Abstract
Blood pressure (BP) and heart rate (HR) were studied in isoflurane-anesthetized Long-Evans rats during sinusoidal galvanic vestibular stimulation (sGVS) and sinusoidal oscillation in pitch to characterize vestibular influences on autonomic control of BP and HR. sGVS was delivered binaurally via Ag/AgCl needle electrodes inserted over the mastoids at stimulus frequencies 0.008–0.4 Hz. Two processes affecting BP and HR were induced by sGVS: 1) a transient drop in BP (≈15–20 mmHg) and HR (≈3 beat*s−1), followed by a slow recovery over 1–6 min; and 2) inhibitory modulations in BP (≈4.5 mmHg/g) and HR (≈0.15 beats*s−1/g) twice in each stimulus cycle. The BP and HR modulations were approximately in-phase with each other and were best evoked by low stimulus frequencies. A wavelet analysis indicated significant energies in BP and HR at scales related to twice and four times the stimulus frequency bands. BP and HR were also modulated by oscillation in pitch at frequencies 0.025–0.5 Hz. Sensitivities at 0.025 Hz were ≈4.5 mmHg/g (BP) and ≈0.17 beat*s−1/g (HR) for pitches of 20–90°. The tilt-induced BP and HR modulations were out-of-phase, but the frequencies at which responses were elicited by tilt and sGVS were the same. The results show that the sGVS-induced responses, which likely originate in the otolith organs, can exert a powerful inhibitory effect on both BP and HR at low frequencies. These responses have a striking resemblance to human vasovagal responses. Thus, sGVS-activated rats can potentially serve as a useful experimental model of the vasovagal response in humans.
Keywords: Otolith organs, Vertical semicircular canals, Autonomic, Heart rate, Blood pressure
Introduction
Vestibular system projections to cardiovascular centers in the CNS modulate blood pressure (BP) and heart rate (HR) in response to changes in head and body position relative to gravity (Yates and Miller 1994; Yates 1996; Balaban and Porter 1998; Yates et al. 1999; Ray 2000; Abe et al. 2008a, b, 2009; Carter and Ray 2008; Tanaka et al. 2009). Upon standing, neural activity initiated in the vestibular system operates in conjunction with the baroreflex to maintain stable blood flow in the head. Concurrently, blood flow to the legs is down-regulated by increases in peripheral resistance in order to combat gravity-dependent blood pooling in the lower extremities (Yavorcik et al. 2009). Kaufmann and colleagues (Kaufman et al. 2002) have shown that muscle sympathetic nerve activity (MSNA), which produces constriction of peripheral blood vessels in the legs, is generated during off-vertical axis rotation (OVAR), a pure otolith and body tilt receptor stimulus in the steady state. Thus, vestibular system activity controlling BP and HR can originate in the otolith organs.
Steps of galvanic vestibular stimulation have been used in many studies to activate the vestibular end organs and cause changes in BP and HR (see Courjon et al. 1987; Abe et al. 2008b, 2009 for review). Although all parts of the end organ are activated by galvanic currents (Goldberg et al. 1982), the finding that ocular roll but not nystagmus is induced by galvanic stimulation (Watson et al. 1998; MacDougall et al. 2002, 2003, 2005) has provided strong evidence that the major effect of the galvanic stimulus is on the otolith organs, not the semicircular canals.
In a series of provocative experiments, Macefield and colleagues (Elam and Macefield 2001; Elam et al. 2002; Bent et al. 2006; Grewal and James 2009; James and Mace-field 2010; James et al. 2010) have demonstrated that sinusoidal galvanic vestibular stimulation (sGVS) has a powerful facilitatory effect on the generation of MSNA in the legs. Given the low frequency of the sinusoidal stimulus and the postural sway evoked by sGVS in standing human subjects, these authors (Bent et al. 2006; Grewal and James 2009) also conclude that it is likely that this stimulus primarily activates the otolith organs.
Although sGVS has been used to evoke MSNA, its effects on BP and HR are not known. The purpose of the present experiments was to explore this in a small animal model, the anesthetized rat. Unexpectedly, we elicited vasovagal responses in many of these animals (Cohen et al. 2010a, b). In humans, a vasovagal response is characterized by a sudden reduction in BP and HR, which is frequently associated with dizziness and syncope (Lewis 1932). It is more common in females than males, frequently occurs in families, and can be provoked by a wide variety of stimuli that activate the autonomic system (Bracic and Stefanovska 1998; Connolly et al. 1999, 2003; Atiga et al. 2007; Alboni et al. 2008, 2010; Alshekhlee et al. 2008; Daas et al. 2009; Lewis 1932; Rea and Thames 1993; Gert van Dijk 2003; Suzuki et al. 2003; Giuseppe and Dinelli 2006; Robertson 2008; Pirodda et al. 2009).
The exact manner in which vestibular activation elicits vaso-vagal syncope is not known, although the diagnosis is frequently supported using a head-up tilt test, which activates vestibular and body tilt receptors. Moreover, although such responses have been described in animals (Gert van Dijk 2003), there is no robust animal model of the response in which the neural mechanisms and physiology of the vaso-vagal response can be investigated adequately. In this study, we used an implanted telemetric BP transducer to assess the impact of sGVS on BP and HR in the anesthetized rat and compared it to the effect of head/body tilt. The data demonstrate that the response to sGVS in the anesthetized rat is a possible animal model of the disorder and that an otolith-autonomic link plays an important role in eliciting this response.
Methods
Seven adult, male Long-Evan rats (Harlan Laboratories, MA) weighing 300–400 g were used in these studies. All experiments were approved by the Institutional Care and Use Committee of the Mount Sinai School of Medicine.
Surgical procedures
The implantation of a blood pressure measurement device and a head fixation mount were accomplished during the same aseptic surgical session. Throughout the surgery, rats were kept on a heating-pad controlled by the feedback of a rectal temperature probe. The surgery and testing were conducted under isoflurane anesthesia (4% induction, 2% maintenance).
Implantation of bolts to allow fixation of the head during experiments
After induction of anesthesia, rats were placed in a stereotaxic frame, and a round patch of skin was removed from the calvarium. The latter was freed from periosteum and four sterile stainless steel screws were secured into small holes drilled around the perimeter of the skull. The screws were secured with dental acrylic cement, and two nuts were encased in semisoft acrylic. These were used to immobilize the head during experiments.
Cannulation of carotid and femoral arteries and implantation of telemetric blood pressure sensors in the aorta
In one rat (#579), a transducer catheter was introduced via a small arteriotomy implanted in the femoral artery and advanced into the abdominal aorta. The catheter was secured with two ties around the femoral artery. In a second rat (#578), the catheter was introduced into the external carotid artery. For this procedure, the neck muscles were retracted laterally to expose the artery, which was retracted downward while the catheter was inserted. The ligature that tied the artery was then released. Blood pressure (BP) was recorded as described below. Both rats were euthanized at the end of the experiments.
In all other rats, a telemetric blood pressure sensor (DSI, St Paul, MN) was implanted in the abdominal aorta. These animals were utilized in a series of experiments performed over the next 2 months. After placing a 2-cm incision in the groin, the femoral artery was isolated and clamped with two mini-clamps. The transducer catheter was inserted into the vessel via a small arteriotomy and, after removing the cranial clamp, was advanced into the abdominal aorta. The catheter was secured with two ties around the artery, and the body of the sensor was placed into a subcutaneous pocket in the animal’s flank. The pocket was closed with a purse-string suture and the skin incision with surgical staples. After recovery from anesthesia, animals were returned to their cages. Post-surgical pain was managed with buprenorphine (0.05 mg/kg BID, SQ) for 3 days, and rats were available for experiments 7–10 days after the surgery.
Sinusoidal galvanic vestibular stimulation (sGVS)
During testing, the heads of the rats were immobilized using the head mounts attached to a cylindrical holder for the animal’s body. Sinusoidal currents generated by a computer-controlled stimulator (Kaufman et al. 2002) were delivered via two Ag/AgCl needle electrodes inserted into the skin over the mastoids, behind the external auditory meati. sGVS was given binaurally in six animals (#578, #579, #588, #602, #618, #619) with currents of 1–4 mA and frequencies of 0.008 to 0.5 Hz. Current and frequencies were randomized, and 15–30 min were allowed to elapse between stimuli to reduce possible effects of habituation.
Head and body tilts
To test responses to tilt, animals (#599, #602) were placed in the body holder, which was secured to the platform of a tilt-table that could be oscillated by a computer-controlled motor. Animals were stimulated in the roll or pitch planes by orienting the head and body around the naso-occipital (roll) or interaural (pitch) axes at frequencies of 0.004 to 0.5 Hz.
Regardless of the nature of the stimuli, the parameters of stimulation such as frequency and angle of tilt were randomized and 15–30 min was allowed to elapse between stimuli to reduce possible effects of habituation.
Data collection and analysis
BP in response to sGVS was recorded continuously using customized A/D conversion hardware (Grass Technologies, West Warwick, RI) and Polyview software (Grass technologies) and stored at a rate of 1 kHz. BP data from the telemetric sensors were collected via a wand receiver (DSI, St Paul, MN) at 1 kHz with 12 bit resolution (Data Translation, Inc) using our data collection program. The data were converted for analysis using our VMF data analysis software.
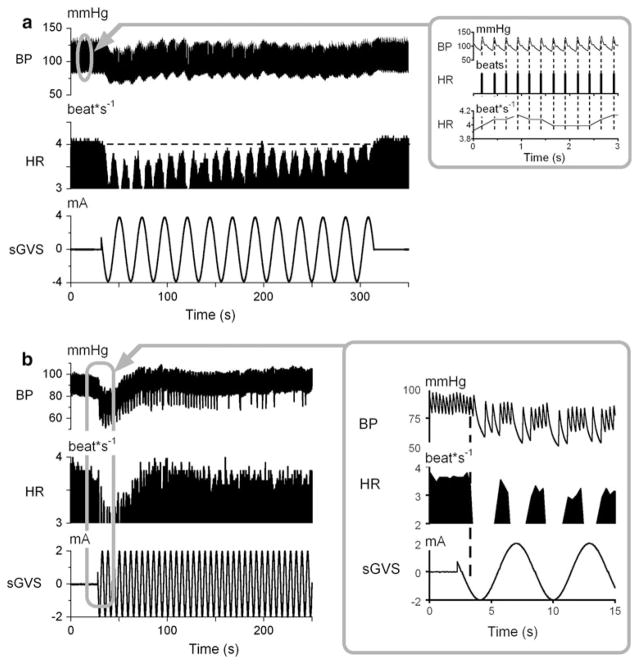
BP was utilized to obtain heart rate (HR) off-line, as shown in the inset of Fig. 1a. The peaks of the rapid increases in BP (top trace) were detected (Fig. 1 inset, vertical dashed lines, 2nd trace). HR was identified from the peaks in BP (Fig. 1 inset, 1st trace). Stored pulses were converted to instantaneous frequency (beat*s−1) and stored in a separate channel for further analysis. Mean square sinusoidal fits to the data were used to estimate variations of BP and HR to the sinusoidal oscillations.
Fig. 1.
a Binaural sGVS in Rat #578 at 0.025 Hz, 4 mA (bottom trace) caused a drop in BP (1st trace) and HR (2nd trace). BP and HR oscillated at twice the stimulus frequency (3rd trace). The horizontal dashed line denotes the initial HR and extends to its recovery after the end of the stimulus. Inset 1a, BP (1st trace), HR (2nd trace), and instantaneous HR (3rd trace) determined from peaks of systolic BP. (See text for description). b sGVS of Rat #588 at 0.17 Hz, 2 mA (bottom trace) caused large modulations in BP (1st trace) and HR (2nd trace) at twice the stimulus frequency (inset). Time base on abscissas in sec
Wavelet transforms of the sGVS stimulus, BP, and HR were utilized to assess the temporal changes of the signals for specific frequency bands or scales. The analysis was performed using Matlab (Mathworks, Inc.) and its implementation of the Daubechies function, db4 as the mother wavelet. This wavelet implementation filters the data using 8 coefficients and can encode functions that have variability of polynomials of the order 3. The order of the function was sufficient to capture the dominant frequency components of the transient signal with the scaling function and the higher frequencies with multiples of this scale.
For analysis of the tilt responses using sine wave fits, the significance of the fits of the data was determined using a reduced case of ANOVA (F statistic) (Yakushin et al. 1995, 2011). The significance of the regression lines for the functional relationships between the stimuli and responses was determined by the critical r value (Daniel 1995). The difference between two groups of data was determined using t test with a Bonferroni correction.
Results
sGVS stimulation
sGVS induced two relatively independent responses, which are demonstrated in the responses of two rats in Fig. 1. At the onset of stimulation, there was a transient drop in BP and HR that slowly recovered over periods that could last as long as 6 min (Fig. 1a). The changes in BP and HR associated with these transient changes were significant (P < 0.001). The largest decreases in BP and HR were in the first hemicycles (≈10 s) of stimulation (Fig. 1b, inset).
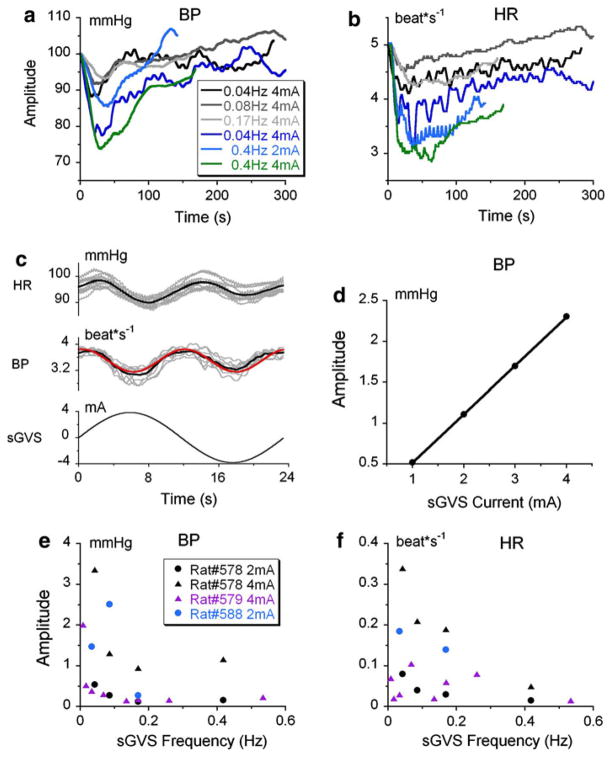
Four animals were tested with various stimulus parameters. Transient responses were defined as 10 mmHg or larger sudden drops in BP in response to sGVS. The transient, slow components of the responses to sGVS were evoked at least once in each of the animals and were elicited many times in others (Fig. 2a, b, Rat 588, Table 1), although overall these transient responses were only present in 20 ± 8% of stimulation trials. One of the first rats tested had distinct transient response when stimulated at 0.025 Hz at 3 mA. Two other animals were repeatedly tested with this stimulus (Rat #618 three and nine times; Rat 619 ten times). No transient responses were observed in these animals. The shortest latency of the responses in BP and HR were ≈2 s. The general profiles of the transient responses were similar in all animals in which the response was evoked, but the amplitude of the initial drop in BP and HR and the time course of recovery varied (Fig. 2a, b). These data indicated marked differences in susceptibility to sGVS between rats and considerable variability in response within individual subjects.
Fig. 2.
Filtered traces (0.05 Hz) of drops in BP (a) and HR (b) produced by stimulation at frequencies of 0.04–0.4 Hz and stimulus currents of 2 and 4 mA, as noted in box in a. There was an initial drop and recovery in both BP and HR in all recordings, but the time of recovery varied. c Modulations in BP (1st trace) and HR (2nd trace) of Rat #578, during 20 cycles of sGVS at 0.025 Hz. The black lines in the 1st and 2nd traces are the means of the responses. The red line is a sinusoidal fit at double frequency. The bottom trace is the stimulating sinusoid. d Increases in amplitude of sinusoids at 0.025 Hz when evoked by stimulus currents of 2–4 mA. e, f Amplitude of the modulations in BP and HR (ordinates) induced by different stimulation frequencies (abscissa)
Table 1.
Transient (vasovagal) responses observed in experiments with random variation of stimulus frequency and amplitude
| Animal’st ID | Number of stimulation | Vasovagal response
|
|
|---|---|---|---|
| Number | (%) | ||
| Rat #578 | 23 | 6 | 26 |
| Rat #579 | 14 | 3 | 21 |
| Rat #588 | 17 | 3 | 18 |
| Rat #602 | 13 | 1 | 8 |
| Average | 20 ± 8% | ||
In the examples shown in Fig. 1b, the mean BP decreased by about 10–20 mmHg and HR decreased from about 3.5–4 to 2.5–3 beat*s−1. When the HR dropped, the subsequent changes in BP during systole were large (Fig. 1b, inset), presumably due to increased filling of the heart in accordance with Starling’s Law (Starling 1918). There was no consistent correlation between the frequencies and current levels of sGVS that were effective in inducing the transient component of the responses. Thus, the variability in evocation of the responses in Fig. 2a, b was typical.
Superimposed on the transient responses were sinusoidal modulations of BP and HR (Fig. 1b inset, Fig. 2c). There were two modulations in BP and HR for each stimulus cycle, and the modulations were close to being in-phase with each other (Fig. 2c). The amplitude of the modulations was largest at the onset of stimulation and decreased or was maintained throughout stimulation. Figure 1a illustrates this: the modulations were generated throughout stimulation, until the slow component had recovered and returned to its pre-stimulation steady state level (Fig. 1a, dashed horizontal line). In some instances (Fig. 1b), the modulations could outlast the recovery of the transient component.
In contrast to the transient changes in BP and HR, in which there was no consistent relationship with either frequency or strength of stimulation, there was a strong correlation between the average amplitude of the modulations and the frequencies and current levels of sGVS (Fig. 2d, e, f). Thus, the amplitudes of the modulations of BP and HR were larger for larger current amplitudes (Fig. 2d). They were also best evoked at the lowest frequencies of stimulation (0.04 Hz), were substantially diminished for stimulation frequencies above 0.5 Hz (Fig. 2e, f) and were never induced at frequencies above 0.4 Hz. The different characteristics of the transient or ‘slow’ response and the modulations suggest that the two components are produced by different neural processes.
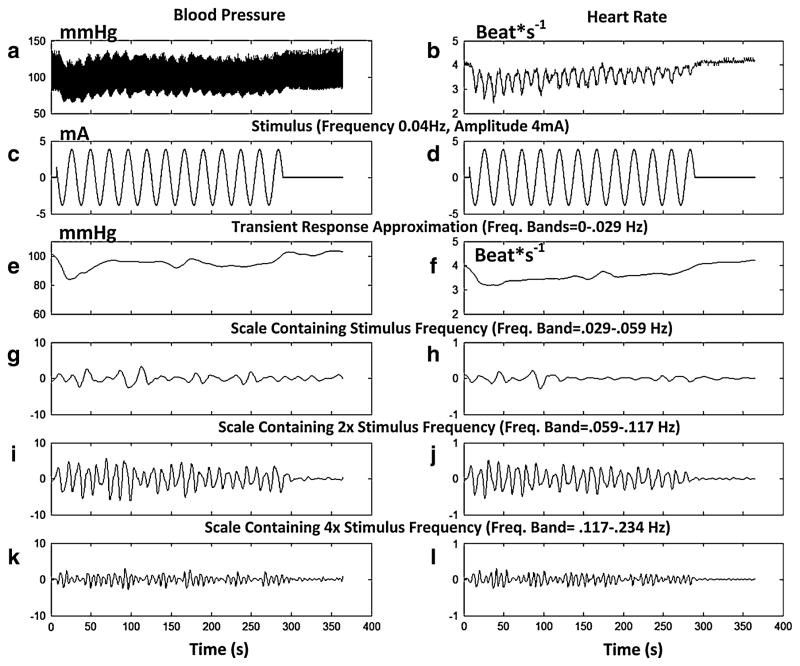
Because the BP and HR signals contained a complex mixture of frequencies in response to the stimulus, a wavelet analysis was performed that allowed better separation of the various time–frequency components that were generated by the stimulation as well as comparisons with the distribution of the stimulus frequency bands (Harris 2004). This analysis also enabled a determination of energy distributions that were evoked in BP and HR by the sGVS (Figs. 3, 4). The wavelet analysis indicated that the temporal variations of BP (Fig. 3a) and HR (Fig. 3b) in response to the stimulus (Fig. 3d) had the largest energy in scales associated with HR and its harmonics (Fig. 4, shaded regions). These scales were not considered further as they were not clearly associated with the stimulus. Rather, BP and HR signals were examined in four frequency bands that were associated with the stimulus and its harmonics (Fig. 3e–l).
Fig. 3.
Wavelet analysis (Daubechies Wavelet, db4) of BP (a) and HR (b) to stimulation at 0.025 Hz and 4 mA. The approximation to the transient response was determined by the highest energy band among the low frequency scales. These scales were combined up to the band that had the highest energy (0–0.029 Hz). c, d Stimulus. The approximation to the transient response had a small and slow initial drop for both BP (e) and HR (f). Three scales contained the stimulus frequency (g, h), twice the stimulus frequency (i, j), and four times the stimulus frequency (k, l). The stimulus activated these bands. Most of the activity for this stimulus frequency (0.025 Hz) was contained in the band containing twice the stimulus frequency (i, j)
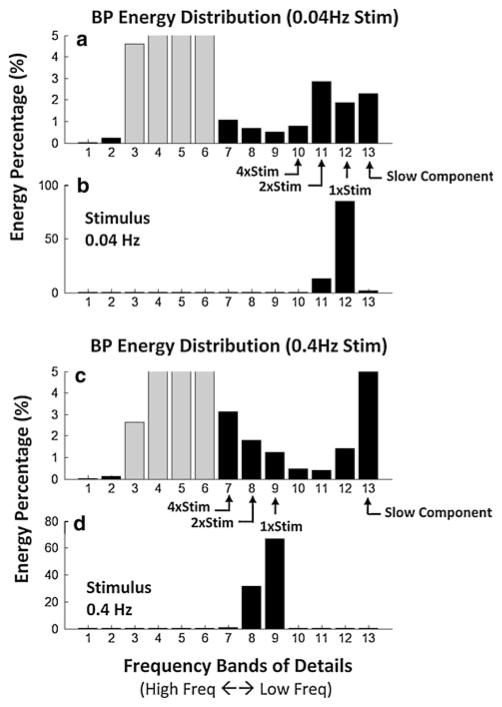
Fig. 4.
Differences in energy distribution of BP in component frequency bands (a, c) between low frequency (b) and high frequency (d) stimuli after wavelet decomposition. The abscissa represents the scales (1–13) associated with specific frequency bands from the highest frequency to the lowest frequency (left to right). The ordinate represents the energy in each band as a percentage of the total. The gray bars are the energies associated with HR and its harmonics. These bands had the largest energies, but are unrelated to the stimulus, and were not considered. The transient response (band > #13) has a larger energy percentage when the stimulus frequency was 0.4 (c) than when it was 0.025 Hz (a). The energy of bands that contain 1×, 2×, and 4× stimulus frequency also have different patterns between the two stimulus frequencies. When the frequency was low (a), bands of BP associated with the stimulus (12, 11, and 10) have peak energy in the band containing 2× stimulus frequency (band 11), whereas when the frequency was high (d), the BP bands associated with the stimulus (d) (bands 9, 8, 7) progressively increase in energy with a peak at 4× stimulus frequency
The first band (Fig. 3e, f) represented a scale comprised of low frequencies (0–0.029 Hz). When these bands were combined, the temporal behavior approximated the transient response, which had a small, slow initial drop followed by an even slower return to a steady state value for both BP and HR (Fig. 3e, f).
Three scales contained the stimulus frequency (0.025 Hz; Fig. 3g, h), twice the stimulus frequency (Fig. 3i, j), and four times the stimulus frequency (Fig. 3k, l). For a stimulus frequency of 0.025 Hz (Fig. 3c, d), which was most effective in generating the modulations in BP and HR (Fig. 2e, f), most of the activity in the BP and HR signals was contained in the bands that had twice the stimulus frequency (0.05 Hz; Fig. 3i, j). Of note, the modulations in BP and HR declined as the stimulus continued in this instance. The same occurred in the wavelet analysis of the trace in Fig. 2b (not shown). This confirms that the stimulus not only evoked activity at the stimulus frequency but also at twice and four times that frequency.
The temporal responses associated with these different bands also showed a separation of the transient and modulatory responses, since the latter persisted until the end of stimulation, while the transient BP response, which essentially covered the low frequency band, lasted only about 50 s (Fig. 3e).
The responses to a stimulus of 0.17 Hz, 2 mA (Fig. 1b) had an altered frequency band distribution (not shown). The initial drop in the transient BP and HR responses induced by stimulation at 0.17 Hz in Rat #588 were larger and faster than the response induced by the 0.04 Hz stimulus in Rat #578 (Fig. 3e, f). As in Fig. 3, there was less activity at scales containing the stimulus frequency than at twice the stimulus frequency, and in this case even greater activity in the band containing four times the stimulus frequency.
The wavelet data were further analyzed by calculating the energy in the different bands for BP and HR for the responses of Fig. 1a, b (Fig. 4). The bands associated with HR and its harmonics had the largest energies (Fig. 4 gray bars), as expected, but were unrelated to the stimulus and were not considered further. The transient response (bands #13) had a larger energy percentage when the stimulus frequency was 0.17 Hz (Fig. 4c) than when it is 0.025 Hz (Fig. 4a). The energy of the bands that contained 1×, 2×, and 4× the stimulus frequency also had different patterns for the two stimuli. When the stimulus frequency was low (Fig. 4a), frequencies in the BP signal associated with the stimulus (scales 12, 11, and 10) had peak energy in the band containing 2× stimulus frequency (scale 11). When the frequency was higher (Fig. 4d), the energy in the bands of the BP signal associated with the stimulus (scales 9, 8, 7) progressively increased to a peak at 4× the stimulus frequency. Thus, frequency bands of BP were activated differently, and had different energy distributions in the various frequency bands, at the different stimulus frequencies (Fig. 4b, d).
Sinusoidal oscillation in pitch and roll
To provide a basis of comparison with the results of sGVS, BP and HR of the anesthetized rats were tested in response to sinusoidal changes in the gravity vector in head coordinates. The animals were oscillated up to ±90° around the naso-occipital (roll) and interaural (pitch) axes. Because activation of the otolith organs and the semicircular canals during tilts was essentially at a single frequency, BP and HR amplitudes were determined at the frequency of oscillation. There were no consistent changes in BP or HR when animals were oscillated in roll, but both BP and HR were modulated by oscillation in pitch (Fig. 5a). The bias level of BP was lower at a higher than a lower anesthesia level (Fig. 5a) although the modulations in BP and HR were not statistically different at both levels (3.2 vs. 3.6 mmHg, 0.052 vs. 0.058 beat*s−1, P > 0.05).
Fig. 5.
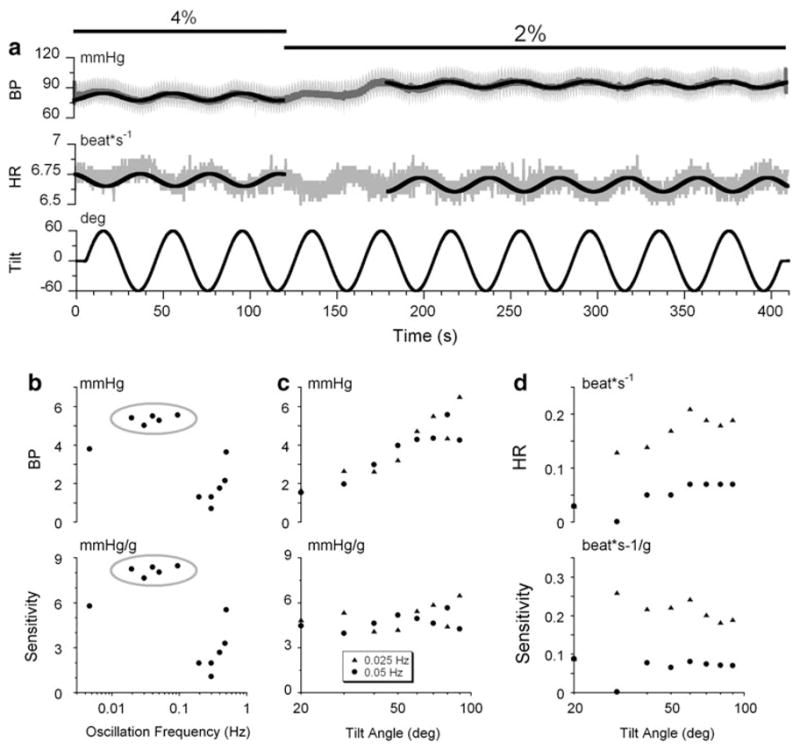
a Modulations in BP (1st trace) and HR (2nd trace) in Rat #599 produced by ± 90°, 0.025 Hz oscillation in pitch at two levels of isoflurane anesthesia (4 and 2%). Note that BP and HR oscillated out-of-phase during changes in head and body position in pitch re gravity. Changes in the level of anesthesia did not affect HR, but caused a rise of about 4 mm Hg in BP. b Effect of frequency of head and body pitch on modulation of BP (b, top graph), and on the sensitivity to gravity (b, bottom graph). The gray ellipses emphasize the frequency range (0.01 < α ≤ 0.1). c Effect of the angle of tilt of the head and body pitch on the modulation in BP (c, top graph), and on sensitivity to gravity (c, bottom graph). BP was modulated as a linear function of the tilt angle (c) and the sensitivity to gravity was the same across angles (d, bottom graph). Effect of changes in the amplitude of modulation of head and body pitch ± 60° at frequencies below 1 Hz in Rat #599 on the modulation of HR (d, top graph), and on the sensitivity to gravity (e, bottom graph)
Oscillations in pitch induced changes in BP and HR that were out-of-phase with each other, whereas they were in-phase during sGVS (Fig 1b, Inset; Fig. 2c). Thus, in contrast to the findings obtained using sGVS, the decreases in HR observed during pitch were not likely to be produced by changes in BP.
The changes in BP and HR during pitch tilt were characterized using the different frequencies of oscillation (Fig. 5b) and different amplitudes of pitch (Fig. 5c. d, Rat #599). BP was maximally altered by oscillation between 0.025 and 0.1 Hz (Fig. 5b, gray ellipse), similar to the best frequencies of induction of the changes in BP and HR using sGVS. There was a weaker response of BP to oscillation in pitch at 0.01 and ≤0.1 Hz. The amplitude of the modulation in BP (Fig. 5c) and HR (Fig. 5d), which were at the frequency of tilt, increased steadily as the angle of tilt was increased. The sensitivity of BP to changes in head and body position re gravity were constant at about 4.5 mmHg/g (Fig. 5e, bottom) at frequencies of 0.025 and 0.05 Hz. HR also increased as a function of the angle of tilt, but with greater variation (Fig. 5d). When the sensitivity to gravity was calculated, the data fell along horizontal lines, suggesting that the sensitivity of BP and HR to head and body position re gravity was constant.
The average sensitivity of HR, contrary to that of BP, was different at two frequencies of sGVS. It was greater at 0.025 Hz (0.17 beat*s−1/g) than at 0.05 Hz (0.054 beat*s−1/g, P = 1.5*10−6, t test) indicating dependence of HR sensitivity on stimulus frequency, but not on tilt angle (Fig. 5d). Similar results were observed in Rat #602 (not shown).
Discussion
The major finding of this study is that sinusoidal galvanic vestibular stimulation (sGVS) at low frequencies produces strong reductions in BP and HR in anesthetized rats. Two types of changes were induced by sGVS: a large initial decrease in BP and HR, which slowly recovered over periods as long as 6 min, and strong inhibitory modulation of BP and HR at twice the frequency of stimulation. Thus, when appropriately stimulated, the portion of the vestibular system processing otolith signals can exert a powerful inhibitory effect on both BP and HR that simulates the changes observed in humans during vasovagal syncope. To our knowledge, the vasovagal response to sGVS has not been described before, and there is no adequate animal model for studying the vasovagal response. Our data suggest that the rat could provide such a model.
The steep initial drops in BP and HR that occurred within approximately 2 s from onset of sGVS were found across rats. The latencies of these responses were consistent with latencies of 1.4 s observed in BP of anesthetized cats in response to nose-up tilts (Woodring et al. 1997). The variability in the time course of recovery may imply that the slow responses were due to differences in the levels of transmitter release and/or balance of sympathetic and parasympathetic activity across animals, or to the release of neuro-modulators with variable rates of uptake.
The dramatic fall in BP and HR during the inhibitory modulations illustrated in the inset of Fig. 1b suggests that the primary cause for the reduction in BP following sGVS was the decrease in HR. This is most likely due to a stimulus-dependent activation of the parasympathetic innervation of the heart, mediated through cells in the dorsal motor vagal nucleus. In fact, axons of cells in the caudal vestibular nuclear complex have been observed in the dorsal motor vagal nucleus and the solitary nucleus (Balaban and Beryozkin 1994; Yates et al. 1994; Porter and Balaban 1997); these vestibular areas are a major target of afferent fibers from the otolith organs (Yates et al. 1993a; Newlands and Perachio 2003) Activation of the pathway to the dorsal motor vagal nucleus would shift the autonomic balance toward increased parasympathetic activity, resulting in a reduction in sympathetic tone and a decrease in HR. In this case, the decline in BP observed in response to sGVS could be a downstream physiological effect of the reduction in HR. In addition, vestibular projections in the solitary nucleus offer a more direct route for the vestibular system to modulate BP through the neural pathways that mediate the baroreflex (for review, see (Balaban 2004)). However, the most direct pathway supporting vestibular influences on the sympathetic control of BP derives from the caudal vestibular nuclei and projects to the vasomotor pre-sympathetic neurons in the rostral ventrolateral medulla (Holstein et al. 2011). It is likely that the tilt stimuli in the present study activated this direct pathway, causing a reduction in BP that was independent of the changes in HR.
The sensitivity of HR and BP to pitch tilts were independent of tilt angle in this study, similar to the response of splanchnic nerve fibers to natural vestibular stimulation (Yates and Miller 1994). Tilts in the roll plane were ineffective in our study, consistent with findings in normal cats, in which only nose-up but not roll stimulation produce cardiovascular responses (Yates and Miller 1994; Yates 1996; Woodring et al. 1997). However, linear oscillation about any axis in the horizontal plane produces similar 7–9 mmHg oscillations of BP (Yates et al. 1999; Zhu et al. 2007). Many studies indicate that BP and HR responses to sinusoidal oscillation in pitch are of otolith origin (Lindsay et al. 1945; Spiegel 1946; Megirian and Manning 1967; Tang and Gernandt 1969; Uchino et al. 1970; Doba and Reis 1974; Ishikawa et al. 1979; Ishikawa and Miyazawa 1980; Yates et al. 1993b; Kerman and Yates 1998; Jian et al. 1999; Kerman et al. 2000). Sympathetic nerve activity is modulated by otolith and not canal-related inputs (Yates and Miller 1994). Furthermore, changes in BP induced by nose-up tilt are held for as long as this position is maintained (Woodring et al. 1997), indicating that activity for these responses was otolith related, not canal related. Body tilt receptors also participate (Lindsay et al. 1945) in sensing tilt in the vestibular nuclei (Yates et al. 2000; Woodring et al. 1997).
In this regard, it is of interest that the gravitationally induced changes in BP and HR were optimally produced at the same low frequencies as the inhibitory modulations in BP and HR that were generated by sGVS (Fig. 2e, f and Fig. 5). These frequencies are also the same optimal frequencies for generating counter-pitch and counter-roll in the rabbit during OVAR and translation while rotating (TWR), which are both pure otolith-induced responses (Maruta et al. 2005, 2008). Although high-frequency eye movements can be induced in frontal eyed species by otolith stimulation (Paige and Tomko 1991), this is not the case in lateral-eyed species and otolith-induced eye movements are best generated by frequencies below 0.1 Hz. Taken together, these studies indicate that BP and HR control through the vestibular system is primarily mediated through the otolith system and has the same frequency bounds as orienting eye movements such as ocular roll and vergence. In this regard, it is of interest that the same low frequencies are critical for the generation of motion sickness, which also involves the otolith system (Dai et al. 2011). Whether the semicircular canals have the same access to BP and HR is not known, but from these results, the major control of BP and HR in the vestibular system is derived from otolith signals.
The wavelet analysis was particularly useful in identifying the frequency bands that were elicited in BP and HR and in comparing the energies in these bands to that of the stimulus. The changes in BP and HR induced by sGVS in the rat were similar to the changes in BP and HR observed in a wavelet analysis of a ‘syncopal youth’ during a faint by Nowak et al. (2008). The slow component in the rat and the wavelet-based frequency band distribution bore a striking resemblance to the human data. As in humans, the slow response was not elicited in all rats, nor could it always be elicited in susceptible rats, but it appeared many times in a susceptible animal (Fig. 2a, b).
The wavelet analysis showed that the transient component of the alterations in BP and HR was confined to low frequency bands, although the energy distribution for BP and HR were somewhat different. Since sGVS mainly activates the otolith organs, the data suggest that the otolithic system can exert a powerful inhibitory effect on both BP and HR whenever activated, independent of direction of activation.
These frequency bands also demonstrated that there was significant energy at 4× the stimulus frequency when the stimulus was 0.17 Hz, at the upper limit of frequencies that could activate the simultaneous inhibitory modulation in BP and HR. Whether the energies distributed in the high frequency band represented dispersion of the responses that were more coherent at the lower frequencies of sGVS (0.025 Hz) or were due to activity generated by the higher stimulus frequencies is not known. Regardless, the wavelet analysis clearly demonstrated that despite the diffuse nature of the sGVS stimulus to the vestibular end organs, the changes in BP and HR were associated with the stimulus frequency.
This study has concentrated on binaural sGVS, mirroring the work of Macefield and associates. For this stimulation, the otolith afferents would be activated on both sides in opposite polarity in each hemicycle of stimulation. The double response to each sinusoid suggests that perhaps alternation in the anodal and cathodal currents between the two ears had alternately activated the labyrinths on each side.
To our knowledge, there is no robust animal model of the vasovagal response (Gert van Dijk 2003). Treatments for vasovagal syncope vary from beta-blockers to implantation of pacemakers (for recent reviews, see (Medow et al. 2008; Aydin et al. 2010), but many of these treatments are not particularly successful in protecting affected individuals (Fenton et al. 2000; Folino 2007; Wieling et al. 2009). The present study suggests that sGVS stimulation in the rat can provide a useful animal model to study these responses.
Acknowledgments
Supported by NIH Grants: DC008846 (GRH), DC004996 (SBY) and Core Center DC05204 (BC).
Contributor Information
Bernard Cohen, Email: Bernard.Cohen@mssm.edu, Department of Neurology, Mount Sinai School of Medicine, Box 1135 1 East 100th Street, New York, NY 10029, USA.
Giorgio P. Martinelli, Department of Neurology, Mount Sinai School of Medicine, Box 1135 1 East 100th Street, New York, NY 10029, USA
Dmitri Ogorodnikov, Department of Neurology, Mount Sinai School of Medicine, Box 1135 1 East 100th Street, New York, NY 10029, USA.
Yongqing Xiang, Department of Computer and Information Sciences, Brooklyn College of the City University of New York, Brooklyn, NY 11210, USA.
Theodore Raphan, Department of Computer and Information Sciences, Brooklyn College of the City University of New York, Brooklyn, NY 11210, USA.
Gay R. Holstein, Department of Neurology, Mount Sinai School of Medicine, Box 1135 1 East 100th Street, New York, NY 10029, USA. Department of Neuroscience, Mount Sinai School of Medicine, New York, NY 10029, USA
Sergei B. Yakushin, Department of Neurology, Mount Sinai School of Medicine, Box 1135 1 East 100th Street, New York, NY 10029, USA
References
- Abe C, Tanaka K, Awazu C, Morita H. Impairment of vestibular-mediated cardiovascular response and motor coordination in rats born and reared under hypergravity. Am J Physiol Regul Integr Comp Physiol. 2008a;295:R173–R180. doi: 10.1152/ajpregu.00120.2008. [DOI] [PubMed] [Google Scholar]
- Abe C, Tanaka K, Awazu C, Morita H. Strong galvanic vestibular stimulation obscures arterial pressure response to gravitational change in conscious rats. J Appl Physiol. 2008b;104:34–40. doi: 10.1152/japplphysiol.00454.2007. [DOI] [PubMed] [Google Scholar]
- Abe C, Tanaka K, Awazu C, Morita H. Galvanic vestibular stimulation conteracts hypergravity-induced plastic alteration of vestibulo cardiovascular reflex in rats. J Appl Physiol. 2009;107:1089–1094. doi: 10.1152/japplphysiol.00400.2009. [DOI] [PubMed] [Google Scholar]
- Alboni P, Alboni M, Bertorelle G. Origin and evolution of vaso-vagal syncope: to protect the heart or to escape predation? Clin Auton Res. 2008;18:170–178. doi: 10.1007/s10286-008-0479-7. [DOI] [PubMed] [Google Scholar]
- Alboni P, Alboni M, Bertorelle G. Origin and evolution of vaso-vagal syncope. G Ital Cardiol (Rome) 2010;11:20–27. [PubMed] [Google Scholar]
- Alshekhlee A, Guerch M, Ridha F, Mcneeley K, Chelimsky T. Postural tachycardia syndrome with asystole on head-up tilt. Clin Auton Res. 2008;18:36–39. doi: 10.1007/s10286-007-0445-9. [DOI] [PubMed] [Google Scholar]
- Atiga WL, Rowe P, Calkins H. Management of vasovagal syncope. J Cardiovasc Electrophysiol. 2007;10:874–886. doi: 10.1111/j.1540-8167.1999.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Aydin MA, Salukhe TV, Wilke I, Willems S. Management and therapy of vasovagal syncope: a review. World J Cardiol. 2010;2:308–315. doi: 10.4330/wjc.v2.i10.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban CD. Projections from the parabrachial nucleus to the vestibular nuclei: potential substrates for autonomic and limbic influences on vestibular responses. Brain Res Bull. 2004;996:126–137. doi: 10.1016/j.brainres.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibulo-autonomic interactions. Exp Brain Res. 1994;98:200–212. doi: 10.1007/BF00228409. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Porter JD. Neuroanatomic substrates for vestibulo-autonomic interactions. Exp Brain Res. 1998;8:200–212. [PubMed] [Google Scholar]
- Bent LR, Bolton PS, Macefield VG. Modulation of muscle sympathetic bursts by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2006;174:701–711. doi: 10.1007/s00221-006-0515-6. [DOI] [PubMed] [Google Scholar]
- Bracic M, Stefanovska A. Wavelet-based analysis of human blood-flow dynamics. Bull Math Biol. 1998;60:919–925. doi: 10.1006/bulm.1998.0047. [DOI] [PubMed] [Google Scholar]
- Carter JR, Ray CA. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R681–R688. doi: 10.1152/ajpregu.00896.2007. [DOI] [PubMed] [Google Scholar]
- Cohen B, Yakushin SB, Martinelli GP, Ogorodnikov D. Blood pressure and heart rate changes induced by sinusoidal galvanic stimulation of the labyrinths in the anesthetized rat. J Vestibular Res. 2010a;20:201. [Google Scholar]
- Cohen B, Yakushin SB, Martinelli GP, Ogorodnikov D, Flores R, Holstein GR. Ass Res in Otolaryngology. Vol. 385. Anaheim; California: 2010b. Feb 6–10, Blood pressure and heart rate changes from labyrinthine stimulation in the anesthetized rat; p. 133. [Google Scholar]
- Connolly SJ, Sheldon R, Roberts RS. The North American Vasovagal Pacemaker Study (VPS). A randomized trial of permanent cardiac pacing for the prevention of vasovagal syncope. J Am Coll Cardiol. 1999;33:16–20. doi: 10.1016/s0735-1097(98)00549-x. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Sheldon R, Thorpe K. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope. JAMA. 2003;289:2224–2229. doi: 10.1001/jama.289.17.2224. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Precht W, Sirkin DW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Exp Brain Res. 1987;66:41–48. doi: 10.1007/BF00236200. [DOI] [PubMed] [Google Scholar]
- Daas A, Mimouni-Bloch A, Rosenthal S, Shuper A. Familial vasovagal syncope associated with mirgraine. Pediatr Neurol. 2009;40:27–30. doi: 10.1016/j.pediatrneurol.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Dai M, Raphan T, Cohen B. Prolonged reduction of motion sickness sensitivity by visual-vestibular interaction. Exp Brain Res. 2011 doi: 10.1007/s00221-011-2548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel WW. Biostatistics. A foundation for analysis in the health sciences. Wiley; New York: 1995. [Google Scholar]
- Doba N, Reis DJ. Role of the cerebellum and the vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res. 1974;40:9–18. doi: 10.1161/01.res.40.4.9. [DOI] [PubMed] [Google Scholar]
- Elam M, Macefield V. Multiple firing of single muscle vasocon-strictor neurons during cardiac dysrhythmias in human heart failure. J Appl Physiol. 2001;91:717–724. doi: 10.1152/jappl.2001.91.2.717. [DOI] [PubMed] [Google Scholar]
- Elam M, McKenzie D, Macefield V. Mechanisms of sympat-hoexcitation: single-unit analysis of muscle vasoconstrictor neurons in awake OSAS subjects. J Appl Physiol. 2002;93:297–303. doi: 10.1152/japplphysiol.00899.2001. [DOI] [PubMed] [Google Scholar]
- Fenton AM, Hammill SC, Rea RF, Low PA, Shen W-K. Vaso-vagal syncope. Ann Intern Med. 2000;133(9):714–725. doi: 10.7326/0003-4819-133-9-200011070-00014. [DOI] [PubMed] [Google Scholar]
- Folino AF. Cerebral autoregulation and syncope. Prog Cardio-vasc Dis. 2007;50:49–80. doi: 10.1016/j.pcad.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Gert van Dijk J. Fainting in animals. Clin Auton Res. 2003;13:247–255. doi: 10.1007/s10286-003-0099-1. [DOI] [PubMed] [Google Scholar]
- Giuseppe F, Dinelli M. The venous system is the main determinant of hypotension in patients with vasovagal syncope. Europace. 2006;8:839–845. doi: 10.1093/europace/eul095. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C, Smith CE. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res. 1982;252:156–160. doi: 10.1016/0006-8993(82)90990-8. [DOI] [PubMed] [Google Scholar]
- Grewal T, James C. Frequency-dependent modulation of muscle sympathetic nerve activity by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2009;197:379–386. doi: 10.1007/s00221-009-1926-y. [DOI] [PubMed] [Google Scholar]
- Harris FJ. Multirate signal processing for communication systems. Prentice Hall PTR; Upper Saddle River, NJ: 2004. p. 478. [Google Scholar]
- Holstein GR, Friedrich VLJ, Kang T, Kukielka E, Martinelli GP. Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience. 2011;175:104–117. doi: 10.1016/j.neuroscience.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Miyazawa T. Sympathetic responses evoked by vestibular stimulation and their interactions with somato-sympathetic reflexes. J Auton Nerv Syst. 1980;1:243–254. doi: 10.1016/0165-1838(80)90020-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Miyazawa T, Shimizu I, Tomita H. Similarity between vestibulo-sympathetic response and supraspinal sympathetic reflex. Nihon Univ J Med. 1979;21:201–210. [Google Scholar]
- James C, Macefield VG. Competitive interactions between vestibular and cardiac rhythms in the modulation of muscle sympathetic nerve activity. Auton Neurosci. 2010;158:127–131. doi: 10.1016/j.autneu.2010.07.005. [DOI] [PubMed] [Google Scholar]
- James C, Stathis A, Macefield VG. Vestibular and pulse-related modulation of skin sympathetic nerve activity during sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2010;202:291–298. doi: 10.1007/s00221-009-2131-8. [DOI] [PubMed] [Google Scholar]
- Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol. 1999;86:1552–1560. doi: 10.1152/jappl.1999.86.5.1552. [DOI] [PubMed] [Google Scholar]
- Kaufman H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B. Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Exp Brain Res. 2002;143:463–469. doi: 10.1007/s00221-002-1002-3. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Yates BJ. Regional and functional differences in the distribution of vestibulosympathetic reflexes. Am J Physiol. 1998;275:R824–R835. doi: 10.1152/ajpregu.1998.275.3.R824. [DOI] [PubMed] [Google Scholar]
- Kerman IA, McAllen RM, Yates BJ. Patterning of sympathetic nerve activity in response to vestibular stimulation. Brain Res Bull. 2000;53:11–16. doi: 10.1016/s0361-9230(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Lewis T. Vasovagal syncope and the carotid sinus mechanism. Br Med J. 1932;3723:873–876. doi: 10.1136/bmj.1.3723.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay JR, Oppenheimer MJ, Wycis HT, Spiegel EA. Receptor apparatus of the vestibulovasomotor reaction. Arch Otolaryngol. 1945;42:257–266. doi: 10.1001/archotol.1945.00680040341004. [DOI] [PubMed] [Google Scholar]
- MacDougall HG, Brizuela AE, Burgess AM, Curthoys IS. Between-subject variability and within-subject reliability of the human eye-movement response to bilateral galvanic (DC) vestibular stimulation. Exp Brain Res. 2002;144:69–78. doi: 10.1007/s00221-002-1038-4. [DOI] [PubMed] [Google Scholar]
- MacDougall HG, Brizuela AE, Curthoys IS. Linearity, symmetry and additivity of the human eye-movement response to maintained unilateral and bilateral surface galvanic (DC) vestibular stimulation. Exp Brain Res. 2003;148:166–175. doi: 10.1007/s00221-002-1289-0. [DOI] [PubMed] [Google Scholar]
- MacDougall HG, Brizuela AE, Burgess AM, Curthoys IS, Halmagyi GM. Patient and normal three-dimensional eye-movement responses to maintained (DC) surface galvanic vestibular stimulation. Otol Neurotol. 2005;26:500–511. doi: 10.1097/01.mao.0000169766.08421.ef. [DOI] [PubMed] [Google Scholar]
- Maruta J, Simpson JI, Raphan T, Cohen B. Orienting eye movements and nystagmus produced by translation while rotating (TWR) Exp Brain Res. 2005;163:273–283. doi: 10.1007/s00221-004-2178-5. [DOI] [PubMed] [Google Scholar]
- Maruta J, Raphan T, Simpson JI, Cohen B. Vertical (Z-axis) acceleration alters the ocular response to linear acceleration in the rabbit. Exp Brain Res. 2008;185:87–99. doi: 10.1007/s00221-007-1138-2. [DOI] [PubMed] [Google Scholar]
- Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. 2008;16:4–20. doi: 10.1097/CRD.0b013e31815c8032. [DOI] [PubMed] [Google Scholar]
- Megirian D, Manning JW. Input-output relations of the vestibular system. Arch Ital Biol. 1967;105:15–30. [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Central projections of the vestibular nerve: a review and single fiber study in the Mongolian gerbil. Brain Res Bull. 2003;60:475–495. doi: 10.1016/s0361-9230(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Ocon A, Taneja I. Multiresolution wavelet analysis of time-dependent physiological responses in syncopal youths. Am J Physiol Heart Circ Physiol. 2008;296:H171–H179. doi: 10.1152/ajpheart.00963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol. 1991;65:1170–1182. doi: 10.1152/jn.1991.65.5.1170. [DOI] [PubMed] [Google Scholar]
- Pirodda A, Brandolini C, Raimondi MC. Tinnitus as a warning for preventing vasovagal syncope. Medical Hypothese. 2009;73:370–371. doi: 10.1016/j.mehy.2009.02.041. [DOI] [PubMed] [Google Scholar]
- Porter JD, Balaban CD. Connections between the vestibular nuclei and regions that mediate autonomic function in the rat. J Vest Res. 1997;7:63–76. [PubMed] [Google Scholar]
- Ray CA. Interaction of the vestibular system and baroreflexes on sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol. 2000;279:H2399–H2404. doi: 10.1152/ajpheart.2000.279.5.H2399. [DOI] [PubMed] [Google Scholar]
- Rea RF, Thames MD. Neural control mechanisms and vasova-gal syncope. J Cardiovasc Electrophysiol. 1993;4:587–595. doi: 10.1111/j.1540-8167.1993.tb01246.x. [DOI] [PubMed] [Google Scholar]
- Robertson D. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res. 2008;18:2–7. doi: 10.1007/s10286-007-1004-0. [DOI] [PubMed] [Google Scholar]
- Spiegel EA. Effect of labyrinthine reflexes on the vegetative nervous system. Arch Otolaryngol. 1946;44:61–72. doi: 10.1001/archotol.1946.00680060072006. [DOI] [PubMed] [Google Scholar]
- Starling EH. The Linacre lecture on the low of the heart. Longmans; London: 1918. [Google Scholar]
- Suzuki M, Hori S, Nakumura I. Role of vagal control in vasova-gal syncope. Pac Clin Electrophysiol. 2003;26:571–578. doi: 10.1046/j.1460-9592.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Abe C, Awazu C, Morita H. Vestibular system plays a significant role in arterial pressure control during head up tilt in young subjects. Auton Neurosci. 2009;148:90–96. doi: 10.1016/j.autneu.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Tang PC, Gernandt BE. Autonomic responses to vestibular stimulation. Exp Neurol. 1969;24:558–578. doi: 10.1016/0014-4886(69)90158-7. [DOI] [PubMed] [Google Scholar]
- Uchino Y, Kudo N, Tsuda K, Iwamura Y. Vestibular inhibition of sympathetic nerve activities. Brain Res Bull. 1970;22:195–206. doi: 10.1016/0006-8993(70)90004-1. [DOI] [PubMed] [Google Scholar]
- Watson SR, Brizuela AE, Curthoys IS, Colebatch JG, MacDougall HG, Halmagyi GM. Maintained ocular torsion produced by bilateral and unilateral galvanic (DC) vestibular stimulation in humans. Exp Brain Res. 1998;122:453–458. doi: 10.1007/s002210050533. [DOI] [PubMed] [Google Scholar]
- Wieling W, Thijs RD, van Dijk N, Wilde AAM, Benditt DG, van Dijk JG. Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain. 2009;132:2630–2642. doi: 10.1093/brain/awp179. [DOI] [PubMed] [Google Scholar]
- Woodring SF, Rossiter CD, Yates BJ. Pressor response elicited by nose-up vestibular stimulation in cats. Exp Brain Res. 1997;113:165–168. doi: 10.1007/BF02454153. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Dai M, Suzuki J-I, Raphan T, Cohen B. Semicircular canal contributions to the three-dimensional vestibuloocular reflex: A model-based approach. J Neurophysiol. 1995;74:2722–2738. doi: 10.1152/jn.1995.74.6.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushin SB, Dai M, Raphan T, Suzuki J-I, Arai Y, Cohen B. Spatial orientation of the angular vestibulo-ocular reflex (aVOR) after semicircular canal plugging and canal nerve section. Exp Brain Res. 2011 doi: 10.1007/s00221-011-2586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates BJ. Vestibular influences on the autonomic nervous system. Ann NY Acad Sci. 1996;781:458–473. doi: 10.1111/j.1749-6632.1996.tb15720.x. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiol. 1994;71:2087–2092. doi: 10.1152/jn.1994.71.6.2087. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Goto T, Bolton PS. Responses of neurons in the rostral ventrolateral medulla of the cat to natural vestibular stimulation. Brain Res Bull. 1993a;601:255–264. doi: 10.1016/0006-8993(93)91718-8. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Jakus J, Miller AD. Vestibular effects on respiratory outflow in the decerebrate cat. Brain Res Bull. 1993b;629:209–217. doi: 10.1016/0006-8993(93)91322-j. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Grélot L, Kerman IA, Balaban CD, Jakus J, Miller AD. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol. 1994;267:R974–R983. doi: 10.1152/ajpregu.1994.267.4.R974. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Aoki M, Burchill P, Bronstein AM, Gresty MA. Cardiovascular responses elicited by linear acceleration in humans. Exp Brain Res. 1999;125:476–484. doi: 10.1007/s002210050705. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Jian BJ, Cotter LA, Cass SP. Responses of vestibular nucleus neurons to tilt following chronic bilateral removal of vestibular inputs. Exp Brain Res. 2000;130(2):151–158. doi: 10.1007/s002219900238. [DOI] [PubMed] [Google Scholar]
- Yavorcik KJ, Reighard DA, Misra SP, Cotter LA, Cass SP, Wilson TD, Yates BJ. Effects of postural changes and removal of vestibular inputs on blood flow to and from the hindlimb of conscious felines. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1777–R1784. doi: 10.1152/ajpregu.00551.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Jordan JR, Hardy S, Fulcher B, Childress C, Varner C, Windham B, Jeffcoat B, Rockhold RW, Zhou W. Linear acceleration-evoked cardiovascular responses in awake rats. J of Appl Physiol. 2007;103(2):646–654. doi: 10.1152/japplphysiol.00328.2007. [DOI] [PubMed] [Google Scholar]