Abstract
In response to Fe-deficiency, various dicots increase their root branching which contributes to the enhancement of ferric-chelate reductase activity. Whether this Fe-deficiency-induced response eventually enhances the ability of the plant to tolerate Fe-deficiency or not is still unclear and evidence is also scarce about the signals triggering it. In this study, it was found that the SPAD-chlorophyll meter values of newly developed leaves of four tomato (Solanum lycocarpum) lines, namely line227/1 and Roza and their two reciprocal F1 hybrid lines, were positively correlated with their root branching under Fe-deficient conditions. It indicates that Fe-deficiency-induced root branching is critical for plant tolerance to Fe-deficiency. In another tomato line, Micro-Tom, the increased root branching in Fe-deficient plants was accompanied by the elevation of endogenous auxin and nitric oxide (NO) levels, and was suppressed either by the auxin transport inhibitors NPA and TIBA or the NO scavenger cPTIO. On the other hand, root branching in Fe-sufficient plants was induced either by the auxin analogues NAA and 2,4-D or the NO donors NONOate or SNP. Further, in Fe-deficient plants, NONOate restored the NPA-terminated root branching, but NAA did not affect the cPTIO-terminated root branching. Fe-deficiency-induced root branching was inhibited by the NO-synthase (NOS) inhibitor L-NAME, but was not affected by the nitrate reductase (NR) inhibitor NH4+, tungstate or glycine. Taking all of these findings together, a novel function and signalling pathway of Fe-deficiency-induced root branching is presented where NOS-generated rather than NR-generated NO acts downstream of auxin in regulating this Fe-deficiency-induced response, which enhances the plant tolerance to Fe-deficiency.
Keywords: Auxin, Fe-deficiency, nitric oxide, nitrate reductase, NO synthase, root branching
Introduction
Although the total Fe content in soil generally exceeds plants’ requirement, its bioavailability is often limited, particularly in calcareous soils (Guerinot and Yi, 1994), which represent 30% of the earth's surface (Imsande, 1998). Severe Fe-deficiency results in growth retardation and stasis. Several specific mechanisms have evolved in plants to optimize Fe acquisition from their surrounding soil environment. In dicots, Fe-deficiency stimulates the activity of the plasmalemma NADPH–ferric chelate reductase (FCR) (Robinson et al., 1999), and enhances the expression of plasmalemma Fe(II) transporters (IRT) (Eide et al., 1996; Vert et al., 2002) and exudation of proton and reduced organic compounds to the extracellular medium (Jin et al., 2006, 2007a; Santi and Schmidt, 2009). It also increases the sub-apical development of root hairs (Jin et al., 2009a) and root branching (Moog et al., 1995; Dasgan et al., 2002; Jin et al., 2008; Long et al., 2010). All of these Fe-deficiency-induced responses have been intensively studied for many years except root branching. Therefore, relatively little information is available on the role of root branching in the Fe nutrition of plants. Recently, it has been demonstrated that Fe-deficiency-induced root branching contributes to the enhancement of FCR activity in red clover (Trifolium pretense L.) and tomato (Solanum lycocarpum) plants (Jin et al., 2008). In dicots, they use a reduction strategy to facilitate Fe uptake, and therefore, FCR has been proved to be the rate-limiting step for Fe uptake under Fe-limiting conditions (Connolly et al., 2003). Collectively, these studies implicate the importance of this Fe-deficiency-induced morphological response.
However, the question arises as to how root branching is increased under Fe-deficiency conditions? Under normal plant-growth conditions, IAA has been demonstrated to be strongly involved in root branching (Benková and Bielach, 2010). It has been reported that the roots of Fe-deficient sunflower contained higher levels of IAA than the Fe-sufficient ones (Römheld and Marschner, 1986). Our recent studies on red clover (Trifolium pratense) and Arabidopsis (Arabidopsis thaliana) also confirmed the accumulation of IAA in Fe-deficient roots (Jin et al., 2007b, Chen et al., 2010). Under Fe-deficient conditions, therefore, IAA could be a chemical signal controlling the increase in root branching. However, direct evidence on this hypothesis has been unavailable until now. On the other hand, in recent years, nitric oxide (NO), a new plant hormone (Shapiro, 2005), has also been demonstrated to be required for the molecular events involved in controlling the development of lateral roots (Correa-Aragunde et al., 2004; Mendez-Bravo et al., 2010) and cluster roots (Wang et al., 2010). Furthermore, it has been shown that the NO level in roots was rapidly and continually elevated when plants were transferred to an Fe-deficient growth medium (Graziano and Lamattina, 2007; Jin et al., 2009a; Chen et al., 2010). Consequently, NO also seems to be involved in regulating Fe-deficiency-induced root branching. It is worth noting that NO has been demonstrated to act as a downstream signal of IAA in regulating many physiological processes (Shapiro, 2005), including the induction of NR activity (Du et al., 2008), the development of lateral roots (Correa-Aragunde et al., 2004), and the enhancement of FCR activity (Chen et al., 2010). Therefore, the connection node between IAA and NO may also function in controlling the stimulative effect of Fe-deficiency on root branching. However, all of above assumptions need to be investigated through experimentation.
Recently, nitrate reductase (NR) and NO-synthase (NOS) enzymes have been recognized as major causes of NO generation in plants (Yamasaki et al., 1999; Meyer et al., 2005; Shapiro, 2005). Interestingly, it has been shown that exogenous auxin could markedly activate the activity of NR in plants (Du et al., 2008), which could be expected to enhance NO synthesis from nitrite. Therefore, NR may act as the connection node between IAA and NO in regulating Fe-deficiency-induced root branching. Further, in another study by Flores et al. (2008), treatment with the NOS inhibitor L-NAME completely prevented the synthetic auxin NAA-induced lateral roots in Arabidopsis. Therefore, NOS also seems to act as a linkage between IAA and NO in regulating Fe-deficiency-induced root branching.
In the present study, tomato plants and pharmacological methods were used to investigate the signalling functions of IAA and NO in the regulation of Fe-deficiency-induced root branching. Whether NR and/or NOS acted as the connection node(s) between IAA and NO in the above signalling pathway or not, was also investigated. Because both IAA and NO have been implicated in regulating FCR activity and the expression of the IRT gene (Graziano and Lamattina, 2007; Chen et al., 2010), in the present study it was also discussed whether root branching and other Fe-deficiency-induced responses were regulated simultaneously or differentially.
Materials and methods
Chemicals
The chemicals used in this study were purchased as NPA (1-naphthylphthalamic acid) from Chemservice (http://www.anal-tech.com/); DAF-FM DA (diaminofluorescein-FM diacetate) from Beyotime Institute of Biotechnology (http://www.beyotime.com/); cPTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) from Dojin Laboratories (http://www.dojindo.com/); L-NAME (Nω-nitro-L-arginine methyl ester hydrochloride) and BPDS (4,7-diphenyl-1,10-phenanhroline-disulphonic acid) from Sigma (http://www.sigmaaldrich.com/); and NAA (α-naphthalene acetic acid), tungstate, glycine (Gly), and MES (4-morpholinoethanesulphonic acid) from Sangon (http://www.sangon.com/).
Plant culture
Tomato (Solanum lycocarpum Mill cv. Micro-Tom) seedlings of uniform size were transferred to aerated hydroponics with full-strength nutrient solution having a nutrient composition (in μM) of KH2PO4, 250; MgSO4, 500; KNO3, 1000; Ca(NO3)2, 500; H3BO3, 10; MnSO4, 0.5; ZnSO4, 0.5; CuSO4, 0.1; (NH4)6Mo7O24, 0.1; and Fe-EDTA, 20. The solution pH was adjusted to 5.8 using 1 M NaOH. All the plants were grown in the controlled-environment growth chamber at 70% relative humidity with a daily cycle of 14 h day at 28 °C, and 10 h night at 22 °C. The daytime light intensity was 200–220 μmol photons m−2 s−1. After 10 d of growth in the nutrient solution, plants were transferred to 100 ml vials containing media either without iron (–Fe) or with 20 μM Fe-EDTA (+Fe). For experiments carried out with treatment of a sole pharmacon, either 0.1 μM NAA, 0.1 μM 2,4-D, 100 μM NONOate or 100 μM SNP were added to the +Fe solution. In the –Fe solution, either 5 μM NPA, 5 μM TIBA, 200 μM cPTIO, 1 mM Gly, 2 mM NH4+, 0.15 mM tungstate, or 0.5 mM L-NAME were added. For experiments carried out with a treatment of double pharmacons, 0.1 μM NAA with 0.5 mM L-NAME was added to the +Fe solution; whereas, either 5 μM NPA with 100 μM NONOate or 200 μM cPTIO with 0.1 μM NAA were added to the –Fe solution. The solutions in all the treatment containers were renewed on alternate days.
Measurement of chlorophyll synthesis, lateral root count, and root biomass
After 6 d of Fe-deficiency treatment, the chlorophyll content of the newly formed leaves was measured as SPAD values with a chlorophyll meter (SPAD-502, Minolta). The root system was then put into a container filled with distilled water. In order to minimize the intercross among the roots during image scanning, the whole root system was carefully cut with scissors and separated into 2–4 portions, and each portion was transferred to respective containers. The number of lateral roots was obtained by scanning with image analysis software (STD 1600+ Scanner, RÉGEN Instruments, Québec, Canada). The scanned root systems were blotted dry with a paper towel and weighed. Further, in another set of plants, the region on the main roots with a length from the root tip of 15 cm (expressed as ‘15 cm root tip’) were also cut by scissors, and the number of lateral roots was recorded as described before.
Analysis of the localization of root ferric chelate reductase
For visualizing the region of the root zone active in ferric-chelate reduction, excised roots were embedded in the ferric-chelate reductase assay solution solidified by the addition of 0.75% (w/v) agarose in a 9 cm diameter Petri dish. The assay solution consisted of 0.5 mM CaSO4, 0.1 mM MES, 0.1 mM BPDS, and 100 μM Fe-EDTA, and the pH was adjusted to 5.5 with 1 M NaOH. Roots were incubated at 23±2 oC for 1 h, and then the colour patterns were recorded using a digital camera.
Measurement of IAA concentration in roots
Extraction, purification, and assay of IAA were undertaken by the modified procedures of Yang et al. (2001). Briefly, about 0.5 g roots were homogenized in 3 ml prechilled 80% methanol on ice in weak light conditions, with the addition of 1 mM 2,6-di-tert-butyl-p-methylohenol as the antioxidant. The homogenate was centrifuged at 5000 rpm for 10 min at 4 °C and the debris was cleaned with 0.5 ml prechilled 80% methanol and centrifuged. The supernatant was combined and purified with C18 columns (C18 Sep-Park Cartridge, Waters Corp., Millford, MA). Then 300 μl of the extract was dried with N2 gas and redissolved in 200 μl methanol. The IAA in the methanol solution was dried in a vacuum freeze-dryer (Christ ALPHA 1-4, Osterode, Germany) and then redissolved in 300 μl phosphate buffer solution (PBS, 1.3 mM NaH2PO4, 8.7 mM Na2HPO4, 0.14 M NaCl, pH 7.4) for the enzyme-linked immunosorbent assay (ELISA). The ELISA kits were purchased from China Agricultural University, Beijing, China. Absorbance of the developed colour was measured at 490 nm using a microplate reader.
In situ measurement of NO in the roots
Nitric oxide was imaged using DAF-FM DA (diaminofluorescein-FM diacetate) and epifluorescence microscopy. Roots were loaded with 10 μM DAF-FM DA in 20 mM HEPES/NaOH buffer (pH 7.4) for 30 min, washed three times in fresh buffer, and observed under a microscope (Nikon Eclipse E600, Nikon, excitation 488 nm, emission 495–575 nm). Exposure settings were constantly maintained during fluorescence microscopy. Signal intensities of green fluorescence in the images were quantified according to the method of Guo and Crawford (2005) by using Photoshop software (Adobe Systems). Data are presented as the mean of fluorescence intensity relative to the root tips of +Fe plants.
Measurement of NR activity in roots
Nitrate reductase activity was assayed as described in our previous study (Jin et al., 2009b). Whole roots system was excised from plants, and about 0.4 g fresh tissues were placed in each test tube. Five millilitres of assay solution, composed of 2% 1-propanol, 100 mM KH2PO4 (pH 7.5), and 30 mM KNO3 were added to each test tube. Samples were vacuum-infiltrated for 5 min and incubated in a shaking water bath at 25 °C for 30 min in the dark. After incubation, the assay solution with roots was filtered and 1 ml aliquot from each sample was transferred to a new tube. One millilitre of sulphanilamide (1% w/v in 1.5 M HCl) and 1 ml N-(1-naphthyl)-ethylenediaminedihydrochloride (0.02% w/v in 0.2 M HCl) was added. The samples were incubated at room temperature for 30 min. The absorbance at 540 nm was measured with a spectrophotometer. The NR activity was expressed as μmol NO2 of produced per hour and per gram of fresh weight.
Statistics
All statistical analyses were conducted with SAS software (SAS Institute, Cary, NC). Means were compared by t test or Fisher's least significant difference test at P <0.05 in all cases.
Results
Effect of Fe-deficiency on root branching
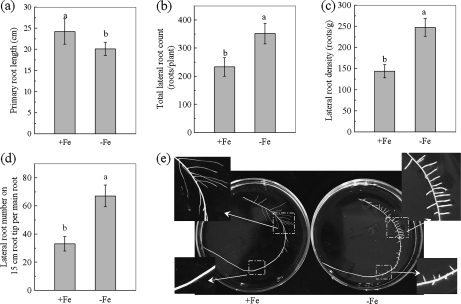
On the 6th day of –Fe culture, the newly formed leaves were chlorotic, and yielded a SPAD reading of only 25, while for leaves of +Fe plants, SPAD readings of approximately 46 were obtained (data not shown). It indicates that the –Fe treatment for 6 d caused Fe-deficiency in tomato plants. For root development, although the length of the primary roots (Fig. 1a) and lateral roots (Fig. 1e) was smaller in –Fe plants, the total lateral root count per plant had increased by 51% (Fig. 1b), and the lateral root density, expressed as the lateral root number per gram fresh weight, had increased by about 72% (Fig. 1c). Furthermore, the lateral roots on the 15 cm root tip (i.e. the region of the main root with a length of 15 cm from the root tip) in the –Fe treatment had a 100% higher lateral root count than that with the +Fe treatment (Fig. 1d). The results suggested that more lateral roots emerge closer to the root tip in response to Fe-deficiency.
Fig. 1.
Effect of Fe-deficiency on root development of tomatoes. (a) The primary root length. (b) The total lateral root number of the whole root system. (c) The lateral root density, expressed as the lateral root number per gram fresh weight. (d) The lateral root number on the 15 cm root tip (i.e. the main root section with a 15 cm length from the root tip). (e) The picture of the tomato roots. The tomato plants were grown for 6 d in nutrient solution that contained (+Fe) or omitted (−Fe) Fe-EDTA. Data are means ±SD (n=8). Different letters indicate significant differences (P <0.05) between treatments.
Localization of FCR and effect of root branching on Fe-deficiency tolerance
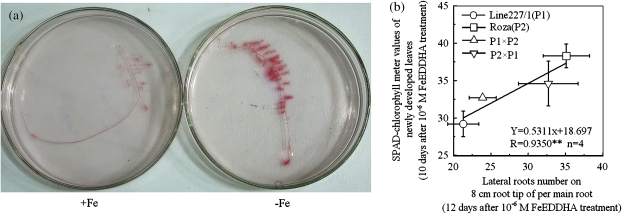
The FCR-catalysed reduction of ferric chelate as indicated by the red colour, was much stronger in the –Fe roots than that in +Fe roots (Fig. 2a). It suggests that the –Fe treatment has induced FCR activity. Furthermore, the FCR-catalysed reduction of ferric chelate was mainly restricted within the lateral root zone, indicating that lateral roots play an important role in Fe uptake by plants from the rhizosphere.
Fig. 2.
The ferric chelate reductase (FCR) activities visualized in roots (a), and the relationship between lateral roots development and SPAD values (b). (a) The tomato plants were grown for 6 d in nutrient solution that contained (+Fe) or omitted (−Fe) Fe-EDTA. (b) The correlation relationship was calculated from the results obtained by the study of Dasagan et al. (2002), in which the four tomato plants were grown in the nutrient solution with the Fe concentration at 10−6 M FeEDDHA. **P<0.05
By analysing the results from Dasgan et al. (2002), it was found that when the four tomato lines, line227/1 (P1) and Roza (P2) and their reciprocal F1 hybrid lines (‘P1×P2’ and ‘P2×P1’), were cultivated under lower Fe conditions (10−6 M Fe-EDDHA), the SPAD-chlorophyll values of their newly developed leaves correlated well with their lateral root counts on the 8 cm root tips (Fig. 2b). These results suggest that increase in root branching enhances the ability of plants to tolerate Fe-deficiency.
Effect of auxin on Fe-deficiency-induced root branching
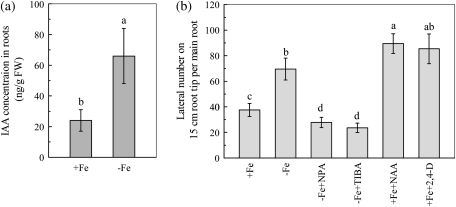
On the 6th day of –Fe treatment, the IAA concentration was significantly higher in the –Fe roots than in the +Fe ones (Fig. 3a). When the polar auxin transport was inhibited with NPA or TIBA, the Fe-deficiency-induced root branching in 15 cm main root tips was terminated. By contrast, treatment with NAA or 2,4-D significantly stimulated the development of lateral roots in the +Fe plants, which mimicked the root branching in the –Fe plants (Fig. 3b). These results suggest that the elevation of IAA accumulation in the roots could be a chemical signal mediating the Fe-deficiency-induced increase of root branching.
Fig. 3.
Accumulation of IAA in Fe-deficient roots (a) and effects of auxin availability on root branching (b). The tomato plants were grown in nutrient solution that contained (+Fe) or omitted (−Fe) Fe-EDTA. For pharmacological treatment, either 0.1 μM NAA (+Fe+NAA) or 0.1 μM 2,4-D (+Fe+2,4-D) were added to the +Fe solution, and either 5 μM NPA (–Fe+NPA) or 5 μM TIBA (–Fe+TIBA) were added to the –Fe solution. The IAA levels and the lateral roots numbers were analysed on the 6th day of treatment. Data are means ±SD (for IAA concentration, n=6; for lateral root, n=8). Different letters indicate significant differences (P <0.05) between treatments.
Effect of NO on Fe-deficiency-induced root branching
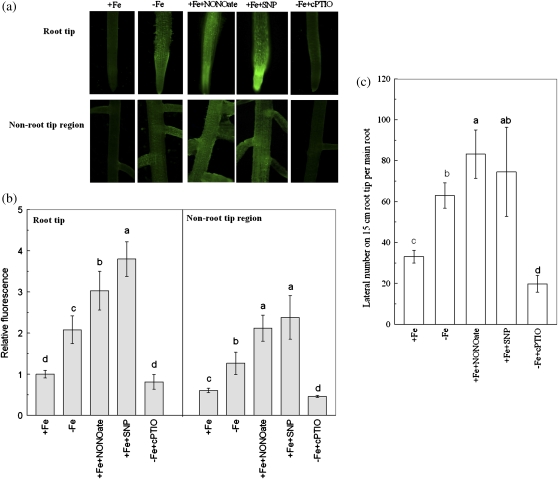
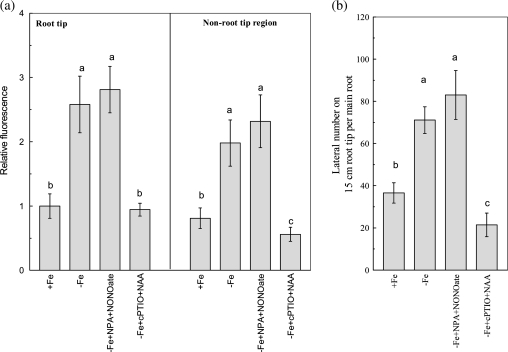
The NO-associated green fluorescence in both root tip and non-root tip regions was clearly increased under –Fe conditions (Fig. 4a). By quantifying the signal intensities of fluorescence, the NO contents in both root tip and non-root tip regions of –Fe plants were increased by about one fold compared with those of +Fe plants (Fig. 4b). When the plant was treated with the NO scavenger cPTIO, the Fe-deficiency-induced root branching in 15 cm main root tips was completely inhibited. However, incubation with the NO donors NONOate or SNP to +Fe plants, closely mimicked the root branching of –Fe plants (Fig. 4c). These results indicated that the elevation of the NO levels in roots could also be a signal involved in the regulation of Fe-deficiency-induced root branching.
Fig. 4.
Accumulation of NO in Fe-deficient roots and effects of NO availability on root branching. (a) Photographs of nitric oxide production shown as green fluorescence in representative roots. (b) The NO production expressed as the fluorescence intensity relative to the root tips of +Fe plants. (c) The lateral root number on the 15 cm root tip. The tomato plants were grown in nutrient solution that contained (+Fe) or omitted (−Fe) Fe-EDTA. For pharmacological treatment, either 100 μM NONOate (+Fe+NONOate) or 100 μM SNP (+Fe+SNP) were added to the +Fe solution, and 200 μM cPTIO (–Fe+cPTIO) was added to the –Fe solution. The NO levels and the lateral roots numbers were analysed on the 6th day of treatments. Data are means ±SD (n=8). Different letters indicate significant differences (P <0.05) between treatments.
Involvement of NO in auxin-mediated Fe-deficiency-induced root branching
Because both NO and IAA were involved in the regulation of Fe-deficiency-induced root branching, their signalling order was therefore investigated. As shown in Fig. 5a, the NONOate co-incubation maintained higher NO accumulation in the roots of NPA-treated –Fe plants. However, the NAA co-incubation did not restore the NO generation in cPTIO-treated –Fe plants. As a consequence, the inhibitory effect of NPA on Fe-deficiency-induced root branching was reversed by NONOate; whereas, the cPTIO-inhibited root branching was not affected by NAA (Fig. 5b). The above results suggested that NO should act as the downstream signal of IAA in mediating Fe-deficiency-induced root branching.
Fig. 5.
The signalling order of IAA and NO in regulating Fe-deficiency-induced root branching. (a) The NO accumulation expressed as the fluorescence intensity relative to the root tips of +Fe plants. (b) The lateral root number on the 15 cm root tip. The tomato plants were grown in nutrient solution that contained (+Fe) or omitted (−Fe) Fe-EDTA. For pharmacological treatment, either 5 μM NPA with 100 μM NONOate (–Fe+NPA+NONOate) or 200 μM cPTIO with 0.1 μM NAA (–Fe+cPTIO+NAA) were added to the –Fe solution. The NO levels and the lateral roots numbers were analysed on the 6th day of treatments. Data are means ±SD (n=8). Different letters indicate significant differences (P <0.05) between treatments.
Effects of NR and NOS on Fe-deficiency-induced root branching
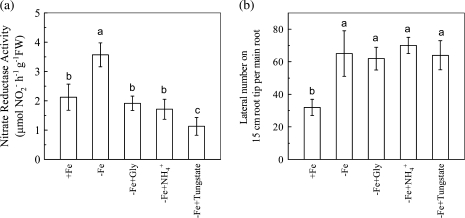
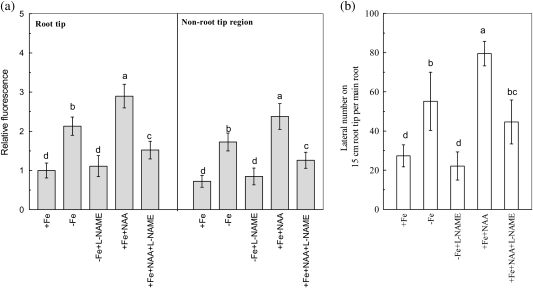
After 6 d of –Fe treatment, the NR activity in roots was increased by about 70% compared with that in +Fe roots. When the –Fe nutrient solution was treated with the NR inhibitor Gly, NH4+ or tungstate, the Fe-deficiency-induced NR activity was completely terminated (Fig. 6a). However, the same treatments did not affect root branching (Fig. 6b). Nevertheless, when the –Fe nutrient solution was supplemented with the NOS inhibitor L-NAME, both NO accumulation and root branching of –Fe plants were terminated (Fig. 7a, b). The results suggest that NOS rather than NR is involved in Fe-deficiency-induced root branching. Further, the NAA-induced NO accumulation and root branching were also greatly inhibited by L-NAME in +Fe plants (Fig. 7a, b).
Fig. 6.
Effect of NR inhibition on Fe-deficiency-induced root branching in tomato plants. (a) The NR activities of roots. (b) The lateral root number on the 15 cm root tip. The plants were grown in nutrient solution that contained (+Fe) or omitted (−Fe) Fe-EDTA. For pharmacological treatment, either 1 mM Gly, 2 mM or 0.15 mM tungstate were added to the –Fe solution. The NR activities and the lateral roots numbers were analysed on the 6th day of treatment. Data are means ±SD (n=8). Different letters indicate significant differences (P <0.05) between treatments.
Fig. 7.
Effects of NOS inhibition on Fe-deficiency-induced NO accumulation and root branching. (a) The NO accumulation expressed as the fluorescence intensity relative to the root tips of +Fe plants. (b) The lateral root number on the 15 cm root tip. The tomato plants were grown in nutrient solution that contained (+Fe) or omitted (−Fe) Fe-EDTA. For pharmacological treatment, 0.5 mM L-NAME was added to the –Fe solution, and either 0.1 μM NAA or 0.1 μM NAA with 0.5 mM L-NAMA were added to the +Fe solution. The NO levels and the lateral roots numbers were analysed on the 6th day of treatments. Data are means ±SD (n=8). Different letters indicate significant differences (P <0.05) between treatments.
Discussion
Fe-deficiency tolerance through Fe-deficiency-induced root branching
Iron deficiency has been demonstrated to have a strong effect on the lateral root development in red clover (Jin et al., 2008), Arabidopsis (Moog et al., 1995; Long et al., 2010), and tomato (Dasgan et al., 2002). It was also found here that the total root count of –Fe plants was significantly increased compared with that of +Fe plants (Fig. 1b). Interestingly, when the number of lateral root was calculated on the basis of per gram fresh weight of root system (i.e. the lateral root density), the stimulatory effect of Fe-deficiency on root branching became more prominent (Fig. 1c). The results indicated that Fe-deficiency makes the plant allocate more root material to develop lateral roots. This behaviour is an energy and/or material economical strategy, which is critically important for stressed plants, because the energy and material demands and also the metabolism in these plants is generally altered. For instance, the energy demand in Fe-deficient Arabidopsis roots was shown to exceed the capacity of oxidative phosphorylation (Thimm et al., 2001).
Generally, a more branched root system is expected to facilitate more nutrient uptake (Drew, 1975). The FCR-catalysed reduction of ferric chelate has been proved to be the rate-limiting step for Fe uptake under Fe-limiting conditions in dicots (Connolly et al., 2003). In our present and previous studies, the FCR was mainly restricted within the lateral root zone (Fig. 2a). Consequently, more lateral roots are expected to provide more sites for Fe uptake. By analysing the results from Dasgan et al. (2002), it was found that the SPAD-chlorophyll meter values of four tomato lines which were grown under lower Fe conditions, correlated well with their lateral root numbers (Fig. 2b). All of these results suggest that the increase of root branching is critically important for plant resistance to Fe-deficiency. Therefore, the regulatory mechanisms related to this Fe-deficiency-induced response were envisaged.
NO acts the downstream of IAA to trigger root branching in response to Fe-deficiency
Because more lateral roots emerge closer to the root tip in response to Fe-deficiency, the investigation of the signalling pathway of this Fe-deficiency-induced response was focused on the region of the main roots with a 15 cm length from the root tip. Several previous reports have demonstrated the importance of both IAA and NO in root development under normal growth condition without nutrient limitation (Correa-Aragunde et al., 2004; Benková and Bielach, 2010). Besides, under phosphorus-deficiency condition, the IAA and NO have also been strongly implicated in the developments of lateral roots (Nacry et al., 2005) and cluster roots (Wang et al., 2010). Therefore, under Fe-deficiency condition, the signalling functions of these two compounds in triggering root branching were emphasized in the present study. As expected, the roots of –Fe tomato contained higher levels of IAA and NO than the Fe-sufficient ones (Figs 3, 4). In order to clarify whether the elevated levels of both IAA and NO were the chemical signals responding for the induction of root branching under –Fe conditions, NPA or TIBA was used to decrease IAA accumulation in roots by inhibiting IAA transport from the shoot to the root, and cPTIO was used to scavenge the NO in the roots. As shown in Figs 3 and 4, both NPA and cPTIO applications completely terminated Fe-deficiency-induced root branching. However, when the IAA analogue or NO donor was co-incubated in the +Fe nutrient solution, root development closely mimicked the bloom of root branching in –Fe plants (Figs 3, 4). These results suggested that both IAA and NO are the primary signals controlling the increase of root branching under –Fe conditions.
However, the question arises as to how the IAA and NO co-ordinately mediate Fe-deficiency-induced root branching? In recent studies, it was found that exogenous auxin treatment could result in a higher level of NO in plants; whereas, NPA treatment decreased the NO production (Ottenschläger et al., 2003; Du et al., 2008; Chen et al., 2010). Consequently, in many signalling processes, NO was demonstrated to act as a downstream signal of IAA (Ötvös et al., 2005; Hu et al., 2005; Chen et al., 2010). More importantly, it was found that IAA-induced NO also controls the adventitious and lateral root formation (Correa-Aragunde et al., 2004; Lanteri et al., 2008). Therefore, it was deduced that this signal pathway may also cause the increase in root branching of plants under –Fe conditions. In the present study, it was found that NPA treatment inhibited the Fe-deficiency-induced NO accumulation in roots, whereas NAA treatment stimulated NO production in the +Fe roots (data not included). Furthermore, the NONOate co-incubation could completely restore the NPA-terminated root branching in –Fe plants, whereas NAA co-incubation did not restore the cPTIO-inhibited root branching (Fig. 5). The results clearly verified the above conclusion that NO acts as the downstream signal of IAA in regulating the Fe-deficiency-induced bloom of root branching.
NOS is responsible for NO generation in mediating Fe-deficiency-induced root branching
Another question that has arisen is that of how IAA linked with NO in the signalling process of Fe-deficiency-induced root branching. In addition, although studies have provided evidence that NO participated in regulating root branching under normal plant growth conditions, the enzymatic source(s) of NO in this signalling pathway has not yet been examined. Because NR and NOS have been recognized as the major origins of NO generation in plants (Yamasaki et al., 1999; Meyer et al., 2005; Shapiro, 2005), their role in IAA linking with NO was therefore investigated here to answer the above two questions. For NR, it has been shown that its activities in roots of tomato and Vaccinium were enhanced when the plants suffered from Fe-deficiency (Brown and Jones, 1976; Poonnachit and Darnell, 2004). It was also observed here that NR activity was significantly increased by Fe-deficiency (Fig. 6). Although treatment with each of the three NR inhibitors, Gly, NH4+ and tungstate completely inhibited Fe-deficiency-increased NR activity, they did not affect root branching in –Fe plants (Fig. 6). It suggests that the Fe-deficiency-activated NR activity do not participate in the signalling pathway of Fe-deficiency-induced root branching.
For NOS, it was found that the NOS inhibitor L-NAME significantly diminished the NAA-induced NO accumulation and root branching in +Fe tomato plants (Fig. 7a, b). It is in accordance with the findings of Flores et al. (2008) for Arabidopsis. Furthermore, when L-NAME was supplied to the –Fe plants, the NO accumulation and the bloom of root branching were also completely inhibited (Fig. 7a, b). Therefore, NOS could be the main source of NO that is used for the induction of root branching under –Fe conditions. However, the connection between IAA and NOS still remains unknown. Therefore, this relationship warrants investigation in future studies.
Do signals regulate root branching and other Fe-deficiency-induced responses in the same way?
Recent studies have shown that both IAA and NO are also involved in regulating other Fe-deficiency-induced responses, including the enhancement of FCR activity and the expression of the IRT gene (Graziano and Lamattina, 2007; Jin et al., 2007b; Chen et al., 2010). Although treatment of either NAA or the NO donor GSNO significantly increased FCR activity and IRT expression in the roots of –Fe plants, only a slight increase or no relation was seen in the roots of +Fe plants with the same treatment (Graziano and Lamattina, 2007; Jin et al., 2007b; Chen et al., 2010). The results suggest that both IAA and NO act as the secondary signals in regulating those Fe-deficiency-induced responses. By contrast, treatment of either of the above chemical compounds could significantly induce the root branching in +Fe plants, which closely mimicked the root development in –Fe plants (Figs 3, 4), indicating that both IAA and NO are probably the primary signals for Fe-deficiency inducing root branching. Therefore, it is possible that root branching and other Fe-deficiency-induced responses are differentially regulated. On the other hand, in the present study it was found that NR-dependent NO generation did not participate in regulating Fe-deficiency-induced root branching (Fig. 6). However, in a study by Graziano and Lamattina (2007) and in our previous study (Chen et al., 2010), NR-dependent NO generation was involved in regulating FCR activity and IRT expression in the roots of –Fe plants. This discrepancy further supported our former deduction that root branching and other Fe-deficiency-induced responses are differentially regulated. This discrepancy also allowed us to suggest that the NO generated by different NO enzymes should function differentially in regulating the Fe-deficiency-induced responses, where the NOS-generated NO participated in regulating root branching whereas the NR-generated NO participated in regulating other Fe-deficiency-induced responses, such as FCR activity and IRT expression.
In all, although it has previously been demonstrated that Fe-deficiency-induced root branching facilitates an increase in FCR activity, whether it eventually enhances the ability of the plant to tolerate Fe-deficiency or not still remains unknown. In addition, although many studies have provided evidence that both auxin and NO are related to root branching under normal plant growth conditions, whether they also participate in the induction of root branching under Fe-deficiency (or not) has not previously been examined. In this study, it has been demonstrated that NOS-generated rather than NR-generated NO acts downstream of auxin in regulating Fe-deficiency-induced root branching, which eventually enhances the plant's tolerance to Fe-deficiency. To the best of our knowledge, this is the first report ever to define the function and signalling pathway for the induction of root branching under Fe-deficient conditions and it also throws light on understanding the mechanism of how plants optimize their Fe acquisition in Fe-limited conditions in a beneficial manner.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (the 973 Program, No. 2007CB109305), the Natural Science Foundation of China (NSFC, No. 30900920, 30900170), a China Postdoctoral Science Foundation funded project (Grant No. 200902643), the Zhejiang Province Natural Science Foundation (No. Y5090106), and IPNI. We appreciate and duly acknowledge the support by these funding agencies.
References
- Benková E, Bielach A. Lateral root organogenesis: from cell to organ. Current Opinion in Plant Biology. 2010;13:677–683. doi: 10.1016/j.pbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Brown JC, Jones WE. Nitrate reductase activity in calcifugous and calcicolous tomatoes as affected by iron stress. Physiologia Plantarum. 1976;38:273–277. [Google Scholar]
- Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiology. 2003;133:1102–1110. doi: 10.1104/pp.103.025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiology. 2010;154:810–819. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgan HY, Römheld V, Cakmak I, Abak K. Physiological root responses of iron deficiency susceptible and tolerant tomato genotypes and their reciprocal F1 hybrids. Plant and Soil. 2002;241:97–104. [Google Scholar]
- Drew MC. Comparison of the effect of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist. 1975;75:479–490. [Google Scholar]
- Du ST, Zhang YS, Lin XY, Wang Y, Tang CX. Regulation of nitrate reductase by its partial product nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L. cv. Baoda) Plant, Cell and Environment. 2008;31:195–204. doi: 10.1111/j.1365-3040.2007.01750.x. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proceedings of the National Academy of Sciences, USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores T, Todd CD, Tovar-Mendez A, Dhano PK, Correa-Aragunde N, Hoyos ME, Brownfield DM, Mullen RT, Lamattina L, Polacco JC. Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiology. 2008;147:1936–1946. doi: 10.1104/pp.108.121459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiology. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. The Plant Journal. 2007;52:949–960. doi: 10.1111/j.1365-313X.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- Guo FQ, Crawford NM. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. The Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Neill SJ, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiology. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J. Iron, sulfur, and chlorophyll deficiencies: a need for an integrative approach in plant physiology. Physiologia Plantarum. 1998;103:139–144. [Google Scholar]
- Jin CW, He YF, Tang CX, Wu P, Zheng SJ. Mechanisms of microbial enhanced iron uptake in red clover. Plant, Cell and Environment. 2006;29:888–897. doi: 10.1111/j.1365-3040.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Jin CW, You GY, Tang CX, Wu P, Zheng SJ. Iron-deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover (Trifolium pratense L.) Plant Physiology. 2007a;144:278–285. doi: 10.1104/pp.107.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, He XX, Zheng SJ. The iron-deficiency induced phenolics accumulation may involve in regulation of Fe(III) chelate reductase in red clover. Plant Signaling and Behavior. 2007b;2(5):327–335. doi: 10.4161/psb.2.5.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Chen WW, Meng ZB, Zheng SJ. The iron-deficiency-induced increase of root branching is important for enhancing ferric chelate reductase activity. Journal of Integrative Plant Biology. 2008;50:1557–1562. doi: 10.1111/j.1744-7909.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. Elevated carbon dioxide improves plant Fe nutrition through enhancing the Fe-deficiency-induced responses under Fe-limited conditions in tomato. Plant Physiology. 2009a;150:272–280. doi: 10.1104/pp.109.136721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Zhang YS, Lin XY, Tang CX. Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum) Annals of Botany. 2009b;104:9–17. doi: 10.1093/aob/mcp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri ML, Laxalt AM, Lamattina L. Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during auxin-Induced adventitious root formation in cucumber. Plant Physiology. 2008;147:188–198. doi: 10.1104/pp.107.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TA, Tsukagoshi H, Busch W, Lahner B, Salt DE, Benfey PN. The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. The Plant Cell. 2010;22:2219–2236. doi: 10.1105/tpc.110.074096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Lea US, Provan F, Kaiser WM, Lillo C. Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynthesis Research. 2005;83:181–189. doi: 10.1007/s11120-004-3548-3. [DOI] [PubMed] [Google Scholar]
- Mendez-Bravo A, Raya-Gonzalez J, Herrera-Estrella L, Lopez-Bucio L. Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant and Cell Physiology. 2010;51:1612–1626. doi: 10.1093/pcp/pcq117. [DOI] [PubMed] [Google Scholar]
- Moog PR, van der Kooij TA, Bruggemann W, Schiefelbein JW, Kuiper PJ. Responses to iron deficiency in Arabidopsis thaliana: the turbo iron reductase does not depend on the formation of root hairs and transfer cells. Planta. 1995;195:505–513. doi: 10.1007/BF00195707. [DOI] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiology. 2005;138:2061–2074. doi: 10.1104/pp.105.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalero RP, Sandberg G, Ishikawa H, Evans ML, Palme K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proceedings of the National Academy of Sciences, USA. 2003;100:2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ötvös K, Pasternak TP, Miskolcz P, Domoki M, Dorjgotov D, Szucs A, Bottka S, Dudits D, Fehér A. Nitric oxide is required for, and promotes auxin-mediated activation of, cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. The Plant Journal. 2005;43:849–860. doi: 10.1111/j.1365-313X.2005.02494.x. [DOI] [PubMed] [Google Scholar]
- Poonnachit U, Darnell R. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Annals of Botany. 2004;93:399–405. doi: 10.1093/aob/mch053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Römheld V, Marschner H Mobilization of iron in the rhizosphere of different plant species. Advance in Plant Nutrition. 1986;2:155–204. [Google Scholar]
- Santi S, Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytologist. 2009;183:1072–1084. doi: 10.1111/j.1469-8137.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- Shapiro AD. Nitric oxide signaling in plants. Vitamins and Hormones. 2005;72:339–398. doi: 10.1016/S0083-6729(05)72010-0. [DOI] [PubMed] [Google Scholar]
- Thimm O, Essigmann B, Kloska S, Altmann T, Buckhout TJ. Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiology. 2001;127:1030–1043. [PMC free article] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. The Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BL, Tang XY, Cheng LY, et al. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytologist. 2010;187:1112–1123. doi: 10.1111/j.1469-8137.2010.03323.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants, new features of an old enzyme. Trends in Plant Science. 1999;4:128–129. doi: 10.1016/s1360-1385(99)01393-x. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Wang W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiology. 2001;127:315–323. doi: 10.1104/pp.127.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]