Abstract
Immediately after unfolding, cotyledons of the tropical platyopuntoid cactus, Opuntia elatior Mill., exhibited a C3-type diel CO2 exchange pattern characterized by net CO2 uptake in the light. Significant nocturnal increases in titratable acidity typical of crassulacean acid metabolism (CAM) were not detected at this early developmental stage. As cotyledons matured and the first cladode (flattened stem) developed, features of CAM were observed and the magnitude of CAM increased. Nonetheless, in well-watered seedlings up to 10 cm tall, C3 photosynthetic CO2 fixation in the light remained the major pathway of carbon fixation. Reduced soil water availability led to an up-regulation of net dark CO2 fixation and greater nocturnal increases in tissue acidity, consistent with facultative CAM. These observations demonstrate that C3 photosynthesis, drought-stress-related facultative CAM, and developmentally controlled constitutive CAM can all contribute to the early growth of O. elatior. The strong C3 component and facultative CAM features expressed in young O. elatior contrast with mature plants in which obligate CAM is the major pathway of carbon acquisition.
Keywords: Carbon dioxide uptake, constitutive CAM, crassulacean acid metabolism, C3 photosynthesis, development, drought stress, environment, facultative CAM, Opuntia
Introduction
Platyopuntias such as Opuntia basilaris, Opuntia ficus-indica, and Opuntia stricta are the most intensely studied cacti (Nobel, 1988; Osmond et al., 2008). They are classic examples of plants that exhibit crassulacean acid metabolism (CAM), a water-conserving mode of photosynthetic CO2 assimilation which is characterized by the uptake of CO2 at night when the driving forces for transpirational water loss are low (Neales et al., 1968; Winter et al., 2005). In these archetypal CAM plants, the CAM cycle is considered to be constitutively expressed, i.e. CAM is the principal pathway of carbon acquisition in mature cladodes, irrespective of seasonal and day-to-day variation of environmental conditions in the native habitat. Indeed, in situ studies of platyopuntias growing in arid semi-desert environments demonstrate net CO2 uptake predominantly at night and little or no CO2 uptake in the light. Under more mesic conditions, significant CO2 uptake may occur during the early morning or in the late afternoon, but the bulk of CO2 is still absorbed in the dark (Gerwick and Williams, 1978; Osmond et al., 1979, 2008). In contrast, in many CAM species from other families C3 photosynthetic CO2 uptake may equal or exceed CO2 uptake in the dark (Holtum and Winter, 1999; Winter and Holtum, 2002). In some species, CO2 may be overwhelmingly fixed by the C3 pathway and CAM is only detectable as a small nocturnal increase in tissue acidity (Silvera et al., 2005). Another photosynthetic phenotype is exhibited by highly flexible species which may operate in either the C3 mode when unstressed, or in the CAM mode when challenged by drought or salinity stress (facultative CAM) (Winter and Holtum, 2005, 2007; Lüttge, 2006; Winter et al., 2008).
Before displaying their full potential for CAM, young plants and young leaf or photosynthetic stem tissue of species with constitutive CAM may exhibit a strong C3 component of net CO2 uptake which decreases as plants and tissues mature. In O. ficus-indica and Hylocereus monocanthus, new photosynthetic stem segments which develop on mature stems may rapidly shift from daily net CO2 loss to CO2 gain via CAM during the sink-to-source transition with little interim use of C3 photosynthesis (Wang et al., 1998; Winter and Holtum, 2002), but these observations do not necessarily apply to autonomous cactus seedlings which fully rely upon CO2 uptake from the atmosphere. Measurements of titratable acidity indicate the presence of CAM in 1-d-old cotyledons of six species of columnar cacti (Hernández-González and Briones Villarreal, 2007), but the relative contributions of day and night CO2 fixation to total carbon gain have not yet been assessed in young developing cactus seedlings (Acevedo et al., 1983; Pimienta-Barrios et al., 2005).
Here CAM and C3 photosynthesis during early growth of the platyopuntia O. elatior were quantified by continuously monitoring net CO2 exchange of seedlings for up to 83 day–night cycles after germination. In addition, long-term CO2 exchange and titratable acidity were used to determine the extent to which seedlings of O. elatior up-regulate CAM in response to drought stress, i.e. are capable of expressing CAM facultatively. If such up-regulation occurs, the current perception of strictly constitutive CAM in platyopuntias would require reassessment. Recent studies with young cladodes of O. ficus-indica developing on mother cladodes were indeed suggestive of a facultative component of CAM expression (Winter et al., 2008), but interpretation of the gas-exchange data was complicated by the contribution to carbon gain by mother cladodes and by possible stress-related reduction in mitochondrial respiration in the young tissues. Furthermore, supporting information on nocturnal increases in acidity in stressed and non-stressed tissues was lacking.
Here it is demonstrated unambiguously that seedlings of O. elatior can exhibit C3 photosynthesis and facultative CAM.
Materials and methods
Plant material and net CO2 exchange
O. elatior Mill. is native to southern Central America and northern South America. Seeds collected from wild plants growing in Sarigua National Park, Republic of Panama, were germinated in moist Jiffy pellets (Hummert International, Earth City, MO, USA; Fig. 1A). The seedlings (plus pellets) were transferred to terracotta pots (1.6 l or 3.7 l) or plastic pots (0.5 l) containing potting mix (Cactus, Palm and Citrus Soil; Miracle-Gro Lawn Products, Marysville, OH, USA) and Osmocote Plus fertilizer (Scotts-Sierra Horticultural Products, Marysville, OH, USA). Cotyledons attached to the plants were enclosed in a Perspex cuvette (internal dimensions of either 11×11×10 cm or 20×20×15 cm) by passing the hypocotyl carrying the two cotyledons through a 1-cm diameter hole in the cuvette base and sealing the hypocotyl–cuvette interface with a non-porous synthetic rubber sealant (Terostat VII; Henkel-Teroson, Heidelberg, Germany). The roots plus pot remained outside the cuvette. Fig. 1B depicts a 5-cm tall seedling enclosed in a cuvette.
Fig. 1.
Seedlings of O. elatior. (A) A seedling with cotyledons, incipient cladode, and remains of the seed coat, and (B) a 5-cm tall cladode with cotyledons at the base inside a gas-exchange cuvette.
Throughout this report, plant age is defined as the number of days following the unfolding of cotyledons.
The gas-exchange cuvette was located inside a controlled-environment chamber (Environmental Growth Chambers, Chagrin Falls, OH, USA) operating under 12-h light (28 °C)/12-h dark (22 °C) cycles. Photon flux density was 420 μmol m−2 s−1 at the top of the cuvette. Ambient air was drawn from a 1 m3 container placed on an eight-storey building to dampen short-term fluctuations in [CO2], and passed through the cuvette at a flow-rate of 2.3 l min−1. In one experiment, the cuvette was supplied at a flow rate of 1.26 l min−1, with air containing 400 ppm CO2 generated by a mass-flow controlled CO2/CO2-free air mixing unit (Walz GmbH, Effeltrich, Germany). The dew-point of the air entering the cuvette was 19 °C or 20 °C. Net CO2 exchange of the cotyledons and the first cladode was measured in a flow-through gas-exchange system consisting of Walz components and a LI-6252 CO2 analyser (Li-Cor, Lincoln, NE, USA) (Holtum and Winter, 2003). Drought treatments were imposed by withholding irrigation.
Titratable acidity
Seedlings were grown in plastic pots inside a controlled environment chamber under conditions similar to those used in the gas-exchange experiments. Cotyledons and cladodes were harvested at the end of the light and dark periods and were frozen in liquid nitrogen. Organic acids were extracted by sequentially boiling samples in 50% ethanol and in water, each for 5 min. Extracts were cooled to room temperature and titrated with 25 mM NaOH to pH 6.5.
Results
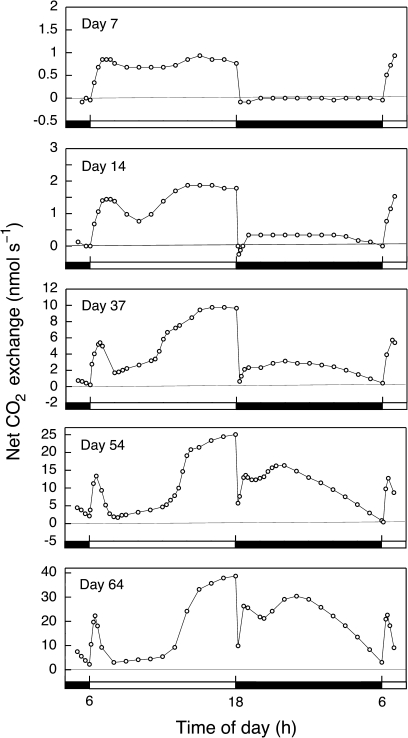
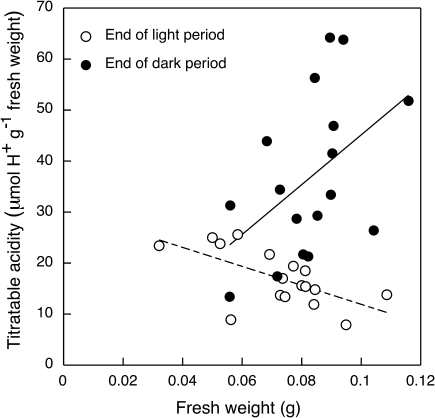
In well-watered 7-d-old seedlings consisting of only the cotyledons (Fig. 1A), net CO2 uptake was restricted to the light and occurred at a more or less constant rate (Fig. 2). Net CO2 uptake was not discernable in the dark. If present, it was below the limits of resolution of the gas-exchange system. In the smallest cotyledons studied (fresh weight <0.08 g per plant), titratable acidities at the end of the light and dark did not differ significantly (Fig. 3).
Fig. 2.
Light–dark patterns of net CO2 exchange during early development of well-watered O. elatior seedlings. Seven days after unfolding the seedling consisted of two cotyledons only. Cladode height was 0, <1, 5, 10, and 15 cm on days 7, 14, 37, 54, and 64, respectively. Open bars, 12-h light periods; closed bars, 12-h dark periods.
Fig. 3.
Relationship between developmental stage of O. elatior and titratable acidity in cotyledons at the end of the light (open circles) and at the end of the dark (closed circles). Cotyledon fresh weight per plant was used as an indicator of developmental stage. There was no significant difference between titratable acidity at the end of light and dark for cotyledons with fresh weights of <0.08 g (two-sample t-test, P>0.05). For cotyledons with fresh weights of ≥0.08 g the titratable acidity at the end of the dark period was significantly greater than that at the end of the light period (two-sample t-test, P<0.01). Lines are linear regressions.
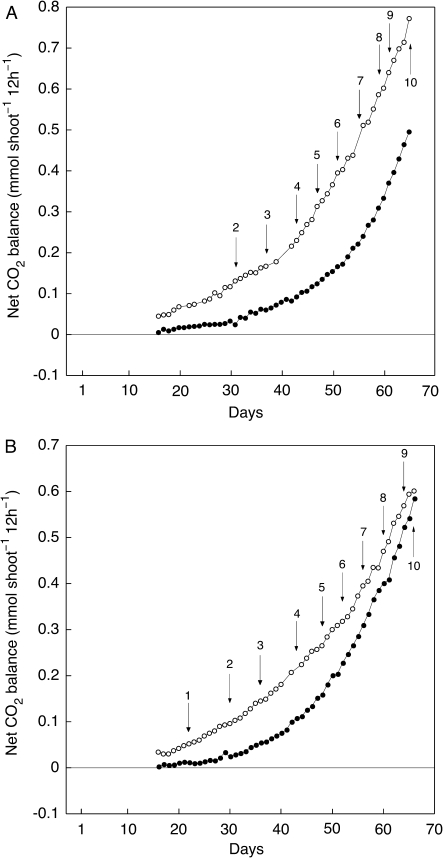
As cotyledons matured and the first cladode developed, CO2 exchange rates increased in the light and the dark (Fig. 2) and nocturnal acidification was observed (Fig. 3). Nocturnal carbon balance became positive after 10–14 d (Figs 2, 4). The proportional contribution of dark CO2 fixation to daily carbon gain increased progressively but only exceeded the level of carbon gain in the light in plants greater than ∼10 cm tall. The time taken to reach this stage of development varied somewhat between individuals (Fig. 4, and four additional experiments for which data are not shown).
Fig. 4.
Net CO2 balance in the dark (closed symbols) and in the light (open symbols) during the early growth of two well-watered seedlings of O. elatior. Two experiments are shown, (A) and (B). Values over arrows indicate cladode height in cm.
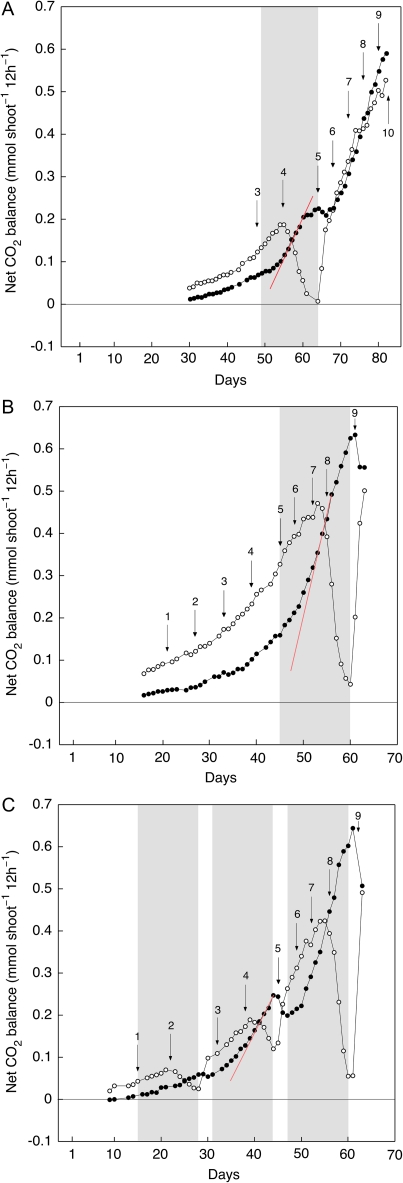
When watering was stopped for 14- to 16-d intervals at different stages of the development of the emerging cladode, carbon gain in the light decreased during the drying cycle but the daily increase in dark CO2 fixation accelerated (Fig. 5). This positive effect of stress on dark CO2 fixation slowed and eventually ceased as water limitation intensified. Upon rewatering, daytime carbon gain recovered rapidly whereas nocturnal CO2 uptake transiently decreased.
Fig. 5.
Effect of drought stress on net CO2 balance of 12-h dark (closed symbols) and 12-h light (open symbols) periods during the early growth of O. elatior. Three experiments are shown, (A), (B), and (C), in which watering was withheld during different stages of development. The red lines highlight accelerated nocturnal CO2 uptake. Shaded areas indicate periods without irrigation and values over arrows are cladode height in cm.
The stimulation of dark CO2 fixation during water stress was accompanied by increased nocturnal acidification in both cotyledons and cladodes (Table 1). On a whole-organ basis, the increase in acidification was 1.3-fold in cotyledons and 1.6-fold in the cladodes. At the stage of development investigated, the cladodes constituted the bulk of plant mass and contributed the most to nocturnal acid storage. The stress-related stimulation of acidification in cladodes as shown in Table 1 on a whole-organ basis is even greater on an area or fresh weight basis as cladode size was marginally smaller in the water-stressed plants (Table 2).
Table 1.
Effect of drought stress on titratable acidity in cotyledons and cladodes of O. elatior seedlings
| Age(d) | Treatment | Titratable acidity (μmol H+ organ−1) |
|||||
| Cotyledons |
Cladode |
||||||
| 18:00 | 06:00 | Δ | 18:00 | 06:00 | Δ | ||
| 23 | Well-watered | 4±1 | 65±7 | 61 | 10±3 | 83±20 | 73 |
| 35 | Well-watered | 4±1 | 92±11 | 88 | 32±8 | 251±23 | 219 |
| 35 | 24 d well-watered; last 11 d not watered | 3±1 | 117±7 | 114 | 21±6 | 362±40 | 341 |
Values are means ±SD of five replicates. End of light 18:00; end of dark 06:00; Δ, nocturnal accumulation of H+.
Table 2.
Effect of drought stress on size of seedlings of O. elatior
| Age (d) | Treatment | Cotyledons |
Cladode |
|||
| Area (cm2) | Fresh weight (g) | Area (cm2) | Fresh weight (g) | Height (cm) | ||
| 23 | Well-watered | 3.3±0.4 | 0.62±0.07 | 3.5±0.6 | 1.30±0.22 | 3.4±0.3 |
| 35 | Well-watered | 3.9±0.3 | 0.77±0.08 | 8.4±1.0 | 3.34±0.41 | 5.9±0.6* |
| 35 | 24 d well-watered; last 11 d not watered | 3.8±1.3 | 0.77±0.09 | 7.7±1.3 | 2.91±0.52 | 5.3±0.6 |
Values are means ±SD of 10 replicates except for the value indicated by an asterisk, for which n=8.
Discussion
This research highlights three features of photosynthetic carbon assimilation previously not fully recognized in platyopuntoid cacti: (i) O. elatior is a C3 plant immediately after germination, (ii) C3 photosynthesis remains the principal pathway of carbon acquisition for several weeks in well-watered plants up to 10 cm tall, and (iii) during this early phase of development seedlings can display a facultative component of CAM when drought stressed.
The contribution of C3 photosynthesis was quantified by direct measurement of daily CO2 uptake in addition to measurements of titratable acidity. In earlier studies of seedlings of cacti, CO2 exchange was not measured and the operation of C3 or CAM photosynthesis was simply inferred from the presence (Hernández-González and Briones Villarreal, 2007) or absence (Altesor et al., 1992; Loza-Cornejo et al., 2003) of fluctuations of titratable acidity, which, although an indicator of CAM, provide no information about rates of C3 photosynthesis or respiration. In the absence of measurements of CO2 exchange it is not possible to determine the point at which photosynthetic CO2 uptake surpasses respiratory CO2 loss and carbon gain becomes positive during early development.
The drought-stress-induced up-regulation of net CO2 fixation in the dark reflects a true facultative stimulation of the CAM pathway, as drought stress also leads to an increase in the nocturnal accumulation of organic acids. Previously, a facultative component of CAM was postulated for young tissues of O. ficus-indica developing on mother cladodes (Winter et al., 2008). It could not be ruled out that in these previous experiments, the transient acceleration of net CO2 fixation in the dark in response to drought stress was the result of a transient reduction in the efflux of respiratory CO2, as water deficit is known to reduce mitochondrial respiration (e.g. Fig. 7 in Winter et al., 2008). There can now be little doubt that a facultative component of CAM expression is present in cactus seedlings.
Remarkably, on most days during the development of well-watered O. elatior there was continuous uptake of CO2 throughout the 24-h cycle, albeit at varying rates that reflected the four phases of CAM gas exchange. This feature of uninterrupted daytime and night-time CO2 uptake has previously been highlighted as a speciality of some tropical woody species of Clusia (Lüttge, 2006). The implications of continuous 24-h CO2 uptake for biomass accumulation warrant further study (Borland et al., 2009; Holtum et al., 2011).
There have been no in situ studies of photosynthetic performance of cactus seedlings under tropical mesic conditions. It is conceivable that the expression of C3 photosynthesis in O. elatior shortly after germination assists establishment at relatively high growth rates when water is still available. The facultative CAM component can speed up the development of constitutive CAM and aid survival if the availability of water is rapidly reduced. Thus, in the field, the contribution of C3 photosynthesis to net carbon gain in seedlings may not endure as long as observed here and the shift to nocturnal CO2 uptake may be accelerated.
Under well-watered laboratory conditions O. elatior attained heights of ∼10 cm after ∼2 months. These growth rates substantially exceed those reported for seedlings of desert cacti in the field (Despain et al., 1970; Jordan and Nobel, 1981; Nobel, 1988) for which the initial C3–CAM shift is probably extremely short compared with that in cacti under more mesic conditions. Similarly when reproduction is vegetative, as is not uncommon in cacti (Nobel, 1988), the supply of carbon to new growth by the C3 phase is largely replaced with carbon supplied from the parent, thus shortening the initial C3 phase (Winter and Holtum, 2002).
This study documents in detail the change from C3 to CAM photosynthesis during the early development of seedlings of a tropical CAM cactus. In the context of the evolution and functional significance of CAM, the observations support the argument that constitutive and facultative CAM are merely extremes on a continuum that ranges from expression of CAM that is fully controlled by ontogeny to the full control of CAM expression by drought stress, and that facultative and constitutive CAM are not mutually exclusive.
Acknowledgments
The authors acknowledge the contribution of Aurelio Virgo who drew the illustrations. The research was supported by funds from the Smithsonian Tropical Research Institute. JAMH was supported by the JCU Special Studies Programme.
Glossary
Abbreviations
- CAM
crassulacean acid metabolism
References
- Acevedo E, Badilla I, Nobel PS. Water relations, diurnal acidity changes, and productivity of a cultivated cactus, Opuntia ficus-indica. Plant Physiology. 1983;72:775–780. doi: 10.1104/pp.72.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altesor A, Ezcurra E, Silva C. Changes in the photosynthetic metabolism during the early ontogeny of four cactus species. Acta Oecologica. 1992;13:777–785. [Google Scholar]
- Borland AM, Griffiths H, Hartwell J, Smith JAC. Exploiting the potential of plants with crassulacean acid metabolism for bioenergy production on marginal lands. Journal of Experimental Botany. 2009;60:2879–2896. doi: 10.1093/jxb/erp118. [DOI] [PubMed] [Google Scholar]
- Despain DG, Bliss LC, Boyer JS. Carbon dioxide exchange in saguaro seedlings. Ecology. 1970;51:912–914. [Google Scholar]
- Gerwick BCC, Williams GJ. Temperature and water regulation of gas exchange of Opuntia polyacantha. Oecologia. 1978;35:149–159. doi: 10.1007/BF00344728. [DOI] [PubMed] [Google Scholar]
- Hernández-González O, Briones Villarreal O. Crassulacean acid metabolism photosynthesis in columnar cactus seedlings during ontogeny: the effect of light on nocturnal acidity accumulation and chlorophyll fluorescence. American Journal of Botany. 2007;94:1344–1351. doi: 10.3732/ajb.94.8.1344. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Chambers D, Morgan T, Tan DY. Agave as a biofuel feedstock in Australia. Global Change Biology, Bioenergy. 2011;3:58–67. [Google Scholar]
- Holtum JAM, Winter K. Degrees of crassulacean acid metabolism in tropical epiphytic and lithophytic ferns. Australian Journal of Plant Physiology. 1999;26:749–757. [Google Scholar]
- Holtum JAM, Winter K. Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta. 2003;218:152–158. doi: 10.1007/s00425-003-1089-1. [DOI] [PubMed] [Google Scholar]
- Jordan PW, Nobel PS. Seedling establishment of Ferocactus acanthodes in relation to drought. Ecology. 1981;62:901–906. [Google Scholar]
- Loza-Cornejo S, Terrazas T, López-Mata L, Trejo C. Características morfo-anatómicas y metabolismo fotosintético en plántulas de Stenocereus queretaroensis (Cactaceae): su significado adaptativo. Interciencia. 2003;28:83–89. [Google Scholar]
- Lüttge U. Photosynthetic flexibility and ecophysiological plasticity: questions and lessons from Clusia, the only CAM tree, in the neotropics. New Phytologist. 2006;171:7–25. doi: 10.1111/j.1469-8137.2006.01755.x. [DOI] [PubMed] [Google Scholar]
- Neales TF, Patterson AA, Hartney VJ. Physiological adaptation to drought in the carbon assimilation and water loss of xerophytes. Nature. 1968;219:469–472. [Google Scholar]
- Nobel PS. Environmental biology of agaves and cacti. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Osmond B, Neales T, Stange G. Curiosity and context revisited: crassulacean acid metabolism in the Anthropocene. Journal of Experimental Botany. 2008;59:1489–1502. doi: 10.1093/jxb/ern052. [DOI] [PubMed] [Google Scholar]
- Osmond CB, Nott DL, Firth PM. Carbon assimilation patterns and growth of the introduced CAM plant Opuntia inermis in eastern Australia. Oecologia. 1979;40:331–350. doi: 10.1007/BF00345329. [DOI] [PubMed] [Google Scholar]
- Pimienta-Barrios E, Zañudo-Hernández J, Nobel PS. Effects of young cladodes on the gas exchange of basal cladodes of Opuntia ficus-indica (Cactaceae) under wet and dry conditions. International Journal of Plant Sciences. 2005;166:961–968. [Google Scholar]
- Silvera K, Santiago LS, Winter K. Distribution of crassulacean acid metabolism in orchids of Panama: evidence for selection of weak and strong modes. Functional Plant Biology. 2005;32:397–407. doi: 10.1071/FP04179. [DOI] [PubMed] [Google Scholar]
- Wang N, Zhang H, Nobel PS. Carbon flow and carbohydrate metabolism during sink-to-source transition for developing cladodes of Opuntia ficus-indica. Journal of Experimental Botany. 1998;49:1835–1843. [Google Scholar]
- Winter K, Aranda J, Holtum JAM. Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Functional Plant Biology. 2005;32:381–388. doi: 10.1071/FP04123. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Holtum JAM. On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. Journal of Experimental Botany. 2008;59:1829–1840. doi: 10.1093/jxb/ern080. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology. 2002;129:1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. The effects of salinity, crassulacean acid metabolism and plant age on the carbon isotope composition of Mesembryanthemum crystallinum L., a halophytic C3-CAM species. Planta. 2005;222:201–209. doi: 10.1007/s00425-005-1516-6. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. Environment or development? Lifetime net CO2 exchange and control of the expression of crassulacean acid metabolism in Mesembryanthemum crystallinum. Plant Physiology. 2007;143:98–107. doi: 10.1104/pp.106.088922. [DOI] [PMC free article] [PubMed] [Google Scholar]