Abstract
Objective
In this article we focus on the role that chemokines and chemokine receptors play in the pathogenesis of multiple myeloma and the associated bone destructive process, and consider their utility as novel therapeutic targets for treating this devastating disease.
Methods
Current research on the role that chemokine and chemokine receptors play in the pathogenesis of myeloma is reviewed.
Results
The chemokines, MIP-1α, MCP-1, IL-8, and SDF-1, and their receptors play important roles in homing of MM cells, tumor growth, and bone destruction in myeloma. They are attractive therapeutic targets for treating myeloma patients.
Conclusion
Addition of chemokine antagonists to current treatment regimens for myeloma should result in better therapeutic responses because of the loss of both the protective effect of the marrow microenvironment on the MM cells and the induction of osteoclast activity.
Multiple myeloma (MM) accounts for 10% of malignant hematological diseases. It is characterized by the clonal proliferation of malignant plasma cells in the bone marrow compartment, secretion of monoclonal immunoglobulins, and suppression of normal immunoglobulin production and hematopoiesis. Myeloma patients often have extensive skeletal destruction with osteolytic lesions, osteopenia, pathological fractures, intractable bone pain, and hypercalcemia. These osteolytic lesions are the major cause of morbidity and possible mortality in multiple myeloma patients [1]. The malignant plasma cells present in the bone marrow (BM) originate from lymph nodes and then migrate across the endothelium of bone marrow sinuses to the BM micro-environment and localize in contact with stromal cells [2,3]. This interaction of plasma cells with BM stromal cells is crucial to the homing and growth of MM cells in the BM microenvironment. Osteoclastic activity also increases in areas adjacent to myeloma cells, resulting in increased bone resorption and decreased bone formation [4]. These observations suggest that both myeloma-derived and stromal cell–produced factors, such as chemokines, participate in the homing and growth of myeloma cells in the marrow and the formation and activation of osteoclasts [5].
Chemokines and their receptors
Chemokines are small (8–11 kDa) chemotactic proteins that are secreted by various cells under influence of inflammatory cytokines, growth factors, and cancer cells [6–8]. Upon release they create a chemical gradient in the local microenvironment that attracts neutrophils, macrophages, and even cancer cells, which move toward higher concentration of chemokines through binding of chemokine receptors on the cells. Chemokines are normally produced by leukocytes and epithelial, endothelial, and stromal cells. They also regulate cell transport, especially recruitment of leukocytes to their target sites, and mediate leukocyte adhesion to endothelial cells, transendothelial migration, and tissue invasion [9–11]. Most chemokines have four characteristic cysteines and are classified based on the arrangement of the first two cytokines in the mature protein. They are subdivided into four classes: 1) CXC; 2) CC; 3) C; and 4) CX3C chemokines. In the CXC chemokines, the first two cysteine molecules are separated by a single amino acid, whereas in the CC chemokines, the first two cysteine molecules are adjacent to each other [8,12]. To date, over 50 chemokines have been identified, out of which 28 are CC chemokines, 16 are CXC chemokines, and one CXXXC (fractalkine) and two C chemokine (lymphotactin) subclasses (Table 1) [9,13].
Table 1.
Chemokine/chemokine receptor nomenclature [13]
| Systematic name | Human ligand | Chemokine receptor |
|---|---|---|
| CXC chemokine/receptor family | ||
| CXCL1 | GROα/MGSA-α | CXCR2 > CXCR1 |
| CXCL2 | GROβ/MGSA-β | CXCR2 |
| CXCL3 | GROγ/MGSA-γ | CXCR2 |
| CXCL4 | PF4 | Unknown |
| CXCL5 | ENA-78 | CXCR2 |
| CXCL6 | GCP-2 | CXCR1, CXCR2 |
| CXCL7 | NAP-2 | CXCR2 |
| CXCL8 | IL-8 | CXCR1, CXCR2 |
| CXCL9 | Mig | CXCR3 |
| CXCL10 | IP-10 | CXCR3 |
| CXCL11 | I-TAC | CXCR3 |
| CXCL12 | SDF-1 α/β | CXCR4 |
| CXCL13 | BCA-1 | CXCR5 |
| CXCL14 | BRAK | Unknown |
| CXCL15 | Unknown | Unknown |
| CXCL16 | Unknown | CXCR6 |
| C chemokine/receptor family | ||
| XCL1 | Lymphotactin | XCR1 |
| XCL2 | SCM1-β | XCR1 |
| CX3C chemokine/receptor family | ||
| CX3CL1 | Fractalkine | CX3CR1 |
| CC chemokine/receptor family | ||
| CCL1 | I-309 | CCR8 |
| CCL2 | MCP-1/MCAF/TDCF | CCR2 |
| CCL3 | MIP-1α/LD78α | CCR1, CCR5 |
| CCL3L1 | LD78β | CCR1, CCR5 |
| CCL4 | MIP-1β | CCR5 |
| CCL5 | RANTES | CCR1, CCR3, CCR5 |
| CCL6 | Unknown | Unknown |
| CCL7 | MCP-3 | CCR1, CCR2, CCR3 |
| CCL8 | MCP-2 | CCR3, CCR5 |
| CCL9/10 | Unknown | CCR1 |
| CCL11 | Eotaxin | CCR3 |
| CCL12 | Unknown | CCR2 |
| CCL13 | MCP-4 | CCR2, CCR3 |
| CCL14 | HCC-1 | CCR1, CCR5 |
| CCL15 | HCC-2/Lkn-1/MIP-1δ | CCR1, CCR3 |
| CCL16 | HCC-4/LEC/LCC-1 | CCR1, CCR2 |
| CCL17 | TARC | CCR4 |
| CCL18 | DC-CK1/PARC/AMAC-1 | Unknown |
| CCL19 | MIP-3β/ELC/exodus-3 | CCR7 |
| CCL20 | MIP-3α/LARC/exodus-1 | CCR6 |
| CCL21 | 6Ckine/SLC/exodus-2 | CCR7 |
| CCL22 | MDC/STCP-1 | CCR4 |
| CCL23 | MPIF-1/CKβ8/CKβ8-1 | CCR1 |
| CCL24 | Eoxatin-2/MPIF-2 | CCR3 |
| CCL25 | TECK | CCR9 |
| CCL26 | Eoxatin-3 | CCR-3 |
| CCL27 | CTACK/ILC | CCR10 |
| CCL28 | MEC | CCR3/CCR10 |
The chemokine receptors are G protein–coupled transmembrane proteins that are expressed on subgroups of leukocytes, and usually bind more than one type of chemokine. They are named on the basis of the chemokine class to which they bind. Receptors CCR1 through CCR10 bind to CC chemokine; receptors CXCR1 through CXCR6 bind CXC chemokines; C chemokine and CX3C chemokine bind to XCR1 and CX3CR1 respectively [9,13–15].
Chemokines in multiple myeloma
Multiple myeloma cells express a variety of chemokine receptors and secrete several chemokines, which participate in cell homing, tumor growth, and progression. Migration of plasma cells to and from bone marrow, as well as their chemotaxis in the bone marrow microenvironment, is directed by the interaction of chemokine receptors and their ligands [16]. MM cell lines express high levels of the chemokine receptors CXCR3, CXCR4, CCR1, CCR5, and CCR6. The ligands of these receptors are MIP-1α, MIP-1β, SDF-1, CXC, and RANTES [7].
MIP-1α and myeloma
Macrophage inflammatory protein 1α (MIP-1α; CCL3) is a low-molecular-weight CC chemokine, which can interact with CCR1, CCR5, or CCR9. It primarily acts as a chemo-attractant and an activator of monocytes and monocyte-like cells [17], and has a role in hematopoiesis and osteoclast recruitment and differentiation in BM [18,19]. Both CCR1 and CCR5 are expressed by human bone marrow stromal cells and osteoclast precursors [20–22]. MIP-1α acts directly on human osteoclast progenitors at the later stages of differentiation [20]. MIP-1α and MIP-1β are constitutively secreted by MM cells and induce the development of osteolytic bone lesions [20–24]. Levels of MIP-1α are elevated in a majority (62%) of BM samples from patients with active myeloma as compared to only 17% of patients with inactive disease, and is not increased in normals [22]. Further, blocking MIP-1α, MIP-1β, CCR1, or CCR5 with neutralizing antibodies inhibited the osteoclast stimulatory factor activity in marrow plasma from MM patients. Recently, increased levels of MIP-1α were reported in the plasma of MM patients with severe bone disease. Patients with higher serum MIP-1α levels had a significantly lower 3-year survival, indicating that high MIP-1α level portends a poor prognosis [25].
Gene expression profiling studies have shown that MIP-1α expression was highly correlated with the extent of bone disease in MM patients [26,27]. In vivo studies showed that the growth of MM cells and bone lesions was decreased in SCID mice transplanted with ARH-77 cells which lacked MIP-1α [28]. Studies with MM patients reported that bone lesions were present in 88% of patients with high levels of MIP-1α, while these lesions were only demonstrated in 46% of patients with low levels of MIP-1α [24]. Further, increases in the levels of MIP-1α and MIP-1β corresponded with elevation of the bone resorption marker, urinary deoxypyridinoline (DPd) [27]. These findings suggest an important role of MIP-1α in the pathogenesis of bone lesions in MM patients.
Interleukin-6 (IL-6) is produced by marrow stromal cells when they bind with myeloma cells. IL-6 is a potent osteoclastogenic factor for human OCL precursors [29]. Han et al. found that MIP-1α enhanced IL-6-induced OCL formation [20]. It is controversial whether MIP-1α directly induces OCL formation. MIP-1α and MIP-1β can induce RANKL expression by murine stromal cells [21]. Serum RANKL levels positively correlated with elevation in MIP-1α levels. These results indirectly support the role of RANKL pathway as the dominant final pathway in the osteoclast stimulation [25]. In contrast, RANK-Fc, a soluble inhibitor of RANKL, did not inhibit human osteoclast activation by MIP-1α, although MIP-1α can enhance the osteoclastogenic effects of RANKL [20]. Further studies are required to clarify this issue.
In addition to its role in the development of osteolytic lesions in MM patients, MIP-1α can stimulate proliferation, migration, and survival of plasma cells by direct activation of the signaling pathways AKT/protein kinase B (PKB) and mitogen-activated protein kinase (MAPK) [30]. MIP-1α can suppress hematopoietic stem cell proliferation and erythropoiesis through the CCR1 receptor expressed on erythroid precursors, suggesting a role for MIP-1α in pathogenesis of anemia in MM patients [31,32]. MIP-1β can also suppress pre–B-cell differentiation in bone marrow, despite the presence of IL-7, which stimulates pre–B-cell differentiation [33].
Role of SDF-1 and CXCR4 in myeloma
Stromal cell–derived factor-1 (SDF1; CXCL12) is a CXC chemokine. It is the sole ligand for CXCR4 [34,35]. SDF-1 is constitutively expressed at high levels by bone marrow stromal cells, and is a chemotactic factor for many cells including hematopoietic progenitor cells [34,36–38]. CXCR4 has a role in B-cell migration and proliferation. It is expressed by myeloma cells and endothelium, but not by marrow stromal cells [6,39]. Migration of myeloma cells across the endothelium lining the bone marrow sinuses is a critical step in the pathogenesis of multiple myeloma, which leads to homing and localization of these cells. Several studies have suggested that SDF-1 is a chemoattractant for human CD34+ hemopoietic progenitor cells, and that it triggers their transendothelial migration by upregulation of the adhesion activity of integrin VLA-4 (α4β1) [37,40]. Similarly, in multiple myeloma, SDF-1/CXCR4 promotes transendothelial migration of myeloma cells by transient upregulation of VLA-4 (α4β1)/VCAM-1, inducing cell adhesion to the endothelium, and contributing to the trafficking of myeloma cells in the bone marrow microenvironment [41]. When SDF-1 expression was downregulated by TGF-β1 in the supernatant from the bone marrow stromal cell line MS-5, there was a parallel decrease in chemotaxis and transendothelial migration of the myeloma cell lines NCl-H929 and Mo7e. The addition of exogenous SDF-1 resulted in a subsequent recovery of cell chemotaxis [16]. This suggests a positive correlation between decreased SDF-1 protein levels and suppression of chemotactic activity. Moreover, treatment with anti-CXCR4 antibodies or the CXCR4 antagonist T134 inhibited SDF-1 activity in MS-5 supernatant [16]. Other studies have also indicated a role for SDF-1 in MM cell migration [42]. Further, low levels of CXCR4 expression in MM correlated with poor prognosis and disease progression [43]. Collectively, these studies confirm the role of SDF-1 and CXCR4 in chemotaxis and homing of MM cells in bone marrow.
Once myeloma cells are within the bone marrow, they localize in close proximity to stromal cells. SDF-1 has been implicated in modulation of VLA-4-dependent myeloma cell adhesion, resulting in homing and localization of MM cells in the bone marrow microenvironment. Integrin VLA-4, its ligand CS-1/fibronectin, and VCAM-1 play a key role in marrow stromal cell interaction with myeloma cells [38]. In addition, SDF-1 secretion by marrow stromal cells is upregulated by adhesion of MM cells to stromal cells, thus promoting greater expression of integrins which enhance homing [42].
CXCR4/SCDF-1 also plays a key role in chemotherapy-based mobilization of hematopoietic stem cells (HSC) and progenitor cells from BM to peripheral blood. Targeted disruption of CXCR4/SCDF-1 signaling results in egress of hematopoietic stem and progenitor cells from the marrow into the peripheral blood [44]. In vivo studies have demonstrated decreased serum levels of SDF-1 and reduced surface expression of CXCR4 in mobilized myeloma cells as compared to premobilized myeloma cells [45]. This is consistent with previous studies that reported that activation through SDF-1/CXCR4 plays a key role in homing of MM cells to the marrow, whereas disruption of SDF-1/CXCR4 signaling is required for mobilization of HSC [44,46,47]. Myeloma cells from peripheral blood had an impaired response to SDF-1. This was reversible on brief incubation with IL-6, indicating that IL-6 upregulates SDF-1/CXCR4. These data support a role for SDF-1/CXCR4 disruption in facilitating the mobilization of myeloma cells [45].
Interestingly, SDF-1 augments the proliferation of both MM cell lines and primary MM cells, although only modest increases in cell proliferation were observed [48,42]. SDF-1 can also be protective against dexamethasone-induced apoptosis through activation of the mitogen-activated protein (MAP) Akt pathway, suggesting a role in drug resistance. SDF-1/CXCR4 induces NF-κB activation in MM cells, which is consistent with previous reports of SDF-1α-induced NF-κB activation in primary osteocytes [42,49]. NF-κB has both growth-inducing and anti-apoptotic roles in normal cells as well as myeloma cells. SDF-1/CXCR4 also plays an indirect role in promoting growth, survival, and migration of MM cells by increased IL-6 and VEGF secretion in marrow stromal cells.
Role of CXCR3 and its ligands in myeloma
CXCR3, a chemokine receptor of CXC family of chemokines, is expressed on activated T cells, tumor T cells, and B cells. It is not expressed on resting T cells or normal B cells [50–54]. It interacts specifically with the CXC chemokines I-TAC (interferon-inducible T-cell α chemoattractant)/CXCL11, Mig (monocyte/macrophage-activating IFN-γ-inducible protein)/CXCL9, and IP10 (IFN-γ-inducible protein)/CXCL10. These chemokines are primarily produced by monocytes and macrophages [55,56]. CXCR3/CXC interactions can contribute to progression, invasion, and metastasis of tumors like melanoma, ovarian cancer, and chronic lymphoid leukemia [50,57]. Recently, CXCR3 was detected on several myeloma cell lines and shown to have a role in chemotaxis of myeloma cells [7,58]. In vivo studies demonstrated that I-TAC, Mig, and IP10/CXCR3 signaling activated tyrosine kinase phosphorylation and chemotaxis in both myeloma cell lines and fresh plasma cells. However, there was no effect on cell proliferation. I-TAC, Mig, and IP10 also stimulated secretion of the metalloproteinases (MMP)-2 and MMP-9 by myeloma cell lines. These MMPs have been associated with tumor progression, invasion, and metastasis in advanced stages of MM [58–60].
Role of IL-8 in multiple myeloma
Interleukin-8 (IL-8; CXCL8) was originally identified as a neutrophil chemoattractant. IL-8 is produced by a variety of cell types, including macrophages, neutrophils, endothelial cells, and various tumor cells. Its receptors, CXCR1 and CXCR2, have been identified on a number of cell types, including neutrophils, T lymphocytes, monocytes, endothelial cells, and some tumor cells [61,62].
Bendre et al. reported IL-8 to be a potent and direct activator of osteoclastic differentiation and bone resorption [63,64]. IL-8 increases RANKL expression in osteoblastic cells, thereby altering the RANKL/OPG ratio in the cells in favor of osteoclast formation [64]. In addition, IL-8 directly stimulated the differentiation of human peripheral blood mononuclear cells into osteoclasts. The bone resorption achieved by IL-8 was independent and comparable to RANKL; however, no additive or synergistic effect was seen on osteoclast numbers or bone resorption areas when both were used together. Identification of CXCR1, for which IL-8 is the only major ligand, on human osteoclast and its precursors further supports the role of IL-8 in osteoclast formation [64]. Bone marrow stromal cell and endothelial cell from myeloma patients secrete IL-8 [48,65]. Tumor cell expression of IL-8 has been linked to metastatic potential in various tumors [66–68]. Endogenous CD28 expressed on myeloma cells was shown to upregulate IL-8 production in patients with multiple myeloma. Since aberrant expression of CD28 on myeloma cells correlates with metastasis, it is possible that IL-8 has some role in promotion of myeloma metastasis [69]. Recently, Pellegrino et al. have shown that IL-8 can induce proliferation and chemotaxis of both MM cell lines and patient plasma cells [48]. IL-8 has also been implicated in tumor progression via its ability to enhance an-giogenesis [67,68]. These observations suggest that IL-8 plays an important role in the disruption of bone homeostasis in various tumors, and anti-IL-8 may be used as a potential target to prevent MM-induced osteolysis.
Role of MCP-1 in multiple myeloma
Monocyte chemoattractant protein-1 (MCP-1; CCL2) acts as potent chemoattractant for monocytes, basophils, eosinophils, endothelial cells, and a subset of T lymphocytes [70,71]. MCP-1 binds with its receptor, CCR2, which is expressed on peripheral blood monocytes as well as activated T and B cells. MCP-1 is typically not expressed in normal bone, but rather its expression is induced by inflammatory mediators. IL-6, a major growth factor in myeloma, can up-regulate the production of MCP-1 in MM [72,73]. Van de Broek et al. have shown functional expression of CCR2 on MM cell lines as well as primary MM cells from bone marrow of MM patients. In addition, MCP-1 is produced by marrow stromal cells from normals and MM patients [74]. Sanz-Rodriguez et al. demonstrated that MCP-1 was a chemoattractant for human MM cells [38]. Blocking antibody against CCR2, as well as a combination of neutralizing antibodies to MCP-1, -2, and -3, significantly reduced the migration of human MM cells to marrow stromal cell–conditioned medium [74]. MCP-1 is upregulated by tumor necrosis factor-α (TNF-α), which is produced by MM cells. Enhanced migration of myeloma cell lines by TNF-α was abrogated by blocking MCP-1. These results show that the increased migration of MM to TNF-α is due to MCP-1.
MCP-1 has also been reported to play a significant role in angiogenesis and osteoblastogenesis [70,75]. Salcedo et al. demonstrated that MCP-1 directly induces blood vessel formation in vivo, which was inhibited by neutralizing antibodies to MCP-1 [76]. This suggests that MCP-1 has a role in homing and tumor progression.
Chemokines as potential therapeutic agents in myeloma
The role of chemokines in the interaction of MM cells with the marrow microenvironment makes them attractive targets for drug development. The CXCR4 antagonist, AMD3100, reversibly blocks binding of CXCR4 with SDF-1 and has no effect on other chemokines. It has been used to enhance mobilization of CD34+ stem cells for transplantation. A Phase 1 study performed recently assessed the effects of this CXCR4 antagonist on mobilization of CD34+ cells from bone marrow to peripheral blood in MM and non-Hodgkin’s lymphoma patients. This study demonstrated statistically significant increases in white blood count and peripheral blood CD34+ cells following single injection of the AMD3100 [77]. The drug was well tolerated and accompanied with mild (Grade I) toxicity. When myeloma cells bind to marrow stromal cells, they are chemoresistant. Blocking CXCR4 on the surface of myeloma cells may also lead to a release of myeloma cells from the bone marrow into peripheral blood, where they may be more responsive to chemotherapeutic agents or other novel agents. In addition, blocking CXCR4 may prevent further homing of MM cells from the circulation into the bone marrow, potentially preventing progression of disease. A potential harmful effect of mobilization in MM cells from marrow into the peripheral circulation is the risk of seeding MM cells into normal marrow sites in other parts of the body, which would result in further tumor growth and progression. However, there were no reports for the development of more aggressive diseases or rapid progression of myeloma in the AMD3100 trial.
MIP-1α is a leading potential target for treating patients with MM and its associated bone disease. MIP-1α enhances myeloma cell growth and osteoclast activation, thereby causing extensive bone destruction. MIP-1α blocking antibodies can decrease the osteoclastogenic activity in mouse models of MM, reduce tumor burden, and decrease adhesion of MM cells to marrow stromal cells. MIP-1α protein and gene expression levels correlate with the extent of myeloma bone disease, and MIP-1α is chemotactic for MM cells. Thus blocking MIP-1α is an attractive therapeutic target for MM. CCR1 and CCR5 antagonists are in clinical trial for other diseases and may be useful for treating MM patients.
Summary
The chemokines, MIP-1α, MCP-1, IL-8, and SDF-1, and their receptors play important roles in MM cell homing, tumor growth, and bone destruction in myeloma. They are emerging as attractive therapeutic targets for treating myeloma patients. Addition of chemokine antagonists to current treatment regimens for myeloma should result in better cytotoxic activity of chemotherapeutic agents or monoclonal antibodies because of the loss of the protective effect of marrow microenvironment on the MM cells, since the malignant cells attached to the stromal cells in the bone marrow are more resistant to apoptosis. However, their beneficial therapeutic potential will have to remain experimental until the side effects of blocking these chemokines are known. Further, understanding of the mechanism of chemokine receptor/ligand interaction in the bone marrow microenvironment, their role in the homing and migration of MM cells, and the signaling pathways that control these interactions, is required. This will help in developing rationally designed novel therapeutic agents for patients with MM. Figure 1 represents a model of our current understanding of the role of chemokines in the growth of myeloma cells and the bone destructive process.
Figure 1.
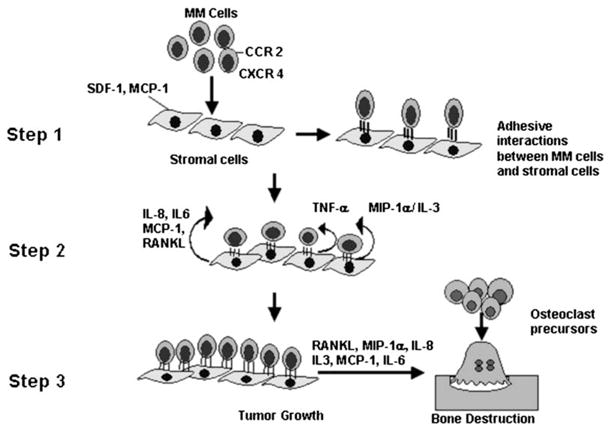
Model for the role of chemokines in myeloma tumor progression in bone. MCP-1 and SDF-1 produced by marrow stromal cells/osteoblasts attract myeloma cells to bone. Myeloma cells then bind to marrow stromal cells through VCAM-1 (Step 1). Marrow stromal cells then increase expression of TNF-α, MCP-1, IL-8, and IL-6. Myeloma cells then increase production of MIP-1α and IL-3, which stimulate their growth (Step 2). These cytokines and chemokines enhance myeloma cell survival and growth and increase angiogenesis. The increased expression of RANKL, IL-3, IL-8, MCP-1, IL-6, and MIP-1α induce osteoclast formation and bone destruction (Step 3).
Acknowledgments
Supported in part by a Multiple Myeloma Research Foundation Grant and a VA Merit Review Grant.
References
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(Suppl):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Billadeau D, Ahmann G, Greipp P, Van Ness B. The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to the malignant plasma cell. J Exp Med. 1993;178:1023–1031. doi: 10.1084/jem.178.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KC, Shaughnessy JD, Jr, Barlogie B, Harousseau JL, Roodman GD. Multiple myeloma. Hematology (Am Soc Hematol Educ Program) 2002:214–240. doi: 10.1182/asheducation-2002.1.214. [DOI] [PubMed] [Google Scholar]
- 5.Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- 6.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 7.Moller C, Stromberg T, Juremalm M, Nilsson K, Nilsson G. Expression and function of chemokine receptors in human multiple myeloma. Leukemia. 2003;17:203–210. doi: 10.1038/sj.leu.2402717. [DOI] [PubMed] [Google Scholar]
- 8.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 9.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 10.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 11.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 12.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 13.IUIS/WHO Subcommittee on Chemokine Nomenclature. Chemokine/chemokine receptor nomenclature. Cytokine. 2003;21:48–49. [Google Scholar]
- 14.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 15.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 16.Wright N, de Lera TL, Garcia-Moruja C, et al. Transforming growth factor-betal down-regulates expression of chemokine stromal cell-derived factor-1: functional consequences in cell migration and adhesion. Blood. 2003;102:1978–1984. doi: 10.1182/blood-2002-10-3190. [DOI] [PubMed] [Google Scholar]
- 17.Wolpe SD, Davatelis G, Sherry B, et al. Macrophages secrete a novel heparin- binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kukita T, Nomiyama H, Ohmoto Y, et al. Macrophage inflammatory protein-1 alpha (LD78) expressed in human bone marrow: its role in regulation of hematopoiesis and osteoclast recruitment. Lab Invest. 1997;76:399–406. [PubMed] [Google Scholar]
- 19.Scheven BA, Milne JS, Hunter I, Robins SP. Macrophage-inflammatory protein- 1 alpha regulates preosteoclast differentiation in vitro. Biochem Biophys Res Commun. 1999;254:773–778. doi: 10.1006/bbrc.1998.9909. [DOI] [PubMed] [Google Scholar]
- 20.Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1 alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97:3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- 21.Abe M, Hiura K, Wilde J, et al. Role for macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–2202. [PubMed] [Google Scholar]
- 22.Choi SJ, Cruz JC, Craig F, et al. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96(2):671–675. [PubMed] [Google Scholar]
- 23.Roodman GD. Mechanisms of bone lesions in multiple myeloma and lymphoma. Cancer. 1997;80(Suppl):1557–1563. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1557::aid-cncr5>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Uneda S, Hata H, Matsuno F, et al. Macrophage inflammatory protein-1 alpha is produced by human multiple myeloma (MM) cells and its expression correlates with bone lesions in patients with MM. Br J Haematol. 2003;120:53–55. doi: 10.1046/j.1365-2141.2003.04040.x. [DOI] [PubMed] [Google Scholar]
- 25.Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1 alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol. 2003;123:106–109. doi: 10.1046/j.1365-2141.2003.04561.x. [DOI] [PubMed] [Google Scholar]
- 26.Magrangeas F, Nasser V, Avet-Loiseau H, et al. Gene expression profiling of multiple myeloma reveals molecular portraits in relation to the pathogenesis of the disease. Blood. 2003;101:4998–5006. doi: 10.1182/blood-2002-11-3385. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T, Abe M, Oshima T, et al. Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1alpha and MIP-1beta correlates with lytic bone lesions in patients with multiple myeloma. Br J Haematol. 2004;125:38–41. doi: 10.1111/j.1365-2141.2004.04864.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi SJ, Oba Y, Gazitt Y, et al. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108:1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy SV, Takahashi S, Dallas M, Williams RE, Neckers L, Roodman GD. Interleukin-6 antisense deoxyoligonucleotides inhibit bone resorption by giant cells from human giant cell tumors of bone. J Bone Miner Res. 1994;9:753–757. doi: 10.1002/jbmr.5650090522. [DOI] [PubMed] [Google Scholar]
- 30.Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101:3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- 31.Su S, Mukaida N, Wang J, et al. Inhibition of immature erythroid progenitor cell proliferation by macrophage inflammatory protein-1 alpha by interacting mainly with a C-C chemokine receptor, CCR1. Blood. 1997;90:605–611. [PubMed] [Google Scholar]
- 32.Graham GJ, Wright EG, Hewick R, et al. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto T, Lisukov IA, Huang N, Mahmoud MS, Kawano MM. Plasma cells induce apoptosis of pre-B cells by interacting with bone marrow stromal cells. Blood. 1996;87:3375–3383. [PubMed] [Google Scholar]
- 34.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 35.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 36.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz-Rodriguez F, Hidalgo A, Teixido J. Chemokine stromal cell-derived factor-1 alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001;97:346–351. doi: 10.1182/blood.v97.2.346. [DOI] [PubMed] [Google Scholar]
- 39.Durig J, Schmucker U, Duhrsen U. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001;15:752–756. doi: 10.1038/sj.leu.2402107. [DOI] [PubMed] [Google Scholar]
- 40.Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 41.Parmo-Cabanas M, Bartolome RA, Wright N, Hidalgo A, Drager AM, Teixido J. Integrin alpha4betal involvement in stromal cell-derived factor-1 alpha-promoted myeloma cell transendothelial migration and adhesion: role of cAMP and the actin cytoskeleton in adhesion. Exp Cell Res. 2004;294:571–580. doi: 10.1016/j.yexcr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Hideshima T, Chauhan D, Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002;1:539–544. [PubMed] [Google Scholar]
- 43.Van de Broek I, Leleu X, Schots R, et al. Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematologica. 2006;91:200–206. [PubMed] [Google Scholar]
- 44.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 45.Gazitt Y, Akay C. Mobilization of myeloma cells involves SDF-1/CXCR4 signaling and downregulation of VLA-4. Stem Cells. 2004;22:65–73. doi: 10.1634/stemcells.22-1-65. [DOI] [PubMed] [Google Scholar]
- 46.Gazitt Y. Recent developments in the regulation of peripheral blood stem cell mobilization and engraftment by cytokines, chemokines, and adhesion molecules. J Hematother Stem Cell Res. 2001;10:229–236. doi: 10.1089/15258160151134908. [DOI] [PubMed] [Google Scholar]
- 47.Gazitt Y. Comparison between granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the mobilization of peripheral blood stem cells. Curr Opin Hematol. 2002;9:190–198. doi: 10.1097/00062752-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrino A, Ria R, Di Pietro G, et al. Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells. Br J Haematol. 2005:248–256. doi: 10.1111/j.1365-2141.2005.05443.x. [DOI] [PubMed] [Google Scholar]
- 49.Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001;108:425–435. doi: 10.1172/JCI12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95:627–632. [PubMed] [Google Scholar]
- 51.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrino A, Vacca A, Scavelli C, Dammacco F. Chemokines and tumors. Recenti Prog Med. 2002;93:642–654. [PubMed] [Google Scholar]
- 53.Piali L, Weber C, LaRosa G, et al. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–972. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Trentin L, Agostini C, Facco M, et al. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J Clin Invest. 1999;104:115–121. doi: 10.1172/JCI7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- 56.Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 57.Oppenheim JJ, Murphy WJ, Chertox O, Schirrmacher V, Wang JM. Prospects for cytokine and chemokine biotherapy. Clin Cancer Res. 1997;3:2682–2686. [PubMed] [Google Scholar]
- 58.Pellegrino A, Antonaci F, Russo F, et al. CXCR3-binding chemokines in multiple myeloma. Cancer Lett. 2004;207:221–227. doi: 10.1016/j.canlet.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 59.Vacca A, Ribatti D, Presta M, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- 60.Vacca A, Ria R, Semeraro F, et al. Endothelial cells in the bone marrow of patients with multiple myeloma. Blood. 2003;102:3340–3348. doi: 10.1182/blood-2003-04-1338. [DOI] [PubMed] [Google Scholar]
- 61.Yoshimura T, Matsushima K, Tanaka S, et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sozzani S, Locati M, Allavena P, Van Damme J, Mantovani A. Chemokines: a superfamily of chemotactic cytokines. Int J Clin Lab Res. 1996:69–82. doi: 10.1007/BF02592349. [DOI] [PubMed] [Google Scholar]
- 63.Bendre MS, Margulies AG, Walser B, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 64.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 65.Merico F, Bergui L, Gregoretti MG, et al. Cytokines involved in the progression of multiple myeloma. Clin Exp Immunol. 1993:27–31. doi: 10.1111/j.1365-2249.1993.tb05943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bendre MS, Gaddy-Kurten D, Mon-Foote T, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002:5571–5579. [PubMed] [Google Scholar]
- 67.Kim SJ, Uehara H, Karashima T, Mccarty M, Shih N, Fidler IJ. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001:33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 69.Shapiro VS, Mollenauer MN, Weiss A. Endogenous CD28 expressed on myeloma cells up-regulates interleukin-8 production: implications for multiple myeloma progression. Blood. 2001:187–193. doi: 10.1182/blood.v98.1.187. [DOI] [PubMed] [Google Scholar]
- 70.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000:34–40. [PubMed] [Google Scholar]
- 71.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Adv Immunol. 1994:97–179. [PubMed] [Google Scholar]
- 72.Arendt BK, Velazquez-Dones A, Tschumper RC, et al. Interleukin 6 induces monocyte chemoattractant protein-1 expression in myeloma cells. Leukemia. 2002:2142–2147. doi: 10.1038/sj.leu.2402714. [DOI] [PubMed] [Google Scholar]
- 73.Biswas P, Delfanti F, Bernasconi S, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998:258–265. [PubMed] [Google Scholar]
- 74.Vande Broek I, Asosingh K, Vanderkerken K, Straetmans N, Van Camp B, Van Riet I. Chemokine receptor CCR2 is expressed by human multiple myeloma cells and mediates migration to bone marrow stromal cell-produced monocyte chemotactic proteins MCP-1, -2 and -3. Br J Cancer. 2003:855–862. doi: 10.1038/sj.bjc.6600833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silvestris F, Cafforio P, Calvani N, Dammacco F. Impaired osteoblastogenesis in myeloma bone disease: role of upregulated apoptosis by cytokines and malignant plasma cells. Br J Haematol. 2004:475–486. doi: 10.1111/j.1365-2141.2004.05084.x. [DOI] [PubMed] [Google Scholar]
- 76.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 77.Tricot G, Jagannath S, Vesole D, et al. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995;85:588–596. [PubMed] [Google Scholar]


