Abstract
Sustainable food production depends critically on the development of crop genotypes that exhibit high yield under reduced nutrient inputs. Rooting traits have been widely advocated as being able to influence optimal plant performance, while breeding-based improvements in yield of spring barley suggest that this species is a good model crop. To date, however, molecular genetics knowledge has not delivered realistic plant ideotypes, while agronomic trials have been unable to identify superior traits. This study explores an intermediate experimental system in which root traits and their effect on plant performance can be quantified. As a test case, four modern semi-dwarf barley varieties, which possess either the ari-e.GP or the sdw1 dwarf allele, were compared with the long-stemmed old variety Kenia under two levels of nutrient supply. The two semi-dwarf types differed from Kenia, exhibiting smaller stem mass and total plant nitrogen (N), and improved partitioning of mass and N to grain. Amongst the semi-dwarfs, the two ari-e.GP genotypes performed better than the two sdw1 genotypes under standard and reduced nutrient supply, particularly in root mass, root investment efficiency, N acquisition, and remobilization of N and mass to grain. However, lack of between-genotype variation in yield and N use efficiency indicated limited potential for exploiting genetic variation in existing varieties to improve barley performance under reduced nutrient inputs. Experimental approaches to test the expression of desirable root and shoot traits are scrutinized, and the potential evaluated for developing a spring barley ideotype for low nutrient conditions.
Keywords: ari.e-GP, nitrogen use efficiency, root, remobilization, sdw1, semi-dwarf barley, trait, uptake
Introduction
Synthetic fertilizer use has enabled crop production to increase in parallel with population growth. However, sustained use of high mineral fertilizer inputs could become compromised by exhaustion of mineral sources and by the energetic costs associated with fertilizer production, particularly nitrogen (N). N fertilizer use for crop production has increased ∼7-fold globally in the last 50 years (Millennium Ecosystem Assessment, 2005). However, the economic and energetic costs of high N inputs to arable systems are not considered sustainable (Royal Society, 2009). Furthermore, mineral fertilizer use can be inefficient and thus a cause of pollution. Globally, around one-third of the N fertilizer applied to cereal crops ends up in harvested grain (Raun and Johnson, 1999). N fertilizer losses contribute to greenhouse gas production through release of nitrous oxides (Mosier et al., 1998) and to water pollution in nitrate-vulnerable areas (Defra, 2008). Thus, alternative fertilizer sources and crop genotypes that yield successfully with reduced nutrient inputs are vital to minimize reliance on inorganic fertilizers.
Root traits are seen as a major focus in the second ‘green revolution’ (Lynch, 2007; Den Herder et al., 2010) to develop crop varieties that perform well on soils with reduced fertility (Ceccarelli, 1996). Root traits have been proposed as selection criteria for breeding for improved nutrient acquisition, but have rarely been used for this purpose (Lynch, 2007). Root traits might even have been subject to neutral or negative selection by modern breeding and testing under high nutrient inputs, but the evidence to support this suggestion is limited (Ceccarelli, 1996; Sylvester-Bradley and Kindred, 2009). Nevertheless, a more directed search for traits, particularly in the roots, that underpin nutrient use efficiency is now imperative.
A combination of complementary methodologies is necessary to search for and test low input crop ideotypes. Plant breeding and molecular biology have both been applied in attempts to identify and understand the genes controlling, for example, N uptake and metabolism (Good et al., 2004) or the shift in biomass allocation to roots when nutrient supply is reduced (Hermans et al., 2006). Good et al. (2004) argued that these disciplines of traditional breeding and molecular biology have themselves been too separate and should work synergistically if crop genotypes with enhanced nutrient use efficiency are to be achieved. Moreover, the experimental work in molecular and genetic studies tends to be in highly controlled conditions, often using model plants such as Arabidopsis thaliana that are related to only a few crop species. To date such work has not led to the definition of realistic crop ideotypes that possess modified root traits or increased nutrient use efficiency. At the other end of the experimental spectrum are agronomic trials that assess nutrient use efficiency mostly on shoot structures in relation to added fertilizer (Sylvester-Bradley and Kindred, 2009; Beatty et al., 2010). Such trials, if conducted in a wide range of environments, provide the ultimate test for a new genotype, but commonly examine only the middle and upper reaches of the nutrient response curve, do not examine roots, and are unable to confirm, for instance, whether the allocation of biomass to roots increases at low nutrient supply with a concomitant effect on N use efficiency (NUE; i.e. grain dry matter yield per unit of available N; Moll et al., 1982; Good et al., 2004). Some intermediate approach is therefore needed that will define the salient root traits and provide the link between genetic and agronomic work in a realistic plant model.
Spring barley (Hordeum vulgare L.) provides a feasible crop model for developing an effective approach. Most research in this crop relevant to nutrient use has been directed at NUE. Yield improvements in barley have already been accompanied by increased NUE, attributed variously to improved N uptake and more efficient conversion of N into dry matter (Muurinen et al., 2007; Sylvester-Bradley and Kindred, 2009). NUE differs among varieties developed for different uses, such as malting and animal feed, and genotypic rankings show some consistency between field and controlled environments (Beatty et al., 2010). Modern varieties have been well characterized genetically and shown to differ in some phenotypic characteristics (Thomas et al., 1995; Ellis et al., 2002), yet shoot traits, such as stem or flag leaf N pools (Montemurro et al., 2006) and efficient N remobilization (Mickelson et al., 2003), have taken precedence in N efficiency studies. Thus, while variation in root traits is likely to exist, the plant traits underlying more efficient N capture and use in modern barley varieties remain unclear (Muurinen et al., 2007).
The most effective combination of approaches to study variation in root traits and their relationship to the use efficiencies of N and other nutrients has yet to be established. Field measurements using methods to assess whole root systems non-destructively by their ‘capacitance’ (Chloupek et al., 2006) indicate that some semi-dwarf modern barley varieties might have a larger root system than tall varieties, but the physiological traits that lead to such differences and the consequences for NUE are unclear. Moreover, root system size inferred by this method suffered from inconsistent genotype×environment interactions, in which, for example, varieties having different dwarfing alleles (ari-e.GP or sdw1) were not ranked the same in different years. Even experiments in controlled environment systems using hydroponics (e.g. Marshall and Ellis, 1998) have seldom examined the root systems of maturing and full-grown barley plants in response to manipulated N supply. Moreover, hydroponic experiments have resulted in apparent N uptake efficiencies of >100% (Beatty et al., 2010). Yet, in studies where root growth has been monitored, small differences in root mass have been detected (e.g. in response to salt stress), including between ari-e.GP, sdw1, and double-dwarf genotypes (Ellis et al., 2002).
In summary, therefore, an appropriate experimental system is still needed to test the promise of barley as a crop model. The first consideration is to select an appropriate nutrient supply regime. Studies of nutrient use efficiency commonly manipulate a single nutrient (e.g. N supply; Marshall and Ellis, 1998), rather than addressing plant responses to overall reductions in nutrients that would typify reduced input or low fertility systems (e.g. Ceccarelli, 1996; Lynch, 2007). While experimental manipulation of a single nutrient can improve understanding of physiological processes specific to that nutrient, interpretation of plant responses could be confounded by changes in the stoichiometric ratio of N to other nutrients, which itself can influence plant growth and productivity (see, for example, Fig. 1 in Elser et al., 2011). In contrast, a proportionate reduction in all nutrients might better reflect the conditions associated with reduced input or nutrient-poor soils. Taking the latter approach, plant responses to an overall reduction in nutrient availability are examined, focusing on NUE as a plant response variable of major importance to crop yield and quality.
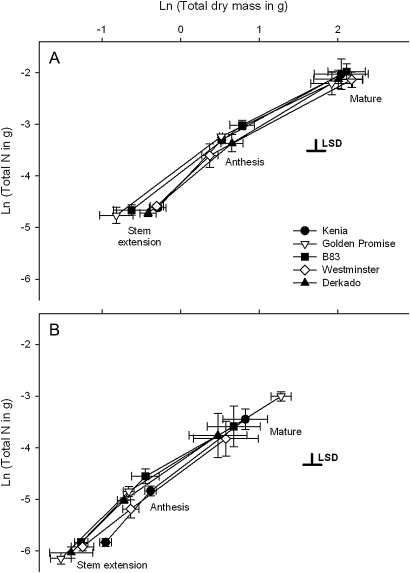
Fig. 1.
Relationship between plant dry mass and N content in (A) standard nutrient supply and (B) reduced (50% standard) nutrient supply at three development stages. Values are the mean (±SE) of ln-transformed data, and least significant difference (LSD) bars are shown for differences between genotypes at the 5% level.
A second consideration is to ensure that the rooting substrate is relevant to growing conditions in the field. The present study, which seeks to determine whether genotypic differences in root and other traits exist, uses an experimental system intermediate between the field and hydroponic chambers. In this experimental system, plants can reach a realistic size and N content, root systems grow to depth in a particulate medium, roots can be extracted and investigated, and the N applied, taken up, and partitioned among the plant parts can be accounted for and measured for individual plants up to maturity.
A comparison is formed by a tall variety of spring barley, introduced in the 1930s and incorporated into many breeding programmes (Russell et al., 2000), and genotypes that possess either the ari-e.GP or the sdw1 dwarfing allele that confer the short-stem traits associated with many modern varieties. The specific aims of the study are to assess genotypic variation in root and shoot traits, and their impact on the components of NUE under standard and low nutrient supply conditions, as a means to identify characteristics suitable for low input barley ideotypes. It is hypothesized that increased NUE in modern semi-dwarf varieties is related, at least in part, to root traits controlling N acquisition, which would influence plant performance under low nutrient supply. The study considers whether it is feasible to use controlled environment systems as a proxy for field trials in the search for root traits and increased NUE, as suggested by Beatty et al. (2010).
Materials and methods
Plant material
Seeds of five spring barley genotypes were obtained from seed stocks at the James Hutton Institute Dundee. Genotype choice was based on a preliminary study of shoot and root traits of 17 spring barley genotypes, which included varieties introduced between 1931 and 2005. Plants were grown to maturity under the standard nutrient conditions described below and the five genotypes selected for the present study represented the range of trait variation exhibited in the genotype screen (TA Valentine et al., unpublished). Genotype identity was confirmed by comparison with known standards using published methods to extract DNA from germinated seedlings and to characterize established genetic markers for spring barley (simple sequence repeats and single nucleotide polymorphism markers; Ramsay et al., 2000 and Close et al., 2009, respectively). The genotypes were: Kenia (introduced 1931; tall variety representative of types before either dwarfing gene was introduced) and two genotypes representative of each of the two dwarfing alleles: Golden Promise (introduced 1966; ari-e.GP); B83-12/4/5 (referred to in this study as B83, introduced 1991; ari-e.GP); Derkado (introduced 1987; sdw1); and Westminster (introduced 2005; sdw1). Seeds were soaked overnight in water, surface-sterilized for 15 min in 2% (w/v) calcium hypochlorite, and rinsed several times with water. Sterilized seeds were soaked for a further hour in water and then placed between layers of wetted filter paper in 230 mm square Petri dishes. The Petri dishes were enclosed in aluminium foil and incubated at 2 °C for 3 d to synchronize germination, followed by 15 °C for 2 d to promote radicle emergence.
Two-day-old seedlings were transferred to a lime-free substrate of grit–sand–gravel (mass ratio of 40:40:20) in open end tubes of length 100 cm and diameter 5 cm. The tubes were lined with a sheet of black polythene, and a layer of nylon gauze covered the base of the tube to prevent loss of the substrate. The tube contents were wetted thoroughly with water to allow the substrate to settle prior to transplanting pairs of germinated seedlings to each tube; one seedling from each pair was removed following successful seedling establishment. The five genotypes were subjected to two nutrient treatments and were harvested at stem elongation, anthesis, or maturity (growth stages 31, 61, and 92, respectively, on Zadoks growth scale: Tottman and Makepeace, 1979). The experiment was randomized in a split-plot design of five blocks to take account of any gradients within the glasshouse. Each block contained three plots corresponding to each of the three development stage harvests, and the two nutrient treatments were allocated randomly to plants within each plot. There were five replicate plants of each genotype at each harvest and under each nutrient treatment. The experiment was surrounded by a guard row of plants of a non-experimental spring barley cultivar.
Nutrient treatment
Nutrients were applied using an automated glasshouse irrigation system (Hortimax Growing Solutions Aqua 500, HortimaX B.V., The Netherlands) linked to a nutrient reservoir via a Dosatron DI 16 (Dosatron International S.A., France) and delivered to each plant through drippers inserted into the substrate with a delivery rate of 9 ml min−1. The nutrient solution contained a final concentration of 1 mmol l−1 K2SO4, 2 mmol l−1 KNO3, 2 mmol l−1 NH4NO3, 2.1 mmol l−1 CaCl2, 0.75 mmol l−1 MgSO4, 0.31 mmol l−1 KH2PO4, 0.03 mmol l−1 K2HPO4, plus trace elements (1 μmol l−1 MnSO4, 1 μmol l−1 ZnSO4, 0.25 μmol l−1 CuSO4, 12.5 μmol l−1 H3BO3, 0.25 μmol l−1 Na2MoO4, and 10 μmol l−1 FeNaEDTA), and had a pH between 5.6 and 5.8. The two nutrient treatments comprised a ‘standard’ treatment consisting of a daily delivery of 72 ml of nutrient solution, which was equivalent to 6 mg N d−1, and a ‘reduced’ nutrient treatment receiving half of the nutrient supplied to the standard treatment (i.e. 36 ml containing 3 mg N d−1). Plants under the reduced nutrient treatment received an additional volume (36 ml d−1) of water, which was applied prior to nutrient addition, to ensure that the amount of liquid applied to tubes in each nutrient treatment was equal. The amount of nutrients delivered to the plants was increased incrementally from zero at the start of the experiment to the rates given above over the first 5 weeks of the experiment. Nutrient treatments continued throughout the experiment until grain ripening when the nutrient and water supply was decreased incrementally to zero at final plant harvesting.
Plant growth and harvest
Plant development was monitored every 2–3 d and plants were harvested at stem elongation, anthesis, or maturity. Prior to harvest, the main stem length was measured and tiller number was recorded. Plant shoots were removed and divided into stems, leaves, and either ears (at anthesis) or grain and chaff (at maturity). Each shoot portion was weighed and oven-dried at 60 °C, except for the leaves which were snap-frozen in liquid nitrogen and stored at –80 °C before freeze-drying for dry mass determination and chemical analysis (see below). Roots were removed by sliding the polythene sheet lining from each tube and transferring the enclosed root system onto a flat surface. The root system was divided into 12 sections at 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, and 100 cm from the shoot base at the top of the substrate. Each root section was washed in sieves of 0.15 mm pore size to separate the root from the substrate and to minimize loss of fine root material. Fresh root material was blotted dry and weighed, then oven-dried at 60 °C. Dry mass was recorded for all plant fractions prior to chemical analysis.
Chemical analysis
Dried plant material was ball-milled to a fine powder. The N and C concentrations of 1 mg samples were determined by continuous flow Dumas combustion using a Europa Scientific (Crewe, UK) ANCA-SL sample converter and mass spectrometric detection (of N2 and CO2) using a Europa Scientific 20-20 mass spectrometer, as described by Scrimgeour and Robinson (2003). The percentage of C and N in the sample was calculated by comparison with known standards.
Statistical analysis
All data analyses were performed using Genstat (13th edition; VSN International Ltd, 2010). Parametric statistical tests were applied to plant trait data that were confirmed to be normally distributed with homogeneous variance. Most data required either natural-log transformation (g plant−1 of tissue dry mass and N) or arcsin-square root transformation (percentage or proportion data) to meet assumptions of normality. Plant traits were analysed with split-plot analyses of variance (ANOVAs) in which factors were growth stage, nutrient treatment, or plant genotype, together with four interaction terms for each combination of factors (i.e. growth stage×nutrient treatment, growth stage×genotype, nutrient treatment×genotype, and growth stage×nutrient treatment×genotype). Linear regression was applied to examine the extent to which (i) tissue N content was determined by tissue mass and (ii) N uptake efficiency was related to the root growth profile. In the following text, all differences discussed are statistically significant at ≤5%, unless otherwise stated.
Results
Dry matter and nitrogen content
Differences in plant total dry matter and N content occurred during development and between the standard and reduced (50% standard) nutrient treatments (Fig. 1; Supplementary Tables S1, S2 available at JXB online). For dry mass, the difference between the treatments increased as growth progressed (Fig. 1; Supplementary Table S1). By maturity, the plants in the reduced nutrient treatment accumulated typically 30% of the dry mass and 26% of the N accumulated by plants under standard nutrient supply.
There were genotypic differences in total N content but not in total dry mass (Supplementary Tables S1, S2). When averaged across growth stages and nutrient supply, B83 (ari-e.GP) and Kenia assimilated the largest amounts of N, between 12% and 35% more than the two genotypes accumulating the smallest amount of N (Derkado and Westminster; both sdw1). Overall, plant N content correlated positively with total plant dry mass [Ln(plant N)=1.08×Ln(plant dry mass)–4.26, R2=0.94; ANOVA F1,142=2295.09, P <0.001].
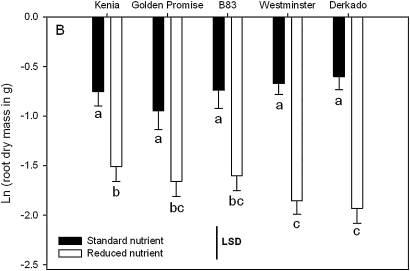
Segmented dry mass of root, stem, leaf, and ear tissue also varied with growth stage and nutrient supply (Supplementary Table S1 at JXB online), with plants in the reduced nutrient treatment accumulating between 28% and 36% of the dry mass accumulated under standard nutrient supply at maturity. For all tissues except the roots, the difference between nutrient treatments increased with growth stage, resulting in a significant interaction term for these two factors (Supplementary Table S1). The only structure showing consistent genotypic differences across treatments was stem mass, which was larger in Kenia than all other genotypes and smallest in Golden Promise, reflecting genotypic differences in plant height rather than number of tillers (data not shown). Genotypic differences in root mass varied with nutrient supply. Derkado and Westminster (both sdw1) exhibited larger root mass differences between nutrient treatments (∼70% smaller root mass under reduced nutrient supply) compared with Kenia (∼50% smaller root mass under reduced nutrient supply: Fig. 2), causing a significant interaction between nutrient supply and genotype (Supplementary Table S1).
Fig. 2.
Genotypic differences in response of root dry mass to standard and reduced (50% standard) nutrient supply. Values are the mean (±SE.) of ln-transformed data across all three development stages. Least significant difference (LSD) bars and letter annotations indicate where differences are significant at the 5% level.
Similarly, N contents of root, stem, leaf, and ear tissue increased as the plants matured and were larger in plants under standard nutrient supply (Supplementary Table S2 at JXB online); plants in the reduced nutrient treatment accumulated between 23% and 27% of the N accumulated in these tissues under standard nutrient supply at maturity. For root, stem, and leaf tissues, N contents did not differ between anthesis and maturity, but these values were larger than those at stem elongation, while ear N content increased between anthesis and maturity. In parallel with the trends in total plant N, the largest values of stem N content were associated with Kenia, while Westminster accumulated smaller leaf N contents than Derkado and B83 (data not shown). Genotypic differences in N transfer between tissues, reflecting N remobilization within the plant, were indicated by a significant interaction term for growth stage and genotype in leaf and stem N content (Supplementary Table S2). Greater N remobilization between anthesis and maturity was detected in Golden Promise (for leaf N) and B83 (for leaf and stem N), belonging to the ari-e.GP genotype, compared with the other genotypes (data not shown).
Root mass and N uptake
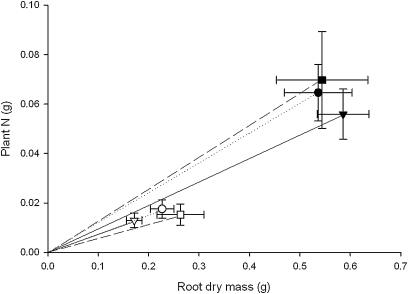
Following the observation that shoot N content correlated positively with root dry mass [(root dry mass)=4.9×(shoot N content)+0.214, R2=0.61; ANOVA F1,142=223.33, P <0.001], the relationship between nutrient supply, root mass, and N accumulation was examined. The mean percentage of dry mass partitioned to the roots decreased from 52.0% to 10.4% as plants matured and increased between the standard and reduced (50% standard) nutrient supply from 29.9% to 32.4% (Supplementary Table S3 at JXB online). The amount of N in the plant per unit of root mass (N return for root investment, or root investment efficiency) increased with plant development stage and with increased nutrient input, although individual genotypes did not differ in this trait (Supplementary Table S3). As the dwarfing allele influenced root mass responses to nutrient supply, the impact of the dwarf allele on N uptake was investigated. When plant responses were grouped by dwarf genotype, smaller amounts of N were acquired per unit root mass by the sdw1 genotypes compared with the ari-e.GP genotypes and Kenia (ANOVA dwarf genotype F2,114=3.68, P <0.05). A significant interaction between dwarf genotype and nutrient supply revealed that genotypic differences were only apparent under standard nutrient supply (ANOVA dwarf genotype×nutrient F2,114=4.38, P <0.05) and there was no significant difference between the groups under reduced nutrient supply (Fig. 3). Thus, more effective N accumulation in the standard nutrient supply was associated with larger amounts of N accumulated per unit of root mass in ari-e.GP and Kenia genotypes compared with sdw1 genotypes (Fig. 3). To investigate potential causal traits for this observation, the root profile was analysed in greater detail.
Fig. 3.
Relationship between mean root dry mass and plant N content for Kenia (squares, dashed line; n=15), ari-e.GP (circles, dotted line; n=28), and sdw1 (triangles, solid line; n=30) plants under standard (filled symbols) and reduced (50% standard; open symbols) nutrient supply. Values are means (±SE) and the slope of the line indicates the N ‘return for investment’ for each value.
Root profile and N uptake efficiency
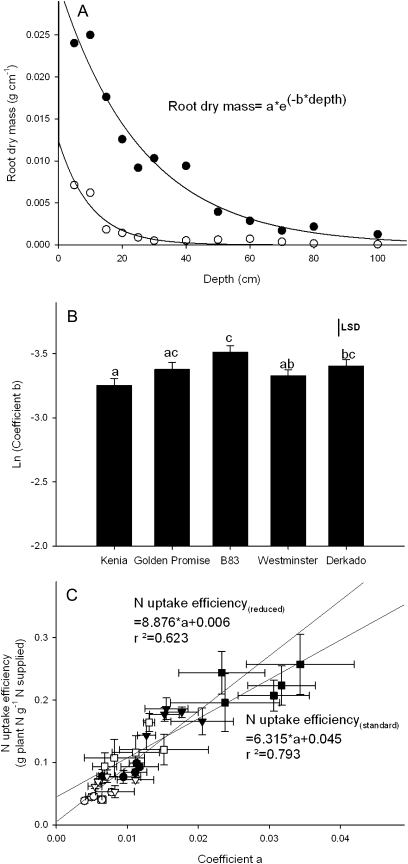
To examine in more detail the relationship between root mass and plant N uptake, the root profile was quantified by fitting an exponential curve to the plot of root mass distribution with depth for each plant (y=aebx: Fig. 4A). Overall root system size (coefficient a) increased between stem elongation and anthesis and was largest in the standard nutrient supply, the difference between the two nutrient treatments increasing as the plants matured (Supplementary Table S3 at JXB online). There were no differences between genotypes in this coefficient. The decrease in root mass with depth (coefficient b) varied with growth stage and nutrient supply and was steepest at stem elongation and maturity in plants receiving reduced (50% standard) nutrient supply, and in mature plants in the standard nutrient treatment (Supplementary Table S3). In addition, coefficient b showed genotypic variation, with the steepest declines in root mass with depth in Kenia and Westminster and the smallest declines in B83 (Fig. 4B). This reflected genotypic differences in the proportion of root mass accumulated in the top 30 cm of the root profile (data not shown).
Fig. 4.
(A) Example exponential fits of root dry mass allocation with depth for two mature Kenia plants under standard nutrient supply (filled circles; y=0.0271e–0.0338x, R2=0.949) and reduced nutrient supply (open circles; y=0.0043e–0.0406x, R2=0.835). (B) Genotypic variation in coefficient ‘b’ of the fitted exponential curve. Values are the mean (±SE) of ln-transformed data, and least significant difference (LSD) bars and letter annotations indicate where differences are significant at the 5% level. (C) Regression of mean nitrogen uptake efficiency on mean root size coefficient ‘a’ of the fitted exponential curve for genotypes grown under standard (filled symbols) and reduced (50% standard; open symbols) nutrient supply, assessed at stem extension (circles), anthesis (triangles), and maturity (squares).
Nitrogen uptake efficiency, or the fraction of supplied N taken up by the plant, was examined in relation to these rooting traits. N uptake efficiency was strongly related to coefficient a (indicative of total root mass) during growth and between nutrient treatments (Fig. 4C), and was similar to the relationship with total root mass (not shown). There was no relationship between coefficient b and N uptake efficiency either between or across growth stages and treatments. N uptake efficiency increased as plants developed, and values in the high nutrient treatment were approximately twice as large as those in the low nutrient treatment (Table 1). There were no genotypic differences in N uptake efficiency (Table 1).
Table 1.
Grain yield and nitrogen use efficiency parameters in mature plants of five barley genotypes
| Nutrient supply | Genotype | Grain dry mass (g)a | Grain N concentration (%)b | N uptake efficiency (g plant N/g N supplied)b | N utilization efficiency (g grain/g plant N) | N use efficiency (g grain/g N supplied) |
| Standard nutrient | Kenia | 3.86±0.81 | 2.77±0.23 | 0.257±0.048 | 24.60±2.62 | 6.60±1.34 |
| Golden Promise | 3.50±0.77 | 2.57±0.17 | 0.196±0.046c | 28.86±2.02 | 5.94±1.58c | |
| B83 | 4.10±0.93 | 2.74±0.44 | 0.244±0.034 | 28.04±5.44 | 6.96±1.62 | |
| Westminster | 4.28±0.70 | 2.04±0.15 | 0.207±0.025 | 34.35±2.35 | 7.20±1.09 | |
| Derkado | 3.53±0.95 | 2.51±0.42 | 0.223±0.031 | 27.04±5.26 | 5.96±1.49 | |
| Reduced nutrientd | Kenia | 0.90±0.28 | 2.41±0.14 | 0.120±0.024 | 25.70±3.48 | 3.08±0.89 |
| Golden Promise | 1.51±0.38 | 2.42±0.22 | 0.164±0.014c | 36.73±4.22c | 5.01±1.20 | |
| B83 | 1.13±0.30 | 2.29±0.24 | 0.116±0.027 | 32.81±2.48 | 3.77±0.92 | |
| Westminster | 1.04±0.34 | 2.16±0.25 | 0.094±0.022 | 34.84±5.73 | 3.54±1.02 | |
| Derkado | 0.81±0.37 | 2.28±0.31 | 0.107±0.030 | 26.98±6.17 | 2.81±1.17 | |
| GLM ANOVA | Nutrient | F1,36=44.66, P <0.001 | F1,36=1.72, P >0.1 | F1,34=28.12, P <0.001 | F1,35=1.30, P >0.1 | F1,35=16.72, P <0.001 |
| Genotype | F4,36=0.47, P >0.1 | F4,36=1.26, P >0.1 | F4,34=0.50, P >0.1 | F4,35=2.21, P <0.1 | F4,35=0.34, P >0.1 | |
| Interaction | F4,36=0.36, P >0.1 | F4,36=0.32, P >0.1 | F4,34=1.52, P >0.1 | F4,35 =0.34, P >0.1 | F4,35=0.53, P >0.1 |
Values are mean ±SE of n=5 plants.
Analysis performed on ln-transformed data.
Analysis performed on arcsin-square root-transformed percentage data.
For these values, n=4 plants.
Reduced nutrient supply was 50% of the standard nutrient supply.
Fractions of dry matter and N allocated to grain
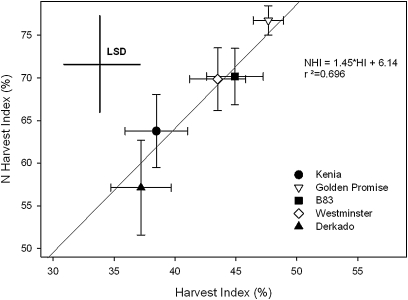
The Harvest Index (grain mass as a fraction of total plant mass) was unaffected by nutrient supply, but varied between 37% in Derkado and 47% in Golden Promise (Fig. 5: ANOVA nutrient F1,35=0.70, P >0.1; genotype F4,35=3.82, P <0.05; interaction F4,35=0.81, P >0.1). Plants that produced more biomass in the grain also allocated more N to the grain (Fig. 5; ANOVA nutrient F1,35=0.08, P >0.1; genotype F4,35=3.62, P <0.05; interaction F4,35=0.63, P >0.1), with the highest values of N Harvest Index in Golden Promise and lowest values in Kenia and Derkado. The Harvest Index and N Harvest Index were positively related (Fig. 5; F1,47=107.6, P <0.001), with an average of 1.45 times more N allocated to grain than dry matter. However, there was no relationship between final plant mass and Harvest Index or between final plant N content and N Harvest Index (analysis not shown). Grain mass was smaller in the reduced (50% standard) nutrient treatment, but grain N concentration was conserved between treatments (Table 1). Overall NUE was low in the reduced nutrient treatment, largely due to the decrease in N uptake efficiency rather than changes in N utilization efficiency (Table 1).
Fig. 5.
Linear regression between Harvest Index and N Harvest Index indicating genotypic differences. Values are the mean (±SE), and least significant difference (LSD) bars indicate where differences are significant at the 5% level.
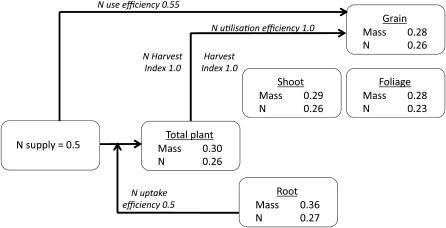
Traits that differed between genotypes (significant at ≤5%) are summarized in Table 2. The impact of nutrient supply on plant growth and N uptake is summarized (Fig. 6) to illustrate that differences between the two nutrient treatments were driven primarily by the disproportionate reduction in root growth and N content in the reduced nutrient treatment (one-third of the values in the standard treatment) relative to the decrease in nutrient availability (one-half of that in the standard nutrient treatment). As mass and N utilization efficiencies were the same regardless of nutrient supply, shoot mass and N contents in the reduced nutrient treatment reflected those in the roots.
Table 2.
Summary of plant traits and variables for five spring barley genotypes indicating where significantly larger (↑) or smaller (↓) values were obtained relative to the other genotypes
| Kenia (tall) | Golden Promise (ari-e.GP) | B83 (ari-e.GP) | Westminster (sdw1) | Derkado (sdw1) | |
| Dry mass (g plant−1) | |||||
| Total | |||||
| Root | ↑Ra | ↓R | ↓R | ||
| Leaf | |||||
| Stem | ↑ | ↓ | ↑ | ||
| Ear | |||||
| Mass remobilization | |||||
| Harvest Index | ↓ | ↑ | ↑ | ↓ | |
| Nitrogen (g plant−1) | |||||
| Total | ↑ | ↑ | ↓ | ↓ | |
| Root | |||||
| Leaf | ↑ | ↓ | ↑ | ||
| Stem | ↑ | ||||
| Ear | |||||
| N remobilization | ↑ | ↑ | |||
| N Harvest Index | ↓ | ↑ | ↓ | ||
| Root investment | |||||
| Coefficient a | |||||
| Coefficient b | ↑ | ↓ | ↑ | ||
| Root mass (% total) | |||||
| Root N (g g−1 dry mass) | ↑ | ↓ | |||
| Root N (% total N) | |||||
| Plant N/root mass (g g−1) | ↑S | ↑S | ↑S | ↓S | ↓S |
| NUE | |||||
| N uptake efficiency | |||||
| N utilization efficiency | |||||
| Overall NUE |
R, in reduced nutrient treatment only; S, in standard nutrient treatment only. Nutrient supply in the reduced nutrient treatment was 50% of that in the standard nutrient treatment.
Fig. 6.
Schematic diagram indicating relative changes in plant mass and N content and in N use efficiencies in the reduced nutrient treatment (50% of standard nutrient supply) as a proportion of the values obtained in the standard nutrient supply.
Discussion
The experimental system in this study, intermediate between hydroponics and field soil, was used successfully to monitor and quantify root systems, and to determine independent values for total plant N uptake efficiency and allocation efficiency within the same experiment. Although absolute root mass was smaller under reduced nutrient supply, greater partitioning of mass to roots was detected, as were several genotypic differences in root and shoot traits and variables. The older tall variety Kenia differed from the semi-dwarf varieties, exhibiting on average larger stem mass and total plant N, and reduced partitioning of mass and N to grain. Between the semi-dwarf types, the ari-e.GP genotypes (compared with sdw1) showed larger root mass at reduced nutrient supply, greater root investment efficiency (N uptake per unit root mass) at standard nutrient supply, and larger N uptake, N remobilization from stem and leaf to grain, and partitioning of mass and N to grain. On the basis of root traits and N uptake, which reflect a number of the traits of interest for optimizing NUE in wheat (Foulkes et al., 2009), the ari-e.GP types might be considered superior under both low and high nutrient supply. However, the genotypic differences detected here were relatively small and none was sufficient to cause differences in overall NUE or its main components, either because increases in individual plant variation as plants matured (see Fig. 4C) outweighed between-genotype trait differences or because desirable traits were not expressed additively in any one genotype. For example, larger root systems under reduced nutrient supply in Kenia and the ari-e.GP genotypes increased neither N uptake per unit root mass nor overall N uptake efficiency compared with sdw1 genotypes.
In contrast, NUE and its components differed greatly between nutrient treatments and over time as plants matured. NUE was smaller under reduced than standard nutrient supply. This observation prompts the question of the expected response by NUE to reduced nutrient supply. NUE is measured as the slope of the relation between grain yield and N supply; a sigmoid relation between these two variables might be expected (see, for example, Fig. 1 in Lynch, 2007), in which grain production is limited at low N supply by small plant biomass and a greater allocation of that biomass to roots or vegetative shoots, followed by a linear phase when yield increases in direct proportion to N supply, and finally a saturation phase as grain yield becomes limited by other factors (e.g. light capture or other abiotic conditions). A sigmoid relationship would invariably lead to variation in NUE: the largest values would be obtained at the top of the linear phase and the smallest values in the regions of low and high N supply. The typical response in agronomic field trials covers only a part of this range—NUE is rarely measured at low N supply in such trials due to the relatively high levels of residual soil N (e.g. Sylvester-Bradley and Kindred, 2009; see also Wacker et al., 2002; Beatty et al., 2010). Therefore, no single or universal response by NUE to a reduction in nutrient supply should be expected: the direction of change would depend on the portion of the response curve examined.
Furthermore, the position of an experimental treatment on the yield–N response surface is likely to vary depending on additional factors that can co-limit grain yield. One such factor could be the stoichiometric ratio between N and other nutrients, which can influence plant performance (Elser et al., 2011) and can alter NUE, as demonstrated in studies that manipulate the supply of more than one nutrient (e.g. N and sulphur in wheat: Fig. 2A in Salvagiotti et al., 2009; for a theoretical discussion, see Sinclair and Park, 1993). In the present study, the move from the standard to the reduced nutrient treatment would be expected (using, as a guide, the example in Salvagiotti et al., 2009) to shift the yield–N response to a lower curve. The effect of this shift on NUE would depend on the particular arrangement of such response curves, but the most likely outcome is no effect or a reduction in NUE.
The result in the present study was a reduction in NUE at low nutrient supply, but, unlike in most other studies showing this response, the experimental system used here enabled the cause to be identified. The root systems of plants in the reduced nutrient treatment were less effective at acquiring N per unit of root mass. Consequently, the increased relative partitioning of plant resources (mass and N) to the roots at low nutrient supply became associated with disproportionate differences between the two treatments in total plant mass, total N content, and grain mass, relative to the change in N supply (Fig. 6). Whether the underlying deficiency was a reduced physiological capacity to take up N in the low nutrient treatment (possibly caused by co-limiting factors) or a reduced accessibility of N to plant roots in the medium of the low nutrient treatment still needs to be determined. Overall, however, the poor return from investment in root mass in the low nutrient treatment was the main factor causing the treatment difference in NUE, since allocation of mass and N to the grain was conserved (Fig. 6), and so N utilization efficiency and grain N concentration were unaffected by nutrient supply.
Genotypic differences in NUE are small
The overall conclusion of this and cited work is that differences in nutrient uptake and NUE are small among current commercial varieties. Agronomic trials provide strong, if indirect, corroborative evidence of the small changes caused by selection and breeding over several recent decades. An analysis of groups of barley varieties introduced ∼30 years apart (Sylvester-Bradley and Kindred, 2009) showed increases in yield, N uptake, and NUE between 1.05 and 1.2 (i.e. mean of new varieties divided by mean of old) and in a comparable analysis extending over 75 years the increase in NUE from oldest to newest barley varieties was ∼1.7 (Bingham et al., 2010). In any single experimental study on individual plants, differences of the order of 1.2–1.7 can be obscured by noise, as occurred in the present study (Table 1). In the field, genotype×environment interactions can be so large that genotypic trends can change direction between years and experimental configurations (e.g. Beatty et al., 2010). Plants are responsive to their nutrient environment, as shown by large changes in mass and N content during development and between nutrient treatments, but conservatism or non-plasticity of certain traits, specifically in the partitioning of N between structures in the present study, seems to restrict genetic differences in NUE.
The implication is that there is little scope for major and rapid improvement in NUE using existing genotypes, particularly in low nutrient input systems. The fact that genotypic trait variation is not pronounced under low nutrient supply might reflect long-term selection for maximal expression of N efficiency traits under high nutrient conditions (Muurinen et al., 2007). Wild barley and landraces are possible alternative sources of genetic variation (Ellis et al., 2000; Russell et al., 2000), although tissue mass allocation and N concentration appear to be highly conserved in wild ancestors and modern relatives (Wacker et al., 2002).
Successful future ideotypes are likely to require a combination of traits. Improvements in NUE and yield resulting from manipulation of individual genes or enzymes for N uptake and assimilation are unlikely (Good et al., 2004; Liu et al., 2009; Masclaux-Daubresse et al., 2010), although more success has been achieved with overexpression of genes involved in N storage and remobilization (Good et al., 2007; Masclaux-Daubresse et al., 2010). Molecular markers for root and shoot traits that improve N efficiency in low input systems will only assist in genetic screens if the selected traits are shown to be effective in field conditions with realistic low input nutrient regimes. Progress may depend on elucidating the reason why plants display very little plasticity in some characteristics (particularly in the allocation of nutrients among plant parts) so that the desired plasticity can be introduced into appropriate germplasm. The ideal plant may be one that operates effectively overall at lower tissue N concentration: in the scheme developed by Greenwood (1982) and adopted by Marshall and Ellis (1998), the ‘minimum nitrogen concentration at which growth is not limited by nitrogen supply’ needs to decrease. One possibility might be to select new varieties based on reduced proteome N content, which tends to be high in domesticated cereal crops relative to non-domesticated plants (Elser et al., 2011). Additionally, any biomass allocated to roots at low nutrient supply must remain effective at taking up nutrients, contrary to the observations here. At present, the genetic control of stability (i.e. non-plasticity) in plant traits is unclear, as is the most effective experimental system for testing their impact on NUE.
A consistent context for laboratory and field testing
If root and whole plant traits can be measured in a system similar to the one used here, the main challenge is to corroborate in field conditions any observed genotypic differences in performance. In the immediate future, there seems no substitute for time-consuming and intensive measurements of root traits in field soil. Root total mass and allocation down the soil profile still need to be measured in a suite of experimental systems to determine whether differences in root traits contribute to improved N uptake, N use, and yield. A productive way forward might be to examine root mass allocation down the soil profile in field conditions of standard and low nutrient inputs. Increased root density at depth has been proposed as a trait of focus for improved N acquisition by wheat (Foulkes et al., 2009), and this study identified differences between Kenia, Westminster, and B83 in the shape of the root mass–depth profile. Expression of this trait in a heterogeneous substrate might influence nutrient acquisition and NUE in barley genotypes differing in root–depth profiles. Ultimately, there may be substitutes for full destructive sampling of roots; Beatty et al. (2010) showed some consistency in the ranking of NUE in barley genotypes across field, glasshouse, and hydroponic systems, using genotypes that differed substantially in phenology and grain quality. In the present study, the lack of a significant interaction between growth stage and genotype for root traits suggests that characteristics at early growth stages might be indicative of the root throughout development, which could reduce the intensity of destructive sampling required to characterize the roots. Alternatively, total above-ground tissue N content, which was a broad indicator of root mass at all growth stages in this study, might be a simple measure of root growth and plant performance in the field.
Concluding remarks
To realize the aim of producing N-efficient crop genotypes for low input systems, a more consistent harmonized approach between molecular and agronomic research is needed, in terms of the traits of interest, the method of nutrient provision to roots, and the ancillary factors that affect nutrient use efficiencies. Notably, the studies cited alongside the present work were each conducted under a particular set of conditions defined by nutrient input, plant traits, and other contextual factors such as solar radiation. Thus, a difference in NUE between or within studies could be due to a factor constraining, for example, total mass rather than a difference in traits responsible for N uptake or metabolism. It is concluded, therefore, that a unified approach is needed in which all components of NUE are isolated, and the underlying traits quantified, based on a defined supply of nutrients that can be translated from controlled to field conditions.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Mean values and ANOVA results for plant dry mass.
Table S2. Mean values and ANOVA results for plant N content.
Table S3. Mean values and ANOVA results for root investment parameters.
Acknowledgments
We are indebted to Kirsty Binnie, Fiona Falconer, Lee Hunt, Hsueh Ling Kuan, Charlie Scrimgeour, Mark Young, and Gladys Wright at JHI Dundee for valuable technical assistance. Thanks also to Allan Booth, Malcolm Macaulay, and Joanne Russell at JHI Dundee for advice and methodology to confirm genotype identities, and to Barry Mulholland and Philip White at JHI Dundee and two anonymous referees for helpful advice on the manuscript. Statistical advice was provided by Jim McNicol at BioSS, JHI Dundee. This work was funded by the 2006–2011 RERAD work package 1.7 Sustainable Crop Systems
References
- Beatty PH, Anbessa Y, Juskiw P, Carroll RT, Wang J, Good AG. Nitrogen use efficiencies of spring barley grown under varying nitrogen conditions in the field and growth chamber. Annals of Botany. 2010;105:1171–1182. doi: 10.1093/aob/mcq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham IJ, Karley AJ, White PJ, Thomas WTB. Montpellier: France; 2010. Analysis of improvements in nitrogen use efficiency associated with 75 years of barley breeding. Proceedings of Agro2010 the XIth European Society of Agronomy Conference; pp. 51–52. [Google Scholar]
- Ceccarelli S. Adaptation to low/high input cultivation. Euphytica. 1996;92:203–214. [Google Scholar]
- Chloupek O, Forster BP, Thomas WTB. The effect of semi-dwarf genes on root system size in field-grown barley. Theoretical and Applied Genetics. 2006;112:779–786. doi: 10.1007/s00122-005-0147-4. [DOI] [PubMed] [Google Scholar]
- Close TJ, Bhat PR, Lonardi S, et al. Development and implementation of high-throughput SNP genotyping in barley. BMC Genomics. 2009;10:1–13. doi: 10.1186/1471-2164-10-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defra . Description of the methodology applied in identifying waters and designating Nitrates Vulnerable Zones in England. London: Water Quality Division, Defra; 2008. Implementation of the Nitrates Directive (91/676/EEC) [Google Scholar]
- Den Herder G, Van Isterdael G, Beeckman T, De Smet I. The roots of a new green revolution. Trends in Plant Science. 2010;15:600–607. doi: 10.1016/j.tplants.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Ellis RP, Forster BP, Gordon DC, et al. Phenotype/genotype associations of yield and salt tolerance in a barley mapping population segregating for two dwarfing genes. Journal of Experimental Botany. 2002;53:1163–1176. doi: 10.1093/jexbot/53.371.1163. [DOI] [PubMed] [Google Scholar]
- Ellis RP, Forster BP, Robinson D, Handley LL, Gordon DC, Russell JR, Powell W. Wild barley: a source of genes for crop improvement in the 21st century? Journal of Experimental Botany. 2000;51:9–17. [PubMed] [Google Scholar]
- Elser JJ, Acquisti C, Kumar S. Stoichiogenomics: the evolutionary ecology of macromolecular elemental composition. Trends in Plant Science. 2011;26:38–44. doi: 10.1016/j.tree.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes MJ, Hawkesford MJ, Barraclough PB, Holdsworth MJ, Kerr S, Kightley S, Shewry PR. Identifying traits to improve the nitrogen economy of wheat: recent advances and future prospects. Field Crops Research. 2009;114:329–342. [Google Scholar]
- Good AG, Johnson SJ, De Pauw M, Carroll RT, Savidov N, Vidmar J, Lu Z, Taylor G, Stroeher V. Engineering nitrogen use efficiency with alanine aminotransferase. Canadian Journal of Botany. 2007;85:252–262. [Google Scholar]
- Good AG, Shrawat AK, Muench DG. Can less yield more? Is reducing nutrient input compatible with maintaining crop production? Trends in Plant Science. 2004;9:597–605. doi: 10.1016/j.tplants.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Greenwood DJ. Modelling of crop response to nitrogen fertiliser. Philosophical Transactions of the Royal Society B: Biological Sciences. 1982;296:351–362. [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science. 2006;11:610–617. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen F, Olokhnuud C, Glass ADM, Tong Y, Zhang F, Mi G. Root size and nitrogen-uptake activity in two maize (Zea mays) inbred lines differing in nitrogen-use efficiency. Journal of Plant Nutrition and Soil Science. 2009;172:230–236. [Google Scholar]
- Lynch JP. Roots of the second green revolution. Australian Journal of Botany. 2007;55:493–512. [Google Scholar]
- Marshall B, Ellis RP. Growth, yield and grain quality of barley (Hordeum vulgare L.) in response to nitrogen uptake. I. A low cost, controlled nutrient supply system. Journal of Experimental Botany. 1998;49:1049–57. [Google Scholar]
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of Botany. 2010;105:1141–1157. doi: 10.1093/aob/mcq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson S, See D, Meyer FD, Garner JP, Foster CR, Blake TK, Fischer AM. Mapping of QTL associated with nitrogen storage and remobilization in barley (Hordeum vulgare L.) leaves. Journal of Experimental Botany. 2003;54:801–812. doi: 10.1093/jxb/erg084. [DOI] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute; 2005. [Google Scholar]
- Moll RH, Kamprath EJ, Jackson WA. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agronomy Journal. 1982;74:562–564. [Google Scholar]
- Montemurro F, Maiorana M, Ferri D, Convertini G. Nitrogen indicators, uptake and utilization efficiency in a maize and barley rotation cropped at different levels and sources of N fertilization. Field Crops Research. 2006;99:114–124. [Google Scholar]
- Mosier A, Kroeze C, Nevison C, Oenema O, Seitzinger S, van Cleemput O. Closing the global atmospheric N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. Nutrient Cycling in Agroecosystems. 1998;52:225–248. [Google Scholar]
- Muurinen S, Kleemola J, Peltonen-Sainio P. Accumulation and translocation of nitrogen in spring cereal cultivars differing in nitrogen use efficiency. Agronomy Journal. 2007;99:441–449. [Google Scholar]
- Ramsay L, Macaulay M, degli Ivanissivich S, et al. A simple sequence repeat-based linkage map of barley. Genetics. 2000;156:1997–2005. doi: 10.1093/genetics/156.4.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raun WR, Johnson GV. Improving nitrogen use efficiency for cereal production. Agronomy Journal. 1999;91:357–363. [Google Scholar]
- Royal Society. Reaping the benefits: science and the sustainable intensification of global agriculture. RS Policy document 11/09. London: The Royal Society; 2009. [Google Scholar]
- Russell JR, Ellis RP, Thomas WTB, Waugh R, Provan J, Booth A, Fuller J, Lawrence P, Young G, Powell W. A retrospective analysis of spring barley germplasm development from ‘foundation genotypes’ to currently successful cultivars. Molecular Breeding. 2000;6:553–568. [Google Scholar]
- Salvagiotti F, Castellarín JM, Miralles DJ, Pedrol HM. Sulphur fertilisation improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crops Research. 2009;113:170–177. [Google Scholar]
- Scrimgeour CM, Robinson D. Stable isotope analysis and applications. In: Smith KA, Cresser MS, editors. Soil and environmental analysis: modern instrumental techniques. New York: Marcel Dekker; 2003. pp. 381–431. [Google Scholar]
- Sinclair TR, Park WI. Inadequacy of the Liebig limiting-factor paradigm for explaining varying crop yields. Agronomy Journal. 1993;85:742–746. [Google Scholar]
- Sylvester-Bradley R, Kindred DR. Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. Journal of Experimental Botany. 2009;60:1939–1951. doi: 10.1093/jxb/erp116. [DOI] [PubMed] [Google Scholar]
- Thomas WTB, Powell W, Waugh R, et al. Detection of quantitative trait loci for agronomic, yield, grain and disease characters in spring barley (Hordeum vulgare L.) Theoretical and Applied Genetics. 1995;91:1037–1047. doi: 10.1007/BF00223917. [DOI] [PubMed] [Google Scholar]
- Tottman DR, Makepeace RJ. An explanation of the decimal code for the growth stages of cereals, with illustrations. Annals of Applied Biology. 1979;93:221–234. [Google Scholar]
- Wacker L, Jacomet S, Körner Ch. Trends in biomass fractionation in wheat and barley wild ancestors to modern cultivars. Plant Biology. 2002;4:258–265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.