Abstract
Later-flowering spikelets in a rice panicle, referred to as the inferior spikelets, are usually poorly filled and often limit the yield potential of some rice cultivars. The physiological and molecular mechanism for such poor grain filling remains unclear. In this study the differentially expressed genes in starch synthesis and hormone signalling between inferior and superior spikelets were comprehensively analysed and their relationships with grain filling was investigated. DNA microarray and real-time PCR analysis revealed that a group of starch metabolism-related genes showed enhanced expression profiles and had higher transcript levels in superior spikelets than in inferior ones at the early and middle grain-filling stages. Expression of the abscisic acid (ABA) synthesis genes, 9-cis-epoxycarotenoid dioxygenase 1 (NCED1) and NCED5, and the ethylene synthesis genes, 1-aminocyclopropane-1-carboxylate oxidase 1 (ACO1) and ACO3, declined with development of the caryopses. Meanwhile, if compared with inferior spikelets, expression of these genes in superior spikelets decreased faster and had lower transcript profiles, especially for ethylene. ABA concentration and ethylene evolution rate showed similar trends to their gene expression. Exogenous supply of ABA reduced the sucrose synthase (SUS) mRNA level and its enzyme activity in detached rice grains, while exogenously supplied ethephon (an ethylene-releasing reagent) suppressed the expression of most starch synthesis genes; that is, SUS, ADP-glucose pyrophosphorylase (AGPase), and soluble starch synthase (SSS), and down-regulated their enzyme activities. In summary, it is concluded that the relatively high concentrations of ethylene and ABA in inferior spikelets suppress the expression of starch synthesis genes and their enzyme activities and consequently lead to a low grain-filling rate.
Keywords: Abscisic acid, ethylene, grain filling, inferior spikelets, rice, starch synthesis, superior spikelets
Introduction
In rice panicles, grain development is asynchronous (Mohapatra et al., 1993; Ishimaru et al., 2003). The degree and rate of grain filling among spikelets can be very different according to their positions on a panicle. In general, caryopses spatially located on the apical primary branches flower earlier, show the highest grain filling, and achieve larger and heavier grains on a panicle, whereas caryopses located on the proximal secondary branches flower relatively later and are either sterile or fill slowly. The former and latter types of caryopses are called superior and inferior spikelets, respectively (Ishimaru et al., 2005; Yang et al., 2006). The poor grain filling of the later-flowering inferior spikelets of rice, especially for some ‘super’ rice cultivars, is a serious problem and frequently limits their yield potential (Yang and Zhang, 2010).
Rice grain filling is a process of starch accumulation since starch contributes 80–90% of the final dry weight of an unpolished grain (Yoshida, 1972; Duan and Sun, 2005). Biosynthesis of starch from sucrose in the developing endosperm primarily determines grain yield and quality of rice. It has been documented that several key enzymes are involved in the pathway of starch synthesis (Emes et al., 2003; Tetlow et al., 2004); that is, sucrose synthase (SUS), UDP-glucose pyrophosphorylase (UGPase), AGPase, and starch synthase. There are two forms of starch synthase, granule-bound starch synthase (GBSS) involved in amylose synthesis, and soluble starch synthase (SSS) and starch branching enzyme (SBE) involved in amylopectin synthesis. In addition, the genes encoding the above enzymes are mostly identified in rice and most of them are multigene families (Duan and Sun, 2005; Ohdan et al., 2005; Sakamoto and Matsuoka, 2008). It has been reported that the low activities of starch synthesis-related enzymes, such as SUS, AGPase, SSS, and SBE, are closely associated with the inferior grain weight (Jiang et al., 2003; Kato et al., 2007; Panda et al., 2009; Tang et al., 2009). Meanwhile, some of them have been shown to be differentially expressed at the transcript level between inferior and superior caryopses during grain filling (Ishimaru et al., 2005). However, to our knowledge, the differences of grain-filling rate between superior and inferior spikelets have not been studied in relation to the comprehensive expression patterns of the starch metabolism genes.
Plant hormones, especially ethylene and abscisic acid (ABA), play important roles in regulating grain filling. A higher ethylene level in developing seeds correlates negatively with starch metabolism-related enzyme activities and always leads to poor grain filling (Yang and Zhang, 2006; Panda et al., 2009; Zhang et al., 2009). Overexpression of the ethylene receptor gene ETHYLENE RESPONSE2 (ETR2) in rice down-regulates the monosaccharide transporter gene and consequently prevents sugar translocation from the stem to grains, leading to reduced grain weight (Wuriyanghan et al., 2009). In contrast, inhibition of ethylene evolution can improve dry matter partitioning and development of later-flowering spikelets on rice panicles (Mohapatra et al., 2000; Yang et al., 2006). The roles of ABA in grain filling are complicated. A high concentration of ABA reduces transport of sucrose into the grains and lowers the ability of grains to synthesize starch (Ahmadi and Baker, 1999; Bhatia and Singh, 2002), while the appropriate concentration of ABA can enhance SUS activity (Tang et al., 2009), increases the expression of genes related to starch metabolism (Akihiro et al., 2005, 2006), and achieves higher grain yield (Yang et al., 2006). Despite these facts, the molecular mechanism by which the hormones regulate the grain filling and their roles in the development of superior and inferior caryopses are still unclear.
Many previous studies have reported the comparative analyses of inferior and superior spikelets and the effects of ethylene and ABA on the phenotypic characteristics during grain filling (Mohapatra et al., 2000; Ishimaru et al., 2003; Yang et al., 2006). In this study, the detailed molecular mechanism was investigated to find the relationships between starch synthesis and hormone signalling. The results show that, if compared with the superior spikelets, the relatively high concentrations of ethylene and ABA in the inferior spikelets suppress the expression of starch synthesis genes and their enzyme activities and consequently lead to their slow grain-filling rate.
Materials and methods
Plant materials and sampling
Rice YD-6 (Yangdao 6, an indica inbred cultivar) was grown in the paddy field. Seedlings were raised at a hill spacing of 0.2×0.2 m and with two seedlings per hill. The soil contained organic matter at 2.45% and available N–P–K at 108, 34.2, and 66.9 mg kg−1, respectively. N (60 kg ha−1 as urea), P (30 kg ha−1 as single superphosphate), and K (40 kg ha−1 as KCl) were applied and incorporated before transplanting. N as urea was also applied at mid-tillering (40 kg ha−1) and at panicle initiation (50 kg ha−1). The time from sowing to heading was ∼90 d, and harvesting took place after another 60 d.
Five hundred panicles that flowered on the same day were chosen and tagged. The duration of anthesis from the first spikelets to the last on a panicle was 7 d. The spikelets that flowered on the first 2 d within a panicle were considered as superior spikelets and those that flowered on the last 2 d within a panicle were considered as inferior spikelets. A total of 15–20 tagged panicles were sampled at 3 d or 6 d intervals according to the experiment. The sampled caryopses were immediately frozen in liquid nitrogen and stored at –80 °C until use, except for the samples for ethylene analysis, which were assayed immediately after sampling.
Assays of grain weight, soluble carbohydrate, and starch content
A total of 80–90 sampled grains were used for measurements of grain dry weight and soluble carbohydrate. These samples were dried at 70 °C to constant weight, dehulled, and weighed. An aliquot of 0.1 g of ground powder was extracted with 10 ml of 80% ethanol at 80 °C for 30 min, and the extraction step was repeated twice. All the supernatants were pooled and used for soluble carbohydrate analysis. The residue was digested with 3% HCl and used for estimation of starch. Soluble carbohydrate was determined as described by Yemm and Willis (1954) and the starch content was determined as described by Buysse 3and Merckx (1993).
DNA microarray and quantitative real-time PCR (qRT-PCR) analysis
The caryopses sampled at 3 DPA (days post-anthesis) and 9 DPA from superior spikelets and inferior spikelets were used for DNA microarray analysis. Total RNA was isolated using TRIzol reagent and purified by RNeasy spin columns (Qiagen, Germany). DNA microarray analysis was performed using an Affymetrix Rice Genome Array. An 8 μg aliquot of total RNA was used to make biotin-labelled cRNA targets. All the processes for cRNA preparation, labelling, hybridization, washing, and scanning were conducted according to the Genechip Standard Protocol (Affymetrix). GCOS software (Affymetrix Genechip Operating software) was used for data collection and normalization. The values were log2 transformed and those whose log2 ratios were ≥1 or less than or equal to –1 were considered as differentially expressed genes. Among 51 279 rice genes on the microarray, Supplementary Table S1 and S2 available at JXB online show the differential genes between the superior and inferior spikelets at 3 DPA and 9 DPA, respectively.
For qRT-PCR, total RNA was isolated and transcribed with oligo(dT) primers using a SuperScript first-strand synthesis system according to the manufacturer's instructions (Invitrogen, USA). Transcript levels of selected genes were measured by qRT-PCR using an iCycler (Bio-Rad, USA) with iQ SYBR Green Supermix (Bio-Rad, USA). The data were normalized to the amplification of the rice ACTIN2 gene. For each sample, the mean value from three qRT-PCRs was used to calculate the transcript abundance. Primer sequences used for qRT-PCR, listed in Supplementary Table S5 at JXB online, were designed according to previous publications (Hirose and Terao, 2004; Iwai et al., 2006; Yamakawa et al., 2007; Zhu et al., 2009).
Assays of ABA concentration and ethylene evolution
For estimation of endogenous ABA levels, 0.2 g of dehulled caryopses were homogenized in 1 ml of distilled water and then shaken at 4 °C overnight. The homogenates were centrifuged and the supernatant were used directly for ABA assay. ABA content was determined using the radioimmunoassay method as previously described (Zhu et al., 2009).
A total of 60–80 freshly sampled caryopses were used for ethylene analysis. Caryopses were sealed in 15 ml glass vials and incubated in the dark for 24 h. Ethylene was assayed by gas chromatography according to Zhang et al. (2009). The ethylene evolution rate was expressed as nmol h−1 g−1 DW.
Exogenous chemical applications
For exogenous ABA and ethylene feeding experiments, panicles from uniformly grown plants at 9 DPA were cut off at 10 cm from the panicle neck node. The detached panicles were further treated and only the four top spikelets were retained, and these were transferred into a 50 ml plastic tube containing 3% sucrose and other essential culture solutions (Sasaki et al., 2005). The tubes were then placed in a growth chamber with 12 h light at a temperature of 28 °C and 55% relative humidity. ABA, ethephon (an ethylene-releasing reagent), cobaltous nitrate (an ethylene synthesis inhibitor) (all from Sigma, USA), and fluridone (an ABA synthesis inhibitor) (Fluka, Germany) were added to the culture solution. Their final concentrations were 20 μM, 5 mM, 50 μM, and 20 μM, respectively. After 2 d treatment, the grains were sampled and used for RNA extraction and enzyme assay.
Enzyme extractions and assays
The preparation of enzyme extracts was similar to that described by Nakamura et al. (1989). About 200 mg of dehulled and frozen grains were homogenized in a pre-cooled mortar containing 1 ml of extraction buffer comprising, 100 mM HEPES-NaOH (pH 7.6), 5 mM MgCl2, 5 mM dithiothreitol (DTT), 2 mM EDTA, 12.5% (v/v) glycerol, and 5% (w/v) insoluble polyvinylpyrrolidone 40. After centrifugation at 12 000 g for 10 min, the supernatant was used for analysis of the SUS, AGPase, and SSS activities. The sediment was re-suspended in 1 ml of extraction buffer for GBSS activity assay.
SUS was assayed in the cleavage direction and analysed as described by Sung et al. (1989). AGPase was assayed by the method of Nakamura et al. (1989). SSS and GBSS were determined according to Schaffer and Petreikov (1997). All the enzyme activities were expressed on a per mg protein basis.
Results
Rice superior and inferior spikelets showed different grain-filling processes and starch content
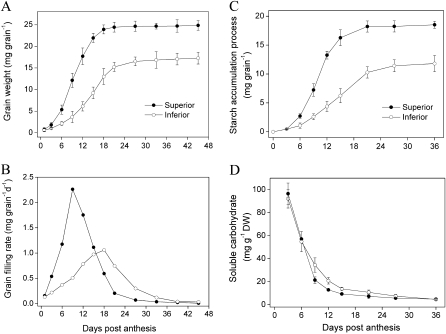
Rice (Yangdao 6) superior and inferior spikelets showed different grain-filling processes. The grain dry weight increased much faster for the earlier-flowering superior spikelets than for the later-flowering inferior ones (Fig. 1A). The maximum grain-filling rate occurred at 9 d for superior spikelets and at 18 d for inferior spikelets (Fig. 1B). Starch accumulation was consistent with the grain-filling process (Fig.1C). Soluble carbohydrate concentrations decreased rapidly for both superior and inferior spikelets, accompanied by the enhancement of starch accumulation. From Fig. 1D, it can be seen that there were no differences in soluble carbohydrate concentrations between the superior and inferior spikelets during the early grain-filling stage (i.e. at 3 DPA and 6 DPA), or even later for inferior spikelets during the middle grain-filling stage (i.e. at 9–21 DPA), indicating that carbohydrate supply may not be the key factor limiting grain filling in inferior spikelets. These results are consistent with previous descriptions about superior and inferior spikelets (Mohapatra et al., 2000; Yang et al., 2006).
Fig. 1.
Grain weight (A), grain filling rate (B), starch accumulation (C), and soluble carbohydrate content (D) in rice spikelets. The indica cultivar YD-6 was field grown. Superior spikelets are those which flowered on the first 2 d within a panicle and inferior spikelets are those which flowered on the last 2 d within a panicle. Vertical bars represent the SEM (n=3).
The differentially expressed genes related to starch synthesis between inferior and superior spikelets
To investigate the metabolic diversities between superior and inferior spikelets during grain filling, differentially expressed genes were identified by Affymetrix genechip analysis from developing caryopses harvested at 3 DPA and 9 DPA. Among 51 279 rice genes on the microarray, 306 genes were up-regulated and 953 genes were down-regulated >2-fold in superior spikelets when compared with inferior spikelets at 3 DPA; these values were 203 genes and 1058 genes at 9 DPA, respectively, including genes associated with carbohydrate metabolism, hormone synthesis, signal transduction, photosynthesis, etc. (Supplementary Tables S1, S2 at JXB online). Among these, genes related to carbohydrate metabolism will be emphasized in this study, as these may play critical roles in determining the grain weight during grain filling.
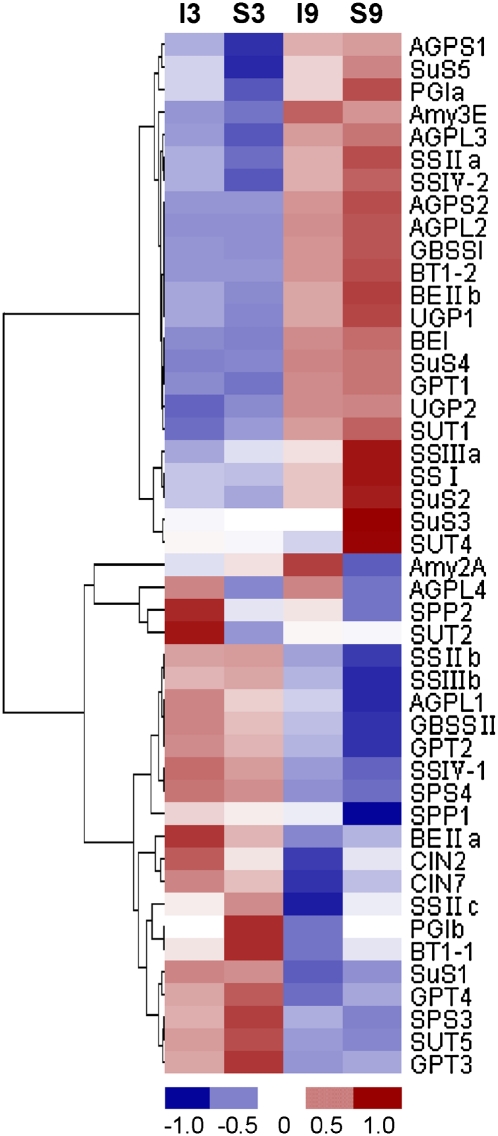
For carbohydrate metabolism, especially starch synthesis, the genes encoding major functional enzymes are mostly known (Hirose and Terao, 2004; Ohdan et al., 2005; Yamakawa et al., 2007). All the available genes related to starch metabolism in the genechip are included in Supplementary Table S3 at JXB online and their mRNA expression values were further clustered and are displayed in a heat map format (Fig. 2). The largest gene expression values are displayed in red and the smallest values in blue. To represent an individual gene expression pattern, these genes were clustered into three groups according to their expression patterns during grain filling (Table 1). The first group was defined as those whose gene expression was up-regulated at least 2-fold at 9 DPA compared with 3 DPA in superior spikelets; this group of genes showed enhanced expression profiles during development of caryopses and, moreover, most of them had higher expression levels in superior spikelets than in inferior spikelets. These genes may play a crucial role in determining the rice grain-filling rate and starch accumulation in superior and inferior spikelets, and include the SUS genes SUS2, SUS3, and SUS4, the UGPase gene UGP1, the AGPase genes AGPS1, AGPS2, AGPL2, and AGPL3, the GBSS gene GBSSI, the SSS genes SSI and SSIIa, the SBE genes BEI and BEIIb, and two protein transporter genes Glc-6-P/phosphate- translocator1 (GPT1) and brittle-1 protein2 (BT1-2). The second group was characterized by gene expression being down-regulated at least 2-fold at 9 DPA compared with 3 DPA in superior spikelets; this group of genes had relatively higher mRNA expression at early stages of seed development which declined during grain filling, as exhibited by SUS1, AGPL1, GBSSII, SSIIIb, and GPT3 genes, which were presumed to be involved in the construction of fundamental cell machinery and initiation of starch granules. The third group was characterized by gene expression being little changed and always at low levels during development of caryopses; these included cell wall invertase2 (CIN2), phospho-Glc isomerase a (PGIa), PGIb, Suc-phosphatase2 (SPP2), Suc-phosphate synthase4 (SPS4), SSIIIa, BEIIa, and GPT4 genes. This group of genes may not be the limiting factors for grain filling.
Fig. 2.
Hierarchical clustering of starch metabolism-related genes of rice during grain filling. Horizontal rows represent individual genes and vertical rows represent individual samples. I3, inferior spikelets at 3 DPA; S3, superior spikelets at 3 DPA; I9, inferior spikelets at 9 DPA; S9, superior spikelets at 9 DPA. Red and blue indicate the transcript level above and below the median, respectively, for that gene across all samples. The clustering is performed on the log2-transformed expression values.
Table 1.
Grouping of rice genes involved in starch synthesis in developmental grains according to their individual expression profiles and patterns
| Starch synthesis process | Changes in the expression level at 9 DPA compared with 3 DPA in superior spikelets | ||
| Group 1: up-regulated 2-fold | Group 2: down-regulated 2-fold | Group 3: within 2-fold | |
| Sucrose transport | SUT1 | SUT5 | SUT2, SUT4 |
| Sucrose to ADPG/G-6-P | SUS2, SUS3, SUS4, UGP1, AGPS2, AGPL2, AGPL3 | SUS1, SPS3 | CIN2, PGIa, PGIb, SPP2, SPS4 |
| ADPG/G-6-P transport | BT1-2, GPT1 | GPT2, GPT3 | GPT4 |
| ADPG/G-6-P to starch | AGPS1, GBSSI,SSI,SSIIa, BEI, BEIIb | AGPL1, GBSSII, SSIIb, SSIIIb | SSIIIa, BEIIa |
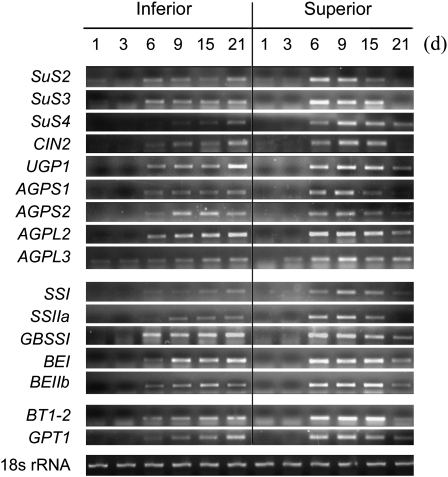
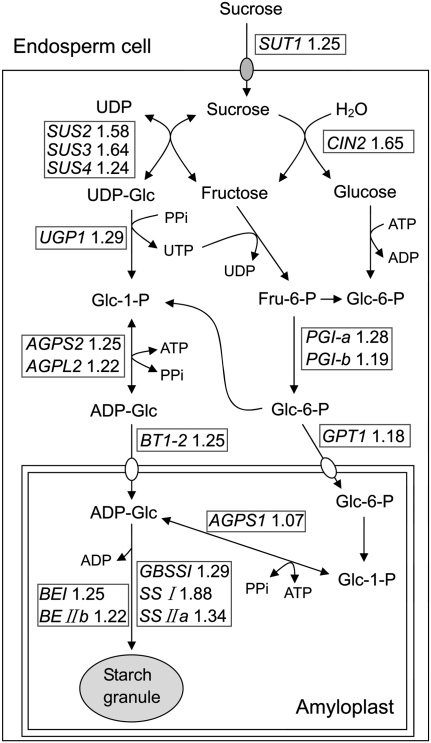
Due to the high expression during grain filling, which could be closely related to the capacity for starch synthesis, the first group of genes was further subjected to time course expression analysis in superior and inferior spikelets by semi-quantitative RT-PCR using gene-specific primers (Supplementary Table S5 at JXB online). As shown in Fig. 3, expression of most genes reached a maximum at the early or middle grain-filling stages for superior spikelets (6 d and 9 d), whereas this occurred at the late stage for inferior spikelets (15 d and 21 d). To clarify the roles of these genes on the pathway of starch synthesis, a metabolic map was created for starch-related metabolism to indicate the reaction steps at the transcriptional level (Fig. 4). The map provides a global comprehension regarding the difference in starch-related metabolism between superior and inferior spikelets at 9 DPA. From Fig. 4 it can be seen that the largest fold changes between superior and inferior grains were in the genes responsible for conversion of sucrose into ADP-glucose, especially SUS genes, and this was also obvious from the RT-PCR data (Fig. 3). The result is consistent with the previous description that a higher SUS activity exists in superior grains compared with inferior grains at the early stage of grain filling in rice (Mohapatra et al., 2009; Tang et al., 2009). These data indicate the great influence of SUS genes on grain filling. However, other differentially expressed genes, which may cooperatively contribute to the differential grain-filling rate between inferior and superior spikelets, cannot be ignored.
Fig. 3.
Expression of genes related to starch metabolism in developing caryopses determined by RT-PCR analysis. RNA was extracted from superior and inferior spikelets at 1, 3, 6, 9, 15, and 21 DPA and then reverse transcribed for RT-PCR analysis. The PCR primers are shown in Supplementary Table S5 at JXB online.
Fig. 4.
A pathway description of the differentially expressed starch metabolism-related genes and their ratios between superior and inferior spikelets at 9 DPA. The ratio means the fold difference in the respective gene expression of superior to inferior spikelets, which was calculated according to microarray data, as described in Supplementary Table S3 at JXB online.
ABA and ethylene synthesis genes were differentially expressed between inferior and superior spikelets
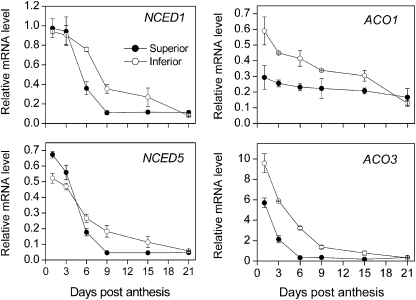
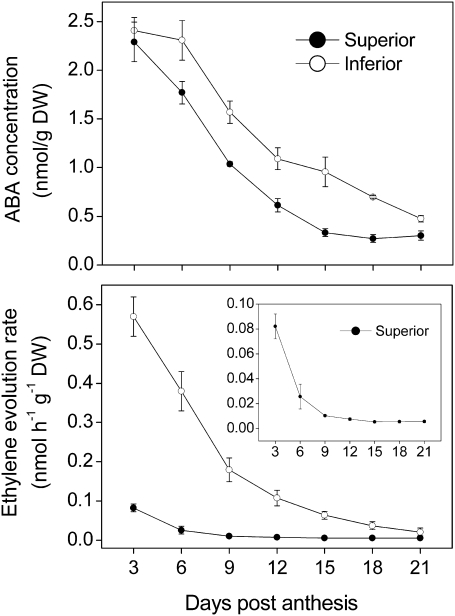
In addition to starch metabolism-related genes, it was also found that some plant hormone metabolism-related genes were differentially expressed between superior and inferior spikelets, especially for ABA and ethylene (Supplementary Table S4). Further qRT-PCR showed that expression of the ABA synthesis key genes, NCED1 and NCED5, and the ethylene synthesis key genes, ACO1 and ACO3, was decreased during grain filling (Fig. 5). However, if compared with inferior spikelets, expression of these genes in superior spikelets decreased faster and had lower transcript profiles, especially for ethylene synthesis genes (Fig. 5). The variation in ABA concentration and ethylene evolution rate (expressed as dry weight) during grain filling were also determined. From Fig. 6, both the ABA concentration and the ethylene evolution rate were decreased and superior spikelets changed faster than inferior spikelets, similar to the results of the transcription analysis described above. These results indicated that inferior spikelets have higher concentrations of ethylene and ABA at the early development stage which may be disadvantageous to finish grain filling. However, the mechanism whereby hormones affect grain filling remains unknown; thus, the relationships between hormones and starch synthesis were further investigated.
Fig. 5.
Changes in the expression profiles of ABA synthesis genes NCED1 and NCED5 and the ethylene synthesis genes ACO1 and ACO3 in superior and inferior spikelets during grain filling of rice. Transcript levels were quantified by qRT-PCR as described in the Materials and methods. Values are means with the SD (n=3).
Fig. 6.
Levels of ABA content and ethylene evolution rate in the superior and inferior spikelets during grain filling of rice. ABA content and the ethylene evolution rate were assayed as described in the Materials and methods. Values are means with the SD (n=3).
Starch metabolic-related genes were regulated by exogenous ABA and ethylene
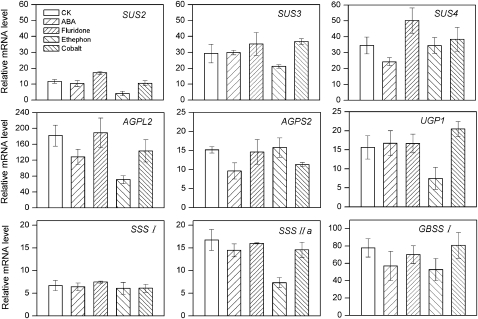
Plant hormones are reported to play important roles in regulating grain filling (Cheikh and Jones, 1994; Mohapatra et al., 2000). In this study, it was found that both starch metabolism-related genes and ABA and ethylene synthesis genes were differentially expressed in superior and inferior spikelets (Figs 3, 5). To investigate whether starch synthesis is regulated by hormones during grain filling, an exogenous supply experiment was performed using 9 DPA detached rice ears. From Fig. 7 it can be seen that application of 20 μM ABA significantly reduced the expression of genes responsible for metabolism of sucrose to ADP-glucose, especially SUS4 and AGPL2, which decreased 29.7% and 29.3%, respectively, while fluridone (an inhibitor of ABA synthesis) increased expression of SUS4 45.3% compared with Control (CK). Application of ethephon (an ethylene-releasing reagent) exhibited more severe inhibition than ABA; expression of most of the starch metabolism-related genes was strongly suppressed by exogenous ethephon, including SUS2, SUS3, AGPL2, UGP1, SSSIIa, and GBSSI. Among them, the relative abundances of AGPL2 and SSSIIa decreased 61.0% and 56.4%, respectively (Fig. 7). Inhibition of ethylene synthesis by cobaltous nitrate had little effect on the expression of starch metabolism genes, which may be because of the intrinsic lower ethylene content in the grains at 9 DPA (Fig. 7).
Fig. 7.
Effects of applied ABA, fluridone, ethephon, and cobaltous nitrate on the expression profiles of starch metabolism-related genes in the grains of detached rice ears. Rice spikelets were detached at 9 DPA and then cultured in solutions with 20 μM ABA, 20 μM fluridone, 5 mM ethephon, or 50 μM cobaltous nitrate. RNAs were extracted from the grains on the top four branches. Values are means with the SD (n=3).
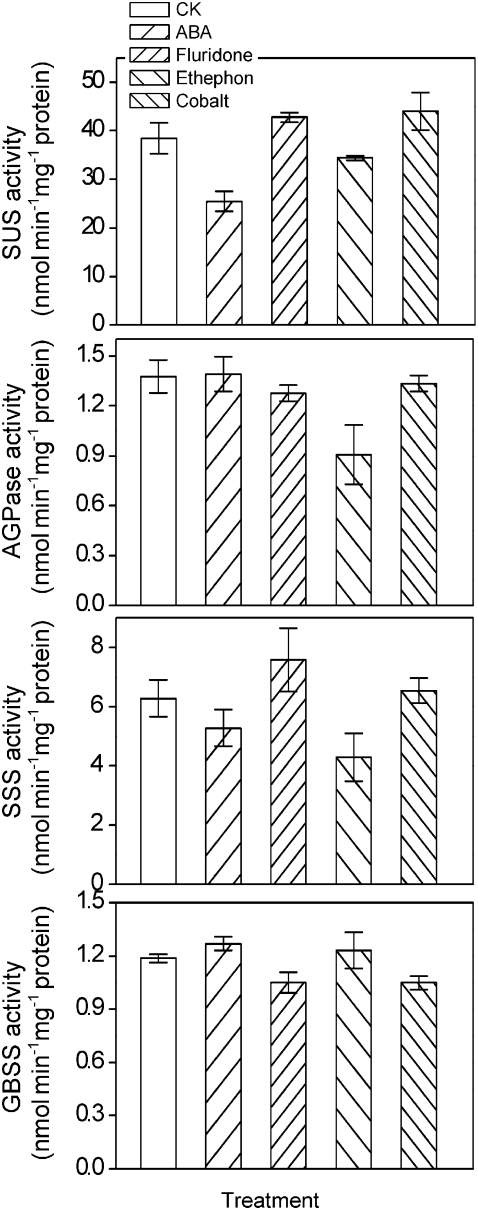
As an indication of the effect of the plant hormones on the activities of enzymes involved in starch synthesis, Fig. 8 shows that the activity of SUS in grains was 33.9% decreased after the supply of ABA in detached rice ears. Meanwhile, the activities of SUS, AGPase, and SSS were negatively regulated by ethylene, which decreased 10.4, 34.1, and 31.8% by exogenous application of ethephon, respectively (Fig. 8). Inhibition of ABA and ethylene synthesis in rice grains showed no significant difference compared with CK, except that fluridone slightly enhanced the activity of SSS (Fig. 8).
Fig. 8.
Effects of applied ABA, fluridone, ethephon, and cobaltous nitrate on the activities of SUS, AGPase, SSS, and GBSS in the grains of detached rice ears. Rice spikelets were detached at 9 DPA and then cultured in solutions with 20 μM ABA, 20 μM fluridone, 5 mM ethephon, or 50 μM cobaltous nitrate. The grains on the top four branches were sampled for the present study. Values are means with the SD (n=3).
Discussion
Poor grain filling of the later-flowering inferior spikelets of rice is a serious problem and often the major reason for yield fluctuation in super rice cultivars (Yang and Zhang, 2010). Conventional thinking of slow grain filling rate and low grain weight of inferior spikelets has been attributed to a limitation of carbohydrate supply (Murty and Murty, 1982; Zhu et al., 1988). However, further work has shown that there is no clear causative relationship between assimilate concentration and spikelet development in rice (Mohapatra et al., 2000; Yang et al., 2006). The present data also show that there is no obvious difference in soluble carbohydrate concentrations between superior and inferior spikelets during grain filling, although a significant difference in the grain-filling rate was observed (Fig. 1).
Poor synthesis of starch may be a crucial factor limiting the grain sink strength during gain filling. Inferior spikelets fill poorly and accumulate less assimilates owing to their poor starch-synthesizing capacity. Mohapatra et al. (2009) and Tang et al. (2009) found that a higher SUS activity in superior grains than in inferior grains at the earlier stage of grain filling in rice is closely related to a higher grain-filling rate and starch accumulation. Dai et al. (2009) and Jiang et al. (2003) reported that wheat superior spikelets have higher starch accumulation rates and activities of enzymes including SUS, AGPase, SSS, and GBSS, and consequently produce much higher grain weight than inferior spikelets. Meanwhile, the transcript expression of these genes in developing caryopses was also analysed. Ishimaru et al. (2005) found that the patterns of gene expression of SUS, AGPL, AGPS, and INV3 in superior spikelets are very different from those of inferior spikelets. In this study, the differentially expressed genes between inferior and superior spikelets were comprehensively analysed using DNA microarray (Supplementary Tables S1, S2 at JXB online). Starch metabolism-related genes were clustered into three groups according to their expression profiles and patterns during grain filling (Table 1). The first group of genes showed enhanced expression profiles and had much higher mRNA levels than the other two groups during grain filling (Supplementary Table S3); thus, it is reasonable to deduce that group 1 genes are the key genes responsible for starch synthesis and play a crucial role in determining the grain-filling rate and starch accumulation in rice grains. These genes were investigated further to clarify their roles in producing superior and inferior spikelets. The data show that this group of genes had very low expression profiles at the initiation stage of seed development (from 1 d to 3 d). However, their expression promptly increased and reached a maximum at the early or middle grain-filling stages in superior spikelets (6 d and 9 d). In contrast, most gene expression, especially for the genes responsible for conversion of sucrose to ADP-glucose (i.e. SUS, UGP, and AGP), was enhanced mildly and slowly and reached a maximum at the late stage in inferior spikelets (15 d and 21 d) (Fig. 3). Thus, photosynthesis assimilates could be efficiently used in superior spikelets, while most of them were wasted in inferior spikelets due to their poor starch-synthesizing capacity at the early/middle grain-filling stages. Although expession of starch synthesis genes was obviously enhanced, assimilate supply may be another limiting factor for inferior spikelets, as they declined to a very low level at the late grain-filling stage (Figs 1, 3).
Plant hormones play important roles in regulating grain filling and are involved in the process determining sink strength and seed weight during development of caryopses. Ethylene is reported to be negatively related to starch metabolism-related enzyme activities and always leads to poor grain filling (Mohapatra et al., 2000; Panda et al., 2009; Zhang et al., 2009). The present data indicate that the ethylene evolution rate, as well as expression of the key genes of ethylene synthesis ACO1 and ACO3, are rapidly decreased during grain filling (Figs 5, 6). Exogenous supply of ethephon, an ethylene-releasing reagent, to detached rice ears strongly suppressed the expression of most starch synthesis genes, including key genes in the conversion of sucrose to ADP-glucose (i.e. SUS2, SUS3, UGP1, and AGPL2) and in the conversion of ADP-glucose into starch granules (i.e. GBSSI and SSIIa). As a result, the activities of SUS, AGPase, and SSS were also down-regulated by ethephon (Figs 7, 8). These data suggested that ethylene negatively regulated development of caryopses probably through non-specifically repressing the expression of starch metabolism-related genes and their enzyme activities. In this sense, it is understandable that inferior spikelets have a lower grain-filling rate and starch content, since they accumulate a higher ethylene level which decreases more slowly than in superior spikelets during grain filling. Thus, it can be concluded that a relatively low ethylene level is indispensable for development of caryopses. A higher ethylene level in inferior spikelets is the crucial limitation for the usage of carbohydrate and ultimately leads to poor grain filling.
ABA has also been known to play an important role in grain filling. A high concentration of ABA in grains was reported to be negatively related to starch synthesis (Ahmadi and Baker, 1999; Bhatia and Singh, 2002). Liu et al. (2003) found that the drought-induced increase in endogenous ABA content in reproductive organs contributed to pod abortion in soybean, presumably via inhibition of cell division in young ovaries. Despite its negative regulatory role, the exogenous application of ABA has been reported to increase tolerance and crop yield. Yang and Zhang (2006) showed that exogenous application of ABA or moderate soil drying leads to faster and better mobilization of carbohydrates from vegetative tissues to the developing grains, and accelerates the grain-filling rate. ABA influences sugar metabolism and transport through regulation of the expression of some genes encoding components of the sugar response pathway (Gibson, 2004; Rook et al., 2006). Akihiro et al. (2005, 2006) found that the gene expression level of AGPase, especially of OsAGPL3, and starch content in the cultured cells are cooperatively controlled by alterations in the concentration of both sucrose and ABA. In the present study, the ABA concentration in both superior and inferior spikelets decreased during grain filling but inferior spikelets had a higher ABA level than superior spikelets at the same grain-filling stage (Fig. 6). Exogenous application of ABA to detached rice ears at 9 DPA decreased both the expression profile of SUS genes and SUS activity, while it had little effect on the genes/enzymes responsible for the conversion of ADP-glucose to starch (Figs 7, 8). It is speculated that SUS may be the primary target gene regulated by ABA. A higher ABA level at the early stage of development of caryopses, or in inferior spikelets, suppresses SUS expression and, consequently, is not favourable for starch synthesis. On the other hand, the grain-filling rate is increased with the decrease in ABA levels, and reached a maximum when the ABA level was maintained in a moderate range, such as 0.5–1.5 nmol g−1 DW in the conditions used here (Fig. 6). Thus, ABA affecting grain filling may function in a dose-dependent manner.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Up-/down-regulated genes in superior compared with inferior spikelets at 3 DPA.
Table S2. Up/down-regulated genes in superior compared with inferior spikelets at 9 DPA.
Table S3. Expression of genes related to starch metabolism in developing caryopses by microarray analysis.
Table S4. Expression of genes related to hormones metabolism in developing caryopses by microarray analysis.
Table S5. Primers used in the present study.
Acknowledgments
This work is supported by the Hong Kong Research Grants Council (HKBU262809), University Grants Committee of Hong Kong (AoE/B-07/99), Hong Kong Baptist University Strategic Development Fund (SDF 090910P03), the National Natural Science Foundation of China (No. 30900873), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20094404120008).
References
- Ahmadi A, Baker D. Effects of abscisic acid (ABA) on grain filling processes in wheat. Plant Growth Regulation. 1999;28:187–197. [Google Scholar]
- Akihiro T, Mizuno K, Fujimura T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant and Cell Physiology. 2005;46:937–946. doi: 10.1093/pcp/pci101. [DOI] [PubMed] [Google Scholar]
- Akihiro T, Umezawa T, Ueki C, Lobna B, Mizuno K, Ohta M, Fujimura T. Genome wide cDNA-AFLP analysis of genes rapidly induced by combined sucrose and ABA treatment in rice cultured cells. FEBS Letters. 2006;580:5947–5952. doi: 10.1016/j.febslet.2006.09.065. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Singh R. Phytohormone-mediated transformation of sugars to starch in relation to the activities of amylases, sucrose-metabolising enzymes in sorghum grain. Plant Growth Regulation. 2002;36:97–104. [Google Scholar]
- Buysse J, Merckx R. An improved colorimetric method to quantify sugar content of plant tissue. Journal of Experimental Botany. 1993;44:1627–1629. [Google Scholar]
- Cheikh N, Jones R. Disruption of maize kernel growth and development by heat stress (role of cytokinin/abscisic acid balance) Plant Physiology. 1994;106:45–51. doi: 10.1104/pp.106.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai ZM, Yin YP, Wang ZL. Comparison of starch accumulation and enzyme activity in grains of wheat cultivars differing in kernel type. Plant Growth Regulation. 2009;57:153–162. [Google Scholar]
- Duan M, Sun S. Profiling the expression of genes controlling rice grain quality. Plant Molecular Biology. 2005;59:165–178. doi: 10.1007/s11103-004-7507-3. [DOI] [PubMed] [Google Scholar]
- Emes M, Bowsher C, Hedley C, Burrell M, Scrase Field E, Tetlow I. Starch synthesis and carbon partitioning in developing endosperm. Journal of Experimental Botany. 2003;54:569–575. doi: 10.1093/jxb/erg089. [DOI] [PubMed] [Google Scholar]
- Gibson S. Sugar and phytohormone response pathways: navigating a signalling network. Journal of Experimental Botany. 2004;55:253–264. doi: 10.1093/jxb/erh048. [DOI] [PubMed] [Google Scholar]
- Hirose T, Terao T. A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.) Planta. 2004;220:9–16. doi: 10.1007/s00425-004-1314-6. [DOI] [PubMed] [Google Scholar]
- Ishimaru T, Hirose T, Matsuda T, Goto A, Takahashi K, Sasaki H, Terao T, Ishii R, Ohsugi R, Yamagishi T. Expression patterns of genes encoding carbohydrate-metabolizing enzymes and their relationship to grain filling in rice (Oryza sativa L.): comparison of caryopses located at different positions in a panicle. Plant and Cell Physiology. 2005;46:620–628. doi: 10.1093/pcp/pci066. [DOI] [PubMed] [Google Scholar]
- Ishimaru T, Ohsugi R, Matsuda T, Yamagishi T. Morphological development of rice caryopses located at the different positions in a panicle from early to middle stage of grain filling. Functional Plant Biology. 2003;30:1139–1149. doi: 10.1071/FP03122. [DOI] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y. Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiology. 2006;142:1202–1215. doi: 10.1104/pp.106.085258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Cao WX, Dai TB, Jing Q. Activities of key enzymes for starch synthesis in relation to growth of superior and inferior grains on winter wheat (Triticum aestivum L.) spike. Plant Growth Regulation. 2003;41:247–257. [Google Scholar]
- Kato T, Shinmura D, Taniguchi A. Activities of enzymes for sucrose–starch conversion in developing endosperm of rice and their association with grain filling in extra-heavy panicle types. Plant Production Science. 2007;10:442–450. [Google Scholar]
- Liu F, Andersen M, Jensen C. Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Functional Plant Biology. 2003;30:271–280. doi: 10.1071/FP02185. [DOI] [PubMed] [Google Scholar]
- Mohapatra P, Naik P, Patel R. Ethylene inhibitors improve dry matter partitioning and development of late flowering spikelets on rice panicles. Functional Plant Biology. 2000;27:311–323. [Google Scholar]
- Mohapatra P, Patel R, Sahu S. Time of flowering affects grain quality and spikelet partitioning within the rice panicle. Functional Plant Biology. 1993;20:231–241. [Google Scholar]
- Mohapatra P, Sarkar R, Kuanar S. Starch synthesizing enzymes and sink strength of grains of contrasting rice cultivars. Plant Science. 2009;176:256–263. [Google Scholar]
- Murty P, Murty K. Spikelet sterility in relation to nitrogen and carbohydrate contents in rice. Indian Journal of Plant Physiology. 1982;25:40–48. [Google Scholar]
- Nakamura Y, Yuki K, Park S, Ohya T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant and Cell Physiology. 1989;30:833–839. [Google Scholar]
- Ohdan T, Francisco P, Jr., Sawada T, Hirose T, Terao T, Satoh H, Nakamura Y. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. Journal of Experimental Botany. 2005;56:3229–3244. doi: 10.1093/jxb/eri292. [DOI] [PubMed] [Google Scholar]
- Panda B, Kariali E, Panigrahi R, Mohapatra P. High ethylene production slackens seed filling in compact panicled rice cultivar. Plant Growth Regulation. 2009;58:141–151. [Google Scholar]
- Rook F, Hadingham S, Li Y, Bevan M. Sugar and ABA response pathways and the control of gene expression. Plant, Cell and Environment. 2006;29:426–434. doi: 10.1111/j.1365-3040.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Matsuoka M. Identifying and exploiting grain yield genes in rice. Current Opinion in Plant Biology. 2008;11:209–214. doi: 10.1016/j.pbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Edo E, Uehara N, Ishimaru T, Kawamitsu Y, Suganuma S, Ueda D, Ohsugi R. Effect of sucrose on activity of starch synthesis enzymes in rice ears in culture. Physiologia Plantarum. 2005;124:301–310. [Google Scholar]
- Schaffer A, Petreikov M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiology. 1997;113:739–746. doi: 10.1104/pp.113.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Xu D, Black C. Identification of actively filling sucrose sinks. Plant Physiology. 1989;89:1117–1121. doi: 10.1104/pp.89.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Xie H, Wang YX, Lu B, Liang JS. The effect of sucrose and abscisic acid interaction on sucrose synthase and its relationship to grain filling of rice (Oryza sativa L.) Journal of Experimental Botany. 2009;60:2641–2652. doi: 10.1093/jxb/erp114. [DOI] [PubMed] [Google Scholar]
- Tetlow I, Morell M, Emes M. Recent developments in understanding the regulation of starch metabolism in higher plants. Journal of Experimental Botany. 2004;55:2131–2145. doi: 10.1093/jxb/erh248. [DOI] [PubMed] [Google Scholar]
- Wuriyanghan H, Zhang B, Cao W, Ma B, Lei G, Liu Y, Wei W, Wu H, Chen L, Chen H. The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. The Plant Cell. 2009;21:1473–1494. doi: 10.1105/tpc.108.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Hirose T, Kuroda M, Yamaguchi T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiology. 2007;144:258–277. doi: 10.1104/pp.107.098665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Zhang JH. Grain filling of cereals under soil drying. New Phytologist. 2006;169:223–236. doi: 10.1111/j.1469-8137.2005.01597.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J. Grain-filling problem in ‘super’ rice. Journal of Experimental Botany. 2010;61:1–5. doi: 10.1093/jxb/erp348. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Wang Z, Liu K, Wang P. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. Journal of Experimental Botany. 2006;57:149–160. doi: 10.1093/jxb/erj018. [DOI] [PubMed] [Google Scholar]
- Yemm E, Willis A. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Physiological aspects of grain yield. Annual Review of Plant Physiology. 1972;23:437–464. [Google Scholar]
- Zhang H, Tan G, Yang L, Yang J, Zhang J, Zhao B. Hormones in the grains and roots in relation to post-anthesis development of inferior and superior spikelets in japonica/indica hybrid rice. Plant Physiology and Biochemistry. 2009;47:195–204. doi: 10.1016/j.plaphy.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Zhu GH, Ye NH, Zhang JH. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant and Cell Physiology. 2009;50:644–651. doi: 10.1093/pcp/pcp022. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Cao X, Luo Y. Growth analysis on the process of grain filling in rice (in Chinese with English abstract) Acta Agronomica Sinica. 1988;14:182–192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.