Abstract
The endoplamic reticulum (ER) is a critical site for intracellular calcium storage as well as protein synthesis, folding, and trafficking. Disruption of these processes is gaining support for contributing to heritable vulnerability of certain diseases. Here, we investigated Bax inhibitor 1 (BI-1), an anti-apoptotic protein that primarily resides in the ER and associates with B-cell lymphoma 2 (Bcl-2) and Bcl-XL, as an affective resiliency factor through its modulation of calcium homeostasis. We found that transgenic (TG) mice with BI-1 reinforced expression, via the neuronal specific enolase promoter, showed protection against the learned helplessness (LH) paradigm, an animal model to test stress coping. TG mice were also protected against anhedonia following both serotonin and catecholamine depletion as measured in two different models, the female urine sniffing test and the saccharine preference test. In addition, we used primary mouse cortical cultures to explore the ability of BI-1 to influence calcium homeostasis under basal conditions and also following challenge with thapsigargin (THPS), an inhibitor of sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) that disrupts calcium homeostasis. TG neurons showed decreased basal cytosolic calcium levels and decreased Ca2+ cytosolic accumulation following challenge with THPS as compared to WT neuronal cultures. Together, these data suggest that BI-1, through its actions on calcium homeostasis, may confer affective resiliency in multiple animal models of depression and anhedonia.
Keywords: Bax inhibitor 1, affective resiliency, endoplasmic reticulum, calcium homeostasis, ER stress
1. Introduction
Bipolar disorder (BD) and major depressive disorder (MDD) are mood disorders that impact approximately 10% of adults in the United States and represent an enormous burden on society. While the causes of mood disorders are unknown and probably diverse, one emerging area of investigation involves intracellular calcium homeostasis through the endoplasmic reticulum (ER). ER stress and its role in maintaining neuronal circuit function is gaining recognition in numerous pathological conditions (Kim et al., 2008), including BD (Kato, 2008) and MDD (Bown et al., 2000).
Disruptions in intracellular calcium homeostasis have been reported for both MDD (Aldenhoff et al., 1997; Konopka et al., 1996; Vollmayr and Aldenhoff, 1994) and BD (Dubovsky et al., 1992; Dubovsky et al., 1994; Emamghoreishi et al., 1997; Hough et al., 1999; Kato, 2008). These disruptions hinder cell viability and function. Anti-apoptotic and pro-apoptotic proteins can associate with organelles, such as the mitochondria or ER, to influence cell survival and death by modulating calcium homeostasis. The anti-apoptotic protein Bcl-2, when over-expressed, has been shown to reduce ER calcium concentrations, and this is a suggested mechanism whereby it may inhibit apoptosis (Foyouzi-Youssefi et al., 2000; Lam et al., 1994; Pinton et al., 2000). The pro-apoptotic proteins Bax and Bak have been shown in double knockout mouse fibroblast cells to reduce resting concentrations of calcium in the ER resulting in less uptake of this calcium by the mitochondria and inhibiting the cell death signaling cascade (Scorrano et al., 2003). This reduction in ER calcium concentration has been attributed to increased calcium leakage by phosphorylation of inositol triphosphate receptor type 1 (IP3R-1) (Oakes et al., 2005).
Like Bcl-2 family proteins, Bax inhibior-1 (BI-1) also regulates ER calcium homeostasis where it has been suggested to act through IP3Rs (Kim et al., 2008). In cell culture studies, augmenting BI-1 in HeLa cells or mouse embryo fibroblasts results in lower basal [Ca2+]er in addition to less calcium release into the cytosol following challenge with thapsigargin (THPS) (Xu et al., 2008). Knock down of BI-1 results in increased basal [Ca2+]er and increased calcium release into the cytosol following THPS challenge (Xu et al., 2008). BI-1’s role in calcium retention may contribute to its neuroprotective effects and has recently been identified as a critical constituent in both ischemia and traumatic brain injury mouse models (Krajewska et al., 2010).
BI-1 associates with the ER and can also inhibit apoptosis induced by the pro-apoptotic protein Bax (BCL2-associated X protein) (Xu and Reed, 1998). Neurons, fibroblasts, and hepatocytes derived from BI-1 deficient mice are more vulnerable to apoptosis induced by ER stress compounds as compared to stimulators of mitochondrial or TNF/Fas-death receptor apoptosis pathways (Chae et al., 2004). In vivo studies show that BI-1 deficient mice have an increased vulnerability to an ER stress agent, hepatic ischemia-reperfusion injury, and stroke (Bailly-Maitre et al., 2006; Chae et al., 2004). Furthermore, increased BI-1 expression protects HT1080 human fibrosarcoma cells and CMS14.1 immortalized rat hippocampal neurons from ER stress-induced apoptosis (Chae et al., 2004). In mice, neuronal enforced expression of HA tagged BI-1 (TG) also protects against ischemia and traumatic brain injury via modulating ER stress proteins (e.g. CHOP) (Krajewska et al., 2010).
In the current study, we utilized BI-1 TG mice (Krajewska et al., 2010) that express the human BI-1 protein under the control of the neuronal specific enolase promoter, which leads to constitutive expression of BI-1 at physiological levels in neurons. We provide the first evidence that BI-1 also confers resiliency in animal models used to screen antidepressant-like efficacy and measure anhedonia, one of the core symptoms of depression. We show that TG BI-1 mice display affective resiliency against three behavioral paradigms used to model depression and anhedonia. Moreover, we are the first to demonstrate BI-1 TG neuronal cortical cell cultures have lower basal cytosolic calcium levels and reduced calcium release from ER following challenge with THPS. These results suggest that BI-1 modulation of ER calcium homeostasis may be a potential target for future psychiatric diseases to enhance affective resiliency.
2. Results
2.1. BI-1 TG mice show normal activity, anxiety, pain, and responses to amphetamine and cocaine
TG mice show normal locomotor activity and anxiety responses comparable to WT littermates as measured by the open field test and elevated plus maze (Supplemental Figure S1). BI-1 TG mice also have comparable pain thresholds as measured by the hot plate test (Supplemental Figure S2). With these data as foundation, we then tested responses using two common animal paradigms to model mania, (1) amphetamine-induced hyperlocomotion and (2) cocaine-induced behavioral sensitization. BI-1 TG mice show comparable responses to WT mice in both of these paradigms (Supplemental Figure S3).
2.2. BI-1 TG mice show enhanced spontaneous recovery in active avoidance test
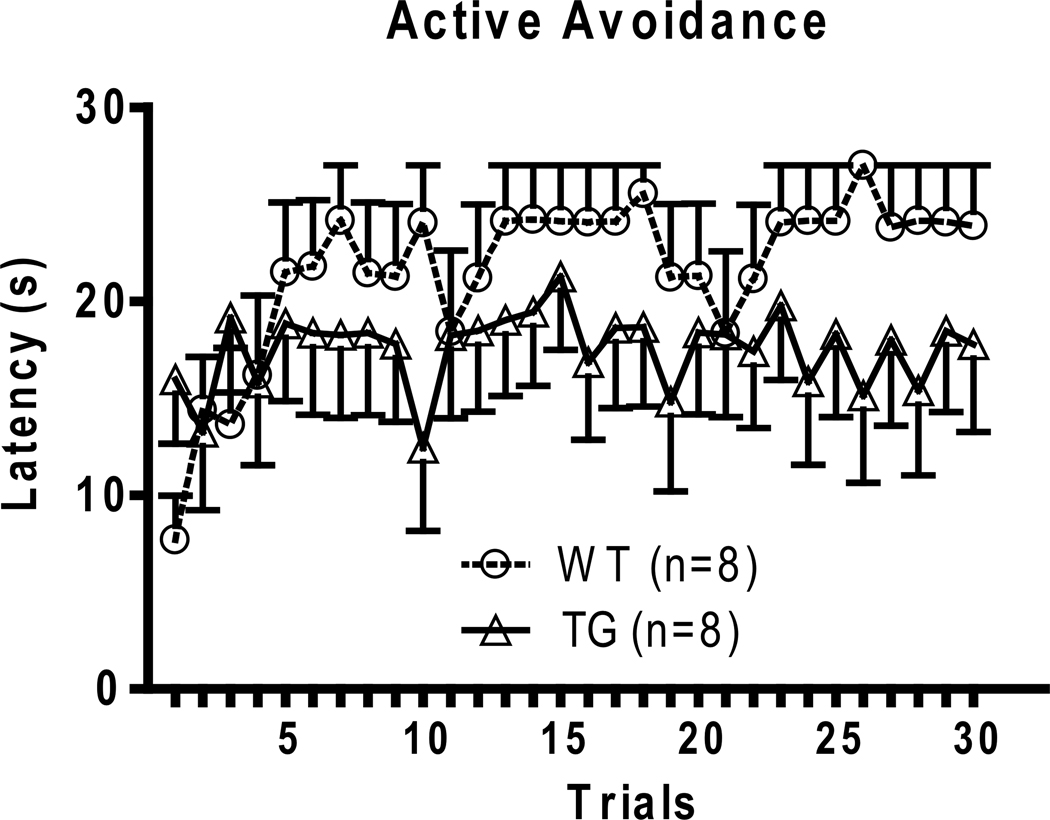
BI-1 TG mice were evaluated in a well-established animal model used to measure stress coping, the learned helplessness (LH) model (Maier, 1984; Seligman and Beagley, 1975). In this paradigm, mice were first exposed to inescapable footshock. On the following day, trained animals were placed in the same environment, except that they were now given the opportunity to escape footshock by shuttling across to a neighboring chamber. Their ability to escape was measured the following day and classified as helpless or non-helpless. Only the helpless animals were tested for in the active avoidance test 8 days following LH induction. BI-1 TG mice exhibit lower escape latencies compared with WT mice in the active avoidance test (Fig. 1).
Fig 1.
Active avoidance testing following 8 days after learned helplessness induction. BI-1 TG mice show enhanced spontaneous recovery measured as reduced escape latencies as compared with wild type (WT) mice. Two-way ANOVA genotype interaction F(1,15) = 18.26, p<0.0001.
2.3. BI-1 TG mice are protected from serotonin depletion as measured by FUST
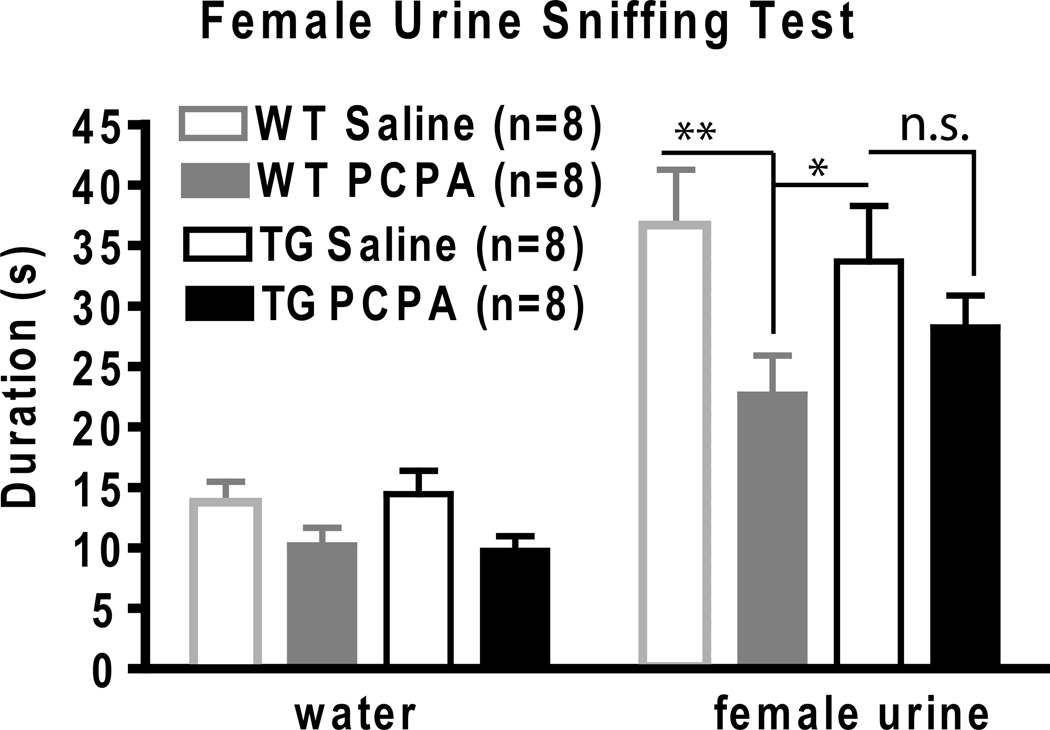
Next, we used the serotonin depletion model to induce a depressive-like phenotype in mice and then monitored their anhedonic response in the female urine sniffing test (FUST). The FUST is a behavioral paradigm recently developed by our group that measures the time a male spends sniffing a cue tip scented with water versus female urine to assess an animal’s reward-seeking behavior (Malkesman et al., 2009). We performed serotonin depletion using tryptophan hydroxylase inhibitor, para-chlorophenylalanine (PCPA), administered the night before testing. No significant differences in sniffing water were seen between groups. As expected, WT male mice injected with PCPA spent less time sniffing female urine than WT animals injected with saline control (Fig. 2). In contrast, BI-1 TG mice injected with PCPA retained normal FUST values with equivalent times spent sniffing female urine observed for the PCPA and saline treated groups (Fig 2). Thus, enforced expression of BI-1 in mouse brain protects against serotonin depletion-induced anhedonia as measured by the FUST.
Fig 2.
Female urine sniffing test (FUST) use to measure anhedonic behavior following serotonin depletion. WT and TG BI-1 mice both spend a longer duration sniffing female urine compared to water. This effect is reduced significantly following serotonin depletion by administration of 300 mg/kg PCPA only in WTs (*p<0.05,** p<0.01, n.s. = non significant).
2.4. BI-1 TG mice are protected from catecholamine depletion as measured by the saccharine preference test
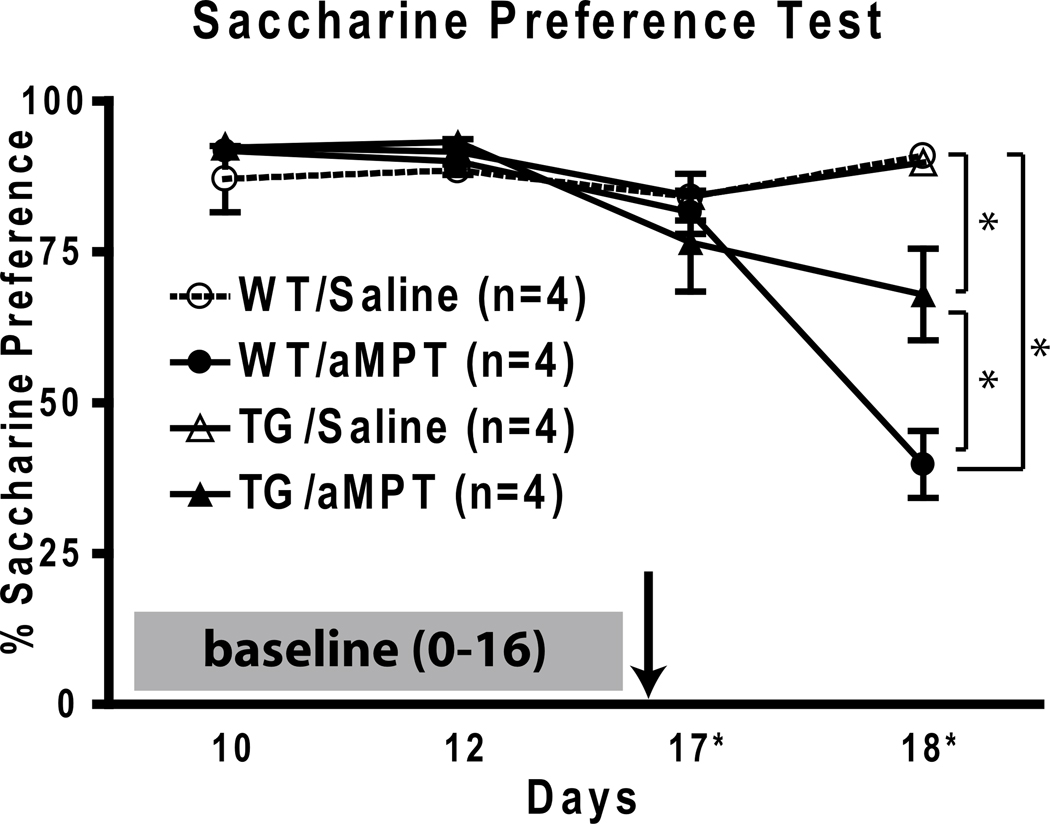
Catecholamine depletion by alpha-methyl para-tyrosine (aMPT) is another model used for inducing a depressive-like phenotype in mice. To monitor the effects of catecholamine depletion, we employed the saccharine preference test. Briefly, this test assesses animal preference for access to water versus saccharine. Normally, mice greatly prefer saccharine solution, which is measured by comparing the volumes of saccharine solution versus water consumed. After a stable saccharine preference of 90.8±1.7% was established in all groups, mice were treated with either saline or aMPT (250 mg/kg i.p.) and saccharine preference was monitored daily. 2 days after aMPT or saline treatment, saccharine preference was reduced to 39.8±5.5% in the WT/aMPT group, while the TG/aMPT group maintained 67.9±7.6% saccharine preference following catecholamine depletion (Fig. 3). In both saline-treated groups, preference was maintained around at 90.8±0.4% for WT and 89.8±1.4% for TG.
Fig 3.
Saccharine preference test to measure anhedonic behavior following catecholamine depletion. A stable saccharin preference (~90%) baseline was established for 16 days before treatment (indicated by arrow) with either aMPT or saline. Saccharin preference was reduced the most in WT/aMPT group (39.8±5.5%) while the TG/aMPT group (67.9±7.6%) illustrates some protection following depletion (* p<0.01). Please note the x-axis is not drawn to scale.
2.5. BI-1 TG neurons show altered calcium dynamics
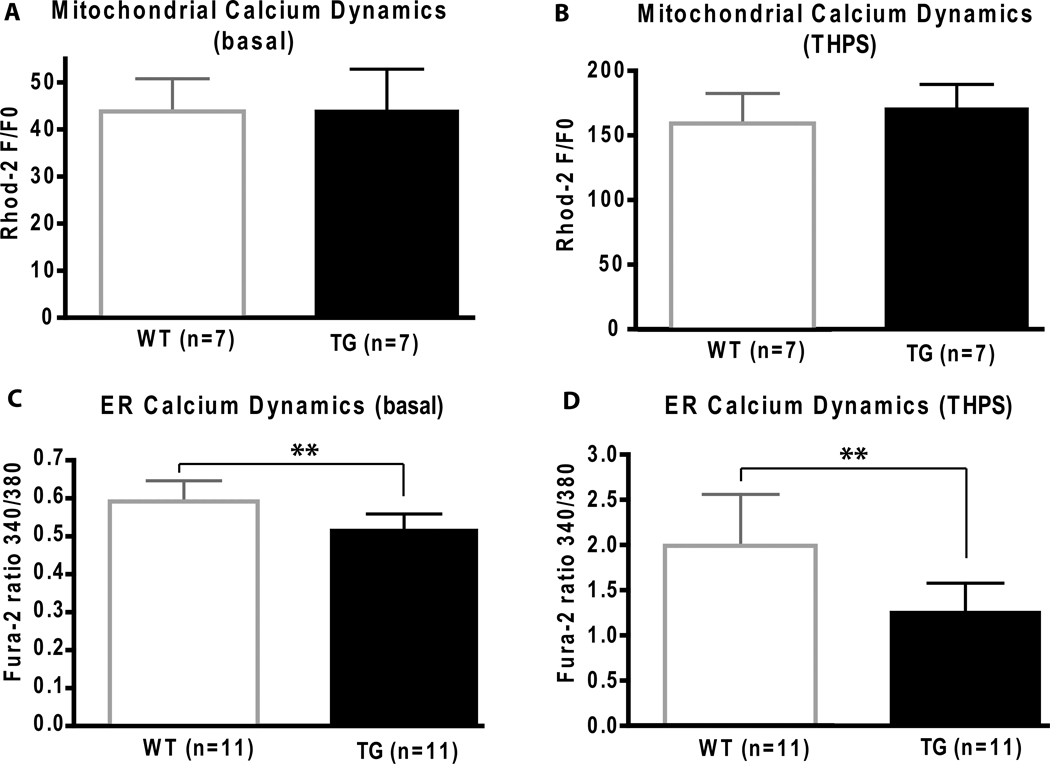
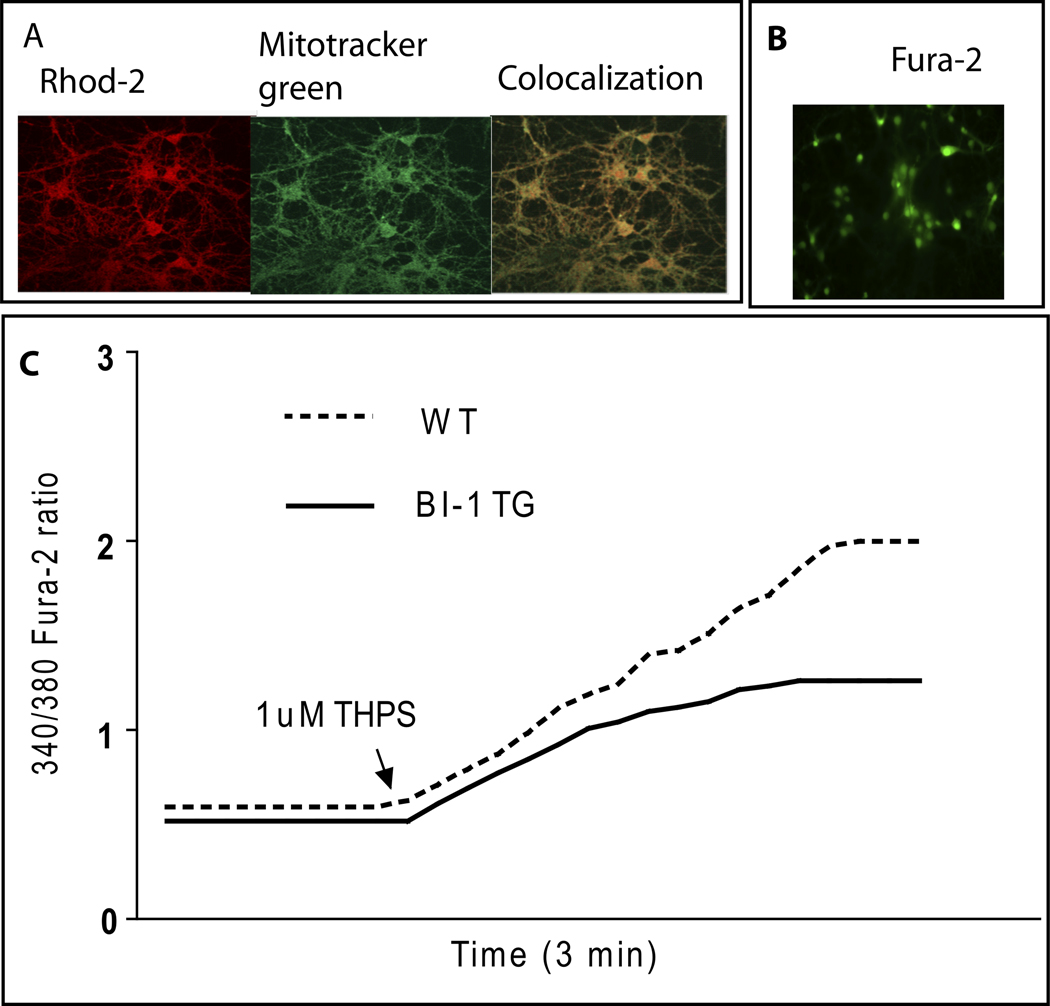
Mitochondrial calcium dynamics was monitored in primary cultures of cortical neurons using Rhod 2, a fluorescent calcium chelator (Minta et al., 1989). Under basal conditions and following challenge with 2 µM THPS, an inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), no differences in mitochondrial calcium handling were observed between WT and TG primary cultures (Fig. 4A & B). The specificity of Rhod-2 for monitoring calcium dynamics in mitochondria is depicted by colocalization with mitotracker green, a mitochondrial-selective fluorescent label (Fig. 5A).
Fig 4.
Mitochondrial and endoplasmic reticulum (ER) calcium dynamics are measured in WT and BI-1 TG cell cultures. In mitochondria (A, B), calcium levels are measured under basal conditions and following response to 2 µM thapsigargin treatment (THPS) where no differences are found between groups. In the ER (C, D), calcium dynamics are measured in cells cultured with or without THPS. Average [Ca2+]i elevations in BI-1 TG neurons after stimulation with 1µM TG shows a decrease in ER Ca2+ release compared to WT. (** p<0.01)
Fig 5.
Monitoring calcium dynamics in mitochondria and the endoplasmic reticulum. Panel A depicts fluorescent calcium chelator (Rhod-2) colocalizes with mitotracker green, a mitochondrial-selective fluorescent label. Scale bar 30µm. Panel B indicates Fura-2 green fluorescent dye used to monitor intracellular calcium in the ER. Panel C depicts a representative cytosolic Ca2+ trace (fura-2) from BI-1 TG or WT neurons following challenge with thapsigargin (THPS).
Calcium dynamics were monitored in both WT and TG primary cortical neuronal cultures using fura-2 AM, a ratiometric fluorescent dye that binds to free intracellular calcium predominantly in the cytosolic compartment (Grynkiewicz et al., 1985) (Fig. 5B). Under basal conditions, TG primary cultures had lower basal cytosolic calcium levels when compared to WT cultures (Fig. 4C). When challenged with 1 µM THPS, the average rise in intracellular calcium concentration, [Ca2+]i, in TG cultures was attenuated in comparison with WT cultures (Fig. 5C). These data in primary cortical neurons thus confirm previous observations made in HeLa cells regarding the role of BI-1 in regulating intracellular calcium dynamics in the ER (Xu et al., 2008).
3. Discussion
Our study shows that reinforced expression of BI-1 under the neuronal specific enolase promoter provides resiliency against LH, serotonin depletion, and catecholamine depletion. These animal paradigms elicit stress coping behavior and model neurotransmitter disruptions believed to underlie some of the causes of mood disorders (Ruhe et al., 2007). How environmental and pharmacological depletion stimuli create depression-like and anhedonic-like states in mice is a subject of considerable controversy (reviewed in (Cryan et al., 2002; Cryan and Mombereau, 2004)). Learned helplessness (situational stress), serotonin depletion, and catecholamine depletion may all share a common mechanism that is modulated by BI-1 expression in neurons. The identity of this common mechanism remains to be determined, but at least three aspects of BI-1 warrant consideration: calcium homeostasis, ER stress mechanisms, and Bcl-2 family-regulated neuroprotection and synaptic plasticity.
Disrupted calcium homeostasis in mood disorders has been identified in a number of studies (Dubovsky et al., 1992; Emamghoreishi et al., 1997; Hough et al., 1999), and the majority of these studies found elevated basal calcium levels and increased calcium release following challenge with THPS in lymphoblast cells. In fact, a recent study examines a Bcl-2 gene SNP on calcium homeostasis in patients with BD and finds disruptions in calcium homeostasis in the AA-variant that expresses reduced levels of Bcl-2 (Machado-Vieira et al., 2010). Elevated cytosolic calcium levels have been associated with apoptosis and necrosis in neuronal cultures (Goldberg and Choi, 1993; Lin et al., 1997), and this disruption may underlie some of the pathological findings of both MDD and BD. For instance, BD patients show decreases in the left cortical subgenular region 24 (SG24) (Drevets et al., 1997) and decreases in gray matter volume in the left dorsolateral prefrontal cortex (Brambilla et al., 2002), combined with increases in protein and mRNA levels of pro-apoptotic factors and decreases in anti-apoptotic factors and synaptic markers (Kim et al., 2010). Together, these data suggest alterations in calcium homeostatic mechanisms may play a role in contributing to some of the pathological findings associated with BD, and treatments that correct this dysregulation could, conceivably, be preventative.
Aberrant ER calcium handling may be corrected by enhancing BI-1 expression. Recent evidence shows that BI-1 regulates ER calcium homeostasis downstream of Bcl-2 family members, suggesting that BI-1 may be capable of controlling the downstream effects of Bcl-2 family members in processes such as neuroprotection and synaptic plasticity (Xu et al., 2008). Curiously, the effects of overexpressing BI-1 on ER calcium handling are similar to the effects of increasing Bcl-2 or knocking out Bax and Bak (Demaurex and Distelhorst, 2003; Foyouzi-Youssefi et al., 2000; Scorrano et al., 2003), which are commonly associated with mitochondrial calcium homeostasis. In addition, knockdown of Bcl-2 or overexpression of Bax has the opposite effect (Chami et al., 2004; Oakes et al., 2005). A previous study showed that calcium load in the ER can direct whether a cell undergoes apoptosis (Scorrano et al., 2003) and suggests a direct interaction between the ER and mitochondria (perhaps through Bcl-2 and Bax/Bak levels) in controlling cytosolic levels of calcium to regulate apoptosis (Demaurex and Distelhorst, 2003).
ER stress has a well documented association with mood disorders (Bown et al., 2000; Cichon et al., 2004; Hayashi et al., 2009; Kakiuchi et al., 2003; Kakiuchi et al., 2007; Yoshida et al., 2006). Notably, certain mood stabilizer treatments for BD modulate ER stress proteins and calcium homeostasis. Valproate (Bown et al., 2002) and lithium (Shao et al., 2006) have both been shown to regulate ER stress proteins including BiP, GRP94, and calreticulin. In addition, lithium and valproate protect against ER stress induced by THPS treatment in studies using cell cultures (Hiroi et al., 2005). Overexpressing ER stress proteins increases the calcium buffering capacity of the ER and may be neuroprotective. For instance, overexpressing the calcium binding protein calreticulin increases intracellular calcium storage in the ER and reduces store-operated calcium influx (Mery et al., 1996). Another study shows that BiP and calreticulin have neuroprotective roles in preventing cell death induced by iodoacetamide, an alkylating toxicant, via modulation of calcium homeostasis (Liu et al., 1997). Our findings support the hypothesis that enhancing ER calcium retention during ER stress may serve as an effective strategy for developing novel mood stabilizer treatments (Kato, 2008).
In pre-clinical rodent models to test antidepressant efficacy, THPS administered intracerebroventricularly (i.c.v.) in mice produced a depressive-like effect in the forced swim test (Galeotti et al., 2006). Also of interest is the observation that disrupting calcium homeostasis and inducing ER stress using the anti-malaria drug, mefloquine (Dow et al., 2003; Dow et al., 2005), induces anxiety and depression in humans (Caillon et al., 1992; Dietz and Frolich, 2002; Tran et al., 2006; Whitworth and Aichhorn, 2005). These studies, together, suggest that disrupting calcium homeostasis and initiating ER stress can produce depressive-like responses in humans and rodents. Reinforcing BI-1 in neurons can protect against disrupting calcium homeostasis, and this mechanism may underlie the effects reported in our study on conferring affective resilience. To access affective resilience, our study used serotonin and catecholamine depletion models in addition to the learned helplessness paradigm to investigate a novel mechanism for treating depression by targeting calcium homeostasis in the ER. The monoamine hypothesis states that depression is caused by the dysregulation of the monoaminergic system in the brain. Common antidepressant agents target monoamines including serotonin (5-HT), norepinephrine (NE), and dopamine (DA). Clinical studies have demonstrated that depleting tryptophan, an essential amino acid required for 5-HT synthesis, in depressed patients blocks the antidepressant effects in most patients treated with selective serotonin reuptake inhibitors (SSRIs) compared with patients treated with a norepinephrine reuptake inhibitors (NRIs) (Delgado et al., 1991; Delgado et al., 1999). Conversely, depleting catecholamines (both NE and DA) by inhibiting tyrosine hydroxylase using aMPT blocked the antidepressant effects in patients treated with NRIs but not with SSRIs (Delgado et al., 1993; Miller et al., 1996). Preclinical studies using PCPA, a selective inhibitor of tryptophan hydroxylase that depletes serotonin, or using aMPT to deplete catecholamines, show both of these drugs to produce depressive-like effects in the tail suspension test, a behavioral model used to predict antidepressant-like activity (O'Leary et al., 2007). These depletion studies provide direct evidence for the importance of monoamines in the antidepressant effects of SSRIs and NRIs, as well as, the potential utility in using these depletion models to induce depressive symptoms in animal models used to predict antidepressant-like activity.
Here, we demonstrate that BI-1 TG mice show facilitated recovery from depletion models and in the well-established LH paradigm. These results suggest that affective resiliency in BI-1 TG mice through modulation of ER calcium homeostasis is capable of protecting against both an environmental stress (unpredictable foot shock) and pharmacological depletion of key neurotransmitters implicated in emotional regulation. We performed cell culture studies to isolate ER from mitochondrial calcium dynamics. We demonstrate, in mouse primary cortical cultures, that TG BI-1 reduces basal cytosolic calcium levels and decreases calcium release from the ER following challenge with the ER stress inducer THPS. We monitored ER stress proteins in the hippocampus of WT and BI-1 TG mice 24 hours following serotonin depletion and found no changes at this time point (Supplemental Figure S4). These results may be due to changes in ER stress proteins that are too transient, small, or cell type/region specific to be detected by western blot analysis. In a recent study using these BI-1 TG mice, the authors found that 24 hours following both ischemia and traumatic brain injury, the ER stress protein CHOP was increased in brain and this increase was attenuated in BI-1 TG mice (Krajewska et al., 2010). This work, combined with the experiments presented here, suggests that severe neurological insults (e.g. traumatic brain injury) may modulate ER stress proteins more robustly than the insults utilized in our study (e.g. serotonin depletion). Clearly, additional investigation is warranted to address these two different mechanisms, calcium homeostasis and ER stress signaling cascades. Future studies should also consider the potential utility for targeting both ER and mitochondria and ultimately how these organelles may interact to achieve synergistic treatments for mood disorders by modulating both calcium homeostasis and ER stress signaling.
In conclusion, our findings provide mechanistic support for BI-1 targeting ER calcium homeostasis to enhance affective resiliency. These findings hold exciting promise for developing new treatments for mood disorders. New treatments that can specifically target neuronal BI-1 and its regulated pathways may provide innovative mechanisms to enhance brain plasticity in psychiatric disease.
4. Experimental Procedure
4.1. Animals
Wild type FVB mice (females) were obtained from Jackson Laboratories (Bar Harbor, Maine), to breed at the NIH with BI-1 TG male mice obtained from Dr. John Reed’s laboratory (Sanford-Burnham Institute for Medical Research, La Jolla, CA) and referenced in this recent manuscript (Krajewska et al., 2010). For the following behavioral paradigms, animals were group-housed 4 per cage: open field test (OFT), elevated plus maze (EPM), hot plate test, learned helplessness (LH) paradigm, amphetamine-induced hyperlocomotion test, and cocaine-induced behavioral sensitization test (CIBS). Animals were singly housed for the female urine sniffing test (FUST) and the saccharine preference test. Animals were housed at constant temperature (22°±1°C) on a 12 hour light/dark cycle (light: 6AM–6PM) with free access to food and water. Behavior testing was performed in a separate behavioral room in the morning (7AM–11AM) for most behavioral experiments except for the learned helplessness inductions and active avoidance which both occurred all day long (7AM–5PM) and the saccharine preference test (free access to water and saccharine day and night). All experimental procedures were approved by the Animal Care and Use Committee of the National Institue of Mental Health and followed the guidelines of the National Institute of Health.
4.2. Learned helplessness (LH) paradigm
LH paradigm was performed similar to those previously described using the Gemini Avoidance System (San Diego, CA) (Shirayama et al., 2002). LH induction conducted on Day 0 and consisted of 50 sessions of inescapable electric foot shocks (unconditioned stimulus- US) at 0.45 mA for 10 seconds with an inter-trial interval (ITI) of 45 seconds on average ranging from 22–38 seconds, accompanied by a cued light (conditioned stimulus- CS) 3 seconds prior to the foot shock delivery. Screening for helplessness, defined as 15 or more failed escapes, was performed the next day in the same chambers; however, the center compartment door opened briefly to allow the animal to escape by shuttling across to the accompanying chamber to avoid the shock. Thirty trials were performed consisting of an initial 300 second acclimation followed by 30 foot shocks (0.45 mA lasting 3 seconds) each preceded by a 3 second light CS and the gate opening (22–38 ITI). Escape latencies and failures were accessed.
4.3. Active avoidance test
Using Gemini Avoidance System (San Diego, CA), mice underwent a series of 30 sessions of electric foot shocks (0.45 mA, 24 second durations, paired with a cued light 3 seconds before shock), with the escaping gate paired to open with the CS light to allow the animal to avoid or escape the shock. Escape latencies and failures were recorded as previously described (Shirayama et al., 2002).
4.4. Serotonin depletion paired with the FUST
para-chlorophenylalanine (PCPA) was used at 300 mg/kg i.p. 10–12 hours prior to testing in a model sensitive to anhedonia developed in our laboratory, FUST(Malkesman et al., 2009). PCPA depletes serotonin by acting as a selective and irreversible inhibitor of tryptophan hydroxylase. Following serotonin depletion, mice were tested in their home cage in a behavioral procedure room under low lighting (20–30 lux) in the FUST consisting of the following procedures: (1) 1-hour habituation to a sterile Q-tip, (2) set up estrus females of same strain as males being tested in a clean cage without bedding to collect urine, (3) record sniffing for 3 minutes to Q-tip dipped in sterile water for all animals, (4) record sniffing for 3 minutes to Q-tip dipped in female urine for all animals.
4.5. Catecholamine depletion paired with saccharin preference
A stable saccharine preference was established in an animal’s home cage over two weeks in mice singly housed and given free access to two bottles, water and saccharine (75 mg/L). Bottle positions were switched to avoid side preferences every Monday, Wednesday, and Friday after bottles were weighed and refilled. Catecholamine depletion was induced using alpha-methyl para-tyrosine (aMPT) injected (250 mg/kg i.p.) as previously described (Hasbani et al., 2005), which causes reduction of catecholamine levels in the brain. Following catecholamine depletion, saccharin preference (75 mg/L) was monitored daily to assess changes in anhedonic behavior.
4.6. Cellular calcium dynamics
Mouse cortical cultures were prepared as described previously (Hao et al., 2004) from embryos at 18 days of gestation, plated at a density of 1×106 cells/mL onto poly-D-lysine coated 15-mm coverslips, and allowed to mature for 10 days prior to calcium handling experiments. For all technical details on measuring calcium dynamics please consult our recent paper (Machado-Vieira et al., 2010). Mitochondrial calcium release was determined by Rhod 2 in BI-1 TG primary cortical cells compared to WT cells. Cultured neurons were placed in MIC buffer (NaCl 130 mM, KCl 5.3 mM, MgSO4 0.8 mM, Na2HPO4 1 mM, Glucose 2 mM, Hepes 20 mM, Na-Pyruvate 1 mM, NaHCO3 2.5 mM, Ascorbic acid 1.0 mM, CaCl2 1.5 mM, BSA 1.5 mg/ml) for 10 minutes at room temperature and loaded with 4.0 mM dihydrorhod-2 in MIC buffer for 30 minutes at 37 °C. After washing twice, the fluorescent signals were measured by Zeiss LSM 510 microscopy (excitation at 543 nm and a 560 nm long pass filter was used for emission). The image-fields were randomly determined and images were captured every 10 sec. After 5 images for baseline, 2 µM THPS was added to evoke ER stress and images were taken for an additional 4 minutes. Average red fluorescent intensity of the cell in each time interval was determined by 510 Metamorph software. The Fi/Fo value was measured before and after THPS treatment. ER calcium release was determined using fura-2 AM (Invitrogen, Carlsbad, CA). Cortical primary cells were pre-incubated for 45–60 minutes at 37°C in an extracellular buffer (ECB) [HBSS plus 20 mM HEPES, 0.5% BSA and 1mM CaCl2] with 1 µM acetoxymethyl esters of the fluorescent calcium indicator fura-2 AM. Following incubation, cells were washed with ECB for 45 minutes at room temperature to permit dye de-esterification. Coverslips were mounted in a perfusion chamber on the stage of a Zeiss Axiovert 200 inverted microscope equipped with a 40X Fluor objective (Zeiss, Thornwood, NY). Images and dual excitation (340/380 nm in wavelength) fluorescence records were continuously acquired with EasyRatioPro hardware and software (PTI, Birmingham, NJ). Cells were randomly selected for each experiment.
4.7. Statistics
Statistical analysis was performed using GraphPad Prism Version 4 (GraphPad Software, La Jolla, California). Two way analysis of variance (ANOVA) was performed for active avoidance, FUST, saccharine preference test, amphetamine-induced hyperlocomotion test, and cocaine-induced behavioral sensitization (CIBS). A paired t-test was performed for the calcium handling experiments, open field test, elevated plus maze, and the hot plate test.
Highlights.
-
>
We reinforce neuronal expression of bax inhibitor 1 in mice.
-
>
These mice demonstrate affective resiliency in multiple animal models.
-
>
Cortical cultures in these mice investigate the role of calcium homeostasis.
-
>
Targeting ER calcium homeostasis may provide novel treatments for mood disorders.
Supplementary Material
Acknowledgments
This work was funded by the NIMH Intramural Program and by NIH grant AG-015393. We thank Danielle Warrinllal and Wenji Xu for technical assistance with mouse breeding and genotyping. We would like to thank Emily Fessler and Peter Leeds for editorial suggestions. We would like to thank S Brian Andrews for use of his facility to perform the calcium experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: None.
References
- Aldenhoff JB, Dumais-Huber C, Fritzsche M, Sulger J, Vollmayr B. Altered Ca(2+)-homeostasis in single T-lymphocytes of depressed patients. J Psychiatr Res. 1997;31:315–322. doi: 10.1016/s0022-3956(96)00045-3. [DOI] [PubMed] [Google Scholar]
- Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW, Farmer D, Reed JC. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:2809–2814. doi: 10.1073/pnas.0506854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown C, Wang JF, MacQueen G, Young LT. Increased temporal cortex ER stress proteins in depressed subjects who died by suicide. Neuropsychopharmacology. 2000;22:327–332. doi: 10.1016/S0893-133X(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Bown CD, Wang JF, Chen B, Young LT. Regulation of ER stress proteins by valproate: therapeutic implications. Bipolar Disord. 2002;4:145–151. doi: 10.1034/j.1399-5618.2002.t01-1-40201.x. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti MA, Harenski K, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27:792–799. doi: 10.1016/S0893-133X(02)00352-4. [DOI] [PubMed] [Google Scholar]
- Caillon E, Schmitt L, Moron P. Acute depressive symptoms after mefloquine treatment. Am J Psychiatry. 1992;149:712. doi: 10.1176/ajp.149.5.712a. [DOI] [PubMed] [Google Scholar]
- Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S, Banares S, Cui J, Digicaylioglu M, Ke N, Kitada S, Monosov E, Thomas M, Kress CL, Babendure JR, Tsien RY, Lipton SA, Reed JC. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15:355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Chami M, Prandini A, Campanella M, Pinton P, Szabadkai G, Reed JC, Rizzuto R. Bcl-2 and Bax exert opposing effects on Ca2+ signaling, which do not depend on their putative pore-forming region. J Biol Chem. 2004;279:54581–54589. doi: 10.1074/jbc.M409663200. [DOI] [PubMed] [Google Scholar]
- Cichon S, Buervenich S, Kirov G, Akula N, Dimitrova A, Green E, Schumacher J, Klopp N, Becker T, Ohlraun S, Schulze TG, Tullius M, Gross MM, Jones L, Krastev S, Nikolov I, Hamshere M, Jones I, Czerski PM, Leszczynska-Rodziewicz A, Kapelski P, Bogaert AV, Illig T, Hauser J, Maier W, Berrettini W, Byerley W, Coryell W, Gershon ES, Kelsoe JR, McInnis MG, Murphy DL, Nurnberger JI, Reich T, Scheftner W, O'Donovan MC, Propping P, Owen MJ, Rietschel M, Nothen MM, McMahon FJ, Craddock N. Lack of support for a genetic association of the XBP1 promoter polymorphism with bipolar disorder in probands of European origin. Nat Genet. 2004;36:783–784. doi: 10.1038/ng0804-783. author reply 784–5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, Licinio J, Krystal JH, Heninger GR, Charney DS. Rapid serotonin depletion as a provocative challenge test for patients with major depression: relevance to antidepressant action and the neurobiology of depression. Psychopharmacol Bull. 1991;27:321–330. [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Heninger GR, Gelenberg AJ, Charney DS. Monoamines and the mechanism of antidepressant action: effects of catecholamine depletion on mood of patients treated with antidepressants. Psychopharmacol Bull. 1993;29:389–396. [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry. 1999;46:212–220. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Distelhorst C. Cell biology. Apoptosis--the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- Dietz A, Frolich L. Mefloquine-induced paranoid psychosis and subsequent major depression in a 25-year-old student. Pharmacopsychiatry. 2002;35:200–202. doi: 10.1055/s-2002-34114. [DOI] [PubMed] [Google Scholar]
- Dow GS, Hudson TH, Vahey M, Koenig ML. The acute neurotoxicity of mefloquine may be mediated through a disruption of calcium homeostasis and ER function in vitro. Malar J. 2003;2:14. doi: 10.1186/1475-2875-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow GS, Caridha D, Goldberg M, Wolf L, Koenig ML, Yourick DL, Wang Z. Transcriptional profiling of mefloquine-induced disruption of calcium homeostasis in neurons in vitro. Genomics. 2005;86:539–550. doi: 10.1016/j.ygeno.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dubovsky SL, Murphy J, Thomas M, Rademacher J. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry. 1992;149:118–120. doi: 10.1176/ajp.149.1.118. [DOI] [PubMed] [Google Scholar]
- Dubovsky SL, Thomas M, Hijazi A, Murphy J. Intracellular calcium signalling in peripheral cells of patients with bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci. 1994;243:229–234. doi: 10.1007/BF02191579. [DOI] [PubMed] [Google Scholar]
- Emamghoreishi M, Schlichter L, Li PP, Parikh S, Sen J, Kamble A, Warsh JJ. High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. Am J Psychiatry. 1997;154:976–982. doi: 10.1176/ajp.154.7.976. [DOI] [PubMed] [Google Scholar]
- Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Bartolini A, Ghelardini C. Blockade of intracellular calcium release induces an antidepressant-like effect in the mouse forced swimming test. Neuropharmacology. 2006;50:309–316. doi: 10.1016/j.neuropharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbani DM, Perez FA, Palmiter RD, O'Malley KL. Dopamine depletion does not protect against acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in vivo. J Neurosci. 2005;25:9428–9433. doi: 10.1523/JNEUROSCI.0130-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Kasahara T, Kametani M, Toyota T, Yoshikawa T, Kato T. Aberrant endoplasmic reticulum stress response in lymphoblastoid cells from patients with bipolar disorder. Int J Neuropsychopharmacol. 2009;12:33–43. doi: 10.1017/S1461145708009358. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Wei H, Hough C, Leeds P, Chuang DM. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J. 2005;5:102–111. doi: 10.1038/sj.tpj.6500296. [DOI] [PubMed] [Google Scholar]
- Hough C, Lu SJ, Davis CL, Chuang DM, Post RM. Elevated basal and thapsigargin-stimulated intracellular calcium of platelets and lymphocytes from bipolar affective disorder patients measured by a fluorometric microassay. Biol Psychiatry. 1999;46:247–255. doi: 10.1016/s0006-3223(98)00308-4. [DOI] [PubMed] [Google Scholar]
- Kakiuchi C, Iwamoto K, Ishiwata M, Bundo M, Kasahara T, Kusumi I, Tsujita T, Okazaki Y, Nanko S, Kunugi H, Sasaki T, Kato T. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat Genet. 2003;35:171–175. doi: 10.1038/ng1235. [DOI] [PubMed] [Google Scholar]
- Kakiuchi C, Ishiwata M, Nanko S, Kunugi H, Minabe Y, Nakamura K, Mori N, Fujii K, Umekage T, Tochigi M, Kohda K, Sasaki T, Yamada K, Yoshikawa T, Kato T. Association analysis of HSP90B1 with bipolar disorder. J Hum Genet. 2007;52:794–803. doi: 10.1007/s10038-007-0188-4. [DOI] [PubMed] [Google Scholar]
- Kato T. Molecular neurobiology of bipolar disorder: a disease of 'mood-stabilizing neurons'? Trends Neurosci. 2008;31:495–503. doi: 10.1016/j.tins.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Konopka LM, Cooper R, Crayton JW. Serotonin-induced increases in platelet cytosolic calcium concentration in depressed, schizophrenic, and substance abuse patients. Biol Psychiatry. 1996;39:708–713. doi: 10.1016/0006-3223(95)00189-1. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Xu L, Xu W, Krajewski S, Kress CL, Cui J, Yang L, Irie F, Yamaguchi Y, Lipton SA, Reed JC. Endoplasmic Reticulum Protein BI-1 Modulates Unfolded Protein Response Signaling and Protects Against Stroke and Traumatic Brain Injury. Brain Res. 2010 doi: 10.1016/j.brainres.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SZ, Yan GM, Koch KE, Paul SM, Irwin RP. Mastoparan-induced apoptosis of cultured cerebellar granule neurons is initiated by calcium release from intracellular stores. Brain Res. 1997;771:184–195. doi: 10.1016/s0006-8993(97)00763-4. [DOI] [PubMed] [Google Scholar]
- Liu H, Bowes RC, 3rd, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Pivovarova NB, Stanika RI, Yuan P, Wang Y, Zhou R, Zarate CA, Jr, Drevets WC, Brantner CA, Baum A, Laje G, McMahon FJ, Chen G, Du J, Manji HK, Andrews SB. The Bcl-2 Gene Polymorphism rs956572AA Increases Inositol 1,4,5-Trisphosphate Receptor-Mediated Endoplasmic Reticulum Calcium Release in Subjects with Bipolar Disorder. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF. Learned helplessness and animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK. The Female Urine Sniffing Test: A Novel Approach for Assessing Reward-Seeking Behavior in Rodents. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biol Chem. 1996;271:9332–9339. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR, Charney DS. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53:117–128. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Beagley G. Learned helplessness in the rat. J Comp Physiol Psychol. 1975;88:534–541. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- Shao L, Sun X, Xu L, Young LT, Wang JF. Mood stabilizing drug lithium increases expression of endoplasmic reticulum stress proteins in primary cultured rat cerebral cortical cells. Life Sci. 2006;78:1317–1323. doi: 10.1016/j.lfs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TM, Browning J, Dell ML. Psychosis with paranoid delusions after a therapeutic dose of mefloquine: a case report. Malar J. 2006;5:74. doi: 10.1186/1475-2875-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Aldenhoff JB. Cytosolic free [Ca2+] in single T-lymphocytes from depressed patients and healthy controls. Eur Arch Psychiatry Clin Neurosci. 1994;243:214–217. doi: 10.1007/BF02191576. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Aichhorn W. First-time diagnosis of severe depression: induced by mefloquine? J Clin Psychopharmacol. 2005;25:399–400. doi: 10.1097/01.jcp.0000169619.07325.ff. [DOI] [PubMed] [Google Scholar]
- Xu C, Xu W, Palmer AE, Reed JC. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem. 2008;283:11477–11484. doi: 10.1074/jbc.M708385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Reed JC. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell. 1998;1:337–346. doi: 10.1016/s1097-2765(00)80034-9. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Nadanaka S, Sato R, Mori K. XBP1 is critical to protect cells from endoplasmic reticulum stress: evidence from Site-2 protease-deficient Chinese hamster ovary cells. Cell Struct Funct. 2006;31:117–125. doi: 10.1247/csf.06016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.