Abstract
Background
Endothelin-1 (ET-1) is a potent vasoconstrictor implicated in the pathogenesis of vasospasm and delayed cerebral ischemia (DCI) in aneurysmal subarachnoid hemorrhage (aSAH) patients. The aim of this study was to investigate the relationship between cerebrospinal fluid (CSF) ET-1 levels and angiographic vasospasm and DCI.
Methods
Patients with aSAH were consented (n = 106). Cerebral vasospasm was determined by angiography. DCI was determined by transcranial Doppler (TCD) results and/or angiogram results with corresponding clinical deterioration. CSF ET-1 levels over 14 days after the initial insult was quantified by ELISA. ET-1 analysis included a group-based trajectory analysis and ET-1 exposure rate during 24, 48, and 72 h prior to, as well as 72 h post angiography, or clinical deterioration.
Results
Trajectory analysis revealed two distinct groups of subjects with 56% of patients in the low ET-1 trajectory group (mean at day 1 = 0.31 pg/ml; SE = 0.04; mean at day 14 = 0.41 pg/ml; SE = 0.15) and 44% of patients in the high ET-1 trajectory group (mean at day 1 = 0.65 pg/ml; SE = 0.08; mean at day 14 = 0.61 pg/ml; SE = 0.06). Furthermore, we observed that ET-1 exposure rate 72 h before angiography and clinical spasm was a significant predictor of both angiographic vasospasm and DCI, whereas, ET-1 exposure after angiography and clinical spasm was not associated with either angiographic vasospasm or DCI.
Conclusion
Based on these results we conclude that ET-1 concentrations are elevated in a sub-group of patients and that the acute (72 h prior to angiography and clinical neurological deterioration), but not chronic, elevations in CSF ET-1 concentrations are indicative of the pathogenic alterations of vasospasm and DCI in aSAH patients.
Keywords: aSAH, CSF, Endothelin-1, Vasospasm, DCI
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating neurological insult affecting approximately 30,000 people per year with a 25% mortality rate. Angiographic vasospasm or arterial vasospasm indicating vascular constriction is present in approximately 70% of patients. Roughly, 20–30% of aSAH patients develop DCI. It is a common sequela of aSAH and is a significant contributor to overall morbidity and mortality. Survivors of the initial insult suffer long-term functional and neuropsychological impairment due to DCI that significantly affects their ability to resume previously held roles [1–3]. Although advances have been made in understanding the pathology of aSAH, there has been limited success in identifying strategies for prevention of DCI or improving neurological outcomes. This is due, in large part, to the paucity of studies evaluating molecular mechanisms in human pathogenesis and the lack of predictive factors involved in DCI. Absent in knowledge of causative mechanisms, it remains difficult to develop interventions to optimally manage this patient population.
Preclinical and clinical studies have implicated metabolites that regulate cerebrovasculature as important mediators of reduced blood flow and subsequent development of vasospasm after aSAH. ET-1 is one such vasoactive substance that has been implicated in the development of vasospasm; elevated levels of ET-1 in cerebrospinal fluid (CSF) have been suggested as causal factors in the pathogenesis of cerebral vasospasm in several clinical studies [4–7]. However, evidence for the association between ET-1 and vasospasm is inconsistent [8–11], in part, due to study design differences in evaluating the temporal profile of ET-1 relative to vasospasm diagnosis. Based on these conflicting reports, the potential relationship between the temporal profile of ET-1 concentrations and pathologic alterations in vascular response remains unclear.
A full understanding of the utility of ET-1 in patients with aSAH remains elusive due to clinical difficulty in measuring ET-1 at the peak interval for clinical vasospasm or over a longer time interval, inadequate power to detect changes of interest due to small sample size, and inconsistencies in the operationalization of DCI by clinical and angiographic evidence. Based on these issues, we evaluated the temporal relationship between CSF ET-1 concentrations and evidence of angiographic vasospasm and DCI in a large cohort of aSAH patients. Specifically, the purpose of this study was to: (1) examine changes in CSF ET-1 levels during the first 14 days following aSAH, (2) determine whether CSF ET-1 levels over the first 14 days following aSAH as well as ET-1 levels during the 24, 48, and 72 h prior to angiography or clinical neurological deterioration were associated with vasospasm and DCI. In this study, we observed that ET-1 CSF exposure 72 h prior to angiography and clinical neurological deterioration is predictive of both angiographic vasospasm and DCI whereas, total ET-1 CSF exposure was not associated with either vasospasm or DCI. These results suggest that the temporal profile of ET-1 CSF concentrations is essential in determining the relationship between ET-1 and vascular alterations after aSAH.
Methods
Patient Sample
Data were obtained from an ongoing NIH trial. Participants were recruited from a neurovascular intensive care unit. Eligibility criteria were: (1) 18–75 years of age, (2) diagnosed with an aSAH via cerebral angiogram and head computed tomography; (3) Fisher grade ≥2 and/or Hunt and Hess grade ≥3; (4) no history of neurological disorders; and (5) ventriculostomy in place. Exclusion criteria were traumatic SAH, mycotic aneurysm, or arteriovenous malformation. The protocol was approved by the Institutional Review Board; informed consent was obtained from the patient/proxy prior to data collection.
Data Collection
A total of 1 cc of CSF was withdrawn from the ventriculostomy port closest to the patient each morning (8 am ± 1 h) and evening (8 pm ± 1 h) until (1) the ventriculostomy was removed, (2) the patient expired/was discharged, or 3) 14 days elapsed from the initial hemorrhage. CSF samples were also collected from patients who received lumbar drains. Specimens were stored at −80° for batch analysis. Socio-demographic and clinical data were collected via chart review.
The majority of patients were Caucasian females with a Hunt and Hess score of 3–5 and a Fisher score of 3–4 (Table 1). Patients (28%) who had Hunt and Hess scores 1 and 2 had ventriculostomy in place because of the presence of hydrocephalus, subsequent neurological deterioration, or mental status change. Mean duration of ventriculostomy placement was 7 ± 3.4 days; 11.7 ± 6.5 CSF samples were obtained per patient. All 106 participants had CSF available for ET-1 analysis over time, 79 patients had CSF drains via an external ventricular catheter (EVD), 13 had lumbar drains (LD), and 14 had initial EVD followed by LD. Angiographic data were available on 71 participants (67%) and DCI data were available on 85 patients (80%).
Table 1.
Socio-demographic and clinical characteristics of total sample, N = 106
| Characteristic | N | % |
|---|---|---|
| Gender | ||
| Female | 75 | 71 |
| Male | 31 | 29 |
| Ethnicity | ||
| Caucasian | 92 | 87 |
| Non-Caucasian | 14 | 13 |
| Hunt and Hess grade upon admission | ||
| I–II | 30 | 28 |
| III–IV | 76 | 72 |
| Fisher grade upon admission | ||
| II | 26 | 25 |
| III | 51 | 48 |
| IV | 29 | 27 |
| Vasospasm | ||
| None or mild | 31 | 29 |
| Moderate or severe | 25 | 24 |
| No post-intervention angiogram performed | 50 | 47 |
| Range | Mean (SD) | |
|---|---|---|
| Age | 24–75 | 53.9 (10.6) |
| Years of education | 3–20 | 12.5 (2.6) |
| Number of days ventriculostomy intact/patient | 1–14 | 7 (3.4) |
| Number of CSF samples available/patient | 1–25 | 11.7 (6.5) |
Measures
Endothelin-1 Levels
ET-1 levels were quantified using QuantiGlo® Human Endothelin-1 Immunoassay kit (R & D Systems, Minneapolis, MN) with a modified standard curve range yielding interday and intraday reproducibility (<15% CV) with a limit of quantitation of 0.195 pg/ml. ET-1 chemiluminescence detection was performed using a Wallace Victor2 1420 MultiLabel Counter (PerkinElmer Wallace, Gaithersburg, MD). Each sample was analyzed separately. A repeated measures model test showed ET-1 levels did not differ significantly between two samples per day, so the mean value was used. The ET-1 exposure rate was determined at 24, 48, and 72 h prior to angiography or clinical neurological deterioration as follows:
Delayed Cerebral Ischemia
Angiography was performed as standard care on admission, as post-operative follow-up care, and/or in the event of neurological deterioration. All patients received standard medical care including nimodipine and triple H therapy, and early surgical or endovascular intervention. Angiographic vasospasm was determined from cerebral angiograms read and coded by neurosurgeons blinded to participant identity and patients were grouped as either “negative” (0–24% narrowing of cerebral blood vessels) or “positive” (≥25% narrowing of cerebral blood vessels). To determine DCI, the following variables were examined: angiographic results when present, daily TCD ultrasound results (mean systolic velocities >200 and/or Lindegaard ratios >3) accompanied by clinical deterioration as recorded by the nurse (decrease of 2 or more from the previous assessment in the National Institutes of Health Stroke Scale (NIHSS)) [12, 13]. Patients were grouped as: (1) DCI positive—elevated vessel velocities on TCD ultrasound and/or angiographic vasospasm with corresponding clinical deterioration, or (2) DCI negative—the absence of neurologic deterioration or neurologic deterioration with no changes in cerebral vessel narrowing.
Data Analysis
Gender, age, race, Hunt and Hess and Fisher grade were obtained from medical records and education information from the participant/proxy. Due to the clinical nature of the disease and standards of care following aSAH (e.g., placement of a ventriculostomy and use of cerebral angiography), different subgroups of the same sample were used for each analysis, allowing the most parsimonious way to present the data. To examine homogeneous latent trajectory classes in CSF ET-1 levels during the first 14 days following aSAH (n = 106), group-based trajectory analysis was performed with the PROC TRAJ macro in SAS version 9.1 [14, 15]. This procedure identifies distinct subgroups within the population, conducts an approximation of the general distribution of the data, and groups similarly behaving patterns together. Trajectory groups are a convenient statistical device for summarizing trajectories. Log transformation which remained consistent with the distribution of the raw data was applied to reduce sample variation and skewness for better model fitting. The Bayesian Information Criterion (BIC) and the substantive utility of the classes (e.g., distinctiveness of the trajectories, proportion assigned to a given class) were used to determine the optimal solution for the number of trajectory groups. Chi-square analyses were completed to determine the overall association between ET-1 levels over time identified by the trajectory analysis and angiographic vasospasm or DCI. Binary logistic regression models were created to examine the association between angiographic vasospasm and ET-1 exposure rates 24, 48, and 72 h prior to detection of angiographic vasospasm and DCI after controlling for sex, age, race, Hunt and Hess grade, and Fisher grade. Independent t-tests were completed after log transformation of the data to determine the association between angiographic vasospasm and ET-1 exposure rate 72 h prior to spasm and after spasm in spasm negative and positive groups. Similar analysis was performed for ET-1 exposure rates in DCI positive and negative patients 72 h prior and after DCI.
Results
CSF ET-1 Levels During the First 14 Days Following aSAH
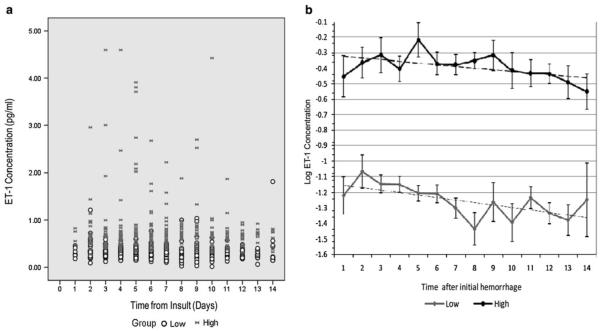
CSF ET-1 concentrations over the first 14 days after initial insult (Fig. 1a, n = 106) differentiated the population into high and low groups as determined by a trajectory analysis (Fig. 1b). Participants in Group 1 (n = 59, 56%) had relatively low ET-1 values (mean at day 1 = 0.31 pg/ml; SE = 0.04; mean at day 14 = 0.41 pg/ml; SE = 0.15); Group 2 (n = 47, 44%) had higher ET-1 values (mean at day 1 = 0.65 pg/ml; SE = 0.08; mean at day 14 = 0.61 pg/ml; SE = 0.06). Within the high and low ET-1 groups, there was no significant change from day 1 to day 14. A two-group trajectory model was defined (Fig. 1b), suggesting two groups of participants with significantly (P < 0.01) different ET-1 values. To explore potential reasons for this finding, chi-square analyses and t tests were used to evaluate potential between-group differences. No significant between-group differences were found related to socio-demographic or clinical characteristics including method of treatment of original aneurysm and CSF drainage methods (Table 2).
Fig. 1.
Raw population values of CSF ET-1 concentrations (pg/ml) over the first 14 days from the time of insult from 106 aSAH patients (a). Raw data are denoted as samples from high ( ) and low (open circle) groups as identified by trajectory analysis (a). Log ET-1 concentrations versus time from insult (days) in high (filled circle) and low (filled diamond) groups as identified by trajectory analysis (b). Data are presented as mean log ET-1 values measured per day. The model shows two groups of patients with significantly different (P < 0.001) ET-1 values. Patients in low group (n = 59, 56%) have relatively low ET-1 values (mean at day 1 = 0.31 pg/ml; SE = 0.04; mean at day 14 = 0.41 pg/ml; SE = 0.15) and those in high group (n = 47, 44%) have higher ET-1 values (mean at day 1 = 0.65 pg/ml; SE = 0.08; mean at day 14 = 0.61 pg/ml; SE = 0.06)
) and low (open circle) groups as identified by trajectory analysis (a). Log ET-1 concentrations versus time from insult (days) in high (filled circle) and low (filled diamond) groups as identified by trajectory analysis (b). Data are presented as mean log ET-1 values measured per day. The model shows two groups of patients with significantly different (P < 0.001) ET-1 values. Patients in low group (n = 59, 56%) have relatively low ET-1 values (mean at day 1 = 0.31 pg/ml; SE = 0.04; mean at day 14 = 0.41 pg/ml; SE = 0.15) and those in high group (n = 47, 44%) have higher ET-1 values (mean at day 1 = 0.65 pg/ml; SE = 0.08; mean at day 14 = 0.61 pg/ml; SE = 0.06)
Table 2.
Socio-demographic and clinical characteristics of patients with high and low CSF ET-1 levels
| Characteristic | Group |
P value | ||
|---|---|---|---|---|
| Low N = 59 N (%) |
High N = 47 N (%) |
|||
| Gender | Male | 16 (27%) | 15 (32%) | 0.59 |
| Female | 43 (73%) | 32 (68%) | ||
| Race | Non-white | 9 (15%) | 5 (11%) | 0.49 |
| White | 50 (85%) | 42 (89%) | ||
| Aneurysm side | Left | 10 (29%) | 10 (40%) | 0.40 |
| Right | 24 (71%) | 15 (60%) | ||
| Aneurysm locale | Anterior | 44 (75%) | 35 (74%) | 0.99 |
| Posterior | 15 (25%) | 12 (26%) | ||
| Intervention | Clipping | 31 (53%) | 32 (68%) | 0.11 |
| Coiling | 28 (47%) | 15 (32%) | ||
| Drainage device | External ventricular (EVD) | 43 (42%) | 36 (34%) | 0.95 |
| Lumbar (LD) | 8 (7%) | 5 (5%) | ||
| EVD then LD | 8 (7%) | 6 (6%) | ||
| Hunt and Hess grade | I–II | 19 (32%) | 11 (23%) | 0.32 |
| III–V | 40 (68%) | 36 (77%) | ||
| Fisher grade | 2 | 16 (27%) | 10 (21%) | 0.66 |
| 3 | 26 (44%) | 25 (53%) | ||
| 4 | 17 (29%) | 12 (26%) | ||
| Vasospasm | Negative | 19 (32%) | 21 (45%) | 0.10 |
| Positive | 16 (27%) | 15 (32%) | ||
| Unavailable | 24 (41%) | 11 (23%) | ||
| DCI | Negative | 28 (48%) | 15 (32%) | 0.21 |
| Positive | 22 (37%) | 20 (43%) | ||
| Unavailable | 9 (15%) | 12 (25%) | ||
| Low |
High |
P value | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Range | N | Mean (SD) | Range | ||
| Age | 59 | 53 (11) | 29–75 | 47 | 55 (10) | 24–74 | 0.50 |
| Years of education | 34 | 12 (3) | 3–20 | 23 | 13 (3) | 3–16 | 0.88 |
Cerebral Vasospasm
To determine whether CSF ET-1 levels were associated with vasospasm, two indicators were utilized: angiographic vasospasm and DCI. First, chi-square analyses were completed to determine the overall association between ET-1 levels over time identified by the trajectory analysis (high versus low ET-1 levels) and angiographic spasm and DCI. No significant differences were found (Table 2). Next, binary logistic regression models were used in a subset of patients to determine the association between positive angiographic vasospasm and ET-1 exposure rates at 24, 48, and 72 h prior to angiographic detection and positive DCI and ET-1 exposure rates at 72 h after positive angiograhic spasm and DCI detection. The subset was chosen based on the availability of CSF collection time data and other covariates. The exposure rate is the actual concentration of ET-1 after controlling for CSF drainage. The rate accounts for the variability in the amount of CSF drainage between patients and provides the concentration of the sample per hour so that a relationship can be drawn with vasospasm. The average CSF output did not significantly differ between spasm positive and negative, DCI positive and negative, and ET-1 high and low patient groups (Table 3). This conclusion is based on independent t test results. After controlling for age and Fisher grade, there were significant relationships between angiographic vasospasm and ET-1 exposure rates during the 48 and 72 h prior to angiography (P = 0.05 and 0.03), and between DCI and exposure rates 72 h prior to DCI (P = 0.05) with odds ratios ranging from 1.23 to 2.95 (Table 4). The average exposure rate for the 72 h before angiography was significantly higher in vasospasm positive patients (n = 16) compared to vasospasm negative (n = 24) patients (P = 0.03). The average ET-1 exposure rate for the 72 h before angiography was 4.35 ± 3.02 pg/h for the vasospasm negative group and 7.94 ± 6.47 pg/h for the vasospasm positive group (Fig. 2a). However, the average exposure rate for the 72 h after angiography did not significantly differ between vasospasm positive (n = 16) and negative groups (n = 19). Similarly, the average exposure rate for the 72 h before DCI was significantly greater in DCI positive patients (n = 26) compared to DCI negative (n = 11) groups (P = 0.02). The average ET-1 exposure rate for the 72 h before DCI was 4.40 ± 4.07 pg/h for the DCI negative group and 7.12 ± 5.43 pg/h for the DCI positive group (Fig. 2b). There was no significant association between DCI and ET-1 exposure rate 72 h after DCI.
Table 3.
Average CSF output in ET-1, spasm, and DCI groups
| Average exposure rate determination time | Group | N | Average CSF volume (ml) |
P value | ||
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| 48 h before angiographic spasm | ET-1 | Low | 19 | 154.46 | 74.89 | 0.12 |
| High | 21 | 119.16 | 67.01 | |||
| Spasm | No | 24 | 124.97 | 72.79 | 0.25 | |
| Yes | 16 | 152.37 | 70.30 | |||
| 72 h before angiographic spasm | ET-1 | Low | 19 | 146.53 | 76.21 | 0.33 |
| High | 21 | 124.29 | 65.59 | |||
| Spasm | No | 24 | 123.85 | 69.53 | 0.23 | |
| Yes | 16 | 151.36 | 71.68 | |||
| 72 h before clinical spasm | ET-1 | Low | 21 | 115.45 | 51.36 | 0.39 |
| High | 23 | 128.82 | 49.67 | |||
| DCI | No | 11 | 107.40 | 49.68 | 0.30 | |
| Yes | 26 | 127.00 | 52.06 | |||
Table 4.
Results from binary logistic regression for spasm positive versus spasm negative groups
| Average exposure rate determination time | N | Odds ratio (with 95% CI) | SE | P value |
|---|---|---|---|---|
| 48 h before angiographic spasm | 40 | 1.23 (1.00–1.51) | 0.11 | 0.05 |
| 72 h before angiographic spasm | 40 | 1.24 (1.03–1.51) | 0.10 | 0.03 |
| 72 h after angiographic spasm | 35 | 1.18 (0.91–1.53) | 0.21 | 0.21 |
| 48 h before clinical spasm | 38 | 1.12 (0.62–2.00) | 0.30 | 0.72 |
| 72 h before clinical spasm | 37 | 2.95 (1.00–8.80) | 0.56 | 0.05 |
| 72 h after clinical spasm | 36 | 1.08 (0.33–3.55) | 0.61 | 0.90 |
Fig. 2.
The average ET-1 exposure rate 72 h prior and after angiography in angiographic cerebral vasospasm (CV) positive and negative patients (a) and the average ET-1 exposure rate 72 h prior and after DCI in DCI positive and negative patients (b). Independent t tests revealed significant differences (P < 0.05) in ET-1 exposure rates between CV positive and negative patients 72 h prior vasospasm and between DCI positive and negative patients 72 h prior DCI development after normalization of data by log transformation
Discussion
aSAH is a complex, multifaceted disease whose pathogenesis progresses over days to weeks. Angiographically evidenced vasospasm, a definable event in the acute course, develops in 70% of the patients between post-bleed days 3 and 14 with a maximum peak at 4–7 days [16]. This window provides an excellent opportunity for protracted CSF sampling for biomarker analysis.
The current study demonstrated that high CSF ET-1 concentrations are associated with vasospasm and DCI. We have observed the following two significant findings. First, our population was clearly dichotomized into high and low groups with respect to CSF ET-1 concentrations over time. This difference was not influenced by gender, race, aneurysm side or locale, method of treatment for original aneurysm, Hunt and Hess or Fisher grade, age, or education level, which suggests that there may be genetic or other predictors of CSF ET-1 concentrations after aSAH. Second, samples obtained 72 h prior to angiographic vasospasm or change in clinical neurological status had higher ET-1 concentrations which suggest that ET-1 levels are temporally related to vascular complications after aSAH. Collectively, these results suggest that ET-1 concentrations are elevated in the time period immediately preceding vascular complications.
Previous research has focused on the role of ET-1 as a pathological mediator of cerebral vasospasm. Clinical and animal studies have shown ET-1 to be a highly potent vasoconstrictive mediator involved in vascular reactivity after aSAH and a likely contributor to the development of vasospasm [17, 18]. Several clinical studies have demonstrated that CSF ET-1 levels are markedly elevated in patients with clinical manifestations of vasospasm compared to patients without vasospasm [6, 8, 18–20]. Specifically, the temporal profiles of ET-1 levels in CSF of aSAH patients have been shown to significantly increase 4–7 days after hemorrhage. In posttraumatic patients (n = 10) with aSAH, CSF ET-1 concentrations showed a significant elevation on day 2 and remained elevated until day 14 [21]. Our findings extend prior investigations by demonstrating the significant relationship between elevated ET-1 exposure rate and cerebral vasospasm in a larger number of patients than previously reported. Based on these findings, ET-1 is an indicator of vascular complications after aSAH.
In our study, we utilized trajectory analysis to determine changes in ET-1 concentrations over time by identifying subpopulations that follow specified patterns of change over that time frame. The analysis approaches a set of individual trajectories by grouping those which closely resembles one another thus simplifying longitudinal data. The identified subpopulations can subsequently be evaluated for their relationship with patient outcomes. This approach has been shown to be a more sensitive method of evaluating biomarker outcome relationships over time as compared to traditional point estimate methods. Recently, a similar approach has been successfully used in identifying trajectories or developmental course in clinical and social research [14, 15, 22–26].
The role of endothelins in spasm associated with SAH and impaired cerebral hemodynamics after traumatic brain injury is well supported by basic science and clinical observations. Selective antagonists of the two ET-1 receptors (ETA and ETB) have been used for the treatment of SAH-induced vasospasm in many animal models, and these studies have proven that vasospasm can be limited by blocking the detrimental effects of endogenous ET-1. Several human clinical studies and experimental animal model studies of SAH have shown that endothelin receptor antagonists reduce vasospasm [27–30]. Preclinical studies suggest that ETA-selective agents might be more efficacious in treating angiographic vasospasm. Specifically, clazosentan, a nonpeptide ETA receptor antagonist, significantly reduced moderate to severe angiographic vasospasm in a randomized phase-2 clinical trial in aSAH patients [31, 32]. A recent meta-analysis of randomized controlled trials assessing the use of ETA antagonists in 867 SAH patients reported marked reduction in radiographic vasospasm without any improvement in neurological outcome [33]. Furthermore, ETA receptor antagonists have been shown to reverse several functional impairments including artery constriction following traumatic brain injury [34–36].
Although ET-1 is implicated in the development of vasospasm and ET-1 antagonist is shown to be effective in the prevention of acute vasospasm, its precise role in DCI is still controversial. The efficacy against angiographic vasospasm did not translate into improved outcome in the clazosentan clinical trial. There was no significant effect on outcome based on the modified Rankin scale at 3 months. The possible explanations are that (1) the study is under-powered to detect relatively small improvements in outcome especially when assessed using relatively insensitive measures such as modified Rankin and Glasgow outcome scales (2) the adverse effects of treatment affected the beneficial effects of the drug. In addition, several studies indicate that the development of DCI cannot be fully attributed to the occurrence of angiographic vasospasm and not all patients with DCI have angiographic vasospasm, and DCI is associated with poor outcome whereas angiographic vasospasm alone is not. Recent advances in elucidating the pathogenesis of DCI have indicated several alternative causes of neurological deterioration and poor outcome after aSAH including microcirculatory dysfunction, cortical spreading depression (CSD), and thromboembolism [37–39]. Apart from being a vasoconstrictor, ET-1 is a neuronal and astroglial modulator. It has been shown that ET-1 is a potent inducer of CSD and ischemia triggered by spreading neuronal activation is induced by ET-1 in rats. It is suggested that the mechanism that triggers spreading neuronal/astroglial depolarization waves is important in the pathogenesis of cortical spreading ischemia [40]. It is hypothesized that this mechanism may be through reduction in Na+–K+-ATPase activity which accounts for half of cerebral energy consumption. Indeed, ET-1 has been shown to inhibit Na+–K+-ATPase from cerebral cortex preparation in vitro [41]. In addition to its action through ETA receptor, ET-1 may elicit deleterious effects through ETB receptor which is the predominant subtype in neuronal/astroglial network. ET-1 has been reported to be released from astrocytes after hypoxia [11]. Therefore, it may be speculated that ET-1 released from neuronal and glial cells after early ischemic damage may later contribute to DCI. ET-1 may also cause microvessel spasm contributing to ischemia after aSAH. However, the question whether ET-1 is a causative factor in the pathogenesis of DCI or an indicator of ischemic cell damage remains an area for future investigation.
These results provide preliminary evidence that acute elevations in CSF ET-1 concentrations are associated with vasospasm and DCI. However, further studies are needed to fully elucidate the role of ET-1 in vasospasm and DCI. Although this is the largest study to evaluate ET-1 and vasospasm and DCI in aSAH patients reported to date, there are a few limitations that should be noted. Patients were enrolled from one center and outcomes may have reflected patient management specific to this setting. The study limited enrollment to patients with a ventriculostomy; more stable patients are less likely to have a ventriculostomy and may have responded differently.
Conclusion
Our results suggest that ET-1 is a pathogenic mediator of aSAH. In combination with results of prior studies demonstrating an association between acute elevations in CSF ET-1 and development of vasospasm and DCI, our findings suggest that therapy to inhibit ET-1 may be beneficial in preventing the acute development of vasospasm and DCI. Future studies are necessary to fully elucidate the effects of ET-1 in aSAH patients.
Acknowledgment
We thank Dr. Patrick M Kochanek for his helpful comments on this article. This study was supported by an NIH funding (RO1NR004339).
Contributor Information
Bhavani P. Thampatty, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA
Paula R. Sherwood, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA
Elizabeth A. Crago, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA
Dianxu Ren, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA.
Chien-Wen J. Kuo, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA
Sheila A. Alexander, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA
Catherine M. Bender, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA
Leslie A. Hoffman, School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA
Samuel M. Poloyac, School of Pharmacy, Department of Pharmaceutical Sciences, University of Pittsburgh, 807 Salk Hall, Pittsburgh, PA 15261, USA
References
- 1.Saveland H, Sonesson B, Ljunggren B, et al. Outcome evaluation following subarachnoid hemorrhage. J Neurosurg. 1986;64:191–6. doi: 10.3171/jns.1986.64.2.0191. [DOI] [PubMed] [Google Scholar]
- 2.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–4. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- 3.Haug T, Sorteberg A, Sorteberg W, Lindegaard KF, Lundar T, Finset A. Cognitive outcome after aneurysmal subarachnoid hemorrhage: time course of recovery and relationship to clinical, radiological, and management parameters. Neurosurgery. 2007;60:649–56. doi: 10.1227/01.NEU.0000255414.70807.A0. discussion 656–7. [DOI] [PubMed] [Google Scholar]
- 4.Fassbender K, Hodapp B, Rossol S, et al. Endothelin-1 in subarachnoid hemorrhage: an acute-phase reactant produced by cerebrospinal fluid leukocytes. Stroke. 2000;31:2971–5. doi: 10.1161/01.str.31.12.2971. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Meguro K, Sakurai T, Saitoh Y, Takeuchi S, Nose T. Endothelin-1 concentration increases in the cerebrospinal fluid in cerebral vasospasm caused by subarachnoid hemorrhage. Surg Neurol. 2000;53:131–5. doi: 10.1016/s0090-3019(99)00179-2. [DOI] [PubMed] [Google Scholar]
- 6.Kastner S, Oertel MF, Scharbrodt W, Krause M, Boker DK, Deinsberger W. Endothelin-1 in plasma, cisternal CSF and microdialysate following aneurysmal SAH. Acta Neurochir (Wien) 2005;147:1271–9. doi: 10.1007/s00701-005-0633-0. discussion 1279. [DOI] [PubMed] [Google Scholar]
- 7.Kessler IM, Pacheco YG, Lozzi SP, de Araujo AS, Jr, Onishi FJ, de Mello PA. Endothelin-1 levels in plasma and cerebrospinal fluid of patients with cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2005;64(Suppl 1):S1:2–5. doi: 10.1016/j.surneu.2005.04.014. discussion S1:5. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Sato S, Suzuki Y, Takekoshi K, Ishihara N, Shimoda S. Increased endothelin concentration in CSF from patients with subarachnoid hemorrhage. Acta Neurol Scand. 1990;81:553–4. doi: 10.1111/j.1600-0404.1990.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenreich H, Lange M, Near KA, et al. Long term monitoring of immunoreactive endothelin-1 and endothelin-3 in ventricular cerebrospinal fluid, plasma, and 24-h urine of patients with subarachnoid hemorrhage. Res Exp Med (Berl) 1992;192:257–68. doi: 10.1007/BF02576282. [DOI] [PubMed] [Google Scholar]
- 10.Gaetani P, Rodriguez y Baena R, Grignani G, Spanu G, Pacchiarini L, Paoletti P. Endothelin and aneurysmal subarachnoid haemorrhage: a study of subarachnoid cisternal cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1994;57:66–72. doi: 10.1136/jnnp.57.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pluta RM, Boock RJ, Afshar JK, et al. Source and cause of endothelin-1 release into cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 1997;87:287–93. doi: 10.3171/jns.1997.87.2.0287. [DOI] [PubMed] [Google Scholar]
- 12.Sloan MA, Haley EC, Jr, Kassell NF, et al. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology. 1989;39:1514–8. doi: 10.1212/wnl.39.11.1514. [DOI] [PubMed] [Google Scholar]
- 13.Spilker J, Kongable G, Barch C, et al. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA Stroke Study Group. J Neurosci Nurs. 1997;29:384–92. doi: 10.1097/01376517-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Nagin D. Analyzing developmental trajectories: a semi-parametric, group-based approach. Psychol Methods. 1999;4:139–57. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Jones BL, Nagin DS, Roeder KA. SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–93. [Google Scholar]
- 16.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–72. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 17.Yamaura I, Tani E, Maeda Y, Minami N, Shindo H. Endothelin-1 of canine basilar artery in vasospasm. J Neurosurg. 1992;76:99–105. doi: 10.3171/jns.1992.76.1.0099. [DOI] [PubMed] [Google Scholar]
- 18.Seifert V, Loffler BM, Zimmermann M, Roux S, Stolke D. Endothelin concentrations in patients with aneurysmal subarachnoid hemorrhage Correlation with cerebral vasospasm, delayed ischemic neurological deficits, and volume of hematoma. J Neurosurg. 1995;82:55–62. doi: 10.3171/jns.1995.82.1.0055. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki R, Masaoka H, Hirata Y, Marumo F, Isotani E, Hirakawa K. The role of endothelin-1 in the origin of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1992;77:96–100. doi: 10.3171/jns.1992.77.1.0096. [DOI] [PubMed] [Google Scholar]
- 20.Mascia L, Fedorko L, Stewart DJ, et al. Temporal relationship between endothelin-1 concentrations and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2001;32:1185–90. doi: 10.1161/01.str.32.5.1185. [DOI] [PubMed] [Google Scholar]
- 21.Maier B, Lehnert M, Laurer HL, Marzi I. Biphasic elevation in cerebrospinal fluid and plasma concentrations of endothelin 1 after traumatic brain injury in human patients. Shock. 2007;27:610–4. doi: 10.1097/shk.0b013e31802f9eaf. [DOI] [PubMed] [Google Scholar]
- 22.Nagin D, Tremblay RE. Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Dev. 1999;70:1181–96. doi: 10.1111/1467-8624.00086. [DOI] [PubMed] [Google Scholar]
- 23.Herrenkohl TI, Hill KG, Hawkins JD, Chung IJ, Nagin DS. Developmental trajectories of family management and risk for violent behavior in adolescence. J Adolesc Health. 2006;39:206–13. doi: 10.1016/j.jadohealth.2005.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broadbent JM, Thomson WM, Poulton R. Trajectory patterns of dental caries experience in the permanent dentition to the fourth decade of life. J Dent Res. 2008;87:69–72. doi: 10.1177/154405910808700112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu MC, Muthen B, Schaffran C, Griesler PC, Kandel DB. Developmental trajectories of criteria of nicotine dependence in adolescence. Drug Alcohol Depend. 2008;98:94–104. doi: 10.1016/j.drugalcdep.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 27.Clozel M, Watanabe H. BQ-123, a peptidic endothelin ETA receptor antagonist, prevents the early cerebral vasospasm following subarachnoid hemorrhage after intracisternal but not intravenous injection. Life Sci. 1993;52:825–34. doi: 10.1016/0024-3205(93)90081-d. [DOI] [PubMed] [Google Scholar]
- 28.Itoh S, Sasaki T, Asai A, Kuchino Y. Prevention of delayed vasospasm by an endothelin ETA receptor antagonist, BQ-123: change of ETA receptor mRNA expression in a canine subarachnoid hemorrhage model. J Neurosurg. 1994;81:759–64. doi: 10.3171/jns.1994.81.5.0759. [DOI] [PubMed] [Google Scholar]
- 29.Shigeno T, Clozel M, Sakai S, Saito A, Goto K. The effect of bosentan, a new potent endothelin receptor antagonist, on the pathogenesis of cerebral vasospasm. Neurosurgery. 1995;37:87–90. doi: 10.1227/00006123-199507000-00013. discussion 90–1. [DOI] [PubMed] [Google Scholar]
- 30.Zuccarello M, Boccaletti R, Romano A, Rapoport RM. Endothelin B receptor antagonists attenuate subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 1998;29:1924–9. doi: 10.1161/01.str.29.9.1924. [DOI] [PubMed] [Google Scholar]
- 31.Vajkoczy P, Meyer B, Weidauer S, et al. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. doi: 10.3171/jns.2005.103.1.0009. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–21. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 33.Kramer A, Fletcher J. Do endothelin-receptor antagonists prevent delayed neurological deficits and poor outcomes after aneurysmal subarachnoid hemorrhage? A meta-analysis. Stroke. 2009;40:3403–6. doi: 10.1161/STROKEAHA.109.560243. [DOI] [PubMed] [Google Scholar]
- 34.Armstead WM. Endothelin-1 contributes to normocapnic hyperoxic pial artery vasoconstriction. Brain Res. 1999;842:252–5. doi: 10.1016/s0006-8993(99)01825-9. [DOI] [PubMed] [Google Scholar]
- 35.Armstead WM. Age-dependent impairment of K(ATP) channel function following brain injury. J Neurotrauma. 1999;16:391–402. doi: 10.1089/neu.1999.16.391. [DOI] [PubMed] [Google Scholar]
- 36.Armstead WM. ET-1 contributes to age-dependent G protein impairment after brain injury. J Neurotrauma. 2003;20:105–10. doi: 10.1089/08977150360517227. [DOI] [PubMed] [Google Scholar]
- 37.Ohkuma H, Manabe H, Tanaka M, Suzuki S. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2000;31:1621–7. doi: 10.1161/01.str.31.7.1621. [DOI] [PubMed] [Google Scholar]
- 38.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–37. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 39.Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–70. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- 40.Dreier JP, Kleeberg J, Petzold G, et al. Endothelin-1 potently induces Leao’s cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura? Brain. 2002;125:102–12. doi: 10.1093/brain/awf007. [DOI] [PubMed] [Google Scholar]
- 41.Meyer-Lehnert H, Wanning C, Predel HG, Backer A, Stelkens H, Kramer HJ. Effects of endothelin on sodium transport mechanisms: potential role in cellular Ca2+ mobilization. Biochem Biophys Res Commun. 1989;163:458–65. doi: 10.1016/0006-291x(89)92158-x. [DOI] [PubMed] [Google Scholar]