Abstract
The purpose of this study was the use of rhodamine 123 (Rho123) accumulation in peripheral blood CD8+cells as a surrogate indicator to evaluate the modulating effect of P-glycoprotein (P-gp) inhibitors in the multidrug resistance (MDR) tumor-bearing mouse model. Rho123 was administered to mice, and the fluorescence level in CD8+ cells was measured. Cepharanthine hydrochloride (CH) and verapamil (VER), two P-gp inhibitors, were administered to mice 1 hour prior to Rho123 administration in vivo or added to peripheral blood 1 hour prior to Rho123 addition ex vivo. The tumor inhibition effect of 5-fluorouracil/adriamycin/cisplatin (FAP) protocol plus CH was also investigated. A concentration- or dose-response relationship was shown between the concentration and dose of CH and Rho123 accumulation or the antitumor activity. In conclusion, the measurement of Rho123 accumulation in CD8+ cells provides a surrogate assay for the screening of candidate P-gp inhibitors in preclinical trials, and CH is effective in modulating P-gp-mediated MDR in vivo.
1. Introduction
Multidrug resistance (MDR) remains the major obstacle to success in a wide variety of advanced malignancies, particularly in solid cancers. The overexpression of P-gp is one of the important mechanisms involved in MDR [1–3]. P-gp-mediated cellular uptake and the efflux of anticancer drugs from cancer cells are responsible for the cell elimination of the drugs which in turn decreases their therapeutic effect. Therefore, one of the best methods of reversing MDR and increasing the efficacy of anticancer drugs is to use the potent P-gp inhibitors modulating P-gp function and/or expression.
It is an important step to use assays to evaluate the effect of candidate drugs as inhibitors of P-gp function in vivo after the drugs were found to have an MDR-reversing effect in vitro. For nonsurgical solid cancer patients, there are currently no direct assays measuring intratumoral drug concentrations or the alteration of P-gp function and/or expression. Thus, alternative approaches to evaluate the degree of functional inhibition of P-gp in vivo will be a useful aid in the development of MDR modulators. P-gp is also expressed in normal tissues in which they seem to have an important role in the protection against xenobiotics [4]. Several authors reported that P-gp was expressed in lymphocytes including CD3+, CD4+, and CD8+ T cells, as well as the CD56+ natural killer (NK) cells, with the highest expression levels and activities observed in CD56+ cells followed by CD8+ cells [5, 6]. However, the physiological roles that P-gp plays in these cells are unclear. The P-gp in the lymphocytes appears functionally identical to that observed in multidrug resistant cells; they have the same substrate and antagonist specificities [5, 7, 8]. Since the expression and function of P-gp in CD56+ cells are the highest, is Rho123 accumulation widely used in CD56+ populations as a surrogate indicator to evaluate the degree of functional inhibition of P-gp in clinical trials of P-gp inhibitors, for example, the work of Tariquidar [9] and Zosuquidar [10]. As mouse NK cells do not express CD56+[11], we used Rho123 accumulation in CD8+ cells as a surrogate indicator to evaluate the reversal activity by P-gp reversors in the mouse MDR tumor-bearing model.
The search for MDR modulators has extended to the natural products and their derivatives; natural source compounds have become the most widely used of fourth-generation P-gp inhibitors because they are less toxic and more potent than the disappointing first- and second-generation MDR modulators [12–14]. CH, manufactured by salification from cepharanthine, which is a biscoclaurine alkaloid, extracted from Stephania cepharantha Hayata has a variety of biological activities (Figure 1) [15–17]. Recently, it has been reported that CH has an MDR-reversal effect, and P-gp inhibition is one of the reversal mechanisms of MDR in vitro [18, 19]; however, the effect was not evaluated in vivo.
Figure 1.
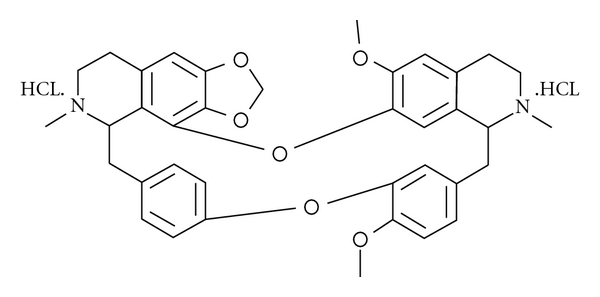
The chemical structure of CH used in the present study.
We present here a method to detect the effect of the inhibitor of the P-gp function on Rho123 accumulations of the CD8+ cells in whole blood from the MDR tumor-bearing mouse. This method was first used to develop and validate the efficacy of CH against P-gp functions in the mouse model for potential use in subsequent clinical evaluations.
2. Materials and Methods
2.1. Animals
The experiments were carried out on 2–4-month-old BALB/c male mice, obtained from the Henan Experimental Animal Center (Zhengzhou, China). The animals had been raised from birth in a specific pathogen-free environment and were handled according to the institutional guidelines complying with Chinese legislation.
2.2. Cell Lines
Hca/FAP variants were developed from the parental Hca hepatocarcinoma cells, which were obtained from KeyGen Biotechnology Co. Ltd. (NanJing, China). By step-by-step, continuous exposure to increasing doses of FAP, the overexpression of P-gp in the variant was confirmed by the Western blot analysis and the cells that have been previously described [20].
2.3. Drugs
CH was supplied by the Henan Academy of Medical and Pharmaceutical Sciences (Zhengzhou, China). VER was purchased from Harvest Pharmaceutical Co., Ltd (Shanghai, China). Adriamycin (ADR) was purchased from Hisun Pharmaceutical Co., Ltd (Zhejiang, China). Cisplatin (CDDP) was purchased from Qilu Pharmaceutical Co., Ltd (Shandong, China). 5-Flurouracil (5-Fu) was purchased from Xudong Haipu Pharmaceutical Co., Ltd (Shanghai, China). Rho123 was purchased from Sigma-Aldrich (St. Louis, Mo, USA) and dissolved in PBS at a concentration of 1 mg/ml. The Ficoll-Histopaque mouse lymphocyte separation medium was obtained from Solarbio Science & Technology Co., Ltd (Beijing, China). The PE antimouse CD8 antibody (clone 53-6.7) and PE Rat IgG2α, κ Isotype Ctrl were obtained from BioLegend (BioLegend Corp., USA). All drugs were freshly prepared.
2.4. Animal Treatment
Hca/FAP cells were collected from the ascitic fluid of BALB/c mice harboring 5–7 day-old ascitic tumor. The 1 × 107 Hca/FAP cells were injected intramuscularly in the right axilla of BALB/c male mice selected for the experiment on Day 0. The next day, the animals were randomized and divided into different groups; each group comprised 10 mice.
To study the effects of Rho123 administration on peripheral blood CD8+ cells, the retention of fluorescence in these cells was investigated as described previously [21] with some modifications and a dose-response curve established. On the 8th day after the Hca/FAP injection, the mice received a single intravenous (i.v.) injection of Rho123. The doses used were 0.5, 1.0, 2.5, 5.0, and 7.5 mg/kg, and the volume of administration was 10 μl/g. The control group was injected with the vehicle alone. One hour after administration, peripheral blood was collected and cell suspension was prepared as described below.
For the investigation of the effects of P-gp inhibitors ex vivo, blood samples were collected as described below on the 8th day after Hca/FAP injection. CH was then added at final concentrations of 10.0, 5.0, and 2.5 μM; VER was added at a final concentration of 5.0 μM, an equal volume of the vehicle was added as control. The samples were incubated at 37°C for one hour before Rho123 was added at a final concentration of 50 ng/mL, and the tubes were incubated further at 37°C for one hour. Cell suspension was prepared as described below.
To study P-gp function in vivo, Rho123 was injected with or without CH or VER as described before [6] with small modifications on the 8th day after Hca/FAP injection. Briefly, mice, respectively, received a single intravenous (i.v.) injection of the vehicle as control; 2.5, 5.0, and 10.0 mg/kg of CH or 2.5 mg/kg of VER followed one hour later by a single i.v. injection of 2.5 mg/kg of Rho123. The volume of administration was 10 μl/g. After one hour, blood samples were collected and cell suspension was prepared as described below.
2.5. Cell Preparation and Rho123 Accumulation by Flow Cytometry
Peripheral blood was collected from the orbital sinus of each mouse in a tube containing K2EDTA as an anticoagulant. Mononuclear cells were obtained by the separation of anticoagulated blood by a Ficoll-Histopaque density-gradient centrifugation and transferred to 1.5 mL Eppendorf tubes and kept on ice. Cells were washed twice with ice-cold phosphate buffered saline (PBS, pH 7.4). After that, the pellet was gently resuspended in ice-cold PBS and adjusted to a concentration of 1 × 106 cells/ml. Single-cell suspensions were incubated with either the PE-conjugated antimouse CD8+ antibody or PE-conjugated Rat IgG2α, κ Isotype Ctrl as a negative control. After staining for 30 minutes in the darkness at 4°C, the cells were washed twice with ice-cold PBS and then resuspended in PBS and kept on ice in the dark until analyzed as previously described [22]. A life-gate based on forward scatter (FSC) and side scatter (SSC) parameters were made to analyze only viable cells; other gates were made to determine the subpopulations. Amplifier settings for FSC and side SSC were used in linear mode and those for fluorescence channels were used in a logarithmic mode. Fluorescence compensation was manually set for FL1 channel (Rho123) and FL2 channel (PE) with single Rho123-stained cells and PE-stained cells separately. At least 30,000 events were acquired per sample. Multicolor flow cytometry analyses were used to evaluate the proportions of the CD8 + population and the mean fluorescence intensity (MFI) of Rho123 in the population. All analyses were performed in duplicate in at least four separate experiments. Cells were analyzed on Epics-XL MCL, and data were analyzed with Expo32 ADC software (Beckman Coulter, Fullerton, Calif, USA).
2.6. Tumor Inhibition of FAP Chemotherapy Protocol plus CH
To evaluate the antitumor effect of FAP chemotherapy protocol plus various concentrations of CH or VER in vivo as previously described [23]. Twenty four-hours after the Hca/FAP injection, the mice were randomly divided into different groups and treated as follows:
-
Group I:control (normal saline i.v. injected consecutively from Day 1 to 9),
-
Group II:10 mg/kg of CH alone i.v. injected from Day 1 to 8,
-
Group III:FAP chemotherapy: 4.2 mg/kg of ADR and 85.0 mg/kg of 5-FU i.v. injected on Day 1, 2.8 mg/kg of CDDP i.v. injected from Day 1 to 8,
-
Group IV:2.5 mg/kg of CH i.v. injected from Day 1 to 8 with FAP chemotherapy,
- Group V: 5.0 mg/kg of CH i.v. injected from Day 1 to 8 with FAP chemotherapy,
-
Group VI:10.0 mg/kg of CH i.v. injected from Day 1 to 8 with FAP chemotherapy,
-
Group VII:2.5 mg/kg of VER i.v. injected from Day 1 to 8 with FAP chemotherapy.
-
Group I:
The volume of administration was 10 μl/g. Tumors were resected on the second day following the last injection. The tumor length (L) and width (W) were measured, and the tumor weight (WR) was calculated as follows:
| (1) |
The percent of tumor growth inhibition was calculated on Day 8 by comparing the average values of the treated groups with those of the tumor-bearing control group. Tumor growth in saline treated control animals was taken to be at 100%.
2.7. Statistical Analysis
For all the experiments, data are presented as the mean ± SD. Statistical comparison between the groups with different doses or concentrations was carried out using two-sided Student's t-tests. Tests for significant differences among the groups were done using one-way ANOVA with multiple comparisons (Fisher's pairwise comparisons) using SPSS 13.0 (IBM software, Somers, NY, USA). A minimum P value of.05 was estimated as the significance level for all tests.
3. Results
The i.v. administration of Rho123 (0.5–7.5 mg/kg) in mice produced a dose-dependent fluorescence fashion in peripheral blood CD8+ cells (Figure 2). All doses were well tolerated without significant toxicity.
Figure 2.
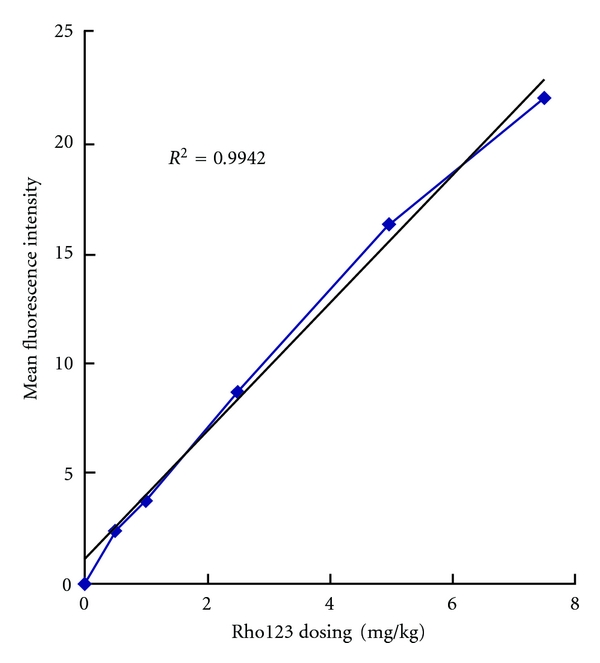
Mean fluorescence intensities of CD8+ cells were linearly correlated to the dose of Rho123 administered (r2 = 0.9942). The treatment protocol and experimental procedures are described in the text. Responses to treatments were significantly different from each other (P < .05).
The lower dose of Rho123, 2.5 mg/kg, was selected to investigate the effects of CH on the accumulation of Rho123 in peripheral blood CD8+ cells. Using this dose, Rho123 accumulations were increased after administration of either VER (positive control) or CH and presented a clear dose-dependent relationship with CH (2.5–10 mg/kg) compared with vehicle control, as shown in Figure 3. The efficacy of the P-gp reversors was not equal; VER was less efficient and potent than CH. There has similar result in ex vivo blood samples; the MFIs of CD8+ cells in the samples treated with the vehicle, 5.0 μM of VER and 2.5, 5.0 and 10.0 μM of CH were 14.4 ± 0.3, 20.0 ± 0.3, 22.9 ± 0.5, 25.4 ± 1.0 and 29.8 ± 0.9, respectively. The MFI was related to the presence of CH in a concentration pattern in ex vivo blood samples, as shown in Figure 4. This demonstrated that P-gp is active in CD8+ cells and can be modulated by CH in vivo and ex vivo.
Figure 3.
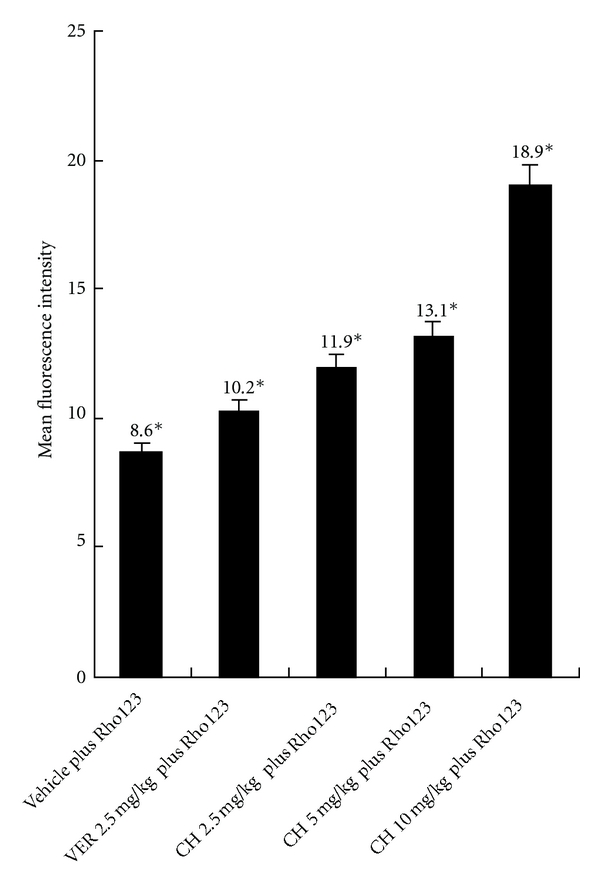
Accumulation of Rho123 in CD8+ cells by different doses of reversors with or without Rho123 at a dose of 2.5 mg/kg in vivo. The increase of the mean fluorescence intensity that reflects the efflux is inhibited. *P < 0.05, different from the control. The results are the mean ± SD of at least 4 independent experiments.
Figure 4.
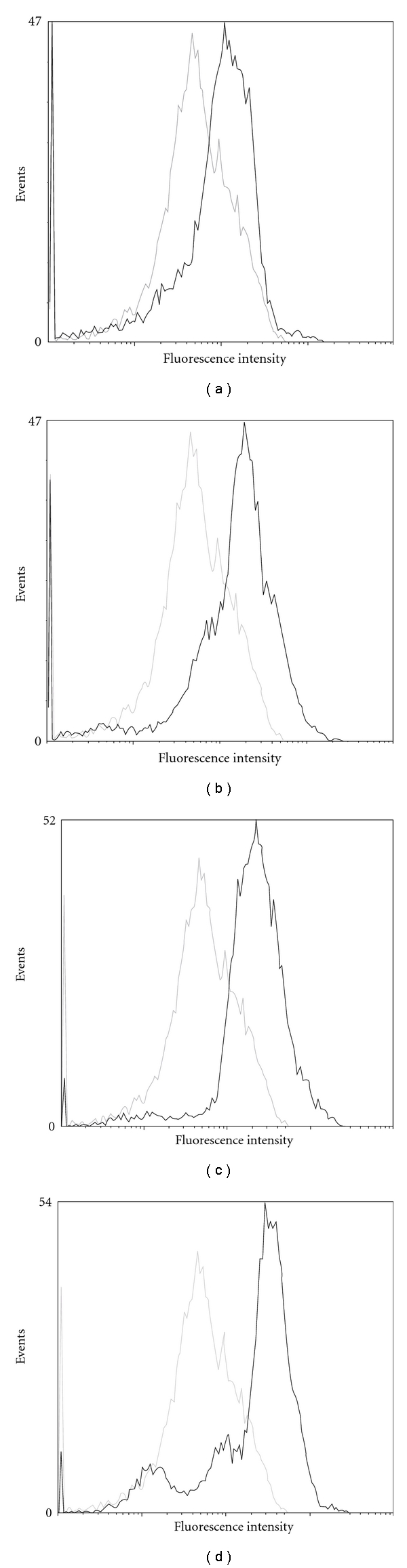
Rho123 accumulations in CD8+ cells ex vivo, where the grey lines stand for cells treated with Rho123 plus the vehicle and it was set as the control in the four sub-figures and the black lines represent cells treated with Rho123 in the presence of various concentrations reversors: A. VER 5.0 μM, B. CH 2.5 μM, C. CH 5.0 μM, D. CH 10.0 μM. This figure is representative of at least four independent experiments.
The antitumor effects of FAP combination chemotherapy plus i.v. 2.5 mg/kg of VER or 2.5, 5.0, and 10.0 mg/kg of CH were evaluated in the Hca/FAP tumor-bearing mouse. The FAP combination chemotherapy plus 10.0 mg/kg of CH provided the maximum antitumor activity of the treatments studied. Compared with the FAP chemotherapy group, the antitumor effect was increased in a dose-dependent manner when the FAP combined chemotherapy plus various concentrations of CH was used, as shown in Table 1. This suggested that the MDR resistance was reversed by CH in vivo.
Table 1.
The Tumor inhibition of the Hca/FAP solid tumor after treatment.
| Treatment groups | Avg. tumor weights (mg) | %Tumor growth inhibition | Mortality |
|---|---|---|---|
| FAP+CH 10.0 mg/kg | 248.0 ± 88.0* | 71.4% | 0/10 |
| FAP+CH 5.0 mg/kg | 364.0 ± 82.2* | 57.9% | 0/10 |
| FAP+CH 2.5 mg/kg | 479.0 ± 96.8∗□ | 44.7% | 0/10 |
| FAP+VER 2.5 mg/kg | 482.8 ± 59.4∗□ | 44.2% | 2/10 |
| FAP | 479.0 ± 187.5* | 44.7% | 0/10 |
| CH 10.0 mg/kg | 689.0 ± 104.8* | 20.4% | 0/10 |
| Control | 866.0 ± 153.3 | — | 0/10 |
*P < .05, different from the control, □no significant difference from each other (P > .05).
4. Discussion
Hepatocellular carcinoma (HCC) represents the third leading cause of cancer-related death [24]. Chemotherapy consisting of FAP was commonly used to treat HCC in clinical situations [25]; however, HCC is often resistant to these drugs. Therefore, one of the key approaches is to overcome drug resistance in the success of chemotherapy against HCC. Previous studies of P-gp inhibitors such as VER, cyclosporine A, and its analog PSC833 [26–28] have been limited because of its serious toxicity at the time of dosage for reversing drug resistance.
An important step to develop the MDR reversors targeting P-gp is to determine the inhibition level of P-gp in the preclinical animal model. An ideal approach is to detect the real concentrations of the investigated drug directly in vivo, but it is almost impossible, even in the animal model. If there is no related data on the inhibition level of P-gp, it is difficult to interpret the efficacy of P-gp inhibitors in pre- or clinical trials. Recently, the functional study of P-gp was of great concern. Among lymphocytes in the mouse, CD8+ cells expressed the highest levels of P-gp followed by CD4+ > CD3+ [6, 21, 29–32]. Since blood samples are easy to be collected, we selected peripheral blood CD8+ cells as a surrogate indicator to investigate the alteration of P-gp activities before and after the administration of P-gp inhibitors and then evaluate the reversal effect of the drug in a solid-tumor-bearing mouse. Rho123 is a fluorescent substrate and has been shown to be a sensitive reagent for the detection of P-gp function by flow cytometry [14, 33]. Some authors reported that Rho123 was a substrate for MRP and P-gp [34], but Leite et al. [6] reported that classical MRP1 reversors were unable to enhance Rho123 accumulation in lymphocytes suggesting that MRP1 might not be active or Rho123 is not a good substrate for MRP1 expressed in these cells. Therefore, the alteration of Rho123 fluorescence intensities in these cells responded to the alteration of the P-gp function. In this study, we demonstrated that the mean fluorescence intensities of CD8+ cells were related to the dose of Rho123 in a dose-dependent manner suggesting that it is a good choice to use the accumulation of Rho123 in CD8+ cells as a surrogate indicator for the study of P-gp inhibitors.
We further studied the modulation effect of CH in vivo and ex vivo; the results showed that the mean fluorescence intensities of CD8+ cells were related to CH in a dose- or concentration-dependent manner either in vivo or ex vivo. This suggested that the activities of P-gp were inhibited by CH. In our previous study, it was shown that the enhancement of cytotoxicity of FAP against cultured Hca/FAP cells by CH occurs in vitro; the efflux rate of Rho123 in Hca/FAP cells was 2 times less than that of Hca cells after 1 hour explosure to CH plus FAP. We have determined the LD50 for i.v. mice is 0.47 times of clinical dosing of FAP protocol, that is, 85.0 mg/kg of 5-FU, 4.2 mg/kg of ADR, and 2.8 mg/kg of CDDP [20]. In the present study, we established an Hca/FAP solid tumor mouse model and assessed the antitumor effect of FAP combination protocol plus CH. The i.v. dosing for a mouse is 42.50 mg/kg of 5-Fu, 2.10 mg/kg of ADR, 1.40 mg/kg of CDDP. The results showed that a single i.v. dose of 10.0 mg/kg CH partly inhibited the tumor growth, but the effect was weak; this is consistent with conclusions drawn from other authors' reports [35]: a single i.v. dose of 5.0 mg/kg CH has no antitumor effect (data not shown). When FAP combined chemotherapy plus various concentrations of CH was used, the antitumor effect was increased in a dose-dependent manner. Therefore, with this correlation with the results of the inhibition of P-gp function by CH in CD8+ cells, we think that the antitumor activity recovered by CH in a dose-dependent manner was related to the inhibition of the P-gp function. All of the efficacious combination protocols with CH appeared to be well tolerated, as indicated by the minimal changes in body weights of the various treatment groups (as shown in Table 2). Since an LD50 of VER for the i.v. administration in mouse was reported to be 7.6 mg/kg [36], but a single i.v. dose of 5.0 mg/kg VER caused the death of 60% of animal in the treated BALB/c mice (data not shown), we selected the dose of 2.5 mg/kg as a positive control. There is no mortality in the FAP treated group; we think the 20% mortality in the FAP plus VER treated group was caused by the increasing toxicity when VER combined with ADR [37].
Table 2.
Body weights of the animal after treatment.
| Treatment groups | Body weights (g) | ||
|---|---|---|---|
| Day 1 | Day 4 | Day 8 | |
| FAP+CH 10.0 mg/kg | 25.9 ± 1.4 | 25.6 ± 1.3 | 25.1 ± 1.2 |
| FAP+CH 5.0 mg/kg | 25.2 ± 1.6 | 25.0 ± 1.6 | 24.7 ± 1.8 |
| FAP+CH 2.5 mg/kg | 25.0 ± 1.9 | 24.5 ± 1.9 | 23.8 ± 2.1 |
| FAP+VER 2.5 mg/kg | 25.1 ± 1.2 | 25.0 ± 1.1 | 24.3 ± 1.3 |
| FAP | 25.5 ± 1.8 | 24.6 ± 1.7 | 24.6 ± 1.5 |
| CH 10.0 mg/kg | 24.9 ± 1.3 | 25.0 ± 1.0 | 24.9 ± 0.9 |
| Control | 25.3 ± 1.2 | 25.8 ± 1.4 | 26.9 ± 1.9 |
Although one can argue that what occurs at the level of peripheral blood CD8+ cells may not accurately reflect the real inhibition level of P-gp in the solid tumor in mouse, 99mTc-sestamibi scans performed in some studies have shown effective P-gp inhibition in both normal tissues and solid tumors [9, 38], suggesting the inhibition level of P-gp was modulated simultaneously. We have to note that no model is perfect and there are obvious differences between humans and mice; the BALB/c mouse is unique for its features, making even more difficult extrapolations to human conditions.
With regard to the results obtained, we conclude that CH is a potent P-gp inhibitor for clinical application. Although limitations may exist in the surrogate assay in CD8+ cells in the mouse model, we are convinced that this assay can provide us with much useful information in screening potential P-gp inhibitors in vivo.
Acknowledgment
This work was supported by grants from social welfare projects for scientific research of Henan Province, China.
References
- 1.Pérez-Tomás R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Current Medicinal Chemistry. 2006;13(16):1859–1876. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 2.Dong X, Mumper RJ. Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine. 2010;5(4):597–615. doi: 10.2217/nnm.10.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu AX. Systemic treatment of hepatocellular carcinoma: dawn of a new era. Annals of Surgical Oncology. 2010;17(5):1247–1256. doi: 10.1245/s10434-010-0975-6. [DOI] [PubMed] [Google Scholar]
- 4.van Waterschoot RA, Lagas JS, Wagenaar E, et al. Absence of both cytochrome P450 3A and P-glycoprotein dramatically increases docetaxel oral bioavailability and risk of intestinal toxicity. Cancer Research. 2009;69(23):8996–9002. doi: 10.1158/0008-5472.CAN-09-2915. [DOI] [PubMed] [Google Scholar]
- 5.Egashira M, Kawamata N, Sugimoto K, Kaneko T, Oshimi K. P-glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of P-glycoprotein function by cyclosporin A and its analogue, PSC833. Blood. 1999;93(2):599–606. [PubMed] [Google Scholar]
- 6.Leite DF, Echevarria-Lima J, Salgado LT, Capella MA, Calixto JB, Rumjanek VM. In vivo and in vitro modulation of MDR molecules in murine thymocytes. International Immunopharmacology. 2006;6(2):204–215. doi: 10.1016/j.intimp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Green LJ, Marder P, Slapak CA. Modulation by LY335979 of P-glycoprotein function in multidrug-resistant cell lines and human natural killer cells. Biochemical Pharmacology. 2001;61(11):1393–1399. doi: 10.1016/s0006-2952(01)00599-8. [DOI] [PubMed] [Google Scholar]
- 8.McCluggage WG, McKenna M, McBride HA. CD56 is a sensitive and diagnostically useful immunohistochemical marker of ovarian sex cord-stromal tumors. International Journal of Gynecological Pathology. 2007;26(3):322–327. doi: 10.1097/01.pgp.0000236947.59463.87. [DOI] [PubMed] [Google Scholar]
- 9.Abraham J, Edgerly M, Wilson R, et al. A phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbine. Clinical Cancer Research. 2009;15(10):3574–3582. doi: 10.1158/1078-0432.CCR-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morschhauser F, Zinzani PL, Burgess M, Sloots L, Bouafia F, Dumontet C. Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leukemia and Lymphoma. 2007;48(4):708–715. doi: 10.1080/10428190701190169. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunological Reviews. 2006;214(1):47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 12.Deferme S, Van Gelder J, Augustijns P. Inhibitory effect of fruit extracts of P-glycoprotein-related efflux carriers: an in-vitro screening. Journal of Pharmacy and Pharmacology. 2002;54(9):1213–1219. doi: 10.1211/002235702320402053. [DOI] [PubMed] [Google Scholar]
- 13.Romiti N, Pellati F, Nieri P, Benvenuti S, Adinolfi B, Chieli E. P-glycoprotein inhibitory activity of lipophilic constituents of Echinacea pallida roots in a human proximal tubular cell line. Planta Medica. 2008;74(3):264–266. doi: 10.1055/s-2008-1034308. [DOI] [PubMed] [Google Scholar]
- 14.Gyémánt N, Engi H, Schelz Z, et al. In vitro and in vivo multidrug resistance reversal activity by a Betti-base derivative of tylosin. British Journal of Cancer. 2010;103(2):178–185. doi: 10.1038/sj.bjc.6605716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemoto K, Yoshida K, Nisimura M, Seki M. The effects of Cepharanthin on the recovery of hematopoietic stem cells after X-ray irradiation. Gan To Kagaku Ryoho. 1991;18(1):81–84. [PubMed] [Google Scholar]
- 16.Furusawa S, Wu J. The effects of biscoclaurine alkaloid cepharanthine on mammalian cells: implications for cancer, shock, and inflammatory diseases. Life Sciences. 2007;80(12):1073–1079. doi: 10.1016/j.lfs.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Igari R, Iseki K, Abe S, et al. Binocular diplopia and ptosis due to snakebite (Agkistrodon blomhoffi “mamushi”)—a case report. Brain and Nerve. 2010;62(3):273–277. [PubMed] [Google Scholar]
- 18.Enokida H, Gotanda T, Oku S, et al. Reversal of P-glycoprotein-mediated paclitaxel resistance by new synthetic isoprenoids in human bladder cancer cell line. Japanese Journal of Cancer Research. 2002;93(9):1037–1046. doi: 10.1111/j.1349-7006.2002.tb02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song YC, Xia W, Jiang JH, Wang QD. Reversal of multidrug resistance in drug-resistant cell line EAC/ADR by cepharanthine hydrochloride and its mechanism. Yaoxue Xue Xue Bao. 2005;40(3):204–207. [PubMed] [Google Scholar]
- 20.Xin W, Yan Z, Ning W, et al. Establishment of Hca multidrug resistance mouse model. Journal of ZhengZhou University (Medical Science) 2010;45(6):920–923. [Google Scholar]
- 21.Marques-Santos LF, Harab RC, de Paula EF, Rumjanek VM. The in vivo effect of the administration of resistance-modulating agents on rhodamine 123 distribution in mice thymus and lymph nodes. Cancer Letters. 1999;137(1):99–106. doi: 10.1016/s0304-3835(98)00348-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim YI, Shin MK, Lee JW, Chung JH, Lee MH. Decreased expression of KAI1/CD82 metastasis suppressor gene is associated with loss of heterozygosity in melanoma cell lines. Oncology Reports. 2009;21(1):159–164. [PubMed] [Google Scholar]
- 23.Jaganathan SK, Mondhe D, Wani ZA, Pal HC, Mandal M. Effect of honey and eugenol on ehrlich ascites and solid carcinoma. Journal of Biomedicine and Biotechnology. 2010;2010 doi: 10.1155/2010/989163. Article ID 989163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wörns MA, Galle PR. Future perspectives in hepatocellular carcinoma. Digestive and Liver Disease. 2010;42(supplement 3):S302–S309. doi: 10.1016/S1590-8658(10)60521-X. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi N, Yano M, Takachi K, et al. Myelotoxicity of preoperative chemoradiotherapy is a significant determinant of poor prognosis in patients with T4 esophageal cancer. Journal of Surgical Oncology. 2009;99(5):302–306. doi: 10.1002/jso.21235. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Chung JB, Park IS, et al. Combined use of tamoxifen, cyclosporin A, and verapamil for modulating multidrug resistance in human hepatocellular carcinoma cell lines. Yonsei Medical Journal. 1993;34(1):35–44. doi: 10.3349/ymj.1993.34.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Shiraga K, Sakaguchi K, Senoh T, et al. Modulation of doxorubicin sensitivity by cyclosporine A in hepatocellular carcinoma cells and their doxorubicin-resistant sublines. Journal of Gastroenterology and Hepatology. 2001;16(4):460–466. doi: 10.1046/j.1440-1746.2001.02457.x. [DOI] [PubMed] [Google Scholar]
- 28.Baer MR, George SL, Dodge RK, et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: cancer and leukemia group B study 9720. Blood. 2002;100(4):1224–1232. [PubMed] [Google Scholar]
- 29.Kyle-Cezar F, Echevarria-Lima J, Rumjanek VM. Independent regulation of ABCB1 and ABCC activities in thymocytes and bone marrow mononuclear cells during aging. Scandinavian Journal of Immunology. 2007;66(2-3):238–248. doi: 10.1111/j.1365-3083.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 30.Valente RC, Capella LS, Nascimento CR, et al. ABCB1 (P-glycoprotein) but not ABCC1 (MRP1) is downregulated in peripheral blood mononuclear cells of spontaneously hypertensive rats. Pflugers Archiv European Journal of Physiology. 2008;456(2):359–368. doi: 10.1007/s00424-007-0397-x. [DOI] [PubMed] [Google Scholar]
- 31.Sada-Ovalle I, Torre-Bouscoulet L, Valdez-VÁzquez R, Lascurain R. In vitro cytotoxicity of CD8+ T cells in multi-drug-resistant tuberculosis. A preliminary report. Respirology. 2009;14(4):574–578. doi: 10.1111/j.1440-1843.2008.01478.x. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Masuda M, Nakajima K, et al. P-glycoprotein function in peripheral T lymphocyte subsets of myasthenia gravis patients: clinical implications and influence of glucocorticoid administration. International Immunopharmacology. 2009;9(3):284–290. doi: 10.1016/j.intimp.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Liang G, Tang A, Lin X, et al. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. International Journal of Oncology. 2010;37(1):111–123. [PubMed] [Google Scholar]
- 34.Daoud R, Kast C, Gros P, Georges E. Rhodamine 123 binds to multiple sites in the multidrug resistance protein (MRP1) Biochemistry. 2000;39(50):15344–15352. doi: 10.1021/bi0020574. [DOI] [PubMed] [Google Scholar]
- 35.Asaumi J, Nishikawa K, Matsuoka H, et al. Direct antitumor effect of cepharanthin and combined effect with adriamycin against Ehrlich ascites tumor in mice. Anticancer Research. 1995;15(1):67–70. [PubMed] [Google Scholar]
- 36.O'Neil MJ. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Whitehouse Station, NJ, USA: Merck and Co; 2006. p. 1772. [Google Scholar]
- 37.Candussio L, Decorti G, Crivellato E, et al. Toxicologic and pharmacokinetic study of low doses of verapamil combined with doxorubicin. Life Sciences. 2002;71(26):3109–3119. doi: 10.1016/s0024-3205(02)02175-6. [DOI] [PubMed] [Google Scholar]
- 38.Bates SE, Bakke S, Kang M, et al. A phase I/II study of infusional vinblastine with the P-glycoprotein antagonist valspodar (PSC 833) in renal cell carcinoma. Clinical Cancer Research. 2004;10(14):4724–4733. doi: 10.1158/1078-0432.CCR-0829-03. [DOI] [PubMed] [Google Scholar]


