Abstract
We compared the effects of yeast-treated human dendritic cells (DCs) with CD40L-matured human DCs for the induction of effector cells and the number and functionality of CD4+CD25+CD127− FoxP3+ regulatory T cells (Tregs). DCs were treated with yeast or CD40L and cocultured with isolated autologous CD4+ T cells. CD4+CD25+CD127− T cells isolated from the coculture of CD4+ T cells plus yeast-treated DCs (yeast coculture) had a lower expression of FoxP3 and decreased suppressive function compared to CD4+CD25+CD127− T cells isolated from the coculture of CD4+ T cells plus CD40L-treated DCs (CD40L coculture). Also, compared to the CD40L coculture, the yeast coculture showed increases in the ratio of CD4+CD25+ activated T cells to Tregs and in the production of Th1-related cytokines (IL-2, TNF-α, IFN-γ) and IL-6. In addition, yeast-treated DCs used as antigen-presenting cells (APCs) incubated with the tumor antigen CEA enhanced the proliferation of CEA-specific CD4+ T cells compared to the use of CD40L-matured DCs used as APCs. This is the first study to report on the role of yeast-treated/matured human DCs in reducing Treg frequency and functionality and in enhancing effector to Treg ratios. These results provide an additional rationale for the use of yeast as a vector in cancer vaccines.
Keywords: Saccharomyces cerevisiae, regulatory T cells, human dendritic cells
1. Introduction
In preclinical and clinical studies, numerous vectors [1–8] have been used to infect or transfect and mature human dendritic cells (DCs) to present a variety of antigens. Recombinant Saccharomyces cerevisiae (yeast) expressing full-length influenza matrix protein has been shown to be a versatile vehicle for presenting antigen to human DCs [1]. Preclinical studies have demonstrated the ability of yeast vectors encoding antigens such as chicken ovalbumin, HIV gag, HCV, and mutant ras to activate specific immune responses [9–12]. We have previously shown that heat-killed recombinant S. cerevisiae containing the transgene encoding carcinoembryonic antigen (yeast-CEA) can activate human DCs, resulting in increased expression of CD80, CD83, CD54, CD58, and MHC class II, and increased production of IL-12p70, TNFα, IFNγ and IL-2, compared to untreated DCs [13]. Yeast-CEA-activated DCs were used as antigen-presenting cells (APCs) to generate specific T-cell lines capable of lysing CEA+ human tumor cells. We found a similar level of activation of a CEA-specific T-cell line between DCs treated with yeast-CEA and DCs sequentially exposed to yeast and CEA peptide. Furthermore, in vivo studies in CEA-transgenic mice have demonstrated that vaccination with a heat-killed recombinant yeast-CEA induces CD4+ and CD8+ CEA-specific immune responses, reduces tumor burden, and extends overall survival [14]. A Phase I study employing yeast-CEA is ongoing.
DCs recognize S. cerevisiae through dectin, mannose-fucose receptors, and toll-like receptors (TLRs) such as TLR-2 and TLR-4 [15]. TLR agonists are potent activators of innate immune responses and DC maturation; as a consequence, they promote an adaptive immune response when administered with antigens [16]. An important property of TLR activation is the capacity to enhance IL-12 production from DCs and, consequently, the ability to promote Th1-type responses, which play a key protective role in tumor immunity [17]. It has also been shown that production of IL-6 by DCs in response to TLR-4 ligation blocks the suppressive effect of T regulatory cells (Tregs), allowing activation of pathogen-specific adaptive immune responses [18].
Dendritic cells, which are potent APCs specialized to initiate immunity, have also been recognized as crucial players in the induction of T-cell tolerance [19]. The mechanism by which DCs induce tolerance is unclear, but could be either direct (induction of anergy or deletion of responding T cells) or indirect (activation of Tregs). In any case, immature DCs are capable of activating Tregs in vitro and in vivo, and fully mature DCs have tolerogenic properties [20,21]. Murine CD4+CD25+ Tregs can proliferate in the absence of added cytokines in vitro and in vivo when stimulated with antigen-loaded mature DCs [22], and human mature DCs are specialized to expand and maintain FoxP3+ Tregs in T-cell cultures from healthy donors and myeloma patients [23]. Human myeloid DCs show greater activity compared with other APCs in increasing the number of functional Tregs and the expression of FoxP3 protein per cell. This expansion in Treg number and function is dependent on DC/T cell contact through CD80/CD86 membrane costimulatory molecules and the presence of endogenous IL-2. Recent studies have also demonstrated human DCs’ capacity to generate antigen-specific Tregs from CD4+CD25− T cells [24].
CD40L-matured DCs were used as a control as CD40L appears to be one of the most effective stimuli to induce DCs to produce massive amounts of bioactive IL-12. In addition, ligation of CD40L on human DCs can induce a marked upregulation of adhesion and costimulatory molecules, thus augmenting T-cell stimulatory capacity. CD40L signals also regulate DC-derived IL-7 production that, in turn, maximizes antigen-specific T-cell yields [25,26]. We show here that in the presence of yeast-treated DCs, CD4+CD25+CD127− T cells had decreased levels of FoxP3 expression, and that this correlated with a decrease in Treg suppressive function. High levels of IL-6 and Th1-related cytokines such as IL-2, TNF-α, and IFN-γ in the supernatant of CD4+ T cells cocultured with yeast-treated DCs also suggest that S. cerevisiae activates human DCs to produce cytokines that alter the balance between CD4+ effector cells and Tregs, resulting in an enhanced immune response to antigens. The study reported here is the first to compare the effect of yeast-treated and CD40L-matured human DCs on the induction of CD4+CD25+CD127− FoxP3+ human Tregs.
2. Materials and Methods
2.1 Generation of DCs
Peripheral blood mononuclear cells (PBMCs) from healthy donors and carcinoma patients were obtained from heparinized blood and separated using lymphocyte separation medium gradient (MP Biomedicals; Aurora, OH) according to the manufacturer’s guidelines. DCs were generated from PBMCs as previously described [27]. Briefly, PBMCs (2 × 107) were resuspended in AIM-V medium (Invitrogen; Carlsbad, CA) and allowed to adhere in a 6-well plate for 2 h. Adherent cells were cultured for 5 days in AIM-V medium containing 100 ng/ml of recombinant human (rh) GM-CSF and 20 ng/ml of rhIL-4. The culture medium was replenished every 3 days.
2.2 Maturation of DCs with yeast or CD40L
An antigen-free S. cerevisiae construct (GlobeImmune; Louisville, CO) was used as a control. Human DCs were treated for 48 h at a yeast:DC ratio of 10:1 in a final volume of 3 ml of complete AIM-V medium/well. As a control, 1 μg/ml of CD40L (Axxora; San Diego, CA) was added to the DC culture 24 h prior to harvesting [13].
2.3 DC/CD4+ T-cell cocultures
Mature DCs were adhered in a 6-well plate (2 × 105 or 4 × 105 DCs/well) for 2 h in AIM-V medium. The medium was then carefully removed and DCs were cocultured with purified autologous CD4+ T cells in RPMI 1640 medium (Mediatech; Manassas, VA) supplemented with 10% human AB serum (Gemini Bio-Products; West Sacramento, CA). CD4+ T cells were obtained using a CD4+ T-cell isolation kit II (Miltenyi Biotec; Auburn, CA). CD4+ T cells (2 × 106) were cocultured with DCs (2 × 105 or 4 × 105) at a CD4+ T cell:DC ratio of 10:1 or 5:1 in a final volume of 4 ml/well for 5 days. In some experiments, DCs were incubated with CEA protein (AspenBio Pharma; Castle Rock, CO) at a concentration of 20 μg/ml and flu protein (USBiological; Swampscott, MA) at a concentration of 10 μg/ml.
2.4 Isolation of CD4+CD25+CD127− Tregs
CD4+CD25+CD127− Tregs were isolated from the DC/CD4+ T-cell cocultures using a CD4+CD25+CD127− Treg isolation kit (Miltenyi Biotec), as previously described [28].
2.5 Flow cytometry analysis
Four-color flow cytometry was used to characterize the phenotype of the CD4+ T-cell population to investigate the balance of CD25+ activated T cells and Tregs in the presence of DCs treated with yeast or CD40L. Cells were resuspended in staining buffer (PBS containing 3% fetal bovine serum) and stained for 30 min at 4°C with FITC-conjugated anti-CD4, PE-Cy7-conjugated anti-CD25 (BD Biosciences; San Jose, CA), and PerCP-Cy5.5-conjugated anti-CD127− (eBioscience; San Diego, CA). Cells were then fixed and permeabilized using a fix/perm kit (eBioscience) according to the manufacturer’s instructions, and labeled with APC-conjugated anti-FoxP3 antibody (236A/E7 clone; eBioscience) or its isotype control antibody (eBioscience) as a negative control. 1 × 105 cells were acquired and analyzed on a Becton Dickinson LSRII (BD Biosciences) using DiVa software (BD Biosciences). CD4+CD25+CD127− Tregs isolated from the DC/CD4+ T-cell coculture were analyzed by 3-color flow cytometry using FITC-conjugated anti-CD4, PE-conjugated anti-CD25 (BD Biosciences) and APC-conjugated anti-FoxP3 antibody.
2.6 Immunosuppression assay
The immunosuppression assay was a modification of a previously described procedure [29]. Briefly, isolated CD4+CD25− T cells (1 × 104 cells/well) were cultured alone or with CD4+CD25+CD127− Tregs isolated from the cocultures at a 1:1 ratio with 1 μg/ml of anti-CD3 antibody (OKT3; eBioscience) in the presence of irradiated (3,500 rad) T cell-depleted PBMCs (5 × 104 cells/well) in a 96-well flat-bottomed plate at 37°C and 5% CO2. Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated human AB serum (Gemini Bio-Products), 100 units/ml of penicillin, 100 μg/ml of streptomycin (Mediatech), and 2 mmol/l of L-glutamine (Mediatech). Proliferation was measured by 1 μCi (0.037 MBq)/well [3H]thymidine (PerkinElmer; Waltham, MA) incorporation pulsed on day 4 and quantified 18 h later using a liquid scintillation counter (Wallac; Waltham, MA). All experiments were done in triplicate. Proliferation of CD4+CD25− T cells without coculturing with Tregs constituted 100% of the proliferation.
2.7 Quantitative DNA Methylation Analysis of FoxP3 by Real-Time PCR
Quantitative DNA methylation analysis of FoxP3 was performed in collaboration with Epiontis GmbH (Berlin, Germany). Briefly, cocultures of CD4+ T cells and yeast-treated DCs or CD40L-treated DCs were established using 3 different healthy donors. CD4+ T cells were collected on day 5. DNA was extracted from 1–2 × 106 CD4+ T cells by Puregene Core Kit A (Qiagen; Valencia, CA), according to the manufacturer’s protocol. Analysis of FoxP3 Treg-specific demethylated region (TSDR) by real-time PCR was performed by Epiontis GmbH, following the protocol described by Wieczorek et al. [30]. Amounts of methylated and unmethylated FoxP3 DNA were estimated from calibration curves by linear regression on crossing points from the second-derivative maximum method. The proportion of unmethylated DNA was computed as the ratio of unmethylated FoxP3 TSDR-DNA and the sum of methylated and unmethylated FoxP3 TSDR-DNA. For female patients, this ratio was corrected with a factor of 2 due to the fact that 1 of the 2 TSDR alleles is methylated as a result of X inactivation.
2.8 Detection of cytokines
Supernatants were collected 48 h after DCs were exposed to yeast or CD40L, and 2 and/or 5 days after DCs were cocultured with CD4+ T cells. All samples were screened for secretion of IFN-γ, IL-2, IL-8, IL-1β, GM-CSF, IL-12p70, IL-6, TNF-α, and IL-10 using a multiplex cytokine/chemokine kit (Meso Scale Discovery; Gaithersburg, MD). TGF-β1 was analyzed by ELISA kit (R&D Systems; Minneapolis, MN and BD Biosciences). Supernatants of CD4+ T-cell lines stimulated for 24 h with peptide-pulsed autologous CD40L-treated DCs in IL-2-free medium were screened for secretion of IFN-γ by ELISA kit (Biosource International; Camarillo, CA).
2.9 CD4+ T-cell proliferation assay
This assay was performed using samples from patients with CEA- or MUC1-expressing metastatic cancers post-vaccination with PANVAC, a poxviral-based vaccine engineered to express the transgenes for CEA and MUC1 and a triad of human T-cell costimulatory molecules (designated TRICOM) composed of B7.1, ICAM-1 and LFA-3. In a study conducted at the National Cancer Institute, patients received recombinant vaccinia (PANVAC-V) as a prime and recombinant fowlpox (PANVAC-F) as multiple booster vaccinations [31]. DCs from these patient samples (1 × 104/well) were cultured for 5 days with GM-CSF and IL-4 and treated with yeast (yeast:DCs = 10:1) for 48 h or with CD40L (1 μg/ml) for 24 h. CD4+ T cells were isolated using Miltenyi columns by negative selection and incubated with DCs (CD4:DCs = 10:1; 1 × 105/well) for 7 days, using PHA (2 μg/ml) as positive control and myoglobulin (20 μg/ml) as negative control (Sigma-Aldrich; St. Louis, MO). CEA protein (AspenBio Pharma) was used at 20 μg/ml. On day 7, [3H]thymidine was added to the culture, incubated for 6 h, and read with a Wallac Trilux B-scintillation counter (fold increase = CD4 proliferation with CEA protein/CD4 proliferation with or without myoglobulin protein). The experiment was performed in triplicate.
2.10 Generation of CEA-specific CD4+ T-cell lines
CD4+ T-cell lines were generated from PBMCs of a vaccinated gastro-esophageal (GE) junction cancer patient. The class II CEA peptide used in this study has been previously described [32]. This CD4+ T-cell epitope was selected from the amino acid sequence of CEA using the algorithm tables from 3 HLA-DR alleles (DRB1*0101, DRB1*0401, and DRB1*0701), as previously described [33]. The CEA peptide selected (YACFVSNLATGRNNS; 653–667; designated p653) was synthesized by Bio-Synthesis, Inc. (Lewisville, TX), with purity > 95%. HIV class II peptide (WIILGLNKIVRMYSPTSI; 133–150) was used as a negative control (Bio-Synthesis). CEA-specific CD4+ cell lines were generated following a previously described protocol [34]. Briefly, yeast-treated and CD40L-treated DCs were pulsed with 10 μg/ml CEA653–667 peptide for 2 h at incubator. The peptide-pulsed DCs were then mixed with 3 × 104 autologous 37°C in a 5% CO2 isolated CD4+ T cells at a CD4:DC ratio of 3:1. Culture medium consisted of RPMI 1640 supplemented with 5% human AB serum. Seven days later, CD4+ T cells were isolated from the cultures and stimulated with autologous yeast-treated DCs or CD40L-treated DCs and pulsed with CEA peptide (at 10 μg/ml) for 2 h. Two days after this second stimulation with peptide, rhIL-2 was added to each well at a final concentration of 10 IU/ml.
2.11 Tetramer staining
PE-labeled CEA653–667/DRB1-0401 tetramer was prepared by the NIH/NIAID MHC Tetramer Core Facility (Atlanta, GA) and PE-labeled hCLIP/DRB1-0401 tetramer was used as a negative control. The staining was performed as previously described [35]. Briefly, cells were stained with tetramer for 3 h at 37°C, washed and stained with CD4-FITC for 30 min at 4°C. Cells (1 × 104) were acquired and analyzed on an LSRII cytometer (BD Biosciences).
2.12 Statistical analysis
Statistical significance was calculated by VassarStats software (Poughkeepsie, NY) using a 2-tailed paired Student’s t test. P < 0.05 was considered statistically significant.
3. Results
We first compared the effect of yeast- and CD40L-treated human DCs on CD4+ T-cells. DCs from three healthy donors were treated with yeast for 48 h at a yeast:DC ratio of 10:1, or with CD40L for 24 h. Isolated autologous CD4+ T cells were cocultured with the yeast or CD40L-treated DCs at CD4+ T cell:DC ratios of 10:1 and 5:1 for 5 days. Cultures were then analyzed by flow cytometry for percent of Tregs, with Tregs defined as CD4+CD25+CD127− FoxP3+-expressing T cells. As shown in Table 1, CD4+ T cells cocultured with yeast-treated DCs at a ratio of 5:1 generated higher levels of CD4+CD25+ T cells than the coculture at a ratio of 10:1 (19.3% and 11.6%, respectively), without a concomitant increase in Tregs. This resulted in a higher ratio of activated CD4+ T cells to Tregs in the coculture (3.8:1 and 2.4:1 at effector T cell:Treg ratios of 5:1 and 10:1, respectively). Effector T cells were defined as CD4+CD25+ T cells minus CD4+CD25+CD127− FoxP3+ T cells. In contrast, levels of Tregs increased in the coculture of CD4+ T cells and CD40L-treated DCs, resulting in a decrease in the ratio of effector T cells to Tregs (0.91:1 and 1.50:1 at CD4+ T cell:DC ratios of 5:1 and 10:1, respectively). Data on 2 additional healthy donors are presented in Supplementary Tables 1A and 1B.
Table 1.
CD4+CD25+:Treg ratio in coculture of CD4+ T cells plus autologous DCs treated with yeast or with CD40L (HD#1)
| Coculture (ratio) | Total number of cells/well | CD4+ (% in total number of cells) | CD4+CD25+ (% in CD4+) | Tregs | Ratio effector cells:Tregs | |
|---|---|---|---|---|---|---|
| (% in CD4+) | (% in CD4+CD25+) | |||||
| CD4+ : CD40L- treated DCs (10:1) | 2.65 × 106 | 2.41 × 106 (91.2%) | 2.12 × 105 (8.8%) | 0.86 × 105 (3.5%) | 40.3% | 1.5:1 |
| CD4+ : YEAST- treated DCs (10:1) | 3.50 × 106 | 3.10 × 106 (88.8%) | 3.59 × 105 (11.6%) | 1.10 × 105 (4.4%) | 30.6% | 2.4:1 |
| CD4+ : CD40L- treated DCs (5:1) | 2.81 × 106 | 2.50 × 106 (89.3%) | 2.12 × 105 (8.5%) | 1.10 × 105 (3.4%) | 51.8% | 0.9:1 |
| CD4+ : YEAST- treated DCs (5:1) | 4.12 × 106 | 3.53 × 106 (85.7%) | 6.81 × 105 (19.3%) | 1.41 × 105 (4.0%) | 20.7% | 3.8:1 |
Tregs = CD4+CD25+CD127−FoxP3+
Effector cells = CD4+CD25+
DCs were treated with yeast at a yeast:DC ratio of 10:1 for 48 h or with CD40L (1 μg/ml) for 24 h. Isolated autologous CD4+ T cells were cocultured with yeast-treated DCs or CD40L-treated DCs at 2 ratios (10:1 or 5:1). FACS analysis was performed on day 5.
Ratio of effector cells:Tregs = ([CD4+CD25+] - [CD4+CD25+CD127-FoxP3+Tregs]/[CD4+CD25+CD127-FoxP3+Tregs]).
HD = healthy donor.
To confirm that the difference in the effector T cell:Treg ratio in the cocultures was not influenced by the lack of signal 1 in the coculture of CD4+ T cells and CD40L-treated DCs, an additional healthy donor sample was analyzed with CEA protein added to the coculture (Table 2). CEA was used as an antigen as we have previously found that some healthy donors respond to CEA [36]. The absolute number of CD4+ T cells and the percentage of CD4+CD25+ T cells in the CD4+ T-cell population were again higher in the coculture of CD4+ T cells and yeast-treated DCs than in the coculture of CD4+ T cells and CD40L-treated DCs, with and without CEA protein. As shown in Table 2, the frequency of Tregs in CD4+CD25+ T cells was higher when CD4+ T cells were cocultured with CD40L-treated DCs compared to yeast-treated DCs with and without CEA. This also translated to comparatively higher ratios of effector T cells:Tregs in the coculture of CD4+ T cells and yeast-treated DCs. Data on 2 additional healthy donors are presented in Supplementary Tables 2A and 2B.
Table 2.
CD4+CD25+:Treg ratio in coculture of CD4+ T cells plus autologous DCs treated with yeast or CD40L and with or without CEA protein (HD#1)
| Coculture (ratio) | Total number of cells/well | CD4+ (% in total number of cells) | CD4+CD25+ (% in CD4+) | Tregs | Ratio effector cells:Tregs | |
|---|---|---|---|---|---|---|
| (% in CD4+) | (% in CD4+CD25+) | |||||
| CD4+ : CD40L-treated DCs (5:1) | 3.46 × 106 | 3.1 × 106 (89.7%) | 5.6 × 105 (18.2%) | 1.9 × 105 (6.4%) | 33.9% | 1.8:1 |
| CD4+ : YEAST-treated DCs (5:1) | 4.11 × 106 | 3.74 × 106 (91.1%) | 9.8 × 105 (26.3%) | 2.3 × 105 (6.3%) | 23.4% | 3.1:1 |
| CD4+ : CD40L-treated DCs (5:1) + CEA | 3.84 × 106 | 3.43 × 106 (89.5%) | 8.3 × 105 (24.3%) | 2.4 × 105 (7.2%) | 28.9% | 2.4:1 |
| CD4+ : YEAST-treated DCs (5:1) + CEA | 4.53 × 106 | 4.17 × 106 (92.0%) | 13.7 × 105 (32.9%) | 2.9 × 105 (7.1%) | 21.1% | 3.6:1 |
Tregs = CD4+CD25+CD127−FoxP3+
Effector cells = CD4+CD25+
DCs were treated with yeast at a yeast:DC ratio of 10:1 for 48 h or with CD40L (1 μg/ml) for 24 h. Isolated autologous CD4+ T cells were cocultured with yeast-treated DCs or CD40L-treated DCs at a ratio of 5:1 with or without CEA protein. FACS analysis was performed on day 5.
Ratio of effector cells:Tregs = ([CD4+CD25+] - [CD4+CD25+CD127-FoxP3+Tregs]/[CD4+CD25+CD127-FoxP3+Tregs]).
HD = healthy donor.
We next investigated the production of cytokines and chemokines in the coculture of CD4+ T cells with yeast- or CD40L-treated DCs. Results showed higher levels of IFN-γ, TNF-α, GM-CSF, IL-2, IL-8, and IL-6 produced in the yeast cocultures, with and without CEA protein (Table 3). This suggested that a strong Th1 response was elicited by concomitant yeast and antigen. Low levels of immunosuppressive cytokines such as IL-10 and TGF-β were detected in cocultures with both yeast- and CD40L-treated DCs (data not shown). Similar results were observed in additional coculture experiments using samples from 5 healthy donors. Data on 2 additional healthy donors are presented in Supplementary Tables 3A and 3B.
Table 3.
Cytokine production from coculture of CD4+ T cells and DCs (HD#1)
| Coculture (ratio) | IL-2 (pg/ml) | IL-8 (pg/ml) | GM-CSF (pg/ml) | IFN-γ (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|---|---|---|
| CD4+ : CD40L-treated DCs (5:1) | 11 | 5,871 | 62 | 682 | 0 | 28 |
| CD4+ : YEAST-treated DCs (5:1) | 154 | 7,976 | 218 | 1,583 | 76 | 139 |
| CD4+ : CD40L-treated DCs (5:1) + CEA | 9 | 8,990 | 81 | 1,857 | 187 | 39 |
| CD4+ : YEAST-treated DCs (5:1) + CEA | 79 | 10,106 | 322 | 4,785 | 288 | 194 |
DCs were treated with yeast at a yeast:DC ratio of 10:1 for 48 h or with CD40L (1 μg/ml) for 24 h. Isolated autologous CD4+ T cells were cocultured with yeast-treated DCs or CD40L-treated DCs at a ratio of 5:1 with or without CEA protein. After 5 days, culture supernatants were collected and screened for cytokine and chemokine production.
HD = healthy donor.
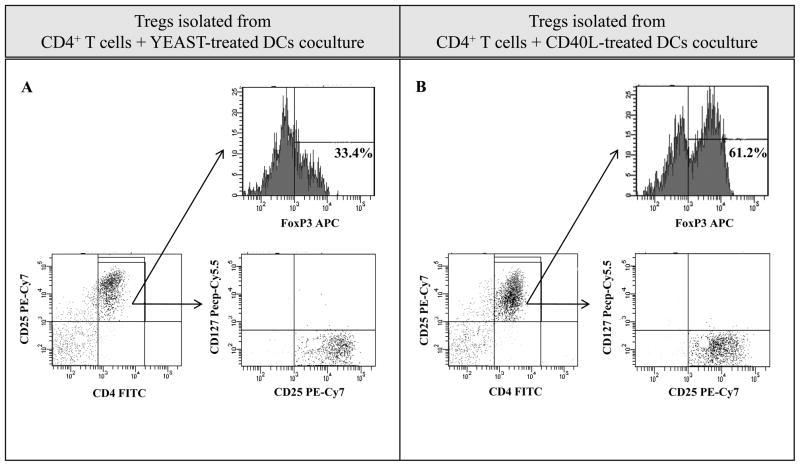
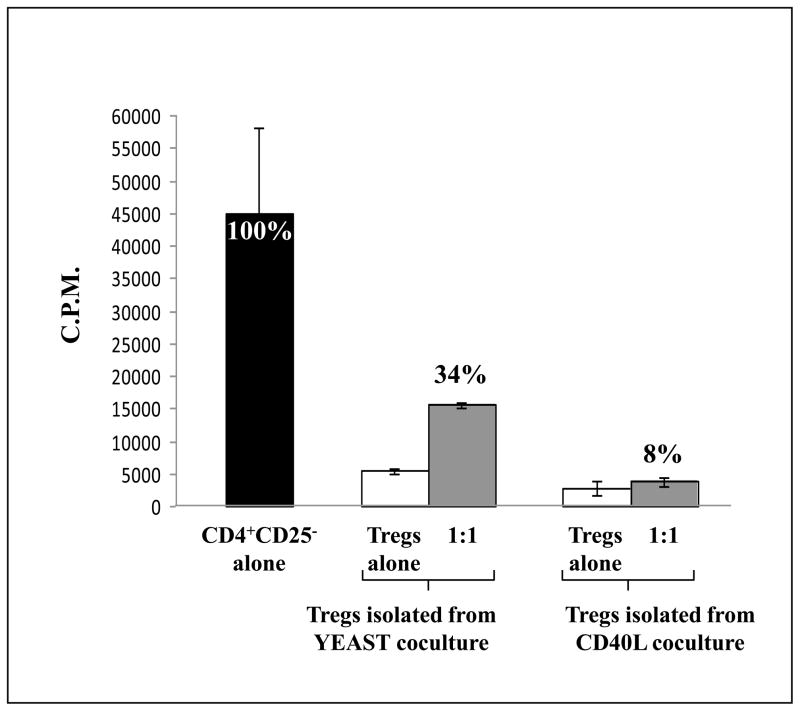
We next evaluated the phenotype and functionality of CD4+CD25+CD127− Tregs isolated from the cocultures of CD4+ T cells and yeast-treated or CD40L-treated DCs from 5 healthy donors. Tregs were isolated using CD4+CD25+CD127− Miltenyi columns, and FoxP3 expression was analyzed by flow cytometry gating on CD4+CD25high cells (Fig. 1). The expression of FoxP3 in CD4+CD25high Tregs generated from the coculture of CD4+ T cells and yeast-treated DCs was significantly decreased compared to CD4+CD25high Tregs generated from the coculture of CD4+ T cells and CD40L-treated DCs (33.4% and 61.2%, respectively). Additional analysis showed that Tregs isolated from both cocultures were CD127−. Similar results as shown in Fig. 1 were obtained from additional experiments using PBMC from 4 different healthy donors. The functional activity of Tregs isolated from the cocultures was determined by suppression assays using autologous CD4+CD25− T cells as effector cells and autologous irradiated T cell-depleted PBMCs as APCs (Fig. 2). Tregs isolated from the coculture of CD4+ T cells and yeast-treated DCs also exhibited a lower suppressive effect on CD4+CD25− effector proliferation than Tregs isolated from the coculture of CD4+ T cells with CD40L-treated DCs.
Fig. 1.
CD4+CD25+CD127− Tregs isolated from the coculture of CD4+ T cells and yeast-treated DCs vs. CD40L-treated DCs showed decreased FoxP3 expression. CD4+ T cells plus yeast-treated DCs (A), and CD4+ T cells plus CD40L-treated DCs (B), were cocultured for 5 days. On day 5, CD4+CD25+CD127− Tregs isolated from the cocultures were analyzed by FACS. Cells were stained with CD4 FITC, CD25 PE, and FoxP3 APC antibodies and acquired on an LSRII. The numbers in the upper right of the graphs represent the % of FoxP3+ cells in CD4+CD25high Tregs. The figure is representative of 3 experiments on 3 healthy donors.
Fig. 2.
Tregs generated from yeast coculture exhibit decreased suppressive function on CD4+CD25− effector cells compared to Tregs generated from CD40L coculture. A representative histogram shows the suppression of CD4+CD25− T-cell proliferation by CD4+CD25+CD127− Tregs in a healthy donor. CD4+CD25+CD127− Tregs generated from yeast and CD40L cocultures were isolated by Miltenyi columns and rested for 24 h. Tregs were then cultured alone or cocultured at a 1:1 ratio with autologous CD4+CD25− T cells, upon stimulation with anti-CD3 and irradiated autologous T-depleted PBMCs. Experiments were performed in triplicate and results are expressed as the mean ± SD (cpm). Percentages indicate the level of proliferation.
Previous studies have demonstrated the possibility that FoxP3 expression could be induced in human naïve CD4+ T cells after cell activation. This transient expression of FoxP3 does not confer any suppressive competence to activated T cells [37]. Recently, Olek et al. have shown that stable expression of the FoxP3 gene in natural Tregs requires DNA demethylation at a highly conserved region of the human FoxP3 gene (Treg-specific demethylated region, TSDR). DNA demethylation was not detected in the FoxP3 gene transiently expressed in activated effector cells [30,38,39]. We therefore analyzed the DNA demethylation in the FoxP3 locus of CD4+ T cells isolated from the yeast- or CD40L-cocultures. In preliminary data using three healthy donors, we found decreased FoxP3 TSDR demethylation levels (% of demethylated FoxP3) in the CD4+ T cells isolated from the yeast coculture compared to those from the CD40L coculture (4.9 vs 7.3, 4.5 vs 5.9, and 5.3 vs 6.0%, respectively). The CD40L cocultures had higher levels of demethylated Tregs. Data from the DNA demethylation analyses were thus similar to results obtained from the yeast coculture, which had a higher ratio of activated CD4+ T cells to Tregs than the CD40L coculture.
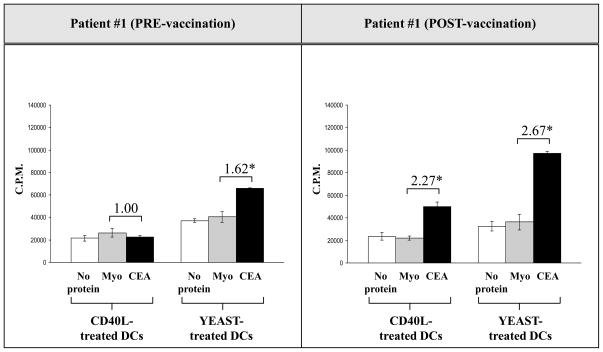
Because the mechanism by which DCs are activated plays a key role in antigen-specific T-cell activity, we further evaluated the effect of yeast-treated DCs on the antigen-specific CD4+ T-cell response. CD4+ T cells isolated from carcinoma patients pre- and post-vaccination with rV-, rF-CEA-MUC1-TRICOM, i.e., PANVAC-V/F were cocultured with irradiated yeast-treated DCs or CD40L-treated DCs with or without CEA protein for 7 days. The CEA-specific response was measured by proliferation assay [31]. As seen in Fig. 3, the CEA-specific CD4+ T-cell response in a patient with carcinoma was higher both pre- and post-vaccination when CD4+ T cells were cocultured with yeast-treated DCs compared to CD40L-treated DCs, with or without the addition of CEA protein. The fold increases of CEA-specific CD4 proliferation (cpm of CD4 response to CEA/cpm of CD4 response to myoglobulin control) were higher in the coculture of CD4+ T cells and yeast-treated DCs compared to the coculture of CD4+ T cells and CD40L-treated DCs. Similar results were observed with T cells isolated from 2 additional cancer patients who had been vaccinated with PANVAC-V/F.
Fig. 3.
CEA-specific CD4+ T-cell proliferation increased in a colorectal cancer patient pre- and post-vaccination when CD4+ T cells were stimulated with DCs treated with yeast and incubated with CEA protein. DCs from a colorectal cancer patient were isolated pre- and post-vaccination and cultured for 5 days with GM-CSF and IL-4. DCs were then treated with yeast (yeast:DCs = 10:1) for 48 h or with CD40L (1 μg/ml) for 24 h. CD4+ T cells were isolated using Miltenyi columns by negative selection and incubated with DCs (CD4+ T cells:DCs = 10:1) for 7 days, using 2 μg/ml PHA as a positive control and 20 μg/ml myoglobulin as a negative control. CEA protein was used at 20 μg/ml. On day 7, 3H was added to the culture, incubated for 6 h, and read with a Wallac Trilux B-scintillation counter. The numbers above the CEA columns represent the fold increase of CD4 proliferation with CEA protein and myoglobulin protein. The experiment was performed in triplicate and the results are expressed as the mean ± SD (cpm).
To further evaluate the ability of yeast-treated DCs to generate antigen-specific T-cell responses, we performed studies to establish a CEA-specific CD4 T-cell line in vitro using yeast-treated DCs as APCs and a class II CEA peptide (p653). PBMCs from a colorectal carcinoma patient were used in the study. Three CD4 T-cell lines were generated and designated as T-A, T-B, and T-C. T-A was generated by stimulating CD4+ T cells with yeast-treated DCs pulsed with peptide p653. T-B was generated by stimulating CD4+ T cells with yeast-treated DCs only. T-C was generated by stimulating CD4+ T cells with CD40L-treated DCs pulsed with peptide p653. The specificity of the T-cell lines was determined by a PE-labeled CEA653–667/DRB1-0401 tetramer. As shown in Table 4, a higher level of CEA tetramer binding T cells was detected in the T-A cell line compared to the T-C cell line. No positive CEA-specific T cells were detected in the T-B cell line. These results suggest that yeast-treated DCs can present CEA peptide and activate CEA-specific CD4+ T cells at a higher level than CD40L-matured DCs. These T-cells were further evaluated by analysis of IFN-γ production by the T-A, T-B, and T-C T-cell lines after stimulation with peptide p653 at IVS-3 (Table 4). IFN-γ production by T-A was higher than by T-C after stimulation with autologous CD40L-matured DCs pulsed with peptide p653. As a negative control, production of IFN-γ from T-A and T-C was < 15.6 pg/ml when stimulated with autologous CD40L-matured DCs pulsed with HIV peptide or no peptide.
Table 4.
Tetramer binding and IFN-γ production from CEA-specific CD4+ T cells
| T-cell line | Treatment of DCs | Tetramer Binding
|
IFN-γ Production
|
|||
|---|---|---|---|---|---|---|
| Control tetramer hCLIP (% of positive cells) | CEA class II tetramer (% of positive cells) | HIV peptide | No peptide | CEA peptide | ||
| T-A | Yeast + CEA peptide | 0.2 | 8.5 | < 15.6 | < 15.6 | 892 pg/ml |
| T-B | Yeast | 0.2 | 0.4 | < 15.6 | < 15.6 | 63 pg/ml |
| T-C | CD40L + CEA peptide | 0.2 | 6.2 | < 15.6 | < 15.6 | 659 pg/ml |
T-A: CEA-specific CD4+ T-cell line generated using DCs treated with yeast and pulsed with class II CEA peptide p653
T-B: CEA-specific CD4+ T-cell line generated using DCs treated with yeast without CEA peptide
T-C: CEA-specific CD4+ T-cell line generated using DCs treated with CD40L and pulsed with class II CEA peptide p653
For the analysis of IFN-γ production, CD4+ T-cell lines at IVS3 were stimulated for 24 h with autologous CD40L-matured DCs pulsed with CEA peptide in IL-2-free medium. HIV peptide or no peptide were used as negative controls. Supernatants were screened for secretion of IFN-γ by ELISA
4. Discussion
Numerous studies have demonstrated that yeast strains such as Candida albicans, Malassezia furfur, and S. cerevisiae can be phagocytosed and processed for antigen presentation by human DCs [15,40,41]. The cell wall components of S. cerevisiae, including β-1,3-D-glucan and mannan, act like pathogen-associated molecular patterns (PAMPs) and interact primarily with TLR-2, TLR-4, mannose receptor, and dectin-1 on DCs. This triggers an innate “danger” signal on DCs and the production of several cytokines (IL-12, IFN-g, IL-2 and TNF-α), which generates a strong Th1 immune response that enhances the proliferation and cytotoxicity of CTLs [10,13,42,43]. We have also demonstrated that human DCs treated with yeast-CEA produce higher levels of IL-7 compared to DCs treated with CD40L (unpublished data). At the same time, yeast can efficiently mature human DCs, leading to increased levels of CD80, CD83, CD54, CD58, and MHC class I and II [10]. We have previously demonstrated that a heat-killed recombinant yeast construct expressing CEA (yeast-CEA) can be successfully processed by human DCs to activate a CEA-specific CD8 T-cell response. Gene expression profiles of human DCs treated with yeast-CEA showed increased expression of numerous genes involved in the production of chemokines and cytokines and their receptors, and genes related to antigen uptake, antigen presentation and signal transduction [13]. A previous murine study from our laboratory found that vaccination with yeast-CEA elicited both a CD4+ and a CD8+ T-cell response. In this study the frequency of Tregs was not significantly altered, but the net effect was an increase in the ratios of CD4+ effector cells to Tregs and CD8+ effector cells to Tregs, thus enhancing the anti-tumor response. Indeed, yeast-CEA vaccinated CEA-transgenic mice displayed reduced tumor burden and increased overall survival compared to mock-treated or control-yeast treated mice in both pulmonary metastasis and pancreatic tumor models [14].
The studies reported here investigated for the first time the effect of yeast-treated DCs on the CD4+ T-cell population, especially on the ratio of CD4+ effector cells to Tregs. Several studies have shown that antigen-loaded mature DCs can induce proliferation of Tregs in vitro and in vivo [19,22]. It has been demonstrated that human myeloid mature DCs (mDCs) are superior APCs for maintaining and expanding CD4+CD25highFoxP3+ Tregs in vitro in healthy donors and patients with myeloma [23]. One of the major challenges of tumor immunotherapy and the use of cancer vaccines is the development of strategies to overcome immune suppression. Evidence that Tregs may play an important role in the suppression of antitumor immunity comes from several clinical investigations showing that a higher frequency of Tregs in tumor correlates with poor clinical outcome [44–46]. We recently demonstrated that the suppressive function of Tregs was enhanced in prostate cancer patients compared to healthy donors [29]. It has also been reported that Tregs can suppress the proliferation of naïve CD4+ T cells and inhibit IL-2 secretion of CD4+ effector cells upon activation by tumor-specific antigen [47].
In this study, we demonstrated that the coculture of human CD4+ T cells and yeast-treated autologous DCs can enhance the CD4+ effector to Treg ratio compared to the coculture of CD4+ T cells and CD40L-treated autologous DCs, with or without signal 1. These results suggest that yeast-treated human DCs can enhance CD4+ T-cell immune responses partly due to an increase in the CD4+ effector to Treg ratio. We also observed that the percentage of CD4+CD25+CD127− FoxP3+ Tregs in CD4+CD25+ T cells decreased in the coculture of CD4+ T cells and yeast-treated DCs compared to the coculture of CD4+ T cells and CD40L-treated DCs, with or without signal 1. These data were further confirmed by the quantitative analysis of DNA demethylation in the FoxP3 locus of the CD4+ T cells collected from the yeast or CD40L cocultures. To ascertain that the decrease in CD4+CD25+CD127− FoxP3+ Tregs was not influenced by the increase in CD4+CD25+ effector cells, we analyzed the phenotype of isolated CD4+CD25+CD127− T cells and found that Tregs generated from the coculture of CD4+ T cells and yeast-treated DCs had lower levels of FoxP3 expression compared to Tregs generated from the coculture of CD4+ T cells and CD40L-treated DCs. FoxP3 is the most reliable molecular marker for natural Tregs, and it has been demonstrated that FoxP3 expression correlates with Treg function [28]. High-level expression of FoxP3 is sufficient to confer suppressive activity to normal non-Treg cells [48]. An evaluation of the functionality of Tregs generated from the 2 cocultures used here showed that Tregs generated from the coculture of CD4+ T cells and yeast-treated DCs exerted a lower suppressor function compared to Tregs from the coculture of CD4+ T cells and CD40L-treated DCs. These results suggest that yeast-induced maturation of DCs leads to decreased FoxP3 expression in Tregs and, consequently, to decreased suppressive function of Tregs, facilitating CD4+ T-cell immune responses.
In this study, we analyzed the production of cytokines and chemokines in 2 separate cocultures. High levels of IFN-γ, TNF-α, IL-8, GM-CSF, and IL-2 were detected in the coculture of CD4+ T cells and yeast-treated DCs, which mediated a high level of Th1 immune response. Higher levels of IL-6 were also detected in the coculture of CD4+ T cells and yeast-treated DCs than in the coculture of CD4+ T cells and CD40L-treated DCs. It has been recently demonstrated that TLR-4 activation on DCs can block the suppressor function of Tregs, due in part to the production of IL-6 [18]. IL-6 induces FoxP3 mRNA down-regulation in Tregs, and acts synergistically with IL-1 to down-regulate FoxP3 expression via a pathway dependent on transcription factor STAT3 [49]. This observation suggests that the decrease in FoxP3 expression and Treg functional activity generated from the coculture of CD4+ T cells and yeast-treated DCs may also be due in part to the presence of IL-6. The addition of anti-IL-6 and anti-IL-6 receptor antibody did not increase the Treg frequency (unpublished data). These results are consistent with findings showing that TLR-4 activation can enhance production of IL-12 and IL-6 by DCs and consequently promote the differentiation of the IFN-γ-secreting Th1 subtype of CD4+ T cells, which in turn can lead to an enhanced CTL response against tumors [50].
To determine whether DCs treated with yeast could increase the antigen-specific CD4+ T-cell response compared to DCs treated with CD40L, we investigated CEA-specific CD4+ T-cell proliferation in cancer patients. The results showed that yeast-treated DCs pulsed with the tumor antigen CEA induced a higher level of CEA-specific CD4+ T-cell immune response compared to CD40L-treated DCs used as APCs. To confirm these data, we generated a CEA-specific CD4+ T-cell line using yeast-treated DCs. We performed a class II tetramer binding assay to identify antigen-specific T-cells, because we did not have enough PBMC to make autologous DCs to be used as APC in a class II ELISPOT assay. IFN-γ and tetramer binding results on CD4+ T-cell lines suggest that yeast-treated DCs can efficiently generate CEA-specific CD4+ T-cell immune responses. These findings also demonstrate that yeast-treated human DCs can decrease the expression of FoxP3 and the suppressive function of CD4+CD25+CD127− Tregs, and thus provide an additional rationale for the clinical evaluation of recombinant yeast constructs such as yeast-CEA in cancer vaccine immunotherapy.
Supplementary Material
Acknowledgments
Grant support: Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
The authors thank Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript. We also thank Udo Baron and Ulrich Hoffmüller from Epiontis GmbH for performing the quantitative DNA methylation analysis of FoxP3 by real-time PCR on our samples.
We also thank Tom King, Yingnian Lu, and Zhimin Guo from GlobeImmune, Inc., for providing the yeast constructs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arthur JF, Butterfield LH, Roth MD, Bui LA, Kiertscher SM, Lau R, et al. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4(1):17–25. [PubMed] [Google Scholar]
- 2.Bello-Fernandez C, Matyash M, Strobl H, Pickl WF, Majdic O, Lyman SD, et al. Efficient retrovirus-mediated gene transfer of dendritic cells generated from CD34+ cord blood cells under serum-free conditions. Hum Gene Ther. 1997;8(14):1651–8. doi: 10.1089/hum.1997.8.14-1651. [DOI] [PubMed] [Google Scholar]
- 3.Neering SJ, Hardy SF, Minamoto D, Spratt SK, Jordan CT. Transduction of primitive human hematopoietic cells with recombinant adenovirus vectors. Blood. 1996;88(4):1147–55. [PubMed] [Google Scholar]
- 4.Philip R, Brunette E, Ashton J, Alters S, Gadea J, Sorich M, et al. Transgene expression in dendritic cells to induce antigen-specific cytotoxic T cells in healthy donors. Cancer Gene Ther. 1998;5(4):236–46. [PubMed] [Google Scholar]
- 5.Szabolcs P, Gallardo HF, Ciocon DH, Sadelain M, Young JW. Retrovirally transduced human dendritic cells express a normal phenotype and potent T-cell stimulatory capacity. Blood. 1997;90(6):2160–7. [PubMed] [Google Scholar]
- 6.Tsang KY, Palena C, Yokokawa J, Arlen PM, Gulley JL, Mazzara GP, et al. Analyses of recombinant vaccinia and fowlpox vaccine vectors expressing transgenes for two human tumor antigens and three human costimulatory molecules. Clin Cancer Res. 2005;11(4):1597–607. doi: 10.1158/1078-0432.CCR-04-1609. [DOI] [PubMed] [Google Scholar]
- 7.Tsang KY, Zhu M, Even J, Gulley J, Arlen P, Schlom J. The infection of human dendritic cells with recombinant avipox vectors expressing a costimulatory molecule transgene (CD80) to enhance the activation of antigen-specific cytolytic T cells. Cancer Res. 2001;61(20):7568–76. [PubMed] [Google Scholar]
- 8.Zhu M, Terasawa H, Gulley J, Panicali D, Arlen P, Schlom J, et al. Enhanced activation of human T cells via avipox vector-mediated hyperexpression of a triad of costimulatory molecules in human dendritic cells. Cancer Res. 2001;61(9):3725–34. [PubMed] [Google Scholar]
- 9.Wadle A, Held G, Neumann F, Kleber S, Wuellner B, Asemissen A, et al. Cross-presentation of HLA class I epitopes from influenza matrix protein produced in Saccharomyces cerevisiae. Vaccine. 2006;24:6272–81. doi: 10.1016/j.vaccine.2006.05.096. [DOI] [PubMed] [Google Scholar]
- 10.Barron MA, Blyveis N, Pan SC, Wilson CC. Human dendritic cell interactions with whole recombinant yeast: implications for HIV-1 vaccine development. J Clin Immunol. 2006;26(3):251–64. doi: 10.1007/s10875-006-9020-8. [DOI] [PubMed] [Google Scholar]
- 11.Haller AA, Lauer GM, King TH, Kemmler C, Fiolkoski V, Lu Y, et al. Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine. 2007;25(8):1452–63. doi: 10.1016/j.vaccine.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7(5):625–9. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 13.Remondo C, Cereda V, Mostbock S, Sabzevari H, Franzusoff A, Schlom J, et al. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27(7):987–94. doi: 10.1016/j.vaccine.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, et al. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14(13):4316–25. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Korting HC. Toll-like receptors and innate antifungal responses. Trends Microbiol. 2004;12(1):44–9. doi: 10.1016/j.tim.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27(2):168–80. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171(10):4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 18.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 19.Coquerelle C, Moser M. Are dendritic cells central to regulatory T cell function? Immunol Lett. 2008;119(1–2):12–6. doi: 10.1016/j.imlet.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101(12):4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 21.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198(2):235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108(8):2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- cells. Proc Natl Acad Sci U S A. 2005;102(11):4103–8. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilliet M, Soumelis V, Watanabe N, Hanabuchi S, Antonenko S, de Waal-Malefyt R, et al. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197(8):1059–63. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loskog A, Ninalga C, Totterman TH. Dendritic cells engineered to express CD40L continuously produce IL12 and resist negative signals from Tr1/Th3 dominated tumors. Cancer Immunol Immunother. 2006;55(5):588–97. doi: 10.1007/s00262-005-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokokawa J, Bera TK, Palena C, Cereda V, Remondo C, Gulley JL, et al. Identification of cytotoxic T-lymphocyte epitope(s) and its agonist epitope(s) of a novel target for vaccine therapy (PAGE4) Int J Cancer. 2007;121(3):595–605. doi: 10.1002/ijc.22698. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14(4):1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 30.Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69(2):599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 31.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14(10):3060–9. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi H, Omiya R, Ruiz M, Huarte E, Sarobe P, Lasarte JJ, et al. Identification of an antigenic epitope for helper T lymphocytes from carcinoembryonic antigen. Clin Cancer Res. 2002;8(10):3219–25. [PubMed] [Google Scholar]
- 33.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160(7):3363–73. [PubMed] [Google Scholar]
- 34.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res. 2000;60(18):5228–36. [PubMed] [Google Scholar]
- 35.Falta MT, Fontenot AP, Rosloniec EF, Crawford F, Roark CL, Bill J, et al. Class II major histocompatibility complex-peptide tetramer staining in relation to functional avidity and T cell receptor diversity in the mouse CD4(+) T cell response to a rheumatoid arthritis-associated antigen. Arthritis Rheum. 2005;52(6):1885–96. doi: 10.1002/art.21098. [DOI] [PubMed] [Google Scholar]
- 36.Yang S, Tsang K, Schlom J. Induction of higher-avidity human CTLs by vector-mediated enhanced costimulation of antigen-presenting cells. Clin Cancer Res. 2005;11:5603–15. doi: 10.1158/1078-0432.CCR-05-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103(17):6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37(9):2378–89. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 39.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9(2):83–9. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 40.Buentke E, Heffler LC, Wallin RP, Lofman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001;31(10):1583–93. doi: 10.1046/j.1365-2222.2001.01199.x. [DOI] [PubMed] [Google Scholar]
- 41.Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46(7):503–12. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 42.Franzusoff A, Duke RC, King TH, Lu Y, Rodell TC. Yeasts encoding tumour antigens in cancer immunotherapy. Expert Opin Biol Ther. 2005;5(4):565–75. doi: 10.1517/14712598.5.4.565. [DOI] [PubMed] [Google Scholar]
- 43.Romagnoli G, Nisini R, Chiani P, Mariotti S, Teloni R, Cassone A, et al. The interaction of human dendritic cells with yeast and germ-tube forms of Candida albicans leads to efficient fungal processing, dendritic cell maturation, and acquisition of a Th1 response-promoting function. J Leukoc Biol. 2004;75(1):117–26. doi: 10.1189/jlb.0503226. [DOI] [PubMed] [Google Scholar]
- 44.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 45.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 46.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–72. [PubMed] [Google Scholar]
- 47.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19(2):217–23. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 49.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212:163–9. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5(5):508–15. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.