Abstract
Using the nucleophilicity of NHCs and aNHCs, as well as the leaving group ability of the former, the carbon-carbon double bond of imidazol-2-ylidenes can be readily mono- and di-functionalized. These results provide also a new light on the formation of abnormal carbene adducts from classical unsaturated NHCs.
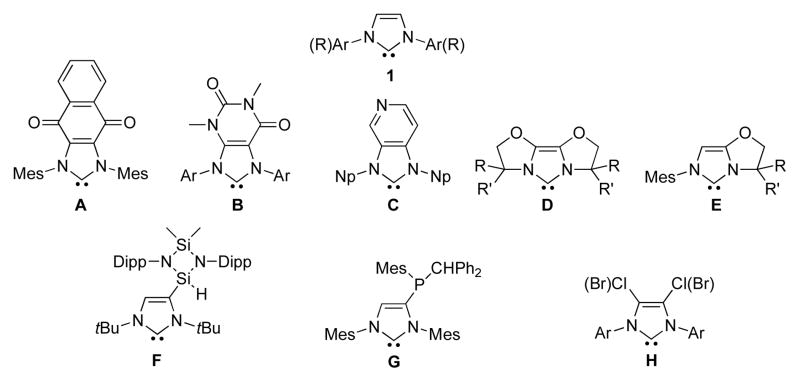
Since the discovery by Arduengo et al. of the stable 1,3-diadamantyl imidazol-2-ylidene (1, R = Ad),1,2 a myriad of the so-called unsaturated N-heterocyclic carbenes (NHCs) has been prepared, and numerous applications have been found.3 Because of the commonly practiced synthetic routes, most unsaturated NHCs feature an unsubstituted carbon-carbon double bond or alternatively alkyl or aryl groups are placed at the 4 and 5 positions.4 The rare exceptions are imidazol-2-ylidenes annulated to a quinone derivative (A)5 or a heterocycle (such as B and C),6 the oxazoline-derivatives (D, E),7 and NHCs featuring one (F, G)8 or two (H)9 heavier main group elements.
Interestingly, it has been shown that the substituents at the carbon-carbon double bond have a dramatic influence on the electronic properties of the carbene center. For example, the dichlorinated derivatives H are exceptionally stable, and are certainly the only carbenes that can be handled in air.9a Therefore, practical synthetic strategies, allowing the access to symmetrically and unsymmetrically 4- and 4,5-functionalized imidazol-2-ylidenes are highly desirable. Herein we report a convenient route to a variety of these compounds from a single precursor, namely a 4,5-unsubstituted imidazol-2-ylidene of type 1 (Ar = 2,6-diisopropylphenyl, Dipp).10 In addition, the mechanism of formation of the so-called abnormal carbene-adducts is discussed.
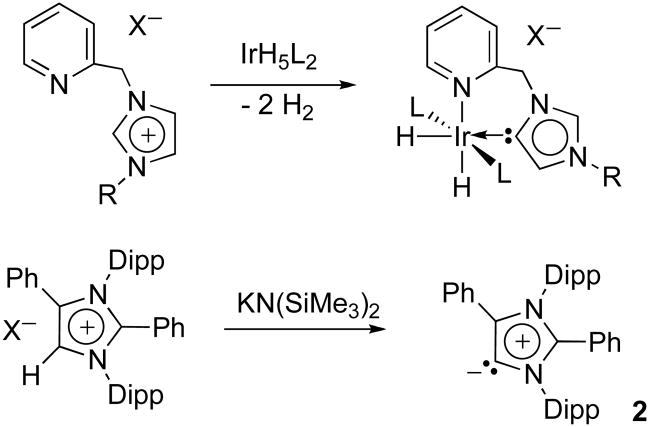
The syntheses of NHCs A–E follow classical methods, using precursors already featuring the desired backbone. In contrast, NHCs F–H are obtained in a single operation from the corresponding 4,5-unsubstituted NHCs of type 1. The latter results are reminiscent of the discovery by Crabtree that 2-pyridylmethylimidazolium salts react with IrH5(PPh3)2 to give a complex in which the imidazole ring bound the “wrong way” at C5 and not at C2 (Scheme 1, top).11 The mechanism of formation of C5-bound adducts is still obscure, whether a transition metal is involved or a main group elements as in F–H.12 These adducts correspond to a formal rearrangement of imidazol-2-ylidene 1 into its isomeric C5-deprotonated imidazolium, a so-called abnormal carbene (aNHC), followed by addition of the electrophile, and finally deprotonation at C-2. However, the rearrangement of 1 is very unlikely since it is well established that the isomeric aNHC is some 70–80 kJmol−1 higher in energy, corresponding to a pKa value for the C5- proton (~ 33) 9 units higher than that for the C2 proton in the parent imidazolium salt;13 moreover, a 1,3-hydrogen shift would certainly be energetically costly.14 Therefore, it is clear that the formation of aNHCs can only be favored if the C2-position is protected, and indeed we have recently shown that aNHC 2 can be prepared and even isolated (Scheme 1, bottom).15
Scheme 1.
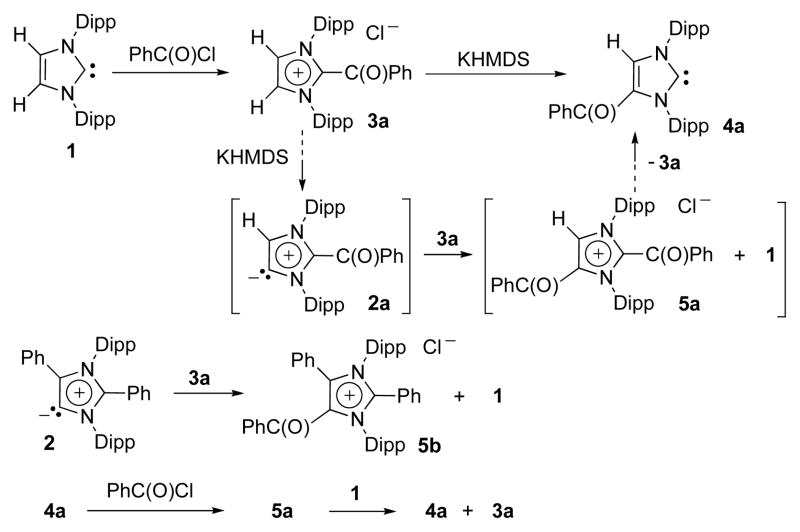
With the aim of tuning the electronic properties of aNHCs, we chose to vary the C2-substituent, using NHC 1 (Ar = Dipp) as a starting material. Addition of one equivalent of benzoyl chloride to 1 cleanly afforded the corresponding adduct 3a. However, deprotonation of 3a with potassium hexamethyldisilazide at −78 °C did not lead to the expected aNHC 2a, but to its isomeric NHC 4a, which was isolated in 64% yield (Scheme 2). Its structure was determined unambiguously by single crystal X-ray diffraction (Fig. 2). A plausible mechanism to rationalize these results involves the deprotonation of 3a with formation of aNHC 2a as a fleeting intermediate. The latter then acts as a nucleophile toward 3a, generating the bis-adduct 5a along with 1. NHC 1 can act as a nucleophile towards the former leading to the observed 4-substituted NHC 4a, and regenerating the starting material 3a. To confirm the viability of this hypothesis, stable aNHC 2 was added to the 2-benzoyl imidazolium 3a, and indeed the formation of the penta-substituted imidazolium salt 5b was observed along with NHC 1. Then, imidazolium salt 5a, prepared by addition of benzoyl chloride to 4a, was reacted with 1, which led to C5-substituted imidazol-2-ylidene 4a and C2-substituted imidazol-2-ylidene 3a.
Scheme 2.
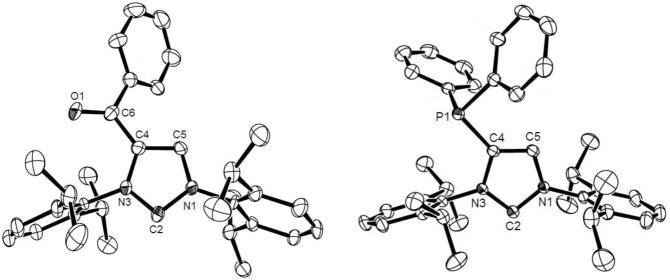
Figure 2.
Molecular structures of 4a (left) and 4f (right) in the solid state (hydrogen atoms are omitted for clarity; ellipsoids are drawn at 50% probability). Selected bond lengths [Å] and angles [°]; 4a: N1-C2 1.372(8), N3-C2 1.351(8), N3-C4 1.389(8), N1-C5 1.380(8), C4–C5 1.366(10), C4–C6 1.464(9), C6-O1 1.229(7), N1-C2-N3 101.9(5), 4f: N3-C2 1.3695(15), N1-C2 1.3714(15), N1-C5 1.3848(15), N3-C4 1.4071(14), C4–C5 1.3510(17), C4-P1 1.8124(12), N3-C2-N1 101.31(9).
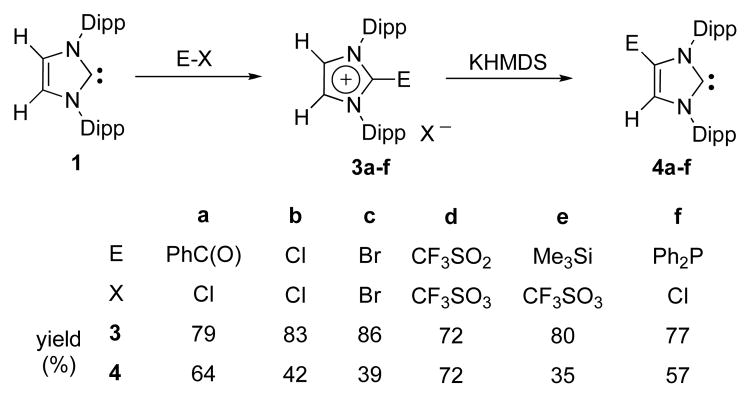
The scope of this reaction is quite general as shown in Scheme 3. A variety of C4-functionalized NHCs 4a–f were prepared in moderate to good isolated yields (not optimized). Of special interest, both electron-withdrawing and -donating groups can be used to functionalize the carbon-carbon double bond of NHCs.
Scheme 3.
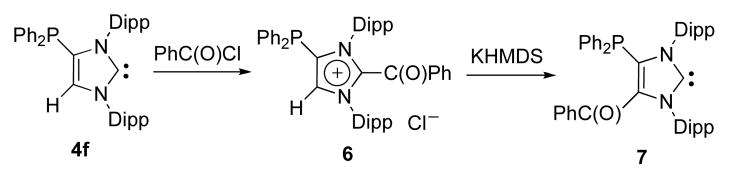
These results prompted us to investigate the possibility of using the same synthetic strategy to place two functional groups at the carbon-carbon double bond. As a proof of principle, 4-diphenylphosphino-NHC 4f was treated with benzoyl chloride, affording the 2-benzoyl-4-diphenylphosphino-imidazolium salt 6 (86% yield). Subsequent treatment with hexamethyldisilazide gave the 4-benzoyl-5-diphenylphosphino imidazol-2-ylidene 7 in 51% isolated yield (Scheme 4).
Scheme 4.
When combined with the recent discovery of modular syntheses of N,N′-unsymmetrically substituted imidazolium salts,4 these results pave the way for the preparation of NHCs with virtually any substitution pattern. Particularly appealing is the possibility of placing strong electron-withdrawing groups, such as trifluoromethane sulfonyl, which should decrease the σ-donor and increase the π-acceptor ability of NHCs. Moreover, these results provide a new light on the formation of abnormal carbene adducts from classical unsaturated NHCs.
Supplementary Material
Figure 1.
Imidazol-2-ylidenes 1 and its derivatives A–H featuring C4 and/or C5 substituents different from H, alkyl, and aryl groups.
Acknowledgments
We are grateful to the NIH (R01 GM 68825) and DOE (DE-FG02-09ER16069) for financial support.
Footnotes
Supporting Information Available. Full experimental details; X-ray crystallographic data for 4a and 4f in CIF format. This material is available free of charge via the internet at htpp://pubs.acs.org.
References
- 1.Arduengo AJ, III, Harlow RL, Kline M. J Am Chem Soc. 1991;113:361. [Google Scholar]
- 2.For the first stable carbene, see: Igau A, Grützmacher H, Baceiredo A, Bertrand G. J Am Chem Soc. 1988;110:6463.Igau A, Baceiredo A, Trinquier G, Bertrand G. Angew Chem Int Ed. 1989;28:621.
- 3.For reviews, see for examples: Tapu D, Dixon DA, Roe C. Chem Rev. 2009;109:3385. doi: 10.1021/cr800521g.Arnold PL, Casely IJ. Chem Rev. 2009;109:3599. doi: 10.1021/cr8005203.Díez-González S, Marion N, Nolan SP. Chem Rev. 2009;109:3612. doi: 10.1021/cr900074m.Poyatos M, Mata JA, Peris E. Chem Rev. 2009;109:3677. doi: 10.1021/cr800501s.Samojlowicz C, Bieniek M, Grela K. Chem Rev. 2009;109:3708. doi: 10.1021/cr800524f.van Otterlo WAL, de Koning CB. Chem Rev. 2009;109:3743. doi: 10.1021/cr900178p.Monfette S, Fogg DE. Chem Rev. 2009;109:3783. doi: 10.1021/cr800541y.Alcaide B, Almendros P, Luna A. Chem Rev. 2009;109:3817. doi: 10.1021/cr9001512.Hindi KM, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ. Chem Rev. 2009;109:3859. doi: 10.1021/cr800500u.Hahn FE, Jahnke MC. Angew Chem Int Ed. 2008;47:3122. doi: 10.1002/anie.200703883.Glorius F. N-Heterocyclic Carbenes in Transition Metal Catalysis; Topics in Organometallic Chemistry. Vol. 21. Springer Verlag; 2007. Bourissou D, Guerret O, Gabbaï FP, Bertrand G. Chem Rev. 2000;100:39. doi: 10.1021/cr940472u.
- 4.a) Fürstner A, Alcarazo M, Cesar V, Lehman CW. Chem Commun. 2006:2176. doi: 10.1039/b604236h. [DOI] [PubMed] [Google Scholar]; b) Hirano K, Urban S, Wang C, Glorius F. Org Lett. 2009;11:1020. doi: 10.1021/ol8029609. [DOI] [PubMed] [Google Scholar]; c) Struble JR, Bode JW. Tetrahedron. 2008;64:6961. [Google Scholar]
- 5.Sanderson MD, Kamplain JW, Bielawski CW. J Am Chem Soc. 2006;128:16514. doi: 10.1021/ja067475w. [DOI] [PubMed] [Google Scholar]
- 6.a) Schütz J, Herrmann WA. J Organomet Chem. 2004;689:2995. [Google Scholar]; b) Ullah F, Bajor G, Veszpremi T, Jones PG, Heinicke JW. Angew Chem Int Ed. 2007;46:2697. doi: 10.1002/anie.200604516. [DOI] [PubMed] [Google Scholar]; c) Saravanakumar S, Kindermann MK, Heinicke J, Kockerling M. Chem Commun. 2006:640. doi: 10.1039/b512884f. [DOI] [PubMed] [Google Scholar]; d) Herrmann WA, Schütz J, Frey GD, Herdweck E. Organometallics. 2006;25:2437. [Google Scholar]
- 7.a) Glorius F, Altenhoff G, Goddard R, Lehmann C. Chem Commun. 2002:2704. doi: 10.1039/b208045a. [DOI] [PubMed] [Google Scholar]; b) Altenhoff G, Goddard R, Lehmann CW, Glorius F. J Am Chem Soc. 2004;126:15195. doi: 10.1021/ja045349r. [DOI] [PubMed] [Google Scholar]; c) Wurtz S, Glorius F. Acc Chem Res. 2008;41:1523. doi: 10.1021/ar8000876. [DOI] [PubMed] [Google Scholar]
- 8.a) Cui H, Shao Y, Li X, Kong L, Cui C. Organometallics. 2009;28:5191. [Google Scholar]; b) Graham TW, Udachin KA, Carty AJ. Chem Comm. 2006:2699. doi: 10.1039/b604150g. [DOI] [PubMed] [Google Scholar]; c) Bates JI, Kennepohl P, Gates DP. Angew Chem Int Ed. 2009;48:9844. doi: 10.1002/anie.200905401. [DOI] [PubMed] [Google Scholar]
- 9.a) Arduengo AJ, III, Davidson F, Dias HVR, Goerlich JR, Khasnis D, Marshall WJ, Prakasha TK. J Am Chem Soc. 1997;119:12742. [Google Scholar]; b) Urban S, Tursky M, Frohlich R, Glorius F. Dalton Trans. 2009:6934. doi: 10.1039/b908981k. [DOI] [PubMed] [Google Scholar]; c) Cole ML, Jones C, Junk PC. New J Chem. 2002;262:1296. [Google Scholar]; d) Huang J, Schanz HJ, Stevens ED, Nolan SP. Organometallics. 1999;18:2370. [Google Scholar]
- 10.Arduengo AJ., III 5077414. U S Patent. 1991
- 11.a) Grundemann S, Kovacevic A, Albrecht M, Faller JW, Crabtree RH. Chem Commun. 2001:2274. doi: 10.1039/b107881j. [DOI] [PubMed] [Google Scholar]; b) Grundemann S, Kovacevic A, Albrecht M, Faller JW, Crabtree RH. J Am Chem Soc. 2002;124:10473. doi: 10.1021/ja026735g. [DOI] [PubMed] [Google Scholar]
- 12.For reviews on C5-bound NHCs, see: Arnold PL, Pearson S. Coord Chem Rev. 2007;251:596.Albrecht M. Chem Commun. 2008:3601. doi: 10.1039/b806924g.Schuster O, Yang L, Raubenheimer HG, Albrecht M. Chem Rev. 2009;109:3445. doi: 10.1021/cr8005087.Albrecht M. Chimia. 2009;63:105.
- 13.a) Magill AM, Cavell KJ, Yates BF. J Am Chem Soc. 2004;126:8717. doi: 10.1021/ja038973x. [DOI] [PubMed] [Google Scholar]; b) Magill AM, Yates BF. Aust J Chem. 2004;57:1205. [Google Scholar]
- 14.Tonner R, Heydenrych G, Frenking G. Chem Asian J. 2007;2:1555. doi: 10.1002/asia.200700235. [DOI] [PubMed] [Google Scholar]
- 15.Aldeco-Perez E, Rosenthal AJ, Donnadieu B, Parameswaran P, Frenking G, Bertrand G. Science. 2009;326:556. doi: 10.1126/science.1178206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.