Abstract
Andrographolide (AND), the diterpene lactone compound, was purified by HPLC from the methanolic fraction of the plant Andrographis paniculata. The compound was found to have potent antiplasmodial activity when tested in isolation and in combination with curcumin and artesunate against the erythrocytic stages of Plasmodium falciparum in vitro and Plasmodium berghei ANKA in vivo. IC50s for artesunate (AS), andrographolide (AND), and curcumin (CUR) were found to be 0.05, 9.1 and 17.4 μM, respectively. The compound (AND) was found synergistic with curcumin (CUR) and addictively interactive with artesunate (AS). In vivo, andrographolide-curcumin exhibited better antimalarial activity, not only by reducing parasitemia (29%), compared to the control (81%), but also by extending the life span by 2-3 folds. Being nontoxic to the in vivo system this agent can be used as template molecule for designing new derivatives with improved antimalarial properties.
1. Introduction
The discoveries leading to information on novel drugs and drug combinations have become a priority to manage the increasing burden of malaria, caused by drug resistant parasites. Artemisinin derivative-based drug combinations that have independent mode of action are seen as a way of enhancing efficacy and simultaneously ensuring protection against resistance [1, 2] among the parasites. The anti-malarial properties of a tropical plant Andrographis paniculata has been studied in details recently by Mishra et al. [3], and also, there are several reports that demonstrate the antimalarial properties of the plant A. paniculata [4–6], but there is hardly any report that describe the active compound responsible for the anti-malarial property of this plant. Andrographolide, the diterpenic lactone compound, is one of the major phytoconstituents of the plant A. paniculata and has been reported to have diverse pharmacological potential including antiviral [7], anti-inflammatory [8–11], and anticancer properties [12]. The present study for the first time establishes andrographolide as the major bioactive anti-malarial constituent of the plant A. paniculata using both in vitro and in vivo approaches. The encouraging in vitro antiplasmodial activities of andrographolide prompted us to evaluate the interaction of andrographolide (AND) with other established anti-malarial compounds such as curcumin (CUR) and artesunate (AS), the hemisuccinate derivative of artemisinin in vitro on Plasmodium falciparum, and in vivo on Plasmodium berghei ANKA strains. This adds new information on combination of potent anti-malarial compounds for novel combinatorial drug therapy against drug-resistant malaria.
2. Methods
2.1. Compounds
Pure grade andrographolide, (Sigma-Aldrich, USA; Cat no. 365645), artesunate (Sigma, USA; Cat no. 3731-100MG) and curcumin (98% Curcuminoid content, Sigma, USA; Cat no. 7727) were purchased commercially.
2.2. Parasite Strain and In Vitro Culture
Chloroquine-resistant strain of Plasmodium falciparum (MRC-pf-303) was obtained from the Malaria Parasite bank, maintained by the National Institute of Malaria Research, New Delhi. Parasites were cultured in human O+ washed erythrocytes using standard protocols [13]. The parasite cultures were synchronized by treatment with 5% D-Sorbitol [14]. A tightly synchronized culture was obtained by two sorbitol treatments that were carried out 12 h apart. The residual schizonts were eliminated by resynchronization, 48 h after the initial synchronization.
2.3. Preparation of Bark Extract
The aerial parts of the plant A. paniculata were crushed in liquid N2 to fine powder. Crude extracts were prepared as per the method reported earlier [3]. Usually, a 0.4% yield of crude extract was obtained invariably from each extraction.
2.4. Isolation of Andrographolide from the Bark Extract
Filter-sterilized crude herbal extracts of A. paniculata were separated by HPLC, using C18 column (Agilent Technologies, 1200 series) in UV range (254 nm) and at 25°C temperatures. Fixed ratio of methanol : H2O (1 : 1) was used as the mobile phase to sieve the phytoconstituents present in the extracts at a flow rate of 1 mL/min as described by Mishra et al. [15]. Different phytoconstituents that were eluted under different peaks a, b, c, d, and e (Figure 1(a)), were collected and pooled. The solvent part (methanol) was evaporated to dryness at 37°C by the help of a Speed Vac (Model 7811001, Labconco, USA), and the compounds were assayed individually on Plasmodium falciparum (MRC-pf-303) for their in vitro antiplasmodial activity.
Figure 1.
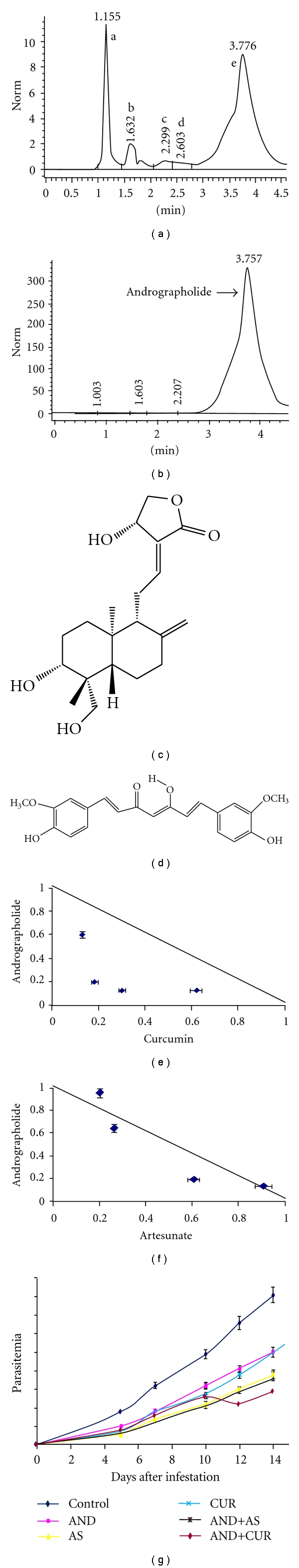
(a) Chromatogram showing purification of andrographolide (AND, retention time, 3.776) from crude bark extracts of Andrographis paniculata. (b) Chromatogram showing the peaks for andrographolide (retention time, 3.757) in standard andrographolide. (c) Structure of andrographolide. (d) Structure of curcumin, (e) and (f) Representative isobolograms of the interactions between andrographolide with curcumin (e) and andrographolide with artesunate (f) against chloroquine-resistant strains (MRC-pf-303) of P. falciparum. The values in X- and Y-axes of the isobolograms represent the FIC50 values of andrographolide-curcumin and andrographolide-artesunate, respectively, at different concentration ratios (1 : 5, 1 : 2, 2 : 1, and 5 : 1). (g) In vivo efficacy of andrographolide (AND), curcumin (CUR) and artesunate (AS), tested in isolation and combination in Plasmodium berghei ANKA-infected mice.
2.5. In Vitro Antiplasmodial Activity and Drug-Interaction Tests
Dose-response assays and in vitro drug-interaction studies were carried out to obtain the 50% inhibitory concentrations (IC50) and 50% fraction inhibitory concentrations (FIC50) of these bioactive compounds, AND, CUR, and AS, respectively, as described earlier [3, 15]. Corresponding isobolograms (Figures 1(d) and 1(e)) were constructed using the method reported earlier [3, 16].
2.6. In Vivo Toxicity Study
In vivo toxicity study was carried out using Balb/C mice (20 ± 3 g, n = 4). Animals were injected intraperitoneally with the test compounds affording doses of 50 mg/kg of body weight with one group being given DMSO (DMSO control) in the same concentration along with serum-free medium, while the other group of control was continued without any solvent treatment. Mice were observed for 96 h to record postinjection discomfort, if any. At the end of 4th day, the gross body weight was recorded. Animals were bled through heart puncture, and the blood, and serum were subjected for hematological and physiological analyses.
2.7. In Vivo Assay of Test Compounds on P. berghei ANKA Strain
In vivo anti-malarial activity was carried out using 5 groups of mice (Balb/C, 28 g ± 4 g, n = 4). Mice were injected intraperitoneally with P. berghei ANKA-infected mouse blood (approximately 40% parasitemia) on day 1, at a density of 1 × 107 parasites (approximately) of the strain. The test compounds (AND, AS, CUR, AND+AS, and AND+CUR) were dissolved in DMSO and serum-free media to make the desired concentrations and were injected (15 mg/kg of body weight) intraperitoneally to individual groups of animals starting from 24 h prior to the parasite challenge (day 0). The control mice were maintained simultaneously without any treatment. Injections were continued daily till the death of the animals in the control group (13–15 days). Parasitemia levels (parasitized erythrocytes/total erythrocytes) were determined with thin blood smears on every alternate day from day 5 onwards of posttreatment (Figure 1(f)). The animal experiments complied with all relevant guidelines and institutional policies on animal ethics and were conducted under environmentally controlled condition, as reported earlier [3].
3. Results
3.1. Purification and Identification of Andrographolide
The phytoconstituents eluted under different peaks were assayed individually in vitro on Plasmodium falciparum. The particular phytoconstituent eluted under peak “e” (retention time 3.78) showed potent anti-malarial activity compared to the compounds eluted under peaks a, b, c, and d (Figure 1(a)). The compound of peak “e” was later identified as andrographolide by comparing the retention time of pure andrographolide (Sigma, Cat no. 365645) which was run as positive control (Figure 1(b)) and finally by using standard methods of compound identification [15, 17, 18]. Approximately, 50 cycles of HPLC purification were carried out to obtain a sufficient amount of test compound, andrographolide (Figure 1(c)) for their in vitro anti-plasmodial assays.
3.2. IC50 Values of AS, AND, and CUR
IC50s for artesunate (AS), andrographolide (AND), and curcumin (CUR) were found to be 0.05, 9.1, and 17.4 μM, respectively. It was noted that the compounds are particularly inhibiting the parasites at their ring stage.
3.3. In Vitro Drug Interaction
The fraction IC50s (FIC50) of the combinations made out of the three compounds was found much below the level of their individual IC50s, as expected. The sums of the FIC values (∑ FIC) are an important parameter that detects and differentiates the classification of the drug interaction. When the sum of putative drug fractions is 1, the combination is said to be additive; when the sum is <1, the combination is synergistic; and when the sum is >1, the combination is antagonistic [19]. Curcumin (Figure 1(d)) was found to be a better combination partner with andrographolide as compared to artesunate with sum FIC50s in the former combination (AND+CUR) remaining within 1 (0.73, 0.38, 0.43, and 0.75) in all 4 ratios (1 : 5, 1 : 2, 2 : 1, and 5 : 1), respectively. This combination (AND+CUR) at lower ratios (1 : 2 and 2 : 1) yielded synergism, while higher ratios (1 : 5 and 5 : 1) exhibited additiveness as shown by the isobologram (Figure 1(e)). Alternatively, AND+AS was found effective only at lower ratios (1 : 2 and 2 : 1) with proven additivity, (sum FIC50s being 0.9 and 0.8), respectively. This combination (AND+AS) exhibited indifference with sum FIC50s exceeding 1 (1.15 and 1.04) at higher ratios (1 : 5 and 5 : 1), respectively (Figure 1(f)).
3.4. In Vivo Antimalarial Activity of Test Compounds without Any Toxicity
The results indicated that andrographolide performed better anti-malarial activity when combined with curcumin than artesunate. All the mice died after attaining the parasitemia level 80% ± 4%. Control group, which died between 13 to 15 days (maximum deaths being on 14th day), were found to harbor 81% ± 2% parasitemia compared to the experimental groups treated with AND, AS, CUR, AND+AS, and AND+CUR, carrying load of parasitemia, 46%, 33%, 46%, 36%, and 29%, respectively (Figure 1(f)). All the treatment groups were kept under observation, and the % parasitemia were monitored till the 30th day (data not shown).
Because of the interesting in vitro anti-malarial properties, in vivo toxicity tests were carried out with mice by intraperitoneal routes during four consecutive days. No mortality or any other abnormality was observed during the treatment period. Treated mice were clinically healthy, and no toxic effects and weight loss were observed with the treatment groups even after 96 hrs. The levels of SGOT (serum glutamic oxaloacetic transaminase) and SGPT (serum glutamic pyruvate transaminase) and the blood parameters of the experimental and control mice were found within the normal range without any significant variation (SGOT, 60.3 ± 3.1; SGPT, 74 ± 4.4 U/mL; urea, 19.6 ±2.1 mg/dL; glucose, 90 ± 3.2 mg/dL; creatinine, 0.77 ± 0.16 mg/dL).
4. Discussion
Andrographolide is a labdane diterpene reported to have potent anti- inflammatory, anticancer and anti-viral activity [9]. As such, the use of antibacterials and antivirals in malaria prophylaxis is also well documented [20]. Identification of anti-plasmodial activity of this compound in view of its efficacious in vitro and in vivo performance opens up several opportunities for further exploration of this compound as a new anti-malarial.
During the erythrocytic life cycle, the period of activity of andrographolide was found evidently on the ring stage of the parasite. The point of action of this compound in the parasite life cycle corresponds with the protein and nucleic acid synthesis. Hence, they could be the “transcription blockers”; more studies focusing on this particular aspect are in progress.
The pharmacological targets of this compound and its mechanism of action on P. falciparum are not known. However, the clue centers on the phenomenon of regulation of a transcription factor. As per Hidalgo et al. [9], the anti-inflammatory action of andrographolide includes inhibition of the nuclear transcription factor-(kappa) B, making it a therapeutic target for the treatment of cancer and autoimmune diseases. The role of NF-kappaB (NF-κB) is also important in malaria as mentioned in a recent study [21]; P. falciparum infected erythrocytes have shown to induce NF-(kappa) B-regulated inflammatory pathways in human cerebral endothelium. The deciphering of anti-malarial activity in andrographolide against the blood stage of the plasmodial life cycle lays foundation to reevaluate its possible role in the regulation of this important transcription factor for the effective control of malaria.
There are several reports on various drug combinations showing their in vitro and in vivo interactions on malaria parasites [16, 22, 23]. The synergy between andrographolide and curcumin is interesting in the fight against the emergence of drug resistance among parasites. Both curcumin and andrographolides being from the plant sources are already documented for their antioxidant [24] and anti-inflammatory properties [25], respectively. This information also generates scope to explore the possibility of modifying the molecular structures of both the plant-derived compounds such as andrographolide and curcumin with the objective of increasing their specific activity against plasmodial species. The semisynthetic modification of artemisinin to artesunate, artemether, and DHA has already yielded compounds with significantly higher activity. The other finding that deciphers the additiveness of andrographolide with artesunate, the most potent artemisinin derivative makes it important in the context that it offers opportunities to further standardize new ACT- (artemisinin-based combination therapies-) based formulae as possible antimalarial combination.
Andrographolide exhibited high level of activity in murine model when administered either with curcumin or artesunate by the intraperitoneal route, without any toxicity. The substantial decrease in level of parasitemia in response to test drug combinations compared to the control and extended periods of life, observed with the mice is the main finding of the present study. This may be attributed to the observed in vitro synergism or addictiveness between the putative drug compounds in different combination ratios. However, studies are in progress for effective dose determination for complete clearance of parasitemia from infected mice using AND+CUR and AND+AS combinations.
Conclusion
Although andrographolide keeps the potency of a suitable lead candidate against malaria, further optimizations are needed for its inclusion in the future prophylaxis as a therapeutic agent. Relatively rapid development of resistance to most of the drugs arises in response to drug pressure [26]. It can also happen in the recent future to the ACT, which is now being promoted as a gold standard for malaria therapy by World Health Organization. The synergistic activity of andrographolide with curcumin could address this issue and generate optimism for further exploration of a new anti-malarial formula by modifying the molecular structures in various possible ways. Of course, equally important will be the investigative line which would study the bioavailability of the said compounds in in vivo system.
Conflict of Interests
The authors do not have any association, relationship, or affiliation that would generate a conflict of interests in future.
Authors' Contributions
K. Mishra has participated in the isolation and purification of the plant-derived compounds and assaying of the compounds in vitro on Plasmodium falciparum and in vivo on Plasmodium berghei ANKA. Both K. Mishra and N. Dey have drafted the paper and revised it. N. Dey and A. P. Dash have coordinated the study and contributed towards the critical revision of the paper. All the authors have read and approved the paper.
Acknowledgments
The authors are grateful to the Director of Institute of Life Sciences for the facilities provided during the work. They thank Dr. Fahima Dilnawaz for her cooperation in andrographolide purification through HPLC. They thankfully acknowledge the contribution of National Institute of Malaria Research, Delhi, particularly for the parasite strains. They thank Red Cross Blood Bank, MKCG Medical College, Berhampur for helping them in procuring human blood serum for the parasite culture work. The DST project to K. Mishra (SR/FT-L-145/2005) and Institution (ILS) core fund to N. Dey for this study are gratefully acknowledged.
References
- 1.WHO facts on ACTs (Artemisinin-Based Combination Therapies) . http://www.rollbackmalaria.org/cmc_upload/0/000/015/364/RBMInfosheet_9.htm.
- 2.Olliaro PL, Taylor WRJ. Antimalarial compounds: from bench to bedside. Journal of Experimental Biology. 2003;206(21):3753–3759. doi: 10.1242/jeb.00653. [DOI] [PubMed] [Google Scholar]
- 3.Mishra K, Dash AP, Swain BK, Dey N. Anti-malarial activities of Andrographis paniculata and Hedyotis corymbosa extracts and their combination with curcumin. Malaria Journal. 2009;8(1, article no. 26) doi: 10.1186/1475-2875-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman NNNA, Furuta T, Kojima S, Takane K, Ali Mohd M. Antimalarial activity of extracts of Malaysian medicinal plants. Journal of Ethnopharmacology. 1999;64(3):249–254. doi: 10.1016/s0378-8741(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 5.Siti Najila MJ, Noor Rain A, Mohamad Kamel AG, et al. The screening of extracts from Goniothalamus scortechinii, Aralidium pinnatifidum and Andrographis paniculata for anti-malarial activity using the lactate dehydrogenase assay. Journal of Ethnopharmacology. 2002;82(2-3):239–242. doi: 10.1016/s0378-8741(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 6.Dua VK, Ojha VP, Roy R, et al. Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata . Journal of Ethnopharmacology. 2004;95(2-3):247–251. doi: 10.1016/j.jep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Coon JT, Ernst E. Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy. Planta Medica. 2004;70(4):293–298. doi: 10.1055/s-2004-818938. [DOI] [PubMed] [Google Scholar]
- 8.Habtemariam S. Andrographolide inhibits the tumour necrosis factor-α-induced upregulation of ICAM-1 expression and endothelial-monocyte adhesion. Phytotherapy Research. 1998;12(1):37–40. [Google Scholar]
- 9.Hidalgo MA, Romero A, Figueroa J, et al. Andrographolide interferes with binding of nuclear factor-κB to DNA in HL-60-derived neutrophilic cells. British Journal of Pharmacology. 2005;144(5):680–686. doi: 10.1038/sj.bjp.0706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pekthong D, Martin H, Abadie C, et al. Differential inhibition of rat and human hepatic cytochrome P450 by Andrographis paniculata extract and andrographolide. Journal of Ethnopharmacology. 2007;115(3):432–440. doi: 10.1016/j.jep.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian R, Asmawi MZ, Sadikun A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochimica Polonica. 2008;55(2):391–398. [PubMed] [Google Scholar]
- 12.Ying H, Bu LIM, Ji X, Liu CY, Wang ZH. Modulation of multidrug resistance by andrographolid in a HCT-8/5-FU multidrug-resistant colorectal cancer cell line. Chinese Journal of Digestive Diseases. 2005;6(2):82–86. doi: 10.1111/j.1443-9573.2005.00197.x. [DOI] [PubMed] [Google Scholar]
- 13.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 14.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. Journal of Parasitology. 1979;65(3):418–420. [PubMed] [Google Scholar]
- 15.Mishra K, Chakraborty D, Pal A, Dey N. Plasmodium falciparum: in vitro interaction of quassin and neo-quassin with artesunate, a hemisuccinate derivative of artemisinin. Experimental Parasitology. 2010;124(4):421–427. doi: 10.1016/j.exppara.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Wiesner J, Henschker D, Hutchinson DB, Beck E, Jomaa H. In vitro and in vivo synergy of fosmidomycin, a novel antimalarial drug, with clindamycin. Antimicrobial Agents and Chemotherapy. 2002;46(9):2889–2894. doi: 10.1128/AAC.46.9.2889-2894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonfill M, Mangas S, Cusidó RM, Osuna L, Piñol MT, Palazón J. Identification of triterpenoid compounds of Centella asiatica by thin-layer chromatography and mass spectrometry. Biomedical Chromatography. 2006;20(2):151–153. doi: 10.1002/bmc.564. [DOI] [PubMed] [Google Scholar]
- 18.Mandal SM, Dey S. LC-MALDI-TOF MS-based rapid identification of phenolic acids. Journal of Biomolecular Techniques. 2008;19(2):116–121. [PMC free article] [PubMed] [Google Scholar]
- 19.Berenbaum MC. A method for testing for synergy with any number of agents. Journal of Infectious Diseases. 1978;137(2):122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 20.Goodman CD, Su V, McFadden GI. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum . Molecular and Biochemical Parasitology. 2007;152(2):181–191. doi: 10.1016/j.molbiopara.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ., Jr. Plasmodium falciparum-infected erythrocytes induce NF-κB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114(19):4243–4252. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nandakumar DN, Nagaraj VA, Vathsala PG, Rangarajan P, Padmanaban G. Curcumin-artemisinin combination therapy for malaria. Antimicrobial Agents and Chemotherapy. 2006;50(5):1859–1860. doi: 10.1128/AAC.50.5.1859-1860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fivelman QL, Adagu IS, Warhurst DC. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum . Antimicrobial Agents and Chemotherapy. 2004;48(11):4097–4102. doi: 10.1128/AAC.48.11.4097-4102.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui L, Miao J, Cui L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrobial Agents and Chemotherapy. 2007;51(2):488–494. doi: 10.1128/AAC.01238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiou WF, Chen CF, Lin JJ. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. British Journal of Pharmacology. 2000;129(8):1553–1560. doi: 10.1038/sj.bjp.0703191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carraz M, Jossang A, Franetich JF, et al. A plant-derived morphinan as a novel lead compound active against malaria liver stages. PLoS Medicine. 2006;3(12):2392–2402. doi: 10.1371/journal.pmed.0030513. [DOI] [PMC free article] [PubMed] [Google Scholar]


