Abstract
The skeleton is one of the most common sites for metastatic cancer, and tumors arising from the breast or prostate possess an increased propensity to spread to this site. The growth of disseminated tumor cells in the skeleton requires tumor cells to inhabit the bone marrow, from which they stimulate local bone cell activity. Crosstalk between tumor cells and resident bone and bone marrow cells disrupts normal bone homeostasis, which leads to tumor growth in bone. The metastatic tumor cells have the ability to elicit responses that stimulate bone resorption, bone formation or both. The net result of these activities is profound skeletal destruction that can have dire consequences for patients. The molecular mechanisms that underlie these painful and often incurable consequences of tumor metastasis to bone are beginning to be recognized, and they represent promising new molecular targets for therapy.
Introduction
The growth of disseminated tumor metastases is a major cause of mortality in patients with cancer. During the formation of a primary lesion, tumor cells undergo a variety of molecular and epigenetic events that eventually permit them to ‘escape’ from the primary tumor site.1 The now well-accepted ‘seed and soil hypothesis’ of Paget2 in essence proposes that tumor metastasis requires a series of specific interactions between tumor cells and normal host stromal cells resident at both the primary and secondary sites.3
Primary tumors constantly release cells that invade the surrounding normal tissue via the production of proteases, which allows tumor cells to cross small blood vessels in the adjacent normal tissue and enter the circulation. Once in the circulation, tumor cells interact with normal circulating cells, such as neutrophils, erythrocytes, T cells, as well as with circulating platelets, and home to distant organ sites, including bone. Although the migration of cancer cells is well-orchestrated and not a random process, the identification of the basic cellular and molecular processes that regulate their movement and subsequent arrival and survival at distant sites remain elusive;4 however, little doubt exists that modulation of both the local host and tumor microenvironments is critical for the completion of the complex, multistep metastatic cascade (Figure 1).5–9 This Review summarizes the cellular and molecular components of the metastatic cascade and highlights potential new directions for future therapeutic strategies to target bone metastasis.
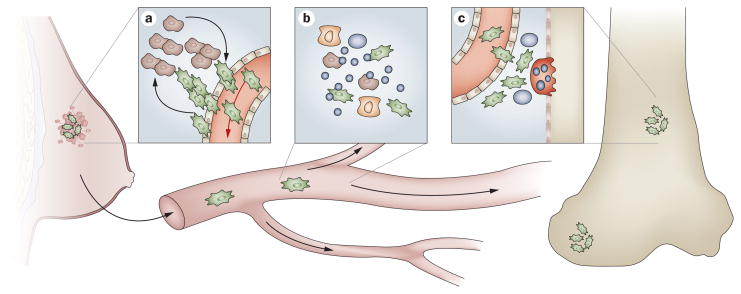
Figure 1.
Steps involved in tumor cell metastasis from a primary site to the skeleton. Each of the steps in the metastatic process offer potential points of therapeutic intervention to reverse or prevent the development of bone metastasis. a | In the primary tumor, both tumor cells (shown in green) and local stroma (shown in brown) interact via a variety of mechanisms to enhance tumor cell migration and escape into the systemic circulation. b | Once in the vasculature, tumor cells interact with resident host blood-borne cells, such as erythrocytes, T cells and neutrophils and with platelets, which facilitate survival in the circulation. c | In the bone marrow, the tumor cells escape from the vasculature (extravasation) into the bone marrow where they interact with resident bone marrow cells for subsequent survival and eventual activation of resident bone cells, such as osteoclasts (shown in red). As a result, a bone metastatic foci is formed.
Bone metastasis
Bone is a common site for metastasis owing to high blood flow in the red marrow; the presence of adhesive molecules on tumor cells that bind them to stromal cells in the bone marrow; and the production of angiogenic factors and bone-resorbing factors that enhance tumor growth, thereby providing access to the resorbed bone matrix for subsequent tumor adhesion and proliferation.10,11
The primary cancers that most frequently metastasize to bone are breast and prostate cancer, amongst many others (Table 1).12–14 Cancer cells that survive the rigors of the systemic circulation invade sinusoids in the bone marrow cavity in preparation for progression to a bone metastasis. These highly specialized cancer cells must possess certain phenotypic characteristics for bone metastasis to occur. To accomplish the complex series of steps of the metastatic cascade (Figure 1) and establish outgrowth in the bone marrow (and eventually in bone), it is critical for tumor cells to migrate across the sinusoidal wall; invade and survive in the bone marrow stroma; stimulate their own vascular supply; and migrate to the relative safety of the bone surface. Once in close proximity to the endosteal bone surface, which is completely covered by lining cells, and in conjunction with local resident bone marrow stromal cells, the tumor cells release agents that stimulate the motility of lining cells and activate bone resorption, thereby providing access to the demineralized bone surface. Each step in the metastatic cascade and the particular phenotypic characteristics of the tumor cell throughout the process represent a valid target for treatments that abrogate the metastatic process.
Table 1.
| Primary tumor type | Postmortem incidence of bone metastasis (%) |
|---|---|
| Breast | 73 |
| Prostate | 68 |
| Thyroid | 42 |
| Lung | 36 |
| Renal | 35 |
| Melanoma | 35 |
| Head and neck | 12 |
| Gastrointestinal tract | 5 |
| Ovarian | 0.1 |
Types of bone metastases
Conventional wisdom has led many to propose that bone metastases are either osteolytic (bone destructive) or osteoblastic (bone forming, sclerotic).9 In this light, osteolytic bone metastases are presumed to be caused by the release of osteoclastogenic agents by tumor cells in the bone microenvironment,9,11,15 whereas osteoblastic metastases are the result of the release of factors that stimulate osteoblast proliferation, differentiation and subsequently uncontrolled bone formation by metastatic cancer cells (Figure 2).16,17 Purely lytic or sclerotic bone lesions are, however, but two extremes of a spectrum of activity that drives tumor destruction of bone, and both processes are typically present in any skeletal site affected by metastases. Accordingly, bone metastases are typically characterized as ‘lytic’, ‘sclerotic’ or ‘mixed’, according to the radiographic and/or pathologic appearance of the lesions.18
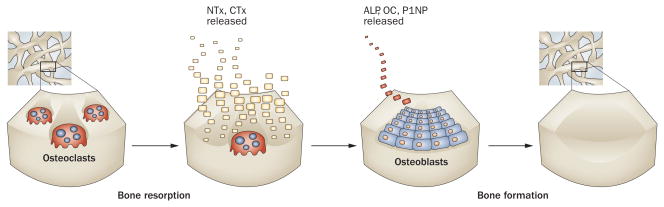
Figure 2.
Biochemical markers of bone turnover are released during bone remodeling. In humans, bone is repeatedly removed and replaced by the continuous and well-managed processes of bone resorption (by osteoclasts) and bone formation (by osteoblasts). Collectively, these coupled processes comprise the bone remodeling cycle that is the fundamental physiologic process responsible for the maintenance of bone mass and strength. The process of bone remodeling is highly ordered and is mediated by changes in the activities of multinucleated osteoclasts (monocyte/macrophage-derived cells) and osteoblasts (mesenchymal-derived cells). Following activation of osteoclastogenesis and recruitment to the bone surface, activated precursors fuse, become multinucleated, form a ruffled border and begin excavation of the bone surface. During the process of bone resorption, catalytic fragments of bone matrix type I collagen (NTx and CTx) are released and enter the systemic circulation. As resorption is completed, mesenchymal osteoblast precursors are recruited to the previously resorbed bone surface and osteoblastogenesis occurs, which results in the differentiation of active, cuboidal osteoblasts. These secretory cells lay down osteoid that is eventually mineralized so that the previously resorbed surface is replaced with an equal quantity of newly formed bone. During the process of bone formation, the osteoblasts secrete characteristic protein markers, alkaline phosphatase (ALP), osteocalcin (OC) and procollagen type 1 aminoterminal propeptide (P1NP), which are the clinical markers correlated with bone formation activity. In cancer patients with bone metastases, tumor cells disrupt the normal process of bone resorption and bone formation, leading to increased bone destruction and/or aberrant bone formation. Abbreviations: CTx, carboxyterminal crosslinking telopeptide of type I collagen; NTx, aminoterminal crosslinking telopeptide of type I collagen.
The tumor-associated activity can be visualized clinically, via the measurement of bone biochemical markers or by radiographs, and pathologically, by measurement of osteoclast numbers and resorption lacunae. These analyses are valuable even in sclerotic, bone-forming lesions.18 Bone resorption and bone formation are tightly linked (Figure 2); although this coupling may become disrupted in cancer, it is nevertheless always present. In fact, as demonstrated by the clinical measurement of bone biochemical markers (Box 1),19 both bone resorption and bone formation activity may be elevated in patients with primary breast cancer but without clinical evidence of bone metastasis.20
Box 1. Biochemical markers of bone remodeling.
During the process of bone remodeling, the activity of osteoblasts and osteoclasts results in the release of proteins or peptides that reflect cellular activity. The peptide products are either proteins released by the cells (alkaline phosphatase, osteocalcin) or degradation products of cellular activity (urinary hydroxyproline, deoxypyridyniline, urinary nonisomerized [αCTX] and β-isomerized [βCTX] carboxyterminal crosslinking telopeptide of type I collagen, procollagen type 1 aminoterminal propeptide [P1NP]). The measurement of these clinical biomarkers, categorized as the biochemical markers of bone remodeling, in serum and urine is widely used as a rapid means by which cellular activity of bone can be monitored.
Osteolytic metastasis
Osteolytic lesions are the most common feature of multiple myeloma—a primary bone tumor—and breast cancer, as well as a variety of other cancers (Table 1).18 As a densely mineralized tissue with high rigidity and modulus, bone represents an especially harsh environment for any tumor cell to establish and grow.21 Osteolysis is caused by tumor stimulation of osteoclast differentiation and activity rather than by any direct effects of cancer cells on the skeleton.22 In other words, invasive capabilities are, of course, essential for tumor progression, but the critical and characteristic phenotype that tumor cells must acquire in order to metastasize to and invade the skeleton is the ability to ultimately stimulate bone resorption.21,23 This function, uniquely performed in mammals by monocyte/macrophage-derived osteoclasts, provides an environment that is receptive to transiting tumor cells and allows them to survive and proliferate.11 In fact, tumor stimulation of osteoclastic bone resorption at the bone marrow–bone interface is required for tumor establishment as a bone metastasis within the strict confines of the mineralized structure of bone.21
Osteoblastic metastasis
Although the dominant lesion type in bone metastases that arise from breast cancer is lytic, an associated local bone formation response almost always occurs. As many as 25% of patients with bone metastases caused by breast cancer also have osteoblastic lesions.18 This phenomenon presumably represents a physiological attempt to activate bone repair.24 The increased bone formation in cancer patients with osteolytic lesions can be measured clinically via increased serum alkaline phosphatase levels, a marker of osteoblast activity, or increased uptake of technetium diphosphonate in clinical bone scans—a diagnostic test that identifies the presence of increased cellular activity and of metastases. However, despite the secondary increases in local tumor-induced bone formation, the predominant effect of bone metastasis in breast cancer remains substantial bone loss. This effect is presumably related to the different efficiencies in bone resorption versus bone formation, as bone resorption is far more rapid than any bone formation response.
In prostate cancer, bone metastases are primarily osteoblastic.25,26 In these metastatic sites, local stimulation of osteoblast activity results in bone formation directly adjacent to the metastatic tumor.25 These lesions avidly take up technetium diphosphonate in bone scans. Parameters of osteoblast activity, such as alkaline phosphatase and osteocalcin levels, are also significantly elevated. Nevertheless, many patients with prostate cancer will also exhibit osteolytic components in bone lesions.27
Bone resorption and formation in metastasis
Coupling of bone turnover
The evidence that both bone resorption and formation are activated in the majority of bone metastases is extensive and includes the following.28 First, the biochemical markers of osteoblast activity are often significantly elevated. Second, the increased uptake of technetium diphosphonate at sites of bone metastasis identifies the presence of increased cellular activity and metastasis. Third, in osteoblastic metastases from prostate cancer, the serum markers of bone resorption are frequently more markedly increased than in osteolytic metastases.29–31
In numerous in vivo studies of tumor progression and metastasis in immunodeficient and immunocompetent animals, histomorphometric evaluation of bone metastases identified coupling between bone resorption and bone formation associated with tumor progression.32–36 The initial activation of osteolysis is characterized by dramatic increases in osteoclast activity that are directly associated with increases in systemic bone resorption markers (Figure 2). The bone resorption activity is then followed by an increase in bone formation, as occurs in normal bone remodeling, where bone resorption always precedes bone formation.37
Uncoupling of bone turnover
As is to be expected given the complexity of human cancer, some clear exceptions to the general concept that tumors contain both lytic and blastic components exist. In some patients, bone lesions can be purely lytic. For example, in multiple myeloma, tumor cells resident in bone marrow cause exclusively osteolytic lesions, with a decrease or even an absence of osteoblastic bone formation adjacent to the tumor.38 The uncoupling of bone resorption and bone formation is a defining feature of multiple myeloma that is reflected by the increased osteoclast number and activity in the regions surrounding the tumor.39 These same regions are also devoid of osteoblasts.39 The selective impairment of osteoblast activity in myeloma results primarily from the blockade of osteogenic differentiation of mesenchymal progenitors to mature osteoblasts,40 and suppression of osteoblast activity plays a key part in the process of bone destruction, as well as tumor progression. The bone marrow microenvironment in which myeloma cells survive is crucial for tumor initiation and expansion and has a pivotal role in the extensive resistance to radiotherapy and chemotherapy that is characteristic of this disorder.10
The effects of ionizing radiation on the growth of myeloma cells was investigated in the presence or absence of the potent antiangiogenic agent anginex in a mouse model of myeloma bone disease.41 Interestingly, radiation or anginex alone significantly diminished tumor growth. Tumor burden remained low 4 weeks after the withdrawal of antiangiogenic therapy and radiation treatment in these mice, whereas it was significantly increased in mice that had received no treatment, radiation alone or antiangiogenic therapy alone. Thus, radiation, primed by an appropriate antiangiogenic agent, may have potential not only as a therapy for focal myeloma bone disease41 but also to treat bone metastasis.
By contrast, some purely osteoblastic lesions such as those seen in osteosarcoma—the third most common malignancy in children and adolescents that accounts for approximately 5% of all cancers in these age groups—have elevated bone formation markers without substantial changes in resorption.42
The bone marrow microenvironment
The development of a bone metastasis replicates the many steps required for metastasis to any distant organ (Figure 1). This series of events is largely inefficient, as the vast majority of tumor cells that enter the circulation do not survive the normal protective host-surveillance mechanisms.8,9,11 In addition to the relatively few meta-static cancer cells that reach and inhabit the bone marrow, cells resident in the bone marrow microenvironment markedly contribute to the development of tumor cell foci. These host cell types are generally considered to be either transient cells or resident stromal cells (Figure 3).43
Figure 3.
The stimulation of bone cell activity by tumors in the bone marrow. Interactions between tumor cells, bone marrow components (for example, stromal cells, platelets, immune cells and hematopoietic progenitors) and resident bone cells results in activation of both osteoclasts and osteoblasts, causing bone resorption, as well as robust bone formation. Tumor-derived activation of host bone cells also supports the aggressive growth and behavior of tumor cells. (1) The release of tumor-derived factors, such as parathyroid hormone-related protein (PTHrP), interleukin 8 (IL-8), tumor necrosis factor (TNF), transforming growth factor β (TGF-β), heparanase and many others, enhances osteoclast activation and bone resorption via RANKL-dependent and RANKL-independent mechanisms. Bone resorption results in the release of bone-derived growth factors that support tumor proliferation. Tumor activation also drives the activation of local stromal cells and platelets that may also enhance tumor proliferation. (2) The production of growth factors such as fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), TGF-β and Wnt family members by metastatic tumors stimulates osteoblast activity, leading to increased bone formation. The result of the enhanced osteoblast proliferation and activity is again tumor stimulation, as well as the normal coupling responses to help enhance osteoclastogenesis and bone resorption. Collectively, these numerous cellular interactions drive all of the well-described skeletal consequences of bone metastasis and result in the inappropriate bone formation and bone resorption characteristic of bone metastasis.
Host cell types
Transient cells
Of the many transient cell types in the metastatic bone marrow microenvironment, such as neutrophils, erythrocytes, T cells (derived from hematopoietic stem cells) and other immune cells, the platelet is among the most interesting. Platelets are small, irregularly-shaped anuclear cell fragments, some 2–3 μm in diameter, that are derived from fragmentation of precursor megakaryocytes. Platelet adhesion to circulating tumor cells was originally thought of as a ‘cloak’ that tumors use to evade the innate immune system during metastasis.44 However, the role of platelets in tumor metastasis has clearly expanded beyond the avoidance of immune surveillance. A role for platelets has emerged with notable platelet contributions evident in tumorigenesis, inflammation and bone resorption. Adhesion between platelets and tumor cells and interactions of tumor cells with other circulating cells support tumor survival in the circulation. It seems reasonable to presume that the increased survival advantage in and outside of the vasculature offered by these interactions is critically important for the ability of tumor cells to colonize distant target organs.
Platelets play a fundamental part in hemostasis and are a natural source of growth factors.45 The adhesion of platelets to MDA-MB-231 breast cancer cells induces the release of lysophosphatidic acid from platelets,46 which subsequently stimulates secretion of the potent angiogenic and pro-osteoclastogenic agent interleukin 8 (IL-8) from tumor cells.47 In other studies using transgenic mice congenic for expression of a dysfunctional platelet-specific glycoprotein GPIb-IX receptor complex, lung metastasis induced by B16F10.1 melanoma cells was reduced 15-fold, indicating that the extracellular domain of the platelet-specific GPIb-IX receptor complex mediates lung metastasis.48 The same mouse model also demonstrated a surprising high bone mass phenotype, the result of decreased osteoclast formation.49 These data strongly implicate the platelet in the regulation of the local bone marrow microenvironment, a finding worthy of further interrogation, and suggest that interactions between platelets and tumor cells might mediate local changes in bone resorption, vascularity and inflammation.49,50
Resident cells
The vascular endothelial cell component of the bone marrow provides a microenvironment that is favorable to the circulating tumor cells. The high vascularity of the bone marrow facilitates the high frequency of bone metastasis and the increased survival of tumor cells.51 Clearly, the presence or initiation of angiogenesis is required for survival of metastatic tumor cells. In addition, many factors that are secreted by tumors, such as IL-8, are potent stimulators of angiogenesis, suggesting a feedback loop that can support tumor cell survival at the primary as well as at distant metastatic sites.43
Resident stromal cells, such as mesenchymal stem cells—the precursors of adipocytes, fibroblasts, chrondrocytes or osteoblasts—support the proliferation, differentiation and survival of bone marrow hematopoetic cells, as well as of circulating metastatic tumor cells.52 The heparan sulfate proteoglycans and carbohydrates that decorate the tumor cell surface and the enzymes that modify these molecules, such as heparanase, act to promote the survival, growth and metastasis of tumor cells via a variety of mechanisms.53–55 Similarly, the expression of activin A by bone marrow stromal cells in multiple myeloma stimulates osteolysis and decreases osteoblastogenesis, and treatment with a soluble activin A receptor (RAP011) significantly inhibits myeloma bone disease.56
Activation of tumor cells
The time frame for activation of disseminated tumor cells and/or local bone marrow and bone cells is highly variable and is a critical concept known as ‘tumor dormancy’. A growing body of evidence supports the rediscovered ‘stem cell theory’ of cancer (Box 2).57 This idea proposes that tumors contain rare cells with infinite growth potential and a variable affinity for distant metastasis.58 However, whether tumor stem cells are bona fide stem cells or a highly malignant and specific cellular subpopulation remains to be determined.59 Some evidence even suggests that tumor-propagating cells, unlike true stem cells, are relatively common.60 Regardless of whether tumor-initiating cells are indeed stem cells that have undergone transformation or some other progenitor or cancer cell that has gained specific stem cell functions, such as self-renewal, tumor-proliferating cells evidently possess certain properties of stem cells.11 Although extremely difficult to define and identify, these elusive cell populations remain an attractive and viable therapeutic target with the potential to radically alter treatment approaches.
Box 2. Models of metastasis.
A number of contrasting theories have re-emerged that provide possible explanations for the metastatic selectivity of cancer.123
The ‘traditional metastasis model’ suggests that select subpopulations of tumor cells acquire metastatic capacity during the late stages of tumorigenesis.123,124 This concept seems improbable, as numerous investigators have demonstrated that the vast majority of tumor cells, independent of tumor stage, have the potential to develop into a metastasis.123,124
The ‘dynamic heterogeneity theory’ suggests that the metastatic potential of tumor cells is determined by the rate at which tumor variants with increased metastatic potential occur within the primary tumor site.125
The ‘clonal selection theory’ proposes that all primary tumors evolve from the same cell, and development of a primary tumor is the consequence of a series of multiple molecular changes resulting in clonal selection. This complex process specifically alters the phenotype of the tumor cell, allowing acquisition of different tumor-specific characteristics, such as the ability for site-specific metastasis.8,59
The ‘stem cell theory’ of cancer proposes that the site selectivity of metastasis is the result of the activation of the so-called cancer stem cell compartment within a specific organ, such as the breast.59
As these metastatic models are not mutually exclusive, it is reasonable to assume that a variety of as yet uncharacterized genetic, molecular, cellular and cell type-specific mechanisms regulate tumor initiation and metastasis to and survival within the skeleton, presumably involving features of all theories.59
Molecular mediators of bone metastases
osteolytic mediators
Transforming growth factor β
Once resident in bone, tumor cells, as well as factors released by the primary tumor, activate bone resorption and release transforming growth factor β (TGF-β) from stores in the bone (Figure 3). TGF-β signaling occurs after specific ligand binding to the type II receptor serine kinase (TGFβRII) on diverse target cells.61 TGFβRII activation results in phosphorylation of the type I receptor (TGFβRI) and signal transduction via phosphorylation of the downstream, regulatory Smad substrates Smad2 and Smad3.61 Subsequent binding of Smad2 or Smad3 to Smad4 results in nuclear translocation and increased transcription of target genes.62
TGF-β released from bone inhibits T-cell proliferation and activity and the function of NK (natural killer) cells,63 thereby suppressing the immune system. In addition, the enhanced release of tumor-secreted factors such as parathyroid hormone related peptide (PTHrP) and IL-8 can activate T cells, thereby increasing the process of bone resorption, while suppressing T-cell function.63 As a result, antibody-producing B cells, so-called plasma cells, upregulate expression of CXC-chemokine receptor 4 (CXCR4) upon completion of differentiation. This upregulation is a signal to which both cancer cells and stromal cells respond. Both cell types commonly express the CXCR4 ligand stromal-derived factor-1 (SDF-1; also known as CXCL12).64 This ligand–receptor interaction has been suggested to facilitate cancer-cell migration throughout the bone microenvironment.65
In addition to its well-described mediation of osteolytic bone metastasis,9,66 TGF-β released from the mineralized bone matrix during osteoclastic bone resorption has direct effects on tumor progression in bone.67 Thus, both tumor cell-derived and bone-derived TGF-β stimulates local cell proliferation in the bone marrow micro-environment.17 TGF-β is also secreted by resident bone marrow cells, contributing further to innate immune suppression and local bone marrow activation.17
As many tumor types secrete TGF-β and respond to it by enhanced invasion and metastasis, targeting of TGF-β signaling pathways via direct antitumor actions or immunomodulation of the tumor microenvironment and/or effects on bone is a valid approach for the treatment of bone metastasis. In support of this concept, small molecule TGFβRI inhibitors have been shown to induce a variety of skeletal changes, including increased bone mass and improved bone material properties;68 results of current ongoing clinical trials are eagerly awaited.
PTHrP and RANKL
Perhaps the most studied mediator of osteoclast stimulation in bone metastasis from breast cancer is tumor-derived PTHrP. The initial cloning and identification of PTHrP from metastatic human lung cancer cells69 and its demonstration as the causal agent of the humoral hypercalcemia of malignancy70 identified it as a previously unrecognized hormone. These observations initiated an ongoing series of studies focused on the role of PTHrP in bone metastasis.
PTHrP stimulates osteoclast activity via the cytokine RANKL (receptor activator of nuclear factor κB ligand), which causes osteoclast formation and activation by binding to its receptor RANK on osteoclasts and their precursors.71,72 RANKL is the primary physiologic mediator of osteoclast formation, function and survival, and the vast majority of pro-osteoclastogenic agents work via upregulation of RANKL.73
The first clinical evidence that PTHrP was an important mediator of bone metastasis in patients with breast cancer was the observation that osteolytic human breast tumors express PTHrP, and PTHrP expression was greater when the tumor cells were present at the metastatic bone site rather than at soft tissue sites or at the primary tumor site in the breast.74,75 As subsequently shown, the vast majority of bone metastases that arise from breast cancer (>90%) express PTHrP, which led to the conclusion that PTHrP is the major osteoclastogenic factor secreted by metastatic tumor cells.21 However, although strong evidence from numerous preclinical studies supports the concept that PTHrP is an important local mediator of osteolytic bone lesions,9,76 clinical data from an antihuman PTHrP antibody trial in the treatment of bone metastases from breast cancer are currently lacking.
By contrast, a large prospective clinical trial demonstrated that the role of PTHrP is not in mediating metastasis but in other stages of cancer progression.77 PTHrP expression by primary breast cancers was associated with improved prognosis and decreased metastasis to all sites, including bone.77 This result implicated an activity that remains unknown but is distinct from the well-characterized and widely accepted osteolytic action of tumor-derived PTHrP in bone.9 In other words, the phenotype of metastatic breast cancer cells in bone, including the expression of PTHrP, is distinct from the phenotype of the tumor cells at the primary tumor site in the breast. Conceivably, the expression of PTHrP at sites of bone metastasis is the result of the tumor cells’ successful completion of the metastatic cascade (Figure 1) and the influence of the bone microenvironment.15,78
RANKL-independent mediators
Based on the complex interactions that mediate all aspects of tumor metastasis, the notion that RANKL activation and the TGF-β–PTHrP axis are the sole mediator of tumor osteolysis is naive. Metastatic breast cancer cells that reside in the bone marrow microenvironment secrete a plethora of osteolytic factors,9,11 capable of stimulating osteoclast formation and bone resorption via both RANKL-dependent and RANKL-independent mechanisms.15,47,79–85
The most compelling evidence that supports RANKL-independent effects on tumor-induced osteoclastogenesis and osteolysis come from clinical trials of the fully human RANKL antibody (denosumab) in patients with bone metastases and elevated bone resorption.86 In one study, patients with bone metastases treated with denosumab every 4 or 12 weeks had significantly decreased urinary N-telopeptide levels more frequently than patients treated with an intravenous bisphosphonate.87 Interestingly, bone resorption was decreased to only about 75–80% of baseline levels, not 100% as might be expected if RANKL alone was driving osteoclast formation and function in these patients. Similar levels of inhibition of resorption were reported in earlier denosumab trials, which used a variety of doses, in patients with myeloma or bone metastasis from breast cancer.87–91
The observations in patients with cancer treated with denosumab are in stark contrast to the level of suppression of bone resorption observed in patients with postmenopausal osteoporosis. In this group of patients, treatment with denosumab elicited reductions in mean levels of serum C-telopeptide of >90%, as might be expected if RANKL is the primary osteoclastogenic agent.92 These data demonstrate the efficacy of the RANKL antibody for the inhibition of osteolysis in patients with cancer, but also reveal that in individuals with elevated bone resorption, tumor-derived factors other than RANKL probably contribute to the increased osteoclast activity.
osteoblastic mediators
As with osteolytic tumors, an accumulating weight of data implicate a variety of factors in the stimulation of bone formation associated with metastatic prostate cancer (Figure 3).
Growth factors
A major challenge in prostate cancer biology is the lack of understanding of the mechanisms of prostate cancer progression and the development of bone metastases. Prostate cancer cells express a large variety of growth factors capable of activating resident bone and bone marrow cells,93–95 for example, acidic and basic fibroblast growth factors (FGFs),96,97 as well as bone morphogenetic proteins (BMPs).98 The expression of these potent osteoblast stimulatory factors is a potential mediator of the profound osteoblast proliferation and bone formation observed in patients with bone metastasis that arise from prostate cancer.
BMPs, originally characterized as inducers of bone formation, were later demonstrated to play an important part in the regulation of cell growth and differentiation.99 Multiple BMPs and their cognate receptors (BMPRs) are expressed in normal prostate and prostate cancer cells.100–102 Altered expression and function of BMPs and BMPRs has been reported during prostate development and prostate cancer progression, and numerous reports have demonstrated effects of BMP on proliferation, invasiveness and growth of prostate cancer cells in bone in vivo.103,104 However, at present, the effects of specific BMPs on osteoblastic tumor progression are inconclusive, given the inconsistency of published results.98
The FGF family currently consists of 22 ligands that bind and activate multiple FGF receptor (FGFR) isoforms. The well-established roles of FGFs and FGFRs were initially identified during embryonic development; the FGF–FGFR2 axis was linked to the embryonic development of both the mammary and prostate glands,105 hence, the interest in these molecules in the regulation of prostate cancer development and progression. Expression of FGFs is elevated in prostate cancer, and these factors can potentially act in either a paracrine or autocrine manner.106 In addition to the ligands, prostate cancer progression has been associated with the expression of specific FGFR isoforms, primarily FGFR1 and FGFR4. Activation of FGFRs results in the stimulation of a variety of signal transduction pathways such as phospholipase Cγ (PLCγ), phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK) and signal transducers and activators of transcription (STAT).106 The finding of overexpression of FGF10 in prostate stroma107 and of FGFR1 in prostate epithelium108 support the idea that FGF10 helps drive prostate cancer tumorigenesis. Similarly, FGFs also seem to regulate the osteoblastic response to bone metastases from prostate cancer. Both acidic and basic FGFs (FGF1 and FGF2) cause the profound stimulation of bone formation in vivo.109–111 FGF2 was also shown to stimulate the proliferation and differentiation of osteoblasts via the concomitant upregulation of both RUNX2 and BMP2.112 Whether this regulation of bone formation via FGF2 also has a role in the formation of bone formation during bone metastasis from prostate cancer remains undetermined.
Wnt signaling
Wnts comprise a large family of secreted glycoproteins that perform a variety of important biologic functions. Members of the Wnt family bind a membrane-bound receptor complex containing the G-protein coupled receptor frizzled (FZD) and an LDL receptor family member. In the past years, canonical Wnt signaling has been shown to play a central part in normal osteoblast development and bone formation.113 This idea has been extended of late to suggest that Wnts also have a paracrine activity to regulate bone formation in bone metastasis from prostate cancer.114
Prostate cancer cells that metastasize to bone have been reported to secrete dickkopf-related protein 1 (DKK1), a secreted Wnt antagonist, early in the development of the skeletal metastasis.115 As the bone metastasis progresses, DKK1 expression decreases, leading to a Wnt-mediated increase in osteoblastic activity, which causes the well-described secondary osteoblastic lesions of prostate cancer.115 In addition, the effects of endothelin-1 to increase osteoblast proliferation and new bone formation also seem to be associated with activation of the Wnt signaling pathway via suppression of the Wnt pathway inhibitor DKK1.116 In sum, these data reinforce the critical need to consider the context of the tumor–stroma microenvironments when investigating pathways relevant to the metastatic progression of cancer.
Metastases and clinical outcome
The growth of disseminated tumor metastases is a major cause of mortality in patients with cancer. For example, the early diagnosis of breast cancer in the absence of metastases is associated with a 5-year survival of around 90%. However, in the presence of metastasis, long-term survival is significantly less (10%).12 Nonetheless, the development of bone metastases from breast cancer is associated with considerably better patient prognosis than a metastasis in other tissues.12
In patients with aggressive tumor growth at the primary site, bone metastases are relatively uncommon. This finding does not mean that, in these particular instances, the tumor cells do not have the ability to grow avidly in bone, but that they may not have had the opportunity to do so. Breast cancer cells can also enter the lymphatic system, where they travel to the sentinel nodes in the axilla and intercostal spaces. This invasive step is considered an indication of the aggressiveness of the particular tumor. Patients with histologically confirmed negative lymph nodes and only the primary tumor (stage I) are considered curable,117 as are patients with later-stage breast cancer. However, 15% of patients with stage I tumors, with little or no lymph node involvement, may go on to later develop metastasis in different tissues.117 Invasion of the lymph nodes should be considered as an early step in the metastatic cascade and one that can lead to bone metastasis by drainage of tumor cells back into the systemic circulation.
Prostate cancer also frequently metastasizes to bone, as approximately 90% of men with high-grade prostate cancer show evidence of skeletal lesions.118 Even if localized to the prostate, a 15–20% incidence of subsequent metastatic disease has been reported.118
Treatment
For patients with overt bone metastases, current treatment objectives are designed to decrease tumor burden, prevent further progression and metastasis and inhibit tumor-associated bone pathology, such as pathologic fracture, pain or hypercalcemia.11 Several local bone metastasis treatment strategies are primarily palliative in nature; individual lesions are surgically excised and the tumor ‘bed’ irradiated, either before or after surgery. The decision for or against surgery and/or radiation, alone or in combination with select bone-targeted agents, is profoundly influenced by the extent of systemic disease at the time of treatment.11
The concept that bone resorption and bone formation are critical for the progression of bone metastasis suggests that, if osteolysis is disrupted, then not only a decrease in bone resorption but also a decrease in tumor burden in bone, and thus potentially an increase in bone mass and strength, will occur. As the bone microenvironment is essential for the growth and aggressive behavior of meta-static cancers in the skeleton, the clinical rationale for the development of specific inhibitors of bone resorption and activators of osteoblastic activity is obvious. To date, other therapeutic regimens, which include but are not limited to bisphosphonates, are available for the treatment of cancer patients with bone metastases.87,119 In fact, treatment with bisphosphonates has shown a decrease in bone resorption and improved disease-free survival even in pre-menopausal patients with estrogen-responsive early-stage breast cancer.120
Similarly, the increased understanding of the role of the bone–tumor microenvironment has been translated into the development of additional bone-targeted therapies, such as denosumab. This agent is FDA-approved for the prevention of fractures and skeletal problems in patients with bone metastases from solid tumors. Denosumab treatment (at higher and more frequent doses than those used in patients with osteoporosis) delayed the time to a first skeletal-related event compared with the bisphosphonate zoledronic acid in patients with bone metastases from breast or prostate cancer.121
Conclusions
The discussion above strongly supports the notion that the bone marrow represents a supportive environment that protects metastatic cancer cells during the process of metastasis. This idea should also be extended to include the notion that the bone marrow microenvironment is a tumor-cell-friendly zone, from which metastatic cells can progress to become a bone metastasis, seed other organs or even reseed the site of the original primary tumor. Indeed, although the interaction between osteoclasts and tumor cells is crucial, the interactions between tumor cells and the cells resident in the bone marrow microenvironment, such as immune cells and the bone marrow stroma, are equally important. If correct, the current concept behind the development of treatments for bone metastasis, namely bone targeting, requires substantial revision.45 This idea also raises several fundamental and important questions. How do tumor cells that manage to metastasize to the bone marrow microenvironment remain dormant, sometimes for decades? What markers can be used to identify meta-static tumor cells in vivo? Does a specific bone marrow microenvironment represent the cancer cell niche? If this niche exists, how can it be identified, targeted, protected or blocked? Will the consequence of the inhibition of bone metastasis be increased soft tissue metastasis?
Increasing evidence supports the idea that particular patient parameters, such as tumor cells in the bone marrow, may mediate the response to therapy and perhaps even the recurrence patterns in individual patients.122 Therefore, the continued elucidation of characteristics of the bone marrow microenvironment responsible for mediating these diverse effects represents a valid therapeutic target, with the potential to improve patient care. Such studies are the focus of a large number of ongoing investigations, the results of which we optimistically and eagerly await. It is our belief that, as the pathways leading to the development of metastatic bone lesions are defined, more effective therapies that target the bone marrow microenvironment and/or tumor progression, in addition to bone destruction, will surely follow.
Key points.
Bone is a common site of metastasis for many tumors, such as breast, lung and prostate cancer
A continuum of bone metastasis exists that extends from primarily osteolytic lesions with limited osteoblast activity to bone metastases that are predominantly osteoblastic, with limited osteoclast activity
The complex interactions between circulating tumor cells, circulating host cells, platelets and cell-derived factors are critical for tumor establishment at metastatic sites
Tumor activation of bone resorption is complex and involves both receptor activator of nuclear factor κB ligand (RANKL)-dependent and RANKL-independent mechanisms
Targeting bone resorption with bisphosphonates reduces osteolytic bone resorption and improves disease-free survival
All steps in the metastatic process and the interaction with host cells are valid therapeutic targets for the treatment of bone metastasis and tumor progression
Review criteria.
A search for original articles listed in PubMed and focusing on bone metastases was performed. The search terms used were “bone”, “cancer”, “metastasis” and “mechanisms”. All articles identified were English-language, full-text, peer-reviewed articles published between 1973 and 2010. In addition, the professional opinion and understanding of all authors was considered. All cited material was considered and reviewed in the preparation of the manuscript.
Acknowledgments
The authors dedicate this manuscript to the memory of Dr Gregory R. Mundy. A fellow ex-patriot Australian, Greg’s boundless energy, determination and drive virtually single-handedly motivated all of us to acknowledge and appreciate the intricacies of the bone–tumor microenvironment. His presence, unique perspective and intellect are sorely missed. Work into the mechanisms of cancer progression and bone metastases described in this Review was supported by NIH 5R01CA107160 (R. J. Griffin) and the Carl L. Nelson endowed Chair in Orthopedic Creativity (L. J. Suva).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
L. J. Suva and C. Washam researched the data for the article. L. J. Suva and R. W. Nicholas provided substantial contributions to discussions of the content. L. J. Suva and R. J. Griffin contributed equally to writing the article. All authors reviewed and/or edited the manuscript before submission.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 3.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 4.Amano H, et al. Roles of a prostaglandin E-type receptor, EP3, in upregulation of matrix metalloproteinase-9 and vascular endothelial growth factor during enhancement of tumor metastasis. Cancer Sci. 2009;100:2318–2324. doi: 10.1111/j.1349-7006.2009.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 8.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 9.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 10.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 11.Suva LJ, Griffin RJ, Makhoul I. Mechanisms of bone metastases of breast cancer. Endocr Relat Cancer. 2009;16:703–713. doi: 10.1677/ERC-09-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243S–6249S. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 13.León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680–686. doi: 10.1002/1097-0347(200010)22:7<680::aid-hed7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Patten RM, Shuman WP, Teefey S. Metastases from malignant melanoma to the axial skeleton: a CT study of frequency and appearance. AJR Am J Roentgenol. 1990;155:109–112. doi: 10.2214/ajr.155.1.2112830. [DOI] [PubMed] [Google Scholar]
- 15.Bendre M, Gaddy D, Nicholas RW, Suva LJ. Breast cancer metastasis to bone: it is not all about PTHrP. Clin Orthop Relat Res. 2003;415 (Suppl):S39–S45. doi: 10.1097/01.blo.0000093844.72468.f4. [DOI] [PubMed] [Google Scholar]
- 16.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310:71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Guise TA, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213S–6216S. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 18.Coleman Re. Skeletal complications of malignancy. Cancer. 1997;80(Suppl):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Coleman R, et al. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat Rev. 2008;34:629–639. doi: 10.1016/j.ctrv.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demers LM, et al. Biochemical markers of bone turnover in patients with metastatic bone disease. Clin Chem. 1995;41:1489–1494. [PubMed] [Google Scholar]
- 21.Martin TJ. Manipulating the environment of cancer cells in bone: a novel therapeutic approach. J Clin Invest. 2002;110:1399–1401. doi: 10.1172/JCI17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyde A, Maconnachie e, Reid SA, Delling G, Mundy GR. Scanning electron microscopy in bone pathology: review of methods, potential and applications. Scan Electron Microsc. 1986;4:1537–1554. [PubMed] [Google Scholar]
- 23.Mundy GR, Martin TJ. Physiology and Pharmacology of Bone. Springer-Verlag; Berlin: 1993. pp. 641–671. [Google Scholar]
- 24.Stewart AF, et al. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab. 1982;55:219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- 25.Charhon SA, et al. Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer. 1983;51:918–924. doi: 10.1002/1097-0142(19830301)51:5<918::aid-cncr2820510526>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Roudier MP, et al. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180:1154–1160. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman RE. Conclusion: Bone markers in metastatic bone disease. Cancer Treat Rev. 2006;32(Suppl 1):27–28. doi: 10.1016/s0305-7372(06)80007-1. [DOI] [PubMed] [Google Scholar]
- 28.Fili S, Karalaki M, Schaller B. Mechanism of bone metastasis: the role of osteoprotegerin and of the host-tissue microenvironment-related survival factors. Cancer Lett. 2009;283:10–19. doi: 10.1016/j.canlet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Percival RC, et al. Biochemical and histological evidence that carcinoma of the prostate is associated with increased bone resorption. Eur J Surg Oncol. 1987;13:41–49. [PubMed] [Google Scholar]
- 30.Pelger RC, Hamdy NA, Zwinderman AH, Lycklama à Nijeholt AA, Papapoulos SE. Effects of the bisphosphonate olpadronate in patients with carcinoma of the prostate metastatic to the skeleton. Bone. 1998;22:403–408. doi: 10.1016/s8756-3282(97)00289-5. [DOI] [PubMed] [Google Scholar]
- 31.Garnero P, et al. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858–864. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi B, Williams PJ, Niewolna M, Wang Y, Yoneda T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 2002;62:917–923. [PubMed] [Google Scholar]
- 33.Yin JJ, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thalmann GN, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 35.LeRoy BE, et al. New bone formation and osteolysis by a metastatic, highly invasive canine prostate carcinoma xenograft. Prostate. 2006;66:1213–1222. doi: 10.1002/pros.20408. [DOI] [PubMed] [Google Scholar]
- 36.Power CA, et al. A novel model of bone-metastatic prostate cancer in immunocompetent mice. Prostate. 2009;69:1613–1623. doi: 10.1002/pros.21010. [DOI] [PubMed] [Google Scholar]
- 37.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 38.Roodman GD. Pathogenesis of myeloma bone disease. Blood Cells Mol Dis. 2004;32:290–292. doi: 10.1016/j.bcmd.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Pennisi A, et al. Inhibitor of DASH proteases affects expression of adhesion molecules in osteoclasts and reduces myeloma growth and bone disease. Br J Haematol. 2009;145:775–787. doi: 10.1111/j.1365-2141.2009.07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian E, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 41.Jia D, et al. Repression of multiple myeloma growth and preservation of bone with combined radiotherapy and anti-angiogenic agent. Radiat Res. 2010;173:809–817. doi: 10.1667/RR1734.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokkel MP, Linthorst MF, Borm JJ, Taminiau AH, Pauwels EK. A reassessment of bone scintigraphy and commonly tested pretreatment biochemical parameters in newly diagnosed osteosarcoma. J Cancer Res Clin Oncol. 2002;128:393–399. doi: 10.1007/s00432-002-0350-5. [DOI] [PubMed] [Google Scholar]
- 43.Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis: what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 44.Fidler IJ, Gersten DM, Hart IR. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- 45.Suva LJ. Adjuvant bisphosphonates in breast cancer: the ABCSG-12 study. Curr Osteoporos Rep. 2010;8:57–59. doi: 10.1007/s11914-010-0012-5. [DOI] [PubMed] [Google Scholar]
- 46.Boucharaba A, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bendre MS, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005;65:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 48.Jain S, et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci USA. 2007;104:9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suva LJ, et al. Platelet dysfunction and a high bone mass phenotype in a murine model of platelet-type von Willebrand disease. Am J Pathol. 2008;172:430–439. doi: 10.2353/ajpath.2008.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boilard E, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chavez-Macgregor M, et al. Angiogenesis in the bone marrow of patients with breast cancer. Clin Cancer Res. 2005;11:5396–5400. doi: 10.1158/1078-0432.CCR-04-2420. [DOI] [PubMed] [Google Scholar]
- 52.Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep. 2005;3:46–51. doi: 10.1007/s11914-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 53.Sanderson RD, Yang Y, Suva LJ, Kelly T. Heparan sulfate proteoglycans and heparanase--partners in osteolytic tumor growth and metastasis. Matrix Biol. 2004;23:341–352. doi: 10.1016/j.matbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Carcel-Trullols J, et al. Characterization of the glycosylation profile of the human breast cancer cell line, MDA-231, and a bone colonizing variant. Int J Oncol. 2006;28:1173–1183. [PubMed] [Google Scholar]
- 55.Yang Y, et al. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallet S, et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci USA. 2010;107:5124–5129. doi: 10.1073/pnas.0911929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheel C, Onder T, Karnoub A, Weinberg RA. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 2007;67:11476–11479. doi: 10.1158/0008-5472.CAN-07-1653. [DOI] [PubMed] [Google Scholar]
- 58.Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 59.Talmadge JE. Clonal selection of metastasis within the life history of a tumor. Cancer Res. 2007;67:11471–11475. doi: 10.1158/0008-5472.CAN-07-2496. [DOI] [PubMed] [Google Scholar]
- 60.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 61.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 62.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 63.Fournier PG, Chirgwin JM, Guise TA. New insights into the role of T cells in the vicious cycle of bone metastases. Curr Opin Rheumatol. 2006;18:396–404. doi: 10.1097/01.bor.0000231909.35043.da. [DOI] [PubMed] [Google Scholar]
- 64.Arya M, Ahmed H, Silhi N, Williamson M, Patel HR. Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration. Tumour Biol. 2007;28:123–131. doi: 10.1159/000102979. [DOI] [PubMed] [Google Scholar]
- 65.Kang H, et al. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005;7:R402–R410. doi: 10.1186/bcr1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massagué J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 67.Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J Biol Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 68.Mohammad KS, et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS ONE. 2009;4:e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suva LJ, et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237:893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 70.Burtis WJ, et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med. 1990;322:1106–1112. doi: 10.1056/NEJM199004193221603. [DOI] [PubMed] [Google Scholar]
- 71.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCe/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 73.Roodman GD, Dougall WC. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat Rev. 2008;34:92–101. doi: 10.1016/j.ctrv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Southby J, et al. Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Res. 1990;50:7710–7716. [PubMed] [Google Scholar]
- 75.Powell GJ, et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51:3059–3061. [PubMed] [Google Scholar]
- 76.Guise TA, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henderson MA, et al. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66:2250–2256. doi: 10.1158/0008-5472.CAN-05-2814. [DOI] [PubMed] [Google Scholar]
- 78.Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis: How the skeleton affects tumor behavior. Bone. doi: 10.1016/j.bone.2010. 07.015. [DOI] [PubMed] [Google Scholar]
- 79.Lu Y, et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 80.Lau YS, et al. RANKL-dependent and RANKL-independent mechanisms of macrophage-osteoclast differentiation in breast cancer. Breast Cancer Res Treat. 2007;105:7–16. doi: 10.1007/s10549-006-9438-y. [DOI] [PubMed] [Google Scholar]
- 81.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]
- 82.Kudo O, et al. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 83.Quinn JM, et al. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J Bone Miner Res. 2001;16:1787–1794. doi: 10.1359/jbmr.2001.16.10.1787. [DOI] [PubMed] [Google Scholar]
- 84.Kelly T, et al. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Res. 2005;65:5778–5784. doi: 10.1158/0008-5472.CAN-05-0749. [DOI] [PubMed] [Google Scholar]
- 85.Kelly T, Suva LJ, Nicks KM, MacLeod V, Sanderson RD. Tumor-derived syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis. J Bone Miner Res. 2010;25:1295–1304. doi: 10.1002/jbmr.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fizazi K, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 87.Vallet S, Smith MR, Raje N. Novel bone-targeted strategies in oncology. Clin Cancer Res. 2010;16:4084–4093. doi: 10.1158/1078-0432.CCR-10-0600. [DOI] [PubMed] [Google Scholar]
- 88.Body JJ, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 89.Lipton A, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 90.Lipton A, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res. 2008;14:6690–6696. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- 91.Vij R, et al. An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. Am J Hematol. 2009;84:650–656. doi: 10.1002/ajh.21509. [DOI] [PubMed] [Google Scholar]
- 92.McClung MR, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 93.Nambi P, Wu HL, Lipshutz D, Prabhakar U. Identification and characterization of endothelin receptors on rat osteoblastic osteosarcoma cells: down-regulation by 1,25-dihydroxy-vitamin D3. Mol Pharmacol. 1995;47:266–271. [PubMed] [Google Scholar]
- 94.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97 (Suppl 3):779–784. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]
- 95.Van Sant C, et al. Endothelin signaling in osteoblasts: global genome view and implication of the calcineurin/NFAT pathway. Mol Cancer Ther. 2007;6:253–261. doi: 10.1158/1535-7163.MCT-06-0574. [DOI] [PubMed] [Google Scholar]
- 96.Mansson PE, Adams P, Kan M, McKeehan WL. Heparin-binding growth factor gene expression and receptor characteristics in normal rat prostate and two transplantable rat prostate tumors. Cancer Res. 1989;49:2485–2494. [PubMed] [Google Scholar]
- 97.Matuo Y, et al. Heparin binding affinity of rat prostatic growth factor in normal and cancerous prostates: partial purification and characterization of rat prostatic growth factor in the Dunning tumor. Cancer Res. 1987;47:188–192. [PubMed] [Google Scholar]
- 98.Morrissey C, Brown LG, Pitts TE, Vessella RL, Corey E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia. 2010;12:192–205. doi: 10.1593/neo.91836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem. 2007;102:829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- 100.Harris SE, et al. Expression of bone morphogenetic protein messenger RNAs by normal rat and human prostate and prostate cancer cells. Prostate. 1994;24:204–211. doi: 10.1002/pros.2990240406. [DOI] [PubMed] [Google Scholar]
- 101.Kim IY, et al. Expression of bone morphogenetic protein receptors type-IA, -IB and -II correlates with tumor grade in human prostate cancer tissues. Cancer Res. 2000;60:2840–2844. [PubMed] [Google Scholar]
- 102.Brubaker KD, Corey E, Brown LG, vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem. 2004;91:151–160. doi: 10.1002/jcb.10679. [DOI] [PubMed] [Google Scholar]
- 103.Feeley BT, et al. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J Bone Miner Res. 2005;20:2189–2199. doi: 10.1359/JBMR.050802. [DOI] [PubMed] [Google Scholar]
- 104.Ye L, Lewis-Russell JM, Kynaston H, Jiang WG. Endogenous bone morphogenetic protein-7 controls the motility of prostate cancer cells through regulation of bone morphogenetic protein antagonists. J Urol. 2007;178:1086–1091. doi: 10.1016/j.juro.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Schwertfeger KL. Fibroblast growth factors in development and cancer: insights from the mammary and prostate glands. Curr Drug Targets. 2009;10:632–644. doi: 10.2174/138945009788680419. [DOI] [PubMed] [Google Scholar]
- 106.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11:709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 107.Memarzadeh S, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acevedo VD, et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell. 2007;12:559–571. doi: 10.1016/j.ccr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 109.Mayahara H, et al. In vivo stimulation of endosteal bone formation by basic fibroblast growth factor in rats. Growth Factors. 1993;9:73–80. doi: 10.3109/08977199308991583. [DOI] [PubMed] [Google Scholar]
- 110.Izbicka E, et al. Human amniotic tumor that induces new bone formation in vivo produces growth-regulatory activity in vitro for osteoblasts identified as an extended form of basic fibroblast growth factor. Cancer Res. 1996;56:633–636. [PubMed] [Google Scholar]
- 111.Dunstan CR, et al. Systemic administration of acidic fibroblast growth factor (FGF-1) prevents bone loss and increases new bone formation in ovariectomized rats. J Bone Miner Res. 1999;14:953–959. doi: 10.1359/jbmr.1999.14.6.953. [DOI] [PubMed] [Google Scholar]
- 112.Kodama N, et al. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone. 2009;44:699–707. doi: 10.1016/j.bone.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 113.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hall CL, Keller ET. The role of Wnts in bone metastases. Cancer Metastasis Rev. 2006;25:551–558. doi: 10.1007/s10555-006-9022-2. [DOI] [PubMed] [Google Scholar]
- 115.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 116.Clines GA, et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol Endocrinol. 2007;21:486–498. doi: 10.1210/me.2006-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gilbey AM, Burnett D, Coleman RE, Holen I. The detection of circulating breast cancer cells in blood. J Clin Pathol. 2004;57:903–911. doi: 10.1136/jcp.2003.013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vessella RL, Corey E. Targeting factors involved in bone remodeling as treatment strategies in prostate cancer bone metastasis. Clin Cancer Res. 2006;12:6285s–6290s. doi: 10.1158/1078-0432.CCR-06-0813. [DOI] [PubMed] [Google Scholar]
- 119.Coleman RE. Adjuvant bisphosphonates in breast cancer: are we witnessing the emergence of a new therapeutic strategy? Eur J Cancer. 2009;45:1909–1915. doi: 10.1016/j.ejca.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 120.Gnant M, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 121.Stopeck AT, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 123.Weigelt B, Peterse JL, van ‘t veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 124.Vaage J. Metastasizing potentials of mouse mammary tumors and their metastases. Int J Cancer. 1988;41:855–858. doi: 10.1002/ijc.2910410614. [DOI] [PubMed] [Google Scholar]
- 125.Hill RP, Chambers AF, Ling V, Harris JF. Dynamic heterogeneity: rapid generation of metastatic variants in mouse B16 melanoma cells. Science. 1984;224:998–1001. doi: 10.1126/science.6719130. [DOI] [PubMed] [Google Scholar]