Abstract
Airway protection is a critically important function that prevents/limits the intrusion of foreign material into the pulmonary tree. A host of different behaviors participate in this process. The control, coordination, and execution of these behaviors is a complex process which has recently received increased attention. Data from human clinical and animal studies support the concept of a coordinated neural control system that governs the appropriate expression and sequencing of airway protective behaviors. Our current knowledge of the proposed neural control network for breathing, cough, swallow and other airway protective behaviors indicates that it is a highly complex system that can “rewire” (reconfigure) itself to perform several different functions. Computational modeling and simulation have been used as tools to investigate this system. The results of modeling efforts have yielded motor output patterns of upper airway and respiratory muscles that are very similar to those recorded in vivo. Regulation and coordination of multiple different airway protective behaviors have been successfully simulated. Outcomes of simulation efforts support the hypothesis that computational modeling of airway protection can yield important testable hypotheses regarding brainstem neural network functions and organization. Modeling of complex systems can be challenging but the open availability of straight-forward computational tools is likely to result in increased implementation of modeling and simulation as adjuncts to traditional methods of investigation of the control of the upper airway.
Keywords: cough, breathing, swallow, computational modeling, airway
Airway protection is the prevention of aspiration and/or reflex clearance of foreign material or mucus from the airways. A host of different reflexive behaviors can be participants in this process including cough, the pharyngeal phase of swallow, expiration reflex, laryngeal adductor reflex, and apnea. Table 1 illustrates a simple classification scheme for these behaviors based on their function in prevention of aspiration or airway clearance.
Table 1.
Functional Classification Scheme for Airway Protective Behaviors
| Aspiration Prevention |
| Swallow |
| Laryngeal adductor reflex |
| Apnea |
| Airway Clearance |
| Cough |
| Expiration Reflex |
Aspiration of saliva or a bolus entering the mouth is prevented by the pharyngeal phase of swallow, which adducts the vocal folds, changes the breathing pattern, and protects the laryngeal orifice by appropriate movement of the epiglottis as the material passes through the pharynx (Jean 2001). Another behavior, the expiration reflex, prevents aspiration by adducting the vocal folds and producing a ballistic-like expiratory airflow to “blow” adherent material from the airway. Other behaviors, such as laryngeal adduction and apnea, also participate in the prevention of aspiration by adduction of the vocal folds and cessation of inspiratory airflow, respectively. If aspiration or mucus accumulation occurs, cough can produce high velocity airflows that generate shear forces to dislodge and eject material from the airway (Leith et al. 1986).
Disorders such as Parkinson’s disease, stroke, and multiple sclerosis which affect excitability of these behaviors are typically associated with significant morbidity and/or mortality (Brookmeyer et al. 1998; Fernandez and Lapane 2002; Stansbury et al. 2005; Freedman 2011). Overexpression of behaviors such as cough can lead to significant declines in quality of life, pneumothorax, syncope, and fractured ribs (Braman and Corrao 1987; McCool 2006). Conversely, under-expression or impairment of these behaviors can contribute to increased risk of aspiration and in some neurologic disorders, profound increases in mortality (Gorell et al. 1994; Wada et al. 2000; Schiermeier et al. 2001; Fernandez and Lapane 2002; Smith Hammond et al. 2009; Pitts et al. 2010).
Each of these airway protective behaviors is produced and regulated by a complex neurogenic system. This system incorporates a diverse array of mechano- and chemo-sensitive afferents located in the pharynx, larynx, and lower airways; a complex set of musculature that also participates in breathing; and multiple distributed networks of brainstem neurons that are organized into cooperative populations whose functions are incompletely understood (Bolser 2009). The influence of neural (central) and/or airway (peripheral) pathology on the function of this complex system also is not well understood.
The functions of complex biological systems can be evaluated by the study of different component elements. These elements can be classified by scale. For example, the functions of individual cells are determined by subcellular processes and metabolic networks. Tissue function is determined by the collective function of its component cell types. Elements across scales can interact in ways that are not easily predictable by investigation of a single level (emergent properties). We have proposed that there is a specific neural control system for coordinating airway protective behaviors and that this system is organized in a holarchical manner (Bolser et al. 2006). The term holarchical means that the system is organized into functional control elements that exhibit emergent properties. One of the proposed emergent properties of this system for airway protection is the concept of behavior selection. Behavior selection describes the process by which the most appropriate airway protective behavior is produced at any moment in time. In effect, the concept of behavior selection describes a decision-making process that integrates moment-by-moment sensory information and the state of central control circuits to generate motor tasks that ensure that the airway is protected from aspiration. This process may result in one or more different behaviors (and different central control networks) being engaged in rapid sequence. This rapid sequencing (or switching from one behavior to another) of different behaviors appears to occur within time frames on the order of 100 ms or less. These time frames fall well within multi-synaptic processing and axonal conduction times associated with the capabilities of known brainstem neural networks that participate in the control of respiratory muscles.
Brainstem functional control elements for airway protection can be termed behavioral control assemblies (BCAs). BCAs are populations of neurons that cooperate to produce a given function. Implicit in the definition is the concept that there may be considerable heterogeneity among the neurons that make up a BCA. For example, this heterogeneity may include (but is not limited to) different activity patterns of these neurons, multiple neurotransmitter phenotypes, and/or varying responsiveness to sensory afferent stimulation. Each of these individual properties of neurons that make up a BCA represents a synthesis of different scales such as molecular, subcellular, cellular, and even tissue properties. This heterogeneity within and across multiple scales can make it very difficult to understand the overall function of a BCA, or even to identify its component elements (individual neurons). As the complexity of multi-scaled systems is revealed through investigation, tools used to further understanding of these interactions must also evolve.
Figure 1 illustrates a brainstem network that is based on a well-accepted representation of the population of neurons that controls breathing, coughing, and swallow. This network model is based on the results of many different types of studies ranging from in vitro investigations of cellular properties to in vivo neuron population dynamics (Rybak et al. 2008). The most basic function of the model is the production of the breathing pattern. Manipulation of selected model parameters allows simulation of the respiratory response to a variety of simulated peripheral stimuli including hypercapnia, increased blood pressure, and hypoxia. The model has been expanded over the last few years to include other respiratory behaviors such as cough and swallow. These behaviors can be simulated in isolation, and we recently have explored modeling these behaviors in sequence to explore their coordination. The model has a number of underlying variables that include activity patterns of component neurons that are based on restricted membrane potential ranges, a range of several biophysical properties of neurons, selectable synaptic strengths between neurons, and controllable numbers of axons between populations to name a few (Rybak et al. 2008).
Figure 1.
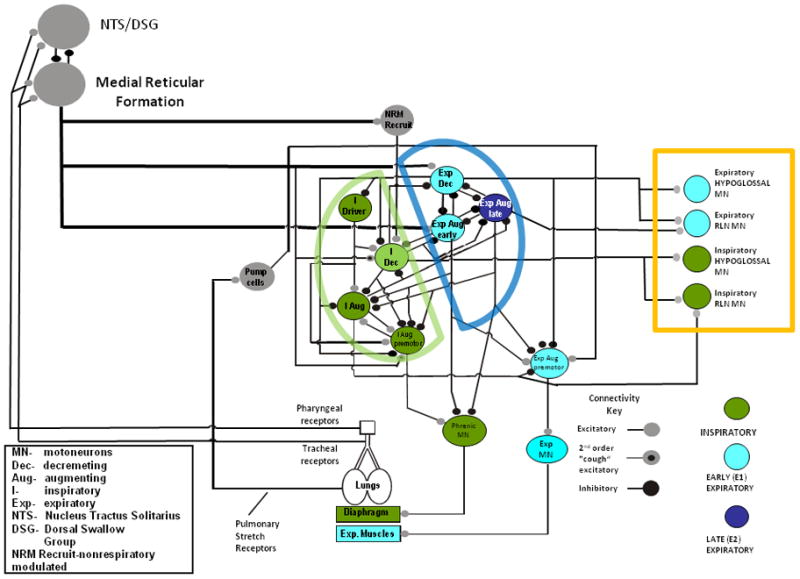
Simpified core synaptic model for the medullary network producing breathing, cough, and swallow. The model is similar to that reported by Rybak and coworkers (Rybak et al. 2008) with several modifications to include elements related to the production of swallow. Populations of neurons are color coded based on major activities during the inspiratory phase, early expiratory phase (E1), and late expiratory phase (E2). colored half-circles show cooperative groups of inspiratory and expiratory neurons. The colored box shows motor outputs specifically related to the production of swallow. Current simulations include recently elucidated participation of modulatory circuits inferred from network connectivity data from the pontine respiratory group and medullary raphe nuclei.
The proposed network is highly complex and defies significant understanding by simple inspection. However, it is at core a reciprocally inhibitory “half-center” oscillator, (see Fig. 1). Connectivity originally inferred from multi-neuron recordings shapes firing profiles of well described sub-populations of inter-neurons as well as pre- and moto-neurons. Several elements of the model have been independently confirmed with spike-triggered averaging of intracellular potentials (Ezure 1990; Jiang and Lipski 1990; Lindsey et al. 1994; Bianchi et al. 1995). Much valuable information about connectivity in the respiratory network has resulted from the of study how the network reconfigures during cough as well as changes in afferent input such as central and peripheral chemoreceptors and blood pressure baroreceptors. Neurons that behave virtually identically during eupnea may completely change character during an airway defensive behavior such as cough (i.e. see Fig. 2, (Morris et al. 2003)).
Figure 2.
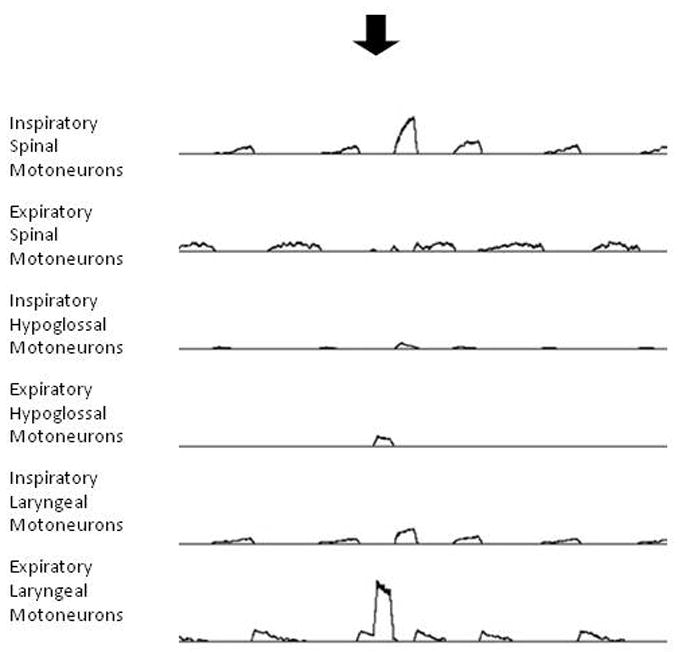
Simulation of breathing and swallow. Traces represent moving averages of spinal and cranial motoneuron populations during simulated breathing and simulated swallow. Arrow indicates stimulation of simulated pharyngeal receptor afferent inputs to the system, as shown in Figure 1. Simulated swallow is depicted as a large burst in expiratory laryngeal (adductor) motoneurons, coincident with an increase in expiratory hypoglossal motoneuron discharge.
Our group and others (Shannon et al. 1998; Rybak et al. 2008), have employed computational modeling and simulation to systematically investigate the behavior and capabilities of this and other similar models. Computational modeling can allow detailed investigation of static or dynamic systems that incorporate multiple variables. There are several important values of computational modeling. First, this method can produce a limited series of testable hypotheses that can optimize in vitro and/or in vivo approaches to discovery. Second, important gaps in our understanding of a complex system can be identified by computational modeling and simulation. For example, if extensive simulation of a model network will not reproduce the behavior of a biological system, the simulated system may be missing critical elements and/or interactions.
When simulated, the model shown in Figure 1, and others like it, can produce motor outputs that closely resemble those observed in vivo. Several studies have shown breathing and coughing to be closely approximated by this model, with inspiratory and expiratory motor bursts that are appropriate for the production of these behaviors. The model will also accurately simulate the behavior of brainstem neurons that are responsible for motor drives to respiratory motoneuron pools (Shannon et al. 1998; Rybak et al. 2008). Figure 1 has two new elements that represent hypothesized BCAs in the system. The first BCA, the DSG/NTS, is intended to represent the collective behavior of the dorsal swallow group and NTS second order interneurons that participate in the production of swallow (Jean 2001). The second BCA, the medullary reticular formation, represents the cooperative behavior of medial medullary neurons that are hypothesized to participate in the production of cough and swallow (Rose 2010).
One or more components of these BCAs are proposed to synaptically interact with specific neuron populations in the lateral medulla (Figure 1, neurons outlined with half circles). This hypothesis illustrates one of the difficulties in modeling across scales in complex neural systems. That is, specifically integrating a functional element, such as a BCA, into a detailed synaptic model. By definition, relatively little is known about the detailed makeup of a BCA. Without this specific information, construction of hypotheses regarding the interactions of the BCAs and the synaptic network remains a comparatively uninformed process. However, simulation can be employed to explore the feasibility of physiological linkages between functional elements (the BCAs in Figure 1) and specific synaptic components of the model. Arrangements that yield simulations resembling appropriate in vivo behaviors provide a framework for the development of informed hypotheses that are testable in vivo.
Figure 2 shows the results of a simulation in which pharyngeal receptors were stimulated during breathing to approximate infusion of water into the oropharynx. The ongoing motor patterns of the spinal and cranial motoneurons before the stimulus were consistent with simulated breathing (Rybak et al. 2008). In response to the stimulus, large increases in laryngeal adductor and expiratory hypoglossal motoneuron discharge were observed, consistent with cranial motor responses observed during in vivo swallow (Oku et al. 1994; Jean 2001). The occurrence of simulated swallow also resulted in shortening of the ongoing expiratory phase, which has been observed in vivo as well (Dick et al. 1993). The shape of the expiratory laryngeal motor burst was ballistic-like (rapid onset and decrement), which is similar to in vivo observations (Dick et al. 1993; Jean 2001). To our knowledge, the large inspiratory spinal motoneuron burst following the simulated swallow has not been routinely observed when swallow is actuated during breathing in vivo. The genesis of this large inspiratory burst is not immediately apparent by inspection of the circuit in Figure 1, but ongoing simulations in which connectivity in the model is systematically altered are likely to suggest hypotheses for the mechanism of this effect.
Modeling and simulation also can be useful to understand mechanisms that underlie clinical treatment efficacy. Surface electrical stimulation is currently being employed as a treatment for dysphagia (Ludlow et al. 2007). The mechanism(s) responsible for proposed treatment effects of this method are a source of contention among proponents of this treatment methodology. Furthermore, some stimulus parameters (such as frequency) of the currently approved device for this use are fixed. Modeling and simulation has the potential to guide evidence-based studies aimed at optimization of these parameters.
Related to this issue is the long standing observation in animal models that behavior selection can occur (cough or swallow) in animal models solely by altering the frequency of electrical stimulation of the superior laryngeal nerve (Bolser 1991; Oku et al. 1994; Jean 2001). Low frequency stimulation of the superior laryngeal nerve produces cough, while higher frequency stimulation, with the same current and pulse width, produces swallow (Oku et al. 1994). Given that the nerve itself has been sectioned distal to the stimulation electrodes, the expression of cough or swallow is solely a central phenomenon. We hypothesize that tuned oscillatory circuits of neurons act as gates by filtering afferent inputs, such as those that produce the cough and swallow motor patterns, to the ventral respiratory network. Such frequency filtering has been previously suggested and recently simulated (Akam and Kullmann 2010). Further, we suggest that feedback from the ventral respiratory column contributes to that gating in order to assure that behaviors are expressed in coordination with inspiratory motor activity (Dick et al. 2008). The gating could be accomplished by neural circuits with firing rate and/or phase dependent synchrony oscillations. By synchrony oscillations, we mean groups of neurons that discharge in a cooperative manner such that the frequency and timing of occurrence of their action potentials is similar. Neuronal populations that behave in this way also can have periods of time in which they are either more or less excitable. These periods may occur at frequencies that “allow” sensory information through to the rest of the network consistent with the function of a filter. By phase dependent synchrony, we mean that this filtering function may preferentially occur during either the inspiratory or expiratory phase of breathing. In effect, the filtering function described above may be restricted to a single phase of the breathing (or cough) cycle. We have previously modeled and simulated phase dependent synchrony as well as described evidence for it in vivo (Lindsey et al. 1992).
Neural circuits, displaying firing rate and/or synchrony oscillations, may act as filters to accomplish behavior selection in such a way that the spatiotemporal features of the behaviors are expressed in correct coordination with breathing or coughing. In this manner, the expression of airway protective behaviors could be controlled to minimize the probability of aspiration. Behaviors such as the pharyngeal phase of swallow may be restricted by such neural filters to occur only when the airway is likely to be closed, or at least not actively opened by another behavior, such as the inspiratory component of cough. This process, which we term phase restriction, has been observed in a preliminary report describing the occurrence of swallow during repetitive coughing (Bolser 2009). When aspiration of foreign material occurs, phase restriction may be a primary tool by which the nervous system corrects or minimizes further aspiration in the face of bombardment of afferent feedback from oral, pharyngeal, laryngeal, and tracheal tissue beds. This process may underlie the orderly initiation, execution, and completion of multiple airway protective behaviors in rapid sequence to effect efficient ejection from the airway and removal of aspirated material from the pharynx surrounding the laryngeal orifice.
Computational models of the respiratory motor control system may become useful in drug discovery. A recent preliminary report (Shevtsova et al. 2011) provided evidence that serotonergic and glycinergic receptor-induced ionic currents can be incorporated into a version of the synaptic model similar to that shown in Figure 1. The modified model produced simulated breathing motor patterns that resembled those observed in vivo after administration of selected serotonin receptor agonists. Given that this model (or models like it) will produce simulated breathing, coughing, and swallow, it is likely that the actions of drugs on multiple behaviors could be predicted by a computational modeling approach. It is well known that pharmacological agents can affect breathing and coughing differently of at least with different potencies (May and Widdicombe 1954; Bolser et al. 1999), even though those behaviors are produced, in part, by a shared brainstem network. New drugs intended for cough suppression may secondarily affect breathing or swallow. Computational modeling may represent an important tool in predicting the range of side effects that a given drug may produce. Focused in vivo studies can then be implemented to address the predicted side effect profile.
Computational modeling can be subject to important limitations. First, computational modeling is its own discipline, requiring expertise that is not easily acquired by a biomedical scientist who is not familiar with it. Second, computational modeling may require significant knowledge of programming languages and platforms that are not typically found in training programs for biomedical scientists. Fortunately, these limitations can be overcome by collaboration with other scientists who routinely engage in computational modeling. There are many open source programs that are available that can be run in a Microsoft Windows environment (http://www.imagwiki.nibib.nih.gov/mediawiki/index.php?title=Modeling_Tools). Quite a few of programs are easily accessible and used by scientists that are not experts in computational modeling. Many of these programs incorporate ordinary differential equations as a mechanistic base.
Highlights.
Protection of the airway from aspiration is a complex process involving multiple behaviors.
These behaviors are subject to coordinated control which is poorly understood.
The central mechanism for regulating airway protection is better understood when computational modeling and simulation are employed as investigative tools.
There are online modeling and simulation resources that can be utilized by investigators interested in adapting these methods to their research.
Acknowledgments
This work was supported by NIH R33 HL89104, R33 HL 89071, and R01 HL103415.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akam T, Kullmann DM. Oscillations and filtering networks support flexible routing of information. Neuron. 2010;67(2):308–320. doi: 10.1016/j.neuron.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75(1):1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bolser DC. Fictive cough in the cat. J Appl Physiol. 1991;71(6):2325–2331. doi: 10.1152/jappl.1991.71.6.2325. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86(3):1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respir Physiol Neurobiol. 2006;152(3):255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Rose MJ, Pitts TE, Davenport PW, Baekey DM, Segers LS, Nuding SC, Lindsey BG, Shannon R, Gestreau C, Morris KF. Neurogenesis of airway protective behaviors in the cat: cough and pharyngeal swallow. FASEB J. 2009;23:1010.1014. [Google Scholar]
- Braman SS, Corrao WM. Cough: differential diagnosis and treatment. Clin Chest Med. 1987;8(2):177–188. [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88(9):1337. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Oku Y, Romaniuk JR, Cherniack NS. Interaction between central pattern generators for breathing and swallowing in the cat. J Physiol. 1993;465:715–730. doi: 10.1113/jphysiol.1993.sp019702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol. 2008;586(Pt 17):4265–4282. doi: 10.1113/jphysiol.2008.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35(6):429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Fernandez H, Lapane K. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Medical science monitor: international medical journal of experimental and clinical research. 2002;8(4) [PubMed] [Google Scholar]
- Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8(4):CR241–246. [PubMed] [Google Scholar]
- Freedman MS. Long-term follow-up of clinical trials of multiple sclerosis therapies. Neurology. 2011;76(1 Suppl 1):S26–34. doi: 10.1212/WNL.0b013e318205051d. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA. Parkinson’s disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology. 1994;44(10):1865–1868. doi: 10.1212/wnl.44.10.1865. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Botzinger complex in the cat. Exp Brain Res. 1990;81(3):639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Leith DE, Butler JP, Sneddon SL, Brain JD. Mechanics of Breathing, Part I. Bethesda: American Physiological Society; 1986. Cough. Handbook of Physiology. The Respiratory System, V. III; pp. 315–336. [Google Scholar]
- Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol. 1992;67(4):923–930. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Segers LS, Morris KF, Hernandez YM, Saporta S, Shannon R. Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis. J Neurophysiol. 1994;72(4):1830–1851. doi: 10.1152/jn.1994.72.4.1830. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal Dysphagia. Dysphagia. 2007;22(1):1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AJ, Widdicombe JG. Depression of the cough reflex by pentobarbitone and some opium derivatives. Br J Pharmacol Chemother. 1954;9(3):335–340. doi: 10.1111/j.1476-5381.1954.tb01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):48S–53S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- Morris KF, Baekey DM, Nuding SC, Dick TE, Shannon R, Lindsey BG. Invited review: Neural network plasticity in respiratory control. J Appl Physiol. 2003;94(3):1242–1252. doi: 10.1152/japplphysiol.00715.2002. [DOI] [PubMed] [Google Scholar]
- Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decerebrate cat. J Physiol. 1994;480(Pt 2):309–324. doi: 10.1113/jphysiol.1994.sp020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431. doi: 10.1378/chest.10-0342. This study showed that selected mechanics of voluntary cough were strongly associated with dysphagia in patients with Parkinson’s disease. This work adds to a growing body of evidence supporting the concept that, just as in animals, the control systems for cough and swallow in humans have shared elements. Further, this putative shared control system in humans is likely to be susceptible to neuropathological mechanisms associated with a variety of neurological diseases. [DOI] [PubMed] [Google Scholar]
- Rose MJ, Pitts TE, Poliacek I, Davenport PW, Morris KF, Bolser DC. Activity patterns of neurons in the caudal medial medulla are modulated during swallow in the cat. FASEB J. 2010;24:1064.1065. [Google Scholar]
- Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100(4):1770–1799. doi: 10.1152/jn.90416.2008. This study clearly shows simulated brainstem neuron and respiratory motor outputs during cough and breathing that are essentially indistinguishable from in vivo recordings. The paper also shows that one neural network can produce several different behaviors by flexible reorganization of its component elements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiermeier S, Schafer D, Schafer T, Greulich W, Schlafke ME. Breathing and locomotion in patients with Parkinson’s disease. Pflugers Arch. 2001;443(1):67–71. doi: 10.1007/s004240100665. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84(6):2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Shevtsova NA, Bischoff A, Molkov YI, Manzke T, Rybak IA, Richter DW. Computational modeling of serotonin-evoked reorganization of the brain stem respiratory network. FASEB Journal. 2011;25:652. doi: 10.1111/j.1460-9568.2011.07825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- Wada H, Nakajoh K, Satoh-Nakagawa T, Suzuki T, Ohrui T, Arai H, Sasaki H. Risk factors of aspiration pneumonia in Alzheimer’s disease patients. Gerontology. 2000;47(5):271–276. doi: 10.1159/000052811. [DOI] [PubMed] [Google Scholar]


