Abstract
Epileptic seizures are traditionally characterized as the ultimate expression of monolithic, hypersynchronous neuronal activity arising from unbalanced runaway excitation. Here we report the first examination of spike train patterns in large ensembles of single neurons during seizures in persons with epilepsy. Contrary to the traditional view, neuronal spiking activity during seizure initiation and spread was highly heterogeneous, not hypersynchronous, suggesting complex interactions among different neuronal groups even at the spatial scale of small cortical patches. In contrast to earlier stages, seizure termination is a nearly homogenous phenomenon followed by an almost complete cessation of spiking across recorded neuronal ensembles. Notably, even neurons outside the region of seizure onset showed significant changes in activity minutes before the seizure. These findings suggest a revision of current thinking about seizure mechanisms and point to the possibility of seizure prevention based on spiking activity in neocortical neurons.
Seizures and epilepsy have been recognized since antiquity, yet we continue to struggle to define and understand these paroxysms of neuronal activity. Epileptic seizures are commonly considered to be the result of monolithic, hypersynchronous activity arising from an imbalance between excitation and inhibition in large populations of cortical neurons1–3. This view of ictal activity is highly simplified, and the level at which it breaks down is unclear. It is largely based on electroencephalogram (EEG) recordings, which reflect the averaged activity of millions of neurons. Whereas some in vitro studies have shown that sparse and asynchronous neuronal activity evolves into a single hypersynchronous cluster with elevated spiking rates at seizure initiation4,5, as the canonical view would suggest, other animal model studies have supported a much less homogeneous progression in neuronal activity during seizures6–8. How well these animal models capture mechanisms operating in human epilepsy remains an open question9,10. Very few human studies have gone beyond macroscopic scalp and intracranial EEG signals to examine neuronal spiking underlying seizures11–14. Hence the behavior of single neurons in human epilepsy remains largely unknown.
The reliance on the macroscopic information of the EEG has also dominated attempts at discovering physiological changes preceding the seizure. The obvious goal of this approach is in predicting and then preventing seizures15,16. While in vitro and in vivo animal models suggest that different neuronal populations might have distinct roles during a preictal period4–8,17–21, reliable seizure prediction based on detection of preictal changes in human scalp and intracranial EEG has remained elusive16. In addition, most seizures end abruptly and spontaneously, followed by a post-seizure attenuation in EEG activity22. The underlying mechanisms governing this behavior are also not understood. Various potential mechanisms, including among others, glutamate depletion, profound inhibition, modulatory effects from subcortical structures and depolarization block, have been hypothesized to underlie seizure termination22,23. Although these mechanisms clearly operate at the level of individual cells, to our knowledge, single-unit activity during this period has not been examined in humans. Such information could be useful in developing better strategies for seizure control and preventing status epilepticus24.
A deeper understanding of neuronal spiking during the different phases of seizure generation would have profound implications for seizure prediction and may provide the basis for new and more effective therapies for people with epilepsy25. Here we studied the spiking activity of hundreds of neurons in four persons with focal epilepsy. We found significant changes in preictal activity in subsets of neurons. During seizure initiation and spread, we observed a high degree of heterogeneity in spiking activity. This heterogeneity did not seem to result purely from differences between interneurons and pyramidal cells; heterogeneity was present even within a class. Spiking evolved into a more homogeneous activity across the recorded neuronal ensemble toward seizure termination, during which we observed an almost complete cessation of spiking across the recorded cortical patch. Further, in our data, depolarization block did not seem to have a primary local role during the end of the seizure.
RESULTS
We used specialized 96-channel microelectrode arrays26–31 to record single-unit spiking activity and local field potentials from a 4 mm × 4 mm region of neocortex in four patients with epilepsy refractory to medical treatments. These patients were implanted with subdural grid electrodes to evaluate their cortical EEG activity (electrocor-ticogram; ECoG) and help localize the onset zone of their seizures for subsequent surgical resection. For research purposes, the micro-electrode arrays were placed in addition to the grids (Fig. 1). We identified a total of 712 single-unit recordings in four participants (A, B, C and D). Single units were sorted using standard techniques (Online Methods). Although we recorded continuously over several days, the consistent sorting of single units over time periods longer than a few hours proved challenging. Over such long periods, waveforms of extracellularly recorded action potentials could change and units appear or disappear from recordings, owing perhaps to array micromotion, changes in brain states and other factors32. For this reason, for each analyzed session we identified for study those single units that were consistently recorded during a time period of ∼2 h centered at the onset of each of eight seizures (see Online Methods). Microelectrode arrays in participants A (three seizures; n = 149, 131 and 131 single units recorded), B (two seizures; n = 57 in each) and D (n = 35 in one seizure) were placed in the middle temporal gyrus ∼2 cm from the anterior tip. The microelectrode array in participant C (two seizures; n = 82 and 70) was placed in the middle frontal gyrus. Microelectrode arrays were positioned both within (participant C) and outside (∼2–4 cm away; participants A, B and D) the seizure onset zone as subsequently defined by the ECoG-electrode locations at which the seizure first appeared (see Online Methods).
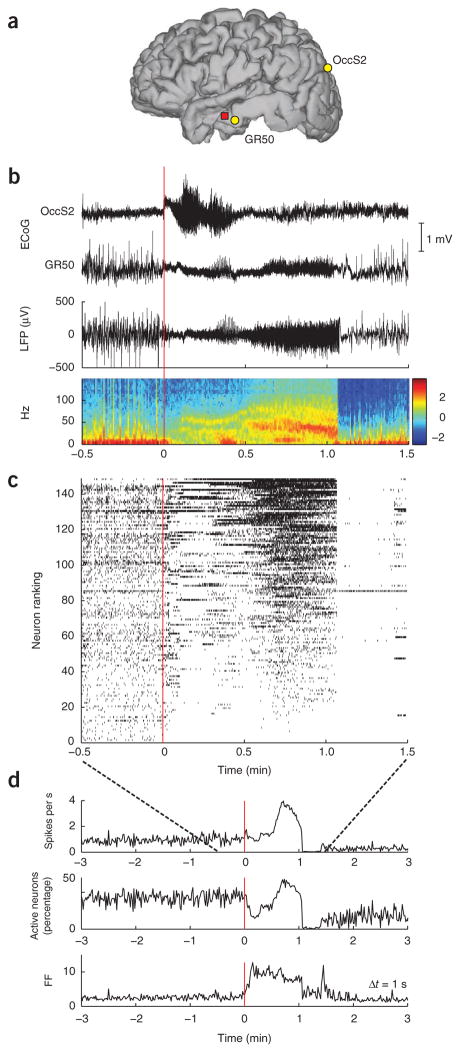
Figure 1.
Heterogeneous neuronal spiking patterns during seizure. (a) Locations of the microelectrode array in participant A (red square), and subdural ECoG electrodes OccS2 and GR50 in occipital and middle temporal cortices, respectively. (b) ECoG traces recorded at the locations shown in a during seizure 1. The ECoG-based onset area was identified to be under the occipital electrode OccS2. Seizure onset is at time 0. The local field potential (LFP) recorded from a single channel in the microelectrode array and the corresponding spectrogram (in dB) are shown below. (c) Neuronal spike raster plot including all recorded neurons (n = 149). Each hash mark represents the occurrence of an action potential. Neurons were ranked (vertical axis) in increasing order according of their mean spiking rate during the seizure. (This ranking number is unrelated to physical location.) Toward the end of the seizure, activity across the population became more homogeneous until spiking was abruptly interrupted at seizure termination. With the exception of a few neurons, spiking in the recorded population remained suppressed for about 20 s. (d) The mean population rate, the percentage of active neurons and the Fano factor (FF) of the spike counts across different neurons at a given time (determined in 1-s time bins). These were roughly stationary during the several minutes preceding the seizure onset. An increase in the Fano factor, reflecting the heterogeneity in neuronal spiking, is observed around seizure onset and precedes an increase in the mean population rate.
Heterogeneous neuronal spiking activity during seizures
Visual inspection of neuronal spike rasters revealed a variety of spiking patterns during seizure initiation, spread and maintenance (Fig. 1c; see also Supplementary Fig. 1 and Supplementary Movie 1 showing spiking rates on the microelectrode array). For example, whereas some neurons increased their spiking rates near the seizure onset, others decreased. These transient spiking rate modulations occurred at different times for different groups of neurons. The Fano factor (variance divided by the mean) of the single neuron spike counts across the population at a given time during the seizure (1-s time bins), which gives an index of spiking heterogeneity in the ensemble, increased substantially after the seizure onset—in some seizures by fivefold (Fig. 1d).
This diversity of neuronal modulation patterns was observed to a greater or lesser extent in all seizures and participants studied (Supplementary Figs. 1–4). Such heterogeneity in spiking rate modulation patterns directly challenges the canonical characterization of epileptic seizures as a simple, widespread and homogeneous runaway excitation leading to a hypersynchronized state. Heterogeneity was present regardless of whether the seizure initiated near (participant C) or far from (the other three participants) the location of the microelectrode array. The admixture of different spiking patterns suggests that heterogeneity is not purely propagation driven but must also reflect local network properties. As based on the Fano factor, this heterogeneity was higher during seizure initiation and decreased toward seizure termination (Fig. 1d and Supplementary Fig. 1).
Seizure termination and suppression of neuronal spiking
In contrast to the beginning of the seizure, seizure termination in participants A, B and C involved widespread, complete cessation of activity for most recorded neurons (Fig. 1c and Supplementary Figs. 1,3). Spiking activity remained suppressed for several seconds (ranging from 5 to 30 s) after seizure termination, until more normal spiking rates gradually returned. Decreases in spiking activity (particularly during either initial or final stages of the seizure) were not due to sorting artifacts such as spike dropout because of obvious alterations in spike waveform. Changes in spike waveforms can be induced by, for example, intense bursting activity. In contrast, Figure 2a,b shows several examples of units that almost completely ceased spiking for 20–30 s after seizure onset, yet did not present any obvious changes in waveform shape and amplitude that would result in spike dropout. Furthermore, toward the end of the seizure, the same units increased substantially their spiking rates, showing that we were still able to detect their spiking even at much higher spiking rates.
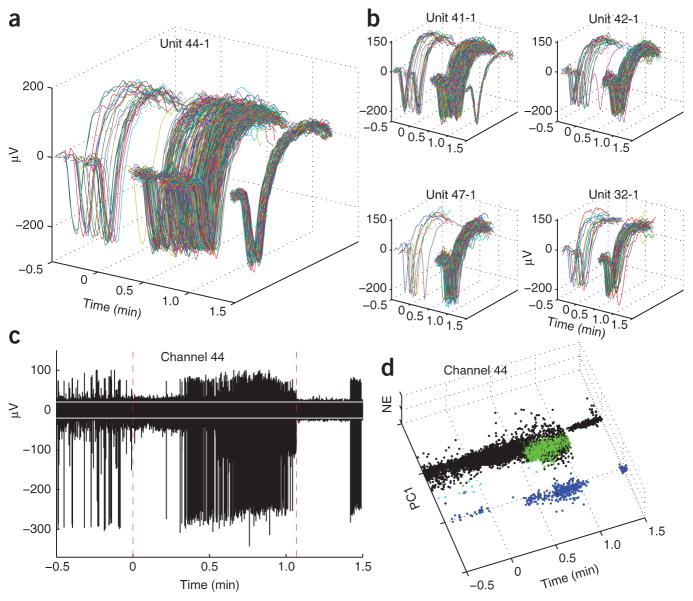
Figure 2.
Transient suppression of neuronal spiking during the seizure and at seizure termination. (a) Spike waveforms from neuron 44–1 (participant A, seizure 1; neuron ranked no. 131 in Fig. 1c). Spiking stopped for ∼ 20 s during the initial seizure phase. The lack of major changes in spike waveform and preceding low spiking rate suggest that suppression was not due to sorting artifacts or depolarization block. (b) Four examples of units with similar behavior, recorded from different sites. All five units were classified as putative principal cells. (c) The high-pass filtered potentials recorded at electrode 44. Larger spikes correspond to unit 44–1, shown in a. Dashed vertical lines show seizure onset and termination, respectively. White lines mark ±3 s.d. of the background noise, estimated from the ‘silent’ period after seizure termination. Another unit with smaller extracellularly recorded action potentials intensifies spiking during the 0.5–1.1 min interval. After seizure termination, both single-unit and multiunit activity were suppressed and the recorded potentials correspond primarily to background noise. Although there is some gradual decrease in spike amplitudes, this decrease is much smaller than what would be expected from depolarization block. See Supplementary Figure 2 for channels 32, 41, 42 and 47. (d) Projection of thresholded waveforms onto a feature space shows clearly separable units. Blue dots represent thresholded spikes from unit 44–1; PC1 and NE denote the first principal component and a nonlinear energy feature, respectively. Green dots correspond to a smaller unit. Black dots correspond to thresholded noise and unsorted multiunit spikes.
Notably, it also seems that the suppression of these units’ spiking either during seizure maintenance or at the end of the seizure was not due to a typical depolarization block scenario18,19,23 where neuronal spike amplitudes decrease gradually until they cannot be detected or spiking is blocked. For example, the examination of the high-pass-filtered potentials (Fig. 2c,d) shows that unit 44–1 stopped spiking at a point where peak-to-peak waveform amplitudes were about 300 μV, whereas the threshold for spike detection was set at around −30 μV. (Examples for other neurons are shown in Supplementary Fig. 2).
Reproducibility of spike patterns in consecutive seizures
In participants A and B, two consecutive seizures occurred within a period of about an hour. In these cases, we were able to ensure that the same units were recorded and consistently identified during both seizures (see Online Methods). This permitted us to examine the reproducibility of neuronal spiking patterns across seizures. For instance, even though the third seizure in participant A lasted slightly longer than the second, the same motifs in the neuronal spiking patterns recurred (Fig. 3). The Pearson correlation coefficient (a measure of similarity) between two spike trains during the initial 30 s of each seizure, for each neuron and averaged across the population, was 0.82. Similar observation was also made for the two consecutive seizures in participant B, with an average correlation coefficient of 0.72 (Supplementary Fig. 3), suggesting a high degree of similarity in both seizures. The probability that these correlation coefficients were statistically zero was extremely small (P < 10−6; see Online Methods).
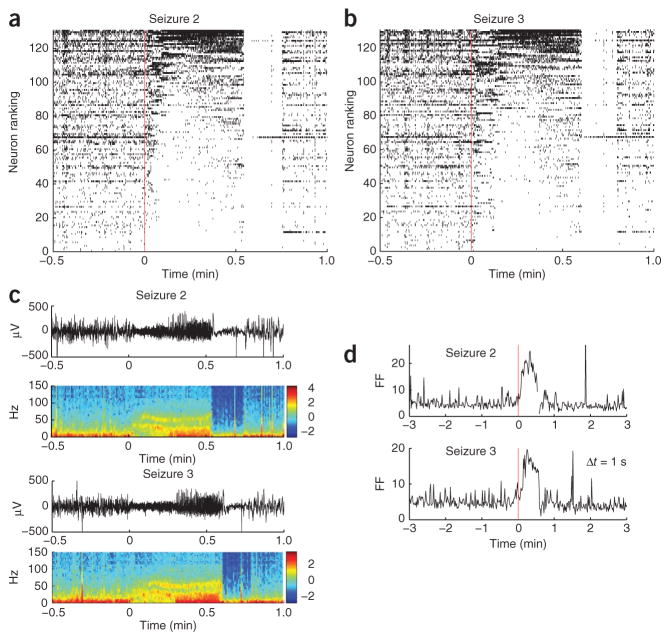
Figure 3.
Reproducibility of neuronal spiking modulation patterns across consecutive seizures. (a,b) An example from participant A with 131 neurons. Following conventions used in Figure 1c, neurons are ranked according to their mean rates measured during the seizure. Seizure 3 (b) follows the same ranking as seizure 2 (a); that is, the single units in any given row of seizures 2 and 3 are the same. Most neurons coarsely preserved the types of spiking rate modulation across the two seizures. For example, the lowest-ranked neurons decreased or stopped spiking; and many of the top-ranked neurons presented similar transient increases in spiking rate modulation. As in seizure 1 (Fig. 1), an almost complete suppression of spiking in the neuronal population occurred abruptly at seizure termination. (c) The corresponding low-pass filtered local field potentials (LFPs) and spectrograms (from the same microelectrode array channel shown in Fig. 1; power in dB). (d) The Fano factor for the spike counts (1-s time bins) in the population of recorded neurons showed similar increase during both seizures, reflecting the increased heterogeneity in neuronal spiking across the population.
We also examined how the similarity between the two ictal events compared to the similarity of random interictal segments preceding the two corresponding consecutive seizures. A random resampling approach using interictal spike rasters each lasting 30 s resulted in significantly smaller correlation coefficients; that is, coefficients whose 95% confidence intervals (mean ± 2 s.d.) corresponded to 0.45 ± 0.14 and 0.16 ± 0.25 for participants A and B, respectively. Despite general similarities across seizures, there were also notable differences in the fraction of active neurons in the population. For example, whereas these fraction increased after a transient decrease at seizure onset in seizure 1 (participant A; Fig. 1d), throughout seizures 2 and 3 it remained lower than during the preictal period (Supplementary Fig. 4).
Preictal and ictal changes in neuronal spiking activity
To characterize preictal and ictal changes in spiking rates, we represented a single neuron’s spike train as a sample path33. A sample path consists of the cumulative number of neuronal spikes as a function of time (Fig. 4a). The sample path representation allows us to preserve information about transient changes in instantaneous spiking rates. We asked whether an observed sample path for a given neuron during the preictal or ictal periods deviated from the collection of sample paths of same time length observed during a preceding interictal period. In this way, we were able to detect transient increases or decreases in spiking rate that were atypical with respect to the preceding interictal activity (Fig. 4b,c).
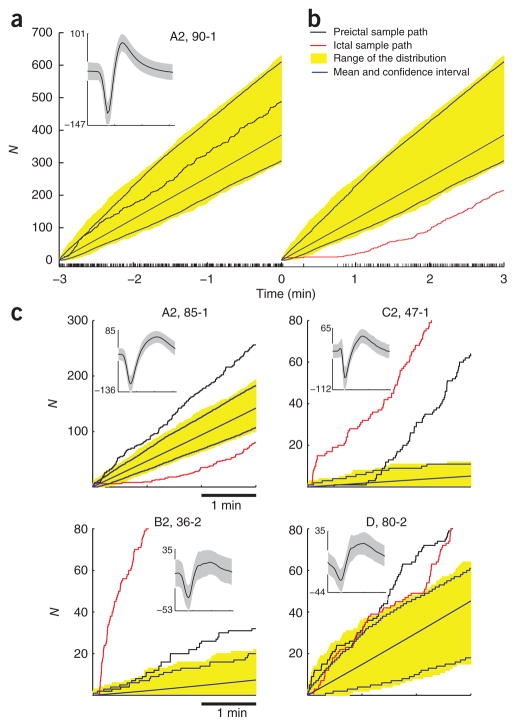
Figure 4.
Preictal and ictal modulations in spiking rates. (a) The neuronal spiking sample path N (neuron 90–1; A2: participant A, seizure 2). The corresponding spike train is shown at the bottom and the inset shows the mean ± 2 s.d. of all recorded spike waveforms. Seizure onset corresponds to time 0. For comparison purposes, the initial value of the sample path is set to 0. The yellow band corresponds to the range of the 3-min-long sample paths observed during a 30-min interictal period preceding the preictal period. Interictal sample paths in this distribution were obtained from an overlapping 3-min-long moving time window, stepped 1 s at a time. Blue curves and surrounding yellow band correspond to the average interictal sample path and the 95% confidence interval, respectively. A sample path was judged to have deviated from the interictal sample paths when it fell outside the range of the collection of interictal sample paths at any given time. (b) Neuron 90–1 transiently stopped spiking for tens of seconds just after the seizure onset. As expected, the sample path during the seizure did deviate from the observed interictal paths. The neuron’s spiking rate gradually recovered and eventually settled at the typical mean rate. (c) Four examples of preictal and ictal sample path deviations, one for each participant. Note that although the preictal and ictal sample paths are plotted along the same axis, they refer to a 3-min period before and after, respectively, the seizure onset.
We found that substantial numbers of neurons significantly changed their activities as the seizure approached. The percentage of neuronal recordings that showed a preictal deviation varied from 20% (7/35, participant D) to 29.9% (123/411, participant A). The percentages of neurons that showed preictal and/or ictal changes are specified separately for each participant and seizure in Figure 5. Observed deviations consisted either of increases or decreases in spiking rate. Across all participants and seizures (712 recordings), 11.8% of recorded neurons increased their spiking rate during the preictal period, and 7.5% decreased. In many cases the onset time for the deviation was earlier than 1 min before the seizure onset time (Fig. 4c). This finding suggests that changes in neuronal spiking activity, even for single neurons recorded well outside the identified epileptic focus, may be detected minutes before the seizure onset defined by ECoG inspection. Several neurons in participants A showed consistent sample path deviations across seizures 2 and 3, the two consecutive seizures where the same single units were recorded (Supplementary Fig. 5).
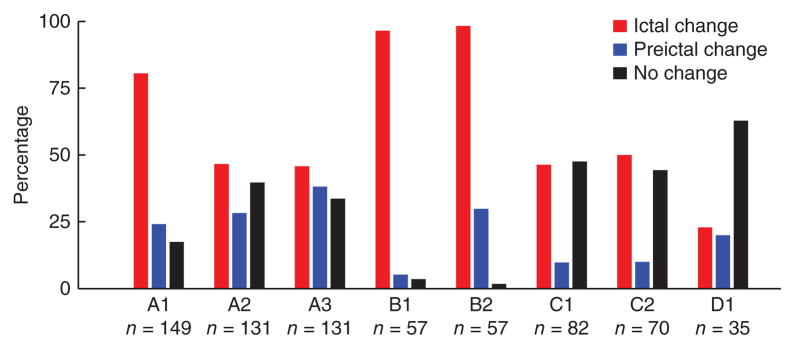
Figure 5.
Preictal and ictal sample path deviations with respect to an interictal period. Each bar indicates the percentage of preictal and ictal sample path deviations in the recorded neuronal population, for each participant and seizure. Sample paths and sample path deviations were defined as in Figure 4.
The percentage of neuronal recordings that showed a sample path deviation during the ictal period varied from 22.8% (8/35, participant D) to 97.4% (111/114, participant B). In line with the heterogeneity observed in raster plots, several types of deviations were observed during the seizure—all from single units recorded simultaneously from a small cortical patch (encompassing different cortical columns). Across all participants and seizures, 45.4% and 9.9% of the neuronal recordings increased and decreased, respectively, their spiking rates during the seizure. Furthermore, a few neurons showed a transient increase (0.1%) followed by a transient decrease in spiking rates, or vice-versa (1.3%). Overall, we found fifteen different patterns of neuronal modulation when taking into consideration both preictal and ictal patterns. (See Supplementary Table 1 for detailed percentages for each participant and seizure.) In addition to these types of sample path deviations, a few neurons in participant A also showed much slower and larger modulations in spiking rates that preceded the seizure onset by tens of minutes (Supplementary Fig. 6) and were not correlated with obvious behavioral or state changes.
Neurons that showed preictal and ictal modulation in spiking rates tended to have statistically higher bursting rates during interictal periods (Kruskal-Wallis test, P < 0.01, Tucker-Kramer correction for multiple comparisons; see Online Methods and Supplementary Figs. 7 and 8). Furthermore, in participant A, the single units recorded could be classified into putative interneurons and principal neurons according to spike half-width and peak-to-valley time width features (see Online Methods and Supplementary Fig. 9; waveforms from the other three participants formed a homogeneous cluster in each case). On the basis of this classification, 79% (45/57) and 68% (240/354) of interneurons and principal cells recordings, respectively, showed some type of preictal or ictal modulation. In addition, the fraction of recorded interneurons that showed a preictal increase was 60% larger than the corresponding fraction of principal neurons (χ2 test, P < 10−6, with Bonferroni correction for multiple comparisons; see Supplementary Fig. 10).
Specificity of preictal changes in neuronal activity
The observed sample path deviations during the preictal period could simply reflect spontaneous or evoked modulations of spiking activity unrelated to the upcoming seizure. Therefore, we also examined how often such sample path deviations could occur during interictal periods. This analysis also provides a preliminary assessment of the specificity of a very simple seizure prediction algorithm that, for example, predicted a seizure every time an observed number of deviations across the recorded neuronal population exceeded a specified threshold. Specifically, we estimated the probability of observing a given number (percentage) of such sample path deviations across the neuronal population during interictal periods. Three different interictal periods were used for participant A, two for participants B and C, and one for participant D. A 3-min-long segment was then randomly selected from a given interictal period and a corresponding (target) sample path was computed for each neuron. Next, we checked whether each sample path deviated from its corresponding distribution derived from paths of same length sampled from a 30-min interictal segment, as done before in Figure 4. This 30-min segment was adjacent to but nonoverlapping with the randomly selected target sample path. The entire procedure was repeated hundreds of times to obtain a distribution of the percentage of deviations across the recorded neuronal population during a given interictal period. Finally, a threshold was defined based on the average number of preictal deviations across the examined seizures for a given participant. (For example, the threshold for participant A was derived from the mean of the percentage of neurons that showed a preictal deviation across the three seizures; Fig. 5.) Given this threshold and a distribution of percentage of deviations, we could then compute the probability that a given percentage of observed deviations across the population during an interictal period was smaller than the specified threshold. As mentioned above, this probability provides an estimate of the specificity of a seizure prediction algorithm based on the defined sample path deviations.
With the exception of participant C, for whom interictal activity showed a very high rate of epileptiform events consisting of slow neuronal bursting and ECoG spike and wave discharges, promising specificity results (0.78–0.94) were obtained for participants A, B and D (Supplementary Fig. 11). Despite these positive preliminary results, we emphasize that a more conclusive assessment will require much larger data sets, to probe a larger range of physiological and behavioral states, as well as corresponding sensitivity analyses.
DISCUSSION
Our findings, based on the most extensive description of single-unit activity in human neocortical seizures yet reported, reveal several important and heterodox points about the nature of epileptic activity. First, the observed heterogeneity in neuronal behavior argues against homogeneous runaway excitation or widespread paroxysmal depolarization as the primary mechanism underlying seizure initiation. Rather, our data indicate that seizures result from a complex interplay among groups of neurons that present different types of spiking behaviors evolving at multiple temporal and spatial scales. We have also observed similar heterogeneity in interictal discharges31. Given the 1-mm microelectrode length, it is likely we recorded from cells in layers 3 and 4. Several studies5,34,35 suggest that epileptiform activity involves and is perhaps initiated by cells in layer 5. Although the potential role of these cells needs to be further explored, they do not seem to be driving homogenous activity. Furthermore, because of the nature of these recordings, we might not have recorded from the initiation site in any of the participants (including participant C). As a result, it is possible that a different form of neuronal dynamics would be observed at the ‘focus’. This is especially likely to be the case in a region of dysplasia in which the neurons and their layering are severely abnormal.
Heterogeneity in neuronal spiking activity during seizures has been previously observed in animal model studies6–8. In particular, it has been hypothesized7 that such heterogeneity could reflect three main sequential stages or states during seizure spreading: ‘depressed’, ‘projected’ and ‘propagated’ states. The fact that the heterogeneity reported here in human epilepsy appears simultaneously in small patches of neocortex would speak against this hypothesis; or, alternatively, it would require that different groups of neurons entered and dwelled in different states with different time constants, perhaps owing to differences in initial conditions or in intrinsic and local network dynamical properties. In addition, our finding based on the classification of units into putative principal cells and interneurons, namely that some putative pyramidal cells increased while other decreased their spiking rates, suggests that such heterogeneity does not simply reflect interleaved spiking of pyramidal cells and interneurons18,19. We also speculate that the observed heterogeneity in the neuronal collective dynamics30 could result from fragmentation into multi-cluster synchronization, which has been studied in various dynamical systems36. The fact that such diverse spiking activity underlies seemingly ‘monomorphic’ EEG waveforms raises the possibility that even normal cortical rhythms might also reflect very heterogeneous underlying neuronal ensemble spiking.
Second, one of the noteworthy features of these data was the abrupt and widespread suppression of neuronal action potentials at seizure end. In participant A, for example, spiking of both putative interneu-rons and principal neurons became suppressed. Previous work has suggested that seizures might self-terminate because of depolarization block resulting from changes in ionic concentrations in the extra-cellular space. A depolarization block of neuronal spikes due to a chain of events that ends with astrocytic release of large amounts of potassium has been hypothesized23. However, several units became suppressed at seizure termination without showing typical signatures of depolarization block (Fig. 2)—an indication that depolarization block was not the primary local factor responsible for the observed marked suppression in spiking activity. Furthermore, our results also argue against massive inhibition from a local source because the suppression of neuronal spiking affected both putative interneurons and other principal cells. Alternatively, distant modulatory inputs involving subcortical structures—for example, thalamic nuclei or substantia nigra pars reticulata22—could lead to seizure termination. There is also the possibility that this critical transition could arise from an emergent property of the large-scale network itself leading to spatially synchronous extinction37.
From a therapeutic perspective, our analysis demonstrates, for the first time in humans, that preictal neuronal spiking reflects a distinct and widely occurring physiological state in focal epilepsies. This is true even outside the region of seizure initiation, suggesting that it may be possible to obtain predictive information from individual neuronal activity without necessarily localizing what has been traditionally considered the seizure focus. Substantiation of this possibility will require large data sets, perhaps only available through multisite collaborative efforts, containing sufficient interictal data for proper specificity and sensitivity analyses16. Promisingly, our data suggests that the clinical care of patients with epilepsy could be revolutionized by using dynamics of ensembles of single neurons to predict seizures.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in this study, as well as the nursing and physician staff at each facility. We also thank A.M. Chan, C.J. Keller, A. Dykstra and J.E. Cormier for technical assistance, and J.P. Donoghue and K.J. Staley for critical reading of the manuscript. This research is funded by a CIMIT grant and US National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) NS062092 to S.S.C.; an NIH–NINDS Career Award (5K01NS057389) to W.T.; NIH NS018741 to E.H.; NINDS K08NS066099-01A1 to W.S.A.; US National Eye Institute EY017658, US National Institute on Drug Abuse NS063249, US National Science Foundation IOB 0645886, Howard Hughes Medical Institute and the Klingenstein Foundation to E.N.E.; NIH Director’s Pioneer Award DP1OD003646 to E.N.B.; US Department of Veterans Affairs Career Development Transition Award, Doris Duke Charitable Foundation–Clinical Scientist Development Award, Massachusetts General Hospital–Deane Institute for Integrated Research on Atrial Fibrillation and Stroke, and NIH-NIDCD R01DC009899 to L.R.H. The contents do not represent the views of the Department of Veterans Affairs or the United States government.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
W.T., S.S.C. and J.A.D. wrote the paper. W.T. and J.A.D. conducted the data analysis. Data collection and preprocessing were done by J.A.D., W.T. and S.S.C. S.S.C., L.R.H., W.T. and E.H. conceived and planned the research. E.N.B. provided guidance on methods of data analysis and interpretation. E.N.E., W.S.A. and J.R.M. performed the surgeries and microelectrode array implantations. All authors participated in editing the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/natureneuroscience/.
Published online at http://www.nature.com/natureneuroscience/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Penfield WG, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. Little, Brown; Boston: 1954. [Google Scholar]
- 2.Schwartzkroin PA. Basic mechanisms of epileptogenesis. In: Wyllie E, editor. The Treatment of Epilepsy. Lea and Febiger; Philadelphia: 1993. pp. 83–98. [Google Scholar]
- 3.Fisher RS, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 4.Jiruska P, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto DJ, Patrick SL, Huang WC, Connors BW. Initiation, propagation, and termination of epileptiform activity in rodent neocortex in vitro involve distinct mechanisms. J Neurosci. 2005;25:8131–8140. doi: 10.1523/JNEUROSCI.2278-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto H, Ajmone Marsan C. Cortical cellular phenomena in experimental epilepsy: ictal manifestations. Exp Neurol. 1964;9:305–326. doi: 10.1016/0014-4886(64)90026-3. [DOI] [PubMed] [Google Scholar]
- 7.Sawa M, Nakamura K, Naito H. Intracellular phenomena and spread of epileptic seizure discharges. Electroencephalogr Clin Neurophysiol. 1968;24:146–154. doi: 10.1016/0013-4694(68)90120-x. [DOI] [PubMed] [Google Scholar]
- 8.Bower M, Buckmaster PS. Changes in granule cell firing rates precede locally recorded spontaneous seizures by minutes in an animal model of temporal lobe epilepsy. J Neurophysiol. 2008;99:2431–2442. doi: 10.1152/jn.01369.2007. [DOI] [PubMed] [Google Scholar]
- 9.Jefferys JGR. Models and mechanisms of experimental epilepsies. Epilepsia. 2003;44 (suppl 12):44–50. doi: 10.1111/j.0013-9580.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- 10.Buckmaster PS. Laboratory animal models of temporal lobe epilepsy. Comp Med. 2004;54:473–485. [PubMed] [Google Scholar]
- 11.Halgren E, Babb TL, Crandall PH. Post-EEG seizure depression of human limbic neurons is not determined by their response to probable hypoxia. Epilepsia. 1977;18:89–93. doi: 10.1111/j.1528-1157.1977.tb05590.x. [DOI] [PubMed] [Google Scholar]
- 12.Wyler AR, Ojemann GA, Ward AA., Jr Neurons in human epileptic cortex: correlation between unit and EEG activity. Ann Neurol. 1982;11:301–308. doi: 10.1002/ana.410110311. [DOI] [PubMed] [Google Scholar]
- 13.Babb TL, Wilson CL, Isokawa-Akesson M. Firing patterns of human limbic neurons during stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol. 1987;66:467–482. doi: 10.1016/0013-4694(87)90093-9. [DOI] [PubMed] [Google Scholar]
- 14.Engel AK, Moll CKE, Fried I, Ojeman GA. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci. 2005;6:35–47. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- 15.Lopes da Silva FH, et al. Dynamical diseases of brain systems: different routes to epilepsy. IEEE Trans Biomed Eng. 2003;50:540–548. doi: 10.1109/TBME.2003.810703. [DOI] [PubMed] [Google Scholar]
- 16.Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–333. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- 17.Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 18.Bikson M, Hahn PJ, Fox JE, Jefferys JGR. Depolarization block of neurons during maintenance of electrographic seizures. J Neurophysiol. 2003;90:2402–2408. doi: 10.1152/jn.00467.2003. [DOI] [PubMed] [Google Scholar]
- 19.Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cymerblit-Sabba A, Schiller Y. Network dynamics during development of pharmacologically induced epileptic seizures in rats in vivo. J Neurosci. 2010;30:1619–1630. doi: 10.1523/JNEUROSCI.5078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaari Y, Beck H. “Epileptic neurons” in temporal lobe epilepsy. Brain Pathol. 2002;12:234–239. doi: 10.1111/j.1750-3639.2002.tb00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lado FA, Moshe SL. How do seizures stop? Epilepsia. 2008;49:1651–1664. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bragin A, Penttonen M, Buzsaki G. Termination of epileptic afterdischarge in the hippocampus. J Neurosci. 1997;17:2567–2579. doi: 10.1523/JNEUROSCI.17-07-02567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treiman DM. Status epilepticus. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practice. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 681–697. [Google Scholar]
- 25.Jacobs MP, et al. Curing epilepsy: progress and future directions. Epilepsy Behav. 2009;14:438–445. doi: 10.1016/j.yebeh.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochberg LR, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 27.Truccolo W, Friehs GM, Donoghue JP, Hochberg LR. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci. 2008;28:1163–1178. doi: 10.1523/JNEUROSCI.4415-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schevon CA, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25:321–330. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng. 2008;5:455–476. doi: 10.1088/1741-2560/5/4/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truccolo W, Hochberg LR, Donoghue JP. Collective dynamics in human and monkey sensorimotor cortex: predicting single neuron spikes. Nat Neurosci. 2010;13:105–111. doi: 10.1038/nn.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller CJ, et al. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain. 2010;133:1668–1681. doi: 10.1093/brain/awq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santhanam G, et al. Hermes B: a continuous neural recording system for freely behaving primates. IEEE Trans Biomed Eng. 2007;54:2037–2050. doi: 10.1109/TBME.2007.895753. [DOI] [PubMed] [Google Scholar]
- 33.Truccolo W, Eden UT, Fellows MR, Donoghue JP, Brown EN. A point process framework for relating neural spiking activity to spiking history, neural ensemble and extrinsic covariate effects. J Neurophysiol. 2005;93:1074–1089. doi: 10.1152/jn.00697.2004. [DOI] [PubMed] [Google Scholar]
- 34.Connors BW. Initiation of synchronized neuronal bursting in neocortex. Nature. 1984;310:685–687. doi: 10.1038/310685a0. [DOI] [PubMed] [Google Scholar]
- 35.Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia. 2004;45 (suppl 4):48–56. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 36.Amritkar RE, Rangarajan G. Stability of multicluster synchronization. Int J Bifurc Chaos. 2009;19:4263–4271. [Google Scholar]
- 37.Amritkar RE, Rangarajan G. Spatially synchronous extinction of species under external forcing. Phys Rev Lett. 2006;96:258102. doi: 10.1103/PhysRevLett.96.258102. [DOI] [PubMed] [Google Scholar]
- 38.Delgado-Escueta AV, Walsh GO. The selection process for surgery of intractable complex partial seizures: surface EEG and depth electrography. In: Ward AA Jr, Penry JK, Purpura DP, editors. Epilepsy. Raven; New York: 1983. pp. 295–326. [PubMed] [Google Scholar]
- 39.Engel J, Crandall PH, Rausch P. Surgical treatment of partial epilepsies. In: Rosenburg RN, Grossman RG, Schoclet S, editors. The Clinical Neurosciences. Churchill Livingston; New York: 1983. pp. 1349–1380. [Google Scholar]
- 40.Lewicki MS. A review of methods for spike sorting: the detection and classification of neural action potentials. Network. 1998;9:53–78. [PubMed] [Google Scholar]
- 41.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Sleep states differentiate single neuron activity recorded from human epileptic hippocampus, entorhinal cortex, and subiculum. J Neurosci. 2002;22:5694–5704. doi: 10.1523/JNEUROSCI.22-13-05694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barthó P, et al. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.