Abstract
Large, Ca-activated K channels (BK) are comprised of an alpha pore (BKα) and one of four beta subunits (BKβ1-4). When the gene for BKβ1 is knocked out (BKβ1-KO) the result is increased myogenic tone of vascular smooth muscle and hypertension. We re-examined whether the hypertension is entirely due to increased vascular tone because most monogenic forms of hypertension have renal origins and BKβ1 resides in renal connecting tubule (CNT) cells. Moreover, BKβ1 is localized in the adrenal glands where it may control production of aldosterone. This review will summarize our report that a majority of the hypertension of BKβ1-KO is the result of insufficient handling of dietary K, resulting in increased plasma K and hyperaldosteronism, the latter promoting Na and fluid retention. The fluid retention and hypertension are exacerbated by a high K diet and reduced by eplerenone, an aldosterone receptor inhibitor. Genetic knock out of the BKβ4 (BKβ4-KO), which resides in intercalated cells, also exhibit deficient K excretion, fluid retention and mild hypertension that is not exacerbated when animals are treated a high K diet. These results show that the hypertension associated with BKβ1-KO occurs because of enhanced fluid retention as well as the previously described vascular dysfunction.
BK channels and a new form of hypertension
Large, Ca-activated K channels (BK) are ubiquitously expressed in nearly all mammalian cells. The BK pore-forming proteins (BKα) are tailored to the functional needs of cells by their differing splice variants and by associating with one of four different accessory subunits (BKβ1-4). Each subunit bestows different pharmacological and biophysical properties to BK. For example, the BKβ1 enhances the Ca and voltage sensitivity of BKα [1]. The role of the BKβ1 subunit in the modulation of blood pressure was first shown by Brenner et al. who reported that the mean arterial pressure (MAP) of mice null for BKβ1 (BKβ1-KO) was elevated by 21 mmHg [2]. Subsequently, several additional studies have shown that the BKβ1 gene (Kcnmb1) is involved in the manifestation of a hypertensive phenotype [3-8].
It has been previously published that vascular smooth muscle tone is increased in BKβ1-KO. This increase is thought to be because, in the absence of BKβ1, the BKα pore is less sensitive to Ca sparks. Normally, BKα/β1 channels open in response to Ca sparks, leading to a compensatory vasorelaxation. However, in BKβ1-KO, BK channel activity becomes uncoupled from Ca sparks, thereby leading to hypertension [2-4;9]. Although sympathetic-dependent increases in vascular tone contribute to hypertension [10], almost all known forms of monogenic hypertension are of renal origin [11]. Therefore, we have recently re-examined the hypertensive phenotype of BKβ1-KO in greater detail. In concert with previous studies, we found an elevated MAP of 137 mmHg in BKβ1-KO versus 116 mmHg in wild type mice (WT) of the C57Bl/6 strain. However, we also observed a unique feature of this hypertension: it is exacerbated by high dietary K to a mean MAP of 145 mmHg. This review will discuss the details of these findings: how a dual effect of the absence of BKβ1 in the connecting tubule and the adrenal medulla causes K retention concomitant with primary aldosteronism and a positive Na balance. In addition, we will discuss the role of the BKβ4 subunit in electrolyte handling in the distal nephron. Based on these findings, we will speculate on the potential relevance of BKβ1-KO hypertension in humans.
Renal localizations of BK/β1 and BKβ4 and their roles in K secretion
The BKβ1 has been considered to be uniquely associated with the BKα in all types of smooth muscle [12-14], as well as in renal mesangial cells [15;16], which have contractile properties similar to smooth muscle. It was therefore surprising to observe BKβ1 in the apical membrane of the mouse and rabbit renal connecting tubule (CNT) [17;18], an important site of K secretion along the nephron. A K secretory role for BKβ1 in the CNT was first indicated by an attenuated kaliuretic response of BKβ1-KO when acutely volume expanded with a physiological saline solution [17]. This result was consistent with the failure of the CNT of BKβ1-KO to secrete K in response to either Na delivery or high distal flow, both of which can stimulate K secretion in this segment by supplying a chemical driving force and stimulating the activation of BKα/β1 [19;20]. The CNT, also known as the late distal tubule, is the nephron site where flow-induced K secretion was initially discovered with in vivo micropuncture techniques [21;22]. Although exclusively localized to the CNT in mouse, the BKβ1 was also identified in the initial part of the CCD in rabbit [17].
It has been generally accepted that basal K secretion is mediated by ROMK (renal outer medullary K channel; Kir1.1), whereas flow-induced K secretion is mediated by BK [23]. Stimulation of K secretion by high flow or increased Na delivery is important clinically, as a reduction in plasma K concentration is a common complication for patients treated with loop or thiazide diuretics. In addition, flow-induced K secretion is physiologically relevant for animals on a high K diet. When fed a high K diet for several days, K secretion is initially stimulated by aldosterone, which increases the driving force for K secretion by enhancing apical ENaC and basolateral Na-K-ATPase. However, due to water reabsorption, K secretion is limited by a rapid build-up of the K concentration in the lumen of the CCD, reversing the chemical gradient in the direction of K reabsorption in the medullary collecting duct [24]. The reabsorbed K recycles (secretes) into the descending limb of Henle's loop causing filtrate K concentration to be very high [25-27]. The medullary interstitial K concentration is high enough (ranging from 35-50mM [4]) to depolarize the basolateral membrane of the thick ascending limb (TAL) to the extent that the driving force for transcellular Cl reabsorption is reduced. The elevated intracellular Cl concentration decreases the passive gradient for Na reabsorption via the apical NKCC, thereby markedly reducing Na reabsorption in the TAL and causing a large increase in Na delivery and flow down the distal nephron. Consuming a high K diet for several days enhances flow by more than four-fold in mice [28;29] and two-fold in rats [30]. Increased Na is delivered to the CNT and CCD to exchange for K. Moreover, a cell now can secrete K into a 4-fold elevation of luminal volume, thereby increasing the cell to lumen K gradient by four-fold. This also suggests that flow-mediated K secretion and the better known aldosterone-induced mechanism of K secretion are interdependent: Aldosterone directly enhances medullary K recycling [31] and initiates the high lumen to plasma K gradient necessary for medullary K recycling. However, aldosterone alone will not effectively eliminate K without the increased filtrate delivery that is necessary to re-establish the plasma to lumen chemical gradients and stimulate the opening of BK.
Eplerenone is an aldosterone receptor blocker, similar to spironolactone. Our data indicated that the loss of K secretion in high K treated BKβ1-KO was primarily an eplerenone-sensitive component. This is consistent with reports that the CNT has several-fold more Na-K-ATPase [32] and ten-fold more aldosterone-regulated ENaC channels than any other segment of the nephron [33]. The driving force for K secretion is potent in this segment, with a transepithelial membrane potentials of -75 mV in K-adapted rats [34]. Thus, the CNT is best-equipped to couple K extrusion in exchange for aldosterone-stimulated Na reabsorption. The mechanism for aldosterone mediated K secretion via BKα/β1 may be partly due to the fact that the large depolarization of the apical membrane both activates the channel and increases the driving force for cell to lumen K exit.
Despite the favorable electrochemical gradient for K secretion, BKα/β1 is predominantly closed at the resting membrane potential. However, the channel can open in response to increased flow. Several studies have shown that flow increases Ca, at least transiently, in both intercalated cells and principal cells in the CCD [20;35]. Therefore, part of the mechanism for flow-mediated activation of BK may be that shear stress induces increased cytosolic Ca that stimulates BKα/β1, which is consistent with the role of BKβ1 to confer more Ca/voltage sensitivity to BKα. [1]. The combination of aldosterone (which would depolarize the apical membrane), as well as elevated intracellular Ca concentration, has the potential to significantly increase the open probability (Po) of BK channels in the apical membrane. Therefore, any defect in K handling by BKβ1-KO could involve either a defective response to the depolarization of the apical membrane or to a failure to respond to the flow-generated increase in intracellular Ca concentration. Both could be directly or indirectly dependent on aldosterone.
High flow serves to activate BK and replenish chemical gradients, and the aldosterone-induced increase in Na-K-ATPase activity is necessary to continually supply the cell with K from the plasma. However, it has been shown that the aldosterone-induced epithelial Na channel (ENaC) and Na-K-ATPase-mediated force is not the only driver of K secretion for mammals on a high K diet. Our study showed that eplerenone eliminated only approximately 50% of the elevated K secretion. However, when high K treated rats are given amiloride, an ENaC blocker, they still exhibit a substantial amount of elevated K secretion [30]. With amiloride treatment, the ENaC – Na-K-ATPase (Na-dependent) driving force for K secretion should be inhibited. Although it is clear that BKα/β1 is a component of aldosterone-mediated K secretion, it is not known whether BKα/β1 is involved in Na-independent K secretion.
BKβ4-KO also have a K secretory defect that results in Na and fluid retention when placed on a high K diet [28]. It is more difficult to understand the role of the BKα/β4 in K secretion because it is localized in IC which contain minimal basolateral Na-K-ATPase and, unlike principal cells, the Na-K-ATPase in IC does not increase with a high K diet. Serial section analysis of immuno-identified intercalated cells (IC) from the connecting tubules and cortical collecting ducts revealed a reduction in IC size when flow-rates in the distal nephron were elevated by feeding mice a high K diet. In the absence of BKβ4 (BKβ4-KO), the ICs were significantly larger and protruded into the lumen. This evidence was consistent with the notion that the shear stress of the high distal flow in the high K fed WT mice normally causes a reduction in IC size by activating BKα/β4, which results in an efflux of intracellular K. It is not completely understood whether the failure of high K fed BKβ4-KO to exhibit a reduction in IC size cell size indirectly affects the Na and K transport of the CNT and CCD or whether BKα/β4 secrete K with an active source of K delivery other than the Na-K-ATPase. In either case, the BKα/β4 in IC is necessary to maintain K balance when animals are presented with a high K diet.
Aldosteronism in BK/β1-KO
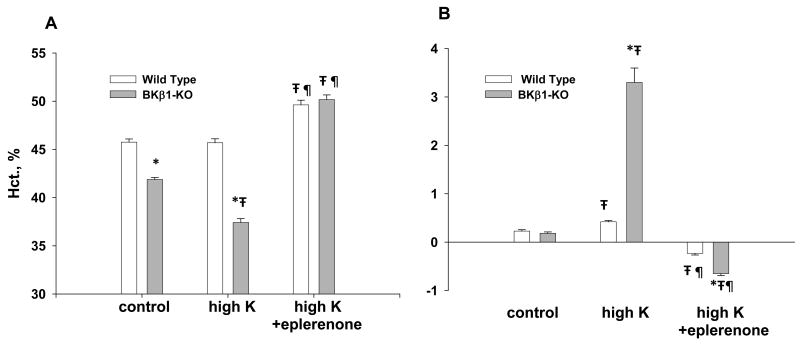
As shown in figure 1 [36], BKβ1-KO on a regular diet were retaining fluid as indicated by the significantly lower hematocrit (45.8% in WT and 41.9% in BKβ1-KO). The fluid retention and volume expansion of BKβ1-KO on a regular diet were enough to be detected by a lower hematocrit but not enough to yield a significant gain of weight (figure 1B). The fluid retention and hypertension were magnified in BKβ1-KO on a high K diet as the hematocrit decreased to 37.4% and the mice gained over 3 gms of weight. The excess fluid, too much to be retained in only the vascular spaces, was observed in the abdominal cavities of high K treated BKβ1-KO. With eplerenone treatment, the hematocrits of WT and BKβ1-KO on high K diets increased to 49.7% and 50.2%, respectively, indicating that eplerenone was causing slight volume depletion in both genotypes and was probably maximally effective. Eplerenone treatment reversed most of the hypertension of BKβ1-KO, without affecting MAP in WT animals. That the edema and most of the hypertension of BKβ1-KO were corrected by eplerenone implicated hyperaldosteronism.
Figure 1.
Illustration of volume status, as determined by hematocrits (A) and weight change (A) of BKβ1-KO and WT on either control (normal K: 0.32% Na and 0.6% K) or high K (0.32% Na and 5.0% K) diets. *denotes significant difference (p<0.05) compared with WT using the unpaired t-test. ∓denotes significant difference compared with normal K (control) using ANOVA plus Student-Newman-Kuels test. ¶denotes significant difference compared with HK using ANOVA plus Student-Newman-Kuels test.
When WT were placed on a high K diet, the plasma aldosterone concentration increased by 34% while the plasma K concentration increased by only 0.3 mM. This compares well with an in vitro study showing that an increase in the plasma K concentration from 4.1 to 5.1 mM caused the steepest increase, with a doubling of aldosterone production from the isolated rat adrenal glomerulosa [37].
The significantly elevated plasma K concentration in BKβ1-KO, compared with WT, indicated a primary defect in renal K secretion. However, part of the BK associated hypertension may be the result of increased sensitivity of the adrenal glomerulosa cells to plasma K, causing an additional elevation of aldosterone. When compared to WT, the slope of the plot of aldosterone produced vs. plasma K concentration for BKβ1-KO was increased by nearly 2-fold 100%. However, surprisingly, we discovered that the BKβ1 was restricted to the adrenal medulla. It is not yet understood how an absence of BKβ1 in the adrenal medulla could cause enhanced aldosterone production in response to an increase in plasma K concentration. However, the glomerulosa cells of the cortex and the chromaffin cells of the adrenal medulla have paracrine effects on each other [38-41].
The aldosteronism was not profound in BKβ1-KO on a high K diet, probably because atrial natriuretic factor, which is stimulated by volume expansion, attenuates the aldosterone response [42;43]. Other investigators found that for BKα knock-outs (BKα-KO) on a high K diet, the plasma aldosterone concentration was elevated to a greater extent, by approximately fourfold, [29]. The more extreme aldosteronism of BKα-KO may reflect the fact that BKα alone is a still a functional channel in BKβ1-KO in the control of aldosterone release.
A surprising finding in our study was the significant elevation of the plasma Na concentration by 4 mM (and the plasma osmolality by 6 mOsm) on a normal diet, compared to wild-type controls. This is counter to the notion that ADH preserves osmolality despite increased renal Na reabsorption. However, it was revealed several years ago that there is a “resetting” of the osmostat in states of primary hyperaldosteronism [44]. The mechanism for osmostat resetting in the face of chronically elevated plasma aldosterone has not been established.
Origin of the BK hypertension – renal vs vascular
Eplerenone reduced the hypertension of both regular diet and HK BKβ1-KO to a value approximately 7 mmHg above MAP of WT. Thus, enhanced vascular tone, independent of fluid retention, contributes to a minor degree of hypertension in BKβ1-KO. This is consistent both with the findings of increased myogenic tone in cerebral arteries of BKβ1-KO [45] and the view that persistent and significant long-term monogenic hypertension has a renal origin [11].
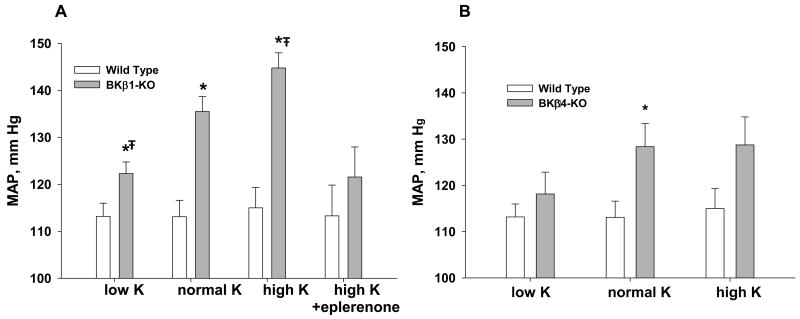
Figure 2 shows how dietary K and Na content affects MAP in BKβ1-KO (A) and BKβ4-KO (B), relative to WT. For BKβ1-KO, the dependency of MAP on dietary K content is dramatic. Eplerenone-treated BKβ1-KO on a high K diet and BKβ1-KO on a low Na diet exhibited MAPs of 8 mmHg and 6 mmHg, respectively, above WT. These results are consistent with the notion that the vascular smooth muscle tone is responsible for about 30% of the hypertension and the fluid retention from the renal/adrenal defect is responsible for 70% of the hypertension in BKβ1-KO. BKβ4-KO also retain Na and fluid but exhibit a milder hypertension than BKβ1-KO. Moreover, the BKβ4-KO did not exhibit a significant increase in MAP when placed on a high K diet. These experiments suggest that BKβ1 has a more critical role than BKβ4 with respect to blood pressure regulation.
Figure 2.
Comparison of differing K and Na diets mean arterial pressures of BKβ1-KO vs WT (A) and BKβ4-KO vs WT (B). Low K contains 0.32% Na and 0.1% K. All other symbols are the same as in figure 1.
Table 1 shows the MAP reported by different studies of BKα-KO and BKβ1-KO. As expected, the anesthetized mice exhibited lower MAPs. One study reported an MAP for BKα-KO that was 19 mm Hg greater than WT. Although these mice were anesthetized, this value was in agreement with average (16.5 mm Hg) of four different studies that determined the difference in MAP between BKβ1-KO and WT.
Table 1. Summary of reported MAPs for BKα-KO and BKβ1-KO.
| Genotype | Genetic background | Method of BP measure | KO-MAP (mm Hg) | WT-MAP (mm Hg) | Difference in MAP (KO–WT) | Reference |
|---|---|---|---|---|---|---|
| BKα-KO | SV129/C57Bl6 | Catheter (anesthetized) | 94±4 | 75±7 | 19 | Rieg et al. 29 |
| BKβ1-KO | SV129 | Catheter (conscious) | 134±5 | 114±6 | 20 | Brenner et al. 2 |
| BKβ1-KO | SV129/C57Bl6 | Catheter (conscious) | 118±3 | 104±2 | 14 | Pluger et al. 3 |
| BKβ1-KO | C57Bl6 | Catheter (anesthetized) | 104±3 | 93±2 | 11 | Pluznick et al. 15 |
| BKβ1-KO | C57Bl6 | Tail-cuff (conscious) | 137±3 | 116±3 | 21 | Grimm et al. 18 |
Abbreviations: BK, Ca-activated K channel; BP, blood pressure; KO, knockout; MAP, mean arterial pressure; WT, wild type.
Perspectives on a human correlation
The important question is whether human polymorphisms in KCNMB1 are associated with hypertension. If so, can the hypertension be attenuated by mineralcorticoid receptor antagonists (spironolactone or eperlenone) or dietary K restriction. A high K diet is considered beneficial in the prevention of stroke and heart disease [46;47]. In fact, a high Na, low K diet has been blamed for the high incidence of cardiovascular disease in Western cultures. A high K diet increases urinary flow by more than four-fold, causing the elimination of Na and lowering blood volume while maintaining a normal concentration of plasma K. The natural diuretic effect of a K rich diet could be beneficial for preventing stroke and cardiovascular disease.
However, a small percentage of the population may have KCNMB1 polymorphisms that contribute to hypertension. These individuals would appear to have resistant essential hypertension. The use of first line pharmacological agents such ACE inhibitors or Ang II receptor antagonist would be ineffective because renin levels would be low in these individuals. Reducing Na intake may lower MAP because it would reduce fluid retention. However, in most instances, the reduction of dietary Na occurs with an increase in K intake, which would likely counter any benefit of Na restriction. The plasma K and aldosterone concentration would be elevated but potentially remain within the range of what is considered normal. The most notable phenotype of such individuals would be the near normalization of MAP following mineralocorticoid receptor antagonism. Such a subset of essential hypertensive subjects are known to have primary hyperaldosteronism with normal plasma K, and are responsive to aldosterone receptor blockers [48]. It would be interesting to determine whether these individuals have loss-of-function polymorphisms in KCNMB1.
Although a loss-of-function polymorphism has not yet been identified, several groups have confirmed a BKβ1 gain-in-function mutation caused by a signal nucleotide polymorphism resulting in an amino acid substitution (E65K). This mutation is associated with lower blood pressures in a variety of population samples [5;7;48-51]. The E65K mutation enhances the gain of the hyperpolarizing feedback mechanism by more effectively coupling the BKα to Ca sparks, thereby reducing vascular tone. It is unclear whether the protection from hypertension of E65K [5;7] would be related to a more efficient elimination of K by the BKα/β1 in the CNT. However, the gain in function would render the vessels more compliant to increases in renal retention of Na and fluid. It would be interesting to determine whether the E65K mutation, specifically incorporated into the CNT, causes the BKβ1-KO to handle a high K load more efficiently than WT.
It may seem that K secretion via BKα/β1 is irrelevant considering that the modern diet consists of high Na and low K and that BKα/β1 may have been more utilized by the “hunter and gatherer” populations [52]. However, it is possible that the consumption of a low K diet for several thousand years has permitted mutations in BKβ1. A sudden change to a vegetarian (high potassium) diet may lead to noticeable hypertension in a small sub-set of subjects.
While a loss-of-function KCNMB1 polymorphism that influenced blood pressure would likely be rare, a large segment of the population could be affected by several conditions that cause a loss of relative expression of BKβ1 in tissues. BKβ1 related changes in K handling and fluid balance might be manifested more profoundly in the elderly because the expression of both BKα and BKβ1 decrease with aging [7;53]. Decreased expression and functionality of BKα/β1 could partially explain the difficulty many of the elderly have controlling their blood pressure and electrolyte concentrations. Evidence suggests that a decrease in perfusion of the retinal arterioles in subjects with type 1 diabetes mellitus is the result of a down-regulation of BKβ1, resulting in increased arteriole myogenic tone [54]. If BKβ1 is similarly decreased in the CNT of diabetic patients, then the ensuing fluid retention would exacerbate the retinopathy and may be partly responsible for the hypertension.
Although the significance of BKβ1-related hypertension to the human population remains to be fully explored, we have learned that the inability of renal K channels in the CNT to excrete a K load will result in a mild form of potassium-sensitive hypertension. As in all other forms of monogenic hypertension, the renal-adrenal axis plays a major role. However, the hypertensive effect of fluid retention is magnified by increased vascular smooth muscle tone.
Summary
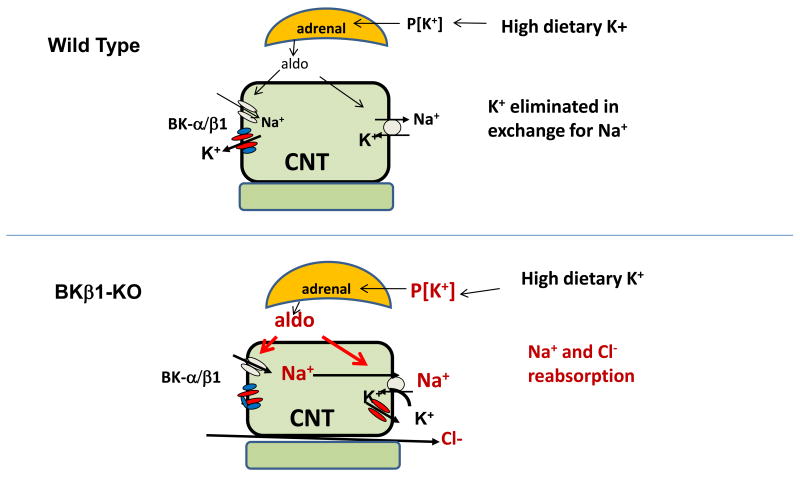
The BKβ1-KO mouse has a hypertensive phenotype that is 30% due to intrinsic tone and 70% due to Na and volume retention. The percentage of hypertension due to fluid retention increases when BKβ1-KO are placed on a high K diet. Figure 3 illustrates how the defect in BK mediated K secretion in the connecting tubule leads to Na and volume retention and hypertension in BKβ1-KO. The latter is the result of deficient K excretion by BKα/β1 in the CNT, resulting in elevated plasma K that stimulates aldosterone production in the adrenal glomerulosa. The aldosteronism may be amplified in an undetermined way by a lack of BKβ1 in the adrenal medulla. The combination of deficient K secretion and a hypersensitive adrenal gland results in the unusual combination of chronic primary aldosteronism with normal to high plasma K. While a high K diet is beneficial to the majority of the population, there may be a small subset of patients who have a KCNMB1 polymorphism with a phenotype of elevated MAP due to fluid retention exaggerated by a high K diet. It may be difficult to identify single nucleotide polymorphisms in this group of individuals because of the multitude of genetic and environmental contributors to hypertension. Nevertheless, identification of subjects with loss-of-function mutations of KCNMB1 may allow for more appropriate antihypertensive treatment regimens for these individuals. The effects of aging and diabetes on the expression of BK and potential consequences of reduced BKβ1 on fluid balance should be explored.
Figure 3.
Illustration of how high K fed BKβ1-KO can develop of Na, Cl and fluid retention. On high dietary K intake, the absence of BKβ1 in the CNT results in relative basolateral K recycling instead of its secretory role. The elevated K stimulates aldosterone production from the adrenals which have enhanced sensitivity to K in BKβ1-KO. The increased plasma aldosterone stimulates Na and Cl reabsorption instead of an exchange of K for Na.
More studies are necessary to examine the K secretory role of the IC-localized BKβ4. Although BKβ4-KO also retains fluid on a high K diet, these mice have much milder hypertension. This is further evidence that the increased intrinsic vascular tone, although accounting for only 6-8 mmHg, is necessary for the high MAP exhibited by the high K diet fed BKβ1-KO. Transgenics with renal, adrenal and VSM cell specific deletions and insertions of BKα and its differing accessory components will address many future questions regarding the hypertension of BKα-KO and BKβ1-KO.
Acknowledgments
We thank Dr. Jennifer L. Pluznick (Johns Hopkins Medical School) for her helpful comments and critical evaluation of this review. This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant RO1 DK71014 (S.C. Sansom), and a fellowship (#610059Z) from the American Heart Association Heartland Affiliate (P.R. Grimm).
Footnotes
Disclosures: None
Reference List
- 1.Nimigean CM, Magleby KL. The beta subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J Gen Physiol. 1999;113:425–440. doi: 10.1085/jgp.113.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner R, Perez GJ, Bonev AD, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 3.Pluger S, Faulhaber J, Furstenau M, et al. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 4.Amberg GC, Bonev AD, Rossow CF, et al. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Fernandez JM, Tomas M, Vazquez E, et al. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest. 2004;113:1032–1039. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotlikoff M, Hall I. Hypertension: beta testing. J Clin Invest. 2003;112:654–656. doi: 10.1172/JCI19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senti M, Fernandez-Fernandez JM, Tomas M, et al. Protective effect of the KCNMB1 E65K genetic polymorphism against diastolic hypertension in aging women and its relevance to cardiovascular risk. Circ Res. 2005;97:1360–1365. doi: 10.1161/01.RES.0000196557.93717.95. [DOI] [PubMed] [Google Scholar]
- 8.Nieves-Cintron M, Amberg GC, Nichols CB, et al. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 9.Lohn M, Lauterbach B, Haller H, et al. beta(1)-Subunit of BK channels regulates arterial wall[Ca(2+)] and diameter in mouse cerebral arteries. J Appl Physiol. 2001;91:1350–1354. doi: 10.1152/jappl.2001.91.3.1350. [DOI] [PubMed] [Google Scholar]
- 10.Fung MM, Viveros OH, O'connor DT. Diseases of the adrenal medulla. Acta Physiol (Oxf) 2008;192:325–335. doi: 10.1111/j.1748-1716.2007.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 12.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel beta1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L802–L810. doi: 10.1152/ajplung.00104.2006. [DOI] [PubMed] [Google Scholar]
- 13.Lu G, Mazet B, Sun C, et al. Inflammatory modulation of calcium-activated potassium channels in canine colonic circular smooth muscle cells. Gastroenterology. 1999;116:884–892. doi: 10.1016/s0016-5085(99)70071-5. [DOI] [PubMed] [Google Scholar]
- 14.Petkov GV, Bonev AD, Heppner TJ, et al. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-beta1-/- mice. Am J Physiol Renal Physiol. 2003;284:F1274–F1279. doi: 10.1152/ajprenal.00010.2003. [DOI] [PubMed] [Google Scholar]
- 16.Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- 17.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-{beta}1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol. 2005;288:F846–F854. doi: 10.1152/ajprenal.00340.2004. [DOI] [PubMed] [Google Scholar]
- 18.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-beta subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol. 2007;293:F350–F359. doi: 10.1152/ajprenal.00018.2007. [DOI] [PubMed] [Google Scholar]
- 19.Stanton BA, Kaissling B. Adaptation of distal tubule and collecting duct to increased Na delivery. II Na+ and K+ transport. Am J Physiol. 1988;255:F1269–F1275. doi: 10.1152/ajprenal.1988.255.6.F1269. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Xu S, Woda C, et al. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285:F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 21.Kunau RT, Jr, Webb HL, Borman SC. Characteristics of the relationship between the flow rate of tubular fluid and potassium transport in the distal tubule of the rat. J Clin Invest. 1974;54:1488–1495. doi: 10.1172/JCI107897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teichert AM, Miller TL, Tai SC, et al. In vivo expression profile of an endothelial nitric oxide synthase promoter-reporter transgene. Am J Physiol Heart Circ Physiol. 2000;278:H1352–H1361. doi: 10.1152/ajpheart.2000.278.4.H1352. [DOI] [PubMed] [Google Scholar]
- 23.Sansom SC, Welling PA. Two channels for one job. Kidney Int. 2007;72:529–530. doi: 10.1038/sj.ki.5002438. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein AM. A mathematical model of rat distal convoluted tubule (II): Potassium secretion along the connecting segment. Am J Physiol Renal Physiol. 2005 doi: 10.1152/ajprenal.00044.2005. [DOI] [PubMed] [Google Scholar]
- 25.Battilana CA, Dobyan DC, Lacy FB, et al. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978;62:1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamison RL, Work J, Schafer JA. New pathways for Potassium transport in the kidney. Am J Physiol. 1982;242:297–312. doi: 10.1152/ajprenal.1982.242.4.F297. [DOI] [PubMed] [Google Scholar]
- 27.Jamison RL, Bennett CM, Berliner RW. Countercurrent multiplication by the thin loops of Henle. Am J Physiol. 1967;212:357–366. doi: 10.1152/ajplegacy.1967.212.2.357. [DOI] [PubMed] [Google Scholar]
- 28.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol. 2010;21:634–645. doi: 10.1681/ASN.2009080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieg T, Vallon V, Sausbier M, et al. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- 30.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol. 2009;297:F389–F396. doi: 10.1152/ajprenal.90528.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higashihara E, Kokko JP. Effects of aldosterone on potassium recycling in the kidney of adrenalectomized rats. Am J Physiol. 1985;248:F219–F227. doi: 10.1152/ajprenal.1985.248.2.F219. [DOI] [PubMed] [Google Scholar]
- 32.Fried MG, Crothers DM. Equilibrium studies of the cyclic AMP receptor protein-DNA interaction. J Mol Biol. 1984;172:241–262. doi: 10.1016/s0022-2836(84)80025-x. [DOI] [PubMed] [Google Scholar]
- 33.Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Renal Physiol. 2004;286:F669–F674. doi: 10.1152/ajprenal.00381.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wright FS, Strieder N, Fowler NB, Giebisch G. Potassium secretion by distal tubule after potassium adaptation. Am J Physiol. 1971;221:437–448. doi: 10.1152/ajplegacy.1971.221.2.437. [DOI] [PubMed] [Google Scholar]
- 35.Woda CB, Leite M, Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca(2+)] (i) in rabbit cortical collecting duct. Am J Physiol Renal Physiol. 2002;283:F437–F446. doi: 10.1152/ajprenal.00316.2001. [DOI] [PubMed] [Google Scholar]
- 36.Grimm PR, Irsik DL, Settles DC, et al. Hypertension of Kcnmb1-/- is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci U S A. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pralong WF, Hunyady L, Varnai P, et al. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1992;89:132–136. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De LA, Racz K, McNicoll N, Desrosiers ML. Direct beta-adrenergic stimulation of aldosterone secretion in cultured bovine adrenal subcapsular cells. Endocrinology. 1984;115:485–492. doi: 10.1210/endo-115-2-485. [DOI] [PubMed] [Google Scholar]
- 39.Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA, et al. Effects of splanchnic nerve stimulation on the adrenal cortex may be mediated by chromaffin cells in a paracrine manner. Endocrinology. 1990;127:900–906. doi: 10.1210/endo-127-2-900. [DOI] [PubMed] [Google Scholar]
- 40.Ehrhart-Bornstein M, Bornstein SR, Gonzalez-Hernandez J, et al. Sympathoadrenal regulation of adrenocortical steroidogenesis. Endocr Res. 1995;21:13–24. doi: 10.3109/07435809509030417. [DOI] [PubMed] [Google Scholar]
- 41.Pratt JH, Turner DA, McAteer JA, Henry DP. Beta-adrenergic stimulation of aldosterone production by rat adrenal capsular explants. Endocrinology. 1985;117:1189–1194. doi: 10.1210/endo-117-3-1189. [DOI] [PubMed] [Google Scholar]
- 42.Kudo T, Baird A. Inhibition of aldosterone production in the adrenal glomerulosa by atrial natriuretic factor. Nature. 1984;312:756–757. doi: 10.1038/312756a0. [DOI] [PubMed] [Google Scholar]
- 43.Atarashi K, Franco-Saenz R, Mulrow PJ, et al. Inhibition of aldosterone production by atrial natriuretic factor. J Hypertens Suppl. 1984;2:S293–S295. [PubMed] [Google Scholar]
- 44.Gregoire JR. Adjustment of the osmostat in primary aldosteronism. Mayo Clin Proc. 1994;69:1108–1110. doi: 10.1016/s0025-6196(12)61380-9. [DOI] [PubMed] [Google Scholar]
- 45.Löhn M, Lauterbach B, Haller H, et al. β1-subunit of BK channels regulates arterial wall [Ca2+] and diameter in mouse cerebral arteries. J Appl Physiol. 2001;91:1350–1354. doi: 10.1152/jappl.2001.91.3.1350. [DOI] [PubMed] [Google Scholar]
- 46.Tobian L. High-potassium diets markedly protect against stroke deaths and kidney disease in hypertensive rats, an echo from prehistoric days. J Hypertens Suppl. 1986;4:S67–S76. [PubMed] [Google Scholar]
- 47.Young DB, Lin H, McCabe RD. Potassium's cardiovascular protective mechanisms. Am J Physiol. 1995;268:R825–R837. doi: 10.1152/ajpregu.1995.268.4.R825. [DOI] [PubMed] [Google Scholar]
- 48.Benchetrit S, Bernheim J, Podjarny E. Normokalemic hyperaldosteronism in patients with resistant hypertension. Isr Med Assoc J. 2002;4:17–20. [PubMed] [Google Scholar]
- 49.Kelley-Hedgepeth A, Peter I, Kip K, et al. The protective effect of KCNMB1 E65K against hypertension is restricted to blood pressure treatment with beta-blockade. J Hum Hypertens. 2008;22:512–515. doi: 10.1038/jhh.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kokubo Y, Iwai N, Tago N, et al. Association analysis between hypertension and CYBA, CLCNKB, and KCNMB1 functional polymorphisms in the Japanese population--the Suita Study. Circ J. 2005;69:138–142. doi: 10.1253/circj.69.138. [DOI] [PubMed] [Google Scholar]
- 51.Kohler R. Single-nucleotide polymorphisms in vascular Ca(2+)-activated K (+)-channel genes and cardiovascular disease. Pflugers Arch. 2009 doi: 10.1007/s00424-009-0768-6. [DOI] [PubMed] [Google Scholar]
- 52.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol. 2004;287:F593–F601. doi: 10.1152/ajprenal.00454.2003. [DOI] [PubMed] [Google Scholar]
- 53.Nishimaru K, Eghbali M, Lu R, et al. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004;559:849–862. doi: 10.1113/jphysiol.2004.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGahon MK, Dash DP, Arora A, et al. Diabetes downregulates large-conductance Ca2+-activated potassium beta1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]