Abstract
Severe asthma in children is a complicated disorder characterized by ongoing symptoms and persistent airway inflammation despite treatment with high doses of inhaled and oral corticosteroids. Although knowledge of asthma and its associated mechanisms has increased substantially over the past decade, significant gaps remain about the determinants of severe asthma in children and the progression of the disorder across the lifespan. This review highlights recent insights into severe asthma in children derived from the National Heart, Lung, and Blood Institute's Severe Asthma Research Program (SARP), with an emphasis on age-specific findings and differences from severe asthma in adults. While the existence of a true severe asthma phenotype in children is subject to some debate, given the results of SARP and other investigators, we conclude that there is indeed a subgroup of children with severe asthma who have extreme morbidity and differentiating clinical features that are identifiable very early in life. However, unlike adults with severe asthma, children with severe asthma are more likely to fall in a more narrow cluster that is characterized by marked atopy and reversible airflow obstruction. While SARP has advanced knowledge of severe asthma in children, considerable gaps remain for which additional studies are needed.
Introduction
Asthma is the most common chronic lung disease of childhood that affects >6.6 million children in the United States.1 Whereas most children with asthma achieve good symptom control when treated with low doses (<500 mcg/day fluticasone equivalents) of inhaled corticosteroids (ICS), children with severe or difficult-to-treat asthma remain highly symptomatic despite treatment with high doses of ICS and even systemic corticosteroids.2,3 The symptoms of severe asthma in these children may be attributed to persistent airway inflammation and relative corticosteroid insensitivity,4,5 although a number of biological, environmental, and/or social factors may also be responsible.6,7 Regardless of the underlying mechanisms, severe asthma in children is a challenging disorder with significant public health implications. Despite the low prevalence of severe asthma in the general population, the significant morbidity associated with the disorder accounts for nearly 50% of all asthma-related expenditures.8,9 This review will focus only on children with severe, therapy-resistant asthma. Unlike children with difficult asthma who may have poor adherence, suboptimal environments, or psychological comorbidities that inhibit the response to asthma medications,10 children with severe, therapy-resistant asthma have ongoing symptoms and airway inflammation despite best attempts at corticosteroid treatment.11 Thus, this definition of severe asthma implies that relevant comorbidities, social issues, and poor medication adherence have fully addressed.11 This review highlights recent insights into severe asthma in children derived from the National Heart, Lung, and Blood Institute's Severe Asthma Research Program (SARP), with an emphasis on age-specific findings and differences from severe asthma in adults. While SARP has advanced knowledge of severe asthma in children, considerable gaps remain for which additional studies are needed.
Overview of SARP and Definition of Severe Asthma in Children
Given the relatively small number of patients with severe asthma at any institution, single-center studies of severe asthma are difficult to conduct. In 2001, the National Heart, Lung, and Blood Institute solicited applications for SARP, a multicenter program focused on the clinical and biological attributes of severe asthma in adults and children. Awards were made to 8 clinical sites at Brigham and Women's Hospital, Imperial College School of Medicine, National Jewish Medical and Research Center, the University of Pittsburgh, the University of Virginia (with coinvestigators at the Cleveland Clinic and Emory University), the University of Wisconsin, Wake Forest University, and Washington University. Recruitment of pediatric subjects is primarily done at Emory University, the University of Virginia, and the University of Pittsburgh. Each of these SARP sites works under a standard definition of severe asthma and follows uniform procedures for asthma characterization. This collaborative approach to the study of severe asthma allows for rigorous phenotyping of the disorder in greater numbers of subjects with diverse backgrounds. Although children enrolled in SARP do not undergo bronchoscopy or imaging for research purposes, they do complete SARP questionnaires and undergo pulmonary function and plethysmography testing, methacholine challenge, exhaled nitric oxide determination, and allergy skin prick testing. Children also submit blood samples for genetic studies and quantification of immunoglobulin E (IgE) and peripheral eosinophils as described previously.3,12 Data are reviewed for accuracy by a centralized Data Coordinating Center. To date, 153 children 6–18 years have completed SARP characterization, including 83 (54%) males and 84 (55%) racial minorities. Ninety-three (61%) children are <12 years of age. Features of a subset of these children have been published previously.3
Although a number of definitions of severe asthma have been proposed by both the National Asthma Education and Prevention Program13 and the Global Initiative on Asthma,14,15 the SARP definition of severe asthma was adopted from the proceedings of an American Thoracic Society Workshop on Refractory Asthma, which were published in 2000.16 According to this American Thoracic Society Workshop definition, severe asthma is present in any individual with persistent asthma who (1) requires treatment with continuous high-dose ICS or continuous oral corticosteroids to maintain asthma control, and (2) has at least 2 minor criteria, including use of asthma controller medications and recent healthcare utilization (Table 1). This definition assumes that comorbid conditions have been treated or addressed and that the patient is compliant with asthma treatment.16 Proposed modifications for the SARP definition include a requirement that the patient has been under the care of an asthma specialist for a minimum period of time so as to address the stability of the severe asthma assignment in a supervised care environment. Thresholds for high-dose ICS for adults and children were established by the SARP Steering Committee according to the relative potency of each drug as expressed by fluticasone equivalents. The list of high-dose ICS appears in Table 2. For the purpose of severity classification in SARP, any subject 12 years and older is considered an adult.
Table 1.
The Severe Asthma Research Program Definition of Severe Asthma, Adapted from the American Thoracic Society Workshop on Refractory Asthma16
| Major criteria for severe asthma (must have at least 1 to achieve asthma control): |
| Treatment with high-dose inhaled corticosteroids |
| Treatment with continuous oral corticosteroids (at least 50% of the year) |
| Minor criteria for severe asthma (must have at least 2): |
| Treatment with additional controller medications to maintain asthma control |
| Daily use of short-acting bronchodilators (5 of 7 days) |
| Persistent airflow obstruction, with baseline FEV1 <80% predicted |
| One or more urgent care visits for asthma in the previous year |
| Three or more oral corticosteroid bursts in the previous year |
| A history of prompt deterioration in asthma symptoms with a reduction in the dose of inhaled corticosteroids or oral corticosteroids |
| A near-fatal asthma event requiring intubation in the past |
Table 2.
Thresholds of High-Dose Inhaled Corticosteroids in Adults and Children
| Adults (12 years and older), minimum mcg/day | Children (<12 years), minimum mcg/day | |
|---|---|---|
| Fluticasone | 880 mcg (Flovent® HFA) | 440 mcg (Flovent® HFA) |
| Fluticasone/salmeterol | 1,000 mcg (Advair® discus) | 500 mcg (Advair® discus) |
| 920 mcg (Advair® HFA) | 460 mcg (Advair® HFA) | |
| Budesonide | 1,600 mcg (Pulmicort® Turbuhaler) | 600 mcg (Pulmicort® Turbuhaler) |
| 1,440 mcg (Pulmicort® Flexhaler) | 450 mcg (Pulmicort® Flexhaler) | |
| 2,000 mcg (Pulmicort® Respules) | ||
| Budesonide/formoterol | 640 mcg (Symbicort® HFA) | 480 mcg (Symbicort® HFA) |
| Flunisolide | 800 mcg (Aerospan®) | 1,250 mcg (Aerobid®) |
| 2,500 mcg (Aerobid®) | ||
| Beclomethasone | 640 mcg (Qvar® HFA) | 160 mcg (Qvar® HFA) |
| Triamcinolone | 2,500 mcg (Azmacort®) | 1,200 mcg (Azmacort®) |
| Mometasone | 880 mcg (Asmanex® Twisthaler) | 440 mcg (Asmanex® Twisthaler) |
The Preschool Years: Birth to Age 5
The Expert Panel Report offers a definition of severe asthma in preschool children with nearly continuous symptoms during the day, night-time awakenings more than once weekly, use of rescue bronchodilators several times per day, and extremely limited daily activities.13 This definition has not been validated by clinical studies and the extent and scope of preschool children who fit this description is unknown. However, many children with severe asthma present in school age recall relatively early onset of symptoms. Although the lower age limit of children enrolled in SARP is 6 years, historical data from these children suggest that the onset of asthma symptoms occurs earlier in life in children with severe versus mild-to-moderate asthma.3 Whereas the average age of asthma symptom onset was 60 months in children with mild-to-moderate asthma, the majority of children with severe asthma had symptoms that appeared within the first 24 months of life.3 Although these data are subject to recall bias, they suggest that features of severe asthma in childhood may be identifiable in the early preschool years. Because children with severe asthma also had a higher prevalence of atopic dermatitis and more skin prick responses to aeroallergens by the early childhood years,3 these findings further suggest that allergic sensitization may be a key factor in distinguishing severe from mild-to-moderate asthma in young children.
The importance of allergic sensitization in asthma persistence has been described previously.17,18 In the Tuscon Children's Respiratory Study, a large birth cohort study of >1,200 newborns and their families,19 only preschool children with atopic wheezing (distinguished by sensitization to aeroallergens and increased serum IgE concentrations) had active asthma symptoms and airflow obstruction between 6 and 13 years of age.20 By contrast, >80% of all nonatopic preschool children had complete remission (ie, cessation) of all asthma symptoms during the school-age years.21 Whereas the majority of these nonatopic preschoolers had respiratory symptoms only with upper respiratory infections during the winter months, preschool children with atopic wheezing had symptoms year-round that occurred both with and without upper respiratory infections.18 Although there are currently no clear markers to distinguish the likelihood of severe asthma in young preschool children, evaluation of atopic markers may prove useful. However, it is unclear whether allergic mechanisms play a causal or supportive role in the development of severe asthma in children.
There is a growing body of literature suggesting that asthma may be present in young children well before the onset of symptoms. In a recent birth cohort of healthy infants in Norway, 3-day-old infants with the lowest pulmonary function (as measured by the fraction of expiratory time to peak tidal expiratory flow) were nearly twice as likely to have current asthma and severe bronchial hyperresponsiveness at 10 years of age.22 Similarly, in the Tuscon Children's Respiratory Study, 2- to 3-month-old infants with the lowest maximal expiratory flow at functional residual capacity at 2–3 months of age had significantly lower FEV1, FEV1:FVC, and FEF25–75 values at 22 years, even after adjustment for smoking status, atopy, parental asthma, and other potential confounders.23 These data highlight the importance of fetal determinants of airway function in some children and argue for additional study of critical periods of lung development in children at high risk for recurrent wheezing.
While the mechanisms of early airflow obstruction in children are unclear, immune dysregulation may play an important role. For instance, young infants with the lowest concentrations of interferon γ (IFN-γ), a Th1 cytokine, have an increased risk of recurrent wheezing during both the preschool and school-age years.24 Because low IFN-γ levels during infancy are also associated with increased aeroallergen skin prick sensitization by 12 months and 6 years of age,25,26 wheezing in these children may be due to enhanced Th2 cell differentiation. Interestingly though, classical hallmarks of airway Th2 activation such as airway eosinophilia and reticular basement membrane thickening are not identifiable in infants 12–24 months of age with wheezing disorders.27 While eosinophilia and basement membrane thickening are present in some preschool children after 24 months of age,28 other preschool children have neutrophilic patterns of inflammation and increased interleukin-8 expression,29–31 perhaps due to respiratory viral infections that may intensify during this time period.32
These findings highlight the phenotypic heterogeneity present in young children with preschool wheezing, which likely accounts for the differential response to asthma treatment that is commonly observed in this population.33–36 Additional studies are critically needed to identify relevant biomarkers of disease persistence and severity in preschool children. Such biomarkers would useful in the refinement of preschool wheezing phenotypes and would also aid in the development of novel therapeutic interventions to slow the progression and severity of wheezing in young preschool children.
The School-Age Years: Age 6–11
Nearly 100 children aged 6–11 years with asthma have participated in SARP to date. The features of this sample are presented in Table 3. Compared to children with mild-to-moderate asthma, children with severe asthma had increased allergic sensitization as measured by serum IgE and exhaled nitric oxide (FENO) and were significantly more likely to be of African American or mixed race. Consistent with the SARP definition, children with severe asthma also had increased medication and healthcare utilization over the previous year. Despite more aggressive treatment with ICS (848 ± 245 versus 328 ± 268 μg of fluticasone per day for severe versus mild-to-moderate asthma, respectively), children with severe asthma also had increased airflow obstruction as reflected by baseline FEV1% predicted values. Although FEV1 improved in both groups of children with maximum bronchodilator administration of up to 720 μg of albuterol sulfate, the best postbronchodilator FEV1 remained significantly lower in children with severe asthma (Table 3). As important, children with severe asthma are differentiated from children with mild-to-moderate asthma by increased presence of air trapping as signified by marked increase in the residual volume to total lung capacity ratio. These findings are similar to those observed in other studies of severe and difficult-to-treat asthma in children2,37–40 and suggest that structural airway changes are present in children with severe asthma as young as 6 years of age.
Table 3.
Features of Children Enrolled in Severe Asthma Research Program, by Age Group
| |
Age 6–11 years |
Age 12–17 years |
||||
|---|---|---|---|---|---|---|
| Mild-to-moderate asthma, n = 45 | Severe asthma, n = 48 | P value | Mild-to-moderate asthma, n = 30 | Severe asthma, n = 30 | P value | |
| Male | 25 (56) | 22 (45) | 0.233 | 17 (59) | 19 (61) | 0.521 |
| Caucasian | 23 (51) | 12 (25) | 0.039 | 17 (59) | 6 (19) | 0.008 |
| Emergency room visita | 20 (46) | 37 (78) | 0.002 | 5 (17) | 25 (81) | <0.001 |
| Hospitalizationa | 7 (16) | 31 (65) | <0.001 | 1 (3.4) | 20 (65) | <0.001 |
| History of intubation | 0 | 11 (23) | 0.031 | 0 | 9 (29) | 0.001 |
| Daily oral corticosteroids | 0 | 4 (8) | 0.067 | 0 | 6 (19) | 0.015 |
| Daily short-acting bronchodilator use | 8 (18) | 29 (60) | <0.001 | 7 (24) | 20 (65) | 0.002 |
| Number of aero-allergen skin prick responses (out of 12) | 1.5 (0–9) | 3 (0–10) | 0.086 | 1 (0–8) | 4 (0–10) | <0.001 |
| Serum immunoglobulin E (kU/L)b | 196 (2–3,484) | 335 (9–3,511) | 0.007 | 117 (7–1,724) | 571 (4–5,458) | 0.006 |
| Blood eosinophils (%)b | 4.2 (0.4–13.2) | 4.2 (0.2–23.8) | 0.389 | 3.0 (0.3–13.0) | 5.1 (0.2–23.6) | 0.273 |
| Exhaled nitric oxide (ppb, offline)b | 7.1 (2.2–28.3) | 13.6 (4.2–45.8) | 0.004 | 7.6 (2.7–27.9) | 11.4 (5.4–30.0) | 0.016 |
| Baseline FEV1 (%) | 98 (78–142) | 87 (57–123) | 0.002 | 95 (70–129) | 71 (37–105) | <0.001 |
| Maximum FEV1 (%) | 105 (89–158) | 101 (65–142) | 0.005 | 99 (78–137) | 90 (50–115) | 0.002 |
Data represent the median (range) or the frequency (%).
Within the previous 12 months.
Data were logarithmically transformed before statistical analysis.
The Dunedin Multidisciplinary Health and Development Study was one of the first to highlight abnormalities in pulmonary function in school-age children according to clinical phenotype.41 In this birth cohort study, children with persistent wheezing or wheezing that relapsed (ie, reappeared after symptom cessation) since the preschool years had significant airflow obstruction at 9 years of age that persisted through early adulthood.41,42 Further, structural airway remodeling in adolescence and early adulthood was 3 times more likely in children with airway hyperresponsiveness at 9 years of age, evidenced by either a methacholine provocative concentration (PC20) of ≤8 mg/mL or an FEV1 bronchodilator response of ≥10%.42 While this birth cohort did not focus specifically on severe asthma, these findings suggest that pulmonary function abnormalities evident during early childhood may persist and even worsen throughout the adult years. However, it is worth noting that pulmonary function declines are also present in a subset of children with mild-to-moderate asthma. In the Childhood Asthma Management Program, a multicenter, longitudinal study of children 5–12 years of age with mild-to-moderate persistent asthma,43 ~30% of all enrolled children had declines of ≥1% or more in postbronchodilator FEV1 regardless of treatment allocation.44 Although the clinical relevance of this finding is unclear, it may be related to altered lung growth in a subset of asthmatic children.45 Alternatively, these lung function declines may also be a feature of airway remodeling and perhaps future asthma severity. A number of recent reviews have highlighted histopathologic evidence of the several mechanisms by which persistent asthma can lead to airway remodeling, including pathological changes in the airway smooth muscle bundle,46 disrupted epithelial barrier integrity and function,47 and altered cross-talk between the airway epithelium and the mesenchyme.48 Although the mechanisms and natural history of this connection are still being discovered, this is a fertile area for ongoing research and a major focus of SARP and related research efforts.
While there are likely a number of different phenotypes of severe asthma in school-age children,49 the majority of children in this age group are characterized by distinct structural airway changes and Th2-mediated patterns of airway inflammation.3,50–52 Whereas reticular basement membrane thickening does not necessarily differentiate severe from mild-to-moderate asthma in children,51,53,54 school-age children with symptomatic severe asthma do have a greater airway smooth muscle surface area and a more dense vascular airway network.51 These features are associated with airflow obstruction51 and suggest that the airway smooth muscle and vessels play an important role in airway remodeling. Children with severe asthma are further characterized by a greater CD4+ T lymphocyte density, a lower ratio of IFN-γ to interleukin-5, and increased numbers of activated eosinophils with increased FENO.52,53,55,56 Although airway neutrophils may also be present in a subset of children with severe asthma,57 they often coexist with eosinophils in the airway tissue and epithelial lining fluid.2,50 Given the limited data on severe asthma in children, further studies of airway inflammatory biomarkers are acutely needed. The development of noninvasive markers would be of particular utility given the ethical and methodological limitations of conducting research in this population.
The Adolescent Years: Age 12–17
Although fewer adolescents (n = 60) aged 12–17 years have been enrolled in SARP, the features of these adolescents are similar to those of the 6- to 11-year-old age groups (Table 3). Compared to adolescents with mild-to-moderate asthma, adolescents with severe asthma were again more likely to be African American or of mixed race and were characterized by higher serum IgE, increased FENO, and greater healthcare utilization despite significantly higher doses of ICS (940 ± 223 versus 326 ± 291 μg of fluticasone per day for severe versus mild-to-moderate asthma, respectively). Baseline FEV1 was also lower in adolescents with severe asthma and remained lower after maximal bronchodilation (Table 3). These findings suggest that patterns of airway remodeling are already established by adolescence and argue for additional study of airway physiology during early development.
The natural history of asthma progression during childhood is complex and not fully understood. There is increasing data to suggest that children who wheeze throughout childhood are more likely to be atopic and are more likely to have increased airflow obstruction and physician-diagnosed asthma in adolescence or early adulthood.58–61 However, the biological and genetic determinants of asthma persistence in adolescents are unclear. Identification of asthma persistence in adolescents is further complicated by different clinical presentations or phenotypes of the disorder. For instance, in the Tuscon Children's Respiratory Study, nearly 63% of patients with newly diagnosed asthma at 22 years of age had episodes of wheezing during the first 3 years of life.60 On retrospective analysis, the patients with newly diagnosed asthma in early adulthood were more likely to have evidence of bronchial hyperresponsiveness at 6 years of age, independent of asthma symptoms.60 A separate study further demonstrated increased airway eosinophilia as measured by FENO and increased bronchial hyperresponsiveness in adolescents in clinical remission of their asthma as compared to healthy controls.62 Although these adolescents had no current asthma symptoms,62 these findings suggest that there are certain clinical features identifiable during the adolescent years that might convey a risk for asthma relapse later in life. In the Dunedin Multidisciplinary Health and Development Study, ~35% of adolescents with asthma in remission at 18 years of age relapsed by 21 or 26 years.63 Factors associated with relapse in these patients included atopy, airflow obstruction, and increased responsiveness to methacholine or bronchodilators.63 These same factors have also been implicated as risk factors for subsequent asthma hospitalizations and may help identify adolescents at risk of poor asthma control.64
Differences Between Children and Adults with Severe Asthma
Because pediatric and adult subjects undergo similar characterization procedures in SARP, it is possible to compare the phenotypic differences between adults and children with severe asthma. Similar to children with severe asthma,3 adults with severe asthma are characterized by air trapping and incomplete reversal with bronchodilation, although the magnitude of this airflow limitation is greater in the adults.65 By contrast, whereas allergic sensitization is a differentiating feature of severe asthma in children,3 positive skin prick responses are less prevalent in adults with severe asthma and there are no significant differences in blood eosinophil counts, FENO, or serum IgE between the severe and mild-to-moderate groups.12 This inverse relationship between atopy and severity was also noted in the European Network for Understanding the Mechanisms of Severe Asthma66 and may be attributable to alterations in the adaptive immune response67 or an increased prevalence of comorbid conditions such as gastroesophageal reflux, sinusitis, and pneumonia.12 Alternatively, because severe asthma in adults is a complex condition associated with several different clinical presentations,49,68 further separation of severe asthma into different phenotypes may be necessary to understand the features of the disorder. Certainly, the vast majority of children with severe asthma and persistent wheezing have asthma that persists well into adult years. However, it is unclear how or whether the features of severe asthma in these children change across the lifespan.
In SARP study, attempts have been made to phenotype adults with severe asthma according to clinical features and inflammatory biomarkers.69 Because previous reports have suggested that severe asthma in adults can be divided into 2 distinct phenotypes based on the age of diagnosis,70,71 adults with severe asthma in SARP were stratified by the self-reported age of asthma onset. Compared to adults with late-onset asthma after 12 years of age, adults with early onset asthma before age 12 had more positive skin prick responses to aeroallergens, more self-reported allergic asthma symptoms, and greater lifelong healthcare utilization,12 similar to previous reports.70,71 In a separate study, atopy also differentiated one phenotype of severe asthma in adults, which was also distinguished by the youngest age of asthma onset and significant baseline airflow obstruction that reversed to the near-normal range after maximum bronchodilator administration,69 similar to our findings in children. Because of the cross-sectional nature of SARP, it is difficult to determine how the features of children with severe asthma enrolled in SARP will manifest in adulthood. However, these early attempts to phenotype severe asthma may be useful in future prospective studies on the natural history of asthma severity in childhood and adulthood.
Summary and Conclusions
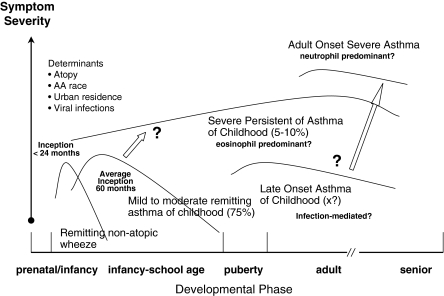
Although knowledge of asthma and its associated mechanisms has increased substantially over the past decade, significant gaps remain about the determinants of severe asthma in children and the progression of the disorder across the lifespan. Based on results from SARP and other investigators, there is indeed a subgroup of children with severe asthma who have extreme morbidity and differentiating clinical features that are identifiable very early in life (Fig. 1). Although phenotypic heterogeneity is an important feature of severe asthma in both children and adults, children with severe asthma are more likely to fall in a more narrow cluster that is characterized by marked atopy and reversible airflow obstruction. The preliminary experience from SARP suggests that over time severe asthma becomes more diverse; that is, a cadre of children with the common phenotype do progress, but are joined by new onset severe asthmatics in which obesity and fixed airflow obstruction are visible characteristics. Whether these new phenotypes evolve from the common one as manifestations of the recurrent “hits” hypothesis due environmental exposures versus new onset disease or both will require long-term longitudinal cohort studies. This knowledge will be critical for the development of new interventions to diagnose and treat severe asthma in children.
FIG. 1.
Hypothesized development of severe asthma in children.
Acknowledgment
This study was funded by NIH/NHLBI SARP RO1 HL69170.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.American Lung Association Epidemiology and Statistics Unit RaPSD. Trends in Asthma Morbidity and Mortality. Feb, 2010. www.lungusa.org www.lungusa.org
- 2.Bossley CJ. Saglani S. Kavanagh C. Payne DN. Wilson N. Tsartsali L, et al. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. Eur Respir J. 2009;34:1052–1059. doi: 10.1183/09031936.00186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick AM. Gaston BM. Erzurum SC. Teague WG. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhavsar P. Hew M. Khorasani N. Torrego A. Barnes PJ. Adcock I, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 5.Hew M. Bhavsar P. Torrego A. Meah S. Khorasani N. Barnes PJ, et al. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med. 2006;174:134–141. doi: 10.1164/rccm.200512-1930OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers DA. Postma DS. Stine OC. Koppelman GH. Ampleford EJ. Jongepier H, et al. Genome screen for asthma and bronchial hyperresponsiveness: interactions with passive smoke exposure. J Allergy Clin Immunol. 2005;115:1169–1175. doi: 10.1016/j.jaci.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 7.ten Brinke A. Ouwerkerk ME. Zwinderman AH. Spinhoven P. Bel EH. Psychopathology in patients with severe asthma is associated with increased health care utilization. Am J Respir Crit Care Med. 2001;163:1093–1096. doi: 10.1164/ajrccm.163.5.2004020. [DOI] [PubMed] [Google Scholar]
- 8.Godard P. Chanez P. Siraudin L. Nicoloyannis N. Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19:61–67. doi: 10.1183/09031936.02.00232001. [DOI] [PubMed] [Google Scholar]
- 9.Smith DH. Malone DC. Lawson KA. Okamoto LJ. Battista C. Saunders WB. A national estimate of the economic costs of asthma. Am J Respir Crit Care Med. 1997;156(3 Pt 1):787–793. doi: 10.1164/ajrccm.156.3.9611072. [DOI] [PubMed] [Google Scholar]
- 10.McKenzie SA. Bush A. Difficult asthma in children. Thorax. 2002;57:915–916. doi: 10.1136/thorax.57.10.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush A. Hedlin G. Carlsen KH. de Benedictis F. Lodrup-Carlsen K. Wilson N. Severe childhood asthma: a common international approach? Lancet. 2008;372:1019–1021. doi: 10.1016/S0140-6736(08)61422-1. [DOI] [PubMed] [Google Scholar]
- 12.Moore WC. Bleecker ER. Curran-Everett D. Erzurum SC. Ameredes BT. Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Liard R. Leynaert B. Zureik M. Beguin FX. Neukirch F. Using Global Initiative for Asthma guidelines to assess asthma severity in populations. Eur Respir J. 2000;16:615–620. doi: 10.1034/j.1399-3003.2000.16d08.x. [DOI] [PubMed] [Google Scholar]
- 15.Von Mutius E. Presentation of new GINA guidelines for paediatrics. The Global Initiative on Asthma. Clin Exp Allergy. 2000;30(Suppl 1):6–10. doi: 10.1046/j.1365-2222.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 16.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 17.Martinez FD. Wright AL. Taussig LM. Holberg CJ. Halonen M. Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 18.Spycher BD. Silverman M. Brooke AM. Minder CE. Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. Eur Respir J. 2008;31:974–981. doi: 10.1183/09031936.00153507. [DOI] [PubMed] [Google Scholar]
- 19.Taussig LM. Wright AL. Morgan WJ. Harrison HR. Ray CG. The Tucson Children's Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129:1219–1231. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 20.Taussig LM. Wright AL. Holberg CJ. Halonen M. Morgan WJ. Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675. doi: 10.1067/mai.2003.162. quiz 76. [DOI] [PubMed] [Google Scholar]
- 21.Castro-Rodriguez JA. Holberg CJ. Wright AL. Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 22.Haland G. Carlsen KC. Sandvik L. Devulapalli CS. Munthe-Kaas MC. Pettersen M, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 23.Stern DA. Morgan WJ. Wright AL. Guerra S. Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerra S. Lohman IC. Halonen M. Martinez FD. Wright AL. Reduced interferon gamma production and soluble CD14 levels in early life predict recurrent wheezing by 1 year of age. Am J Respir Crit Care Med. 2004;169:70–76. doi: 10.1164/rccm.200304-499OC. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FD. Stern DA. Wright AL. Holberg CJ. Taussig LM. Halonen M. Association of interleukin-2 and interferon-gamma production by blood mononuclear cells in infancy with parental allergy skin tests and with subsequent development of atopy. J Allergy Clin Immunol. 1995;96(5 Pt 1):652–660. doi: 10.1016/s0091-6749(95)70264-4. [DOI] [PubMed] [Google Scholar]
- 26.Tang ML. Kemp AS. Thorburn J. Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–985. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 27.Saglani S. Malmstrom K. Pelkonen AS. Malmberg LP. Lindahl H. Kajosaari M, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 28.Saglani S. Payne DN. Zhu J. Wang Z. Nicholson AG. Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 29.Hauk PJ. Krawiec M. Murphy J. Boguniewicz J. Schiltz A. Goleva E, et al. Neutrophilic airway inflammation and association with bacterial lipopolysaccharide in children with asthma and wheezing. Pediatr Pulmonol. 2008;43:916–923. doi: 10.1002/ppul.20880. [DOI] [PubMed] [Google Scholar]
- 30.Marguet C. Bocquel N. Benichou J. Basuyau JP. Hellot MF. Couderc L, et al. Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr Allergy Immunol. 2008;19:157–165. doi: 10.1111/j.1399-3038.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 31.Marguet C. Jouen-Boedes F. Dean TP. Warner JO. Bronchoalveolar cell profiles in children with asthma, infantile wheeze, chronic cough, or cystic fibrosis. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1533–1540. doi: 10.1164/ajrccm.159.5.9805028. [DOI] [PubMed] [Google Scholar]
- 32.Heymann PW. Platts-Mills TA. Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J. 2005;24(11 Suppl):S217–S222. doi: 10.1097/01.inf.0000188164.33856.f9. discussion S20–S21. [DOI] [PubMed] [Google Scholar]
- 33.Panickar J. Lakhanpaul M. Lambert PC. Kenia P. Stephenson T. Smyth A, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–338. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 34.Guilbert TW. Morgan WJ. Zeiger RS. Mauger DT. Boehmer SJ. Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 35.Bacharier LB. Guilbert TW. Zeiger RS. Strunk RC. Morgan WJ. Lemanske RF, Jr, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009;123:1077–1082. doi: 10.1016/j.jaci.2008.12.1120. 1082.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacharier LB. Phillips BR. Bloomberg GR. Zeiger RS. Paul IM. Krawiec M, et al. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol. 2007;119:604–610. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 37.Chipps BE. Szefler SJ. Simons FE. Haselkorn T. Mink DR. Deniz Y, et al. Demographic and clinical characteristics of children and adolescents with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2007;119:1156–1163. doi: 10.1016/j.jaci.2006.12.668. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins HA. Cherniack R. Szefler SJ. Covar R. Gelfand EW. Spahn JD. A comparison of the clinical characteristics of children and adults with severe asthma. Chest. 2003;124:1318–1324. doi: 10.1378/chest.124.4.1318. [DOI] [PubMed] [Google Scholar]
- 39.Zeiger RS. Chipps BE. Haselkorn T. Rasouliyan L. Simons FE. Fish JE. Comparison of asthma exacerbations in pediatric and adult patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2009;124:1106–1108. doi: 10.1016/j.jaci.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 40.Payne DN. Wilson NM. James A. Hablas H. Agrafioti C. Bush A. Evidence for different subgroups of difficult asthma in children. Thorax. 2001;56:345–350. doi: 10.1136/thorax.56.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sears MR. Greene JM. Willan AR. Wiecek EM. Taylor DR. Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen F. Taylor DR. Flannery EM. Cowan JO. Greene JM. Herbison GP, et al. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002;165:1480–1488. doi: 10.1164/rccm.2108009. [DOI] [PubMed] [Google Scholar]
- 43.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 44.Covar RA. Spahn JD. Murphy JR. Szefler SJ. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med. 2004;170:234–241. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 45.Strunk RC. Weiss ST. Yates KP. Tonascia J. Zeiger RS. Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006;118:1040–1047. doi: 10.1016/j.jaci.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 46.Dekkers BG. Maarsingh H. Meurs H. Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc. 2009;6:683–692. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- 47.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. quiz 45–46. [DOI] [PubMed] [Google Scholar]
- 48.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc. 2009;6:678–682. doi: 10.1513/pats.200907-067DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bush A. Menzies-Gow A. Phenotypic differences between pediatric and adult asthma. Proc Am Thorac Soc. 2009;6:712–719. doi: 10.1513/pats.200906-046DP. [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick AM. Higgins M. Holguin F. Brown LA. Teague WG. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–857.e18. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tillie-Leblond I. de Blic J. Jaubert F. Wallaert B. Scheinmann P. Gosset P. Airway remodeling is correlated with obstruction in children with severe asthma. Allergy. 2008;63:533–541. doi: 10.1111/j.1398-9995.2008.01656.x. [DOI] [PubMed] [Google Scholar]
- 52.Just J. Fournier L. Momas I. Zambetti C. Sahraoui F. Grimfeld A. Clinical significance of bronchoalveolar eosinophils in childhood asthma. J Allergy Clin Immunol. 2002;110:42–44. doi: 10.1067/mai.2002.123304. [DOI] [PubMed] [Google Scholar]
- 53.de Blic J. Tillie-Leblond I. Tonnel AB. Jaubert F. Scheinmann P. Gosset P. Difficult asthma in children: an analysis of airway inflammation. J Allergy Clin Immunol. 2004;113:94–100. doi: 10.1016/j.jaci.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 54.Payne DN. Rogers AV. Adelroth E. Bandi V. Guntupalli KK. Bush A, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 55.Payne DN. Qiu Y. Zhu J. Peachey L. Scallan M. Bush A, et al. Airway inflammation in children with difficult asthma: relationships with airflow limitation and persistent symptoms. Thorax. 2004;59:862–869. doi: 10.1136/thx.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Payne DN. Adcock IM. Wilson NM. Oates T. Scallan M. Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1376–1381. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 57.Lex C. Payne DN. Zacharasiewicz A. Li AM. Wilson NM. Hansel TT, et al. Sputum induction in children with difficult asthma: safety, feasibility, and inflammatory cell pattern. Pediatr Pulmonol. 2005;39:318–324. doi: 10.1002/ppul.20159. [DOI] [PubMed] [Google Scholar]
- 58.Covar RA. Strunk R. Zeiger RS. Wilson LA. Liu AH. Weiss S, et al. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010;125:359–366.e3. doi: 10.1016/j.jaci.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Limb SL. Brown KC. Wood RA. Wise RA. Eggleston PA. Tonascia J, et al. Adult asthma severity in individuals with a history of childhood asthma. J Allergy Clin Immunol. 2005;115:61–66. doi: 10.1016/j.jaci.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 60.Stern DA. Morgan WJ. Halonen M. Wright AL. Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vonk JM. Postma DS. Boezen HM. Grol MH. Schouten JP. Koeter GH, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004;59:925–929. doi: 10.1136/thx.2003.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Den Toorn LM. Prins JB. Overbeek SE. Hoogsteden HC. de Jongste JC. Adolescents in clinical remission of atopic asthma have elevated exhaled nitric oxide levels and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000;162(3 Pt 1):953–957. doi: 10.1164/ajrccm.162.3.9909033. [DOI] [PubMed] [Google Scholar]
- 63.Taylor DR. Cowan JO. Greene JM. Willan AR. Sears MR. Asthma in remission: can relapse in early adulthood be predicted at 18 years of age? Chest. 2005;127:845–850. doi: 10.1378/chest.127.3.845. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen F. Taylor DR. Flannery EM. Cowan JO. Greene JM. Herbison GP, et al. Risk factors for hospital admission for asthma from childhood to young adulthood: a longitudinal population study. J Allergy Clin Immunol. 2002;110:220–227. doi: 10.1067/mai.2002.125295. [DOI] [PubMed] [Google Scholar]
- 65.Sorkness RL. Bleecker ER. Busse WW. Calhoun WJ. Castro M. Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 66.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22:470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 67.Vrugt B. Wilson S. Underwood J. Bron A. de Bruyn R. Bradding P, et al. Mucosal inflammation in severe glucocorticoid-dependent asthma. Eur Respir J. 1999;13:1245–1252. doi: 10.1183/09031936.99.13612539. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 69.Moore WC. Meyers DA. Wenzel SE. Teague WG. Li H. Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miranda C. Busacker A. Balzar S. Trudeau J. Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 71.Wenzel SE. Schwartz LB. Langmack EL. Halliday JL. Trudeau JB. Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]