Abstract
In all vertebrates the cell nucleus becomes highly condensed and transcriptionally inactive during the final stages of red cell biogenesis. Enucleation – the process by which the nucleus is extruded by budding off from the erythroblast – is unique to mammals. Enucleation has critical physiological and evolutionary significance in that it allows an elevation of hemoglobin levels in the blood and also gives red cells their flexible biconcave shape. Recent experiments reveal that enucleation involves multiple molecular and cellular pathways that include histone deacetylation, actin polymerization, cytokinesis, cell-matrix interactions, specific microRNAs, and vesicle trafficking; many evolutionarily conserved proteins and genes have been recruited to participate in this uniquely mammalian process. Here, we review recent advances in mammalian erythroblast chromatin condensation and enucleation, and conclude with our perspectives on future studies.
Mammalian erythroblast enucleation
Red blood cells are continuously replenished; in humans their half-life is 120 days. Erythropoietin (Epo), a cytokine produced by the kidney in response to low oxygen pressure, is the principal regulator of red blood cell production. Epo binds to cognate receptors on Colony Forming Unit Erythroid (CFU-E) progenitor cells and activates the JAK2 protein tyrosine kinase and several downstream signal transduction pathways. These prevent CFU-E apoptosis and stimulate its terminal proliferation and differentiation; during the ensuing three days each CFU-E produces 30–50 enucleated reticulocytes that are released into the circulation. The first phase of CFU-E erythroid differentiation is highly Epo dependent, whereas later stages are no longer dependent on Epo but are enhanced by adhesion of erythroblasts to a fibronectin substratum [1]. Consistent with this, Epo receptors are lost as erythroid progenitors undergo terminal proliferation and differentiation [2].
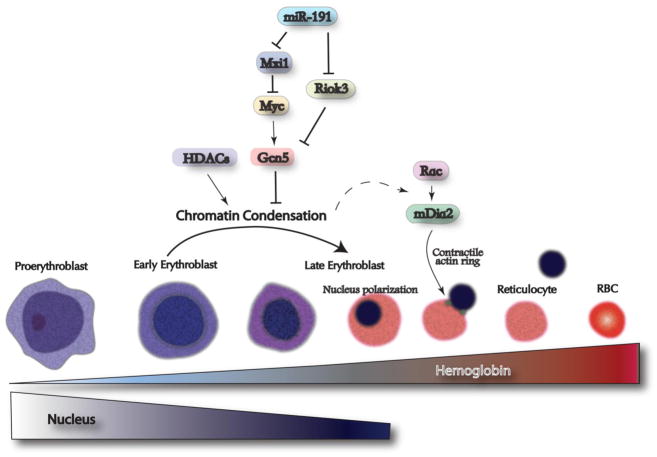
As judged by high throughput mRNA sequencing, expression of about 600 genes increases greater than two-fold during terminal erythroid differentiation ( Wong P, Hattangadi S, and Lodish H, unpublished data). These include many erythroid-important genes including α and β globins, heme biosynthetic enzymes, erythroid membrane and cytoskeletal proteins, and erythroid-important transcription factors. Concomitantly the volume of the nucleus gradually decreases, ultimately becoming about 1/10th its original volume with highly condensed chromatin (Figure 1). Although in all vertebrates the erythrocyte nucleus becomes highly condensed, only mammalian erythroblasts enucleate (Box 1). Red cells in many non-mammalian vertebrates contain a special linker histone H5 that is not seen in mammals [3–5].
Figure 1.
Enucleation of mammalian erythroblast requires multiple molecular and cellular pathways. During mammalian erythroblast differentiation, the chromatin gradually condenses while the hemoglobin concentration gradually increases. Chromatin condensation involves histone deacetylation that is controlled by up-regulation of histone deacetylases (HDACs) and down-regulation of histone acetyltransferases (Gcn5). Gcn5 is directly regulated by c-myc, whose level gradually decreases during erythropoiesis. In addition, miR-191, an erythroid specific microRNA, targets Mxi1 and Riok3 to regulate c-myc and Gcn5, respectively. Subsequently, chromatin condensation may provide unknown signals to activate the Rac-GTPases-mDia2 pathway, which is required for contractile actin ring formation and enucleation.
Box 1. Vertebrate erythrocytes and evolution of mammalian erythroblast enucleation.
Erythroid cells in all vertebrates undergo gradual chromatin condensation during erythropoiesis. The mature circulating red blood cells vary in size across the vertebrates, ranging from over 50μM in diameter in certain species of amphibians to less than 10μM in mammals [73]. The size of the circulating red blood cells correlates with the diameter of capillaries, as well as the capacity of the vertebrate heart to pump blood to the extremities. In mammals, the presence of four heart ventricles enables sufficient blood flow through the microcirculation, where many capillaries possess a diameter less than that of mature red blood cells. Therefore, circulating mammalian red blood cells must change or deform their shape in order to migrate through these small capillaries. Enucleation is hypothesized to have been selected for during mammalian evolution in order to enhance blood cell circulation and prevent possible blockage of small capillaries by deformed red cells. The lack of a nucleus is also thought to provide more intracellular space for hemoglobin. However, blood hemoglobin content and mean cell hemoglobin concentration are similar between mammals and birds [73], which could be due to the fact that in birds hemoglobin is also localized in the nuclear space [73].
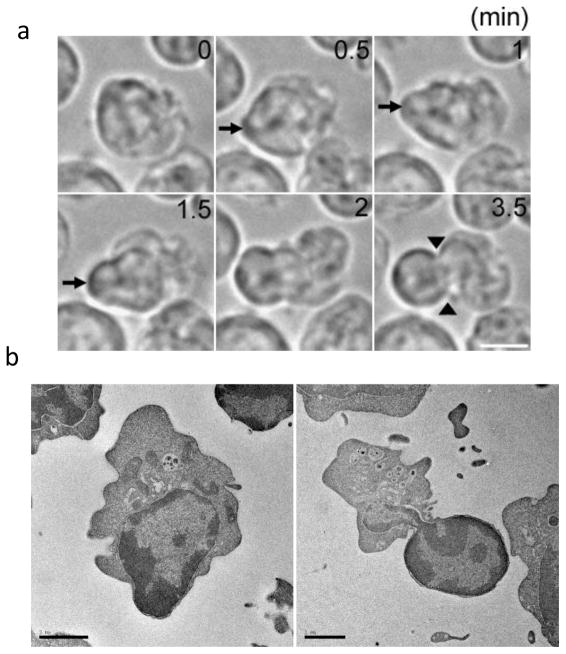
Our understanding of mammalian erythroid enucleation has increased significantly since morphological studies decades ago [6–11]. Time-lapse live-cell imaging, for instance, shows that the erythroblast quickly extrudes its nucleus, surrounded by and closely apposed to the plasma membrane through a bleb-like structure from a limited area of the cortex adjacent to the nucleus (Figure 2a). Notably, the nucleus becomes largely deformed during nuclear extrusion and the outer nuclear membrane becomes closely apposed to the plasma membrane (Figure 2b), suggesting a possible requirement for remodeling of the nuclear envelop prior to enucleation. The process is completed by a type of cytokinesis that requires actin and other cytoskeletal proteins discussed later. Here, we discuss the many recent advances in understanding mammalian erythroblast chromatin condensation and enucleation, and conclude with a discussion of future studies that can clarify our understanding of these processes.
Figure 2.
During enucleation the nucleus is rapidly squeezed out from the cell, forming a bleb-like structure. (a) Time-lapse live-cell imaging of late erythroblasts undergoing nuclear extrusion. The nucleus is quickly squeezed out from a limited area of the cortex facing the nucleus, forming the bleb-like structure (arrows). Note that the nucleus becomes largely deformed during enucleation, and that a cytokinetic-like furrow is observed at the neck region of the bleb-like protrusion (arrowheads). Scale bar, 5 μm.(b) Electron microscope images of late erythroblasts undergoing enucleation in an in vitro culture system. Shown are late erythroblasts at early (left) and late (right) stages of nuclear extrusion. These images show deformed nuclei and cytokinesis-like furrows similar to what has been seen in erythroid cells from mice [10], indicating that late erythroblasts in the in vitro culture system reproduce the in vivo situation. The images were taken by T. Ramirez and M. Murata-Hori. Scale bar, 2 μm.
Chromatin condensation and apoptosis
During mammalian erythropoiesis the chromatin gradually undergoes condensation; nuclear and chromatin condensation are thought to be critical for enucleation. Chromatin condensation occurs during other cellular processes such as apoptosis and apoptotic mechanisms are known to play important roles in erythropoiesis (reviewed in [12]). Apoptotic mechanisms have been implicated in the loss of the nucleus in mammalian lens epithelia and keratinocytes [13, 14], although these processes are morphologically dissimilar to erythroid cell enucleation.
shRNA knockdown of caspase 3 in human erythroid cells leads to a significant reduction in enucleated cells with no change in the fraction of apoptotic cells [15]. However, this phenotype could be a consequence of the blockage of erythroid differentiation from pronormoblasts to basophilic normoblasts after down-regulation of caspase 3 [15, 16]. In addition, treatment of mouse spleen erythroblasts with caspase inhibitors fails to block enucleation [17]. Furthermore, there is no evidence for caspase-induced cleavage of target proteins during the late stages of erythropoiesis [18]. These results indicate that erythroid chromatin condensation and enucleation may not require caspases or other apoptotic proteins.
Modifications of the flexible “tails” of several histones do play important roles in the structure and stability of chromatin. For example, histone acetylation by histone acetyl transferases (HATs) tends to destabilize chromatin, which makes the DNA more accessible to transcription factors. In contrast, histone deacetylation by histone deacetylases (HDACs) stabilizes chromatin and is critical for heterochromatin formation. A recent study demonstrated that global levels of several acetylated histones drop during the differentiation of cultured mouse fetal liver erythroblasts, especially H3K9Ac, H4K5Ac, H4K12Ac, and H4K8Ac [19]. Furthermore, the level of Gcn5 protein, a histone acetyltransferase, gradually decreases during erythropoiesis. Ectopic expression of Gcn5, which makes chromatin more open, partially blocks chromatin condensation and subsequent enucleation [19]. In addition, Gcn5 is transcriptionally activated by c-Myc, whose level also gradually decreases during erythropoiesis. Expression of c-Myc at physiological levels during the terminal stages of erythropoiesis also blocks chromatin condensation and enucleation [19].
Consistent with this result, histone deacetylase 2 (HDAC2), whose level gradually increases during erythropoiesis, is required for chromatin condensation and enucleation; pharmacological inhibition of HDAC2 activity or shRNA knockdown of HDAC2 blocks chromatin condensation and enucleation of cultured mouse fetal erythroblasts [20]. Similarly, treatment of Friend-virus infected murine spleen erythroblasts with histone deacetylase inhibitors blocks enucleation [21]. These studies establish the critical role of inhibiting histone acetylation and activating histone deacetylation in terminal erythroid cell chromatin condensation and enucleation. We do not know whether the histones that are deacetylated are localized in specific gene or chromosome regions or are dispersed throughout the genome.
Membrane and cytoskeletal proteins that mediate enucleation and asymmetrical cell division
Early studies indicated that enucleation involves a process of cytokinesis involving several membrane and cytoskeleton proteins [9, 10, 22, 23]. Mature red cells possess a unique submembrane cytoskeleton that maintains the stability and flexibility characteristic of these cells. Essentially, the heterodimeric α and β-spectrins self-associate into α2 β2 tetramers, which are connected by actins to form pentagonal or hexagonal lattices [24]. Ankyrin-protein 4.2 and protein 4.1-adducin complexes further mediate the interaction of the spectrin-actin network with plasma membrane proteins Band 3 (also called Anion Exchanger 1, AE1) and glycophorin, respectively. Altogether, these membrane and cytoskeletal proteins ensure the integrity of the mature erythrocyte membrane (reviewed in [25]).
Functional disruptions of these proteins in humans can lead to hereditary spherocytosis (HS), hereditary elliptocytosis (HE) or hereditary pyropoikilocytosis (HPP) [26]. In light of the significance of these proteins, recent work showed that the number of normoblasts arrested at the stage of nuclear extrusion dramatically increases when a rat is treated with colchicine, a microtubule-disrupting substance [23]. Additionally, the F-actin inhibitor cytochalasin D causes a complete inhibition of enucleation that could be reversed by washing out the cytochalasin D [22]. Furthermore, studies using yolk sac-derived erythroblasts showed that actin and myosin become accumulated at the area between the extruding nucleus and incipient reticulocyte, the so-called cortical actin ring [27].
One initial step in the enucleation process is that late erythroblasts establish a polarization such that the nucleus is displaced to one side of the cytoplasm (Figure 2; [9, 10]). Little is known about how this asymmetry is established. This process may involve extensive cytoskeletal and membrane changes, including changes in the microtubule networks, and appears to share many features with those seen in migrating cells (Wang J, Ramirez T, and Murata-Hori M, unpublished data).
Rac GTPases play essential roles in enucleation through the activation of a downstream target, the formin mDia2, and subsequent formation of the contractile actin ring (Figure 1). Deregulation of Rac GTPases during the late stages of erythropoiesis completely blocked enucleation of cultured mouse fetal erythroblasts without affecting normal proliferation and differentiation [28]. The contractile actin ring formed on the plasma membrane of the late-stage erythroblasts at the boundary between the cytoplasm and the nucleus of enucleating cells was disrupted when Rac GTPases were inhibited in the late stages of erythropoiesis. The activities of Rac GTPases are mediated by the downstream target protein, mDia2, a formin protein required for nucleation of unbranched actin filaments. These results reveal important roles for Rac GTPases and mDia2 in enucleation of mammalian erythroblasts [28] (Figure 1). Many other membrane and cytoskeletal proteins undergo reorganization during terminal erythropoiesis. Prior to and during enucleation, spectrin, actin, tubulin, ankyrin, glycophorin A and protein 4.1 all partition to the incipient reticulocyte, whilst no major erythroid membrane or cytoskeletal proteins are found in the plasma membrane surrounding the extruded nucleus [22, 29–31]. Temporal synthesis and degradation of individual membrane and cytoskeletal proteins are thought to play crucial roles in the reorganization process [30].
Questions remain regarding the dynamic aspects of membrane and cytoskeletal protein reorganizations during erythropoiesis and little is known of the contribution of these changes to enucleation. These reorganizations may facilitate the enucleation process, because morphological studies imply that the nucleus in the late stages of erythropoiesis moves to a patch of membrane that is deficient in the spectrin network [29]. However, mice bearing mutations in or deletions of α-spectrin, β-spectrin, ankyrin, band 3, protein 4.1, or protein 4.2 do not exhibit significant increases in circulating nucleated red blood cells [32–36]. In addition, fetal liver erythroblasts purified from these mice show no obvious defects of enucleation in in vitro culture (Ji P, and Lodish H unpublished data). It remains to be determined whether or not a deficiency of any these membrane or cytoskeletal proteins, individually or together, causes a drop in the rate or extent of enucleation.
Earlier studies using mammalian erythroid cell lines showed that the intermediate filament vimentin is absent or becomes rapidly lost during maturation [37, 38]. In contrast, in other vertebrates intermediate filaments appear to be important in anchoring the condensed nucleus and preventing it from being extruded [39, 40]. Hypothetically, depletion of vimentin or other intermediate filament proteins could contribute to nuclear displacement but not be sufficient for mammalian erythroblast enucleation. It is not clear whether other cytoskeleton proteins may be involved in this complex process.
There is still debate as to precisely how the reticulocyte and nucleus undergo final separation. The findings noted above concerning the cortical actin ring suggest a process akin to cytokinesis during asymmetric cell division. However, early electron micrographic studies revealed multiple vesicles forming in the region between the extruding nucleus and incipient reticulocyte [22]. Recent evidence suggests that endocytic vesicle trafficking is essential for enucleation. Inhibition of clathrin-dependent vesicle trafficking blocked enucleation of primary fetal liver erythroblasts without affecting normal differentiation or proliferation [41]. Clearly much needs to be learned about the role of cytoskeletal proteins and membranes in this process.
Erythropoietic microenvironment and enucleation
Studies in the 1970s showed that the extruded nucleus is enveloped by a portion of the plasma membrane with distinctive cell surface proteins different from those on the reticulocyte [29, 42]. The exoplasmic leaflet of the membrane enveloping the nucleus contains high levels of phosphatidylserine, which serves as a signal for macrophage engulfment; masking phosphatidylserine on the nucleus prevents macrophage phagocytosis of the extruded nuclei [17]. Correspondingly, the cell surface of mature red blood cells contains CD47, which serves as a “self” marker and prevents elimination by macrophages [43].
Macrophages also play important roles in the development of erythroid cells. Erythropoiesis in fetal liver and spleen and adult bone marrow is regulated by its microenvironment, a domain known as the “erythroblastic island” (see review [44]). Morphologically, the island contains central macrophages surrounded by a ring of erythroblasts. The central macrophages are also called nursing cells, in that they provide iron and developmental signals to the surrounding erythroblasts [45].
Many cell surface proteins have been implicated in the macrophage-erythroblast interaction. Among these, Emp (Erythroblast macrophage protein) seems to be particularly important for erythroblast enucleation. Emp is normally expressed on the cell surface of both macrophages and erythroblasts [46, 47]. Genetic ablation of Emp in the mouse leads to a dramatic increase of nucleated red blood cells in the peripheral blood, and no fetal liver erythroblastic islands are observed in Emp-deficient mice [48]. Importantly, a deficiency of Emp leads to a disruption of actin localization during terminal erythroblast differentiation [48]. It would be interesting to determine whether Emp plays any role in the Rac GTPases-mDia2 or other pathways in enucleation. However, primary erythroid progenitors do undergo differentiation and enucleation when cultured in the absence of macrophages or other non-erythroid cells [20].
Other proteins that are involved in the macrophage-erythroblast interaction include the tumor suppressor retinoblastoma (Rb) protein. Rb deficiency in the mouse fetal liver somehow prevents interactions between macrophages and erythroblasts and blocks erythroblast enucleation [49]. This is likely due to the fact that loss of Rb blocks normal macrophage differentiation, because wild type macrophages can bind to Rb-deficient erythroblasts and induce their enucleation [49]. However, Rb also plays a cell-intrinsic role in erythropoiesis, as loss of Rb blocks erythroblast differentiation at an early stage [50]. Clearly we need to learn more about the function of Rb and the genes it regulates in both erythroblasts and macrophages.
Mammalian erythroblasts also interact with other cellular and matrix components during development. In vitro studies indicated that enucleated murine erythroleukemia cells are significantly increased when cultured in fibronectin-coated dishes [51]. Fibronectin, through interactions with α4β1 integrin on the erythroblast cell surface, is also important for the survival of the late-stage erythroblast by preventing apoptosis [1]. How these interactions affect enucleation is unclear.
During embryogenesis, prior to “definitive” erythropoiesis in the fetal liver, red cell formation begins in the yolk sac with production of “primitive” erythrocytes that express embryonic globins and that retain their nuclei in the embryonic circulation [52]. It was long believed that circulating primitive erythroid cells never lose their nuclei and thus share morphological similarities with those of other vertebrates [53]. However, a recent report demonstrated that primitive mammalian erythroid cells do undergo enucleation; in contrast to definitive erythroid cells in the fetal liver, terminal differentiation and enucleation of primitive erythroid cells take place in the circulation [54]. This raises questions concerning how nucleated circulating primitive erythroid cells maintain their interactions with the microenvironment.
MicroRNAs in enucleation
MicroRNAs are a class of small RNAs that downregulate expression of specific target genes post-transcriptionally [55]. They play a wide variety of roles in hematopoiesis [56, 57], specifically in erythropoiesis [58]. Among these microRNAs, the miR-144/451 cluster is directly activated by the erythroid-important GATA1 transcription factor and is required for erythropoiesis [59]. Specifically, in Zebrafish miR-144 directly suppresses the levels of Klfd, an erythroid specific Kruppel-like transcription factor, to regulate α-hemoglobin synthesis [60]. On the other hand, miR-451 targets GATA2 mRNA to promote Zebrafish erythroid cell maturation [61]. Recent data from the miR-144/451 knockout mouse revealed that murine erythrocytes with miR-144/451 deficiency exhibit a cell autonomous impairment of late erythroblast maturation and increased susceptibility to oxidant damage [62].
In contrast, over-expression of miR-191, a microRNA that is normally down-regulated during erythropoiesis, blocks chromatin condensation and enucleation with minor effects on proliferation and differentiation [63]. Riok3 and Mxi1, two erythroid enriched and developmentally up-regulated genes, were found to be principal downstream targets of miR-191. Knocking down either of these two proteins mimicked the effects of miR-191 over-expression, blocking both chromatin condensation and enucleation. In this aspect, it is interesting to note that Mxi1 is an antagonist of c-Myc [64], whose level we noted earlier gradually decreases during erythropoiesis [19]. In addition, knockdown of either Mxi1 or Riok3 blocked Gcn5 down-regulation in erythroblasts [63]. This and other studies illustrate the importance of the miR-191-Mxi1-c-myc-Gcn5 and miR-191-RioK3-Gcn5 pathways in the regulation of terminal erythroid cell chromatin condensation (Figure 1). Indeed, the significance of this pathway needs to be further investigated, as do the roles of many other abundantly expressed microRNAs in erythropoiesis.
Concluding remarks
Enucleation of mammalian erythroblasts is a complex process that involves a series of morphological and structural changes. In this review we summarized current progress in understanding mammalian erythroid cell enucleation. Most of these studies utilized in vitro erythroblast culture systems, since the extruded nucleus is released into the culture medium and not immediately engulfed by macrophages. The Emp-deficient mouse might provide an intriguing system to study enucleation in vivo. However, it is not clear whether Emp plays a specific role in enucleation since the Emp-deficient mouse has significant amounts of immature proerythroblasts [48], which indicates that Emp is critical for early stages of erythropoiesis as well. Similar to Emp, Rac GTPases are also important for the early stage of erythropoiesis [65], in addition to their functions in enucleation [28]. These facts raise difficulties in associating proteins with chromatin condensation or enucleation per se, as any inhibition of early stages of erythroblast differentiation will likely reduce formation of enucleated reticulocytes. Furthermore, possible lethality associated with enucleation failure and the redundancy of proteins required for key steps in this complex pathway add to the dilemma of in vivo investigations. In this respect, it would be desirable to establish inducible knockout mouse models to study enucleation. Examples would be mice in which a gene is inactivated solely in late erythroblasts. SSignificant numbers of circulating nucleated red blood cells are seen when the human body is under chronic stress or in certain pathological processes [66]. Studies of available mouse models in these conditions might provide informative insights into the enucleation process.
Enhancing erythroblast enucleation has a practical significance clinically in producing red blood cells in culture for transfusion. Current practice in transfusion medicine faces constant difficulties in obtaining sufficient supplies of specific red blood cell subtypes [67]. Recently, ex vivo or in vitro production of human red blood cells from hematopoietic stem cells, embryonic stem cell, and induced pluripotent stem cells have been extensively explored [68–72]. In addition to the challenges of generating sufficient amount of cells for transfusion purposes, the reduced ability of these cultured cells to fully enucleate remains an obstacle. Future endeavors focusing on the efficiency of enucleation in late erythroblasts derived in culture from various types of stem or progenitor cells, as well as the understanding of the basic biology of red cell enucleation, will help in transferring these studies from bench to bedside.
Clearly other major questions concerning enucleation need to be addressed. First, the significance of the erythroid microenvironment, including macrophages, in enucleation needs to be further investigated. For example, as we discussed above, Emp deficiency may affect early stages of erythropoiesis, which in turn are required for later enucleation. Second, we do not know whether or how chromatin condensation triggers or facilitates the enucleation process. As an example, could chromatin condensation or a modified nuclear envelope induce the Rac GTPases-mDia2 pathway to promote enucleation? (Figure 1) Third, why is enucleation only seen in mammals? In “lower” vertebrates intermediate filaments prevent enucleation by anchoring the nucleus. Are other subcellular structures involved as well? Could loss of expression of these genes in erythroid cells during vertebrate evolution promote enucleation? Comparative genomic or proteomic studies across species would provide clues.
Acknowledgments
This study was supported by NIH grant P01 HL 32262 and a research grant from Amgen, Inc. to H.F.L.; and by intramural funds from the Temasek Life Sciences Laboratory to M. M.-H. P.J. is a recipient of a Leukemia and Lymphoma Society fellowship and a National Institutes of Health (NIH) Pathway to Independence Award (K99/R00).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eshghi S, et al. Alpha4beta1 integrin and erythropoietin mediate temporally distinct steps in erythropoiesis: integrins in red cell development. J Cell Biol. 2007;177:871–880. doi: 10.1083/jcb.200702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, et al. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 3.Neelin JM, et al. The Histones of Chicken Erythrocyte Nuclei. Can J Biochem Physiol. 1964;42:1743–1752. doi: 10.1139/o64-185. [DOI] [PubMed] [Google Scholar]
- 4.Hnilica LS. The specificity of histones in chicken erythrocytes. Experientia. 1964;20:13–14. doi: 10.1007/BF02146014. [DOI] [PubMed] [Google Scholar]
- 5.Miki BL, Neelin JM. The histones of rainbow trout erythrocytes include an erythrocyte-specific histone. Can J Biochem. 1975;53:1158–1169. doi: 10.1139/o75-161. [DOI] [PubMed] [Google Scholar]
- 6.Awai M, et al. Studies on the mechanism of denucleation of the erythroblast. Acta Haematol. 1968;39:193–202. doi: 10.1159/000208962. [DOI] [PubMed] [Google Scholar]
- 7.Muir AR, Kerr DN. Erythropoiesis: an electron microscopical study. Q J Exp Physiol Cogn Med Sci. 1958;43:106–114. doi: 10.1113/expphysiol.1958.sp001295. [DOI] [PubMed] [Google Scholar]
- 8.Orlic D, et al. An ultrastructural study of erythropoietin-induced red cell formation in mouse spleen. J Ultrastruct Res. 1965;13:516–542. doi: 10.1016/s0022-5320(65)90012-2. [DOI] [PubMed] [Google Scholar]
- 9.Simpson CF, Kling JM. The mechanism of denucleation in circulating erythroblasts. J Cell Biol. 1967;35:237–245. doi: 10.1083/jcb.35.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skutelsky E, Danon D. An electron microscopic study of nuclear elimination from the late erythroblast. J Cell Biol. 1967;33:625–635. doi: 10.1083/jcb.33.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skutelsky E, Danon D. Comparative study of nuclear expulsion from the late erythroblast and cytokinesis. Exp Cell Res. 1970;60:427–436. doi: 10.1016/0014-4827(70)90536-7. [DOI] [PubMed] [Google Scholar]
- 12.Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004;18:1176–1199. doi: 10.1038/sj.leu.2403383. [DOI] [PubMed] [Google Scholar]
- 13.Ishizaki Y, et al. A role for caspases in lens fiber differentiation. J Cell Biol. 1998;140:153–158. doi: 10.1083/jcb.140.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weil M, et al. Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr Biol. 1999;9:361–364. doi: 10.1016/s0960-9822(99)80162-6. [DOI] [PubMed] [Google Scholar]
- 15.Carlile GW, et al. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004;103:4310–4316. doi: 10.1182/blood-2003-09-3362. [DOI] [PubMed] [Google Scholar]
- 16.Kolbus A, et al. Raf-1 antagonizes erythroid differentiation by restraining caspase activation. J Exp Med. 2002;196:1347–1353. doi: 10.1084/jem.20020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida H, et al. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–758. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 18.Krauss SW, et al. Nuclear substructure reorganization during late-stage erythropoiesis is selective and does not involve caspase cleavage of major nuclear substructural proteins. Blood. 2005;106:2200–2205. doi: 10.1182/blood-2005-04-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayapal SR, et al. Down-regulation of Myc is essential for terminal erythroid maturation. J Biol Chem. 2010;285:40252–40265. doi: 10.1074/jbc.M110.181073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji P, et al. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica. 2010;95:2013–2021. doi: 10.3324/haematol.2010.029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popova EY, et al. Chromatin condensation in terminally differentiating mouse erythroblasts does not involve special architectural proteins but depends on histone deacetylation. Chromosome Res. 2009;17:47–64. doi: 10.1007/s10577-008-9005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koury ST, et al. Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J Cell Biol. 1989;109:3005–3013. doi: 10.1083/jcb.109.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chasis JA, et al. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74:1112–1120. [PubMed] [Google Scholar]
- 24.Byers TJ, Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985;82:6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gratzer WB. The red cell membrane and its cytoskeleton. Biochem J. 1981;198:1–8. doi: 10.1042/bj1980001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tse WT, Lux SE. Red blood cell membrane disorders. Br J Haematol. 1999;104:2–13. doi: 10.1111/j.1365-2141.1999.01130.x. [DOI] [PubMed] [Google Scholar]
- 27.Takano-Ohmuro H, et al. Distribution of actin, myosin, and spectrin during enucleation in erythroid cells of hamster embryo. Cell Motil Cytoskeleton. 1996;34:95–107. doi: 10.1002/(SICI)1097-0169(1996)34:2<95::AID-CM2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 28.Ji P, et al. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol. 2008;10:314–321. doi: 10.1038/ncb1693. [DOI] [PubMed] [Google Scholar]
- 29.Geiduschek JB, Singer SJ. Molecular changes in the membranes of mouse erythroid cells accompanying differentiation. Cell. 1979;16:149–163. doi: 10.1016/0092-8674(79)90196-x. [DOI] [PubMed] [Google Scholar]
- 30.Wickrema A, et al. Changes in cytoskeletal proteins and their mRNAs during maturation of human erythroid progenitor cells. J Cell Physiol. 1994;160:417–426. doi: 10.1002/jcp.1041600304. [DOI] [PubMed] [Google Scholar]
- 31.Lee JC, et al. Mechanism of protein sorting during erythroblast enucleation: role of cytoskeletal connectivity. Blood. 2004;103:1912–1919. doi: 10.1182/blood-2003-03-0928. [DOI] [PubMed] [Google Scholar]
- 32.Wandersee NJ, et al. Defective spectrin integrity and neonatal thrombosis in the first mouse model for severe hereditary elliptocytosis. Blood. 2001;97:543–550. doi: 10.1182/blood.v97.2.543. [DOI] [PubMed] [Google Scholar]
- 33.Yi SJ, et al. Red cell membranes of ankyrin-deficient nb/nb mice lack band 3 tetramers but contain normal membrane skeletons. Biochemistry. 1997;36:9596–9604. doi: 10.1021/bi9704966. [DOI] [PubMed] [Google Scholar]
- 34.Peters LL, et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 35.Shi ZT, et al. Protein 4.1R-deficient mice are viable but have erythroid membrane skeleton abnormalities. J Clin Invest. 1999;103:331–340. doi: 10.1172/JCI3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters LL, et al. Mild spherocytosis and altered red cell ion transport in protein 4. 2-null mice. J Clin Invest. 1999;103:1527–1537. doi: 10.1172/JCI5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dellagi K, et al. Alteration of vimentin intermediate filament expression during differentiation of human hemopoietic cells. EMBO J. 1983;2:1509–1514. doi: 10.1002/j.1460-2075.1983.tb01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngai J, et al. Differentiation of murine erythroleukemia cells results in the rapid repression of vimentin gene expression. J Cell Biol. 1984;99:306–314. doi: 10.1083/jcb.99.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granger BL, et al. Synemin and vimentin are components of intermediate filaments in avian erythrocytes. J Cell Biol. 1982;92:299–312. doi: 10.1083/jcb.92.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virtanen I, et al. Nucleus-anchoring cytoskeleton in chicken red blood cells. Cell Biol Int Rep. 1979;3:157–162. doi: 10.1016/0309-1651(79)90121-8. [DOI] [PubMed] [Google Scholar]
- 41.Keerthivasan G, et al. Vesicle trafficking plays a novel role in erythroblast enucleation. Blood. 2010;116:3331–3340. doi: 10.1182/blood-2010-03-277426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skutelsky E, Farquhar MG. Variations in distribution of con A receptor sites and anionic groups during red blood cell differentiation in the rat. J Cell Biol. 1976;71:218–231. doi: 10.1083/jcb.71.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 44.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manwani D, Bieker JJ. The erythroblastic island. Curr Top Dev Biol. 2008;82:23–53. doi: 10.1016/S0070-2153(07)00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanspal M, Hanspal JS. The association of erythroblasts with macrophages promotes erythroid proliferation and maturation: a 30-kD heparin-binding protein is involved in this contact. Blood. 1994;84:3494–3504. [PubMed] [Google Scholar]
- 47.Hanspal M, et al. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. 1998;92:2940–2950. [PubMed] [Google Scholar]
- 48.Soni S, et al. Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. J Biol Chem. 2006;281:20181–20189. doi: 10.1074/jbc.M603226200. [DOI] [PubMed] [Google Scholar]
- 49.Iavarone A, et al. Retinoblastoma promotes definitive erythropoiesis by repressing Id2 in fetal liver macrophages. Nature. 2004;432:1040–1045. doi: 10.1038/nature03068. [DOI] [PubMed] [Google Scholar]
- 50.Sankaran VG, et al. Rb intrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev. 2008;22:463–475. doi: 10.1101/gad.1627208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel VP, Lodish HF. A fibronectin matrix is required for differentiation of murine erythroleukemia cells into reticulocytes. J Cell Biol. 1987;105:3105–3118. doi: 10.1083/jcb.105.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 53.Lazarides E. From genes to structural morphogenesis: the genesis and epigenesis of a red blood cell. Cell. 1987;51:345–356. doi: 10.1016/0092-8674(87)90631-3. [DOI] [PubMed] [Google Scholar]
- 54.Kingsley PD, et al. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 55.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen CZ, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 57.Chen CZ, Lodish HF. MicroRNAs as regulators of mammalian hematopoiesis. Semin Immunol. 2005;17:155–165. doi: 10.1016/j.smim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Zhao G, et al. MicroRNAs in erythropoiesis. Curr Opin Hematol. 2010;17:155–162. doi: 10.1097/MOH.0b013e328337ba6c. [DOI] [PubMed] [Google Scholar]
- 59.Dore LC, et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc Natl Acad Sci U S A. 2008;105:3333–3338. doi: 10.1073/pnas.0712312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu YF, et al. Mir-144 selectively regulates embryonic alpha-hemoglobin synthesis during primitive erythropoiesis. Blood. 2009;113:1340–1349. doi: 10.1182/blood-2008-08-174854. [DOI] [PubMed] [Google Scholar]
- 61.Pase L, et al. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood. 2009;113:1794–1804. doi: 10.1182/blood-2008-05-155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasmussen KD, et al. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med. 2010;207:1351–1358. doi: 10.1084/jem.20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, et al. miR-191 regulates mouse erythroblast enucleation by down-regulating Riok3 and Mxi1. Genes Dev. 2011;25:119–124. doi: 10.1101/gad.1998711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schreiber-Agus N, et al. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast transcriptional repressor SIN3. Cell. 1995;80:777–786. doi: 10.1016/0092-8674(95)90356-9. [DOI] [PubMed] [Google Scholar]
- 65.Kalfa TA, et al. Rac1 and Rac2 GTPases are necessary for early erythropoietic expansion in the bone marrow but not in the spleen. Haematologica. 2010;95:27–35. doi: 10.3324/haematol.2009.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch Dis Child Fetal Neonatal Ed. 2001;84:F211–215. doi: 10.1136/fn.84.3.F211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Douay L, Andreu G. Ex vivo production of human red blood cells from hematopoietic stem cells: what is the future in transfusion? Transfus Med Rev. 2007;21:91–100. doi: 10.1016/j.tmrv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Panzenbock B, et al. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92:3658–3668. [PubMed] [Google Scholar]
- 69.Giarratana MC, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 70.Anstee DJ. Production of erythroid cells from human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPS) Transfus Clin Biol. 2010;17:104–109. doi: 10.1016/j.tracli.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Fujimi A, et al. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int J Hematol. 2008;87:339–350. doi: 10.1007/s12185-008-0062-y. [DOI] [PubMed] [Google Scholar]
- 72.Lu SJ, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snyder GK, Sheafor BA. Red blood cells: Centerpiece in the evolution of the vertebrate circulatory system. Am Zool. 1999;39:189–198. [Google Scholar]