Abstract
In immunocompetent persons, cytomegalovirus (CMV) is thought to persist primarily in monocytes and myeloid progenitor cells, establishing a chronic infection. In older adults, chronic CMV infection is typically diagnosed by a positive IgG serology. While many studies have shown CMV-specific T-cell expansion in CMV seropositive older individuals, significant heterogeneity has also been observed in this elderly population. In a study of 71 community-dwelling older adults, we assessed CMV viral DNA in peripheral monocytes by nested PCR and compared the relationships of detectable CMV DNA and IgG serology with serum levels of neopterin, a marker for monocyte/macrophage-mediated immune activation. The results showed that 52 (73.2%) participants were CMV seropositive, of whom 30 (57.5%) had detectable CMV DNA. CMV seropositive and seronegative participants did not differ in their neopterin levels, but individuals with detectable CMV DNA had higher neopterin than those without (10.6±4.4 vs 8.0±1.9nM, respectively, p<.0001) adjusting for demographic and clinical covariates and interferon (IFN)-γ levels. In addition, there was no association between IgG titers and neopterin. These findings suggest that detection of CMV viral DNA in monocytes may be an informative tool to evaluate chronic CMV infection and its potential role in monocyte/macrophage-mediated immune activation in the elderly.
Keywords: Detectable CMV DNAin peripheral monocytes, neopterin, immune activation, older adults
1. INTRODUCTION
Human cytomegalovirus (CMV) is a ubiquitous β herpesvirus that causes significant morbidity and mortality in immunocompromized patients and neonates (1). In immunocompetent individuals, primary CMV infection is typically subclinical and asymptomatic (1;2). The virus is thought to persist in a latent state with its DNA genome harbored primarily in monocytes and myeloid progenitor cells, establishing a chronic infection with intermittent reactivations (3;4). Positive CMV IgG titer is conventionally recognized as the diagnostic marker for chronic CMV infection in older adults. Based on this diagnostic criterion, chronic CMV infection is extremely common with estimated seroprevalence of 70–99% in this population (5;6). A large number of studies have shown CMV-specific T-cell clonal expansion and shrinking T-cell repertoire in CMV seropositive older individuals, suggesting a role of chronic CMV infection in age-related T-cell immunosenescence (7–10). However, significant heterogeneity has been observed in the CMV seropositive elderly population. For example, in the Swedish NONA immune longitudinal study, Wikby and colleagues observed that some elderly individuals with” immune risk profile (IRP)” for which CMV seropositivity is a major criterion had less expansion of late-stage differentiated CD8+ T cells at 2-year follow-up from baseline (11). In the same study, Strindhall, et al reported no such T-cell expansion in participants 90 years and older, likely due to survivor selection (12). Because a positive CMV IgG titer merely indicates prior exposure to the virus, it is not known if seropositive older adults continue to harbor CMV, and, if so, whether the presence of CMV is associated with immunological consequences.
Neopterin, a guanosine triphosphate (GTP) metabolite, is exclusively produced by monocytes and macrophages in humans and primates. Interferon (IFN)-γ is known to induce neopterin production (13). Elevated serum neopterin levels have been demonstrated in conditions with significant immune activation, such as acute infections, autoimmune diseases, and transplant rejection (14–18). Hence, neopterin is recognized as a marker of immune activation and monocyte/macrophage activity (13;19;20). We have recently observed that community-dwelling older adults have elevated serum neopterin levels (21), and those who are frail manifest upregulated expression of specific immune activating/modulating genes by purified monocytes (22;23). While CMV is known to modulate the function and differentiation of monocytes and macrophages, much remains to be learned about a potential role of chronic CMV infection in immune activation in the elderly.
The objective of this study was to investigate CMV DNA detection in peripheral monocytes, CMV IgG serology, and their relationship with serum neopterin levels in older adults. We hypothesized that detectable CMV DNA rather than positive IgG serology is associated with elevated neopterin levels. Testing this hypothesis will assess the utility of CMV DNA detection as an important additional tool for better evaluation of chronic CMV infection in the elderly. It will also provide initial insight into the role of chronic CMV infection in monocyte/macrophage-mediated immune activation in this population. To achieve this, we evaluated CMV viral DNA in peripheral monocytes by nested PCR and compared the relationships of detectable CMV DNA and positive IgG serology with serum levels of neopterin in community-dwelling older adults over 70 years of age. We also evaluated if IFN-γ would have a mediating role in the relationships between CMV measurements and neopterin levels.
2. MATERIALS AND METHODS
2.1. Human subjects
Community-dwelling older adults over 70 years were recruited in two settings. About two-thirds of the study participants (n=49) were recruited via collaborating physicians and community newspaper advertisement and flyers at outpatient clinics, senior centers, residential retirement communities, and residential areas in Baltimore, Maryland. Potential candidates who consented to participate were screened by experienced clinical research coordinators. The remaining one-third (n=22) were recruited from the Women’s Health and Aging Study (WHAS) II, an established observational cohort study of community-dwelling older women in the same area (24) with demographic and clinical data available to determine consented individuals’ eligibility for enrollment to the present study. Exclusion criteria for this study include chronic inflammatory conditions (e.g. rheumatoid arthritis and inflammatory bowel disease), active malignancy, acute illness such as bacterial or viral infections or acute exacerbation of chronic conditions, or use of immune modulating agents (oral steroids, methotrexate, etc) or chemotherapy. These exclusion criteria were intended to minimize the impact of potential immune activation or suppression from the listed acute or chronic systemic conditions or immune modulating agents. A detailed medical history and brief physical examination were performed by a physician investigator to ensure enrollees’ eligibility and ascertain clinical diagnoses and medication use. Seventy-eight volunteers who met the entry criteria (age and community-dwelling status) were initially screened with 7 individuals excluded based on the exclusion criteria described above, yielding the final sample size of 71 for this study. The Johns Hopkins Institutional Review Board approved the study protocol, and written informed consent was obtained from all participants.
2.2. CMV viral DNA detection in peripheral monocytes by nested polymerase chain reaction (PCR)
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood samples by centrifugation over Ficoll–Hypaque density gradient (specific density, 1.077 g/ml) for 10 min at 600 × g at room temperature, washed three times with phosphate buffered saline (PBS) containing 2 mM EDTA and 0.5% bovine albumin (pH 7.4), and stored in liquid nitrogen. Monocytes were enriched from freshly thawed PBMC samples via 2 h-incubation in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Gaithersberg, MD) at 37°C in a humidified 5% CO2 incubator, after which non-adherent cells were removed by repeated rinsing with serum-free RPMI 1640. The number of CD14+ monocytes were assessed by flow cytometry and standardized as previously described (22;23). DNA was extracted from isolated peripheral monocytes using a Qiagen kit (Qiagen, Valencia, CA) and quantified using standard laboratory protocol. Nested PCR with primers targeted to the CMV UL123 gene was performed using Tapbead hot start polymerase (Promega, Madison, WI) with 1.5 mM MgCl2. The primers included a first set: forward 5′-CAATACACTTCATCTCCTCGAAAGG-3′ and reverse 5′-ATGGAGTCCTCTGCCAAGAGAAAGATGGAC-3′ and second set: forward 5′-TCTGCCAGGACATCTTTCTC-3′ and reverse 5′-GTGACCAAGGCCACGACGTT-3′) as previously reported (25;26). Sample DNA (50 ng) was added to the first round PCR from which 2 μl of the product mix was added to the second round PCR with a thermal cycling program of enzyme activation for 5 min at 95°C and 40 cycles of 1 min denaturation at 94°C, 1 min annealing at 45°C, and 2 min extension at 72°C for both PCR reactions. A 167-bp CMV viral DNA fragment was visualized by gel electrophoresis and confirmed by DNA sequencing. The quality of input sample DNA was confirmed by amplification of a cellular house-keeping gene glyceraldehydes 3 phosphate dehydrogenase (GAPDH). Purified human CMV laboratory strain AD169 (CMVAD169, Advanced Biotech Inc, Paterson, NJ) was employed as a positive control. In the pilot experiments, CMV DNA was detected in all experiments when 2 or more copies of CMVAD169 were in the sample DNA after series of dilutions and at about half of the times when 1 copy of CMVAD169 was calculated to be present. Negative results for CMV DNA detection were confirmed by increasing the amount of input sample DNA to 500 ng.
2.3. Measurements of serum anti-CMV IgG antibody titers, neopterin and IFN-γ levels
Serum samples were obtained from each participant according to the standard protocol, and stored in aliquots at −80°C until analysis. Anti-CMV IgG titers were determined by commercially available enzyme-linked immunosorbent assay (ELISA, United Biotech Inc., Mountain View, CA) with an interassay coefficient of variance (CV) of 5.2%; a titer of 15 ELISA Units (EU)/ml or greater was pre-determined by the manufacturer as CMV-seropositive. Neopterin was measured using a competitive ELISA (ALPCO Diagnostics; Salem, NH) with a sensitivity of 0.8nM and an interassay CV of 5.29%. IFN-γ was measured using ultra sensitive ELISA human cytokine kit (Meso Scale Discovery, Gaithersburg, MD) with a sensitivity of 0.39pg/ml and an interassay CV of 6.4%.
2.4. Statistical analysis
Summary statistics of demographic characteristics, CMV serology status and absolute titers, and serum neopterin and IFN-γ levels were constructed for all 71 study participants and distributions of these characteristics were compared by the CMV viral DNA detection. Data on serum neopterin levels, the outcome measure of the present study, were determined as normally distributed. CMV DNA detection (detectable vs undetectable) and serology status (seropositive vs seronegative) were modeled as binary predictors. Absolute CMV antibody titers and serum IFN-γ concentrations were log transformed to approximate normality. Linear regression analyses were performed to determine 1) statistical significance of differences in neopterin levels between participants with detectable CMV DNA and those without, adjusting for IFN-γ and 2) relationship between log-transformed CMV IgG titers and neopterin levels. All analyses except for the above regression analysis of the relationship between log-transformed CMV IgG titers and neopterin levels were adjusted for age, race, sex, body mass index (BMI), education, major disease categories including hypertension, other cardiovascular diseases (coronary artery disease, congestive heart failure, and stroke), diabetes mellitus, and anemia, as well as medication use such as lipid-lowering drugs. Intercooled Stata software, version 9 was used for model estimation and diagnostics (Stata Corporation, College Station, TX).
3. RESULTS
3.1. Demographic and clinical characteristics of the study participants
Seventy-one community-dwelling older adults with complete data on CMV DNA detection, CMV IgG antibody titers, and serum neopterin and IFN-γ levels were included in the study. Table 1 summarizes major demographic and clinical characteristics of the participants. The mean age of the participants was 84.5 (range of 72–95 years). The majority of them were female and Caucasians with education level of high school and above. Participants had an average of 3 medical diagnoses including the following common chronic conditions: hypertension, other cardiovascular diseases (coronary artery disease, congestive heart failure, and stroke), diabetes mellitus, and anemia. On average, participants took 2–3 medications such as lipid-lowering drugs including HMG-CoA reductase inhibitors. No significant difference was observed between participants with detectable CMV DNA and those without in age, race, sex, education, BMI, total of medical diagnoses and total number of medications, prevalence of hypertension, other cardiovascular diseases, diabetes mellitus, and anemia. Participants with detectable CMV DNA had a higher usage of lipid-lowering drugs than those with no detectable CMV DNA (60% vs 31.7%, respectively, p= .02). In our exploratory analyses, we observed no significant difference in demographic and clinical characteristics by gender (data not shown).
Table 1.
Selected demographic and clinical characteristics of all participants and by CMV DNA detection status
| Characteristics | All participants (N=71) | CMV DNA (+) (N=30) | CMV DNA (−) (N=41) | P value |
|---|---|---|---|---|
| Age (yrs): mean±SD | 84.5±4.6 | 84.4±4.3 | 84.6±4.8 | .85 |
|
| ||||
| Race (White), % | 91.6% | 93.3% | 90.2% | .64 |
|
| ||||
| Sex (female), % | 77.5% | 83.3% | 73.2% | .31 |
|
| ||||
| Education (high school or above), % | 94.4% | 93.3% | 95.1% | .23 |
|
| ||||
| BMI (kg/m2), % | .68 | |||
| <21.5 | 16.4% | 17.2% | 15.8% | |
| 21.5–24.9 | 41.8% | 37.9% | 44.7% | |
| 25–29.9 | 31.3% | 37.9% | 26.3% | |
| ≥30 | 10.5% | 6.9% | 13.2% | |
|
| ||||
| Total # of diagnoses, mean±SD | 3.2±1.6 | 3.1±1.6 | 3.2±1.6 | .69 |
|
| ||||
| Hypertension, % | 67.1% | 76.7% | 60% | .14 |
|
| ||||
| Other cardiovascular diseases, % | 38.0% | 36.7% | 39.0% | .84 |
|
| ||||
| Diabetes mellitus, % | 11.3% | 13.3% | 9.8% | .64 |
|
| ||||
| Anemia, % | 11.3% | 13.3% | 9.8% | .64 |
|
| ||||
| Total # of medications, mean±SD | 2.4±1.5 | 2.7±1.4 | 2.2±1.6 | .15 |
|
| ||||
| Use of lipid lowering drug, % | 43.7% | 60.0% | 31.7% | .02 |
3.2. CMV DNA detection in peripheral monocytes and CMV IgG serology
Fig. 1 shows results from a typical nested PCR experiment in which a 167-bp CMV viral DNA fragment was detected in two out of three participants tested (panel A). The quality of sample DNA from three participants was confirmed by amplification of a cellular house-keeping gene GAPDH (panel B). Pilot experiments with purified genomic DNAof human CMVAD169 strain showed specific viral DNA detection as low as 5 copies of CMVAD169 added to the sample DNA (data not shown), indicating the sensitivity of the nested PCR assay under our experimental conditions. Out of 71 participants tested, CMV DNA was detected in the peripheral monocytes of 30 (42.3%) participants.
Fig. 1.
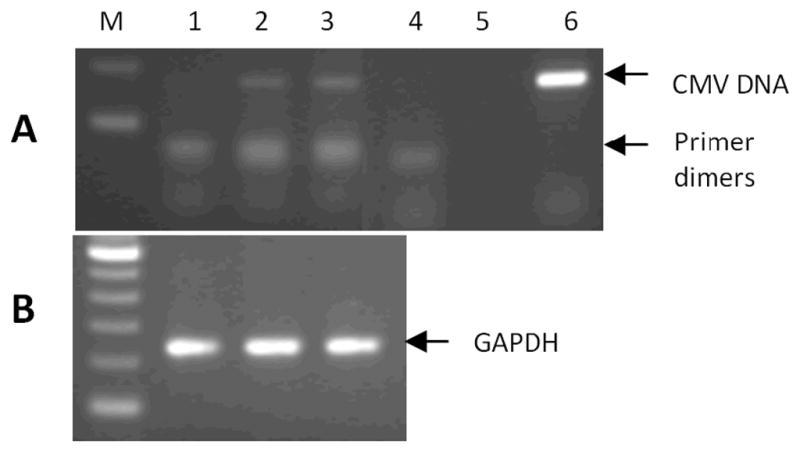
CMV viral DNA detection in peripheral monocytes by nested PCR targeted to the CMV UL123 gene. (A) Positive control (lane 6): A 167-bp PCR product from purified genomic DNA of human CMV strain AD169 (CMV AD169, Advanced Biotech Inc, Paterson, NJ); Negative controls (lanes 4–5) with no input sample DNA (lane 4 – primers for both rounds of PCR and lane 5 – primer for first round PCR only); Two participants with detectable 167-bp CMV DNA (lanes 2–3) and one with none (lane 1). (B) PCR amplification of cellular gene GAPDH with the same amount of input DNA from three participants shown in lanes 1–3, respectively. M: DNA marker.
The median absolute serum anti-CMV IgG antibody titer of all study participants was 40.6 EU/ml (range of 0.9–71.4). Based on the cut-off of 15 EU/ml pre-determined by the assay, 52 (73.2%) were CMV seropositive. All participants with detectable CMV DNA were CMV seropositive. However, only 30 out of 52 (57.7%) CMV seropositive individuals had detectable CMV DNA and 22 out of 41 (53.7%) participants who had no detectable CMV viral DNA were CMV seropositive. Absolute CMV IgG antibody titers did not differ significantly between CMV DNA-positive and –negative participants (medians=42.5 vs 39.1 EU/ml, respectively, p=.23, Table 2).
Table 2.
CMV IgG seropositivity, absolute IgG titers, and levels of neopterin and IFN-γ of all subjects and by CMV DNA detection status
| Study variables | All participants (N=71) | CMV DNA (+)(N=30) | CMV DNA (−) (N=41) | P Values |
|---|---|---|---|---|
| CMV seropositivity, % | 73.2% | 100% | 53.7% | .01 |
| Absolute CMV IgG titer (EU/ml), median (range) | 40.6 (0.9–71.4) | 42.5 (0.9–71.4) | 39.1 (0.9–65.9) | .23* |
| Neopterin (nM), mean±SD | 9.1±3.4 | 10.6±4.4 | 8.0±1.9 | < .0001 |
| IFN-γ (pg/ml), median (range) | 1.09 (0.40 – 10.37) | 1.09 (0.43 – 5.20) | 1.09 (0.40 – 10.37) | .77* |
These P values were obtained from Mann-Whitney test.
3.3. Serum neopterin levels and their relationships with detectable CMV DNA and CMV IgG serology
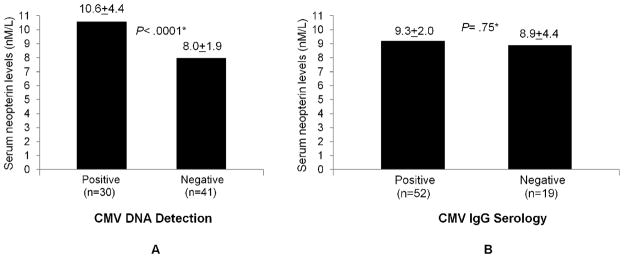
Serum neopterin data in the study participants had nearly normal distribution with a mean of 9.1nM after exclusion of one outlier measurement (data not shown). As shown in Table 2, participants with detectable CMV DNA had significantly higher neopterin levels than those without (10.6±4.4 vs 8.0±1.9 nM, respectively, p< .0001). Fig. 2 shows this data and the difference between the two groups remained statistically significant after adjusting for age, race, sex, education, BMI, hypertension, other cardiovascular diseases, diabetes mellitus, anemia, frailty, and use of lipid-lowering medications (p< .0001, panel A). There was no significant difference in neopterin levels between CMV seropositive and seronegative groups (9.3±2.0 vs 8.9±4.4, respectively, p= .46, panel B).
Fig. 2.
Serum neopterin levels between participants with detectable monocytic CMV viral DNA and those without (A) and between CMV seropositive and seronegative participants (B). *Adjusted for age, race, sex, education, BMI, hypertension, other cardiovascular diseases, diabetes, anemia, and use of lipid-lowering medications.
Next, we evaluated whether neopterin levels were associated with absolute CMV IgG titers. We first modeled log(CMV titers) as a continuous variable, linear regression analysis showed no significant association between neopterin levels and log(CMV titers) (p= .46). Since our exploratory analysis showed possible non-linear relationship between neopterin levels and log(CMV titers) (data not shown), we then tested the quadratic term of log(CMV titers), which again showed no significant association (p= .13). In addition, we modeled CMV titers in tertiles (bottom tertile <30.9 EU/ml, mid tertile ≥30.9 EU/ml and <26.4 EU/ml, and top tertile ≥26.4 EU/ml), and there was no significant difference in neopterin levels between the tertiles (p= .72, ANOVA). These results indicate no statistically significant association between neopterin levels and absolute CMV IgG titers in the study participants. Taken together, these results demonstrate a significant association of detectable CMV DNA rather than CMV seropositivity nor absolute IgG titers with neopterin levels in community-dwelling older adults.
3.4. The association of detectable CMV DNA with neopterin: Independent of serum IFN-γ levels
Since IFN-γ is known to induce neopterin production by monocytes/macrophages, we measured serum IFN-γ levels and evaluated if the observed neopterin difference between the CMV DNA detection study groups was due to the difference in IFN-γ levels. All study participants had detectable IFN-γ in their sera with a median level of 1.09pg/ml (range: 0.40 – 10.37pg/ml). There was no significant difference in IFN-γ levels between participants with detectable CMV DNA and those without (median = 1.09, range = 0.43 – 5.20pg/ml vs median = 1.09, range = 0.40 – 10.37pg/ml, respectively, p=.77) (Table 2). Moreover, the difference in neopterin levels between the two study groups remained significant after adjusting for levels of IFN-γ in the regression model (p< .0001).
4. DISCUSSION
To the best of our knowledge, this study is the first to evaluate the presence of CMV DNA in peripheral monocytes and its relationship with CMV IgG serology and serum neopterin levels in community-dwelling older adults. The results demonstrate that while the majority of participants (73.2%) had positive CMV IgG serology, 42.3% of all participants and 57.7% of seropositive participants had detectable CMV DNA. In addition, detectable CMV DNA rather than positive IgG titers had significant association with elevated neopterin levels. This association was independent of serum IFN-γ levels.
Chronic CMV infection is conventionally diagnosed by positive IgG serology in older adults. In the present study, we sought physical evidence of CMV infection beyond positive IgG serology through detection of CMV DNA by nested PCR in peripheral monocytes, one of the known primary host cell types for CMV infection. Nested PCR is a highly sensitive and specific technique that has been applied to test blood donors for CMV infection (25;26). As expected, all participants with detectable CMV DNA were CMV seropositive. To our surprise, however, nearly half of seropositive older individuals did not have detectable CMV DNA in peripheral monocytes. This is unlikely due to lack of sensitivity of the nested PCR assay because CMV DNA was detected as low as 1–2 copies of CMVAD169 in the sample DNA and negative results were confirmed by increasing the amount of input sample DNA to 500 ng. As positive IgG serology does not distinguish between past and persistent infections, these results suggest that CMV seropositive older adult population is heterogeneous in regard to the status of chronic CMV infection. Whether detectable CMV DNA in monocytes indicates CMV reactivation or persistent CMV infection in this otherwise immunocompetent elderly population requires further investigation.
CMV is known to modulate the function and differentiation of monocytes and macrophages in vitro. However, the relationship between chronic CMV infection and monocyte/macrophage-mediated immune activation in older adults has not been adequately evaluated. Neopterin, whose production is primarily induced by IFN-γ stimulation, is a well established immune activation marker produced by monocytes and macrophages. Serum neopterin levels increase with age (13;21;27–29). Our results are consistent with previously reported neopterin levels of 9 nM or higher in persons over 75 years of age (13;21). Elevated neopterin levels have been reported in the setting of acute CMV infection with IgM seroconversion in blood donors (30). Novel findings in this study include that elevated neopterin levels are associated with detectable monocytic CMV DNA rather than CMV IgG titers in older adults after adjusting for major demographic and clinical covariates. To address the possibility that the difference in IFN-γ could account for the observed difference in neopterin levels, we measured serum IFN-γ levels and demonstrated no significant difference between participants with detectable monocytic CMV DNA and those without. In addition, the association between elevated neopterin levels and detectable CMV DNA in monocytes remained significant after adjusted for IFN-γ levels. While IFN-γ is known to induce neopterin production, IFN-γ is produced by a number of different cell types – T cells, NK cells, dendric cells, etc. Thus, its levels reflect the balance of production at many different sites and cells, not necessarily linked to direct stimulation of neopterin production at specific tissue sites (macrophages) or niches (monocytes). Alternatively, CMV infection may induce neopterin production by monocytes and macrophages independent of IFN-γ, which is under active investigation in our laboratory. Of note, participants with detectable CMV DNA had more frequent use of HMG-CoA reductase inhibitors (statins) than those without. These drugs have been shown to suppress neopterin production and to be associated with decreased neopterin levels in stable coronary artery disease (31;32). Whether chronic CMV infection can overcome the suppressive effect of statins in its contribution to elevated neopterin levels in older adults deserves further investigation.
A number of studies have shown CMV-specific CD4+ and CD8+ T-cell clonal expansion in CMV seropositive older adults, suggesting a role of chronic CMV infection in age-related T-cell immunosenescence (10;33;34). CMV seropositivity has been associated with increased risk for frailty and mortality in older adults (7;35;36). In addition, absolute CMV IgG titer appears to be superior than seropositivity in detecting its association with frailty and mortality in the elderly (37). Our results showed significant association of elevated neopterin levels only with detectable CMV DNA and no such association with either CMV seropositivity or absolute IgG titers, suggesting that CMV DNA detection in monocytes may provide additional information about the status of chronic CMV infection in the elderly. Contrary to CMV DNA detection in peripheral monocytes, which requires isolation of PBMCs and advanced nested PCR technique, CMV IgG serology is usually measured by standard ELISA assays in small amount of serum sample with established sensitivity and specificity and thus, appears to be a useful marker that is easy to obtain in large cohort studies. However, recent studies have shown detection of CMV DNA by PCR in dried blood spot (DBS) as a useful tool in screening congenital CMV infection in the pediatric population (38;39). Whether the PCR methodology employed in this study can be optimized and applied for CMV DNA detection via DBS in large-scale aging research deserves further evaluation.
We acknowledge several limitations of the study. First, there is a possibility that CMV could be harbored in other cells and tissues in seropostiive older individuals. While we cannot rule out this possibility, peripheral monocytes have been shown to be the principal cell types that harbor the CMV DNA genome (4;40–42). Secondly, serum neopterin levels are likely influenced by many factors other than chronic CMV infection. While acute bacterial and viral infections were excluded from this study and age, BMI, IFN-γ and other potential demographic and clinical covariates were adjusted in our regression analyses, potential influence from unrecognized or subclinical infections and other unidentified factors could not be completely eliminated. Lastly, this is a cross-sectional study with limited sample size. The biologically plausible causal relationship between chronic CMV infection and elevated neopterin levels will need to be confirmed in larger older adult cohort studies. Despite these limitations, results from this study support our original hypothesis and provide initial evidence suggesting CMV DNA detection in monocytes as an additional informative tool for better understanding of the status of chronic CMV infection in older adults. These findings also provide a basis for further investigation into underlying mechanisms through which chronic CMV infection contributes to elevated neopterin levels and monocyte/macrophage-mediated immune activation in the elderly.
Reference List
- 1.Mocarski E, Shenk T, Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Philadelphia: Lippincott, Williams & Wilkins; 2007. pp. 2701–72. [Google Scholar]
- 2.Wreghitt TG, Teare EL, Sule O, Devi R, Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003 December 15;37(12):1603–6. doi: 10.1086/379711. [DOI] [PubMed] [Google Scholar]
- 3.Sissons JG, Bain M, Wills MR. Latency and reactivation of human cytomegalovirus. J Infect. 2002 February;44(2):73–7. doi: 10.1053/jinf.2001.0948. [DOI] [PubMed] [Google Scholar]
- 4.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 September;72(Pt 9):2059–64. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 5.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006 November 1;43(9):1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 6.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009 January;137(1):58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000 December 20;121(1–3):187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 8.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002 January;37(2–3):445–53. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003 August;38(8):911–20. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 10.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005 June;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 11.Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Lofgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006 August;127(8):695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Strindhall J, Nilsson BO, Lofgren S, Ernerudh J, Pawelec G, Johansson B, Wikby A. No Immune Risk Profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp Gerontol. 2007 August;42(8):753–61. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs D. Immune activatoin marker neopterin. scitopoics com. 2008 August 7; Available from: URL: http://www.scitopics.com/Immune_activation_marker_neopterin.html.
- 14.Denz H, Fuchs D, Hausen A, Huber H, Nachbaur D, Reibnegger G, Thaler J, Werner ER, Wachter H. Value of urinary neopterin in the differential diagnosis of bacterial and viral infections. Klin Wochenschr. 1990 February 15;68(4):218–22. doi: 10.1007/BF01662720. [DOI] [PubMed] [Google Scholar]
- 15.Sheldon J, Riches PG, Soni N, Jurges E, Gore M, Dadian G, Hobbs JR. Plasma neopterin as an adjunct to C-reactive protein in assessment of infection. Clin Chem. 1991 December;37(12):2038–42. [PubMed] [Google Scholar]
- 16.Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J Clin Pharm Ther. 2001 October;26(5):319–29. doi: 10.1046/j.1365-2710.2001.00358.x. [DOI] [PubMed] [Google Scholar]
- 17.Nasonov EL, Samsonov MI, Tilz G, Fuchs D. Neopterin: new immunological marker of autoimmune rheumatic disease. Klin Med (Mosk) 2000;78(8):43–6. [PubMed] [Google Scholar]
- 18.Reibnegger G, Fuchs D, Fuith LC, Hausen A, Werner ER, Werner-Felmayer G, Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in malignant disease. Cancer Detect Prev. 1991;15(6):483–90. [PubMed] [Google Scholar]
- 19.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002 April;3(2):175–87. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 20.Melichar B, Solichova D, Freedman RS. Neopterin as an indicator of immune activation and prognosis in patients with gynecological malignancies. Int J Gynecol Cancer. 2006 January;16(1):240–52. doi: 10.1111/j.1525-1438.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 21.Spencer ME, Jain A, Matteini A, Beamer B, Wang NY, Leng S, Punjabi NM, Walston J, Fedarko N. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI and percentage body fat. J Gerontol A Biol Sci Med Sci. 2010 doi: 10.1093/gerona/glq066. doi: 10 1093/gerona/glq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA, Ferrucci L, Rose NR, Leng SX. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009 March;130(3):161–6. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009 June;46(3):319–24. doi: 10.1016/j.cyto.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000 January;55(1):M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 25.Slobedman B, Mocarski ES. Quantitative analysis of latent human cytomegalovirus. J Virol. 1999 June;73(6):4806–12. doi: 10.1128/jvi.73.6.4806-4812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roback JD, Hillyer CD, Drew WL, Laycock ME, Luka J, Mocarski ES, Slobedman B, Smith JW, Soderberg-Naucler C, Todd DS, Woxenius S, Busch MP. Multicenter evaluation of PCR methods for detecting CMV DNAin blood donors. Transfusion. 2001 October;41(10):1249–57. doi: 10.1046/j.1537-2995.2001.41101249.x. [DOI] [PubMed] [Google Scholar]
- 27.Reibnegger G, Huber LA, Jurgens G, Schonitzer D, Werner ER, Wachter H, Wick G, Traill KN. Approach to define “normal aging” in man. Immune function, serum lipids, lipoproteins and neopterin levels. Mech Ageing Dev. 1988 December;46(1–3):67–82. doi: 10.1016/0047-6374(88)90115-7. [DOI] [PubMed] [Google Scholar]
- 28.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004 August;37(8):684–7. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Solichova D, Melichar B, Blaha V, Klejna M, Vavrova J, Palicka V, Zadak Z. Biochemical profile and survival in nonagenarians. Clin Biochem. 2001 October;34(7):563–9. doi: 10.1016/s0009-9120(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 30.Schennach H, Hessenberger G, Mayersbach P, Schonitzer D, Fuchs D. Acute cytomegalovirus infections in blood donors are indicated by increased serum neopterin concentrations. Med Microbiol Immunol. 2002 October;191(2):115–8. doi: 10.1007/s00430-002-0148-8. [DOI] [PubMed] [Google Scholar]
- 31.Walter RB, Fuchs D, Weiss G, Walter TR, Reinhart WH. HMG-CoA reductase inhibitors are associated with decreased serum neopterin levels in stable coronary artery disease. Clin Chem Lab Med. 2003 October;41(10):1314–9. doi: 10.1515/CCLM.2003.200. [DOI] [PubMed] [Google Scholar]
- 32.Neurauter G, Wirleitner B, Laich A, Schennach H, Weiss G, Fuchs D. Atorvastatin suppresses interferon-gamma -induced neopterin formation and tryptophan degradation in human peripheral blood mononuclear cells and in monocytic cell lines. Clin Exp Immunol. 2003 February;131(2):264–7. doi: 10.1046/j.1365-2249.2003.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L, Pedrazzoni M, Bucci L, Monti D, Medici MC, Chezzi C, Franceschi C, Sansoni P. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol. 2007 September 15;179(6):4283–91. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- 34.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002 August 15;169(4):1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 35.Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009 January;19(1):47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- 36.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005 May;53(5):747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010 May 15;171(10):1144–52. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Jr, Palmer AL, Ahmed A, Michaels MG, Sanchez PJ, Bernstein DI, Britt WJ, Fowler KB. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010 April 14;303(14):1375–82. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharrazi M, Hyde T, Young S, Amin MM, Cannon MJ, Dollard SC. Use of screening dried blood spots for estimation of prevalence, risk factors, and birth outcomes of congenital cytomegalovirus infection. J Pediatr. 2010 August;157(2):191–7. doi: 10.1016/j.jpeds.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Bolovan-Fritts CA, Mocarski ES, Wiedeman JA. Peripheral blood CD14(+) cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood. 1999 January 1;93(1):394–8. [PubMed] [Google Scholar]
- 41.Taylor-Wiedeman J, Hayhurst GP, Sissons JG, Sinclair JH. Polymorphonuclear cells are not sites of persistence of human cytomegalovirus in healthy individuals. J Gen Virol. 1993 February;74(Pt 2):265–8. doi: 10.1099/0022-1317-74-2-265. [DOI] [PubMed] [Google Scholar]
- 42.Sinclair J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J Clin Virol. 2008 March;41(3):180–5. doi: 10.1016/j.jcv.2007.11.014. [DOI] [PubMed] [Google Scholar]