Abstract
Borrelia burgdorferi is the etiological agent for Lyme disease (LD), the most common vector borne disease in the United States. There is no human vaccine against LD currently available. Our approach to a vaccine is based on its surface-exposed glycolipids. One group of these glycolipids termed BBGL-2 consists of 1,2-di-O-acyl-3-O-(α-D-galactopyranosyl)-sn-glycerol congeners having palmitic, oleic, stearic, linoleic, and myristic acids. In order to delineate the immunodominant region(s) of the BBGL-2 components, we embarked on a synthetic project to provide available structurally defined, homogeneous analogs of BBGL-2 that might help identify the best vaccine candidate. The antigenicity of the synthetic glycolipids was examined by dot-blot analysis using mice sera obtained by immunization with killed B. burgdorferi cells, with native BBGL-2 in complete Freund's adjuvant, as well as sera obtained from patients with Lyme disease. We found that the presence of two acyl groups in the glycerol moiety was essential for antigenicity. At least one of these groups must be an oleoyl moiety. Neither the anomeric configuration of the galactose nor the configuration of the glycerol at C-2 was a decisive factor. Based on these findings we designed an `unnatural' BBGL-2 analog having the structure 3-O-(β-D-galactopyranosyl)-1,2-di-O-oleoyl-DL-glycerol which is easier and less expensive to synthesize than the other BBGL-2 congeners prepared in this study. This substance proved to be antigenic and is considered a candidate vaccine for Lyme disease.
Keywords: Antigen, Borrelia burgdorferi, Vaccine, Diacylglycerol, Dot-blot, Glycolipid
1. Introduction
Lyme disease is a multisystem infection caused by the spirochete Borrelia burgdorferi that can involve the skin, nervous system, heart, and joints.1 It is transmitted by some of the Lyxodes tick species, and it is the most common vector borne disease in the United States with more than 304,000 LD cases have been reported to the Centers for Disease Control and Prevention from 1995 to 2009. The majority of cases occur in the Middle Atlantic, Northeastern, and North Central states, reaching 30,000 confirmed cases in 2009 together with more than 8000 probable cases in the same year.2,3 A licensed vaccine (LYMErix™) containing a lipidated recombinant surface protein of B. burgdorferi designated as L-OspA, although effective above the age of 12 years when administered with aluminum hydroxide as the adjuvant4 was withdrawn from the market in early 2002 after less than 5 years of use, because of inadequate market results. Moreover, there was also the contentious issue of the vaccine's hypothetical potential to induce autoimmunity because of OspA's partial homology to the human leukocyte function-associated antigen 1 in persons with certain HLA-DR alleles5,6 but studies have shown no increase in the development of arthritis or other adverse effects.7
Currently, there is no vaccine for human use against LD, and prevention of the disease is limited to protective measures to avoid tick bites. An effective vaccine to prevent human Lyme disease would be of great benefit for populations with high risk of acquiring the infection.8,9
B. burgdorferi produces neither a lipopolysaccharide nor a capsular polysaccharide.10,11 On the other hand, immunoreactive glycolipids were isolated from B. burgdorferi that were shown to be α-galactosyl diacylglycerols.12 However, neither the location of the acyl groups nor the stereochemistry of the glycerol residue was defined in the early studies. Our laboratory reported the isolation and structural characterization of two groups of surface-exposed glycolipids termed BBGL-1 and BBGL-2.11 Using a variety of chemical and spectroscopic methods, BBGL-1 was identified as 6-O-acyl-β-D-galactopyranosyl-cholesterol and BBGL-2 as 1,2-di-O-acyl-3-O-α-D-galactopyranosyl-sn-glycerol.11 These findings were confirmed by other workers who also reported the isolation of cholesteryl β-D-galactopyranoside without an acyl group at the galactose unit as well as 6-O-acyl-β-D-glucopyranosyl-cholesterol.13,14 In BBGL-2 the most common fatty acid is palmitic acid (1.00 mol), followed by oleic and stearic acids (approx. 0.65 and 0.25 mol, respectively). Myristic and linoleic acids were also detected in 0.15 and 0.12 mol amounts. Based on the relative proportions of the palmitic and oleic acids it was proposed that the major components of BBGL-2 are 3-O-α-D-galactopyranosyl-1-O-oleoyl-2-O-palmitoyl-sn-glycerol (4) and/or 3-O-α-D-galactopyranosyl-2-O-oleoyl-1-O-palmitoyl-sn-glycerol (3) whereas the other fatty acids detected in the BBGL-2 fraction remain unaccounted for.11 Because the individual components of the BBGL-2 complex could not be separated, no single structure could be identified. Therefore, the presence of galactosyl-homodiacyl-glycerol derivatives and of those incorporating the minor fatty acids cannot be excluded. Because of the lack of genes in B. burgdorferi for the synthesis or elongation of fatty acids,15 its fatty acids are incorporated from the host or from the environment. This may explain the differences in fatty acid composition reported by different laboratories for in vitro cultivated B. burgdorferi cells.11,12 In mice and rabbits, BBGL-2 elicited antibodies that reacted with both BBGL-1 and -2, and the sera of LD patients had a strong IgG reaction with BBGL-2.11,16 These propensities make the BBGL-2 glycolipids candidates for developing diagnostics and vaccines against B. burgdorferi devoid of any immunogenic proteins such as L-OspA that might have the potential to elicit autoantibodies.5
Chemical syntheses of BBGL-2 glycolipids having one oleoyl and one palmitoyl group on their glycerol moieties have been reported, but the published synthetic protocols lack rigorous proof of their homogeneity.17–20 An approach by α-galactosylation of the commercially available diglyceride 2-O-oleoyl-1-O-palmitoyl-sn-glycerol (Avanti Polar Lipids) has recently been described.19,20 Examination of the 13C NMR spectrum of the commercial material, obtained from the same source revealed that it contains up to 25% of an accompanying compound that is most likely 1-O-oleoyl-2-O-palmitoyl-sn-glycerol. The basis of this assumption is that while the 1H NMR spectrum of the commercial material is fully consistent with the proposed structure, the 13C NMR spectrum exhibits, in addition to two major carbonyl carbon signals, two additional ones that are approximately 1/4th of the major ones, together with several similarly low-intensity peaks. Because in our experience separation of 3 and 4 is not possible, we are tempted to assume that the material reported in Refs. 19 and 20 as compound 3 is a ca. 4:1 mixture of 3 and 4. An alternative approach17,19 starts with the commercially available 3-O-benzyl-sn-glycerol which is regio-selectively acylated at O-1 followed by a second acylation at O-2 and removal of the O-benzyl group with trichloroborane to afford the targeted 1-O-acyl(1)-2-O-acyl(2)-sn-glycerols17,19,21 which are then galactosylated. In applying this approach to 1,2-diacyl-sn-glycerol precursors, care has to be taken to avoid the well-documented acyl migration that can take place under both acidic and basic conditions.22–27 We did not observe acyl migration from the O-1 to the O-2 position of the glycerol moieties, but we did observe migration from O-2 to O-3 over extended periods at room temperature, or even during silica gel column chromatography. In order to prevent undesirable acyl migration, short reaction times and quick chromatographic procedures have been suggested.25 It has been proposed that the regioisomeric purity of acyl glycerols can best be determined by integration of the carbonyl signals of the acyl moieties in high field 13C NMR spectra.22,23 Because of the low natural abundance (1.1%) of 13C combined with the low NMR sensitivity of carbonyl carbons, this method requires long acquisition times to obtain good-quality spectra. Unfortunately, the signal-to-noise ratio of the carbonyl region is insufficient in most reports to allow rigorous assessment of homogeneity.
We are examining aproaches to producing BBGL components for use in a vaccine against B. burgdorferi. Growing B. burgdorferi to produce BBGL's in sufficient quantities for immunization experiments is difficult. In addition, isolation of the glycolipids in a homogeneous form has not been possible, raising reproducibility concerns. To circumvent these difficulties, we are preparing BBGL components by using synthetic chemical methods. So far, we have reported the synthesis of the major BBGL-1 components in their native and bioconjugatable forms28 and prepared a semisynthetic experimental vaccine against B. burgdorferi consisting of the BBGL-1 glycolipids covalently linked to bovine serum albumin through an oxime linkage.29 The aim of the present work is to delineate the immunodominant region of BBGL-2 components by assessing their antigenicity. It is expected that such recognition will facilitate the design of a vaccine against LD.
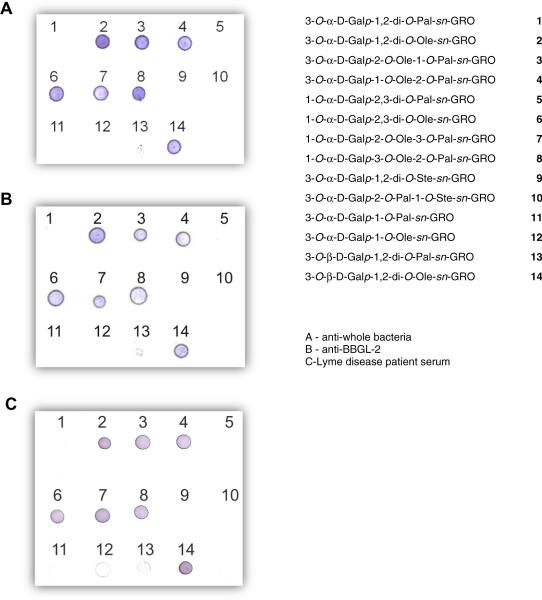
Here we describe experiments aimed at synthesizing the putative BBGL-2 components 1–4. In order to evaluate the biological importance of various structural features we also describe the synthesis of the diastereoisomers 5–8 that differ from the native ones only in the stereochemistry at C-2 of the glycerol moiety, the saturated analogs 9 and 10, the mono-O-acyl derivatives 11 and 12, as well as two additional BBGL-2 analogs (13 and 14) that contain the galactose moiety in the unnatural, beta glycosidic linkage. (Table 1).
Table 1.
Synthetic BBGL-2 analogs
| 3-O-α-D-Galp-1,2-di-O-Pal-sn-GRO | 1 |
| 3-O-α-D-Galp-1,2-di-O-Ole-sn-GRO | 2 |
| 3-O-α-D-Galp-2-O-Ole-1-O-Pal-sn-GRO | 3 |
| 3-O-α-D-Galp-1-O-Ole-2-O-Pal-sn-GRO | 4 |
| 1-O-α-D-Galp-2,3-di-O-Pal-sn-GRO | 5 |
| 1-O-α-D-Galp-2,3-di-O-Ole-sn-GRO | 6 |
| 1-O-α-D-Galp-2-O-Ole-3-O-Pal-sn-GRO | 7 |
| 1-O-α-D-Galp-3-O-Ole-2-O-Pal-sn-GRO | 8 |
| 3-O-α-D-Galp-1,2-di-O-Ste-sn-GRO | 9 |
| 3-O-α-D-Galp-2-O-Pal-1-O-Ste-sn-GRO | 10 |
| 3-O-α-D-Galp-1-O-Pal-sn-GRO | 11 |
| 3-O-α-D-Galp-1-O-Ole-sn-GRO | 12 |
| 3-O-β-D-Galp-1,2-di-O-Pal-sn-GRO | 13 |
| 3-O-β-D-Galp-1,2-di-O-Ole-sn-GRO | 14 |
Following the synthetic studies, we disclose salient NMR features of several of the synthetic glycolipids, and discuss the antigenicity of the synthesized glycolipids.
2. Results and discussion
2.1. Synthetic studies
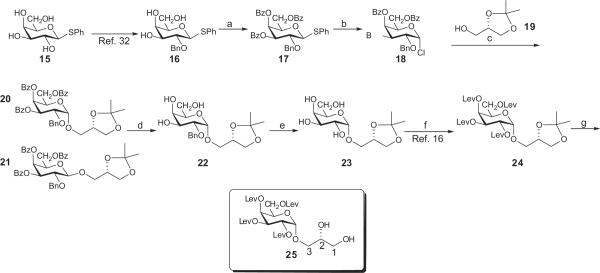
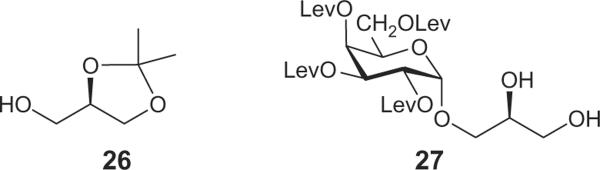
We explored an approach in which the introduction of the α-D-galactopyranosyl moiety to sn-3-O of the glycerol unit precedes the attachment of the fatty acyl groups, thereby preventing acyl migration to that site.18 This concept is shown in Scheme 1. Precursor to the target compounds was phenyl 1-thio-β-D-galactopyranoside30,3115. In preliminary trials, compound 15 was converted into its tetra-O-benzyl derivative, which was reacted with the commercially available glycerol-sn-1,2-acetonide 19 under a variety of published thioglycoside activation protocols (not described in the experimental part). Unexpectedly, the products contained mostly β-interglycosidic linkages, despite the presence of the non-participating benzyl protecting group at the O-2 position of the galactose moiety. We hypothesized that tuning down the reactivity of the galactosyl donor would improve this situation. After attempts with various tri-O-acyl derivatives of the thiogalactoside triol 16, including levulinoyl,32 pentafluoropropionyl,33 and trifluoroacetyl33 groups we eventually selected the benzoylated galactosyl chloride 18 obtained by chlorinolysis of thiogalactoside 17, which in turn was prepared by conventional benzoylation of 16.34 Compound 18 was reacted with acetonide 19 under AgOTf activation to afford the desired a-linked intermediate 20 in 61% yield. The 1,2-cis (α) interglycosidic linkage was proven by the value of the J1,2 coupling constant being 3.6 Hz (see Section 4). The corresponding β-linked glycoside 21 was also isolated, in 19% yield. Its anomeric configuration was indicated by the doublet in its 1H NMR spectrum at 4.71 ppm, with the J1,2 coupling constant being 7.7 Hz. Next, the benzoyl groups were cleaved from 20 by sodium methoxide uneventfully, to afford the triol 22 in excellent yield, followed by careful hydrogenolytic removal of the benzyl group, using ethyl acetate as solvent and a commercial palladium-on-charcoal catalyst, in admixture with 2,4,6-tri-tert-butylpyrimidine, thus affording 23 in 87% yield. In our experience, extended hydrogenolysis often leads to a significant drop in pH. A likely reason for this is the presence of residual palladium chloride in the catalyst, from which hydrogen chloride may be generated upon hydrogenation. Treatment of the tetraol 23 with levulinic acid and DCC in the presence of DMAP afforded a nearly stoichiometric yield of the fully protected galactoside 24.18 Subsequently, the isopropylidene group was removed from the glycerol moiety by hydrolysis in acetic acid to yield diol 25 in 83% yield, for incorporation of the lipid chains. Employing identical reaction conditions, diastereomer 27 having the galactose moiety at the sn-1 position was also synthesized, using the commercially available glycerol derivative 26, the enantiomer of 19.(Chart 1).
Scheme 1.
Reagents and conditions: (a) 4.9 equiv BzCl, CH2Cl2,C6H5N, DMAP (cat), rt, 24 h, 91%; (b) Cl2 in CCl4 (excess), CH2Cl2,0 °C, 30 min, hex-1-ene (excess), 69%); (c) 1.4 equiv 19, 2,4,6-tri-tert-butylpyrimidine (22.5 g, 184 mmol), 4 Å molecular sieves, AgOTf (15 g, 58.2 mmol), −40 °C, 15 min, 61%); (d) NaOMe (excess), CH2Cl2, MeOH, Dowex 50WX8, CH2N2, 91%); (e) H2, Pd/C, 2,4,6-tri-tert-butylpyrimidine, EtOAc, 15 min, 87%; (f) levulinic acid (6 equiv), DCC (7 equiv), 4-dimethylaminopyridine (cat), EtOAc, 94%); (g) AcOH, MeOH, reflux, 5 min, 83%.
Chart 1.
The synthetic sequence toward BBGL-2 analogs 13 and 14, that contain the galactose moiety in the unnatural, beta glycosidic linkage started from imidate 2835 that was reacted with alcohol 19 under activation by TMSOTf, to afford disaccharide 29 in which the β interlycosidic linkage was proven by the value J1,2 7.9 Hz. A two-step replacement of the acyl groups in 29 by levulinoyl groups afforded the fully protected intermediate 30, from which acetic acid hydrolysis yielded diol 31 in high yield. (Scheme 2).
Scheme 2.
Reagents and conditions: (a) 2 equiv 19,CH2Cl2, TMSOTf (cat), 0 °C, 1 h, 80%); (b) NaOMe (excess), MeOH, 24 h, Dowex 50WX8 (H+); (c) levulinic acid (8 equiv), DCC (8 equiv), EtOAc, 93%); (d) AcOH, MeOH, reflux, 5 min, 85%.
With the availability of compounds 25, 27, and 31 incorporating both the galactose and the glycerol moieties in the required stereo-chemistries, the stage was set for the introduction of the oleoyl, palmitoyl, and stearoyl groups.
For the synthesis of the sn-1-O and sn-2-O hetero-di-substituted Gro-moieties, we opted initially to adapt a published protocol18 that, as we eventually found out by 13C NMR spectroscopy, afforded regioisomeric mixtures. In Ref. 18, diol 25 was treated with one equivalent of palmitic or oleic acid in the presence of DCC, apparently without the use of the nucleophilic catalyst DMAP that in our experience is essential to induce O-acylation. Hexane-EtOAc mixtures were used to follow the course of the reaction by TLC, and to isolate the required product `singly acylated' at sn-1-O.18 Using 95:5 and 99:1 v/v mixtures of CH2Cl2 and MeOH as the TLC developing solvent allowed us to observe that the `singly acylated' product is, in fact, a mixture of sn-1-O and sn-2-O mono-acylated isomers, with the former predominating in a range of approximately 4:1–9:1. The formation of this mixture remained unnoticed in an earlier protocol,18 and no physical data have been reported for the `singly acylated' derivatives either.18 All of our efforts to separate the `singly acylated' sn-1-O and 2-O products by TLC or column chromatography using a range of hexane-EtOAc mixtures, reported in the published protocol,18 proved to be futile.
We also noted the formation of a di-O-Gro acylated product before the disappearance of the starting diol, an observation that required the termination of the reaction when some of the diols 25, 27, or 31 were still present.
The faster-moving mono-O-acylated component was invariably the targeted sn-1 acyl product identified by the presence of a characteristic doublet of doublets in its 1H NMR spectrum at ca. 4.37 ppm, indicating that acylation took place at the primary hydroxyl group of the glycerol moiety, leaving HO-2 unsubstituted. The slightly slower-migrating product was the undesired sn-2 acyl isomer showing a diagnostic one-proton multiplet at about 5.1 ppm.
With the homogeneous O-1 (sn) acylated derivatives in hand, the second acyl group was introduced at O-2 of the glycerol moiety, in uneventful reactions using the DCC/DMAP method.
Treatment of the diols 25, 27, and 31 with various FAs used in excess under the agency of DCC/DMAP proceeded as expected, and produced the fully substituted intermediates featuring two identical fatty acids on the glycerol moiety.
The singly and the doubly glycerol-acylated protected BBGL-2 congeners prepared in this study are listed in Tables 2 and 3, respectively. In the final stage of the syntheses, the levulinoyl protecting groups were conventionally removed from the galactose moiety by hydrazine acetate in pyridine32 to afford 1–14.
Table 2.
Singly Gro-acylated protected BBGL-2 congeners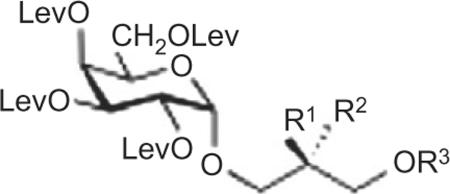
| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 32 | H | OH | Pal |
| 33 | H | OH | Ole |
| 34 | H | OH | Ste |
| 35 | OH | H | Pal |
| 36 | OH | H | Ole |
Table 3.
Doubly Gro-acylated protected BBGL-2 congeners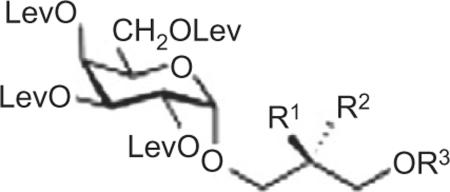
| Compound | R1 | R2 | R3 |
|---|---|---|---|
| 37 | H | OPal | Pal |
| 38 | H | OOle | Ole |
| 39 | H | OOle | Pal |
| 40 | H | OPal | Ole |
| 41 | H | OSte | Ste |
| 42 | OPal | OH | Pal |
| 43 | OOle | OH | Ole |
| 44 | OOle | OH | Pal |
| 45 | OPal | OH | Ole |
2.2. Nuclear magnetic resonance studies
A detailed NMR investigation was performed on compounds 1–4. 1H NMR spectra were assigned using 2D COSY and high-resolution, 2D TOCSY, the latter technique being used to generate separate 1D sub-spectra for the Gal, Gro, Ole, and Pal residues. Selected 1H NMR chemical shifts are reported in Table 4 and coupling constants in Table 6. The small J4,5 value of the Gal residue resulted in inefficient magnetization transfer from H-4 to H-5 and beyond, so thatthe assignments for H-5, H-6, and H-6′ were obtained from the one-bond, 1H–13C correlations established by 2D HSQC. Selected 13C NMR chemical shifts are listed in Table 5. Substituent positions in compounds 1–4 were verified by the inter-residue connectivities observed by high-resolution, 2D HMBC. Anomeric 1JC-1,H-1 coupling constants of 1–4 were measured by 1H-coupled, 2D HSQC, and were found to lie in the range of 170.1–170.4 Hz, which together with the small values of J1,2 3.7–3.8 Hz confirms the a configuration of the glycosidic linkages, as designed.
Table 4.
Selected 1H NMR chemical shifts (ppm) of galactopyranosyl diglyceride analogs 1–4 in CDCl3
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Gal H-1 | 4.954 | 4.925 | 4.940 | 4.937 |
| H-2 | 3.829 | 3.868 | 3.850 | 3.856 |
| H-3 | 3.758 | 3.788 | 3.774 | 3.776a |
| H-4 | 4.098 | 4.087 | 4.095 | 4.093a |
| H-5 | 3.824 | 3.798 | 3.808 | 3.806 |
| H-6 | 3.962 | 3.833 | 3.914 | 3.905 |
| H-6′ | 3.851 | 3.799 | 3.837 | 3.834 |
| Gro H-1 | 4.379 | 4.3794 | 4.377 | 4.373 |
| H-1′ | 4.117 | 4.148 | 4.132 | 4.135 |
| H-2 | 5.254 | 5.250 | 5.252 | 5.254 |
| H-3 | 3.856 | 3.812 | 3.834 | 5.254 |
| H-3′ | 3.641 | 3.624 | 3.632 | 3.631 |
| Ole H-2,2′ | — | 2.318, 2.309 | 2.321 | 2.313 |
| H-8,8′ | — | 2.009, 2.008 | 2.010 | 2.009 |
| H-9,10 | — | 5.342, 5.342 | 5.344 | 5.343 |
| H-11,11′ | — | 2.009, 2.008 | 2.010 | 2.009 |
| H-18 | — | 0.880, 0.880 | 0.880 | 0.880 |
| Pal H-2,2′ | 2.324, 2.317 | — | 2.313 | 0.880 |
| H-16 | 0.881, 0.881 | — | 0.880 | 0.880 |
Our assignments are at variance with those published in Ref. 19 for compound 4: H-3 of Gal was reported in the range of 4.03–4.12 ppm, while H-4 was in the 3.79–3.94 ppm range, that is, reversed from the data shown above.
Table 6.
Selected two and three bond homonuclear H-H and one-bond heteronuclear C-H NMR coupling constants (Hz) of galactopyranosyl diglyceride analogs 1–4 in CDCl3
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Gal J1,2 | 3.8 | 3.7 | 3.8 | 3.8 |
| J 2,3 | 9.7 | 10.3 | 9.8 | 9.9 |
| J 3,4 | 3.3 | 3.2 | 3.2 | 3.3 |
| J 4,5 | 0.9 | <0.5 | 1.1 | 1.3 |
| J 5,6 | 5.1 | Nra | 5.0 | 5.0 |
| J 5,6′ | Nr | Nr | 4.6 | 4.7 |
| J 6,6′ | 11.6 | nr | 11.4 | 11.3 |
| J C-1,H-1 | 170.1 | 170.1 | 170.4 | 170.1 |
| Gro J1,1′ | 11.8 | 12.0 | 11.9 | 12.0 |
| J 1,2 | 4.2 | 3.6 | 4.0 | 3.9 |
| J 1′,2 | 5.7 | 6.2 | 5.9 | 6.0 |
| J 2,3 | 4.6 | 5.4 | 5.0 | 5.0 |
| J 2,3′ | 6.3 | 5.8 | 6.1 | 6.0 |
| J 3,3′ | 11.0 | 10.8 | 10.9 | 10.9 |
Nr = not resolved.
Table 5.
Selected 13C NMR chemical shifts (ppm) of galactopyranosyl diglyceride analogs 1–4 in CDCl3
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Gal C-1 | 99.25 | 99.37 | 99.31 | 99.34 |
| C-2 | 69.45 | 69.13 | 69.30 | 69.28 |
| C-3 | 71.00 | 70.64 | 70.81 | 70.79 |
| C-4 | 70.31 | 70.14 | 70.29 | 70.29 |
| C-5 | 69.98 | 70.14 | 70.03 | 70.06 |
| C-6 | 63.28 | 62.45 | 62.93 | 62.85 |
| Gro C-1 | 61.95 | 62.42 | 62.17 | 62.24 |
| C-2 | 69.87 | 69.88 | 69.87 | 69.87 |
| C-3 | 66.88 | 66.46 | 66.67 | 66.65 |
| Ole C-1 | — | 173.73, 173.35 173.29 | 173.63 | |
| C-2 | — | 34.28, 34.11 | 34.28 | 34.30 |
| C-9 | — | 130.03 | 130.05 | 130.04 |
| C-10 | — | 129.69 | 129.69 | 129.69 |
| C-18 | — | 14.13, 14.13 | 14.13 | 14.13 |
| Pal C-1 | 173.50, 173.25 | — | 173.65 | 173.34 |
| C-2 | 34.29, 34.10 | — | 34.16 | 34.10 |
| C-16 | 14.13, 14.13 | — | 14.13 | 14.13 |
The structures of regioisomers 3 and 4 are not easily differentiated by 1H NMR at 500 MHz, but we have found that 1D 13CNMR provides an excellent method for distinguishing the structures of the compounds, and assessing their purity. The key parameter is the 13C=O chemical shift (see Table 5), which appears to be quite sensitive to the type and chemical environment of the acyl groups. In this regard we note that HMBC was less suitable to detect connectivities in minor isomers. This is because of the limited spectral resolution imposed by small numbers of data points in 2D HMBC, but also because of the restricted signal:noise ratio and dynamic range caused by the small number of scans per FID in 2D NMR. By contrast, 1D 13C NMR has better spectral resolution because of the far greater number of points sampled in the FID, as compared with those in the indirectly detected, 13C dimension of 2D HMBC.
Analysis of the 13C NMR data of pure oleoyl and palmitoyl diglycerides and their mixtures suggests the following rules:
-
(a)
The 13C=O chemical shift is primarily influenced by the substituent's position on the glycerol: the 1-sn-substituent 13C=O's resonate at lower field than the 2-sn-substituent 13C=O's, for example, 173.65 versus 173.34 ppm for the Pal substituent at O-1 versus O-2.
-
(b)
The origin of the minor influence derives from the type of the substituent: the 13C=O's of the palmitoyl groups resonate at lower field than those of the oleoyl groups, for example, 173.34 versus 173.29 for the Pal versus the Ole group at sn O-2.
These differences have proved to be extremely useful in detecting the presence of glyceride mixtures produced by acylation reactions of imperfect regioselectivity, a facet that has not previously been recognized.
2.3. Immunochemistry
That sera from LD patients react with carbohydrate and lipid-containing substances extracted from B. burgdorferi was demonstrated many years ago,36–38 but not until 2003 was it shown that the surface of this pathogen contains two groups of glycolipids, which were termed BBGL-1 and 2.11 BBGL-2 was reported to contain several FA's of which palmitate and oleate were shown to be the major components, but their exact location on the glycerol moiety is unknown.11 This knowledge may be crucial for the preparation of a chemically well-characterized, synthetic vaccine. In order to determine the structural features necessary for recognition by antibodies, 14 glycolipids have been synthesized, of which 12 contain the Gal residue in the native, a anomeric configuration, and 2 in the unnatural, β one. The synthetic glycolipids were reacted with mouse sera induced by (1) formaldehyde killed B. burgdorferi cells and (2) by native purified BBGL-2, and also with serum of patients with late Lyme neuroborreliosis (Fig. 1). There was no reaction of any glycolipid with control sera (not shown). All of the glycolipids exhibited similar antigenic activities to all three sera. No binding occurred with the glycolipids containing only a single FA moiety (compounds 11 and 12). The lipids containing two saturated FA's were also unreactive: no binding occurred with the compounds containing two palmitates (1, 5, and 13), two stearates (compound 9), or one palmitate and one stearate (compound 10). The observed non-reactivity might be related to the low solubility of these compounds as compared to the rest of the glycolipids in Table 1. All of the compounds having two FA's of which at least one is an oleoyl residue reacted with each of the three sera (3, 4, 7, and 8). The glycolipids having two oleates (2, 6, and 14) were also reactive. Unexpectedly, compounds with the Gal moiety in the unnatural β configuration exhibited the same reactivities as did their natural counterparts: the di-palmitoyl derivative 13 was unreactive, whereas the di-oleoyl congener was seemingly as reactive as compound 2.
Figure 1.
Immunoblotting of BBGL-2 derivatives 1–14 with mice sera induced against killed B. burgdorferi cells (A), against purified BBGL-II (B), and with LD patient serum (C). No reaction was observed with control serum (picture not shown).
These observations led us to the following conclusions:
-
(a)
The presence in the glycerol part of two fatty acid moieties is required for antigenicity, leading to the hypothesis that the binding to antibodies involves two fatty acids and two closely located binding sites. Alternatively, one FA may position the other in order to achieve the proper conformation for binding with antibodies.
-
(b)
Of the two fatty acids, at least one should be oleic acid.
-
(c)
The position of the oleic acid moiety is not critical.
-
(d)
Replacement of the oleic acid by stearic acid abolishes antigenicity.
-
(e)
The anomeric configuration has no influence on antigenicity: α and β-galactoside derivatives can be equally antigenic.
-
(f)
The galactose moiety may be linked to either O–1 or to O–3 of sn-glycerol to maintain antigenicity.
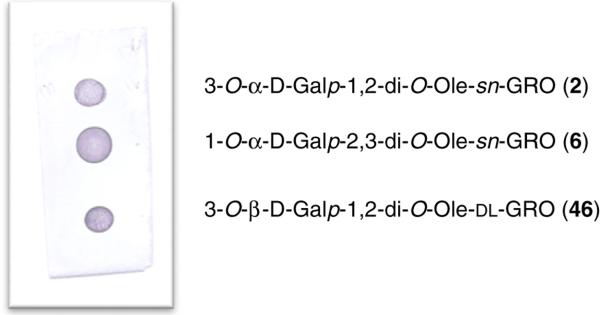
Based on the above observations, we prepared an `unnatural' BBGL-2 analog, using the chemistry described in Section 2.1 having the structure 3-O-(β-D-galactopyranosyl)-1,2-di-O-oleoyl-DL-glycerol 46, which is much easier and less expensive to synthesize than the native analogs, by starting from DL-glycerol. Figure 2. shows that the `unnatural' congener is just as good an antigen as are the reactive native analogs, and is a candidate for a vaccine against LD.
Figure 2.
Dot-blot of BBGL-2 derivatives 2, 6, and 46 with mice sera induced by native purified BBGL-2 injected in Freund's adjuvant.
There is some similarity between our results on antigenic specificity and those published recently by others demonstrating that iNKT cells react with BBGL-2 glycolipids in a FA structure-dependent manner, and that the difference in fatty acids influences the steric position of the sugar epitope.18,39 The highest response, assessed by the level of induced IL-2 was to compound 4 having Ole and Pal residues in the sn-1 and sn-2 positions, respectively, whereas the reverse arrangement produced less than half of that. In our present study, compound 4 also showed a high level of antigenicity. On the other hand, compound 1 produced only slightly more than baseline IL-2 levels, as shown by others.18,39 In a similar fashion, in our study compound 1 was not recognized by sera induced to B. burgdorferi, or to purified native BBGL-2.
3. Conclusions
We have described synthetic schemes to homogeneous BBGL-2 congeners that make up a distinctive group of major glycolipids on the surface of B. burgdorferi, the causative organism of Lyme disease, comprising α-galactopyranosyl-diacyl glycerols. We have presented evidence that the diastereomeric purity of these glycolipids can best be ascertained by examination of the carbonyl carbon resonances in their 13C NMR spectra, whereas the 1H NMR spectra are less informative. We found that the antigenicity of the synthetic BBGL-2 glycolipids depends on the unsaturation of the lipid components, but is independent of the anomeric configuration of the sn-3 linked hydrophilic galactose head-group. The presence of two FAs in the glycerol part is necessary, of which at least one has to be an oleic acid residue for antigenicity, a property that is lost completely upon saturation.
4. Experimental
4.1. General methods
All chemicals were of commercial grade and used without purification. Solvents for chromatography were distilled prior to use. Anhydrous solvents were obtained from Aldrich. Column chromatography was performed on Silica Gel 60 (0.040–0.063 mm) and thin layer chromatography was performed on glass-supported silica gel layers obtained from Analtech (Uniplate) or on HPTLC plates from Merck. Visualization was carried out by inspection under UV light (254 nm), by iodine adsorption, and by charring using a solution of ammonium cerium(IV) sulfate and ammonium molybdate in sulfuric acid. 2-O-Oleoyl-1-O-palmitoyl-sn-glycerol (lot #160-181DG-22) was obtained from Avanti Polar Lipids Inc., Alabaster, AL. API-ES mass spectra were recorded on an Agilent Technologies LC MDS CL spectrometer.
NMR spectra were recorded at 300 K, using a Bruker DRX-500 spectrometer equipped with a 5 mm, HCN cryoprobe. Solutions containing ~20 mg of glycolipid in CDCl3 unless indicated otherwise (0.5 mL) were used, except for compounds 1, 5, 9, and 13 which were not soluble to this extent, and for which a saturated solution was employed. Tetramethylsilane was used as an internal chemical shift reference at δ = 0, for 1H and 13C NMR spectra. The NMR data were acquired and processed by means of the Bruker Topspin program, version 1.3.
One-dimensional (1D) 1H NMR spectra were acquired in 32,768 data points, zero-filled to 65,536 points, with use of a spectral width of 4.01 kHz, a 30° pulse (2.67 μs), and a recycle time of 6 s. For integration, the free induction decay (FID) was subjected to Gaussian multiplication, using a line-broadening of −0.5 Hz and a truncation fraction of 0.3. However, coupling constants were measured by using a line-broadening of −1.0 to −2.0 Hz. 1D 13CNMR spectra were acquired in 65,536 data points, zero-filled to 131,072 points, by using a 25.15 kHz spectral width, a 45° pulse (7.7 μs), and a recycle time of 2 s. The free induction decays were subjected either to exponential multiplication using a line-broadening of 1.0 Hz, or to Gaussian multiplication with a line-broadening of −0.1 to −0.5 Hz.
2D COSY NMR spectra were acquired by using a 30° 1H pulse, and 2048 × 512 point datasets, zero-filled to 2048 × 2048 points. The data were processed by using an unshifted sine-bell squared window in both dimensions, a magnitude calculation in F1, and symmetrization to remove artifacts. 2D TOCSY spectra were collected in 16,384 × 512 point datasets, zero-filled to 32,768 × 2048 points. Resolution enhancement by Gaussian multiplication was used in F2 with a line-broadening of −1.5 Hz, and in F1, a sine-bell squared window, shifted by π/2 rad. The spectra were analyzed by means of extraction of 1D slices in the F2 dimension. 2D HSQC spectra were recorded in 2048 × 512 point datasets, zero-filled to 8192 × 4096 points. A sine-bell squared window shifted by π/4 rad was used in both dimensions. 2D HMBC was conducted with an evolution delay of 83 ms, corresponding to optimization for 2,3JCH 6 Hz, together with 2048 × 512 or 2048 point datasets, zero-filled to 4096 × 4096 points. A magnitude calculation was used in F1, with sine-bell squared windows shifted by π/2 rad in both dimensions. 1H-coupled 2D HSQC spectra were acquired in 8192 × 512 point datasets, zero-filled to 8192 × 2048 points, with a sine-bell squared window in both dimensions, shifted by π/2 rad. All 2D pulse sequences were field-gradient selected. 1H and 13C spectral widths were 3.005 and 25.15 kHz, respectively. The phase-sensitive, echo–anti-echo protocol was used for 2D TOCSY, HSQC, and 1H-coupled HSQC.
The synthetic glycolipids were dissolved in CHCl3/MeOH 80:20 (v/v) at 1 mg/ml concentration. Then, 3 μL of the solution was directly pipetted onto stripes of a PVDF membrane (Millipore, Bedford, MA). After 10 min, the membranes were blocked with 5% BSA in PBS for 1 h at rt and washed three times with PBS. Next, the membranes were incubated for 2 h at rt with the following sera: (1) induced to heat killed B. burgdorferi cells (strain B31, ATCC 35210), diluted 1:100 in blocking buffer; (2) induced to purified BBGL-2, diluted 1:50 in blocking buffer; (3) serum from human patients diagnosed with Lyme disease, diluted 1:50 in blocking buffer; (4) control mouse and human sera. Mouse sera were prepared as described in Refs. 11 and 40. After washing as above, the membranes were incubated with goat anti-mouse or anti-human IgG conjugated to alkaline phosphatase (KPL, Gaithersburg, MD) for 1 h at rt, then washed and developed with BCIP/NBT phosphate substrate (KPL, Gaithersburg, MD).
4.2. 3-O-α-D-Galactopyranosyl-1,2-di-O-palmitoyl-sn-glycerol18 (1)
TLC: CH2Cl2–MeOH (95:5). For the 1H and 13C NMR spectra see Tables 4–6. API-ES-MS: m/z calcd for [C41H78O10]NH4+: 748.6. Found 748.4.
4.3. 3-O-α-D-Galactopyranosyl-1,2-di-O-oleoyl-sn-glycerol (2)
TLC: EtOAc–hexanes (1:1, 4:1). For the 1H and 13C NMR spectra see Tables 4–6. API-ES-MS: m/z calcd for [C45H82O10]NH4+: 800.6. Found 800.6.
4.4. 3-O-α-D-Galactopyranosyl-2-O-oleoyl-1-O-palmitoyl-sn-glycerol17–20 (3)
TLC: EtOAc–MeOH (99:1, 95:5), CH2Cl2–MeOH (95:5). For the 1H and 13C NMR spectra see Tables 4–6. API-ES-MS: m/z calcd for [C43H80O10]NH4+: 774.6. Found 774.4.
4.5. 3-O-α-D-Galactopyranosyl-1-O-oleoyl-2-O-palmitoyl-sn-glycerol18–20 (4)
TLC: EtOAc–MeOH (99:1, 95:5), CH2Cl2–MeOH (95:5). For the 1H and 13C NMR spectra see Tables 4–6. API-ES-MS: m/z calcd for [C43H80O10]NH4+: 774.6. Found 774.6.
4.6. 1-O-α-D-Galactopyranosyl 2,3-di-O-palmitoyl-sn-glycerol (5)
TLC: CH2Cl2–MeOH (95:5); 1H NMR (CDCl3): δ 5.24 (m, 1H), 4.97 (d, 1H, J = 3.7 Hz), 4.29 (m, 1H, J = 4.1 Hz, J = 11.9 Hz), 4.20 (dd, 1H, J = 6.0 Hz, J = 11.9 Hz), 4.09 (br d, 1H, J = 2.1 Hz), 3.94 (dd, 1H, J = 6.3 Hz), J = 11.6 Hz), 3.87–3.81 (m, 4H), 3.74 (dd, 1H, J = 3.1 Hz, J = 9.8 Hz), 3.63 (dd, 1H, J = 4.8 Hz, J = 11.1 Hz); 13C NMR (CDCl3): δ 173.55, 173.35, 99.29, 70.89, 70.28, 70.11, 69.92, 69.36, 66.89, 63.14, 62.17, 34.31, 34.10, 31.94, 29.71, 29.69, 29.67, 29.66, 29.51, 29.37, 29.30, 29.15, 29.12, 24.89, 22.70, 14.13. API-ES-MS: m/z calcd for [C41H78O10]NH4+: 748.6. Found 748.4.
4.7. 1-O-α-D-Galactopyranosyl-2,3-di-O-oleoyl-sn-glycerol (6)
TLC: EtOAc–hexanes (1:1, 4:1); 1H NMR (CDCl3): δ 5.39–5.29 (m, 4H), 5.25 (m, 1H), 4.97 (d, 1H, J = 3.6 Hz), 4.30 (dd, 1H, J = 3.9 Hz, J = 11.9 Hz), 4.19 (dd, 1H, J = 6.2 Hz, J = 11.9 Hz), 4.09 (d, 1H, J = 3.2 Hz), 3.93 (dd, 1H, J = 5.0 Hz, J = 11.6 Hz), 3.88–3.78 (m, 4H), 3.75 (dd, 1H, J = 3.3 Hz, J = 10.3 Hz), 3.62 (dd, 1H, J = 5.0 Hz, J = 11.1 Hz), 2.35–2.27 (m, 4H), 2.04–1.95 (m, 8H), 1.66–1.54 (m. 4H), 1.37–1.19 (m, 40H), 0.91–0.82 (m, 6H); 13C NMR (CDCl3): δ 173.60, 173.34, 129.98, 129.64, 129.62, 99.37, 70.58, 70.18, 70.09, 69.95, 69.09, 66.63, 62.45, 62.41, 34.24, 34.04, 31.87, 29.73, 29.70, 29.70, 29.49, 29.28, 29.19, 29.13, 29.09, 29.06, 27.19, 27.16, 24.89, 24.82, 22.65, 14.08. API-ES-MS: m/z calcd for [C45H82O10]NH4+: 800.6. Found 800.4.
4.8. 1-O-α-D-Galactopyranosyl-2-O-oleoyl-3-O-palmitoyl-sn-glycerol (7)
TLC: EtOAc–MeOH (99:1, 95:5), CH2Cl2–MeOH (95:5); 1H NMR (CDCl3): δ 5.38–5.31 (m, 2H), 5.24 (m, 1H), 4.30 (dd, 1H, J = 4.9 Hz, J = 11.8 Hz), 4.19 (dd, 1H, J = 6.2 Hz, J = 11.8 Hz), 4.09 (d, 1H, J = 2.8 Hz), 3.93 (dd, 1H, J = 5.3 Hz, J = 11.8 Hz), 3.86–3.79 (m, 4H), 3.75 (dd, 1H, J = 2.8 Hz, J = 9.6 Hz), 3.62 (dd, 1H, J = 4.9 Hz, J = 10.9 Hz), 2.33 (t, 1H, J = 6.9 Hz), 2.31 (t, 2H, J = 7.4 Hz), 2.05–1.99 (m, 4H), 2.05–1.99 (m, 2H), 1.65–1.56 (m, 4H), 1.38–1.19 (m), 0.88 (t, 6H, J = 7.0 Hz); 13C NMR (CDCl3): δ 173.69, 173.38, 130.02, 129.66, 99.39, 70.84, 70.28, 70.13, 69.94, 69.34, 66.83, 63.00, 62.23, 34.28, 34.10, 31.93, 31.91, 29.76, 29.71, 29.68, 29.67, 29.65, 29.54, 29.51, 29.37, 29.33, 29.32, 29.30, 29.21, 29.15, 29.09, 27.19, 27.18, 24.92, 24.88, 22.70, 22.69, 14.13. APIES-MS: m/z calcd for [C43H80O10]NH4+: 774.6. Found 774.6.
4.9. 1-O-α-D-Galactopyranosyl-3-O-oleoyl-2-O-palmitoyl-sn-glycerol (8)
TLC: EtOAc–MeOH (99:1, 95:5), CH2Cl2–MeOH (95:5); 1H NMR (CDCl3–MeOH-d4): δ 5.30–5.23 (m, 2H), 5.15 (m, 1H), 4.85 (d, 1H, J = 3.4 Hz), 4.27 (dd, 1H, J = 3.7 Hz, J = 12.0 Hz), 4.11 (dd, 1H, J = 6.5 Hz, J = 12.0 Hz), 4.01 (d, 1H, J = 3.3 Hz), 3.48–3.65 (m, 5H), 3.54 (dd, 1H, J = 5.4 Hz, J = 10.8 Hz), 2.25 and 2.23 (2t, 2 × 2H, J = 7.2 Hz for each), 1.99–1.91 (m, 4H), 1.60–1.48 (m, 4H), 1.31–1.14 (m), 0.86–0.77 (m, 6H); 13C NMR (CDCl3–MeOH-d4): δ 173.68, 173.35, 129.96, 129.6, 99.37, 70.51, 70.19, 69.96, 68.99, 66.56, 62.54, 62.16, 34.24, 34.07, 31.89, 31.86, 29.72, 29.71, 29.68, 29.63, 29.51, 29.49, 29.42, 29.33, 29.29, 29.28, 29.21, 29.14, 29.06, 28.18, 27.15, 24.88, 24.84, 22.65, 22.64, 14.07. APIES-MS: m/z calcd for [C43H80O10]NH4+: 774.6. Found 774.6.
4.10. 3-O-α-D-Galactopyranosyl-1,2-di-O-stearoyl-sn-glycerol17 (9)
TLC: EtOAc–MeOH (99:1, 95:5), CH2Cl2–MeOH (95:5); 1H NMR (CDCl3–MeOH-d4): δ 5.26 (m, 1H), 4.88 (d, 1H, J = 3.7 Hz), 4.39 (dd, 1H, J = 3.6 Hz, J = 12.0 Hz), 4.16 (dd, 1H, J = 6.4 Hz, J = 12.0 Hz), 3.99 (d, 1H, J = 3.3 Hz), 3.85–3.75 (m), 3.73 (dd, 1H, J = 3.3 Hz), J = 10.0 Hz), 3.63 (dd, 1H, J = 5.9 Hz, J = 10.9 Hz), 2.53 (m, 1H), 2.45 (m, 1H), 2.35–2.30 (m, 4H), 1.65–1.56 (m, 4H), 1.36–1.19 (m, 56H), 0.88 (t, 6H, J ~6.8 Hz); 13C NMR (CDCl3–MeOH-d4): δ 173.82, 173.48, 99.34, 70.27, 70.03, 69.85, 69.62, 68.82, 66.15, 62.34, 61.71, 34.06, 33.89, 31.70–28.89, 25.62, 24.67, 24.65, 22.45, 13.79. API-ES-MS: m/z calcd for [C45H86O10]NH4+: 804.6. Found 804.7.
4.11. 3-O-α-D-Galactopyranosyl-2-O-palmitoyl-1-O-stearoyl-sn-glycerol (10)
TLC: EtOAc–MeOH (99:1, 95:5), CH2Cl2–MeOH (95:5); 1H NMR (CDCl3–MeOH-d4): δ 5.28 (m, 1H), 4.87 (d, 1H, J = 3.6 Hz), 4.44 (dd, 1H, J = 3.2 Hz, J = 12. Hz), 4.19 (dd, 1H, J = 6.4 Hz, J = 12.0 Hz, 3.95 (d, 1H, J = 3.2 Hz), 3.86 (dd, 1H, J = 5.5 Hz, J = 10.8 Hz), 3.83–3.71 (m, 6H), 3.65 (dd, 1H, J = 5.7 Hz, J = 10.8 Hz), 2.35 and 2.34 (2t, 2 × 2H, J ~7.1 Hz), 1.68–1.57 (m, 4H), 1.40–1.20 (52H), 0.89 (t, 6H, J ~7 Hz); 13C NMR (CDCl3–MeOH-d4): δ 173.54, 173.19, 99.03, 70.37, 69.64, 69.58, 69.19, 68.36, 65.53, 62.09, 61.06, 33.64, 33.47, 31.32, 29.07–28.49, 24.30, 22.03, 13.16. API-ES-MS: m/z calcd for [C43H82O10]NH4+: 776.6. Found 776.6.
4.12. 3-O-α-D-Galactopyranosyl-1-O-palmitoyl-sn-glycerol (11)
TLC: EtOAc–MeOH (95:5), CH2Cl2–MeOH (95:5, 9:1); 1H NMR (CDCl3–MeOH-d4): δ 4.88 (d, 1H, J = 3.6 Hz), 4.17 (dd, 1H, J = 4.6 Hz, J = 11.3 Hz), 4.10 (dd, 1H, J = 5.9 Hz, J = 11.3 Hz), 4.06 (m, 1H), 3.95 (d, 1H, J = 3.1 Hz), 3.85–3.72 (m, 6H), 3.42 (dd, 1H, J = 7.6 Hz, J = 10.3 Hz), 2.36 (t, 2H, J = 7.5 Hz), 1.63 (m, 2H), 1.36–1.24 (m, 24H), 0.89 (t, 3H, J ~6.9 Hz); 13C NMR (CDCl3MeOH-d4): δ 173.86, 99.01, 70.26, 69.61, 69.21, 68.97, 68.50, 67.85, 64.44, 61.07, 33.38, 31.27, 29.01, 28.98, 28.95, 28.82, 28.69, 28.63, 28.48, 24.22, 21.99, 13.11. API-ES-MS: m/z calcd for [C25H48O9]NH4+: 510.4. Found 510.5.
4.13. 3-O-α-D-Galactopyranosyl-1-O-oleoyl-sn-glycerol (12)
TLC: EtOAc–MeOH (95:5), CH2Cl2–MeOH (95:5, 9:1); 1H NMR (CDCl3–MeOH-d4): δ 5.38–5.30 (m, 2H), 4.89 (d, 1H, J = 3.7 Hz), 4.17 (dd, 1H, J = 4.1 Hz, J = 11.3 Hz), 4.10 (dd, 1H, J = 5.8 Hz, J = 11.3 Hz), 4.05 (m 1H), 3.95 (d, 1H, J = 3.4 Hz), 3.89–3.69 (m, 6H), 3.42 (dd, 1H, J = 7.6 Hz, J = 10.4 Hz), 2.37 (t, 2H, J = 7.4 Hz), 2.08–1.98 (m, 4H), 1.69–1.58 (m, 2H), 1.40–1.20 (m, 20H), 0.89 (t, 3H, J ~6.8 Hz); 13C NMR (CDCl3-MeOH–d4): δ 173.89, 129.36, 129.12, 99.07, 70.26, 69.67, 69.25, 69.10, 68.57, 67.93, 64.50, 61.13, 33.45, 31.32, 29.15, 29.11, 28.91, 28.72, 28.69, 28.61, 28.53, 28.51, 26.56, 26.54, 24.27, 22.06, 13.23. API-ES-MS: m/z calcd for [C27H48O9]NH4+: 536.4. Found 536.5.
4.14. 3-O-β-D-Galactopyranosyl-1,2-di-O-palmitoyl-sn-glycerol (13)
TLC: EtOAc–MeOH (95:5), CH2Cl2–MeOH (95:5, 9:1); 1HNMR (CDCl3–MeOH-d4): δ 5.28 (m, 1H), 4.43 (dd, 1H, J = 3.0 Hz, J = 12.0 Hz), 4.25 (d, 1H, J = 7.5 Hz), 4.24 (dd, 1H, J = 6.8 Hz, J = 12.0 Hz), 3.99 (dd, 1H, J = 5.4 Hz, J = 12.0 Hz), 3.88 (dd, 1H, J = 1.1 Hz, J = 3.3 Hz), 3.81 (dd, 1H, J = 6.6, J = 11.5 Hz), 3.75 (dd, 1H, J = 5.4 Hz, J = 11.5 Hz), 3.74 (dd, 1H, 5.9 Hz, J = 11.0 Hz), 3.54 (dd, 1H, J = 7.5 Hz, J = 9.7 Hz), 3.89 (dd, 1H, J = 3.4 Hz, J = 9.7 Hz), 2.35–2.31 (m, 4H), 1.61 (m, 4H), 1.36–1.22 (m, 48H), 0.89 (t, 6H, J ~7.1 Hz); 13C NMR (CDCl3–MeOH-d4): 173.44, 173.11, 103.39, 74.60, 72.82, 70.47, 69.79, 68.19, 67.00, 62.17, 60.57, 33.50, 33.36, 31.21, 28.96–28.37, 24.19, 21.92, 12.98. API-ES-MS: m/z calcd for [C41H78O10]NH4+: 748.6. Found 748.7.
4.15. 3-O-β-D-Galactopyranosyl-1,2-di-O-oleoyl-sn-glycerol (14)
TLC: EtOAc–MeOH (95:5), CH2Cl2–MeOH (95:5, 9:1); 1H NMR (CDCl3–MeOH-d4): δ 5.37–5.31 (m, 4H), 5.29 (m, 1H), 4.39 (dd, 1H, J = 3.1 Hz, J = 12.2 Hz), 4.27 (d, 1H, J = 7.6 Hz), 4.21 (dd, 1H, J = 6.6 Hz, J = 12.1 Hz), 4.02 (d, 1H, J = 2.6 Hz), 3.92 (dd, 1H, J = 5.4 Hz, J = 11.0 Hz), 3.84 (dd, 1H, J = 4.9 Hz, J = 12.0 Hz), 3.74–3.65 (m, 3H), 3.59 (dd, 1H, J = 2.9 Hz, J = 9.6 Hz), 3.54 (t, 1H, J ~4.6 Hz), 2.32 and 2.32 (2t, 2 × 2H, J ~7.4 Hz), 2.08–1.94 (m, 8H), 1.65–1.54 4H), 1.37–1.19 (m, 40H), 0.88 (t, 6H, J ~6.8 Hz); 13C NMR (CDCl3–MeOH-d4): δ 173.88, 173.47, 129.97, 129.64, 104.00, 74.52, 73.37, 71.11, 70.12, 68.88, 68.04, 62.87, 61.52, 34.24, 34.11, 31.88, 27.78–29.08, 27.20, 27.18, 24.86, 24.84, 22.65, 14.09. API-ES-MS m/z calcd for [C45H82O10]NH4+: 800.6. Found 800.4.
4.16. Phenyl 3,4,6-tri-O-benzoyl-2-O-benzyl-1-thio-β-D-galactopyranoside (17)
To a solution of compound 16 (32 g, 88.4 mmol) in a mixture of anhydrous CH2Cl2 (300 mL) and C5H5N (160 mL) containing a catalytic amount of DMAP was added benzoyl chloride (50 mL, 60.5 g, 430 mmol), dropwise at room temperature under stirring. After 24 h, the solution was treated with MeOH (70 mL). The solution was concentrated under reduced pressure. The residue was equilibrated between CHCl3 and water. The organic layer was extracted with 4 N hydrochloric acid followed by water and saturated aq NaHCO3. Column chromatographic purification of the product using a hexanes-EtOAc gradient 10:1→4:1 afforded 17 (54.0 g, 91%) as a solid: 1H NMR (CDCl3): δ 8.03–7.13 (m, 25H), 5.92 (ddd, 1H, J3,4 = 3.5 Hz, J4,5 = 0.9 Hz, H-4), 5.50 (dd, J2,3 = 9.5 Hz, J3,4 = 3.5 Hz, H-3), 4.89 (d, 1H, J1,2 = 9.6 Hz, H-1), 4.84 and 4.61 [2d, 2 × 1H J = 10.8 Hz each, CH2 (Bn)], 4.62 (dd, 1H, J5,6 = 7.1 Hz, J6,6′ = 11.5 Hz, H-6), 4.41 (dd, 1H, J5,6′ = 5.7 Hz, J6,6′ = 11.5 Hz), 4.27 (ddd, 1 J, J4,5 = 0.9 Hz, J5,6 = 7.1 Hz, J5,6′ = 5.7 Hz, H-5), 4.01 (t, 1H, J1,2 = J = 9.5 Hz); 13C NMR (CDCl3): δ 166.01, 165.35, 137.36, 133.50, 133.22, 133.18, 132.81, 132.71, 129.92, 129.79, 129.64, 129.46, 129.29, 129.21, 128.99, 128.55, 128.41, 128.29, 128.27, 128.10, 127.93, 127.83, 87.51, 75.48, 75.22, 74.80, 74.77, 68.79, 62.58, 62.52. API-ES-MS: m/z calcd for [C40H34O8S]NH4+: 692.2. Found 692.2.
4.17. 3,4,6-Tri-O-benzoyl-2-O-benzyl-α-D-galactopyranosyl chloride (18)
To a solution of compound 17 (25.0 g, 41.6 mol) in anhydrous CH2Cl2 (250 mL) was added a saturated solution of chlorine in CCl4 at 0 °C. After 30 min TLC (hexanes-EtOAc, 6:1) showed complete conversion to a slightly faster-moving compound. The reaction mixture was treated with 1-hexene (excess) followed by concentration. Column chromatographic purification of the residue (hexanes-EtOAc, 5:1) afforded amorphous 18 (15.4 g, 69%): 1H NMR (CDCl3, δ): 8.04–7.19 (m, 20H), 6.29 (d, 1H, J1,2 = 3.8 Hz, H-1), 5.99 (dd, 1H, J3,4 = 3.4 Hz, J4,5 = 1.2 Hz, H-4), 5.81 (dd, 1H, J2,3 = 10.3 Hz, J3,4 = 3.4 Hz, H-3), 4.84 (m, 1H, H-5), 4.70 and 4.64 [2d, 2 × 1H J = 12.0 Hz each, CH2 (Bn)], 4.56 (dd, 1H, J5,6 = 6.8 Hz, J6,6′ = 11.5 Hz, H-6), 4.34 (dd, 1H, J5,6 = 6.0 Hz, J6,6′ = 11.5 Hz, H-6′), 4.32 (dd, 1H, J1,2 = 3.8 Hz, J2,3 = 10.3 Hz, H-2); 13C NMR (CDCl3): δ 165.90, 165.26, 165.21, 136.88, 133.55, 133.24, 133.18, 129.79, 129.76, 129.66, 129.33, 129.28, 129.08, 128.58, 128.51, 128.40, 128.28, 128.17, 128.04, 93.01, 73.09, 72.81, 69.89, 69.71, 68.59, 61.84.
4.18. (3,4,6-Tri-O-benzoyl-2-O-benzyl-α-D-galactopyranosyl)-1,2-O-isopropylidene-sn-glycerol (20) and (3,4,6-tri-O-benzoyl-2-O-benzyl-β-D-galactopyranosyl)-1,2-O-isopropylidene-sn-glycerol (21)
To a stirred mixture of chloride 18 (15.2 g, 25.3 mmol), 2,4,6-tri-tert-butylpyrimidine41 (22.5 g), crushed 4 Å molecular sieves (15 g), 1,2-O-isopropylidene-sn-glycerol 19 (4.5 mL, 4.8 g, 36.5 mmol), and anhydrous CH2Cl2 (125 mL) was added AgOTf (15 g, 58.2 mmol) at −40 °C. After 15 min TLC (3:1 hexanes–EtOAc) indicated the disappearance of 18 and the formation of two closely migrating products. To the reaction mixture were added Bu4NBr (11.5 g) and saturated aqueous NaHCO3 and the resulting mixture was filtered through a layer of Celite. The organic layer was concentrated and the residue was chromatographed on silica gel using a 20:1→2:1 hexanes-EtOAc gradient to afford 20 (10.8 g, 61%) as an amorphous substance: 1H NMR (CDCl3, δ): 8.01–7.25 (m, 20H), 5.94 (dd, 1H, J3,4 = 3.0 Hz, J4,5 = 1.2 Hz, H-4), 5.76 (dd, 1H, J2,3 = 10.5 Hz, J3,4 = 3.0 Hz, H-3), 5.14 (d, 1H, J1,2 = 3.6 Hz, H-1), 4.71 and 4.63 [2d, 2 × 1H J = 12.3 Hz each, CH2 (Bn)], 4.58 (m, 1H, H-5), 4.53 (dd, 1H, J7.5 Hz, J = 11.1), 4.36 (m, 1H, H-2), 4.32 (dd, 1H, J = 5.2 Hz, J = 11.0 Hz), 4.16 (dd, 1H, J = 3.5 Hz, J = 10.5 Hz, H-2), 4.09 (dd, J = 6.5 Hz, 8.4 Hz), 3.78 (dd, J = 6.5 Hz, J = 8.4 Hz), 3.74 (dd, 1H, J = 5.5 Hz, J = 10.5 Hz), 3.64 (dd, 1H, J = 5.5 Hz, J = 10.5 Hz), 1.42 and 1.37 (2s, 2 × 3H, 2CH3); 13C NMR (CDCl3): δ 166.02, 165.47, 165.46, 137.78, 133.40, 133.18, 133.02, 129.83, 129.72, 129.66, 129.53, 129.41, 128.54, 128.41, 128.25, 127.96, 127.93, 109.54, 97.85, 74.55, 73.44, 72.87, 70.05, 69.51, 69.16, 67.10, 66.85, 62.72, 26.81, 25.50. API-ES-MS: m/z calcd for [C40H40O11]NH4+: 714.3. Found 714.2.
Also isolated was (3,4,6-tri-O-benzoyl-2-O-benzyl-β-D-galactopyranosyl)-1,2-O-isopropylidene-sn-glycerol 21 (3.35 g, 19%): 1H NMR (CDCl3): δ 8.03–7.09 (m, 20H), 5.88 (br d, 1H, J3,4 = 3.5 Hz, H-4), 5.44 (dd, 1H, J2,3 = 10.1 Hz, J3,4 = 3.5 Hz, H-3), 4.87 and 4.69 [2d, 2 × 1H J = 11.8 Hz each, CH2 (Bn)], 4.71 (d, [2d, 2 × 1H J = 12.3 Hz each, CH2 (Bn)], 4.71 (d, 1H, J1,2 = 7.7 Hz, H-1), 4.39 (m, 1H, H-2′), 4.36 (dd, 1H, J = 6.5 Hz, J = 11.3 Hz), 4.10 (dd, 1H, J = 6.5 Hz, J = 8.3 Hz), 4.08 (dd, 1H, J = 5.0 Hz, J = 10.4 Hz), 3.94 (dd, 1H, J = 7.8 Hz, J = 10.4 Hz), 3.90 (dd, 1H, J = 6.0 Hz, J = 8.2 Hz), 3.72 (dd, 1H, J = 6.0 Hz, J = 10.4 Hz), 1.45 and 1.37 (2s, 2 × 3H, 2CH3); 13C NMR (CDCl3): δ 165.89, 165.41, 165.33, 137.65, 133.32, 133.13, 133.00, 129.80, 129.62, 129.60, 129.33, 129.23, 129.18, 128.41, 128.34, 128.11, 128.09, 127.97, 127.53, 109.43, 104.18, 76.08, 74.56, 74.24, 72.62, 70.93, 70.60, 68.39, 66.56, 61.97, 26.71, 25.21. API-ES-MS: m/z calcd for [C40H40O11]NH4+: 714.3. Found 714.3.
4.19. 3-O-(2-O-Benzyl-α-D-galactopyranosyl)-1,2-O-isopropylidene-sn-glycerol (22)
To a stirred solution of tribenzoate 20 (8.0 g, 11.4 mmol), anhydrous CH2Cl2 (50 mL) and MeOH (50 mL) was added a 25% solution of NaOMe in MeOH (10 mL) at rt. After 12 h, the solution was carefully treated with Dowex 50WX8 to approx. pH 5 as seen by indicator paper. The resin was removed by filtration and the filtrate was treated with an ethereal solution of CH2N2 until the yellow color persisted. Purification of the residue by silica gel chromatography using EtOAc as the eluant afforded solid 22 (5.0 g, 91%): 1H NMR (CDCl3): δ 7.38–7.29 (m, 5H), 4.95 (d, 1H, J = 3.5 Hz, H-1), 4.66 [s, 2H, CH2 (Bn)] 4.32 (m, 1H), 4.08 (dd, 1H, J = 1.1 Hz, J = 3.4 Hz, H-4), 4.05 (dd, 1H, J = 6.5 Hz, J = 8.3 Hz), 3.99 (dd, 1H, J = 3.4 Hz, J = 9.8 Hz), 3.91 (dd, 1H, J = 5.2 Hz, J = 11.2 Hz), 3.85 (dt, 1H, J = 1.2 Hz, J = 4.3 Hz, J = 5.4 Hz), 3.81 (dd, 1H, J = 4.2 Hz, J = 11.2 Hz, 3.76 (dd, 1H, J = 3.5 Hz, J = 9.7 Hz), 3.73 (dd, 1H, J = 6.2 Hz, J = 8.2 Hz), 3.65 (dd, 1H, J = 4.9 Hz, J = 10.7 Hz), 3.46 (dd, 1H, J = 6.2 Hz, J = 10.7 Hz), 1.43 and 1.36 (2s, 2 × 3H, 2CH3); 13C NMR (CDCl3): δ 137.88, 128.63, 128.20, 128.15, 109.63, 97.11, 76.48, 74.63, 72.67, 70.29, 69.32, 68.98, 68.75, 66.47, 63.02, 26.78, 25.39. API-ES-MS: m/z calcd for [C19H28O8]NH4+: 402.2. Found 402.2.
4.20. 3-O-(α-D-Galactopyranosyl)-1,2-O-isopropylidene-sn-glycerol (23)
A mixture of compound 22 (480 mg), 2,4,6-tri-tert-butylpyrimidine (500 mg), 10% palladium-on-charcoal (0.5 g), EtOAc (10 mL) was stirred under hydrogen at 200 psi. After 10 min, the mixture was filtered and the solids were washed with MeOH. Concentration of the combined solutions under reduced pressure afforded a semisolid which was purified by silica gel column chromatography using 9:1 CH2Cl2–MeOH as the eluant to afford amorphous 23 (320 mg, 87%): 1H NMR (CDCl3): δ 4.91 (d, 1H, J = 3.5 Hz), 4.37 (m, 1H, H-2′), 4.11 (dd, 1H, J = 6.8 Hz, J = 8.5 Hz), 3.97 (br d, 1H, J = 3.2 Hz), 3.85–3.73 (m, 7H), 3.50 (dd, 1H, J = 6.8 Hz, J = 10.5 Hz), 1.46 and 1.39 (2s, 2 × 3H, 2CH3); 13C NMR (CDCl3): δ 109.45, 98.89, 74.39, 70.25, 69.92, 69.39, 68.78, 68.68, 65.85, 61.37, 26.07, 24.72. API-ES-MS: m/z calcd for [C12H22O8]NH4+: 312.2. Found 312.2.
4.21. 1,2-O-Isopropylidene-3-O-(2,3,4,6-tetra-O-levulinoyl-α-D-galactopyranosyl)-sn-glycerol (24)
To a stirred solution of tetraol 23 (320 mg, 1.09 mmol) in EtOAc (10 mL) were added sequentially levulinic acid (760 mg, 6.55 mmol), N,N-dicyclohexylcarbodiimide (1.6 g, 7.7 mmol) and a catalytic amount of 4-dimethylaminopyridine at rt. After 24 h, the solids were removed by filtration and washed with EtOAc). The solutions were combined and concentrated under reduced pressure. The residue was purified by silica gel column chromatography using 1:1→1:4 hexanes–EtOAc as the eluant to afford 24 (702 mg, 94%) as a syrup: 1H NMR (CDCl3): δ 5.43 (dd, 1H, J = 1.2 Hz, J = 3.7 Hz), 5.34 (dd, 1H, J = 3.7 Hz, J = 10.6 Hz), 5.13 (dd, 1H, J = 3.7 Hz, J = 9.5 Hz), 5.12 (br s, 1H), 4.30 (dd, 1H, J = 5.8 Hz, J = 11.6 Hz), 4.26 (t, J = 6.6 Hz), 4.11 (br s, 1H), 4.04 (br s, 1H), 4.08 (dd, 1H, J = 6.4 Hz, J = 8.3 Hz), 3.76 (dd, 1H, J = 6.4 Hz, J = 8.3 Hz), 3.72 (dd, 1H, J = 5.2 Hz, J = 10.5 Hz), 3.56 (dd, 1H, J = 5.2 Hz, J = 10.5 Hz), 2.82–2.41 (m, 16H), 2.19, 2.177, 2.172, 2.171 (4s, each 3H); 13C NMR (CDCl3): δ 206.51, 206.36, 206.31, 206.06, 172.18, 172.08, 171.98, 171.84, 109.52, 96.63, 74.38, 69.15, 68.20, 67.97, 67.52, 66.49, 66.45, 61.84, 37.80, 37.78, 37.75, 37.70, 29.79, 29.78, 29.76, 27.92, 27.77, 27.75, 26.71, 25.47. API-ES-MS: m/z calcd for [C32H46O16]NH4+: 704.3. Found 704.2.
4.22. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-sn-glycerol (25)
A stirred solution of 24 (1.25 g, 1.8 mmol) in a mixture of MeOH (5 mL) and AcOH (5 mL) was heated at reflux for 5 min when TLC (EtOAc) indicated disappearance of 24. The solution was concentrated and the residue purified by column chromatography using a 1:1→1:0 EtOAc–hexanes gradient as the eluant to afford 25 (1.0 g, 83%) as a syrup: 1H NMR (CDCl3): δ 5.42 (dd, 1H, J = 1.3 Hz, J = 3.8 Hz), 5.37 (dd, 1H, J = 3.8 Hz, J = 10.6 Hz), 5.14 (d, 1H, J = 3.8 Hz), 5.09 (dd, 1H, J = 3.8 Hz, J = 10.6 Hz), 4.26 (br t, 1H, J = 6.7 Hz), 4.15–4.07 (m, 2H), 3.92 (m, 1H), 3.81 (dd, 1H, J = 3.8 Hz, J = 10.7 Hz), 3.72 (dd, 1H, J = 4.3 Hz, J = 11.2 Hz), 3.62 (dd, 1H, J = 5.6 Hz, J = 11.2 Hz), 3.53 (dd, 1H, J = 6.5 Hz, J = 10.6 Hz), 2.84–2.41 (m, 16H), 2.20 (s, 3H), 2.19 (s, 6H), 2.17 (s, 3H); 13C NMR (CDCl3): δ 207.77, 207.20, 206.62, 206.19, 172.18, 171.88, 171.79, 171.73, 96.40, 70.50, 69.93, 68.22, 68.10, 67.30, 66.37, 63.30, 61.92, 37.63, 37.58, 37.50, 29.65, 29.63, 29.60, 29.58, 29.50, 27.94, 27.76, 27.62, 27.59, 27.43. API-ES-MS: m/z calcd for [C29H42O16]NH4+: 664.3. Found 664.2.
4.23. 1-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-sn-glycerol (27)
1H NMR (CDCl3): δ 5.42 (d, 1H, J = 3.5 Hz), 5.35 (dd, 1H, J = 3.5 Hz, J = 10.8 Hz), 5.17 (d, 1H, J = 3.6 Hz), 5.08 (dd, 1H, J = 3.6 Hz, J = 10.8 Hz), 4.24 (m, 1H), 4.16 (dd, 1H, J = 5.2 Hz, J = 11.3 Hz), 4.08 (dd, 1H, J = 7.6 Hz, 11.3 Hz), 3.88 (m, 1H), 3.79 (dd, 1H, J = 5.7 Hz, J = 10.4 Hz), 3.72 (d, 1H, J = 4.5 Hz), 3.62 (dd, 1H, J = 5.1 Hz, J = 10.4 Hz), 2.83–2.41 (m, 16H), 2.19 (s, 6H), 2.18 and 2.17 (2s, 3H each); 13C NMR (CDCl3): δ 207.75, 207.29, 206.46, 206.03, 172.31, 171.98, 171.93, 171.77, 96.19, 70.15, 69.33, 68.37, 68.28, 67.40, 66.65, 63.33, 62.31, 37.80, 37.76, 37.74, 37.66, 29.86, 29.78, 29.76, 27.87, 27.74, 27.70. API-ES-MS: m/z calcd for [C29H42O16]NH4+: 664.3. Found 664.2.
4.24. 3-O-(2,3,4,6-Tetra-O-acetyl-β-D-galactopyranosyl)-1,2-O-isopropylidene-sn-glycerol (29)
To a stirred solution of imidate3528 (4.0 g, 8 mmol) and alcohol 19 (2.1 g, 16 mmol) in anhydrous CH2Cl2 was added at 0 °C TMSOTf (20 μL). After 1 h the solution was treated with a saturated aqueous solution of NaHCO3. The usual processing followed by column chromatographic purification afforded 29 as a syrup (3.0 g, 80%): 1H NMR (CDCl3): δ 5.38 (dd, 1H, J = 1.1 Hz, J = 3.5 Hz), 5.21 (dd, 1H, J = 7.9 Hz, J = 10.4 Hz), 5.01 (dd, 1H, J = 3.5 Hz, J = 10.4 Hz), 4.58 (d, 1H, J = 7.9 Hz), 4.26 (m, 1H), 4.18 (dd, 1H, J = 6.7 Hz, J = 11.2 Hz), 4.13 (dd, 1H, J = 6.7 Hz, J = 11.2 Hz), 4.02 (dd, 1H, J = 6.5 Hz, J = 8.3 Hz), 3.92 (dt, 1H, J = 1.1 Hz, J = 6.8 Hz), 3.89 (dd, 1H, J = 4.4 Hz, J = 10.8 Hz), 3.81 (dd, 1H, J = 5.9 Hz, J = 8.3 Hz), 3.63 (dd, 1H, J = 6.0 Hz, J = 10.7 Hz), 2.15, 2.07, 2.05, 1.99, 1.42, 1.35 (6s, 6 × 3H); 13C NMR (CDCl3): δ 170.32, 170.17, 170.08, 169.35, 109.26, 101.35, 74.19, 70.82, 70.66, 69.03, 68.66, 66.96, 66.16, 61.21, 26.53, 25.09, 20.68, 20.60, 20.59, 20.51. API-ES-MS: m/z calcd for [C20H30O12]NH4+: 480.2. Found 480.2.
4.25. 1,2-O-Isopropylidene-3-O-(2,3,4,6-tetra-O-levulinoyl-β-D-galactopyranosyl)-sn-glycerol (30)
To a solution of compound 29 (3.5 g, 7.6 mmol) in anhydrous MeOH was added NaOMe in MeOH (excess) until the pH of the solution reached 12 as seen with an indicator paper. After 24 h, the solution was carefully treated with Dowex 50WX8 (H+) ion exchange resin until the pH reached approx. 7, then the solution was concentrated and the residue dried under vacuum to obtain a semisolid. A solution of this material in EtOAc (50 mL) was treated under stirring with levulinic acid (7.1 g, 61 mmol) followed by N,N-dicyclohexylcarbodiimide (12.5 g, 61 mmol). After 3 h, MeOH (5 mL) was added and stirring was continued for an additional 2 h. The solids were filtered and the filtrate concentrated followed by column chromatographic purification using a hexane–EtOAc 10:1→2:1 gradient to yield 30 (4.8 g, 93%) as a syrup: 1HNMR (CDCl3): δ 5.36 (d, 1H, J = 3.6 Hz), 5.17 (dd, 1H, J = 8.0 Hz, J = 10.4 Hz), 5.02 (dd, 1H, J = 3.6 Hz, J = 10.4 Hz), 4.57 (d, 1H, J = 8.2 Hz), 4.27 (m, 1H), 4.20 (dd, 1H, J = 6.7 Hz), J = 11.4 Hz), 4.11 (dd, 1H, J = 6.7 Hz, J = 11.6 Hz), 4.04 (dd, 1H, J = 6.7 Hz, J = 8.2 Hz), 3.91–3.87 (m, 2H), 3.83 (dd, 1H, J = 5.9 Hz, J = 8.2 Hz), 3.63 (dd, 1H, J = 6.3 Hz, J = 10.8 Hz), 2.83–2.42 (m, 16H), 2.19, 2.18, 2.17, 2.16, 1.41, 1.34 (6s, 6 × 3H); 13C NMR (CDCl3): δ 207.74, 206.82, 206.64, 205.30, 172.16, 171.89, 171.88, 171.41, 109.29, 101.26, 74.24, 70.82, 70.75, 69.39, 68.85, 67.23, 66.39, 61.38, 37.85, 37.78, 37.76, 37.68, 29.76, 29.75, 29.67, 27.79, 27.76, 26.67, 25.19. API-ES-MS: m/z calcd for [C32H46O16]NH4+: 704.3. Found 704.3.
4.26. 3-O-(2,3,4,6-Tetra-O-levulinoyl-β-D-galactopyranosyl)-sn-glycerol (31)
Compound 30 was treated with acetic acid as described for 24 to afford 31 as a syrup: 1H NMR (CDCl3): δ 5.36 (d, 1H, J = 3.7 Hz), 5.17 (dd, 1H, J = 7.9 Hz, J = 10.5 Hz), 5.04 (dd, 1H, J = 3.4 Hz, J = 10.5 Hz), 4.53 (d, 1H, J = 8.1 Hz), 4.21 (dd, 1H, J = 7.2 Hz, J = 11.4 Hz), 4.11 (dd, 1H, J = 6.0 Hz, J = 11.4 Hz), 3.94–3.90 (m, 2H), 3.86 (m, 1H), 3.77 (dd, 1H, J = 3.6 Hz, J = 10.3 Hz), 3.68 (dd, 1H, J = 4.3 Hz, J = 11.5 Hz), 3.65 (dd, 1H, J = 5.1 Hz, J = 11.5 Hz), 2.91–2.41 (m, 16H), 2.20, 2.19, 2.18, 2.16 (4s, 4 × 3H); 13C NMR (CDCl3): δ 207.71, 206.76, 206.56, 206.09, 172.18, 171.87, 171.72, 171.67, 101.49, 72.04, 70.83, 70.54, 70.34, 68.92, 67.24, 63.24, 61.54, 37.76, 37.74, 37.69, 37.57, 29.76, 29.73, 29.69, 29.61, 27.71, 27.68, 27.66. API-ES-MS: m/z calcd for [C29H42O16]NH4+: 664.3. Found 664.5.
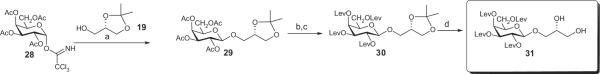
4.27. General procedure for the preparation of singly Gro-substituted intermediates featuring tetra-levulinoylated Gal moieties
To a solution of the diol 3-O-(2,3,4,6-tetra-O-levulinoyl-α-D-galactopyranosyl)-sn-glycerol 25 (129 mg, 0.2 mmol) in anhydrous CH2Cl2 (3 mL) was added fatty acid (0.2 mmol) followed by DCC (60 mg, 0.29 mmol) and a catalytic amount of DMAP at room temperature. After 16 h MeOH (0.5 mL) was added followed by stirring for 1 h. TLC (CH2Cl2–MeOH 100:4) indicated that most of the starting diol had disappeared and two closely migrating mono-substituted products had formed. Some less polar material was also formed that was the doubly-substituted product by mass spectrometry. The mixture was concentrated, then CH2Cl2 was added, followed by removal of the solids by filtration. The residue was applied to a silica gel column made in CH2Cl2 which was then eluted with CH2Cl2–MeOH 100:1 to afford the mono-substituted product in 65–75% yield, free of the slower-migrating isomer.
4.28. General procedure for the preparation of fully substituted intermediates having two different fatty acyl groups in the Gro moiety
To a stirred solution of the singly Gro-substituted intermediate in anhydrous CH2Cl2 was added fatty acid (1.3 equiv) followed by DCC (1.5 equiv) and a catalytic amount of DMAP. After 3 h, the solution was treated with MeOH (excess). After 1 h, the mixture was concentrated. To the residue was added CH2Cl2 followed by removal of the solids by filtration. Column chromatographic purification of the residue with a CH2Cl2–MeOH mixture of appropriate polarity afforded a near quantitative yield of the doubly Gro-substituted product.
4.29. General procedure for the preparation of fully protected intermediates having two identical fatty acyl groups in the Gro moiety
To a stirred solution of 3-O-(2,3,4,6-tetra-O-levulinoyl-α-D-galactopyranosyl)-sn-glycerol (25) in anhydrous CH2Cl2 was added fatty acid (3 equiv) followed by DCC (6 equiv) and a catalytic amount of DMAP. After 3 h, the solution was treated with MeOH (excess). After 1 h, the mixture was concentrated and processed as described in Section 4.28.
4.30. General procedure for the removal of the levulinoyl groups
The levulinylated material is dissolved in a 1 M solution of hydrazine hydrate in pyridine–AcOH (3:2) at rt. After 3 h, the solution is treated with 2,4-pentanedione (excess). The solution is concentrated under reduced pressure and the residue is purified by silica gel column chromatography. Depending on the acyl groups attached to the Gro moiety, the product may precipitate as a solid material during work-up.
4.31. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-1-O-palmitoyl-sn-glycerol (32)
1H NMR (CDCl3): δ 5.42 (dd, 1H, J = 1.4 Hz, J = 3.5 Hz), 5.36 (dd, 1H, J = 3.5 Hz, J = 10.7 Hz), 5.16 (d, 1H, J = 3.7 Hz), 5.09 (dd, 1H, J = 3.7 Hz, J = 10.7 Hz), 4.25 (ddd, 1H), 4.18 (dd, 1H, J = 4.6 Hz, J = 11.3 Hz), 4.13 (dd, 1H, J = 6.1 Hz, J = 11.2 Hz), 4.09 (dd, 1H, J = 7.0 Hz, J = 11.2 Hz), 3.81 (dd, 1H, J = 3.4 Hz, J = 10.9 Hz), 3.57 (d, 1H, J = 4.4 Hz), 3.51 (dd, 1H, J = 7.0 Hz, J = 10.9 Hz), 2.84–2.41 (m, 16H), 2.34 (dd, 1H, J = 7.3 Hz, J = 7.8 Hz), 2.189, 2.187, 2.181, 2.17 (4s, 4 × 3H), 1.66–1.58)m, 2H), 1.35–1.20 (m, 24H), 0.91–0.84 (m, 3H); 13C NMR (CDCl3): δ 207.35, 206.63, 206.40, 205.97, 173.71, 172.11, 171.86, 171.72, 171.67, 96.76, 70.18, 68.68, 68.15, 68.07, 67.27, 66.51, 64.91, 61.89, 37.66, 37.62, 37.59, 37.55, 33.99 31.79, 29.66, 29.63, 29.62, 29.56, 29.53, 29.49, 29.43, 29.36, 29.23, 29.17, 29.04, 27.84, 27.66, 27.64, 27.60, 24.78, 22.56, 14.00. API-ES-MS: m/z calcd for [C45H72O17]NH4+: 902.5. Found 902.4.
4.32. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-1-O-oleoyl-sn-glycerol (33)
1H NMR (CDCl3): δ 5.42 (d, 1H, J = 3.4 Hz), 5.36 (dd, 1H, J = 3.6 Hz, J = 10.4 Hz), 5.36–5.32 (m, 2H), 5.16 (d, 1H, J = 3.7 Hz), 5.09 (dd, 1H, J = 3.7 Hz, J = 10.7 Hz), 4.24 (ddd,1H), 4.18 (dd, 1H, J = 4.4 Hz, J = 11.3 Hz), 4.13 (dd, 1H, J = 5.8 Hz, J = 10.7 Hz), 4.09 (dd, 1H, J = 7.2 Hz), J = 11.3 Hz), 3.81 (dd, 1H, J = 3.4 Hz, J = 10.7 Hz), 3.51 (dd, 1H, J = 6.8 Hz, J = 10.7 Hz), 2.84–2.41 (m, 16H), 2.34 (t, 2H, J = 7.6 Hz), 2.188 (s, 6H), 2.181 (s, 3H), 2.17 (s, 3H), 2.04–1.98 (m, 2H), 1.65–1.59 (m, 2H), 1.36–1.24 (m), 0.89–0.86 (m, 3H); 13C NMR (CDCl3): δ 206.50, 206.42, 206.22, 205.98, 173.78, 172.20, 171.95, 171.80, 171.76, 129.96, 129.72, 96.86, 70.31, 68.82, 68.25, 68.16, 67.33, 66.60, 65.03, 61.99, 53.42, 37.76, 37.72, 37.69, 37.66, 34.08, 31.88, 29.77, 29.76, 29.74, 29.72, 29.69, 29.49, 29.29, 29.18, 29.10, 27.94, 27.74, 27.72, 27.69, 27.19, 27.16, 24.86, 22.66, 14.09. API-ES-MS: m/z calcd for [C47H74O17]NH4+: 928.5. Found 928.4.
4.33. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-1-O-stearoyl-sn-glycerol (34)
1H NMR (CDCl3): δ 5.42 (d, 1H, J = 3.6 Hz), 5.36 (dd, 1H, J = 3.6 Hz, J = 11.0 Hz), 5.16 (d, 1H, J = 3.7 Hz), 5.09 (dd, 1H, J = 3.7 Hz, J = 11.0 Hz), 4.25 (t, 1H, J = 6.6 Hz), 4.19–4.07 (m, 5H), 3.81 (dd, 1H, J = 3.1 Hz, J = 10.6 Hz), 3.58 (d, 1H, J = 3.9 Hz), 3.51 (dd, 1H, J = 6.7 Hz, J = 10.6 Hz), 2.84–2.41 (m, 16H), 2.34 (t, 2H, J ~7.6 Hz), 2.19 (s, 6H), 2.18 and 2.17 (2s, 2 × 3H), 1.62 (m, 2H), 1.38–1.18 (m, 28H), 0.88 (t, 3H, J ~ 6.7 Hz); 13C NMR (CDCl3): δ 207.31, 206.59, 206.37, 205.93, 173.67, 172.07, 171.83, 171.69, 171.63, 96.73, 70.16, 68.65, 68.13, 68.04, 67.25, 66.48, 64.88, 61.86, 37.63, 37.60, 37.57, 37.53, 33.97, 31.77, 29.69–29.02, 27.82, 27.63, 27.61, 27.57, 24.76, 22.54, 13.98. API-ES-MS: m/z calcd for [C47H76O17]NH4+: 930.5. Found 930.4.
4.34. 1-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-3-O-palmitoyl-sn-glycerol (35)
1H NMR (CDCl3): δ 5.42 (dd, 1H, J = 1.2 Hz, J = 3.4 Hz), 5.34 (dd, 1H, J = 3.4 Hz, J = 10.7 Hz), 5.15 (d, 1H, J = 3.8 Hz), 5.10 (dd, 1H, J = 3.8 Hz, J = 10.7 Hz), 4.24 (t, 1H, J ~6.5 Hz), 4.18–4.14 (m, 2H), 4.08 (dd, 1H, J = 7.5 Hz, J = 11.4 Hz), 4.05–4.01 (m, 1H), 3.75 (dd, 1H, J = 5.7 Hz, J = 11.0 Hz), 3.60 (dd, 1H, J = 4.4 Hz, J = 10.8 Hz), 3.46 (d, 1H, J = 5.6 Hz), 2.83–2.41 (m, 16H), 2.34 (d, 2H, J ~7.6 Hz), 2.19 (s, 9H), 2.17 (s, 3H), 1.66–1.58 (m, 2H), 1.35–1.21 (m, 24H), 0.88 (t, 3H, J ~7 Hz); 13C NMR (CDCl3): δ 207.18, 206.98, 206.45, 206.02, 173.86, 172.23, 171.96, 171.94, 171.76, 96.39, 76.51, 69.53, 68.46, 6.31, 68.13, 67.39, 66.68, 65.12, 62.17, 37.76, 37.71, 37.69, 34.14, 31.92, 29.83, 29.75, 29.69, 29.66, 29.48, 29.35, 29.30, 29.18, 27.92, 27.75, 29.69, 24.91, 22.69, 14.12. API-ES-MS: m/z calcd for [C45H72O17]NH4+: 902.5. Found 902.4.
4.35. 1-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-3-O-oleoyl-sn-glycerol (36)
1H NMR (CDCl3): δ 5.42 (d, 1H, J = 3.3 Hz), 5.37–5.30 (m, 3H), 5.14 (d, 1H, J = 3.6 Hz), 5.10 (dd, 1H, J = 3.6 Hz, J = 10.6 Hz), 3.24 (m, 1H), 4.20–4.11 (m, 3H), 4.07 (dd, 1H, J = 7.4 Hz, J = 11.3 Hz), 3.76 (dd, 1H, J = 5.5 Hz, J = 10.8 Hz), 2.83–2.41 (m, 16H), 2.32 (t, 2H, J ~7.6 Hz), 2.19 (s, 9H), 2.17 (s, 3H), 2.03–1.99 (m 4H), 1.67–1.28 (m, 2H), 1.39–1.20 (m, 20H), 0.89–0.86 (m, 3H); 13C NMR (CDCl3): δ 207.13, 206.94, 206.42, 205.93, 173.74, 172.16, 171.90, 171.87, 171.69, 129.91, 129.67, 96.31, 69.44, 68.36, 68.22, 58.06, 67.32, 66.59, 65.05, 62/08, 37.69, 37.68, 37.64, 37.60, 34.04, 35.00, 31.81, 29.74, 29.68, 29.64, 29.43, 29.23, 29.18, 29.12, 29.06, 29.05, 27.85, 27.69, 27.67, 27.62, 27.13, 27.10, 24.82, 22.59, 14.04. API-ES-MS: m/z calcd for [C47H74O17]NH4+: 928.5. Found 928.4.
4.36. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-1,2-di-O-palmitoyl-sn-glycerol (37)
1H NMR (CDCl3): δ 5.43 (dd, 1H, J = 1.2 Hz, J = 3.4 Hz), 5.32 (dd, 1H, J = 3.4 Hz, J = 10.8 Hz), 5.22 (m, 1H), 5.12 (dd, 1H, J = 3.7 Hz, J = 10.8 Hz), 5.06 (d, 1H, J = 3.7 Hz), 4.37 (dd, 1H, 3.9 Hz, J = 11.8 Hz), 4.20 (ddd, 1H), 4.18–4.06 (m, 3H), 3.83 (dd, 1H, J = 4.5 Hz, J = 11.2 Hz), 3.65 (dd, 1H, J = 5.0 Hz, J = 11.0 Hz), 2.83–2.42 (m, 16H), 2.33 and 2.30 (2t, 2 × 2H, J ~7.8 Hz), 1.66–1.56 (m, 4H), 1.36–1.20 (m, 44H), 0.92–0.85 (m, 6H); 13C NMR (CDCl3): δ 206.40, 206.35, 206.30, 206.00, 173.25, 172.97, 172.09, 172.08, 171.90, 171.79, 96.58, 69.73, 67.99, 67.81, 67.44, 66.63, 66.47, 62.19, 61.65, 37.73, 37.68, 37.66, 34.20, 34.01, 31.88, 29.69, 29.66, 29.61, 29.49, 29.47, 29.31, 29.29, 29.27, 29.11, 29.10, 27.81, 27.74, 27.71, 27.65, 24.89, 24.84, 22.64, 14.07. API-ES-MS: m/z calcd for [C61H102O18]NH4+: 1140.7. Found 1160.6.
4.37. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-1,2-di-O-oleoyl-sn-glycerol (38)
1H NMR (CDCl3): δ 5.43 (d, 1H, J = 3.7 Hz), 5.38–5.30 (m 5H), 5.22 (m, 1H), 5.13 (dd, 1H, J = 3.7 Hz, J = 10.8 Hz), 5.06 (d, 1H, J = 3.7 Hz), 4.37 (dd, 1H, J = 4.0 Hz, J = 11.8 Hz), 4.22–4.06 (m, 4H), 3.83 (dd, 1H, J = 4.5 Hz, J = 11.2 Hz), 3.65 (dd, 1H, J = 5.0 Hz, J = 11.2 Hz), 2.83–2.42 (m, 16H), 2.33 and 2.30 (2t, 2 × 3H, J ~7.7 Hz), 2.18 (s, 3H), 2.177 (s, 3H), 2.170 (s, 6H), 2.05–1.97 (m, 8H), 1.66–1.56 (m, 4H), 1.38–1.20 (m, 42H), 0.88 (t, 6H, J ~7.0 Hz); 13C NMR (CDCl3): δ 206.35, 206.12, 205.90, 173.17, 172.89, 172.04, 172.03, 171.87, 171.75, 129.90, 129.63, 96.55, 69.70, 67.95, 67.77, 67.40, 66.58, 66.44, 62.15, 61.61, 37.68, 37.64, 37.62, 34.13, 33.94, 31.81, 29.67, 29.66, 29.65, 29.43, 29.22, 29.15, 29.13, 29.09, 29.06, 29.03, 29.02, 27.77, 27.70, 27.67, 27.62, 27.13, 27.11, 27.10, 24.82, 24.77, 22.59, 14.03. APIES-MS: m/z calcd for [C65H106O18]NH4+: 1192.8. Found 1192.6.
4.38. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-2-O-oleoyl-1-O-palmitoyl-sn-glycerol (39)
1H NMR (CDCl3): δ 5.43 (d, 1H, J = 1.2 Hz, J = 3.5 Hz), 5.38–5.30 (m, 3H), 5.22 (m, 1H), 5.12 (dd, 1H, J = 3.7 Hz, J = 10.7 Hz), 4.37 (dd, 1H, J = 4.0 Hz, 12.0 Hz), 4.22–4.06 (m, 4H), 3.83 (dd, 1H, J = 4.6 Hz, J = 11.1 Hz), 3.65 (4.9 Hz, J = 11.3 Hz), 2.82–2.42 (m, 16H), 2.33 and 2.30 (2t, s × 2H, J = 7.5 Hz for each), 2.05–1.96 (m, 4H), 1.65–1.55 (m, 4H), 1.37–1.19 (m), 0.88 (t, 6H, J = 6.7 Hz); 13C NMR (CDCl3): δ 206.41, 206.18, 206.96, 173.25, 172.94, 172.09, 172.08, 171.91, 171.80, 129.94, 129.66, 96.58, 69.73, 67.99, 67.81, 67.44, 66.61, 66.47, 62.17, 61.65, 37.72, 37.67, 37.66, 34.17, 34.00, 31.87, 31.85, 29.71, 29.70, 29.69, 29.68, 29.65, 29.62, 29.60, 29.47, 29.46, 29.31, 29.26, 29.19, 29.12, 29.10, 29.06, 27.80, 27.73, 27.70, 27.65, 27.17, 27.15, 24.86, 24.83, 22.64, 22.63, 14.06. API-ES-MS: m/z calcd for [C63H104O18]NH4+: 1166.8. Found 1166.6.
4.39. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-1-O-oleoyl-2-O-palmitoyl-sn-glycerol (40)
1H NMR (CDCl3): δ 5.43 (d, 1H, J = 3.7 Hz), 5.36–5.29 (m, 3H), 5.22 (m, 1H), 5.12 (dd, 1H, J = 3.9 Hz, J = 10.6 Hz), 5.06 (d, 1H, J = 3.5 Hz), 4.36 (dd, 1H, J = 4.1 Hz, J = 12.3 Hz), 4.21–4.06 (m, 4H), 3.83 (dd, 1H, J = 4.5 Hz, J = 11.3 Hz), 3.65 (dd, 1H, J = 5.0 Hz, J = 11.0 Hz), 2.82–2.42 (m, 16H), 2.18 and 2.176 (2s, 2 × 3H), 2.172 (s, 3H), 2.33 and 2.30 (2t, 2 × 2H, J = 7.5 Hz), 2.01 (m, 4H), 1.62 (m, 4H), 1.37–1.21 (m), 0.88 (t, 6H, J = 7.3 Hz); 13C NMR (CDCl3): δ 206.45, 206.23, 206.22, 205.99, 173.29, 173.03, 172.15, 172.14, 171.97, 171.86, 130.01, 129.73, 96.64, 69.79, 68.05, 67.87, 67.50, 66.70, 66.53, 62.26, 61.71, 37.79, 36.74, 37.73, 34.25, 34.05, 31.93, 31.91, 31.79, 29.77, 29.75, 29.72, 29.71, 29.68, 29.55, 29.54, 29.46, 29.38, 29.35, 29.34, 29.33, 29.24, 29.17, 29.14, 27.86, 27.80, 27.76, 27.71, 27.23, 27.20, 24.95, 24.86, 22.70, 22.69, 14.13. API-ES-MS: m/z calcd for [C63H104O18]NH4+: 1166.8. Found 1166.6.
4.40. 3-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-1,2-di-O-stearoyl-sn-glycerol (41)
1H NMR (CDCl3): δ 5.43 (d, 1H, J = 3.4 Hz), 5.32 (dd, 1H, J = 3.4 Hz, J = 10.7 Hz), 5.22 (m, 1H), 5.12 (dd, 1H, J = 3.6 Hz, J = 10.7 Hz), 5.06 (d, 1H, J = 3.6 Hz), 4.37 (dd, 1H, J = 3.9 Hz, J = 12.0 Hz), 4.22–4.06 (m, 4H), 3.83 (d, 1H, J = 4.6 Hz, J = 11.2 Hz), 3.66 (dd, 1H, J = 5.1 Hz, J = 11.2 Hz), 2.86–2.41 (m, 16H), 2.33 and 2.30 (2t, 2 × 2H, J ~7.5 Hz), 2.18 and 2.17 (2s, 2 × 3H), 2.16 (s, 6H), 1.66–1.56 (m, 4H), 1.59–1.21 (m, 56H), 0.88 (t, 6H, J ~7.0 Hz); 13C NMR (CDCl3): δ 206.32, 206.10, 205.87, 173.18, 172.91, 172.03, 172.01, 171.85, 171.74, 96.52, 69.68, 67.94, 67.76, 67.39, 66.58, 66.42, 62.14, 61.60, 37.67, 37.62, 37.61, 34.14, 33.96, 31.82, 29.74–29.04, 27.76, 27.69, 27.66, 27.60, 24.84, 24.78, 22.59, 14.02. API-ES-MS: m/z calcd for [C65H110O18]NH4+: 1196.8. Found 1197.0.
4.41. 1-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-2,3-di-O-palmitoyl-sn-glycerol (42)
1H NMR (CDCl3): δ 5.42 (dd, 1H, J = 1.3 Hz, J = 3.5 Hz), 5.30 (dd, 1H, J = 3.5 Hz, J = 10.7 Hz), 5.20 (m, 1H), 5.12 (dd, 1H, J = 3.7 Hz, J = 10.7 Hz), 5.08 (d, 1H, J = 3.7 Hz), 4.32 (dd, 1H, J = 3.7 Hz, J = 12.5 Hz), 4.21–4.17 (m, 2H), 4.11 (s, 1H), 4.09 (m, 1H), 3.82 (dd, 1H, J = 5.4 Hz, J = 11.1 Hz), 3.63 (dd, 1H, J = 5.4 Hz, J = 11.1 Hz), 2.83–2.42 (m, 16H), 2.31 and 2.30 (2t, 2 × 2H, J ~7.5 Hz), 2.18, 2.176, 2.174, 2.171 (4s, 3 × 3H), 1.65–1.56 (m, 4H), 1.34–1.21 (m, 48H), 0.89–0.86 (m, 6H); 13C NMR (CDCl3): δ 206.37, 206.26, 206.20, 206.19, 173.21, 172.78, 172.07, 172.03, 171.85, 171.71, 96.61, 69.67, 67.97, 67.75, 67.31, 66.55, 66.28, 62.18, 61.63, 37.70, 37.68, 37.63, 37.61, 34.10, 33.97, 31.83, 29.66, 29.64, 29.61, 29.58, 29.57, 29.43, 29.42, 29.27, 29.23, 29.22, 29.06, 29.04, 27.77, 27.69, 27.66, 27.63, 24.81, 24.78, 22.60, 14.03. API-ES-MS: m/z calcd for [C61H102O18]NH4+: 1140.7. Found 1140.6.
4.42. 1-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-2,3-di-O-oleoyl-sn-glycerol (43)
1H NMR (CDCl3): δ 5.42 (dd, 1H, J = 1.1 Hz, J = 3.4 Hz), 5.37–5.31 (m, 4H), 5.30 (dd, 1H, J = 3.4 Hz, J = 10.7 Hz), 5.20 (m, 1H), 5.12 (dd, 1H, J = 3.8 Hz, J = 10.7 Hz), 5.08 (d, 1H, J = 3.8 Hz), 4.32 (dd, 1H, J = 3.8 Hz, J = 12.0 Hz), 4.1–4.17 (m, 2H), 4.09 (m, 1H), 3.82 (dd, 1H, 5.3 Hz, J = 11.2 Hz), 3.63 (dd, 1H, J = 3.63 Hz, J = 11.2 Hz), 2.82–2.42 (m, 16H), 2.31 and 2.30 (2t, 2 × 2H, J ~7.5 Hz), 2.183, 2/176, 2.173, 2.170 (4s, 3H each), 2.03–1.99 (m, 4H), 1.36–1.22 (m, 40H), 0.89–0.86 (m, 6H); 13C NMR (CDCl3): δ 206.48, 206.25, 206.20, 206.17, 173.21, 172.77, 172.09, 172.05, 171.87, 172.77, 172.09, 172.05, 171.88, 171.74, 129.92, 129.64, 96.63, 69.70, 67.99, 7.77, 67.33, 66.57, 66.30, 62.20, 61.64, 37.72, 37.70, 37.66, 37.63, 34.09, 33.96, 31.83, 29.69, 29.66, 29.45, 29.24, 29.15, 29.09, 29.07, 29.05, 29.02, 27.79, 27.70, 27.68, 27.65, 27.15, 27.12, 27.11, 24.80, 24.78, 22.60, 14.04. API-ES-MS: m/z calcd for [C65H106O18]NH4+: 1192.8. Found 1192.6.
4.43. 1-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-2-O-oleoyl-3-O-palmitoyl-sn-glycerol (44)
1H NMR (CDCl3): δ 5.43 (d, 1H, J = 3.4 Hz), 5.37–5.27 (m, 3H), 5.20 (m, 1H), 5.12 (dd, 1H, J = 3.7 Hz, J = 10.7 Hz), 5.08 (d, 1H, J = 3.7 Hz), 4.32 (dd, 1H, J = 3.7 Hz, J = 11.9 Hz), 4.22–4.16 (m, 2H), 4.12–4.08 (m, 2H), 3.82 (dd, 1H, J = 5.3 Hz, J = 11.1 Hz), 3.63 (dd, 1H, J = 5.3 Hz, J = 11.1 Hz), 2.82–2.42 (m, 16H), 2.31 and 2.30 (2t, 2H, J ~7.6 Hz), 2.180 (s, 3H), 2.175 (s, 6H), 2.172 (s, 3H), 2.06–1.98 (m, 4H), 1.66–1.56 (m, 4H), 1.38–1.20 (m, 44H), 0.92–086 (m, 3H); 13C NMR (CDCl3): δ 206.44, 206.26, 206.18, 205.99, 173.33, 172.86, 172.18, 172.14, 171.96, 171.83, 130.01, 129.72, 96.73, 69.78, 68.07, 67.86, 67.41, 66.65, 66.38, 62.27, 61.73, 37.81, 37.79, 37.74, 37.72, 34.18, 34.06, 31.93, 31.91, 29.78, 29.76, 29.71, 29.69, 29.67, 29.54, 29.52, 29.37, 29.34, 29.33, 29.24, 29.18, 29.11, 27.87, 27.78, 27.76, 27.73, 27.24, 27.21, 24.89, 22.70, 14.13. API-ES-MS: m/z calcd for [C63H104O18]NH4+: 1166.8. Found 1166.6.
4.44. 1-O-(2,3,4,6-Tetra-O-levulinoyl-α-D-galactopyranosyl)-3-O-oleoyl-2-O-palmitoyl-sn-glycerol (45)
1H NMR (CDCl3): δ 5.42 (d, 1H, J = 3.4 Hz), 5.38–5.32 (m, 2H), 5.30 (dd, 1H, J = 3.4 Hz, J = 10.6 Hz), 5.20 (m, 1H), 5.12 (dd, 1H, J = 3.6 Hz, J = 10.6 Hz), 5.08 (dd, 1H, J = 3.6 Hz), 4.32 (dd, 1H, J = 3.6 Hz, J = 11.8 Hz), 4.22–4.17 (m, 2H), 4.12–4.08 (m, 2H), 3.82 (dd, 1H, J = 5.3 Hz, J = 11.1 Hz), 3.63 (dd, 1H, J = 5.3 Hz, J = 11.1 Hz), 2.83–2.42 (m, 16H), 2.307 and 2.305 (2t, 2 × 2H, J ~7.4 Hz each), 2.18 (s, 3H), 2.175 (s, 6H), 2.171 (s, 3H), 2.06–1.97 (m, 4H), 1.67–1.56 (m, 4H), 1.42–1.19 (m, 44H), 0.94–0.85 (m, 6H); 13C NMR (CDCl3): δ 206.44, 206.26, 206.18, 205.99, 173.29, 172.89, 172.18, 172.14, 171.96, 171.82, 130.01, 129.73, 96.72, 69.77, 68.07, 67.86, 67.41, 66.65, 66.39, 62.29, 61.73, 37.81, 37.79, 37.74, 37.72, 34.20, 34.05, 31.93, 31.91, 29.78, 29.75, 29.72, 29.70, 29.67, 29.54, 29.37, 29.33, 29.23, 29.16, 29.14, 27.87, 27.78, 27.76, 27.73, 27.23, 27.20, 24.91, 24.87, 22.70, 22.69, 14.13. API-ES-MS: m/z calcd for [C63H104O18]NH4+: 1166.8. Found 1166.6.
4.45. 3-O-α-D-Galactopyranosyl)-1,2-di-O-oleoyl-DL-glycerol (46)
1H NMR (CDCl3): δ 5.39–5.28 (m, 5H), 4.40 (dd, 1H, 3.6 Hz, J = 12.5 Hz), 4.28 (d, 1H, J = 7.6 Hz), 4.21 (dd, 1H, J = 6.7 Hz, J = 12.0 Hz, 4.14 (dd, 1H, J = 6.0 Hz, J = 12.0 Hz), 4.02–3.84 (m, 4H), 3.77 (dd, 1H, J = 6.4 Hz, J = 11.5 Hz), 3.75 (dd, 1H, J = 6.4 Hz, J = 11.5 Hz), 3.66 (t, 1H, J = 8.4 Hz), 3.61–3.57 (m, 1H), 3.55 (m, 2H), 2.35–2.29 (m, 4H), 2.06–1.96 (m, 8H), 1.67–1.54 (m, 6H), 1.38–1.19 (m, 38H), 0.88 (t, 6H, J ~7.2 Hz); 13C NMR (CDCl3): δ 173.76, 173.70, 173.52, 173.51, 130.06, 129.72, 104.06, 103.65, 74.53, 74.40, 73.46, 73.36, 71.76, 71.67, 70.19, 70.14, 69.58, 68.49, 68.26, 63.02, 62.96, 62.70, 62.44, 34.33, 34.31, 34.14, 34.13, 31.92, 29.79, 29.74, 29.55, 29.35, 29.34, 29.21, 29.15, 29.12, 29.08, 27.25, 27.20, 24.91, 24.86, 22.70, 14.13. API-ES-MS: m/z calcd for [C45H82O10]NH4+: 800.6. Found 800.5.
5. Note added after the review process
An anonymous referee suggested the use of Lemieux's halideion catalysis protocol. Accordingly, tetra-O-benzyl-D-galactopyranosyl bromide, obtained in situ by the action of bromine on the corresponding ethylthio-galactoside, was treated with 1,2-O-isopropylidene-sn-glycerol (6 molar equiv) in the presence of Bu4NBr and Hünig's base for 4 days at rt to afford the expected α-galactopyanosyl-glycerol derivative together with an inseparable minor product (<5%, NMR) in a combined yield of 78% yield (for two steps).
Acknowledgments
This work was supported by the intramural programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Abbreviations
- API-ES-MS
atmospheric pressure ionization electrospray mass spectrometry
- BBGL
Borrelia burgdorferi glyco lipid
- DCC
N,N-dicyclohexylcarbodiimide
- DMAP
4-dimethylaminopyridine
- FA
fatty acid
- Gal
galactosyl
- Gro
glycerol
- IL
interleukin
- iNKR
invariant natural killer T cell
- LD
Lyme disease
- Ole
oleoyl
- Pal
palmitoyl
- sn
stereochemical nomenclature
- Ste
stearoyl
- TLC
thin layer chromatography
Footnotes
Dedicated to Professor Dr. András Lipták on the occasion of his 75th birthday
References
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2. http://www.cdc.gov.
- 3.Bacon RM, Kugeler KJ, Mead PS. MMWR. 2008;57:1–9. [PubMed] [Google Scholar]
- 4.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, Krause DS. Lyme Dis Vaccine Study Grp. N. Eng. J. Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 5.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, Field JA, Steere AC, Huber BT. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 6.Nigrovic LE, Thompson KM. Epidemiol. Infect. 2007;135:1–8. doi: 10.1017/S0950268806007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball R, Shadomy SV, Meyer A, Huber BT, Leffell MS, Zachary A, Belotto M, Hilton E, Bryant-Genevier M, Schriefer ME, Miller FW, Braun MM. Arthritis Rheum. 2009;60:1179–1186. doi: 10.1002/art.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen AK, Mead PS, Beard C. Clin. Infect. Dis. 2011;52:S247–S252. doi: 10.1093/cid/ciq115. [DOI] [PubMed] [Google Scholar]
- 9.Plotkin SA. Clin. Infect. Dis. 2011;52:S271–S275. doi: 10.1093/cid/ciq119. [DOI] [PubMed] [Google Scholar]
- 10.Takayama K, Rothenberg RJ, Barbour AG. Infect. Immun. 1987;55:2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain H, Wellensiek HJ, Geyer R, Lochnit G. Biochimie. 2001;83:683–692. doi: 10.1016/s0300-9084(01)01296-2. [DOI] [PubMed] [Google Scholar]
- 13.Schroder NWJ, Schombel U, Heine H, Gobel UB, Zähringer U, Schumann RRJ. Biol. Chem. 2003;278:33645–33653. doi: 10.1074/jbc.M305799200. [DOI] [PubMed] [Google Scholar]
- 14.Stubs G, Fingerle V, Wilske B, Gobel UB, Zähringer U, Schumann RR, Schroder NWJ. J. Biol. Chem. 2009;284:13326–13334. doi: 10.1074/jbc.M809575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 16.Jones KL, Seward RJ, Ben-Menachem G, Glickstein LJ, Costello CE, Steere AC. Clin. Immunol. 2009;132:93–102. doi: 10.1016/j.clim.2009.03.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MREI, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Nat. Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 19.Du W, Kulkarni SS, Gervay-Hague J. Chem. Commun. 2007:2336–2338. doi: 10.1039/b702551c. [DOI] [PubMed] [Google Scholar]
- 20.Schombs M, Park FE, Du WJ, Kulkarni SS, Gervay-Hague J. J. Org. Chem. 2010;75:4891–4898. doi: 10.1021/jo100366v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia J, Hui YZ. Tetrahedron: Asymmetry. 1997;8:3019–3021. [Google Scholar]
- 22.Fraser BH, Perlmutter P, Wijesundera C. J. Am. Oil Chem. Soc. 2007;84:11–21. [Google Scholar]
- 23.Andrews PC, Fraser BH, Junk PC, Massi M, Perlmutter P, Thienthong N, Wijesundera C. Tetrahedron. 2008;64:9197–9202. [Google Scholar]
- 24.Fureby AM, Virto C, Adlercreutz P, Mattiasson B. Biocatal. Biotransform. 1996;14:89–111. [Google Scholar]
- 25.Greimel P, Ito Y. Tetrahedron Lett. 2008;49:3562–3566. [Google Scholar]
- 26.Martin SF, Josey JA, Wong YL, Dean DW. J. Org. Chem. 1994;59:4805–4820. [Google Scholar]
- 27.Rosseto R, Bibak N, Hajdu J. Org. Biomol. Chem. 2006;4:2358–2360. doi: 10.1039/b603788g. [DOI] [PubMed] [Google Scholar]
- 28.Pozsgay V, Kubler-Kielb J, Coxon B, Ekborg G. Tetrahedron. 2005;61:10470–10481. [Google Scholar]
- 29.Pozsgay V, Kubler-Kielb J. Carbohydr. Res. 2007;342:621–626. doi: 10.1016/j.carres.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khiar N, Martin-Lomas M. J. Org. Chem. 1995;60:7017–7021. [Google Scholar]
- 31.Osswald M, Lang U, Friedrich-Bochnitschek S, Pfrengle W, Kunz HZ. Naturforsch., B. 2003;58:764–774. [Google Scholar]
- 32.van Boom JH, Burgers PMJ. Tetrahedron Lett. 1976:4875–4878. [Google Scholar]
- 33.Takatani M, Matsuo I, Ito Y. Carbohydr. Res. 2003;338:1073–1081. doi: 10.1016/s0008-6215(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 34.Pozsgay V. Tetrahedron: Asymmetry. 2000;11:151–172. [Google Scholar]
- 35.Eisele T, Windmüller R, Schmidt RR. Carbohydr. Res. 1998;306:81–91. doi: 10.1016/s0008-6215(97)10044-1. [DOI] [PubMed] [Google Scholar]
- 36.Cinco M, Banfi E, Balanzin D, Godeas C, Panfili E. FEMS Microbiol. Immunol. 1991;3:33–38. doi: 10.1111/j.1574-6968.1991.tb04160.x. [DOI] [PubMed] [Google Scholar]
- 37.Eiffert H, Lotter H, Jareckikhan K, Thomssen R. Med. Microbiol. Immunol. 1991;180:229–237. doi: 10.1007/BF00202557. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler CM, Monco JCG, Benach JL, Golightly MG, Habicht GS, Steere AC. J. Infect. Dis. 1993;167:665–674. doi: 10.1093/infdis/167.3.665. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Li YL, Kinjo Y, Mac TT, Gibson D, Painter GF, Kronenberg M, Zajonc DM. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu C, Liu B, Watson D, Szu S, Bryla D, Shiloach J, Schneerson R, Robbins JB. Infect. Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crich D, Smith M, Yao QJ, Picione J. Synthesis-Stuttgart. 2001:323–326. [Google Scholar]