Abstract
The family of poly(2-oxazoline)s (POx) is being increasingly investigated in the context of biomedical applications. We tested the relative cytotoxicity of POx and were able to confirm that these polymers are typically not cytotoxic even at high concentrations. Furthermore, we report structure-uptake relationships of a series of amphiphilic POx block copolymers that have different architectures, molar mass and chain termini. The rate of endocytosis can be fine-tuned over a broad range by changing the polymer structure. The cellular uptake increases with the hydrophobic character of the polymers and is observed even at nanomolar concentrations. Considering the structural versatility of this class of polymers, the relative ease of preparation and their stability underlines the potential of POx as a promising platform candidate for the preparation of next-generation polymer therapeutics.
Keywords: amphiphilic block copolymer, endocytosis, biocompatibility, drug delivery, flow cytometry
1. Introduction
Poly(2-oxazoline)s (POx) are a family of polymers that can be obtained via living cationic ring opening polymerization of 2-oxazoline monomers, which allows for excellent synthetic control over the molar mass and preparation of multi-block copolymers.[1,2] POx are structurally versatile, for example, both chain termini can be independently equipped with a chemical (e.g., –OH, -NH, etc.) or structural (e.g. lipid) functionality.[3] A number of 2-oxazoline monomers are commercially available (e.g. 2-methyl-, 2-ethyl-, 2-isopropyl, 2-phenyl-2-oxazoline) and a wide range of 2-oxazoline monomers carrying other aliphatic side chains and functional side chains are readily accessible. Recently, in addition to the long known side functionalities such as –OH and –COOH,[4] we and other researchers introduced a number of functional side chains, which can be employed in chemoselective ligations, such as oxime formation [5] and click chemistry [6] as well as the possibility to obtain star-like POx using pluritriflate initiators.[7] Moreover, molecular brushes based on POx have been introduced and complete the structural tool kit available with POx.[8,9]
POx are attractive for biomedical applications.[10] For therapeutic and analytical purposes, Saegusa and co-workers have suggested the use of POx for catalase conjugation (POxylation) already in 1990 [11] and more recently a similar approach has been used to conjugate trypsin and cytosine arabinose.[12] We recently reported on the modification of cellular uptake of horseradish peroxidase by attachment of amphiphilic POx.[13] Also, we discovered recently that in particular 2-butyl-2-oxazoline (BuOx) based POx amphiphiles are interesting candidates for formulation and delivery of highly water-insoluble drugs.[14]
POx with C2 and C3 side chains are thermosensitive in aqueous solutions and can be tumed to yield cloud points over a broad range of temperatures.[10,15,16,17]
Hydrophilic poly(2-methyl-2-oxazoline) (PMeOx) and poly(2-ethyl-2-oxazoline) (PEtOx) can impose a stealth effect when grafted on liposomes and surfaces similar to PEG.[18,19] Also, amphiphilic POx block copolymers exhibit only very limited and weak interactions with human serum proteins.[20] Accordingly, low molar mass PMeOx and PEtOx are excreted very rapidly via the kidneys and show no significant unspecific uptake in vivo after intravenous administration.[21] At the same time, POx can be tailored to exhibit antimicrobial effects.[22,23] As a result, POx are discussed as a potential alternative for the current biomaterial “gold standard” poly(ethylene glycol) (PEG) to overcome its limitations.[10,24] Overall, these polymer carriers are chemically well-defined, non-toxic, exhibit low immunogenicity and their sufficiently small size allows them to be easily excreted from the body.
Cellular uptake of synthetic polymers and nanomaterials is a central interest in drug delivery. The vast structural diversity among POx makes this platform an ideal candidate to decipher structure-uptake relationships in cells. Therefore, we synthesized a number of POx amphiphiles comprising 2-butyl-2-oxazoline (BuOx), 2-nonyl-2-oxazoline (NOx), 2-n-propyl-2-oxazoline (nPrOx), 2-isopropyl-2-oxazoline (iPrOx) as the hydrophobic blocks and investigated their cytotoxicity in human cancer cell lines (MCF7, MCF7-ADR) and immortalized canine kidney epithelial cells (Madin-Darby Canine Kidney, MDCK). After conjugation with fluorescent labels (tetramethylrhodamine isothiocyanate (TRITC) or ATTO425, respectively) we investigated the cellular uptake of these polymer amphiphiles by flow cytometry and laser scanning confocal microscopy to evaluate their feasibilty for intracellular drug delivery. Our results suggest that POx based amphiphiles are generally non-toxic even at high concentrations of up to 20 g/L and show, depending on their structure, rapid, energy dependent cellular uptake.
2. Materials and Methods
2.1 Materials and Instrumentations
All substances were purchased from Aldrich (München, Germany) and Acros (Geel, Belgium) and were used as received unless otherwise stated. Methyl trifluoromethylsulfonate (MeOTf), 2-methyl-2-oxazoline (MeOx), 2-ethyl-2-oxazoline (EtOx), acetonitrile (ACN) and other solvents were dried by refluxing over CaH2 under dry nitrogen atmosphere and subsequent distillation prior to use. NMR spectra were recorded on a Bruker ARX 300 (1H: 300.13 MHz) or a Bruker AC 250 (1H: 250.13 MHz) at room temperature. The spectra were calibrated using the solvent signals (CDCl3: 7.26 ppm; D2O: 4.67 ppm). Gel permeation chromatography (GPC) was performed on a Waters system (pump mod. 510, RI-detector mod. 410, precolumn PLgel and two PL Resipore colunms (3 µm, 300 × 7,5 mm)) with N,N-dimethyl acetamide (DMAc) (57 mmol/L LiBr, 80 °C, 1 mL/min) as eluent and calibrated against polystyrene standards. Microwave supported polymerizations were performed using a CEM Discover microwave with a maximum power setting to 150 W. The microwave was set to reaction temperature of 130 °C that was continuously monitored by an external infra-red detector.
MCF7-ADR cells derived from human breast carcinoma cell line, MCF7 (ATCC HT-B22) by selection with Doxorubicin, were kindly presented by Y.L. Lee (William Beaumont Hospital, Royal Oak, MI). Cells were maintained in Dulbecco´s Modified Eagle´s Medium (DMEM), containing 10% heat inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin as described elsewhere. All cell culture materials were obtained from Gibco Life Technologies, Inc. (Grand Island, NY) unless otherwise stated. Cells were used 2 days after seeded unless otherwise stated.
2.2 Synthesis of polymer amphiphiles
2.2.1 Preparation of bifunctional initiator
The bisoxazoline 1,2-bisoxazolinylethane was prepared according to literature procedure.[25]
2.2.2 Preparation of polymer amphiphiles
The polymerizations and work-up procedures were carried out according to the procedure described previously for H1 [21], T1–T3 and D3 [14].
Exemplary, the preparation of T1 is described as follows.
Under dry and inert conditions 32.2 mg (0.2 mmol, 1 eq) of methyl trifluoromethylsulfonate (methyl triflate, MeOTf) and 440 mg (5.17 mmol, 26 eq) of 2-methyl-2-oxazoline (MeOx) were dissolved in 3 mL dry acetonitrile at room temperature. The mixture was subjected to microwave irradiation (150 W maximum, 130 °C) for 15 min. After cooling to room temperature, the monomer for the second block, 2-butyl-2-oxazoline (256 mg, 2.01 mmol, 10 eq) was added and the mixture was irradiated the same way as for the first block. The procedure was repeated for the third block with 442 mg (5.19 mmol, 26 eq). Finally, T1 was terminated by addition of 0.1 mL piperidine (1.01 mmol, 5 eq) at room temperature. After stirring over night, an access of K2CO3 was added and the mixture was allowed to stir for several hours. After centrifugation, the solvent was removed from the supernatant under reduced pressure, the residue was taken up by addition of 3 mL of chloroform. After precipitation from cold diethyl ether (approx. 10 times the amount of polymer solution) the product was obtained by centrifugation. The precipitation was repeated twice and the polymer was obtained as colorless powder (792 mg, 67 %, Mth = 5.8 kg/mol) after lyophilization from water. GPC (DMAc): Mn = 8.5 kg/mol (Đ = 1.21).
Accordingly, the other polymers were prepared with the appropriate intiators, monomers and terminating reagents.
2.2.3 Fluorescent labeling of polymer
Fluorescent labels (tetramethylrhodamine isothiocyanate and ATTO425-NHS ester) were coupled to amine terminated polymers in dry dimethylformamide using a 1.2 fold excess of dye and diisopropylethlyamine as base. Free dye was removed by repeated gel filtration (Sephadex LH20, mobile phase methanol). Typically after 2–3 filtrations, no free dye band was observed. Degree of labeling was obtained spectrophotometrically and was found to be typically 30–60 %.
2.3 Cytotoxicity assay
Cells were plated in 96 well plates (Corning Inc., Corning NY) (1×104 cells/well) and incubated with polymer solutions in assay buffer (2 h incubations) (containing 122 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 10 mM HEPES, 3 mM KCl, 1.2 mM MgSO4, 1.4 mM CaCl2, and 0.4 mM K2HPO4, pH 7.4) or with polymer solutions in full media for longer incubations. After incubation, cells were washed with phosphate buffered saline (PBS) and supplemented for additional 48 h – 72 h with media. The media was removed, 100 µL of fresh media without FBS and 25 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Invitrogen, Eugene, Oregon) solution in PBS (5 g/L) was added. After incubation at 37 °C for 2 h, the solution was removed and 100 µL of solvent (25% v/v DMF, 20% w/v sodium dodecylsulfate in H2O) were added. The formazan salt was allowed to dissolve for 4 h or over night at 37 °C and absorbance was read at 570 nm using a plate reader (SpectraMax M5, Molecular Devices). As negative control, wells which had no cells plated onto were used, for positive control, cells were incubated with media alone. All experiments were performed in quadruplicate and data presented in means ± standard error means (SEM).
2.4 Flow cytometry
For flow cytometric analysis cells were plated in 24 well plates (5×104 cells/well). Subsequent incubation with 200 µL of polymer solutions of indicated concentrations in media without FBS was performed for 1 h at 37 °C. After washing thrice with ice-cold PBS, cells were trypsinized and centrifuged. The pellet was resuspended in PBS containing bovine serum albumin (1 g/L). The mean fluorescence intensity was analyzed using a Becton Dickinson LSRII flow cytometer operating under FACSDiva software (San Jose, CA). Data were acquired in linear mode and visualized in logarithmic mode. Data from 10,000 events were gated using forward and side scatter parameters to exclude debris and dead cells as well as control cells incubated with media alone as control for autofluorescence.
All experiments were conducted in triplicates and data presented as means ± SEM. The data were fitted with Boltzmann equations from which the concentration at 50% gated cells (EC50) was calculated.
2.5 Laser scanning confocal microscopy on live cells
Cells were seeded and grown (48 h) in Lab-Tek Chambered Coverglass wells (Nalge Nunc Int. Rochester, NY) (8 wells, 4×104 cells/well). Cells were incubation with 200µL of labeled polymer solutions (1 g/L) in FBS free media for indicated times, subsequent washing (3×) with PBS and addition of media using a laser scanning confocal microscope (Carl Zeiss LSM 510 Meta, Peabody, MA)
3. Results
3.1. Polymer synthesis and fluorescent labeling
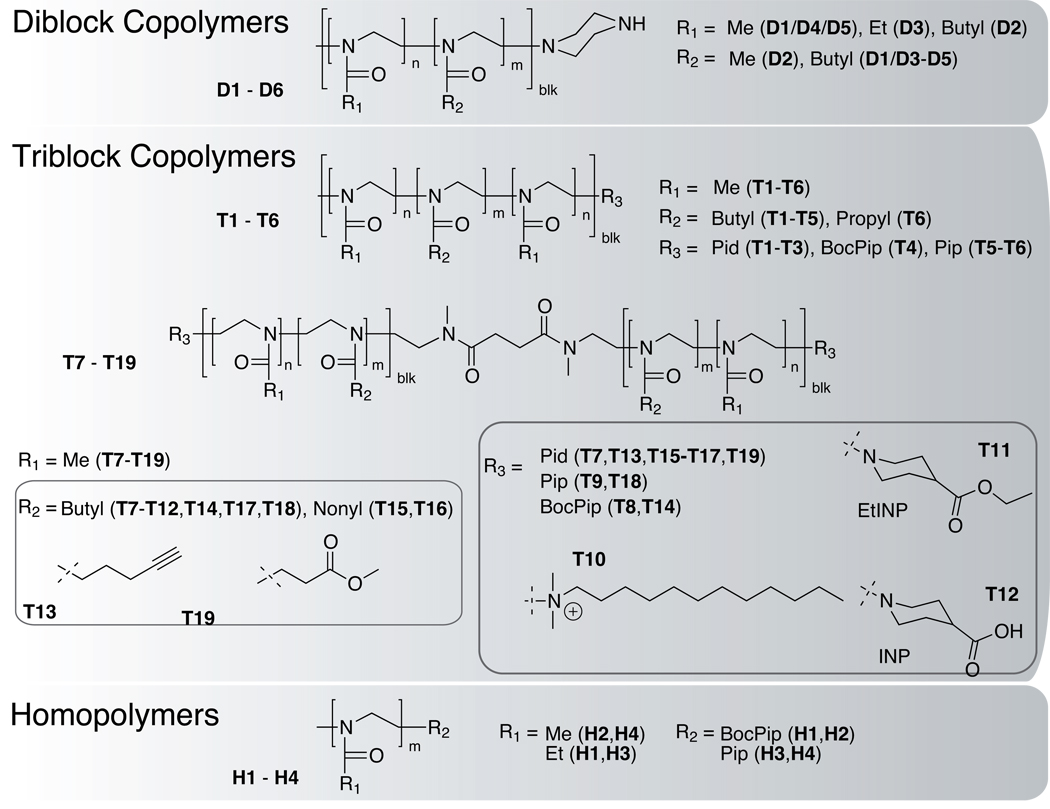
In this study we investigate homopolymers and block copolymers of different architectures, molar masses and hydrophilic/lipophilic balance (HLB) (Figure 1, Table 1).
Figure 1.
Structures of investigated polymers. Different architectures of diblock, triblock copolymers and homopolymers were investigated. Triblock copolymers were prepared from bisfunctional initiators as well as monofunctional initiators. Moreover, polymers with different termini were prepared and studied (T7–T12). Water soluble homopolyers were synthesized from 2-methyl-2-oxazoline and 2-ethyl-2-oxazoline.
Table 1.
Compositions of the polymers investigated in the present study (T denotes triblock, D diblock copolymers. H stands for homopolymers.
| Polymer | 1st Block | 2nd Block | 3rd Block | Terminus | Polymer | 1st Block | 2nd Block | Terminus |
|---|---|---|---|---|---|---|---|---|
| T1 | MeOx | BuOx | MeOx | Pid | T15 | NOx | MeOx | Pid |
| T2 | MeOx | BuOx | MeOx | Pid | T16 | NOx | MeOx | Pid |
| T3 | MeOx | BuOx | MeOx | Pid | T17 | BuOx | MeOx | Pid |
| T4 | MeOx | BuOx | MeOx | BocPip | T18 | BuOx | MeOx | Pip |
| T5 | MeOx | BuOx | MeOx | Pip | T19 | MOP | MeOx | Pid |
| T6 | MeOx | nPrOx | MeOx | Pip | D1 | MeOx | BuOx | Pip |
| T7 | BuOx | MeOx | n.a. | Pid | D2 | BuOx | MeOx | Pip |
| T8 | BuOx | MeOx | n.a. | BocPip | D3 | EtOx | BuOx | Pip |
| T9 | BuOx | MeOx | n.a. | Pip | D4 | MeOx | iPrOx | Pip |
| T10 | BuOx | MeOx | n.a. | DMDod | D5 | MeOx | nPrOx | Pip |
| T11 | BuOx | MeOx | n.a. | EtINP | H1 | EtOx | n.a. | BocPip |
| T12 | BuOx | MeOx | n.a. | INP | H2 | MeOx | n.a. | BocPip |
| T13 | PynOx | MeOx | n.a. | Pid | H3 | EtOx | n.a. | Pip |
| T14 | BuOx | MeOx | n.a. | BocPip | H4 | MeOx | n.a. | Pip |
Monomers: MeOx:2-methyl-2-oxazoline, EtOx:2-ethyl-2-oxazoline, nPrOx:2-n-propyl-2-oxazolin, iPrOx:2-iso-propyl-2-oxazolin, NOx:2-nonyl-2-oxazoline, PynxOx:2-(4-pentynyl)-2-oxazoline, MOP: methyl-3-(oxazol-2-yl)propionate.
Endgroups: Pid:piperidine, Pip:piperazine, BocPip:N-Boc-piperazine, INP:isonipecotinic acid, EtINP:ethyl isonipecotate, DMDod:N,N-dimethly-N-dodecylamine.
For example, triblock copolymers of ABA type (e.g. T5) are compared with diblock copolymers AB and BA (i.e. D1 and D2) of the same monomer composition, in which A comprises MeOx or EtOx whereas B represents a more hydrophobic block (e.g., comprising nPrOx, iPrOx, BuOx etc.). In addition, we prepared polymers with different termini. For example, polymer T7–T12 are derived from one batch of polymerization, but obtained by addition of different amines to aliquots of the mixture after the polymerization. In particular, T10 is a polymer terminated with N,N-dimethyl-N-dodecylamine, generating an amphiphilic block copolymer with a hydrophobic terminus bearing a quaternized amine. Tiller and co-workers have shown in previous accounts that similar structures are effective antimicrobials.[22,23] We were interested in their ability to integrate into mammalian cell membranes leading to cytotoxicity. Other termini at the very same polymers included N-Boc-piperazine (a protected amine terminal group) (T8), piperidine (T7) and ethyl isonipecotate (a protected carboxylic acid terminal group) (T11). T9 represents the same polymer bearing a piperazine terminus, obtained by deprotection of T8. Accordingly, T12 is saponified T11 (Figure 1).
In general, the polymers are of good to medium definition (Đ < 1.3) with some exceptions where we obtained relatively broad distributions (Đ = 1.3–1.5) (Table 2).
Table 2.
Analytical data of polymers investigated in this study.
| Polymer | Mtheoa | MnGPC,b | Đb | MnNMR,c | DPHc | DPLc | Yield |
|---|---|---|---|---|---|---|---|
| T1 | 5.8 | 8.5 | 1.21 | 6.6 | 54 | 12 | 67 |
| T2 | 6.4 | 10.4 | 1.18 | 7.3 | 51 | 19 | 80 |
| T3 | 7.1 | 9.9 | 1.23 | 7.0 | 48 | 20 | 55 |
| T4 | 5.9 | 5.8 | 1.19 | n.d. | n.d. | n.d. | n.d. |
| T5 | 8.0 | 14.7 | 1.22 | 10.0 | 88 | 18 | 69 |
| T6 | 8.2 | 6.2 | 1.47 | 6.7 | 47 | 23 | 83 |
| T7 | 6.7 | n.d. | n.d. | 6.7 | 52 | 16 | >95% |
| T8 | 6.9 | 10.0 | 1.19 | 6.9 | 52 | 16 | >95% |
| T9 | 6.7 | 10.2 | 1.18 | 6.7 | 52 | 16 | n.d. |
| T10 | 7.0 | n.d. | n.d. | 7.0 | 52 | 16 | >95% |
| T11 | 6.8 | 7.5 | 1.24 | 6.8 | 52 | 16 | >95% |
| D1 | 7.7 | 11.3 | 1.35 | 6.9 | 51 | 19 | 80 |
| D2 | 7.7 | 10.0 | 1.19 | 6.9 | 54 | 17 | >95% |
| D3 | 7.8 | 11.5 | 1.09 | 7.9 | 54 | 20 | 77 |
| D4 | 12.4 | 13.1 | 1.35 | n.d. | n.d. | n.d. | 50 |
| D5 | 12.4 | 10.4 | 1.29 | 8.7 | 94 | 5 | 48 |
| H1 | 4.5 | 6.2 | 1.15 | 5.7 | 60 | n.a. | 88 |
| H2 | 4.3 | 6.6 | 1.16 | 5.3 | 56 | n.a. | 84 |
| H3 | 4.4 | 6.7 | 1.18 | 5.6 | 60 | n.a. | 88 |
| H4 | 4.2 | 6.4 | 1.22 | 5.2 | 56 | n.a. | 66 |
as determined from [M]0/[I]0.
as determined by gel permeation chromatography (solvent DMAc, PMMA calibration).
as estimated by 1H-NMR by end-group and side chain analysis.
To study the endocytosis of the polymers piperazine-terminated polymers were modified with Atto425 (polymers indexed with †) or TRITC (index *). These dyes were chosen due to their small molar mass and zero net charge. Thus, we expected minimal influence of the fluorophore on the HLB of the polymer-dye conjugate. However, some influence on the interaction of the polymers with cell membranes cannot be ruled out.
Five different diblock copolymers were investigated with respect to their concentration-dependent cellular uptake. Four comprised MeOx as hydrophilic monomer (D1†, D2†, D4† and D5†) while D3† comprised EtOx.
The hydrophobic block varied from BuOx (e.g. D1† – D3†) to iPrOx and nPrOx (D4†, D5†). The triblock copolymers included in the flow cytometry study were T5†, T6†, T9† and T9*. In addition, endocytosis of H3*, a homopolymer of EtOx was studied by flow cytometry.
3.2. Cytotoxicity studies
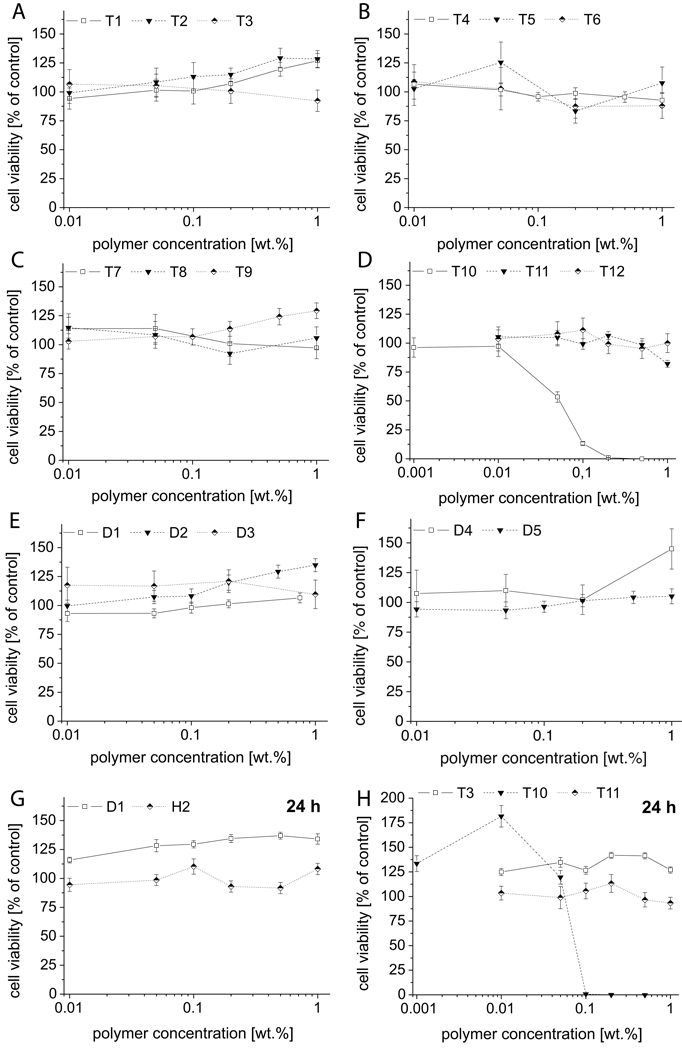
The cytotoxicity of the polymers was investigated in different cell lines. We investigated the cell viability using the MTT assay after treatment with polymer solution at different concentrations for 2h and 24h, in either human breast cancer cells (MCF7 and MCF7-ADR) or immortalized canine kidney cells (MDCK). Corroborating earlier studies, we found that POx are in general well tolerated at polymer concentrations of up to 20 g/L with some exceptions. Initially, we studied the cytotoxicity profile of POx amphiphiles in MDCK cells (Figure 2). As can be clearly seen, only one polymer sample, the quaternized triblock copolymer T10 showed a marked inhibition of the cells proliferation after 2 h incubation with a IC50 of approx. 0.05 wt.% (0.07 mM). After 24 hours of incubation, the IC50 value of T10 remained practically unchanged. It should be noted that T10 is a triblock copolymer obtained from a bisfunctional initiator and therefore also bears two termini.
Figure 2.
Cell viability of blockco- and homo poly(2-oxazoline)s in MDCK cells as determined by MTT assay after 2h (A–F) and 24h incubation (G,H). Experiments were performed in quadruplicate and data is expressed as means ± standard error means (SEM).
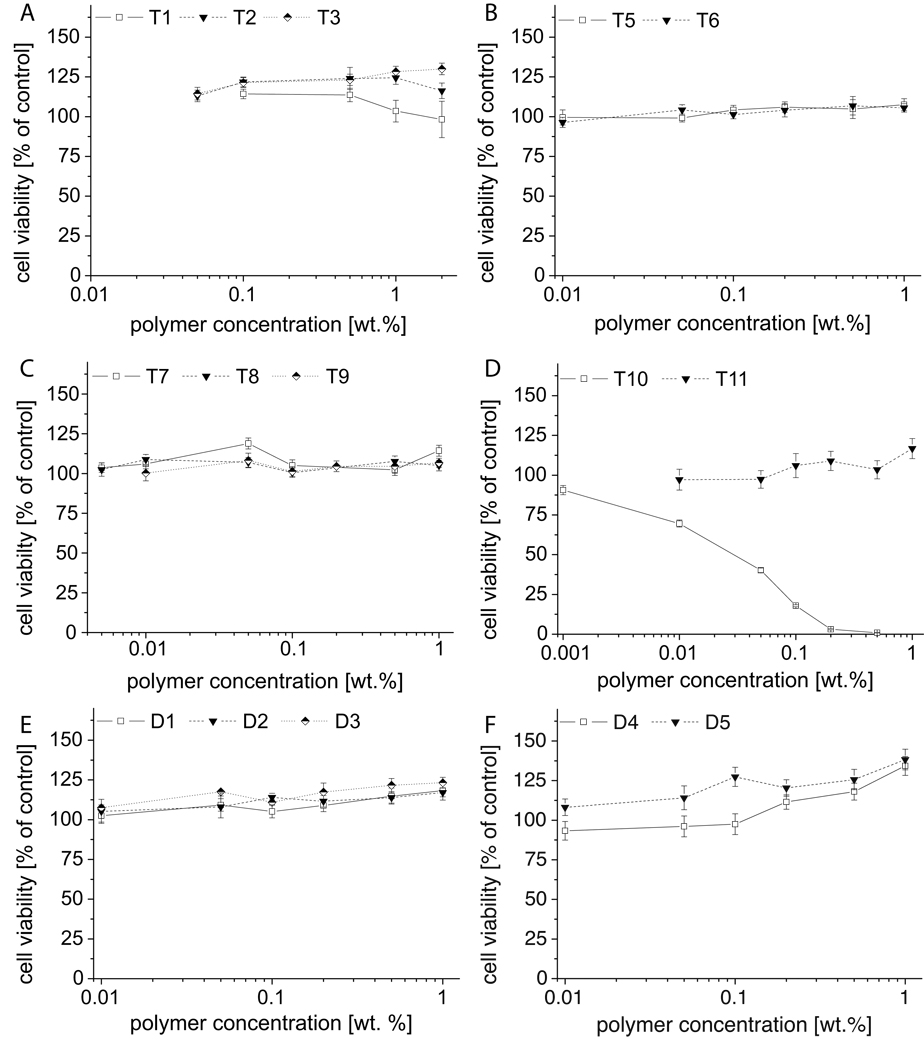
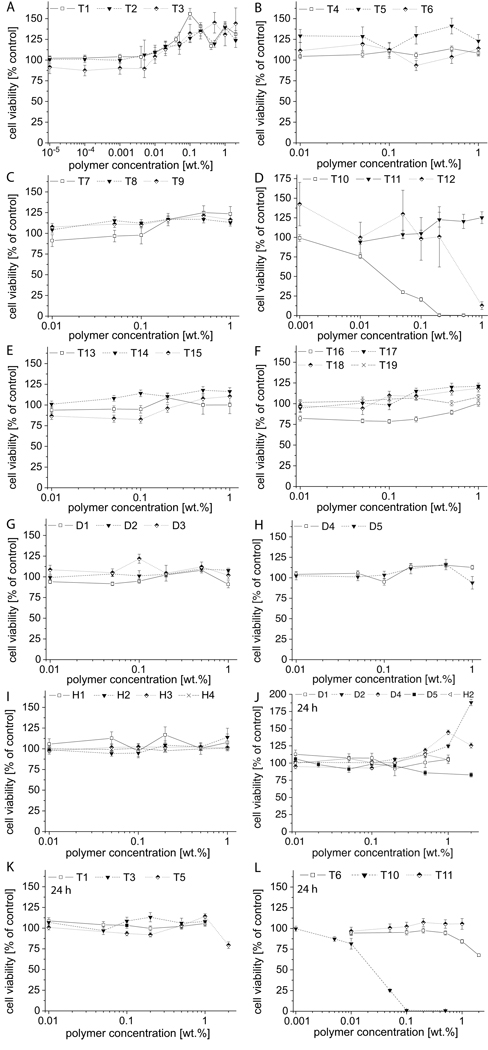
Also for MCF7 cells, no adverse effects were observed with up to 1 wt.% or 2 wt.% (for example T1–T3, 20 g/L; 3.6 mM, 3.1 mM and 2.6 mM, respectively) (Figure 3). Finally, we investigated cytotoxicity of polymers in adriamycin-resistant cell line MCF7-ADR (Figure 4). Again, after 2h incubation, we observed no signs of cytotoxicity in the majority of samples, with notable exceptions of T10 and T12. In contrast, we found that starting from concentrations of 0.01 wt.% (0.1 g/L, 0.02 mM - 0.01 mM, respectively) the apparent cell viability increased slightly with T1 – T3 and several other polymers. The results after 24 h incubation in MCF7-ADR cells show no cytotoxicity for the majority of polymers. Again T10 is the exception with IC50 values of approx. 0.7 wt.% and 0.04 wt.%, after 2h and 24h incubation, respectively. Some reduced viability at higher concentrations is observed for T5 and T6. The observed increase of apparent cell viability in a number of experiments was particularly pronounced for polymer D2 exhibiting an increase to almost 200 % after 24 h incubation.
Figure 3.
Cell viability of blockco- and homo poly(2-oxazoline)s in MCF7 cells after 2h incubation as determined by MTT assay. Experiments were performed in quadruplicate and data is expressed as means ± SEM.
Figure 4.
Cell viability of block co- and homo poly(2-oxazoline)s in MCF7-ADR cells after 2h (A–I) or 24h (J–L) incubation as determined by MTT assay. Experiments were performed in quadruplicate and data is expressed as means ± SEM.
3.3. Cellular uptake of fluorescently labeled polymers
In general it is of importance to understand the fate of non-degradable materials after injection. Besides pharmacokinetic studies in animals, studies of the endocytosis of such materials are warranted. Although hydrophobic drugs can enter cells by diffusion once the carrier reaches the target, it is also of interest whether the carrier itself can enter the target cells. If this is the case, it may help drugs to bypass drug resistance mechanisms, increasing the effective drug concentration within the cells.[26,27] We therefore used fluorescently labeled polymers and investigated their cellular uptake using flow cytometry in the multi-drug resistant cell line MCF7-ADR.
3.3.1. Flow cytometry study of temperature dependent uptake of copolymers
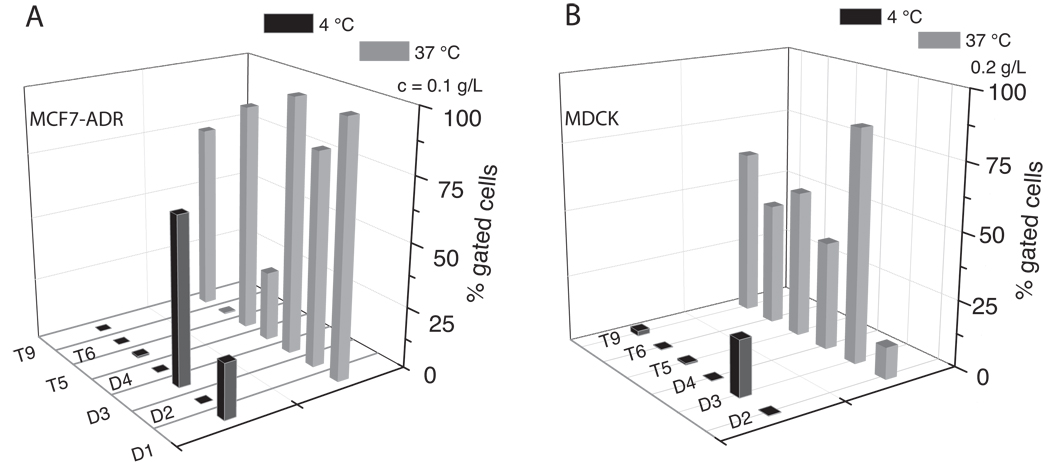
Comparison of the uptake at 37 °C and 4 °C after 60 min exposure revealed that the uptake was strongly inhibited at 4 °C for all polymers investigated (Figure 5). For the majority of polymers the uptake appears to be completely diminished as about 0% gated cells are observed. In contrast, two polymers, D1† and D3† still show considerable uptake at 4 °C with 25 % and 65 % gated cells, respectively.
Figure 5.
Cellular uptake in MCF7-ADR (A) and MDCK (B) cells of fluorescently labeled block copolymers at 4 °C (black) and 37 °C (grey) after 60 min incubation as investigated by flow cytometry and expressed as % gated cells. Experiments were performed in triplicate and data is expressed as means.
3.3.2. Flow cytometry study of concentration dependent uptake of copolymers
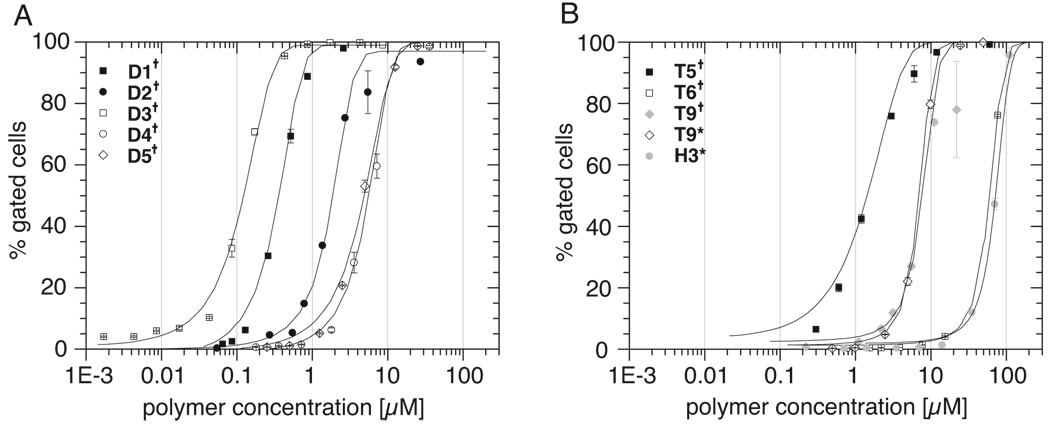
The uptake study was performed after 60 min incubation. All polymers showed a pronounced concentration dependent cellular uptake, albeit with markedly differing uptake behavior (Figure 6A, Table 3). The effective concentration resulting in 50% gated cells (EC50) ranges from high nanomolar (D1†, D3†) to micromolar (D2†, D4†, D5†) values, with D3† having the lowest value of 0.1 µM. In contrast, block copolymers with the 2-propyl-2-oxazoline blocks (D4†/D5†), which are partly water soluble enter the cells only at 50 – 60 times higher concentrations (EC50 5–6 µM). Also the point of attachment of the fluorescent dye may have some influence, as the EC50 values of D1† and D2† differ somewhat (0.4 vs 2 µM).
Figure 6.
Concentration dependence of uptake of polymers in MCF7-ADR cells expressed in % gated cells. Cells were incubated at 37 °C with solutions of different concentrations of fluorescently labeled diblock- (A) and triblock copolymers as well as EtOx homopolymer (both B). Incubation time 60 min. Concentrations of polymers are expressed as concentration of labeled polymers.
Experiments were performed in triplicate and data is expressed as means ± SEM. Data points were fitted with Boltzmann functions from which the EC50 values (concentration at 50 % gated cells) were calculated.
Table 3.
Comparison of EC50 values for the different di- and triblock copolymers (D1–D5, T5, T6, T9) and EtOx homopolymer H3 as obtained from flow cytometry and subsequent Boltzmann fitting of data.
| Polymer ID | Polymer composition | EC50 (µM) | EC50 (g/L) |
|---|---|---|---|
| D1† | MeOx52-b-BuOx20 | 0.4 | 0.003 |
| D2† | BuOx20-b-MeOx54 | 2 | 0.01 |
| D3† | EtOx53-b-BuOx20 | 0.1 | 0.0008 |
| D4† | MeOx93-b-iPOx38 | 6 | 0.07 |
| D5† | MeOx93-b-nPOx38 | 5 | 0.06 |
| T5† | MeOx25-b-BuOx22-b-MeOx27 | 1 | 0.01 |
| T6† | MeOx25-b-nPOx35-b-MeOx25 | 60 | 0.4 |
| T9†,* | MeOx26-b-BuOx15-b-MeOx26 | 8 | 0.06 |
| H3† | EtOx43 | 72 | 0.4 |
It should be noted that the cells reacted as one relatively homogenous population, i.e. we were unable to detect subpopulations of cells, which differ in their uptake behavior, e.g. taking up much more or much less material than the main population.
Also in the case of triblock copolymers (T5†, T6† and T9†/T9*) a marked difference with respect to the concentration dependence of the endocytosis was observed (Figure 6B). The EC50 values range from 1 µM to approx 0.06 mM. T5†, the polymer with the lowest EC50 values also comprised the longest hydrophobic block. T9†/T9*, with the hydrophobic block length of only approx. 2/3 of the hydrophobic block in T5†, had a slightly higher EC50 of approx. 6 µM, irrespective of the nature of the fluorescent dye. As with the diblock copolymers, the triblock copolymer with moderately hydrophobic nPrOx (T6†) block exhibited the highest EC50 value of approx. 0.06 mM.
Finally, H3* was studied by flow cytometry. Being itself slightly amphiphilic in nature, it was able to enter the cells, albeit only at rather high concentrations with an EC50 value of approx. 0.07 mM.
3.3.3. Flow cytometry study of time dependent uptake of copolymers
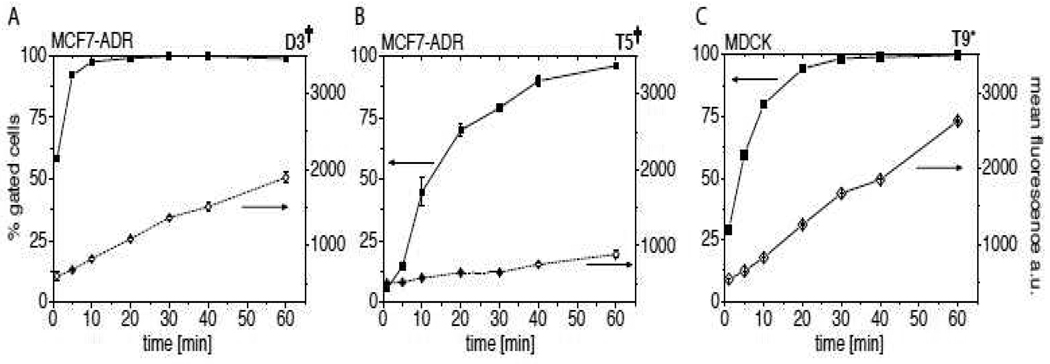
To determine the kinetics of cellular uptake of POx in greater detail we also performed a time course experiment. Cells were incubated with the polymers for predetermined times of 1 min to up to 60 min. D3† showed an extremely fast uptake as already after 1 min more than 50 % gated cells were obtained and after only 10 min all cells were gated (Figure 7A). As observed by the mean fluorescence it becomes clear that the uptake continues after that with an essentially linear increase of the mean fluorescence over time. In comparison, the time course of the uptake of T5† appears somewhat slower (Figure 7B). Even after 60 min, 100 % gated cells were not obtained while 50 % gated cells were reached after around 12 min. In the example of T9* it took about 30 min to obtain 100 % gated cells in this cell line with 50 % gated cells (MDCK) reached within less than 5 min (Figure 7C).
Figure 7.
Cellular uptake (expressed in % gated cells and mean fluorescence) of fluorescently labeled block copolymers D3†, T5† and T9* in MCF7-ADR (A,B) and MDCK (C) in dependence of incubation time at 37 °C as investigated by flow cytometry. Experiments were performed in triplicate and data is expressed as means ± SEM.
3.3.4. Confocal study of cellular uptake of amphiphilic block copolymers
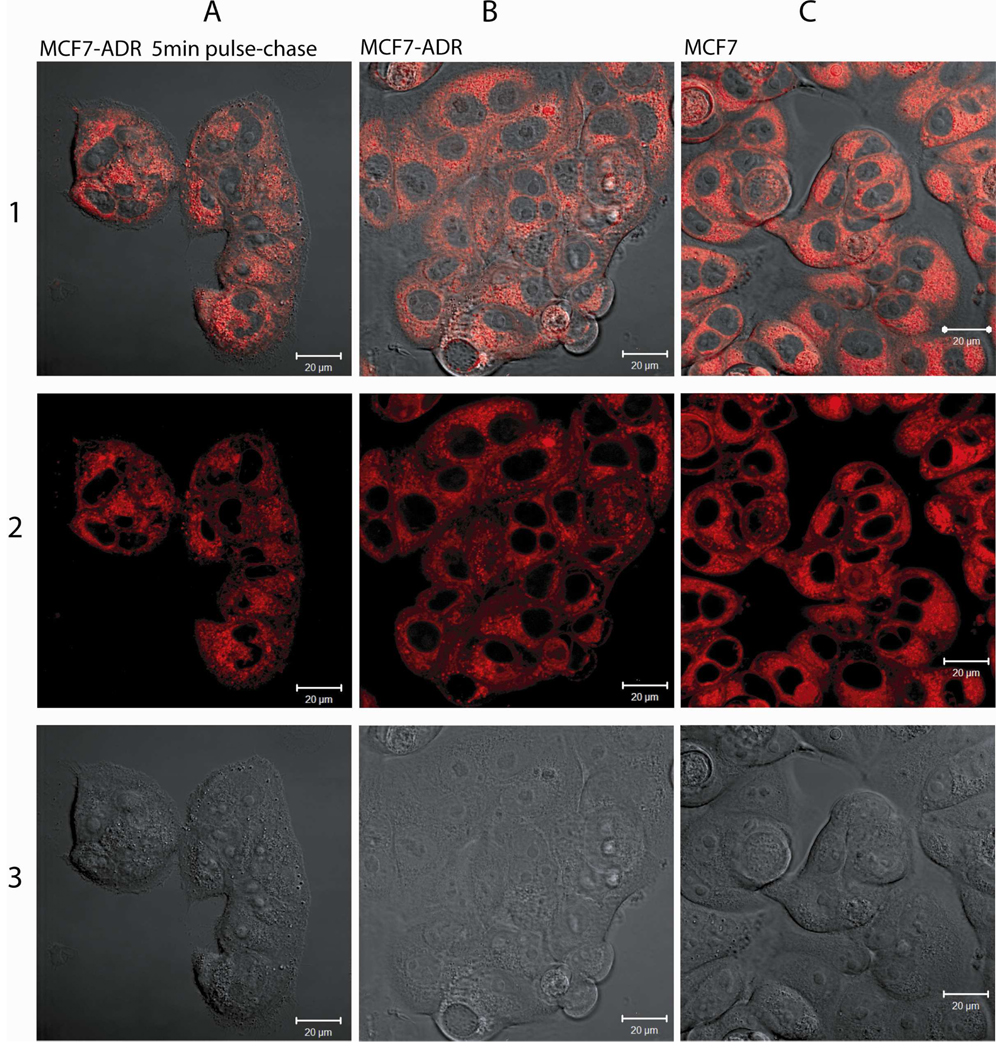
In order to confirm that the fluorescence signals obtained in flow cytometry experiment do not stem from merely cell membrane-associated polymers, laser scanning confocal microscopy of live cells was performed. In no case we observed a considerable amount of membrane-associated polymer. In the case of T9* we performed a 5 min pulse, 55 min chase experiment (with unlabeled polymer T9) as well as a 60 min incubation experiment in MCF7-ADR cells and a 60 min incubation experiment in MCF7 cells (Figure 8).
Figure 8.
Representative laser scanning confocal microscopy images of MCF7-ADR (columns A and B) and MCF7 (column C) cells incubated with T9*. Row 1 shows overlay of fluorescence observed at 561 nm (row 2) and DIC (row 3). Scale bars represent 20 µm.
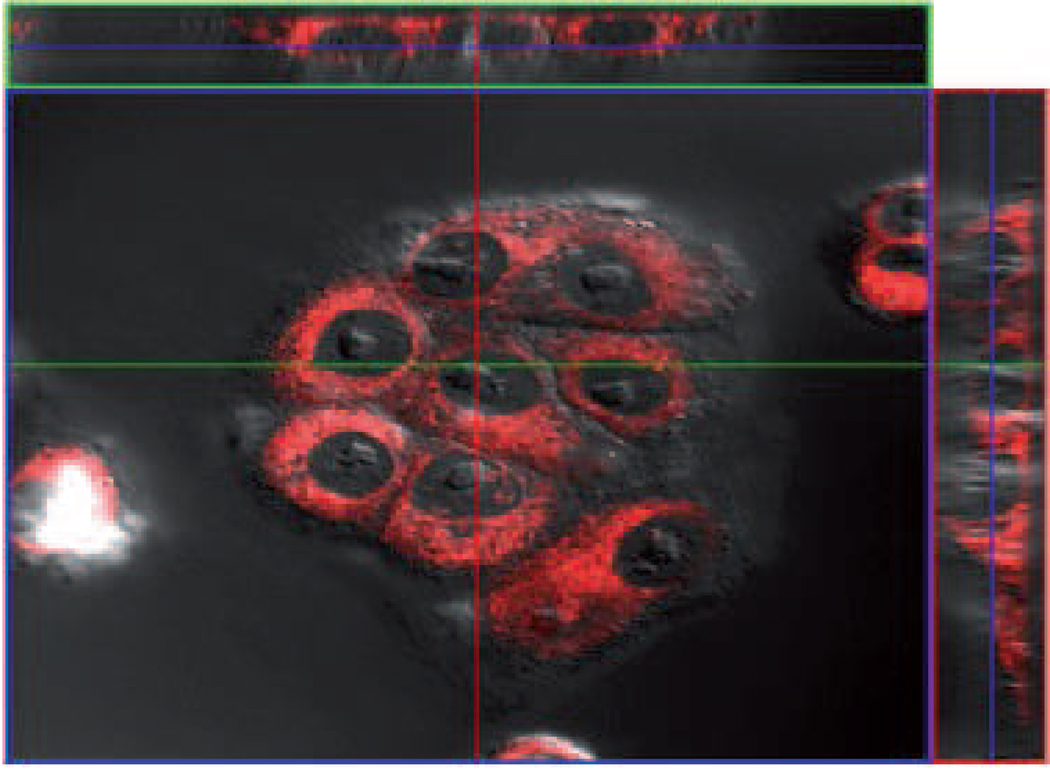
It is evident that little portion of the fluorescent material is bound with cell membranes. In contrast, the fluorescence appears to be distributed over the entire cell especially in the perinuclear region but did not show in the nucleus. No apparent differences in the fluorescence patterns between the pulse-chase experiment, the 60 min incubation and the non-resistant cell line can be observed. A z-stack of a cell cluster of MCF7-ADR cells incubated with T9* confirms the distribution over the entire cytoplasm with the exception of the nucleus as well as the perinuclear enrichment (Figure 9).
Figure 9.
Representative laser scanning confocal microscopy z-stack image of MCF7-ADR cells incubated with T9*.
4. Discussion
4.1. Cytotoxicity
Although several publications have dealt with biomedical use of POx based polymers, any systematic investigation of the toxicity of these polymers is still lacking. Here, we studied a panel of POx based polymers of different structure (homo-, block copolymers) in three different immortal mammalian cell lines. In general, it was found that POx are very well tolerated even at rather high concentrations of ≥ 10 g/L as we did not observe any significant effects with the great majority of samples. We tested water-soluble homopolymers and block copolymers (di- and triblock copolymers) and could not observe a general effect of the respective polymer structures. Some notable exceptions must be mentioned. Using different terminating reagents during POx synthesis, identical polymers with different polymer termini are accessible (T7 through T12). Only T10, bearing a quaternized amine along with a long alkyl chains exhibits any cytotoxicity at concentrations below 10 g/L (Figures 1–3). The IC50 values of T10 range between 0.1 and 1 g/L (i.e. 14 – 140 µmol/L) which is in the same order of magnitude as the minimal inhibitory concentrations (of bacterial growth) observed by Tiller and co-workers for similar polymers.[22]
On the contrary, in several experiments in different cell lines we observed a minor or marked (exceptional cases) increase in cell viability as observed by the MTT assay (e.g. Figures 2A,F;3E,F;4A,F,L). We believe that the repeated observation of this effect makes it unlikely to be an experimental error although the reason for this tendency of apparent increased cell viability remains unclear at the moment. However, it should be kept in mind that the MTT assay is a measure of the metabolic activity of the cells and as such a function of cell number and their metabolic activity. More detailed investigations to elucidate this effect are currently under way. However, in conclusion, we can state that the vast majority of samples studied showed no cytotoxicity even at very high concentrations of up to 20 g/L which indicates that in general POx seem to be well tolerated by mammalian cells, confirming the general trend reported up to date.
4.2 Endocytosis
We recently described the cellular uptake of POxylated horseradish peroxidase, which was dependend on the structure POx.[13] Preliminary results suggested that amphiphilic POx alone also entered cells rather efficiently. To understand this uptake in detail we decided to investigate the temperature, concentration and time dependence of a series of fluorescently labeled polymers (Table 3).
The fluorescently labeled polymers show a drastically different temperature dependence of cellular uptake as compared to the free dye (TRITC) as verified for T6*. While the polymer appears to be taken up by endocytosis (strongly diminished at 4 °C), the small dye molecule freely diffuses into MCF7-ADR cells as expected (no temperature dependence observed, data not shown). This difference shows that no or only negligible amount of free dye is present in the incubation solution of T9*. Moreover, this results demonstrates how drug loaded POx micelles could help to overcome multi-drug resistance in cancer therapy. While the uptake of free small molecule and Pgp substrate TRITC is markedly reduced in the MDR phenotype, the POx micelles are still entering the cells effectively.
Comparing the uptake of diblock and triblock copolymers with different block sizes and different hydrophobic blocks we were able to identify clear structure-property relationships, supporting previous endocytosis studies with homo- and random and block copolymers of 2-hydroxypropylmethacrylamide (HPMA) in MCF7-ADR cells.[28] Within the structural limits we investigated, the more hydrophobic the polymer is, the more readily it enters the cells. It should be noted, however, that all polymers were excellently water-soluble with solubility exceeding 100 g/L in all cases. The polymers with the lowest EC50 values were D3† (0.1 µM) and D1† (0.4 µM), both comprising 20 units of BuOx as the hydrophobic block and about 50 units of EtOx or MeOx as the hydrophilic block. Using the concentration dependence of the endocytosis of D1† and D3† it can be estimated that the uptake of the polymers at 4 °C as compared to 37 °C is comparable to a 50-fold decrease of the polymer concentration. Interestingly, the structural isomer of D1† with the opposite sequence of hydrophilic and hydrophobic block (D2†) has a slightly higher EC50 of 2 µM. A possible explanation of this may be the resulting different attachment point of the fluorescent dye. In the case of D1†, the dye is attached to the hydrophobic block while in D2† it is attached to the hydrophilic block. It is known that terminal groups can have significant effects on the physicochemical properties of POx.[16] In this context, one can argue that the hydrophobic dye elongates the hydrophobic block in D1†. In contrast, the hydrophobic character of the dye may not influence the uptake as much when it is attached to the hydrophilic MeOx block in the case of D2†.
Reducing the hydrophobicity of the hydrophobic block further also diminishes the uptake of the polymers as observed with D4† and D5†, which bear iPrOx and nPrOx, respectively. Both monomers yield thermoresponsive polymers with cloud points around 45 °C and 25 °C (at 20 g/L), respectively.[13] At the investigated temperature and low concentrations, both polymers are presumably fully water-soluble. Accordingly, their uptake was observed only at higher concentrations with EC50 values being 6 and 5 µM, respectively.
A similar trend was observed for triblock copolymers T5†, T6† and T9†/T9*. The monomer composition of T5† is similar to the one of D1†. However, T5† is an ABA type triblock with a central hydrophobic core and flanking hydrophilic blocks. The effect of this structural difference appears to be minor with virtually identical EC50 values of 0.4 and 1 µM observed for D1† and T5†, respectively. As the size of the hydrophobic block was decreased by approx. 25% (22 vs 15 units), the uptake was shifted towards higher concentrations (T9†/T9*; EC50 = 8 µM). T6† exhibited the highest EC50 values of all copolymers investigated (0.06 mM). Interestingly, here a pronounced difference between a diblock copolymer and triblock copolymer (D5† vs T6†) was observed with the EC50 value of D5† (5 µM) being about one order of magnitude less than that of the T6†. D5† has a significantly longer hydrophilic block as compared to T6†. Therefore, one could expect a higher EC50 value for D5†, contrary to what is observed. This observation suggests that the length of the hydrophobic blocks play a minor role for the cellular uptake, similarly as the length of hydrophilic blocks has limited influence on the critical micelle concentration or critical aggregation concentration. Finally, a homopolymer of EtOx was studied and we found very low endocytosis even at rather high concentrations (0.07 mM). In summary, the order of EC50 values of the polymers investigated in this study was D3† < D1† < T5† < D4† ≈ D5† < T9† = T9* < T6† ≈ H3* and are summarized in Table 3.
It should be noted that the cmc values for all polymers are higher than the respective EC50 values. Moreover, under the current experimental conditions, we were unable to observe any apparent change in the uptake behavior when crossing into the micellar region. Thus, it is safe to assume that the individual unimers and micelles are taken into the cells. However, to investigate whether unimers and micelles use different uptake mechanisms, more detailed studies with endocytosis inhibitors and markers of individual uptake mechanisms would be necessary.[29,30]
In the present study, we found the uptake of POx based amphiphiles to be rather fast. In the case of D3†, the polymer with the lowest EC50 value, the uptake was also particularly fast with 50% of cells positively gated after only 1 min of incubation.
Direct comparison to other studies is difficult as only relatively few groups studied the endocytosis of POx based materials. For example, Schubert recently reported endocytosis of PEtOx based nanoparticles. Uptake was studied after 24 hrs and even at relatively low concentrations of 0.1 µg/L the material entered the cells effectively.[31]
Similar structure-property relationship of the cellular uptake were observed earlier with Pluronic® blockcopolymers, although this study was qualitative in nature. Polymers with very high or very low HLB values exhibited less uptake into bovine brain microvasculature endothelial cells.[32] In this study we could confirm the limited uptake of hydrophilic polymers, while we are unable to confirm the lower uptake for polymers of a more pronounced hydrophobic character. Future studies should therefore include polymers with a considerably stronger hydrophobic character than D3†.
A comparison with the previously published concentration dependent uptake of HPMA based random and block copolymers and HPMA homopolymer reveals that the EC50 values of POx-based polymers are in the similar range. A random copolymer of HPMA and laurylacrylate (molar monomer ratio 80/20) of 15 kg/mol had an EC50 of 0.2 µM while a block copolymer of comparable size and monomer composition gave an EC50 = 7 µM. Similar as in the present report HPMA homopolymers had increased EC50 values of about 30 µM.[28]
DeSimone and co-workers reported recently on the uptake of PEG-containing particles synthesized by the PRINT technique. It was observed that the concentration dependent uptake of the nanoparticles was cell-type dependent, suggesting further uptake studies of the introduced POx block copolymers in a wider range of cell lines. Although no EC50 values are given in this account, it is apparent that the internalization of the 1 µm large particles occurred in the same order of magnitude of concentration.[33] The same group also studied the size effects on the internalization. In this particular case, no concentration (dependence) is given, but fastest uptake is observed for particles with a diameter of 150 nm and length of 450 nm (> 50 % gated cells in < 15 min).[34] Haag and co-workers report on the cellular uptake of dendritic polyglycerols in A549 lung epithelial cells. At low concentrations of 10 µM (4h incubation), a pronounced difference between charged and non-charged derivatives was observed, the latter giving only little uptake while the former where internalized efficiently.[35]
Laser scanning confocal microscopy of live cells clearly shows that all polymers enter the cells and no fluorescence is fixed to cell membranes (Figures 8 and 9). Although the fluorescence is distributed throughout the entire cells, the polymers appear to be enriched in vesicular compartment(s) of the cells. However, in detail investigation of the subcellular distribution and intracellular trafficking of POx amphiphiles is beyond the scope of this article and will be published in a separate account. We hypothesize a specific uptake mechanism for these rapidly incorporated POx amphiphiles similar but possibly distinct to the uptake mechanism we postulated for Pluronic® P85, a triblock copolymer of similar molar mass (4.6 kg/mol) of a central poly(propylene oxide) and two flanking PEG blocks.[29]
5. Conclusion
In summary, we studied the cytotoxicity of a range of POx based homo and block copolymers. We could confirm that in general POx appear to be well tolerated by mammalian cells, a result suggested earlier in a number of studies, albeit using only a few examples. More importantly, we performed a quantitative screening of the endocytosis of amphiphilic POx which already have demonstrated great potential in micellar drug delivery.[14] The synthetic possibilities given by the living cationic ring-opening polymerization of POx give an ideal tool to manufacture defined polymers and investigate structure property relationships of their interaction with biological entities. The results of our study underline the importance of thorough investigation of the concentration dependent uptake of nanomaterials, in particular to allow for a comparison of nanomaterials of different origin, structure and properties.
Acknowledgement
RL gratefully acknowledges a postdoctoral fellowship of the Deutscher Akademischer Austauschdienst (DAAD) and the King Abdullah University of Science and Technology (KAUST, Award No. KUK-F1-029-32) for partial salary support. This study was supported by National Institutes of Health grants 1P20 RR021937, UO1 CA151806 and 2RO1 CA89225 awarded to AVK and the DFG Forschergruppe FOR411 “Radionuklidtherapie” awarded to RJ (project P12). We would also like to thank the flow cytometry and confocal microscopy core facilities at UNMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Aoi K, Okada M. Polymerization of oxazolines. Prog. Polym. Sci. 1996;21:151–208. [Google Scholar]
- 2.Kobayashi S, Uyama H. Polymerization of cyclic imino ethers: from its discovery to the present state of the art. J. Polym. Sci., Part A: Polym. Chem. 2002;40:192–209. [Google Scholar]
- 3.Lüdtke K, Jordan R, Hommes P, Nuyken O, Naumann CA. Lipopolymers from new 2-substituted-2-oxazolines for artificial cell membrane constructs. Macromol. Biosci. 2005;5:384–393. doi: 10.1002/mabi.200500004. [DOI] [PubMed] [Google Scholar]
- 4.Levy A, Litt M. Polymerization of cyclic iminoethers. V. 1,3-Oxazolines with hydroxy-, acetoxy-, and carboxymethly-alkyl groups in the 2-position and their polymers. J. Polym. Sci. A1. 1968;6:1883–1894. [Google Scholar]
- 5.Taubmann C, Luxenhofer R, Cesana S, Jordan R. First Aldehyde-Functionalized Poly(2-oxazoline)s for Chemoselective Ligation. Macromol. Biosci. 2005;5:603–612. doi: 10.1002/mabi.200500059. [DOI] [PubMed] [Google Scholar]
- 6.Luxenhofer R, Jordan R. Click chemistry with poly (2-oxazoline)s. Macromolecules. 2006;39:3509–3516. [Google Scholar]
- 7.Luxenhofer R, Bezen M, Jordan R. Kinetic Investigations on the Polymerization of 2-Oxazolines Using Pluritriflate Initators. Macromol. Rapid Commun. 2008;29:1509–1513. [Google Scholar]
- 8.Zhang N, Huber S, Schulz A, Luxenhofer R, Jordan R. Cylindrical Molecular Brushes of Poly (2-oxazoline) s from 2-Isopropenyl-2-oxazoline. Macromolecules. 2009;42:2215–2221. [Google Scholar]
- 9.Zhang N, Steenackers M, Luxenhofer R, Jordan R. Bottle-Brush Brushes: Cylindrical Molecular Brushes of Poly (2-oxazoline) on Glassy Carbon. Macromolecules. 2009;42:5345–5351. [Google Scholar]
- 10.Barz M, Luxenhofer R, Zentel R, Vicent MJ. Overcoming the PEG-addiction: Well-defined Alternatives to PEG, from Structure-Property Relationships to better defined Therapeutics. Polym. Chem. 2011 [Google Scholar]
- 11.Miyamoto M, Naka K, Shiozaki M, Chujo Y, Saegusa T. Preparation and enzymatic activity of poly[(N-acylimino)ethylene-modified catalase. Macromolecules. 1990;23:3201–3205. [Google Scholar]
- 12.Mero A, Pasut G, Via LD, Fijten MWM, Schubert US, Hoogenboom R, Veronese FM. Synthesis and characterization of poly(2-ethyl 2-oxazoline)-conjugates with protein and drugs: Suitable alternatives to PEG-conjugates? J. Control. Release. 2008;125:87–95. doi: 10.1016/j.jconrel.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Tong J, Luxenhofer R, Yi X, Jordan R, Kabanov AV. Protein Modification with Amphiphilic Block Copoly(2-oxazoline)s as a New Platform for Enhanced Cellular Delivery. Mol. Pharm. 2010;7:984–992. doi: 10.1021/mp100102p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luxenhofer R, Schulz A, Roques C, Li S, Bronich TK, Batrakova EV, et al. Doubly amphiphilic poly(2-oxazoline)s as high-capacity delivery systems for hydrophobic drugs. Biomaterials. 2010;31:4972–4979. doi: 10.1016/j.biomaterials.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber S, Hutter N, Jordan R. Effect of end group polarity upon the lower critical solution temperature of poly(2-isopropyl-2-oxazoline) Colloid Polym. Sci. 2008;286:1653–1661. [Google Scholar]
- 16.Huber S, Jordan R. Modulation of the lower critical solution temperature of 2-Alkyl-2-oxazoline copolymers. Colloid Polym. Sci. 2008;286:395–402. [Google Scholar]
- 17.Park J-S, Kataoka K. Precise Control of Lower Critical Solution Temperature of Thermosensitive Poly(2-isopropyl-2-oxazoline) via Gradient Copolymerization with 2-Ethyl-2-oxazoline as a Hydrophilic Comonomer. Macromolecules. 2006;39:6622–6630. [Google Scholar]
- 18.Zalipsky S, Hansen CB, Oaks JM, Allen TM. Evaluation of blood clearance rates and biodistribution of poly(2-oxazoline)-grafted liposomes. J. Pharm. Sci. 1996;85:133–137. doi: 10.1021/js9504043. [DOI] [PubMed] [Google Scholar]
- 19.Konradi R, Pidhatika B, Mühlebach A, Textor M. Poly-2-methyl-2-oxazoline: A Peptide-like Polymer for Protein-Repellent Surfaces. Langmuir. 2008;24:613–616. doi: 10.1021/la702917z. [DOI] [PubMed] [Google Scholar]
- 20.Naka K, Nakamura T, Ohki A, Maeda S. Aggregation behavior and interaction with human serum albumin of 2-oxazoline block copolymers in aqueous solutions. Macromol. Chem. Phys. 1997;198:101–116. [Google Scholar]
- 21.Gaertner FC, Luxenhofer R, Blechert B, Jordan R, Essler M. Synthesis, biodistribution and excretion of radiolabeled poly(2-alkyl-2-oxazoline)s. J. Control. Release. 2007;119:291–300. doi: 10.1016/j.jconrel.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Waschinski CJ, Tiller JC. Poly(2-oxazoline)s with Telechelic Antimicrobial Functions. Biomacromolecules. 2005;6:235–243. doi: 10.1021/bm049553i. [DOI] [PubMed] [Google Scholar]
- 23.Waschinski CJ, Herdes V, Schueler F, Tiller JC. Influence of Satellite Groups on Telechelic Antimicrobial Functions of Polyoxazolines. Macromol. Biosci. 2005;5:149–156. doi: 10.1002/mabi.200400169. [DOI] [PubMed] [Google Scholar]
- 24.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 25.Loontjens T, Bel W, Stanssens D, Weerts P. Synthesis of 1,2-bis(2-oxazolinyl-2)ethane and its application as chain extenders for poly(ethylene terephtalate) Polym. Bull. 1993;30:13–18. [Google Scholar]
- 26.Lee SC, Kim C, Kwon IC, Chung H, Jeong SY. Polymeric micelles of poly(2-ethyl-2-oxazoline)-block-poly(epsilon-caprolactone) copolymer as a carrier for paclitaxel. J. Control. Release. 2003;89:437–446. doi: 10.1016/s0168-3659(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 27.Miller DW, Batrakova EV, Kabanov AV. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm. Res. 1999;16:396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- 28.Barz M, Luxenhofer R, Zentel R, Kabanov AV. The uptake of N-(2-hydroxypropyl)-methacrylamide based homo, random and block copolymers by human multi-drug resistant breast adenocarcinoma cells. Biomaterials. 2009;30:5682–5690. doi: 10.1016/j.biomaterials.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahay G, Batrakova EV, Kabanov AV. Different internalization pathways of polymeric micelles and unimers and their effects on vesicular transport. Bioconjug. Chem. 2008;19:2023–2029. doi: 10.1021/bc8002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J. Control. Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kempe K, Vollrath A, Schaefer HW, Poehlmann TG, Biskup C, Hoogenboom R, et al. Multifunctional Poly(2-oxazoline) Nanoparticles for Biological Applications. Macromolecular Rapid Communications. 2010;31:1869–1873. doi: 10.1002/marc.201000283. [DOI] [PubMed] [Google Scholar]
- 32.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 2003;304:845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 33.Gratton SE, Napier ME, Ropp PA, Tian S, DeSimone JM. Microfabricated particles for engineered drug therapies: elucidation into the mechanisms of cellular internalization of PRINT particles. Pharm. Res. 2008;25:2845–2852. doi: 10.1007/s11095-008-9654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khandare J, Mohr A, Calderón M, Welker P, Licha K, Haag R. Structure-biocompatibility relationship of dendritic polyglycerol derivatives. Biomaterials. 2010;31:4268–4277. doi: 10.1016/j.biomaterials.2010.02.001. [DOI] [PubMed] [Google Scholar]