and ≥ 70% during the first FR were on the appropriate lever, the training condition was changed for the next session. Discrimination training was complete when these criteria were satisfied for five consecutive sessions. For monkeys failing to acquire ethanol discrimination, the number of training sessions was limited by the time required to complete substitution testing in the other monkeys.
Discrimination testing
Tests were conducted after two sessions with criterion performance. If the performance criteria were not met during a single session, three consecutive sessions with criterion performance were required prior to the next test session. A maximum of 50% of doses tested were administered after both ethanol and water training sessions (by chance, 65% of tests after ethanol training).
Blood-Ethanol Concentration
As in Green et al. (1999), blood-ethanol concentration time-course studies overlapped the pre-treatment period for ethanol discrimination training sessions after ≥ 48-h abstinence from ethanol in the fasted state, i.e., the most recent meal was the evening prior to the day of the time-course. Five-hour BEC time-course studies were repeated in individual monkeys to study ethanol pharmacokinetics by menstrual cycle (measured serum progesterone within 24 h of BEC). Blood samples (20 μl) were collected from the saphenous vein 15, 30, 60, 90, 120, 180, 240, and 300 min post-gavage (1.0 g/kg ethanol, i.g.). When monkeys were in the discrimination chamber, the door was opened and the sample was obtained without removing the monkey (< 2 min). The blood was sealed in an air-tight vial (20 μl 10% isopropanol internal standard and 0.5 ml distilled water) and stored at 4°C until assayed via headspace gas chromatography (Hewlett-Packard 5890 Series II, with flame ionization detector and Hewlett-Packard 3392A integrator).
Menstrual Cycle Characterization
Menstrual cycle data were recorded to determine whether any age differences in ethanol discrimination or substitution efficacy or potency were due to hormonal changes. The monkeys were trained with positive reinforcement to participate in daily vaginal swabbing for menses while in their home cage. Menstrual cycle days were determined by counting backward from menses (Grant et al. 1997) because the duration of the luteal phase (ovulation to menses, reverse cycle days 1-14) is very consistent (Leibenluft et al. 1994). In accordance with Green et al. (1999), the late follicular phase included the days before the luteal phase, including ovulation, and up to reverse cycle day 22 ( reverse cycle days 15-22). The remaining days in the cycle, plus menses, corresponded to the early follicular phase (reverse cycle days 23-37). Approximately three days per week, blood (1 ml) was collected from the femoral vein (22-g needle, EDTA-coated Vacutainer tube) while monkeys were seated in the primate chair. The blood was allowed to clot and then centrifuged (3000 rpm, 15 min) and frozen at 4°C until assayed. Serum progesterone < 2 ng/ml indicated early or late follicular, whereas > 4 ng/ml indicated luteal phase. Serum concentrations of progesterone were measured by the Endocrine Technology and Support Core at the Oregon National Primate Research Center using an Immulite 2000, a chemiluminescence-based automatic clinical platform (sensitivity, 0.2 ng/ml; intra- and inter-assay variation < 15%; Siemens Healthcare Diagnostics, Deerfield, IL). Validation of progesterone includes a comparison of monkey serum samples analyzed coordinately by an Immulite 2000 and a Roche Elecsys 2010 analyzer (chemiluminescence-based clinical platform; Hoffmann-La Roche, Basel, Switzerland) (Jensen et al. 2008).
Drug Treatment
Ethanol (95%) was diluted to 20% (w/v) with tap water and administered in a 5-10 ml/kg (i.g.) volume, followed by 3 ml tap water. Allopregnanolone (0.30-3.0 mg/kg), pregnanolone (1.0-30 mg/kg) and androsterone (1.0-30 mg/kg; Steraloids, Newport, RI) were dissolved in 45% hydroxypropyl beta-cyclodextrin (Sigma, St. Louis, MO) (≤ 5 ml/kg, s.c.). Purified allopregnanolone and pregnanolone were obtained from R.H. Purdy. Pentobarbital (Sigma) and midazolam (Sigma) were dissolved in saline (5 ml/kg, i.g.).
Statistical Analyses
The percentage of total responding on the ethanol-appropriate lever (total responses on the ethanol-appropriate lever/total responses) was calculated for each monkey and test session during which ≥ 1 reinforcer was obtained (≥ 82% of sessions). When a drug completely substituted for ethanol, the dose resulting in 50% ethanol-appropriate responding (ED50) was calculated via linear interpolation between the two doses encompassing the 50% effect (Excel). Some sessions (0-13/monkey) were omitted from the analysis due to equipment malfunction, interfering behavior of the monkey (e.g., dismantling the tubing that delivers banana pellets), or experimenter error. Percent ethanol-appropriate responding, progesterone and BEC were analyzed across menstrual cycle phase and pre-treatment drug with repeated-measures ANOVAs. Sex differences in peak BEC were analyzed using independent-samples t-test. When variance in BEC differed between the sexes, the Sattherwaite approximation of standard error was used, as reflected in the degrees of freedom. Main effects and interactions were evaluated with Bonferroni-corrected pairwise comparisons. Statistical analyses were conducted using SAS 9.2. For all tests, α < 0.05.
Results
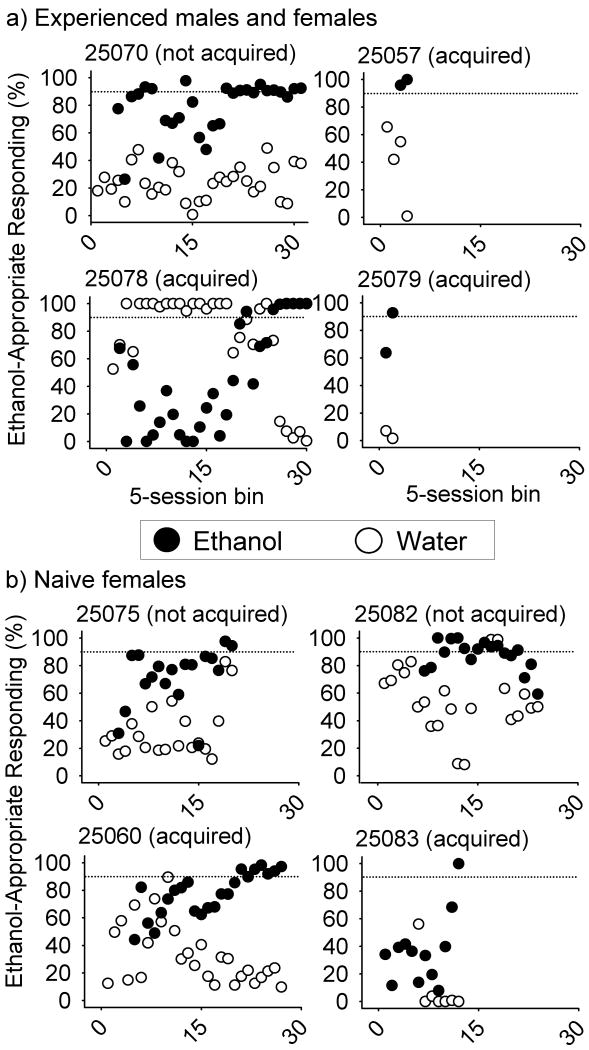
Figure 1 shows ethanol-appropriate responding aggregated across 5-session bins for each monkey, counting backwards from the last training session. Since the total number of training sessions was not necessarily divisible by five, the first data point for each monkey may represent fewer than five sessions. Also, because the maximum number of consecutive ethanol or water training sessions was two, sessions contributing to the same session bin did not necessarily occur on consecutive days. Lastly, 4/8 monkeys attained criterion performance during more ethanol than water training sessions, resulting in a greater number of water training sessions. Previously-trained monkeys completed discrimination training in 27, 177, and 29 sessions for 25057, 25078, and 25079, respectively. Monkey 25070, previously trained to discriminate 2.0 g/kg ethanol, failed to acquire 1.0 g/kg ethanol discrimination after 283 sessions. Two naïve monkeys (25075, 25082) failed to acquire ethanol discrimination after 189 and 204 sessions, respectively. The other two naïve monkeys (25060, 25083) completed discrimination training in 250 and 90 sessions, respectively, and testing occurred three times in 47 sessions and 13 times in 197 sessions (15 sessions/test). In contrast, previously-trained monkeys had 39 tests in 216 sessions (25057, 5.5 sessions/test), 10 tests in 74 sessions (25078, 7.4 sessions/test) and 31 tests in 234 sessions (25079, 7.5 sessions/test). As described in the Methods, the number of training sessions allowed was determined for each monkey by health issues (25075) or the time required for the other monkeys to complete substitution testing.
Fig. 1.
Percentage of responses on the ethanol-appropriate lever averaged across five-session bins in which water (open circles) or 1.0 g/kg ethanol (closed circles) was administered during discrimination training of middle-aged cynomolgus monkeys. a) Monkeys 25057, 25078 and 25079 were previously trained to discriminate 1.0 g/kg ethanol and monkey 25070 was previously trained to discriminate 2.0 g/kg ethanol; b) Naïve females
A majority of menstrual cycles in 4/6 middle-aged female monkeys were ovulatory (peak progesterone > 4 ng/ml) and of normal duration (Table 1). Menstrual cycle phase could not be determined due to absence of menses for 25082 in two BEC time-courses. Due to ovariectomy, one BEC time-course determined in 25057 could not be associated with a specific menstrual cycle phase. Excluding these data, 55% and 45% of BEC time-courses were collected in the follicular (average day: early, 25; late, 20) and luteal phases (average day, 8), respectively.
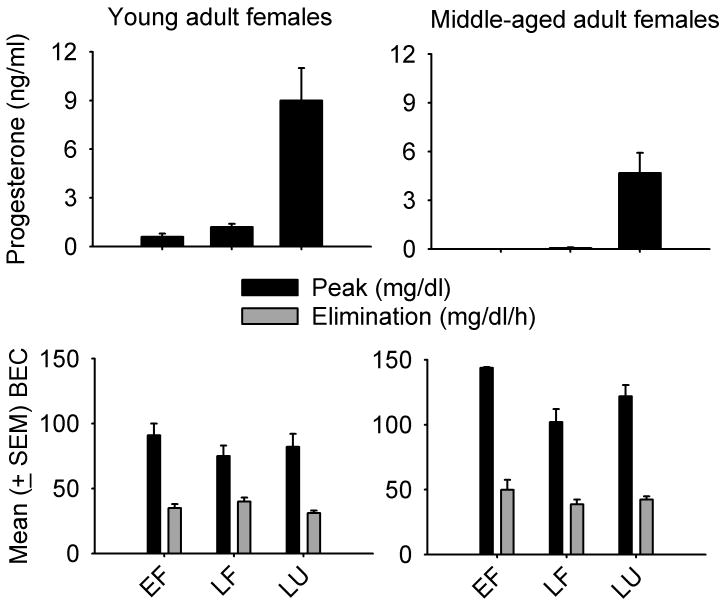
Since BEC time-course was not measured for all cycle phases in all monkeys, the data from early and late follicular phases were collapsed together. A repeated measures ANOVA for mean progesterone revealed a main effect of cycle phase, F(1, 4) = 30.6, p < 0.01, with greater progesterone in the luteal compared to the follicular phase. In contrast, repeated measures ANOVAs for elimination rate, F(1, 4) = 0.40, and peak (60-min) BEC, F(1, 4) = 0.88, showed similar ethanol pharmacokinetics in the follicular and luteal phases (Figure 2). Middle-aged female monkeys showed greater peak (60-min) BECs (mean ± SEM, 103.9 ± 7.2 mg/dl) compared to the two middle-aged male monkeys tested [63.2 ± 0.2 mg/dl; t(5.0) = 5.7, p = 0.002].
Fig. 2.
Mean (± SEM) progesterone, peak blood-ethanol concentration (BEC) and elimination rate by menstrual cycle phase (early follicular, EF; late follicular, LF; luteal, LU) in 13-21-year old female cynomolgus monkeys in the current study and young adult female cynomolgus monkeys (Green et al. 1999)
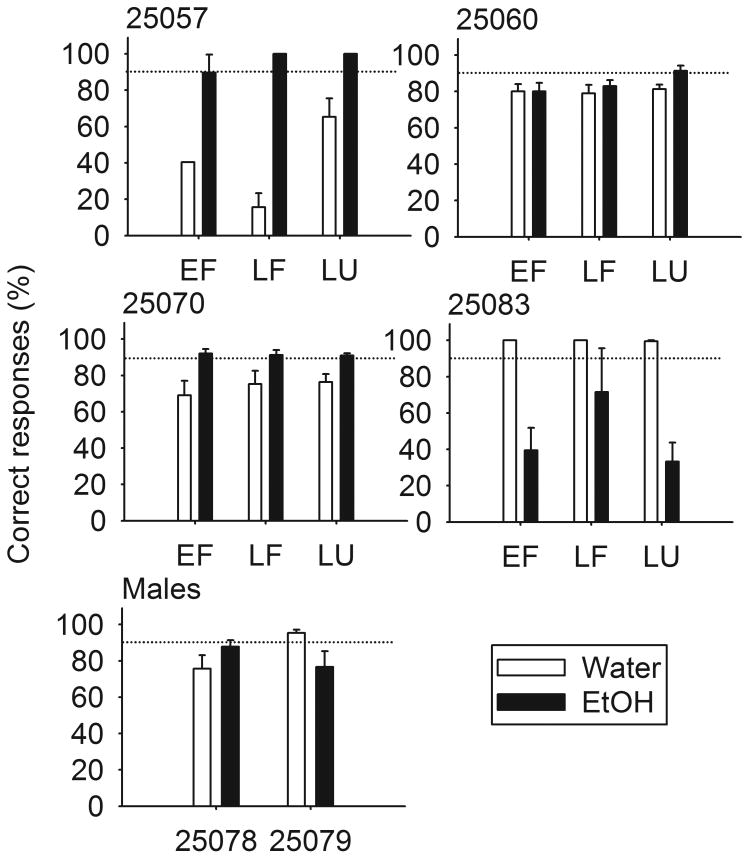
Discrimination accuracy did not vary with menstrual cycle phase, F(2, 8) = 0.07, or pre-treatment drug, F(1, 7) = 0.07 (Figure 3). However, all except male 25079 and female 25083 were more accurate during ethanol compared to water training sessions. Figure 1 suggests that performance during ethanol and water training sessions began to diverge half-way into training, so the data in Figure 3 were taken from the last 50% of training sessions, except for male 25079 and female 25057 for whom all discrimination training sessions were used. Only 25057 showed slightly greater water discrimination accuracy during the luteal compared to the follicular phase of the menstrual cycle.
Fig. 3.
Mean (± SEM) percentage correct responses across menstrual cycle phase during water (open) and ethanol (closed) discrimination training sessions prior to generalization testing in individual female and male cynomolgus monkeys. Discrimination for female monkeys is split by menstrual cycle phase (EF, early follicular; LF, late follicular; LU, luteal; see Methods for details). For all monkeys excluding 25057 and 25079, for whom discrimination was re-acquired in < 30 sessions, the data plotted represent the last half of all training sessions
Comparison of test drug substitution potency between young and middle-aged adult cynomolgus monkeys is shown in Table 2. The sex difference in ED50 for ethanol substitution for 1.0 g/kg ethanol observed in young adult monkeys was also observed in the middle-aged monkeys, although not confirmed statistically, t(7.9) = -1.9, p = 0.09. Young adult and middle-aged monkeys' ED50 did not differ for any test drug except midazolam, t(7) = -3.7, p < 0.01 (ethanol, t(11) = -0.02; allopregnanolone, t(10) = -0.40; pregnanolone, t(9) = 1.0; androsterone, t(8) = -0.48; pentobarbital, t(8) = -1.96, p = 0.09). For midazolam substitution, the middle-aged male and female were also tested as young adults (Table 3) and these values were included in the means for young adults in Table 2.
Table 2.
Mean (± SEM) ED50 for test drugs that completely substitute for the discriminative stimulus effects of 1.0 g/kg ethanol (60-min pre-treatment) in young and middle-aged adult cynomolgus monkeys
| Young Adult Female | Middle-Aged Female | Young Adult Male | Middle-Aged Male | |
|---|---|---|---|---|
| Ethanol | 330 ± 30 (n=4)a | 352 ± 60 (n=3) | 560 ± 14 (n=4)a | 503 ± 64 (n=2) |
| Pentobarbital | 2.6 ± 1.6 (n=4)a | 5.01 ± 4.41 (n=2)c,d | 2.5 ± 0.4 (n=4)a | 7.80 (n=1) |
| Midazolam | 1.2 ± 0.8 (n=4)a | 2.42 (n=1)c | 1.5 ± 0.5 (n=4)a | 2.43 (n=1) |
| Allopregnanolone | 0.75 ± 0.21 (n=4)b | 1.04 ± 0.13 (n=2)c,d | 0.83 ± 0.37 (n=5) | 0.73 ± 0.05 (n=2) |
| Pregnanolone | 3.83 ± 1.03 (n=4)b | 2.94 ± 1.55 (n=2)c,d | 4.70 ± 1.15 (n=6) | 2.00 (n=1) |
| Androsterone | 10.90 ± 1.17 (n=3)b | 22.94 (n=1)c,d | 16.69 ± 3.90 (n=4) | 13.73 ± 9.44 (n=2) |
Data previously published in Grant et al., 2000;
Data previously published in Grant et al., 2008;
one monkey ovariectomized;
saline vehicle not tested, water test data used for 25083
Table 3.
Maximum percent ethanol-appropriate responding and ED50 (mg/kg, parentheses) for test drugs to substitute for 1.0 g/kg ethanol with a 60-min pre-treatment longitudinally in cynomolgus monkeys (female, 25057; males, 25078 and 25079).
| Test Drug | 25057 | Year | 25078 | Year | 25079 | Year |
|---|---|---|---|---|---|---|
| Ethanol | 100 (352) | 1997 | 100 (262) | 1999 | 100 (829) | 1997 |
| 100 (671) | 2007 | 100 (632) | 2008 | 100 (375) | 2007 | |
| 100 (830) | 2008a | |||||
| Pentobarbital | 98 (6.94) | 1997 | NA | 100 (1.41) | 1998 | |
| 97 (3.82) | 2008a | NA | 100 (7.8) | 2008 | ||
| Midazolam | 100 (0.57) | 1997 | NA | 100 (0.51) | 1997 | |
| 89 (2.43) | 2008a | NA | 97 (1.68) | 2003 | ||
| 89 (2.43) | 2008 | |||||
| Allopregnanolone | 97 (0.73) | 1999 | 100 (2.28) | 2002 | 100 (0.78) | 1998 |
| 100 (1.32) | 2008a | 100 (1.35) | 2008 | 100 (0.83) | 2008 | |
| Pregnanolone | 59 (NA) | 1999 | NA | 90 (3.0) | 1998 | |
| 90 (1.39) | 2008a | NA | 100 (1.37) | 2008 | ||
| Androsterone | 67 (NA) | 1999 | 0 (NA) | 1998 | 100 (5.60) | 1998 |
| 100 (22.94) | 2008a | 100 (23.17) | 2008 | 100 (4.29) | 2008 |
NA, data not available;
post-ovariectomy
In 12/15 within-subjects comparisons (Table 3), the test drugs substituted with similar efficacy for the discriminative stimulus effects of 1.0 g/kg ethanol up to 11 years after the first dose-response curves were measured. In three cases in which neuroactive steroids showed low efficacy in young adulthood, neuroactive steroids showed greater substitution efficacy when the monkeys were older. Specifically, for 25057, pregnanolone and androsterone partially-substituted for ethanol during 1999 and 2002, but completely substituted in 2008. For 25078, androsterone did not substitute for ethanol during 1998, but completely substituted in 2008.
Within subjects, ethanol substitution potency did not significantly differ between young adulthood and middle age according to repeated measures ANOVA, F(1, 2) = 0.07. The substitution potency of pentobarbital, F(1, 1) = 0.11, and midazolam, F(1, 1) = 0.11, did not differ significantly between young adulthood and middle age according to repeated measures ANOVA. Likewise, the potency of allopregnanolone did not vary with age, F(1, 2) = 0.07. In 2001, 17.0 mg/kg pregnanolone partially-substituted for ethanol in 25057 and increased response rate by approximately 30%. After this same dose in 2008, 25057 was not responsive in the chamber. Due to low efficacy of pregnanolone and androsterone in young adulthood, statistical comparison of ED50 for these test drugs between young adulthood and middle age could not be conducted.
Discussion
These studies show consistent GABAA receptor involvement in the discriminative stimulus effects of ethanol over approximately one-third of monkeys' life spans (averages 30 years in captivity). Of 15 dose-response curves characterized in middle-aged monkeys, zero show reduced substitution efficacy and only three showed increased efficacy (i.e., neuroactive steroid) compared to data from the same monkeys in young adulthood (Table 3). Pregnanolone, allopregnanolone and androsterone positively-modulate GABAA receptors (Park-Chung et al. 1999). The expression of GABAA receptors changes with age in rats (6-24-month old, Mhatre and Ticku 1992; 10-d to 18-month old, Yu et al. 2006). Reproductive hormones decline with age (e.g., dehydroepiandrosterone, Kemnitz et al. 2000; Schumacher et al., 2003), and gonadectomized male (but not female) mice showed nearly 4-fold greater GABAA receptor binding in forebrain membranes compared to mice that underwent sham surgery (Akinci and Johnston 1997). In female rhesus monkeys, brain expression of GABAA receptor subunits varies with ovariectomy, or ovariectomy with 17β-estradiol or 17β-estradiol plus progesterone replacement (Noriega et al. 2010). Thus, altered potency or efficacy of neuroactive steroids with age could be due to hormonal-related changes in GABAA receptor expression.
The current data, however, suggest that age-related differences in progesterone across the menstrual cycle did not greatly influence ethanol discrimination, substitution potency or efficacy. Within subjects, variation in endogenous hormones across the menstrual cycle did not influence accuracy of water or ethanol discrimination (Figure 3). Serum progesterone in middle-aged females in the current study was approximately 2-fold lower than young adult female monkeys described by Green et al. (1999). However, the efficacy and potency of ethanol and GABAA receptor positive modulators to substitute for the discriminative stimulus effects of ethanol was similar between the middle-aged monkeys tested in the current study and young adult monkeys (Table 2). Thus, although endogenous progesterone-derived neuroactive steroids contribute additively to ethanol-like discriminative stimulus effects (i.e., allopregnanolone, Grant et al. 1997), the GABAA receptor mechanisms mediating these effects are stable despite age-related changes in progesterone.
Peak BEC was slightly greater in middle-aged monkeys in the current study compared to data from young adult monkeys (Green et al. 1999; Figure 2). Training dose influences substitution potency (e.g., Grant et al. 2000, 2008) and affects brain concentrations of the drug during training. If age differences in ethanol pharmacokinetics affected substitution potency, all potency changes would be expected to be in the same direction (i.e., leftward or rightward). In contrast, the current study observed increases and decreases in potency of test drugs within the same individuals, implicating pharmacodynamic factors.
Changes in pharmacokinetics could account for age-related changes in the potency of test drugs to substitute for ethanol, i.e., the lower potency of midazolam in middle-aged monkeys compared to young adults (Table 2). Albrecht et al. (1999) reported that 24-28-year old and 67-81-year old men did not differ in a majority of pharmacokinetic parameters, but midazolam was distributed more slowly in aged men. Intestinal metabolism of oral midazolam is greater in cynomolgus monkeys compared to humans (Takahashi et al. 2010), suggesting that humans may be more sensitive to midazolam compared to monkeys. However, both cynomolgus monkeys (Kanazu et al. 2004) and humans (Thummel and Wilkinson 1998) metabolize midazolam via cytochrome P450 3A enzymes, with no difference between young and aged adult humans (Klotz 2009).
The naïve middle-aged monkeys in the current study showed weak ethanol stimulus control despite greater peak BECs compared to previously-published data from young adult monkeys (Green et al. 1999; Figure 2). Twice as many trials were required for 28-34-year old rhesus monkeys to discriminate exteroceptive visual stimuli with 85% accuracy compared to 8-13-year old monkeys. In contrast, aged and young adult monkeys required similar numbers of trials to acquire a spatial discrimination (Voytko et al. 1999). An inverse correlation is observed between sensitivity to an interoceptive stimulus (i.e., heart beat) and age in humans (Khalsa et al. 2009). Thus, interoceptive ethanol-related stimuli may be less salient in aged compared to young adult individuals.
Exposure to ethanol or ethanol discrimination training in young adulthood may facilitate behavioral control by ethanol-like discriminative stimulus effects in middle age, consistent with the decreased odds of alcohol dependence when drinking begins at a later age (Grant et al. 2001). Two previously-trained monkeys attained criterion performance after < 30 sessions, whereas naïve young adult (Grant et al. 2000) and middle-aged (present study) monkeys require > 100 sessions to criterion performance, indicating memory for ethanol discrimination despite an approximately three-year lapse in training. The present study suggests that GABAA receptors are likely to mediate the discriminative stimulus effects of ethanol for at least one-third of the human lifespan, which is approximately 78 years in the United States (Central Intelligence Agency World Fact Book). This is an important consideration given that alcoholism is a long-term chronically-relapsing disease that often begins in young adulthood (O'Brien and McLellan 1996).
Acknowledgments
This research was supported by RR000163, NIH/NIAAA AA013510, AA13541, AA017040, and NIH/NIA AG023477.
References
- Akinci MK, Johnston GAR. Sex differences in the effects of gonadectomy and acute swim stress on GABAA receptor binding in mouse forebrain membranes. Neurochem Int. 1997;31:1–10. doi: 10.1016/s0197-0186(96)00143-x. [DOI] [PubMed] [Google Scholar]
- Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, Schüttler J. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. 1999 doi: 10.1016/S0009-9236(99)90084-X. [DOI] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Blow FC, Barry KL. Use and misuse of alcohol among older women. Alcohol Res Health. 2002;26:308–315. [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Vital and Health Statistics, Series 10, No 245 Health Behaviors of Adults: United States, 2005-2007; DHHS Publication No (PHS) 2010-1573. 2010. [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou P, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV Alchol Abuse and Dependence: United States, 1991-1992 and 2001-2002. Alcohol Res Health. 2006;29:79–993. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Dep. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3α-hydroxy-5α-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Helms CM, Rogers LSM, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008;326:354–361. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABAA receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology. 2000;152:181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis) Alcohol Clin Exp Res. 1999;23:611–616. [PubMed] [Google Scholar]
- Groerer JC, Penne MA, Pemberton MR, Folsom RE. Substance abuse treatment need among older adults in 2020: the impact of the aging baby-boom cohort. Drug Alcohol Abuse. 2003;69:127–135. doi: 10.1016/s0376-8716(02)00307-1. [DOI] [PubMed] [Google Scholar]
- Groseclose CH, Middaugh LD. The discrimination and durability of an ethanol cue in young and mid-aged female mice. Alcohol. 1997;14:191–197. doi: 10.1016/s0741-8329(96)00145-0. [DOI] [PubMed] [Google Scholar]
- Helms CM, Rogers LSM, Waters CA, Grant KA. Zolpidem generalization and antagonism in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol. Alcohol Clin Exp Res. 2008;32:1197–1206. doi: 10.1111/j.1530-0277.2008.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception. 2008;77:303–307. doi: 10.1016/j.contraception.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, Jones BM. The relationship of age and drinking habits to the effects of alcohol on memory in women. J Stud Alcohol. 1980;41:179–186. doi: 10.15288/jsa.1980.41.179. [DOI] [PubMed] [Google Scholar]
- Jung S, Harris RA. Sites in TM2 and 3 are critical for alcohol-induced conformational changes in GABAA receptors. J Neurochem. 2006;96:885–892. doi: 10.1111/j.1471-4159.2005.03617.x. [DOI] [PubMed] [Google Scholar]
- Kanazu T, Yamaguchi Y, Okamura N, Baba T, Koike M. Model for the drug-drug interaction responsible for CYP3A enzyme inhibition. I: evaluation of cynomolgus monkeys as surrogates for humans. Xenobiotica. 2004;34:391–402. doi: 10.1080/00498250410001685755. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Stoops WW, Perry AS, Prendergast MA, Rush CR. Clinical neuropharmacology of drugs of abuse: a comparison of drug-discrimination and subject-report measures. Behav Cog Neurosci Rev. 2003;2:227–260. doi: 10.1177/1534582303262095. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Roecker EB, Haffa ALM, Pinheiro J, Kurzman I, Ramsey JJ, MacEwen EG. Serum dehydroepiandrosterone sulfate concentrations across the life span of laboratory-housed rhesus monkeys. J Med Primatol. 2000;29:330–337. doi: 10.1034/j.1600-0684.2000.290504.x. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Tranel D. Interoceptive awareness declines with age. Psychophysiol. 2009;46:1130–1136. doi: 10.1111/j.1469-8986.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Fiero PL, Rubinow DR. Effects of menstrual cycle on dependent variables in mood disorder research. Arch Gen Psychiatry. 1994;51:761–781. doi: 10.1001/archpsyc.1994.03950100009002. [DOI] [PubMed] [Google Scholar]
- Lucey MR, Hill EM, Young JP, Demo-Dananberg L, Beresford TP. The influences of age and gender on blood ethanol concentrations in health humans. J Stud Alcohol. 1999;60:103–110. doi: 10.15288/jsa.1999.60.103. [DOI] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF. Selective antagonism of 5α-reduced neurosteroid effects at GABAA receptors. Mol Pharmacol. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Aging related alterations in GABAA receptor subunit mRNA levels in Fischer rats. Mol Brain Res. 1992;14:71–78. doi: 10.1016/0169-328x(92)90012-z. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. p. 125. [Google Scholar]
- Noriega NC, Eghlidi DH, Garyfallou VT, Kohama SG, Kryger SG, Urbanski HF. Influence of 17β-estradiol and progesterone on GABAergic gene expression in the arcuate nucleus, amygdala and hippocampus of the rhesus macaque. Brain Res. 2010;1307:28–42. doi: 10.1016/j.brainres.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Nocera R, Fuller LM, Kordower JH, Armstrong DM. Age-related alterations in GABAA receptor subunits in the nonhuman primate hippocampus. Brain Res. 2006;1073-1074:120–130. doi: 10.1016/j.brainres.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Signs SA, Boja JW. Stability of the stimulus properties of drugs over time. Pharmacol Biochem Behav. 1989;32:361–364. doi: 10.1016/0091-3057(89)90256-6. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJM, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Washio T, Suzuki N, Igeta K, Yamashita S. Investigation of the intestinal permeability and first-pass metabolism of drugs in cynomolgus monkeys using single-pass intestinal perfusion. Biol Pharm Bull. 2010;33:111–116. doi: 10.1248/bpb.33.111. [DOI] [PubMed] [Google Scholar]
- The World Factbook 2009. Washington, DC: Central Intelligence Agency; 2009. https://www.cia.gov/library/publications/the-world-factbook/index.html. [Google Scholar]
- Thompson SA, Whiting PJ, Wafford KA. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br J Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Wilkinson GR. In vitro and in vivo drug interaction involving human CYP3A. Ann Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol Aging. 1999;20:617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]