Abstract
The impact of water deficit on stomatal conductance (gs), petiole hydraulic conductance (Kpetiole), and vulnerability to cavitation (PLC, percentage loss of hydraulic conductivity) in leaf petioles has been observed on field-grown vines (Vitis vinifera L. cv. Chasselas). Petioles were highly vulnerable to cavitation, with a 50% loss of hydraulic conductivity at a stem xylem water potential (Ψx) of –0.95 MPa, and up to 90% loss of conductivity at a Ψx of –1.5 MPa. Kpetiole described a daily cycle, decreasing during the day as water stress and evapotranspiration increased, then rising again in the early evening up to the previous morning's Kpetiole levels. In water-stressed vines, PLC increased sharply during the daytime and reached maximum values (70–90%) in the middle of the afternoon. Embolism repair occurred in petioles from the end of the day through the night. Indeed, PLC decreased in darkness in water-stressed vines. PLC variation in irrigated plants showed the same tendency, but with a smaller amplitude. The Chasselas cultivar appears to develop hydraulic segmentation, in which petiole cavitation plays an important role as a ‘hydraulic fuse’, thereby limiting leaf transpiration and the propagation of embolism and preserving the integrity of other organs (shoots and roots) during water stress. In the present study, progressive stomatal closure responded to a decrease in Kpetiole and an increase in cavitation events. Almost total closure of stomata (90%) was measured when PLC in petioles reached >90%.
Keywords: grapevines (Vitis vinifera L.), hydraulic conductivity, stomatal conductance, water relations, xylem embolism
Introduction
Water deficit, due to environmental limitations of soil available water and high evapotranspiration demand, generally impacts on leaf gas exchanges (carbon assimilation and transpiration) and growth (Hsiao, 1973). In order to avoid water stress, plants have developed long- and short-term acclimation such as root development, leaf surface area reduction, specialized leaf anatomy, and conducting elements (Schultz and Matthews, 1988). In addition, short-term strategies allow the plant to react rapidly in a changing, restrictive environment.
Amongst short-term strategies, stomatal regulation of leaf gas exchange plays a key role in plant response to water stress. Nevertheless, stomatal behaviour is a complex phenomenon involving feedback controls which interact with a wide range of environmental conditions (Zweifel et al., 2007). A general distinction is made between CO2 feedbacks, which probably operate either by substomatal CO2 concentration (Ci) (Mott, 1988) or by assimilation rates (Wong et al., 1985), and hydraulic feedbacks that are dependent on specific aspects of stomatal or whole-plant water status (Monteith, 1995; Jarvis and Davies, 1998) and hydraulic continuity in the soil–plant–atmosphere system (Sperry et al., 2002; Brodribb and Jordan, 2008). Various studies have indeed shown that a relationship exists between stomatal closure during water restriction and the maintenance of xylem integrity (Tyree and Sperry, 1988; Jones and Sutherland, 1991). Under conditions of water restriction, the progressive closure of stomata can be observed when the vapour pressure deficit (VPD) increases and hydraulic conductance along the soil–plant–atmosphere pathway decreases, so that Ψleaf (leaf water potential) remains above a critical threshold required to prevent xylem cavitation and hydraulic rupture (Tyree and Sperry, 1988; Hubbard et al., 2001; Cochard et al., 2002).
The regulation of xylem pressure by stomatal closure may play a role in limiting the risk of cavitation and, consequently, the loss of hydraulic conductivity in the plant (Bucci et al., 2003; Brodridd and Holbrook, 2003; Brodribb et al., 2003). A positive relationship between hydraulic conductance and stomatal conductance (gs) has been described in a number of studies (Meinzer and Grantz, 1990; Saliendra et al., 1995; Comstock, 2000), when Ψleaf remains constant, which would suggest a feedback link between gs and some form of hydraulic signal (Nardini et al., 2001). Moreover, the vulnerability of vessels to cavitation depends on the balance between embolism formation and its possible repair. Recent works have highlighted diurnal changes in xylem hydraulic conductance and water refilling, in spite of the presence of significant tension inside xylem vessels in roots (McCully et al., 1998; McCully, 1999; Domec et al., 2006; Lovisolo et al., 2008), in shoots (Salleo et al., 1996, 2004; Zwieniecki and Holbrook, 1998; Melcher et al., 2001), in petioles (Zwieniecki et al., 2000; Bucci et al., 2003; Hacke and Sperry, 2003; Loviosolo et al., 2008), and in leaves (Lo Gullo et al., 2003; Brodribb and Holbrook, 2004; Johnson et al., 2009). The first in vivo visualization and quantification of the refilling process in grapevines were carried out by Holbrook et al. (2001) using magnetic resonance imaging, and were recently improved using high-resolution X-ray computed tomography (CT) (Brodersen et al., 2010). Furthermore, although cavitation appears to be reversible within a relatively short time lapse (from tens of minutes to several hours), the diurnal cycles of embolism and leaf-specific hydraulic conductance (Kplant), followed by the refilling of leaves and petioles, are considered to play an important role in the regulation of gas exchanges (Lo Gullo et al., 2003). Moreover, other studies (Tyree et al., 1993; Salleo et al., 2001) have been able to show that petioles and leaves from various plant species were more vulnerable to cavitation than stems. In grapevines, the findings of Choat et al. (2010) and Alsina et al. (2007) demonstrated a relative resistance in stems to xylem embolism as water stress rose, whereas the petioles were found to be more vulnerable (Schultz, 2003). Zimmermann (1983) put forward the hypothesis of hydraulic segmentation, according to which distal organs, such as petioles and leaves, were more vulnerable to embolism. Acting as hydraulic fuses, they would be able to prevent the propagation of embolism in the stems, trunk, or roots, and constitute an effective means of resistance to drought and diminish the risks of hydraulic rupture.
Even though the precise mechanisms responsible for embolism recovery are still unknown (Tyree et al., 1999), various hypotheses have been proposed (Clearwater and Goldstein, 2005) and partially confirmed by different studies. Some authors, for example, have suggested that the refilling of vessels possibly occurs by reverse osmosis, induced by rising pressure in petiole or shoot tissues, established by the hydrolysis of starch into osmotically active sugars (Canny, 1997; Bucci et al., 2003). Other theories were advanced (Hacke and Sperry, 2003) including the activity of several tissues (phloem and xylem), the hydraulic compartmentalization of conducting elements during refilling, or even complex properties specific to membranes and perforations. All appear to indicate that a finely balanced equilibrium exists between xylem pressure and the loss of hydraulic conductivity, and that vulnerability to cavitation would be part of a feedback mechanism linking stomatal regulation to hydraulic conductance and plant water status (Bond and Kavanagh, 1999). Other authors (Zwieniecki and Holbrook, 1998; Salleo et al., 2004) have pointed out that the process of water refilling is energy demanding and, consequently, requires phloem activity and an adequate supply of carbohydrates. Brodersen et al. (2010) clearly presented embolism repair as a dynamic process using solutes, parenchyma water, pressure gradients, and conduit surface wettability. Under these conditions, recovery from an embolism may be facilitated in organs where close contacts are present between phloem, xylem, and living parenchyma cells. Such would be the case in leaves and petioles, where narrow vessels would favour refilling, as opposed to those in stems (Vesala et al., 2003).
The goal of the present study was to determine the vulnerability to cavitation of a drought-susceptible grapevine cultivar (Vitis vinifera L. cv. Chasselas) when subjected to water restriction. The importance of hydraulic conductance in the soil–plant–atmosphere continuum and, more specifically, of hydraulic conductance in petioles in controlling stomatal behaviour, was evaluated during water stress. Does stomatal regulation of leaf gas exchanges in grapevines provide a sufficient guarantee against cavitation development and hydraulic rupture? Is the appearance of midday wilting symptoms in Chasselas leaves, observed during the day when climatic and edaphic constraints are very high, and their disappearance at the end of the day linked to diurnal changes in hydraulic conductance in the plant, and, more especially, in the petioles? To answer these questions, different levels of water stress were imposed upon grapevines growing in the field, and the resulting hydraulic behaviours during the season were studied.
Materials and methods
Study site and plant material
The experiments were conducted in 2009 at the experimental station of Agroscope Changins-Wädenswil ACW in Leytron (Switzerland), located in an Alpine valley. The planting material was the cultivar Chasselas, clone 14/33-4, grafted on Vitis berlandieri×Vitis riparia cv. Kober 5BB rootstock. Vines were planted in the Guyot training system (vertical shoot-positioned) with a planting density of 5500 vines ha−1 (planting distance: 1.8×1.0 m). Six shoots per vine were maintained. The experimental site in Leytron lies on very stony (peyrosol >60% large elements, stones, blocks, and gravel) and deep soil (>2.5 m vine root depth), with a water-holding capacity estimated at 150 mm. In 2009, the annual rainfall was 560 mm, corresponding to the average annual rainfall over the last 30 years. August, September, and October were particularly dry (Table 1). Maximum daily temperatures measured locally were ∼30–35 °C during the period of physiological study (July–August). Maximum daily VPDs ranged from 2 kPa to 4.5 kPa.
Table 1.
Monthly rates of precipitation (mm) at the experimental site of Leytron (CH) during the 2009 season in comparison with the long-term averages (1960–1990)
| 2009 | Long term | |
| January | 55 | 54 |
| February | 32 | 46 |
| March | 17 | 39 |
| April | 35 | 36 |
| May | 22 | 39 |
| June | 40 | 47 |
| July | 58 | 49 |
| August | 2 | 62 |
| September | 16 | 47 |
| October | 11 | 46 |
| November | 60 | 52 |
| December | 105 | 60 |
| Year | 560 | 570 |
Irrigation treatments
Three different irrigation treatments were established. In the first treatment, 9.0 l m−2 soil (16.0 l per vine) were drip-fed weekly from bloom (152 DOY) to fruit ripening (216 DOY). This level of irrigation corresponded to an approximately weekly compensation of 30% evapotranspiration potential (ETP). The second treatment was not irrigated at all throughout the whole growing season. Neither was the third treatment. In addition, a waterproof and non-reflecting plastic was laid on the soil of the third treatment, from bloom (152 DOY), in order to eliminate the infiltration of water from precipitation events. The trial was carried out using 40 plants per treatment, set out in four split-plot randomized blocks of 10 vines each.
Measurements of water relations
Pre-dawn water potential (ΨPD), Ψleaf, and stem xylem water potential (Ψx) were measured using a pressure chamber (Scholander et al., 1965) according to Turner (1988). ΨPD was measured between 04:00 h and 05:00 h in complete darkness, on eight mature, undamaged and non-senescent leaves. Ψleaf and Ψx measurements were carried out between 14:00 h and 15:00 h when evapotranspiration was at the greatest. Ψleaf was determined on eight mature non-senescent leaves exposed at photon flux density (PFD) levels >1200 μmol m−2s−1. Ψx was measured on eight leaves bagged with a plastic sheet and covered with aluminium foil to stop transpiration at least 1 h before measurements were taken (Fulton et al., 2001).
The differences in stomatal behaviour and hydraulic conductance in the whole plant were estimated using the Kplant. Kplant was estimated from the relationship between the rates of single-leaf transpiration and soil–leaf water potential difference. The soil water potential (Ψsoil) was considered to be very close to the ΨPD in the leaves (Breda et al., 1995). In this way, ΨPD could be considered to be equivalent to Ψsoil and it was possible to estimate Kplant from the rate of single-leaf transpiration (E) and the difference in water potential between the soil and the leaf with the formula Kplant=E/(ΨPD–Ψleaf) (Sperry and Pockman, 1993).
Xylem embolism
Xylem embolism, induced by the presence of air bubbles in vessels, was measured using the method introduced by Sperry et al. (1988) and established by Cochard et al. (2000). The apparatus XYL'EM (Xylem Embolism meter, Instructec, Montigny-les-Cormeilles, France), based on a high-resolution liquid mass flowmeter, was used to determine the percentage loss in conductivity (PLC) in leaf petioles.
For each determination of PLC, the leaf petioles and their leaf blades were cut under water from the shoots bent in a container filled with water in the field: the petioles and their leaf blades were swiftly covered with aluminium foil (kept in darkness), then taken immediately to the laboratory in situ. Lengths and diameters of petioles, and the leaf blade surface area were measured. The petioles were attached to the XYL'EM apparatus tubes and their initial hydraulic conductance (Kinit) was determined with a hydrostatic pressure gradient of ∼3–4 kPa. Distilled and degassed water was used as perfusion liquid for all measurements. Since changes in the value of K may be caused by the absence or presence of certain ions in the solution, 10 mmol l−1 KCl was added to the solution. To measure the maximum conductance (Kmax), the petioles were then flushed twice during a period of 2 min with water pressurized at 0.15 MPa. Ψx was controlled on shoots at regular intervals during the day as well as just before petiole sampling.
Furthermore, with a view to amplifying water tensions artificially, some PLC measurements were carried out on petioles taken from shoots which were sectioned a few minutes in advance then progressively dehydrated in room conditions (temperatures of 22 °C, relative air humidity 60%).
PLC figures were constructed using the average values obtained from four petioles and as many measurements representing the xylem water potential Ψx. The loss of conductivity versus Ψx curves were utilized to determine vulnerability to cavitation, expressed as ΨPLC50, the xylem water potential inducing 50% loss in hydraulic conductivity. The ΨPLC50 was estimated by adjusting a sigmoid PLC curve versus Ψx data: PLC=100/{1+exp [a (Ψx–ΨPLC50)]}, where the parameter ‘a’ represents the slope of the curve at the point of inflection. The petiole hydraulic conductance (Kpetiole) was estimated from the following formula: Kpetiole=Kh×L/LA, Kh being the initial hydraulic conductance described above, L the length of the petiole and LA the surface area of the leaf blade.
Leaf gas exchange measurements
Leaf gas exchange measurements [net photosynthesis (A) and transpiration (E)] and gs measurements were carried out on adult, non-senescent leaves with a leaf plastochon index (LPI) >15, and well exposed to direct sunlight (PFD >1200 μmol m−2 s−1). Gas exchanges were measured using an open gas exchange system (ADC-System, LCA-4, Hoddesdon, UK) equipped with a Parkinson leaf chamber. Maximum photosynthetic rates (Amax) and maximum stomatal conductance (gmax) to ambient CO2 concentration were observed between 10:00 h and 11:00 h on different days throughout the season. Supplementary gs measurements were carried out during the day on adult non-senescent leaves located on the same shoots as those used for PLC and Kpetiole measurements.
Results
Cavitation vulnerability
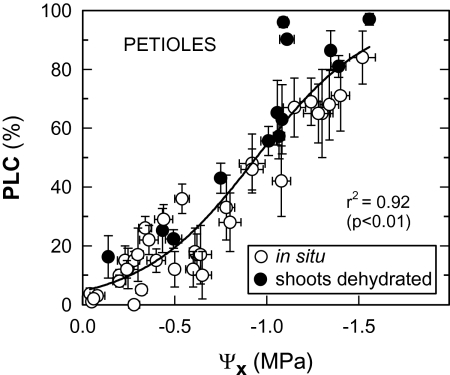
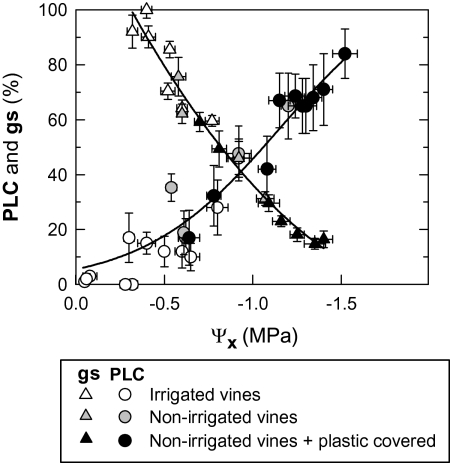
The cavitation vulnerability curves (Figs 1, 2) were constructed by plotting the changes in the PLC in relation to Ψx. At Ψx of around –0.3 MPa, a PLC of 10% was observed (Fig. 1). The 50% PLC threshold was reached when Ψx was at –0.95 MPa, according to the parameters of the function employed. Non-irrigated vines with covered soils presented values of PLC >60% in petioles when Ψx fell below –1.1 MPa (Fig. 2). Irrigated vine plants showed a lower vulnerability to cavitation (PLC <30%) with values of Ψx greater than –0.8 MPa. An intermediate situation was observed in non-irrigated vines.
Fig. 1.
Vulnerability to cavitation (PLC, percentage loss of conductivity) versus stem xylem water potential (Ψx) for grapevine leaf petioles measured in situ and of dehydrated shoots. Means ±SE of four petioles. Chasselas, Leytron (CH), 2009.
Fig. 2.
Vulnerability to cavitation (PLC, percentage loss of conductivity) and stomatal conductance (gs) versus stem xylem water potential (Ψx) for grapevine leaf petioles. Means ±SE of four petioles (for PLC), and eight leaves (for gs). Chasselas, Leytron (CH), 2009.
The leaf gs decreased with increasing xylem tension (low xylem water potentials) (Fig. 2). Stomatal closure reached a level of 50% when the values of Ψx were of the order of –0.8 MPa, and of 90% with values of Ψx at –1.5 MPa.
Diurnal variations of Kpetiole and PLC
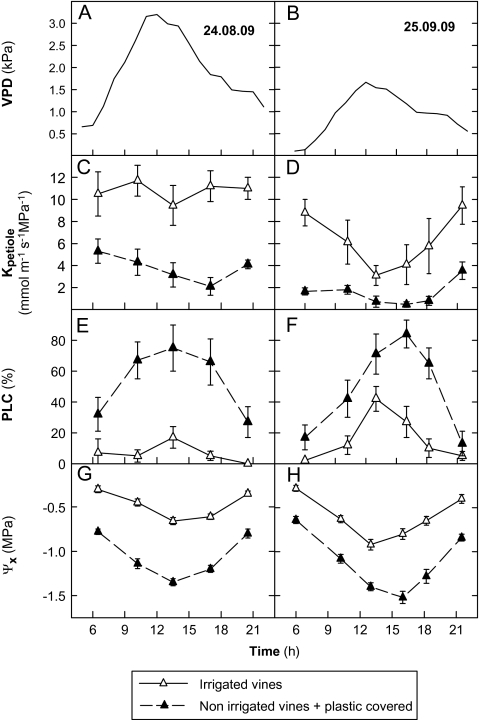
The Kpetiole (Fig. 3C–D) decreased progressively during the first hours of the day as water stress increased. The lowest values of Kpetiole were observed in the afternoon when the evapotranspiration was at its highest (high VPD) and water stress at its greatest. At the end of the afternoon and in the early evening, Kpetiole rose again, reaching levels close to those measured during the earliest hours of the day. This type of behaviour in Kpetiole variation was observed mainly in the irrigated vines, which, incidentally, presented Kpetiole values 2–3 times greater than those in the non-irrigated and soil-covered vines where the water stress was very high (Ψx – 1.5 MPa) (Fig. 3G, H).
Fig. 3.
Diurnal courses of leaf-atmospheric (VPD) (A, B), petiole hydraulic conductance (Kpetiole) (C, D), vulnerability of petioles to cavitation (PLC, percentage loss of conductivity) (E, F), and stem xylem water potential (Ψx) (G, H) during two days (24 August and 25 September) for different irrigation treatments. Chasselas, Leytron (CH), 2009.
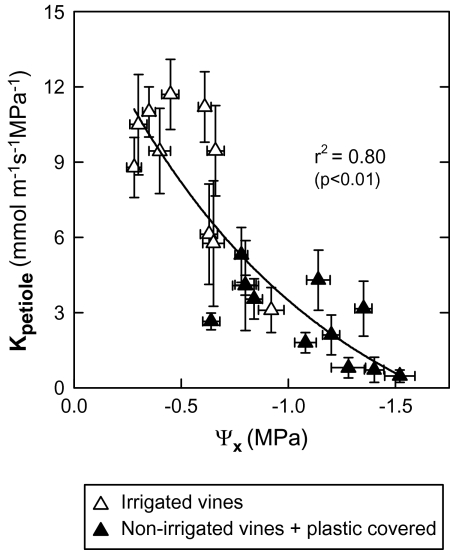
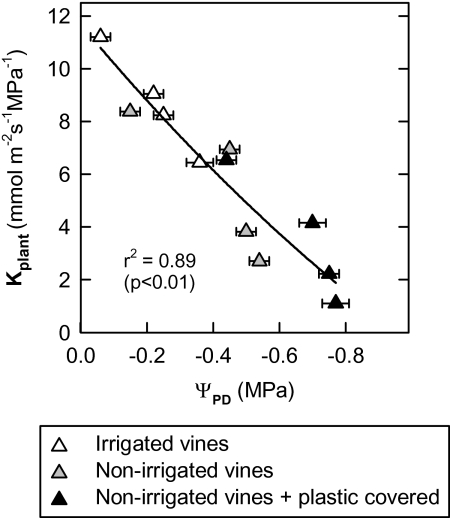
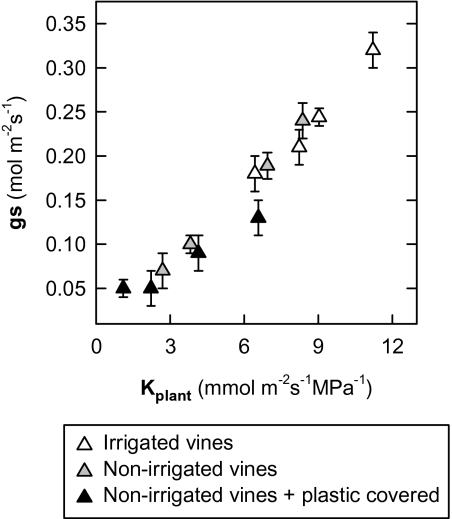
The PLC increased sharply during the daytime to reach maximum values (70–90%) in the non-irrigated and soil-covered vines in the middle of the afternoon (Fig. 3E, F). At the end of the afternoon, it then decreased, reaching minimum values during the night, identical to those measured at dawn. The same kind of PLC behaviour was monitored over several days, of which only two days (24 August and 25 September) are presented herein. In the irrigated treatment, the pattern of the PLC daily evolution was found to be identical to that of the non-irrigated vines but with smaller variations. Moreover, a good correlation exists between the Kpetiole and Ψx (Fig. 4) measured in vines subjected to different levels of irrigation. In the same way, a relationship between Kplant (=E/ΨPD–Ψleaf), estimated according to Sperry and Pockman (1993), and the level of water stress estimated by ΨPD was established in the present study (Fig. 5). The latter decreases in a linear fashion as water stress rises. Finally, leaf gs is closely related to Kplant, which was measured several times around midday, whether in vines with or without water restriction (Fig. 6).
Fig. 4.
Relationship between the stem xylem water potential (Ψx) and the petiole hydraulic conductance (Kpetiole) for adult leaves and different irrigation treatments. Means ±SE of four petioles/leaves. Chasselas, Leytron (CH), 2009.
Fig. 5.
Relationship between mid-morning leaf-specific hydraulic conductance [Kplant=E/(ΨPD–Ψleaf), equation from Sperry and Pockman (1993)] and the pre-dawn water potential (ΨPD) for different irrigation treatments. Means ±SE of eight leaves. Chasselas, Leytron (CH), 2009.
Fig. 6.
Stomatal conductance (gs) as a function of mid-morning leaf-specific hydraulic conductance (Kplant) for different irrigation treatments. Means ±SE of eight leaves. Chasselas, Leytron (CH), 2009.
Water and gas exchange relationships
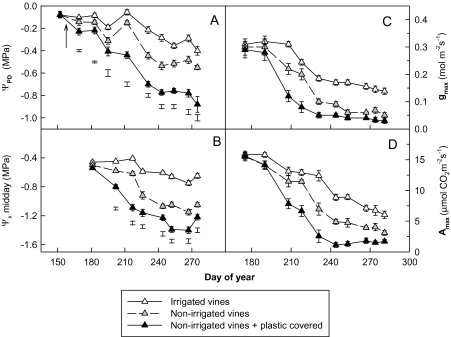
The different irrigation treatments tested in the present study on grapevines resulted in markedly different conditions of water stress (Fig. 7). Drip-irrigated vines presented no water stress throughout the whole growing period, with values of ΨPD greater than –0.3 MPa (Fig. 7A) and of Ψx greater than –0.8 MPa (Fig. 7B). The absence of irrigation led to a more noticeable water stress from the end of July (210 DOY) with ΨPD values between –0.3 MPa and –0.6 MPa, and Ψx values around –1.0 MPa during August and September. Finally, an elevated water stress was observed (ΨPD less than –0.6 MPa, and Ψx less than –1.1 MPa) from mid-August (DOY 230) until harvesting time in non-irrigated vines where the soil was covered with impermeable plastic. Figure 7 illustrates the impact of the different watering regimes on gmax (Fig. 7C) and Amax (Fig. 7D) observed mid-morning. The non-irrigated vines presented lower stomatal conductances and photosynthetic rates than the irrigated vines from the end of July (DOY 210), and more especially during September, with a reduction in gas exchange rates of almost 40%. In non-irrigated vines with covered soil, a drop in gas exchanges (gmax and Amax) was observed from the end of July down to very low levels of gs and A in the following months, which corresponded more or less to the residual conductance gres (0.03 mol m−2 s1) and a photosynthetic rate of ∼2 μmol CO2 m−2 s−1.
Fig. 7.
Changes in (A) pre-dawn water potential (ΨPD), (B) stem xylem water potential (Ψx), (C) maximum stomatal conductance (gmax), and (D) maximum photosynthetic rate (Amax) during the 2009 season with different irrigation treatments. The arrow indicates irrigation onset. Means ±SE for eight leaves. Bars: statistically significant at the 5% level of probability. Chasselas, Leytron (CH).
Discussion
Vulnerability to cavitation and diurnal change in PLC and Kpetiole
The different irrigation treatments set up in these trials enabled the development of well-contrasted water regimes in grapevines, which had in turn a strong influence on leaf gas exchanges, hydraulic conductance in petioles, and cavitation vulnerability. The present observations showed that the petioles of the cultivar Chasselas were highly vulnerable to cavitation, with values of ΨPLC50 measured at –0.9 MPa and an almost complete cavitation (90% PLC) with xylem water potential at around –1.5 MPa. Furthermore, a daily depression in Kpetiole and in Ψx became clear over the daily course, coinciding with the highest evapotranspiration (although moderate in the present study). At the end of the afternoon, Kpetiole and Ψx recovered and this trend continued overnight. In parallel, the rate of petiole cavitation increased during the day, regardless of the level of water status (high or low water restriction), followed by repair of embolism during the afternoon and the night.
Several studies have also mentioned this cavitation daily cycle in petioles (Zwieniecki et al., 2000; Bucci et al., 2003) and in leaf blades (Brodribb and Holbrook, 2003, 2004), which shows a recovery in hydraulic conductance at the end of the day. Brodersen et al. (2010) recently succeeded in in vivo visualization of the xylem embolism refilling phenomenon in grapevines, using high-resolution X-ray CT. This unequivocal method of visualization demonstrates that water refilling can occur in embolized vessels within a few hours and depends on the water flow from surrounding parenchyma. Brodersen et al. (2010) mentioned that water refilling can happen under water tensions between –0.6 MPa and –0.8 MPa, which correspond to the present results (Ψx measured in the evening at around –0.8 MPa with PLC values of ∼20–30%). Accordingly, the grapevine does appear to recuperate from embolism and very rapidly re-establishes water flow in the petioles during mid-afternoon and the night. In addition, Choat et al. (2010) showed that stems of grapevines, in contrast to petioles, were relatively resistant to cavitation, with embolism rates remaining under 30% even with high hydraulic tensions measured in stem vessels (Ψx less than –1.5 MPa), confirming Alsina's results on different cultivars (Alsina et al., 2007). These authors have not, however, excluded the possibility of cavitation phenomena occurring on a wider scale and to a greater extent in the roots, petioles, or leaves, when the grapevine is subjected to water stress, which would concord with the present results and those of Lovisolo et al. (2008). Indeed, it seems that the grapevine develops a hydraulic segmentation in which petiole cavitation would play an important part as ‘hydraulic fuses’, thus limiting leaf transpiration and the spread of embolism, and preserving the hydraulic integrity of shoots and grapes during periods of high water restriction and/or evapotranspiration. This hypothesis of hydraulic segmentation, originally put forward by Zimmermann (1983), would account for the maintenance of a water flux in basal organs, such as grapevine stems, which represent a greater investment in carbon and in which embolism repair would be more difficult due to higher metabolic cost. Furthermore, in some tree species, the distal organs (petioles and leaves) have been observed to be more vulnerable to cavitation than the stems (Tyree et al., 1993; Salleo et al., 2001), the trunk, and the roots (Choat et al., 2005). The findings of Choat et al. (2005) have shown, in addition, that the primary xylem/secondary xylem ratio of the organs is of greater importance in the vulnerability to cavitation than the diameter of vessels. The petioles, effectively possessing a sparse secondary thickening of primary xylem conduits, would thus be more likely to be subjected to hydraulic rupture (lower air seeding threshold; Choat et al., 2005). The hypothesis according to which the petioles may act as hydraulic fuses is partly proved by the studies carried out on the xylem structure in grapevines by Chatelet et al. (2006). The latter have shown that the continuity of open and continuous conduits is guaranteed for three internodes only through the primary xylem of grapevine shoots. Pit connections towards the secondary xylem, on the other hand, restrict the displacement of air from the primary xylem towards the secondary xylem, and thus represent an effective segmentation for the propagation of embolism throughout the stems.
In different plants, the combination of a very rapid stomatal response to high evaporation, coupled with a very large maximum hydraulic conductance, may help to minimize the risks of cavitation development and xylem failure, after a decrease in Kplant over the daily course (Brodribb and Holbrook, 2003). In grapevines, Kplant and Kpetiole present maximum values which are relatively modest in comparison with other species. Moreover, decreases in Kpetiole might not be directly related to cavitation and refilling, but could well also be influenced by environmental factors, such as the temperature, VPD, or light intensity. Brodribb and Holbrook (2004) have suggested that the reversible loss of leaf hydraulic conductivity could be a means of amplifying the signal of evaporative demand to the stomata in order to trigger a stomatal response. The major benefit would be protection of upstream xylem. The results of the present study clearly illustrate that the phenomena of daily variations of cavitation and embolism are linked to rising xylem water tension in the conducting elements and high evaporative demand (VPD). In the presence of low xylem water potentials, between –1.3 MPa and –1.5 MPa, the observed cavitation rates were at the highest, in the order of 70–90% PLC. The relationship established between Kplant and Kpetiole (r2=0.75, P <0.01) demonstrates that changes in hydraulic conductance in the leaves themselves were mainly influenced by the efficiency of water transport in sites of evaporation. Furthermore, gs decreased progressively from the mid-morning throughout the afternoon, in both experimental treatments (with and without water restriction), to reach its lowest level when Kpetiole was also at its lowest (unpublished results). At the end of the afternoon, a slight increase in gs was observed, in parallel with Kpetiole rising as well, even though no variation in VPD was recorded. With present knowledge, it was not possible to determine whether shoots and roots have the same faculty for re-establishing hydraulic conductivity as quickly as petioles.
Water xylem refilling under tension
It has been difficult to explain xylem water refilling in the presence of adjacent conduits under high tensions, requiring large positive pressures to clear the air bubbles or to cause their re-absorption in the solution (Tyree, 1997). Although several hypotheses have been formulated on the capacity of various plant organs to refill xylem vessels rapidly (Clearwater and Goldstein, 2005), it appears that a pre-existing condition is necessary. This condition requires the hydraulic compartmentalization of vessels so that local pressurization is possible (Zwieniecki and Holbrook, 2000) in order to enable embolism repair in transpiring plants. In other words, without an effective compartmentalization, water and solute flows, which have been transferred temporarily to the embolized vessel, would rapidly be lost and would be redirected towards neighbouring vessels with more negative hydraulic potentials. Brodersen et al. (2010), using high-resolution X-ray CT, pointed out the requirement of vessels for hydraulic compartmentalization towards surrounding vessels with higher water tensions. Thus, embolism repair would be a dynamic process. From this perspective, Brodersen et al. (2010) put forward a model for vessel refilling, based on the phloem load of water and solutes, as well as pressure gradients and vessel surface wettability. Various authors (Canny, 1997; Bucci et al., 2003) have been able to demonstrate that the hydrolysis of starch into osmotically active sugars in the daytime may well participate in the water refilling of xylem by means of a reverse osmotic mechanism. Recent research projects on grapevines undergoing rehydration after a drought (Lovisolo et al., 2008) confirm that the phenomenon of embolism repair is quite frequent in V. vinifera L. These authors have further suggested that the control of transpiration, induced by abscisic acid, is likely to be favourable to the gradual repair of embolisms in rehydrated grapevines.
Cavitation and stomatal behaviour
The cavitation phenomena may act as a signal for progressive stomatal closure (Nardini et al., 2001) or stomatal closure could occur to prevent cavitation (Tyree and Sperry, 1988). Under conditions of high climatic demand (high VPD) and dehydrated soils (restricted water), the stomata move towards closure by limiting evapotranspiration of the guard cells, while maintaining the plant water status above a threshold value of Ψ in order to avoid the development of xylem cavitation (Jones and Sutherland, 1991). In walnut (Cochard et al., 2002), a high restriction in gs (∼90% stomatal closure) precedes the onset of xylem cavitation. In this case, the effective closure of stomata in response to the water stress allows the leaf rachis xylem pressure to be maintained above the value of –1.4 MPa and the leaf water potential (Ψleaf) above the value of –1.6 MPa. These values represent thresholds of water status in the walnut, below which phenomena of embolism begin to develop. With regards to avoiding the risks of a rapidly developing embolism, the strategy of efficient stomatal closure performed well in this case. However, the major disadvantage of such a strategy resides in the concomitant limitation of CO2 fixation and a reduction in plant growth. For the Chasselas cultivar, on the other hand, stomatal control of xylem cavitation would appear to be less effective since almost complete closure of stomata (90%) intervenes only when the loss in hydraulic conductivity has already reached 90%, with a xylem potential recorded at –1.5 MPa. In grapevines, stomatal closure alone does not appear capable of regulating the increasing risk of cavitation as water stress rises. The cultivated grapevine (V. vinifera L.) is generally considered to be a ‘drought-avoiding’ plant (Smart and Combe, 1983) or, according to a more eco-physiological classification, an isohydric plant (Tardieu and Simmoneau, 1998), in accordance with its stomatal behaviour. Some V. vinifera L. cultivars of different geographical origin may display very diverse Ψleaf responses under water stress (Schultz, 2003), indicating that both iso- and anisohydric behaviours can co-exist within a single species. Nevertheless, Chouzouri and Schultz (2005) showed that there was a good correlation between drought tolerance and vulnerability to embolism in four grapevine cultivars of different geographic origin, and that the decrease in gs occurred concomitantly with an increase in cavitation events for all varieties, as soil water availability became restricted.
In the present study, the Ψx and Ψleaf (non-presented results) of Chasselas dropped steeply in the daytime and were lower in water-stressed plants than in well-watered plants, indicating a more anisohydric behaviour. The most negative values of ΨPD and Ψx were stabilized during the growing season at around –0.8 MPa and –1.5 MPa, respectively, in the most stressed situation (soil covered over, no irrigation). The significant defoliation (50% of leaf surface per vine, non-presented results) observed under highly restricted water conditions meant that the available water reserves in the soil were not used up too rapidly, by adaptation of water flux along the soil–plant–atmosphere continuum, while more or less stable values of Ψx and ΨPD were able to be maintained.
The stomatal closure in the present study probably responded to a decrease in petiole hydraulic conductance and an increase in cavitation events, but other factors cannot be totally excluded. Feed-forward behaviour of stomata with respect to regulation of Ψleaf has been attributed to the presence of chemical signals which modify stomatal opening and which are brought to the leaf in the transpiration flow (Davies et al., 1994).
Acknowledgments
We thank Jill Béchet for her assistance with the English translation. We would like to thank Dr Tété Barigah (INRA Clermont-Ferrand, France) for technical help and advice. We are also grateful to Eirini Pantazopoulou and Léa Delaporte (Master students) and the personnel of the group Viticulture and Enology of Agroscope Changins-Wädenswil ACW for help in physiological studies and field assistance.
Glossary
Abbreviations
- Amax
maximum net photosynthetic rate at ambient CO2 concentration
- E
transpiration rate
- gmax
maximum stomatal conductance to water vapour
- gres
residual stomatal conductance
- gs
stomatal conductance to water vapour
- Kpetiole
petiole hydraulic conductance
- Kplant
leaf-specific hydraulic conductance (whole plants)
- LPI
leaf plastochron index
- PLC
percentage loss of conductivity
- ΨPD
pre-dawn leaf water potential
- ΨPLC50
stem water potential at 50% of PLC
- Ψx
stem xylem water potential
- VPD
leaf–air vapour pressure deficit
References
- Alsina MM, De Herralde F, Aranda X, Save R, Biel C. Water relations and vulnerability to embolism are not related: experiments with eight grapevine cultivars. Vitis. 2007;46:1–6. [Google Scholar]
- Bond BJ, Kavanagh KL. Stomatal behaviour of four woody species in relation to leaf-specific hydraulic conductance and threshold water potential. Tree Physiology. 1999;19:503–510. doi: 10.1093/treephys/19.8.503. [DOI] [PubMed] [Google Scholar]
- Breda N, Granier A, Barataud F, Moyne C. Soil water dynamics in an oak stand. I. Soil moisture, water potentials and water uptake by roots. Plant and Soil. 1995;172:17–27. [Google Scholar]
- Brodersen CR, McElrone AJ, Choat B, Matthews MA, Shackel KA. The dynamics of embolism repair in xylem: in vivo visualizations using high resolution computed tomography. Plant Physiology. 2010;154:1088–1095. doi: 10.1104/pp.110.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Stomatal closure during leaf dehydratation, correlation with other leaf physiological traits. Plant Physiology. 2003;132:2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant, Cell and Environment. 2004;27:820–827. [Google Scholar]
- Brodribb TJ, Holbrook NM, Edward EJ, Gutiérrez MV. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant, Cell and Environment. 2003;26:443–450. [Google Scholar]
- Brodribb TJ, Jordan GJ. Internal coordination between hydraulics and stomatal control in leaves. Plant, Cell and Environment. 2008;31:1557–1564. doi: 10.1111/j.1365-3040.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- Bucci SJ, Scholtz FG, Goldstein G, Meinzer FC, Sternberg L. Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant, Cell and Environment. 2003;26:1633–1645. [Google Scholar]
- Canny MJ. Vessel contents during transpiration: embolisms and refilling. American Journal of Botany. 1997;84:1223–1230. [PubMed] [Google Scholar]
- Chatelet DS, Matthews MA, Rost TL. Xylem structure and connectivity in grapevine (Vitis vinifera) shoots provides a passive mechanism for the spread of bacteria in grape plants. Annals of Botany. 2006;98:483–494. doi: 10.1093/aob/mcl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Lahr EC, Melcher PJ, Zwieniecki MA, Holbrook NM. The special pattern of air seeding thresholds in mature sugar maple trees. Plant, Cell and Environment. 2005;28:1082–1089. [Google Scholar]
- Choat B, Drayton WM, Brodersen CR, Matthews MA, Shackel KA, Wada H, McElrone AJ. Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to long-vesseled species. Plant, Cell and Environment. 2010;33:1502–1512. doi: 10.1111/j.1365-3040.2010.02160.x. [DOI] [PubMed] [Google Scholar]
- Chouzouri A, Schultz HR. Hydraulic anatomy, cavitation susceptibility and gas-exchange of several grapevine cultivars of different geographical origin. Acta Horticulturae. 2005;689:325–331. [Google Scholar]
- Clearwater MJ, Goldstein G. Embolism repair and long distance water transport. In: Holbrook NM, Zwieniecki MA, editors. Vascular transport in plants. Burlington, MA: Elsevier Academic Press; 2005. pp. 375–399. [Google Scholar]
- Cochard H, Bodet C, Ameglio T, Cruziat P. Cryo-scanning electron microscopic observations of vessel content during transpiration in walnut petioles: facts or artefacts? Plant Physiology. 2000;124:1191–1202. doi: 10.1104/pp.124.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Coll L, Le Roux X, Ameglio T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiology. 2002;128:282–290. [PMC free article] [PubMed] [Google Scholar]
- Comstock JP. Variation in hydraulic architecture and gas exchange in two desert sub-shrubs, Hymenoclea salsola (T.&G.) and Ambrosia dumosa. Oecologia. 2000;125:1–10. doi: 10.1007/PL00008879. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Tardieu F, Trejo CL. How do chemical signals work in plants that grow in drying soil? Plant Physiology. 1994;104:309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domec JC, Scholz FG, Bucci SJ, Meinzer FC, Goldstein G, Villalobos-Vega R. Diurnal and seasonal variation in root xylem embolism in neotropical savanna woody species: impact on stomatal control of plant water status. Plant, Cell and Environment. 2006;29:26–35. doi: 10.1111/j.1365-3040.2005.01397.x. [DOI] [PubMed] [Google Scholar]
- Fulton AR, Bucher R, Olson B, Schwankl L, Gilles C, Bertagna N, Walton J, Shackel K. Rapid equilibration of leaf and stem water potential under field conditions in almonds, walnuts and prunes. HortTechnology. 2001;11:609–615. [Google Scholar]
- Hacke UG, Sperry JS. Limits to xylem refilling under negative pressure in Laurus nobilis and Acer negundo. Plant, Cell and Environment. 2003;26:303–311. [Google Scholar]
- Hsiao TC. Plant response to water stress. Annual Review of Plant Physiology. 1973;24:519–570. [Google Scholar]
- Holbrook NM, Ahrens ET, Burns MJ, Zwieniecki MA. In vivo observation of cavitation and embolism repair using magnetic resonance imaging. Plant Physiology. 2001;126:27–31. doi: 10.1104/pp.126.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RM, Ryan MG, Stiller V, Sperry JS. Stomatal conductance and photosynthesis vary lineary with plant hydraulic conductance in Ponderosa pine. Plant, Cell and Environment. 2001;24:113–121. [Google Scholar]
- Jarvis AJ, Davies WJ. Modeling stomatal responses to soil and atmospheric drought. Journal of Experimental Botany. 1998;49:399–406. [Google Scholar]
- Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC. Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiology. 2009;29:879–887. doi: 10.1093/treephys/tpp031. [DOI] [PubMed] [Google Scholar]
- Jones HG, Sutherland RA. Stomatal control of xylem embolism. Plant, Cell and Environment. 1991;14:607–612. [Google Scholar]
- Lo Gullo M, Nardini A, Trifilo P, Salleo S. Changes in leaf hydraulics and stomatal conductance following drought stress and irrigation in Ceratonia siliqua (Carob tree). Physiologia Plantarum. 2003;117:186–194. [Google Scholar]
- Lovisolo C, Perrone I, Hartung W, Schubert A. An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytologist. 2008;180:642–651. doi: 10.1111/j.1469-8137.2008.02592.x. [DOI] [PubMed] [Google Scholar]
- McCully ME. Root xylem embolisms and refilling. Relation to water potentials of soil, roots, and leaves, and osmotic potentials of root xylem sap. Plant Physiology. 1999;119:1001–1008. doi: 10.1104/pp.119.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully ME, Huang CX, Ling LE. Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytologist. 1998;138:327–342. doi: 10.1046/j.1469-8137.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Grantz DA. Stomatal and hydraulic conductance in growing sugarecane: stomatal adjustment to water transport capacity. Plant, Cell and Environment. 1990;13:383–388. [Google Scholar]
- Melcher PJ, Goldstein G, Meinzer FC, Yount DE, Jones TJ, Holbrook MN, Huang CX. Water relations of coastal and estuarine Rhizosphora mangle xylem pressure potential and dynamics of embolism formation and repair. Oecologia. 2001;126:182–192. doi: 10.1007/s004420000519. [DOI] [PubMed] [Google Scholar]
- Monteith JL. A reinterpretation of stomatal responses to humidity. Plant, Cell and Environment. 1995;18:357–364. [Google Scholar]
- Mott KA. Do stomata respond to CO2 concentrations other than intercellular? Plant Physiology. 1988;86:200–203. doi: 10.1104/pp.86.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Tyree MT, Salleo S. Xylem cavitation in the leaf of Prunus laurocerasus and its impact on leaf hydraulics. Plant Physiology. 2001;125:1700–1709. doi: 10.1104/pp.125.4.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, LoGullo MA, De Paoli D, Zippo M. Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytologist. 1996;132:47–56. doi: 10.1111/j.1469-8137.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Salleo S, LoGullo MA, Raimondo F, Nardini A. Vulnerability to cavitation of leaf minor veins: any impact on leaf gas exchange? Plant, Cell and Environment. 2001;24:851–859. [Google Scholar]
- Salleo S, LoGullo MA, Trifilo P, Nardini A. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant, Cell and Environment. 2004;27:1065–1076. [Google Scholar]
- Saliendra NZ, Sperry JS, Comstock JP. Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta. 1995;196:357–366. [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Schultz HR. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant, Cell and Environment. 2003;26:1393–1405. [Google Scholar]
- Schultz HR, Matthews MA. Resistance to water transport in shoots of Vitis vinifera L. Relation to growth at low water potential. Plant Physiology. 1988;88:718–724. doi: 10.1104/pp.88.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart RE, Coombe BG. Water relations of grapevines. In: Kozlowski TT, editor. Water deficits and plant growth. Vol. VII. New York: Academic Press; 1983. pp. 137–196. [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficit and hydraulic limits to leaf water supply. Plant, Cell and Environment. 2002;25:251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Pockman WT. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis. Plant, Cell and Environment. 1993;16:279–287. [Google Scholar]
- Tardieu F, Simonneau T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modeling isohydric and anisohydric behaviours. Journal of Experimental Botany. 1998;49:419–432. [Google Scholar]
- Turner NC. Measurement of plant water status by the pressure chamber technique. Irrigation Science. 1988;9:289–308. [Google Scholar]
- Tyree MT. The cohesion–tension theory of sap ascent: current controversies. Journal of Experimental Botany. 1997;48:1753–1765. [Google Scholar]
- Tyree MT, Cochard H, Cruiziat P, Sinclair B, Ameglio T. Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant, Cell and Environment. 1993;16:879–882. [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Lo Gullo MA, Mosca R. Refilling of embolic vessels in young stems of laurel. Do we need a new paradigm? Plant Physiology. 1999;120:11–21. doi: 10.1104/pp.120.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answer from a model. Plant Physiology. 1988;88:574–580. doi: 10.1104/pp.88.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesala T, Holtta T, Peramaki M, Nikinmaa E. Refilling of a hydraulically isolated embolized xylem vessel: model calculation. Annals of Botany. 2003;91:419–428. doi: 10.1093/aob/mcg022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. Leaf conductance in relation to rate of CO2 assimilation. I. Influence of nitrogen nutrition, phosphorous nutrition, photon flux density and ambient partial pressure of CO2 during ontogeny. Plant Physiology. 1985;78:821–825. doi: 10.1104/pp.78.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MH. Xylem structure and the ascent of sap. Berlin: Springer-Verlag; 1983. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Steppe K, Sterck FJ. Stomatal regulation by microclimate and tree water relations: interpreting eco-physiological field data with a hydraulic plant model. Journal of Experimental Botany. 2007;58:2113–2131. doi: 10.1093/jxb/erm050. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Holbrook NM. Diurnal variation in xylem hydraulic conductivity in white ash (Fraxinus americana L.), red maple (Acer rubrum L.) and red spruce (Picea rubens Sarg.) Plant, Cell and Environment. 1998;21:1173–1180. [Google Scholar]
- Zwieniecki MA, Hutyra L, Thompson MV, Holbrook NM. Dynamic changes in petiole specific conductivity in red maple (Acer rubrum L.), tulip tree (Liriodendron tulipifera L.) and northern fox grape (Vitis labrusca L.) Plant, Cell and Environment. 2000;23:407–414. [Google Scholar]