Abstract
In an era of limited healthcare budgets, mathematical models can be useful tools to identify cost-effective programs and to support policymakers in informed decision making. This paper reports results of our work carried out over several years with the Asian Liver Center at Stanford University, a nonprofit outreach and advocacy organization that is an international leader in the fight against hepatitis B and liver cancer. Hepatitis B is a vaccine-preventable viral disease that, if untreated, can lead to death from cirrhosis and liver cancer. Infection with hepatitis B is a major public health problem, particularly in Asian populations. We used new combinations of decision analysis and Markov models to analyze the cost-effectiveness of several interventions to combat hepatitis B in the United States and China. The results of our OR-based analyses have helped change United States public health policy on hepatitis B screening for millions of people and have helped encourage policymakers in China to enact legislation to provide free catch-up vaccination for hundreds of millions of children. These policies are an important step in eliminating health disparities, reducing discrimination, and ensuring that millions of people who need it can now receive hepatitis B vaccination or lifesaving treatment.
Keywords: Health care, Economics, Cost-benefit analysis, Decision analysis, Epidemiology, Government services
Hepatitis B is a major public health problem, particularly in Asian populations. Approximately 8-10 percent of people in China and other east Asian countries are chronically infected with hepatitis B (World Health Organization 2008c). A similar fraction of Americans of Asian descent are infected (Centers for Disease Control and Prevention 2006, Chao et al. 2004, Cotler et al. 2009, Guane et al. 2004, Lee et al. 2005, Lin et al. 2007). Hepatitis B typically infects newborns and children, often becoming a chronic lifelong disease that may cause serious health problems decades later in life (World Health Organization 2008c). The disease is particularly likely to become a lifelong infection if the person is initially exposed to the virus at birth or in childhood (Edmunds et al. 1993). Chronic infection is typically asymptomatic for decades. However, if left untreated, about 25 percent of those who are chronically infected will die of liver diseases such as cirrhosis or liver cancer (World Health Organization 2008c). A hepatitis B vaccine has been available since the 1980s, but is considered costly in many low-income countries. Moreover, although treatment is available, it is long term, does not cure the disease, and costs thousands of dollars per year.
In the United States, vaccination of children for hepatitis B is relatively widespread, and prevalence of chronic hepatitis B infection is only about 0.5 percent in the general population (Custer et al. 2004, McQuillan et al. 1999). However, approximately 10 percent of adult Asian and Pacific Islanders in the United States have chronic hepatitis B infection (Centers for Disease Control and Prevention 2006, Chao et al. 2004, Cotler et al. 2009, Guane et al. 2004, Lee et al. 2005, Lin et al. 2007). Rates of liver cancer among Asian and Pacific Islanders are more than three times higher than in the general population (American Cancer Society California Division 2008, Chang et al. 2007). Unfortunately, about two-thirds of individuals who are chronically infected are unaware of their infection (Lin et al. 2007). This prevents them from properly monitoring their health and receiving effective treatment.
In the United States, policies for prevention and control of hepatitis B must be targeted to the appropriate risk groups, such as Asian and Pacific Islander adults. But what is the correct combination of screening, vaccination, and treatment for this group? As healthcare budgets in the United States become strained, public health officials are increasingly looking for evidence-based analysis to determine what programs to fund. Specifically, they want to know not only the effectiveness of potential public health programs (that is, the health benefit), but also the cost-effectiveness of such programs (that is, the health benefit achieved per dollar spent).
China has the largest burden of hepatitis B disease in the world. Recent reports estimate that liver disease caused by hepatitis B causes approximately 300,000 deaths each year in China (Centers for Disease Control and Prevention 2007, Liu and Fan 2007, Sun et al. 2002). That total exceeds the combined number of annual deaths in China from tuberculosis (200,000 deaths), HIV/AIDS (38,000 deaths), and malaria (37 deaths) – three diseases that have received significant attention and funding in recent years (The Global Fund to Fight AIDS Tuberculosis and Malaria 2010, World Health Organization 2004, World Health Organization 2006, World Health Organization 2008a, World Health Organization 2008b). In addition to the impact on their health, hepatitis B carriers in China face significant discrimination. Many children are denied admission to schools if they are infected with hepatitis B (Reuters 2008). A recent survey of 115 branches in China of multinational companies showed that 80 percent would not hire individuals who are hepatitis B carriers (China Daily 2007).
Rates of newborn vaccination coverage in China have increased from an estimated 70.7 percent in 1997 to 89.8 percent in 2003 (Centers for Disease Control and Prevention 2007). However, data from a recent national serologic survey suggest that about 20 percent of 1- to 4-year olds and 40 percent of 5- to 19-year olds still remain unprotected from hepatitis B (Chinese Ministry of Health 2008). Since infection at an early age is more likely to result in a lifelong, chronic infection than infection later in life, it is most important to vaccinate children while they are young.
A recent pilot program of hepatitis B catch-up vaccination in the Qinghai province in China vaccinated more than 500,000 school-age children who had not been previously vaccinated (Chen 2008, People’s Daily Online 2006). The program demonstrated that catch-up vaccination programs are feasible: this program was able to achieve 99.4 percent coverage of the three required doses of hepatitis B vaccine (Chen 2008). However, the costs of healthcare programs are a concern in China, and therefore public health officials want assurance that potential hepatitis B control programs are not only effective, but cost-effective.
Working with the Asian Liver Center at Stanford University, a nonprofit outreach and advocacy organization that is an international leader in the fight against hepatitis B and liver cancer, we used OR-based analyses to address two policy questions related to hepatitis B prevention and control:
What combination of hepatitis B screening, treatment, and vaccination for adult Asian and Pacific Islanders in the United States is cost-effective?
Is it cost-effective to provide hepatitis B catch-up vaccination for children and adolescents in China?
Assembling a Multidisciplinary Team
Multidisciplinary teams are especially important when analyzing health policy problems because such problems tend to be complex and interdisciplinary. To address the hepatitis B policy questions, we needed knowledge not only of operations research and cost-effectiveness analysis, but also knowledge of the clinical details of hepatitis B infection and the policy issues surrounding hepatitis B prevention and control in the United States and China. We assembled a multidisciplinary team with this knowledge. Dr. Samuel So, a liver transplant surgeon and Director of the Asian Liver Center at Stanford University, helped provide the motivation behind the problem, clinical and policy expertise, and connections with policy makers. He was also an invaluable expert ensuring that our models accurately reflected the “real world”. Professor Margaret Brandeau of Stanford’s Department of Management Science and Engineering provided valuable input, guidance, and supervision regarding the modeling and cost-effectiveness analysis and the reporting of results. David Hutton, a doctoral student in Management Science and Engineering, created the mathematical models, reviewed more than 250 published and unpublished studies to obtain data for the models, implemented the models and interpreted the results, and drafted the manuscripts containing our results. Together our multidisciplinary team was able to accomplish research and policy changes that none of us could have effected individually.
Models
In the medical community, clinical trials are the gold standard for decision making: evidence that an intervention is effective and cost-effective in a cohort of patients provides a strong foundation for decision making. However, since hepatitis B usually takes decades to progress, a trial evaluating these policies would be very expensive, and any results – and valuable policy interventions – would be delayed for decades. We instead used operations research-based modeling to estimate the likely health and economic impacts of alternative hepatitis B prevention and control programs.
We evaluated the health and economic impacts using a cost-effectiveness framework. We present our results in the standard health economics format of an incremental cost-effectiveness ratio, where the strategies are compared, incrementally to each other, in terms of the incremental cost to gain an incremental unit of health, and health units are expressed in terms of quality-adjusted life years (QALYs) (Drummond et al. 2005, Gold et al. 1996, Muennig 2008). This allows for ready comparison of alternative hepatitis B control strategies as well as comparison with other potential public health interventions. When thought of in a traditional operations research optimization framework, the incremental cost-effectiveness ratio is the value/cost metric used to solve a fractional knapsack problem (Hillier and Lieberman 2005). Interventions that cost less per QALY gained than three times a country’s per capita GDP are considered to be cost-effective, and interventions that cost less than a country’s per capita GDP are considered to be very cost-effective (Murray and Lopez 2002, World Health Organization 2009).
To estimate the economic and health impacts of hepatitis B screening, treatment, and vaccination programs, we created a decision model of alternative policies (Figure 1) along with a Markov model of hepatitis B disease (Figure 2) (Hutton et al. 2010, Hutton et al. 2007). This allowed us to capture the long-term health and economic outcomes among chronically infected individuals with and without treatment. We modified the model (policy alternatives considered, epidemiological factors, costs, etc.) as appropriate for our analyses of policies in the United States and China.
Figure 1. Schematic of decision model*.
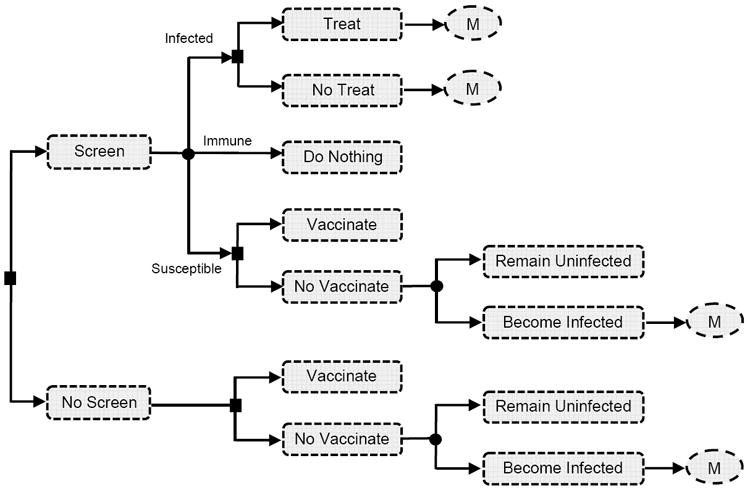
* This figure shows the decision model for our analysis of hepatitis B screening, vaccination, and treatment in the United States. The circles with “M” in them denote our Markov model of disease progression in treated and untreated individuals (Figure 2). For our analysis of catch-up vaccination in China, the decision tree consists only of Screen with Vaccinate or No Vaccinate and No Screen with Vaccinate or No Vaccinate (along with the Markov model of infection).
Figure 2. Schematic of Markov model of hepatitis B infection and progression*.
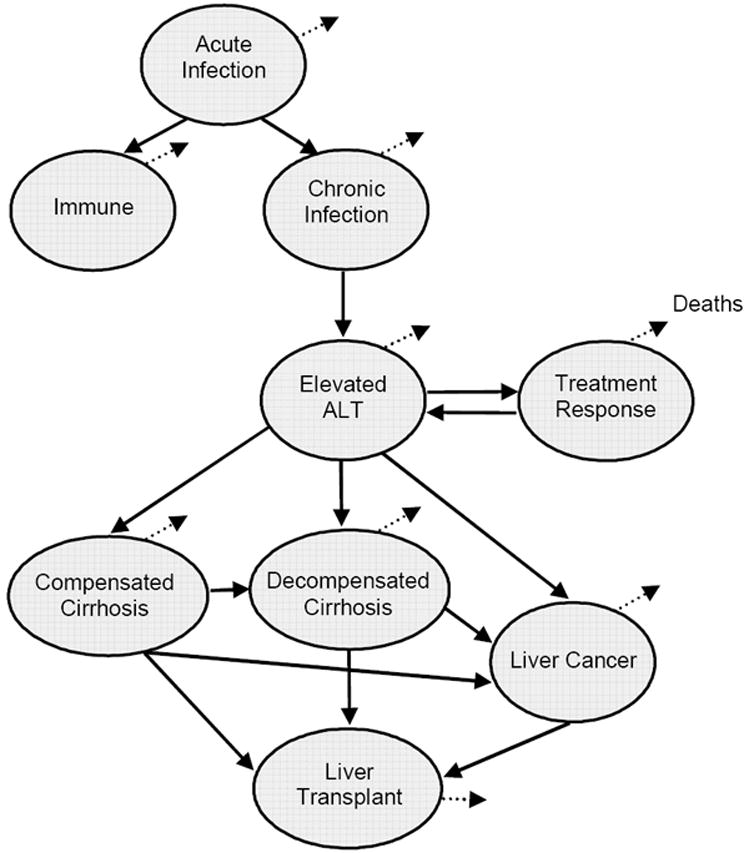
* ALT = alanine aminotransferase, a blood enzyme measuring the health of the liver. Circles represent health states. Lines represent transitions between those states. Dotted lines represent death. When exposed to hepatitis B, individuals develop acute infection. Acute infection causes individuals to either become immune (if their immune system is able to resolve the infection) or to develop chronic HBV infection. The first sign of progression to liver disease is an elevated ALT level (“Elevated ALT”), at which point individuals are eligible for treatment. Treatment may be successful in suppressing the virus (“Treatment Response”) or liver disease may progress to cirrhosis or liver cancer. Patients with cirrhosis or liver cancer are eligible for a liver transplant. Individuals in all states can die, at a rate equal to the background mortality based on the age of the individual plus the incremental mortality related to being in that particular health state.
The decision model (Figure 1) captures various possibilities for screening, treatment, and vaccination. We consider a cohort of 10,000 individuals of a given age (e.g., age 40 for adult Asian and Pacific Islanders in the United States, or age 10 for school-age children in China). Individuals can be screened (via blood tests) to determine their infection status. If an individual is screened and found to be infected, the individual can be treated or not; if the individual is found to be susceptible, the individual can be vaccinated or not; and if the individual is found to be immune (either from previous vaccination or from previously resolving the infection), no further action is taken. If an individual is not screened, the individual can still be vaccinated or not vaccinated. For all individuals who were already infected, or who were susceptible and unvaccinated and subsequently acquired the infection, we model disease progression over the future lifetime of those individuals using a Markov model.
Our Markov model of hepatitis B disease (Figure 2) is a discrete time, time-inhomogeneous model. To construct the model, we examined previously published models of hepatitis B infection in the literature and used the expertise of Dr. So to create a model that reflects current knowledge of hepatitis B infection and treatment. Individuals who acquire acute hepatitis B infection can become immune by resolving the infection; if their immune system cannot resolve the infection, they develop chronic hepatitis B infection. Chronic infection can be asymptomatic for many years. For many individuals, chronic infection does not cause liver disease; in such cases an individual remains in the chronic infection state for his or her lifetime. Some individuals do develop liver disease, which is often asymptomatic until serious liver disease develops. Individuals identified as having liver disease (either through screening or via the development of symptoms) can receive treatment, which may successfully suppress the virus. Otherwise, the disease can progress to cirrhosis and/or liver cancer. We chose yearly transitions for the Markov model since chronic hepatitis B tends to progress slowly. Transitions follow the Markov property of being memoryless, with one exception: background mortality is based on the age of the individuals in the cohort, year by year.
The model calculates net present costs and health outcomes (measured in discounted quality-adjusted life years (QALYs)) of each policy, over the lifetime of the cohort of individuals. Quality-adjusted life years are a measure of both quality and length of life. Each year of life is multiplied by a utility value between 0 (representing death) and 1 (representing perfect health); this utility value is intended to reflect the relative “quality” of a given health state. In each time period, costs and utilities are assigned to individuals in each health state. The costs incurred and QALYs experienced are measured for each health state and time period over the time horizon of the problem (in this case, the remaining lifetime of all individuals) and then discounted back to the present.
Our model has several novel features. First, we combined a decision model of alternative screening and vaccination policies with probabilistic trees of infection and a Markov model of disease progression and treatment. No previous model has examined hepatitis B screening and treatment together. Some analyses have considered the cost-effectiveness of screening, without considering the effects of subsequent treatment for infected individuals (Jacobs et al. 2003, Margolis et al. 1995, Pisu et al. 2002), while other analyses have considered only the cost-effectiveness of treatment for infected individuals who have already been identified (Kanwal et al. 2005, Shepherd et al. 2006). Second, our Markov model of hepatitis B infection captures the effects of treatment with recently developed antiviral drugs. These drugs, developed as an outgrowth of advances in treatment for HIV infection, are more effective than previous treatments for hepatitis B infection, but also more expensive. Although treatment with some of the newer drugs has been shown to be cost-effective (Kanwal et al. 2005, Rajendra and Wong 2007, Shepherd et al. 2006), they have not been considered in conjunction with population-based screening to identify people needing treatment.
Because our intent was to make a model that could be shared with policy makers, we developed the model in Microsoft Excel (Figure 3). We incorporated sufficient detail to capture important characteristics of hepatitis B disease progression and treatment so that it would be believable to a clinical audience – but we also tried to keep the model simple enough so that it could be easily understood by non-modelers. In fact, during the course of model development we shared the model directly with the Centers for Disease Control and Prevention (CDC) and reviewed and interpreted the results with them, as described below. In developing the model we used an iterative process: we began with a very simple representation of patient health states and then added details incrementally as suggested by Dr. So, our colleagues at the CDC, and other hepatitis B experts with whom we consulted. We continued to add detail until the clinicians and hepatitis B experts were satisfied that our model was an appropriate representation of the policy problem. In this way, we kept the model as simple as possible while still capturing the salient elements of the problem.
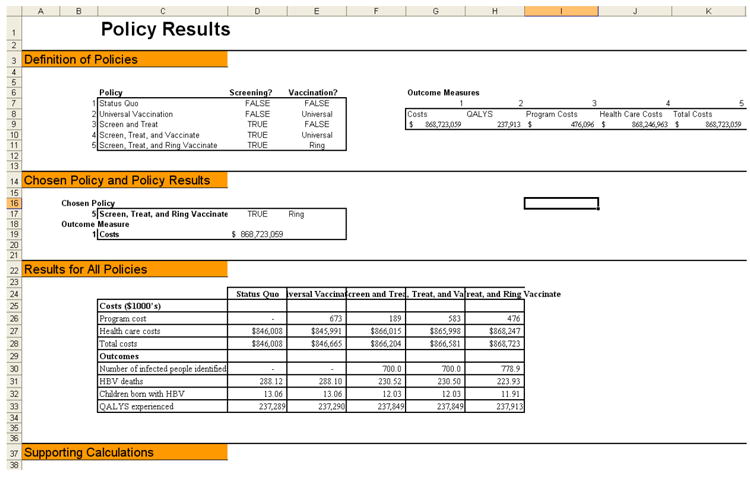
Figure 3. Screenshots from the Excel model.
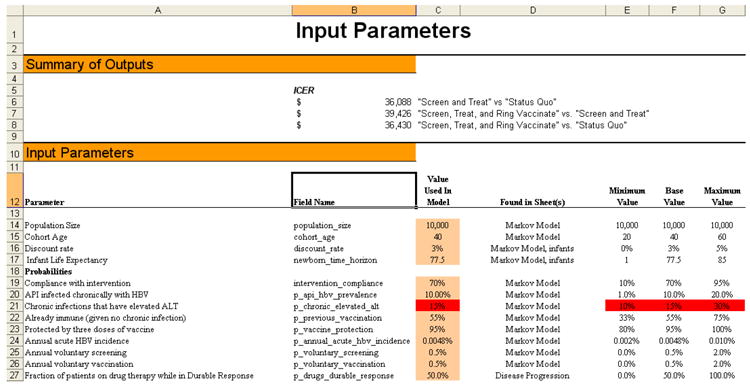
a: Input parameters screen shot
b: Policy results screen shot
Results of the Analyses
Hepatitis B Policy in the United States
We analyzed several alternative programs for hepatitis B screening and vaccination of adult Asian and Pacific Islanders in the United States (Hutton et al. 2007). We examined the status quo and four clinically relevant strategies: a universal vaccination strategy in which all individuals are vaccinated; a screen-and-treat strategy in which individuals are given blood tests to determine whether they are chronically infected with hepatitis B; a screen, treat, and ring vaccinate strategy in which all individuals are given blood tests to determine whether they are chronically infected, and close contacts of persons found to be infected are also screened and vaccinated if needed; and a screen, treat, and vaccinate strategy in which all individuals are screened to determine whether they are chronically infected or should be vaccinated, and are then vaccinated if needed. In all cases, identified chronically infected persons are monitored and treated.
We found that it is cost-effective to screen adult Asian and Pacific Islanders for chronic hepatitis B infection so that they can receive treatment; and it is cost-effective to vaccinate those in close contact with the infected individuals so that they can be protected from infection. We estimated that it costs about $36,000-$40,000 per QALY gained to screen adult Asian and Pacific Islanders for hepatitis B and provide treatment for them. An intervention that costs less than about $50,000 to $100,000 per QALY gained is generally considered to be a cost-effective intervention in the United States (Murray and Lopez 2002, Owens 1998, World Health Organization 2009). Interestingly, we did not find it to be cost-effective to provide universal vaccination to all adult Asian and Pacific Islanders in the United States. This is because the risk of being exposed to hepatitis B for adults in the United States is low and, if exposed to the virus, adults are highly likely to resolve the infection.
The analysis showed that the cost-effectiveness is mainly driven by the cost of treatment, and that the cost of screening to identify infected individuals is of much less importance. For the policies that involved screening and treatment, the total costs of treating individuals identified as being chronically infected with hepatitis B were more 100 times greater than the costs of the initial screening program. Our sensitivity analyses showed that screening is also cost-effective in population groups with much lower prevalence of the disease – for example, in population groups where the prevalence of chronic hepatitis B infection is only 2 percent.
Hepatitis B Policy in China
To analyze the cost-effectiveness of implementing catch-up hepatitis B vaccination for children and adolescents in China, we examined the status quo (which assumes current levels of vaccination coverage) and a strategy of catch-up hepatitis B vaccination for children and adolescents who missed newborn vaccination (Hutton et al. 2010). We calculated the costs and health effects for various aged cohorts of 10,000 hypothetical children. We found that catch-up vaccination improves health outcomes and also saves costs for children up to age 19. For a cohort of 10,000 children aged 1-19, we estimate that more than 4,000 children would receive vaccinations, and this would avert almost 200 hepatitis B infections and save $21,000 over the lifetime of the children in the cohort.
We conducted sensitivity analyses using the model and determined that catch-up vaccination might not be cost-saving if the chance of a child becoming infected is one-fifth as high as our base case estimate of 100/100,000 per year. This may be the case in certain urban areas of China that have achieved sustained high levels of newborn vaccination coverage. Additionally, if treatment becomes cheaper and more effective in the future, the benefit of vaccination will be lower. However, we determined that treatment costs would have to be halved (to approximately $1,000 per year) and infection risk would have to be five times lower than in the base case before the cost of catch-up vaccination would exceed $2,500 per QALY gained (an amount roughly equal to the GDP per capita in China); above this cost, catch-up vaccination could be considered cost-effective but would no longer be considered highly cost-effective according to international standards for cost-effectiveness (Murray and Lopez 2002, World Health Organization 2009).
Dissemination and Impact
The overriding goal of our work was to publish and disseminate our results in a way that would enable our analyses to have real impact on health policy, both in China and the United States. In public health, there is typically no single decision maker; rather, public health policy is arrived at through consensus among multiple decision makers, including lawmakers, public health officials, nongovernmental agencies, and healthcare providers (Kahn et al. 1998). Thus, dissemination of research results in order to effectively influence policy is a multi-pronged effort that involves publication of results in widely read health and medical journals and presentation of results to a broad variety of policy makers.
Hepatitis B Policy in the United States
As we worked through our analysis of screening, treatment, and vaccination of adult Asian and Pacific Islanders in the United States, we shared our model and results directly with policymakers at the Centers for Disease Control and Prevention (CDC). We spent time with analysts at the CDC describing our assumptions and explaining the model’s results. This process provided us with valuable suggestions for improving the model and analysis, and helped them develop confidence in our findings. We published our findings in a widely read general medical journal, Annals of Internal Medicine (Hutton et al. 2007), so that the general physician community could see our results and become aware of the need to test their Asian and Pacific Islander patients for hepatitis B. Publishing our results in a top medical journal, and personally explaining the workings and results of our model to experts at the CDC, helped convince the CDC to update their recommendations. Consistent with our findings, the CDC’s most recent hepatitis B screening guidelines recommend screening all adult Asian and Pacific Islanders for hepatitis B as well as all adults born in areas of intermediate (2-7 percent) hepatitis B prevalence (Weinbaum et al. 2009, Weinbaum et al. 2008).
The changed screening policy could have significant public health impact. In the United States, there are about 9.2 million adult Asian and Pacific Islanders. With about 10 percent of this group chronically infected with hepatitis B and two of three unaware of their infection, there are more than 600,000 people who could benefit from knowing their chronic hepatitis B infection status. If all of these people knew they were infected and received appropriate medical monitoring and treatment, almost 50,000 premature deaths caused by hepatitis B would be averted. Although it may not be possible to test and treat every single person indicated under the new CDC guidelines, our hope is that the new guidelines will encourage physicians to test and treat their Asian and Pacific Islander patients as well as patients born in countries such as Egypt and Somalia where hepatitis B prevalence is above 2 percent. Such screening (and subsequent treatment) is an important step toward eliminating a major health disparity in the United States.
Hepatitis B Policy in China
We published the results of our catch-up vaccination analysis in the internationally recognized journal on liver disease, Hepatology (Hutton et al. 2010). Subsequently, the journal selected the article for translation into Chinese so that it can reach a broader audience in Asia. While conducting our analysis, we shared our interim results in the fall of 2008 with academics in China, members of China’s Center for Disease Control and Prevention, and members of the World Health Organization. At the time, China was considering providing free hepatitis B catch-up vaccination for children and adolescents. One area of concern for them was the cost-effectiveness of the program, since they have many competing health interventions and are concerned about value for their health funds that are spent. Our analysis of the cost-effectiveness of the program influenced their recent decision in April 2009 to expand free hepatitis B catch-up vaccination to all children in China under the age of 15.
We estimate that providing catch-up hepatitis B vaccination for all children under the age of 15 in China would involve vaccinating almost 170 million children, and would prevent almost 8 million acute infections, 400,000 chronic infections, and almost 70,000 deaths due to hepatitis B. This would cost the equivalent of $540 million US dollars. However, it would save the equivalent of $1.4 billion US dollars over the lifetime of these children (net present value, discounted at 3 percent annually), for a net present savings of approximately $900 million US dollars. Additionally, by preventing infection with hepatitis B, such catch-up vaccination will spare hundreds of thousands of children in China from a lifetime of discrimination.
Ongoing Hepatitis B Policy Projects
Our success in examining these programs has helped us gain support for new projects related to hepatitis B prevention and control. Partially due to the success of our work on screening Asian and Pacific Islander adults, Dr. So and Prof. Brandeau were selected to participate in an Institute of Medicine Committee examining prevention and control of viral hepatitis infections in the United States (Institute of Medicine of the National Academies 2009). The committee examined public health strategies to combat hepatitis B and hepatitis C (another viral infection that causes liver disease). We used our model several times to provide analyses to inform the committee’s recommendations (Institute of Medicine Committee on Prevention and Control of Viral Hepatitis Infections in the United States 2010). For example, analyses we carried out with our model provided evidence to the committee that it is not cost-effective to vaccinate the general adult population in the United States against hepatitis B infection.
We are continuing to share the results of our analysis with policy makers from the World Health Organization in hopes of expanding hepatitis B catch-up vaccination to other similarly affected countries in the region, such as Vietnam and Thailand, and we are working with decision makers to analyze new programs to combat hepatitis B both in the United States and abroad. Approximately one-third of San Franciscans are of Asian descent (United States Census Bureau 2008) and there is a high rate of liver cancer due to hepatitis B in the city (Chang et al. 2007). The SF Hep B Free initiative, a collaborative undertaking by more than 50 healthcare groups, aims to provide free or low-cost testing and vaccination to all adult Asian and Pacific Islanders in San Francisco (San Francisco Hep B Free Campaign 2009). Their ultimate goal is “to turn San Francisco into the first hepatitis B free city in the nation” (San Francisco Hep B Free Campaign 2009). We are working with California Pacific Medical Center (CPMC), one participant in the SF Hep B Free initiative, to evaluate the cost-effectiveness of the program. Over a 13-month period, CPMC screened 1,885 Asian and Pacific Islanders, identifying 88 chronically infected individuals who can now receive appropriate medical attention (Tana et al. 2009). Although CPMC has thus far identified fewer chronically infected individuals than originally expected, our analysis shows that the program is nonetheless a cost-effective use of public health funds (Tana et al. 2009).
Knowledge of hepatitis B infection among vulnerable populations is poor, both in the United States and in China: infected individuals may not know of their chronic infection or about the availability of lifesaving treatments, and uninfected individuals may not know about vaccination and possible routes of transmission (Ma et al. 2007, Ma et al. 2008, Wu et al. 2007). In many cases, healthcare providers also have poor knowledge of hepatitis B: they may not know which groups are at highest risk of hepatitis B infection, and may fail to provide screening and/or vaccination to those high-risk individuals (Chao et al. 2009, Ferrante et al. 2008, Lai et al. 2007). In conjunction with the Asian Liver Center, we are currently implementing and evaluating a CDC-funded pilot program in the San Francisco Bay Area that aims to reduce perinatal transmission of hepatitis B by providing educational materials to increase knowledge of hepatitis B among pregnant women and their healthcare providers. With proper screening procedures, chronically infected women can be identified before they give birth; and with proper immunization procedures, newborns of these mothers will receive a birth dose of the hepatitis B vaccine within 24 hours of birth, thus avoiding infection with hepatitis B. We are also working with public health officials from Shandong Province, China to develop similar educational interventions for healthcare providers there. If successful (and cost-effective), we hope that such programs will eventually be expanded nationally, both in the United States and China.
Discussion
We have successfully used novel operations research models to assist policy makers in the United States and China in improving health policies related to hepatitis B. They have changed policy in the United States to encourage screening of millions of high-risk people and have enacted guidelines in China to provide free catch-up vaccination for hundreds of millions of children. These policies are an important step in eliminating health disparities, reducing discrimination, and ensuring that millions of people who need it can now receive hepatitis B vaccination or lifesaving treatment.
Three keys to the success of our projects were the creation of a multidisciplinary team with the appropriate expertise, the development of a model with the right level of complexity for the problem and in a modeling platform that is accessible to policy makers, and extensive outreach to and connections with policy makers. Collectively, our research team had expertise in OR modeling, cost-effectiveness modeling, data analysis, hepatitis B infection, and hepatitis B policy in the United States and China – all skills that were needed to effectively carry out our projects. Our models were complex enough to provide sufficient realism for clinical experts, but simple enough so that our assumptions and results could be successfully communicated to clinicians, journal reviewers, and public health officials. We also used a modeling platform, Microsoft Excel, which is familiar to most of our policymaking audience. Having an understandable model with the appropriate level of detail was critical to gaining traction with policymakers. Most importantly, we worked with public health officials in the United States and China over a period of several years to understand their concerns and priorities and to communicate the findings from our analyses. It is only through sustained efforts of this type that policy changes can be effected.
We are gratified that our analyses have been able to influence national and international health policy and have led to changes that can improve the lives of millions of people and help save thousands of lives. We hope that projects such as ours can raise awareness in the medical community of the insight into complex health policy questions that operations research methods can provide, and the value of such analyses. Working together with policy makers and public health officials, operations researchers can indeed “do good with good operations research.”
Acknowledgments
For their valuable input and suggestions during the projects, the authors thank Dr. Cynthia Weinbaum and Dr. Eric Mast from the US Centers for Disease Control and Prevention; Dr. John Wong from the Tufts New England Medical Center; the China Foundation for Hepatitis Prevention and Control; Professor Ji-Dong Jia from the Capital University of Medical Science in Beijing and president of the Asian Pacific Association in the Study of the Liver; Dr. Stephen Hadler, Medical Officer in the World Health Organization and China-GAVI program officer, Beijing, China office; and Dr. Ellen Chang of the Northern California Cancer Center and the Asian Liver Center at Stanford University. David Hutton is supported by a Stanford Graduate Fellowship and Grant Number R18PS000830 from the US Centers for Disease Control and Prevention. Samuel So is supported by Grant Number R18PS000830 from the US Centers for Disease Control and Prevention, R21CA122317 from the National Institute of Health, and CP1MP071051 from the Office of Minority Health. Margaret Brandeau is supported by Grant Number R01-DA15612 from the National Institute on Drug Abuse and Grant Number R18PS000830 from the US Centers for Disease Control and Prevention.
Contributor Information
David W. Hutton, Email: david.hutton@stanford.edu.
Margaret L. Brandeau, Email: brandeau@stanford.edu.
Samuel K. So, Email: samso@stanford.edu.
References
- American Cancer Society California Division. California Cancer Facts and Figures 2008. 2008 Retrieved October 30, 2009, http://www.ccrcal.org/PDF/ACS2008.pdf.
- Centers for Disease Control and Prevention. Screening for chronic hepatitis B among Asian/Pacific Islander populations--New York City, 2005. Morbidity and Mortality Weekly Rep. 2006;55(18):505–9. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Progress in hepatitis B prevention through universal infant vaccination--China, 1997-2006. Morbidity and Mortality Weekly Rep. 2007;56(18):441–5. [PubMed] [Google Scholar]
- Chang ET, Keegan THM, Gomez SL, Le GM, Clarke CA, So SK, Glaser SL. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109(10):2100–2108. doi: 10.1002/cncr.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S, Lee PV, Prapong W, Su J, So SK. High prevalence of chronic hepatitis B (HBV) infection in adult Chinese Americans living in California. Hepatology. 2004;40(Suppl 1):717A. Abstract. [Google Scholar]
- Chao SD, Cheung C, Yue A, So SK. Low hepatitis B knowledge among perinatal healthcare providers serving county with nation’s highest rate of births to mothers chronically infected with hepatitis B. Presented at 13th International Symposium on Viral Hepatitis and Liver Disease; March 20-24, 2009; Washington, DC. 2009. [Google Scholar]
- Chen JJ. A model HBV catch-up immunization and education project in Qinghai, China. National Immunization Conference, Centers for Disease Control and Prevention. 2008 Retrieved September 9, 2009, http://cdc.confex.com/cdc/nic2008/techprogram/P15494.HTM.
- China Daily. Nearly 80 percent foreign companies won’t hire hepatitis B carriers. 2007 Retrieved October 29, 2009, http://www.chinadaily.com.cn/bizchina/2007-06/28/content_5421170.htm.
- Chinese Ministry of Health. The Ministry of Health conference on planning and hepatitis B immunization, malaria prevention and control work [Chinese] 2008 Retrieved August 1, 2008, http://www.gov.cn/xwfb/2008-04/21/content_950425.htm.
- Cotler SJ, Dhamija MK, Siqueira F, Bartram AH, Luc BJ, Layden TJ, Wong SS. Hepatitis B seroprevalence and disease characteristics in an urban Chinatown community. Clinical Gastroenterol Hepatology. 2009;7(7):776–80. doi: 10.1016/j.cgh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clinical Gastroenterol. 2004;38(10 Suppl):S158–68. doi: 10.1097/00004836-200411003-00008. [DOI] [PubMed] [Google Scholar]
- Drummond MF, O’Brien BJ, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press; Oxford: 2005. [Google Scholar]
- Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253(1337):197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- Ferrante JM, Winston DG, Chen PH, de la Torre AN. Family physicians’ knowledge and screening of chronic hepatitis and liver cancer. Family Medicine. 2008;40(5):345–351. [PubMed] [Google Scholar]
- Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; Oxford: 1996. [Google Scholar]
- Guane R, Siu P, Lam K, Kim KE, Warren V, Liu H, et al. Prevalence of HBV and risk for HBV acquisition in hepatitis B screening programs in large metropolitan cities in the United States. Hepatology. 2004;40(S4):716A. Abstract. [Google Scholar]
- Hillier FS, Lieberman GJ. Introduction to Operations Research. McGraw-Hill; New York: 2005. [Google Scholar]
- Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology. 2010;51(2):405–414. doi: 10.1002/hep.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Internal Medicine. 2007;147(7):460–9. doi: 10.7326/0003-4819-147-7-200710020-00004. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Prevention and Control of Viral Hepatitis Infections in the United States. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. National Academies Press; Washington, DC: 2010. [Google Scholar]
- Institute of Medicine of the National Academies. Prevention and Control of Viral Hepatitis Infections in the United States. 2009 Retrieved September 9, 2009, http://iom.edu/CMS/3793/59310.aspx.
- Jacobs RJ, Saab S, Meyerhoff AS. The cost effectiveness of hepatitis immunization for US college students. J Amer College Health. 2003;51(6):227–236. doi: 10.1080/07448480309596355. [DOI] [PubMed] [Google Scholar]
- Kahn JG, Brandeau ML, Dunn-Mortimer J. OR modeling and AIDS policy: From theory to practice. Interfaces. 1998 May-June;28:3–22. [Google Scholar]
- Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: A cost-effectiveness analysis. Ann Internal Medicine. 2005;142(10):821–31. doi: 10.7326/0003-4819-142-10-200505170-00007. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Nguyen TT, Hwang J, Stewart SL, Kwan A, McPhee SJ. Provider knowledge and practice regarding hepatitis B screening in Chinese-speaking patients. J Cancer Educ. 2007;22(1):37–41. doi: 10.1007/BF03174373. [DOI] [PubMed] [Google Scholar]
- Lee HO, Hontz I, Warner A, Park SJ. Hepatitis B infection among Asian American Pacific Islanders in the Rocky Mountain area. Appl Nurs Res. 2005;18(1):2–6. doi: 10.1016/j.apnr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: A cross-sectional study of 3,163 Asians in California. Hepatology. 2007;46(4):1034–40. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369(9573):1582–1583. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- Ma GX, Fang CY, Shive SE, Toubbeh J, Tan Y, Siu P. Risk perceptions and barriers to hepatitis b screening and vaccination among Vietnamese immigrants. J Immigrant Minority Health. 2007;9(3):213–220. doi: 10.1007/s10903-006-9028-4. [DOI] [PubMed] [Google Scholar]
- Ma GX, Shive SE, Toubbeh JI, Tan Y, Wu D. Knowledge, attitudes, and behaviors of Chinese [regarding] hepatitis b screening and vaccination. Amer J Health Behav. 2008;32(2):178–187. doi: 10.5555/ajhb.2008.32.2.178. [DOI] [PubMed] [Google Scholar]
- Margolis HS, Coleman PH, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization: An economic analysis of current recommendations. J Amer Medical Assoc. 1995;274(15):1201–1208. [PubMed] [Google Scholar]
- McQuillan GM, Coleman PJ, Kruszon-Moran D. Prevalence of hepatitis B virus infection in the United States: The National Health and Nutrition Examination Surveys, 1976 through 1994. Amer J Public Health. 1999;89(1):14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muennig P. Cost-Effectiveness Analyses in Health: A Practical Approach. Jossey-Bass; San Francisco: 2008. [Google Scholar]
- Murray CJ, Lopez A. World Health Report 2002: Reducing risks, promoting healthy life. World Health Organization; Geneva: 2002. [Google Scholar]
- Owens DK. Interpretation of cost-effectiveness analyses [Editorial] J General Internal Medicine. 1998;13(10):716–717. doi: 10.1046/j.1525-1497.1998.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- People’s Daily Online. Chinese, U.S. agencies help hepatitis B prevention in northwest China. 2006 Retrieved November 18, 2008, http://english.peopledaily.com.cn/200609/11/eng20060911_301654.html.
- Pisu M, Meltzer MI, Lyerla R. Cost-effectiveness of hepatitis B vaccination of prison inmates. Vaccine. 2002;21(3-4):312–321. doi: 10.1016/s0264-410x(02)00457-7. [DOI] [PubMed] [Google Scholar]
- Rajendra A, Wong JB. Economics of chronic hepatitis B and hepatitis C. J Hepatology. 2007;47(4):608–617. doi: 10.1016/j.jhep.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Reuters. China’s hepatitis B carriers face gloomy future. 2008 Retrieved October 29, 2009, http://www.reuters.com/article/healthNews/idUST25713820080319.
- San Francisco Hep B Free Campaign. San Francisco Hep B Free. 2009 Retrieved September 9, 2009, http://sfhepbfree.org/
- Shepherd J, Jones J, Takeda A, Davidson P, Price A. Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: A systematic review and economic evaluation. Health Technol Assess. 2006;10(28):1–200. doi: 10.3310/hta10280. [DOI] [PubMed] [Google Scholar]
- Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Medical Virology. 2002;67(3):447–50. doi: 10.1002/jmv.10094. [DOI] [PubMed] [Google Scholar]
- Tana M, Hoda K, Hutton DW, Wong J, Wei Y, Liu W, Hinojales N, Lykins P, et al. HBV screening in Asians and Pacific Islanders: An interim cost-effectiveness analysis of a single-center San Francisco program. Working paper, California Pacific Medical Center, Department of Gastroenterology and Hepatology; San Francisco: 2009. [Google Scholar]
- The Global Fund to Fight AIDS Tuberculosis and Malaria. 2010 Retrieved March 12, 2010, http://www.theglobalfund.org/en/
- United States Census Bureau. “San Francisco city”. 2005-2007 American Community Survey 3-Year Estimates. 2008 Retrieved September 9, 2009, http://factfinder.census.gov/servlet/ACSSAFFFacts?_event=ChangeGeoContext&geo_id=16000US0667000&_geoContext=01000US|04000US06|16000US0665028&_street=&_county=san+francisco&_cityTown=san+francisco&_state=04000US06&_zip=&_lang=en&_sse=on&ActiveGeoDiv=geoSelect&_useEV=&pctxt=fph&pgsl=010&_submenuId=factsheet_1&ds_name=ACS_2007_3YR_SAFF&_ci_nbr=null&qr_name=null®=null:null&_keyword=&_industry=
- Weinbaum CM, Mast EE, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology. 2009;49(5 Suppl):S35–44. doi: 10.1002/hep.22882. [DOI] [PubMed] [Google Scholar]
- Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Morbidity and Mortality Weekly Rep. 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- World Health Organization. WHO Disease and injury country estimates. 2004 Retrieved October 30, 2008, http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html.
- World Health Organization. Malaria annual data. 2006 Retrieved November 18, 2008, http://www.wpro.who.int/sites/mvp/data/malaria/2006.htm.
- World Health Organization. Epidemiological fact sheet on HIV and AIDS: China. 2008a Retrieved November 18, 2008, http://www.who.int/globalatlas/predefinedreports/EFS2008/full/EFS2008_CN.pdf.
- World Health Organization. Global tuberculosis control - Surveillance, planning, financing. 2008b Retrieved November 18, 2008, http://www.who.int/tb/publications/global_report/2008/pdf/annex_3.pdf.
- World Health Organization. Hepatitis B. Fact sheet no. 204. 2008c Retrieved October 29. 2009, http://www.who.int/mediacentre/factsheets/fs204/en/
- World Health Organization. Threshold values for intervention cost-effectiveness by region. 2009 Retrieved November 4, 2009, http://www.who.int/choice/costs/CER_levels/en/index.html.
- Wu CA, Lin SY, So SK, Chang ET. Hepatitis b and liver cancer knowledge and preventive practices among Asian Americans in the San Francisco Bay Area, California. Asian Pacific J Cancer Prev. 2007;8(1):127–134. [PubMed] [Google Scholar]


