Abstract
An indica variety Takanari is known as one of the most productive rice varieties in Japan and consistently produces 20–30% heavier dry matter during ripening than Japanese commercial varieties in the field. The higher rate of photosynthesis of individual leaves during ripening has been recognized in Takanari. By using pot-grown plants under conditions of minimal mutual shading, it was confirmed that the higher rate of leaf photosynthesis is responsible for the higher dry matter production after heading in Takanari as compared with a japonica variety, Koshihikari. The rate of leaf photosynthesis and shoot dry weight became larger in Takanari after the panicle formation and heading stages, respectively, than in Koshihikari. Roots grew rapidly in the panicle formation stage until heading in Takanari compared with Koshihikari. The higher rate of leaf photosynthesis in Takanari resulted not only from the higher content of leaf nitrogen, which was caused by its elevated capacity for nitrogen accumulation, but also from higher stomatal conductance. When measured under light-saturated conditions, stomatal conductance was already decreased due to the reduction in leaf water potential in Koshihikari even under conditions of a relatively small difference in leaf–air vapour pressure difference. In contrast, the higher stomatal conductance was supported by the maintenance of higher leaf water potential through the higher hydraulic conductance in Takanari with the larger area of root surface. However, no increase in root hydraulic conductivity was expected in Takanari. The larger root surface area of Takanari might be a target trait in future rice breeding for increasing dry matter production.
Keywords: Dry matter production, high-yielding variety, hydraulic conductance, hydraulic conductivity, leaf nitrogen, photosynthesis, rice (Oryza sativa), ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco), root surface area, stomatal conductance
Introduction
Rice is one of the most important staple foods in the world. Improvements in grain yield from existing available land to meet the demands of rapidly growing populations present a major challenge (Juliano, 1993; Hubbart et al., 2007; IRRI, 2008, www.irri.org). Given that the land area that is available for rice cultivation is unlikely to increase significantly, there is a need to increase yields significantly. Breakthroughs in genetic improvement of rice have resulted in the availability of semi-dwarf high-yielding varieties, which have increased potential yields of rice grains worldwide (Yoshida, 1981; Osada, 1995; Hubbart et al., 2007). In recently improved varieties, top-dressing with nitrogen fertilizer at the panicle formation stage increases levels of leaf nitrogen without increasing leaf area, and suppresses the reduction in leaf area index (LAI) after heading (Yoshida, 1981; Kumura, 1995), resulting in improvements in light-intercepting characteristics (Tanaka et al., 1968; Hayami, 1982; Takeda et al., 1983) and the rate of leaf photosynthesis during ripening (Kuroda and Kumura, 1990; Sasaki and Ishii, 1992).
An indica variety Takanari is well known as one of the most productive rice varieties in Japan. It consistently attains higher grain yield and dry matter production than new and old commercial japonica varieties in Japan (Taylaran et al., 2009). Takanari produces 8–9 t of grain (brown rice) and 19–21 t of total dry matter per hectare under normal field conditions (San-oh et al., 2004; Taylaran et al., 2009). The superiority of Takanari with respect to dry matter production is recognizable after heading. This is a cause of the higher grain yield of Takanari because ∼70% of the final carbohydrates in rice grains are derived from photosynthates that are generated after heading. This would also increase the harvest index to as high as 0.5 in Takanari compared with the japonica varieties examined. It was suggested that higher rates of canopy photosynthesis from heading through ripening in Takanari resulted from the enhanced light-intercepting characteristics of the canopy (Xu et al., 1997; Taylaran et al., 2009) and the higher rate of photosynthesis of individual leaves which form the canopy (Xu et al., 1997).
Takanari maintains a high level of leaf nitrogen and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Xu et al., 1997; Ohsumi et al., 2008; Hirasawa et al., 2010), and, therefore, a high rate of leaf photosynthesis compared with common varieties in Japan even at the same rate of nitrogen application. The level of leaf nitrogen is not only affected by the rate of nitrogen application but it is also influenced by the capacity for absorption of nitrogen and the partitioning of absorbed nitrogen within the plant. There are several reports on varietal differences among these properties (Peng et al., 1995; Ookawa et al., 2003; Shiratsuchi et al., 2006).
Stomatal conductance also affects the rate of leaf photosynthesis (Ishihara and Saito, 1987;Hirasawa et al., 1988). It was far larger in Takanari than some japonica varieties (Xu et al., 1997; Ohsumi et al., 2008; Hirasawa et al., 2010). High hydraulic conductance is responsible for a larger stomatal conductance under intense transpiration in rice plants (Hirasawa et al., 1992b). Root hydraulic conductance controls a plant's hydraulic conductance (Boyer, 1971; Hirasawa et al., 1992a) and is influenced by root surface area and root hydraulic conductivity (Kramer, 1983; Steudle and Peterson, 1998). When evaluating the traits of a higher rate of leaf photosynthesis in Takanari, it would be necessary to clarify the cause of the higher level of leaf nitrogen and the larger stomatal conductance.
Identification of traits that contribute to greater production of dry matter in high-yielding varieties is vital since this would contribute significantly to the effective use of newly developed breeding methods, such as marker-assisted selection (Yamamoto et al., 2009). To apply quantitative trait locus (QTL) analysis effectively to rice breeding, it would be important to identify the traits responsible for the greater production of dry matter in high-yielding variety and to know when the significant difference in the traits appears between parents. For the future application of QTL analysis to incorporate the trait of a higher rate of leaf photosynthesis of Takanari into Japanese commercial varieties, the higher rate of leaf photosynthesis in Takanari has to be characterized by comparing it with that of Japanese varieties and also the heritability of the trait has to be clarified.
In the present study, rates of photosynthesis were compared as a possible explanation for varietal differences in dry matter production between Takanari and the most popular Japanese variety, Koshihikari. Plants were examined from tillering through ripening, and efforts were made to identify factors responsible for the difference by comparing the amount of accumulated nitrogen, by comparing the respective level of leaf nitrogen and the rate of leaf photosynthesis and by comparing root mass and hydraulic conductance. Then, possible reasons for the higher capacity for nitrogen accumulation in leaves and the higher capacity of stomatal conductance responsible for the higher rates of leaf photosynthesis were investigated.
Materials and methods
Materials and cultivation of plants
A high-yielding variety of rice (Oryza sativa L.), Takanari, and the most popular Japanese rice variety, Koshihikari, were the main varieties used. Nipponbare, Asanohikari, and Sasanishiki were also employed as Japanese commercial rice varieties when necessary. They were grown in pots with ∼12.0 l of soil per pot (12.0 l pots). Rice seedlings at the fourth leaf expansion stage were transplanted to pots filled with a mixture of alluvial soil from the Tama River and Kanto diluvial soil (1:1, v/v) at a density of four plants per hill, with three hills per pot, and grown under submerged conditions. Fertilizer was applied at rates of 1.0, 1.0, and 1.0 g per pot of N, P2O5, and K2O, respectively, as a basal dressing, and 0.5 g of N per pot was applied as top-dressing at the booting stage. They were grown outdoors. Pots were arranged in rows. The distance between rows and between pots in a row was ∼100 cm and 30 cm, respectively. For comparisons of the relationships between leaf nitrogen content and rates of photosynthesis, plants were grown at lower rates of basal application of nitrogen, namely 0.1 g and 0.5 g per pot, for measurements at the tillering and heading stages, respectively, and leaf nitrogen content was manipulated by top-dressing with nitrogen at different rates, ∼10 d before measurements were made. The experiment was laid out in a completely randomized design, with five replicates.
Rice plants were also grown in pots with ∼3.0 l of soil per pot (3.0 l pots) in a growth chamber (KG-50HLA;Koito Manufacturing Co. Ltd, Tokyo, Japan) under the conditions of 28 °C/23 °C day/night temperature, 60%/80% relative humidity, a 12 h photoperiod, and ∼1000 μmol m−2 s−1 photosynthetic photon flux density (PPFD) at the top of the canopy. Basal fertilizer was applied at a rate of 0.5, 0.5, and 0.5 g per pot for N, P2O5, and K2O, respectively, and 0.1 g of nitrogen per pot was applied to Koshihikari 10 d before measurements at the heading stage to bring the leaf nitrogen content of Koshihikari to the same as that of Takanari.
Plants which were grown in the paddy field of the University Farm at a density of 22.2 (30 cm×15 cm) hills m−2 with three plants per hill were also used. Barnyard manure was applied at a rate of ∼2 kg m−2 each year in the field. Chemical fertilizer was applied at rates of 5.0, 5.0, and 5.0 g m−2 of N, P2O5, and K2O, respectively, as basal fertilizer, and 3.0 g m−2 of N and K2O, respectively, were added as top-dressing at the panicle formation stage.
Measurements of dry weight
Plants were separated into leaves, leaf sheaths plus stems, panicles (after heading), and roots. The roots were washed carefully with tap water after separation from above-ground parts. Leaf area was measured with an automatic area meter (AAM-8; Hayashi Denko Co., Tokyo, Japan) immediately after separation of leaves from plants. Each group of plant parts was dried in a ventilated oven at 80 °C for >4 d or to constant weight. The plant growth rate (PGR), net assimilation rate (NAR), and the mean leaf area (mean LA) were calculated according to Beadle (1993).
Measurements of the CO2 assimilation rate and stomatal conductance
Rates of photosynthesis and stomatal conductance were measured with a portable photosynthesis system (LI-6400; LI-COR Inc., Lincoln, NE, USA) in leaves attached to the main stem on cloudy days between 8:00 h and 14:00 h. On clear days, measurements were completed prior to 11:00 h, before a marked reduction in the rate of photosynthesis occurred. The quantum flux density at the leaf surface, the concentration of CO2 in the chamber, the leaf–air vapour pressure difference, and leaf temperature were maintained at 2000 μmol m−2 s−1, 370 μmol mol−1, ∼1.5 kPa, and 30 °C (for plants grown outdoors) and 28 °C (for plants grown in the growth chamber), respectively. To compare the photosynthetic activity of a leaf without the effect of stomatal conductance on the rate of photosynthesis (stomatal factor), the rate of photosynthesis at the same intercellular CO2 concentration (Ci) was also measured. The Ci was calculated as described by von Caemmerer and Farquhar (1981).
Measurement of the nitrogen content of plants and leaves
Plant samples were dried in an oven at 80 °C to constant weight. The total nitrogen concentration of each plant was determined with a CN analyser (MT-600 and MT-700 II; Yanako Inc., Kyoto, Japan) and the plant nitrogen content was expressed as the product of the plant dry weight and the nitrogen concentration.
Quantitation of the Rubisco content and the nitrogen content of individual leaves
Leaves were collected immediately after completion of measurement of the rate of photosynthesis and stomatal conductance, and they were stored at –80 °C prior to analysis. The area and fresh weight of each leaf were determined and each leaf was separated into two equal parts for separate quantification of Rubisco and nitrogen. The halves of leaves were homogenized separately with a mortar and pestle in a solution that contained 50 mM TRIS-HCl (pH 7.5), 1 mM EDTA, 10 mM MgCl2, 10 mM 2-mercaptoethanol, and 5% (w/w) insoluble polyvinylpyrrolidone (Polyclar VT; Wako Chem., Tokyo, Japan). Each homogenate was centrifuged at 10 000 g for 10 min at 4 °C. The supernatant was used for quantitation of Rubisco by the single radial immunodiffusion method (Sugiyama and Hirayama, 1983) with rabbit polyclonal antibodies raised against purified Rubisco from rice. Nitrogen was quantified from the other halves of the leaves with a CN analyser (MT-700 II).
Determination of the hydraulic conductance and hydraulic conductivity of plants
For plants grown in 3.0 l pots, plant hydraulic conductance from roots to leaves (Cpp) was calculated as follows:
where S is the number of stems of the plants, Uw is the water uptake rate of the whole plant,Ψs is the water potential of the soil immediately outside the root, and Ψl is the water potential of the uppermost three leaves. Hydraulic conductance per root surface area, referred to here as the hydraulic conductivity of a plant (Lp), was measured for plants grown in pots. Lp was calculated as follows:
where RA is the root surface area.
Measurements were made in the environment-controlled chamber mentioned above (KG-50HLA; Koito Manufacturing Co. Ltd) during a day (air temperature, 28 °C; relative humidity, 58–62%; and PPFD at the top leaves, ∼1000 μmol m−2 s−1). The Uw was determined from the rate of weight loss of the pot after a steady state had been reached. To prevent evaporation from the surface of the pot, the top of the pot was covered with polystyrene foam and oily clay was used to seal the gap between the foam and the stem. When the water uptake rate reached a constant value, the leaf was excised at the leaf collar immediately after covering it with a polyethylene bag. The leaf water potential of the covered whole leaf blade was measured with the pressure chamber (model 3005, Soil Moisture Equipment Inc., Santa Barbara, CA, USA) according to Hirasawa and Ishihara (1991). Since rice plants were grown under submerged conditions, Ψs could be regarded as 0 when compared with Ψl. After roots had been washed gently with water, the root surface area was measured with an image analyser (Win-Rhizo REG V 2004 b, Regent Inc., Quebec, Canada).
For plants grown in the paddy field, the hydraulic conductance from the soil through the roots to the flag leaf (Cpf) was calculated from the following equation (Hirasawa and Ishihara, 1991):
where T is the transpiration rate per leaf area at steady state. The transpiration rate of a single intact whole leaf was measured in an air-sealed acrylic assimilation chamber (Hirasawa and Ishihara, 1991) under natural sunlight. Air, with the dewpoint controlled to 10 (±0.1) °C, was pumped into the chamber at a rate of 6.67×10−5 m3 s−1. The humidity of the air that was pumped into and out of the chamber was measured with a dewpoint hygrometer (model 660, EG&G Inc., Waltham, MA, USA). When the transpiration rate reached a constant value, the water potential of the leaf was measured with a pressure chamber (model 3005, Soil Moisture Equipment Inc.).
Treatments with a polyethylene bag and leaf excision
To increase the stomatal conductance of the leaves of Koshihikari, polyethylene bag and leaf excision treatments were conducted. For the polyethylene bag treatment, individual leaves were covered with a transparent polyethylene bag and illuminated with an artificial cool fibre light (LA-180Me, Hayashi Watch Works, Tokyo, Japan) for 20 min. The PPFD at the surface of the bag was ∼1000 μmol m−2 s−1. The maximum leaf temperature during the polyethylene bag treatment was ∼34 °C. Immediately after removal of the bag and blotting of water drops with gauze, the rate of photosynthesis of the leaf was measured with an LI-6400 system at a CO2 concentration of ∼400 μmol mol−1 in the reference chamber. For leaf excision, after the leaf gas exchange had reached a steady state, the leaf was excised at its base with a razor blade. The rate of photosynthesis was recorded continuously after both treatments at 5 s intervals and the maximum value was determined as the value of the treatment.
Statistical analysis
The statistical significance of the difference between two varieties and among four varieties was determined by Student's t-test and Tukey–Kramer test, respectively.
Results
Dry matter and nitrogen accumulation and nitrogen partitioning to leaves
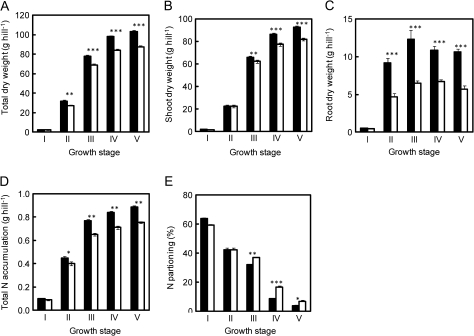
In both varieties, total dry weight increased rapidly until the heading stage and it continued to increase, but more slowly, after heading (Fig. 1A). The superiority of Takanari over Koshihikari in terms of total dry matter production was evident from the panicle formation stage and was maintained through the ripening stage. No significant difference in shoot dry weight was apparent before the heading stage (Fig. 1B) but Takanari had significantly greater root dry weight from the panicle formation stage through ripening than that of Koshihikari (Fig. 1C). These observations indicated that the difference in dry weight at the panicle formation stage was a consequence of a difference in root growth.
Fig. 1.
Changes in total dry weight (A), shoot dry weight (B), root dry weight (C), total nitrogen accumulation (D), and nitrogen partitioning to the leaves (E) in Takanari (filled squares) and Koshihikari (open squares) grown in 12.0 l pots. I, II, III, IV, and V represent the tillering stage (3 weeks after transplanting), the panicle formation stage (7 weeks after transplanting), the heading stage, 2.5 weeks after heading, and 4.5 weeks after heading, respectively. August 14 and 21 were the heading dates of Koshihikari and Takanari, respectively. Vertical bars represent the standard deviation (n=5). *, **, and ***: values are significantly different at the 5, 1, and 0.1% level, respectively.
Both varieties accumulated nitrogen rapidly before heading and slowly during ripening (Fig. 1D). Differences in nitrogen accumulation resembled differences in dry matter accumulation. Takanari accumulated a larger amount of nitrogen from the panicle formation stage, and the amount remained higher than in Koshihikari through the ripening stage. The relative partitioning of nitrogen to leaves decreased with growth and was somewhat lower in Takanari than in Koshihikari after heading (Fig. 1E). To compare the capacities for nitrogen accumulation of Takanari and Koshihikari, the effects of nitrogen top-dressing on the accumulation of nitrogen from tillering through ripening were examined by comparing plants with and without top-dressing (Supplementary Table S1 available at JXB online). The effects of nitrogen top-dressing at the tillering stage did not differ between the two varieties. A varietal difference in the effects of nitrogen top-dressing became evident at the heading and ripening stages. The increase in accumulated nitrogen was larger in Takanari than in Koshihikari.
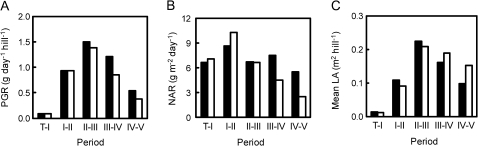
The PGR of Takanari was higher than that of Koshihikari from the panicle formation stage through ripening (Fig. 2A). The higher PGR of Takanari was supported by the larger leaf area before heading (Fig. 2C) and by the higher net assimilation rate after heading (Fig. 2B).
Fig. 2.
Plant growth rate (PGR; A), net assimilation rate (NAR; B), and mean leaf area (mean LA; C) in Takanari (filled squares) and Koshihikari (open squares) grown in 12.0 l pots. In this figure, T-I, I-II, II-III, III-IV, and IV-V represent the growth period from transplanting to the tillering stage (3 weeks after transplanting), from tillering to the panicle formation stage (7 weeks after transplanting), from panicle formation to the heading stage, from heading to 2.5 weeks after heading, and from 2.5 weeks after heading to 4.5 weeks after heading, respectively. A statistical comparison could not be applied to the growth analysis because the experiments were conducted with five replicates and one replicate consists of one pot with no correspondence among pots.
Levels of leaf nitrogen and rates of leaf photosynthesis
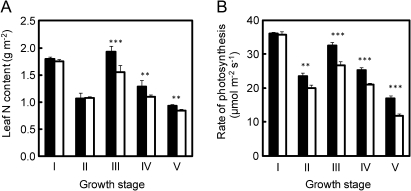
Leaf nitrogen content decreased at 6 weeks after transplanting, but it increased again at heading due to the nitrogen top-dressing at the booting stage (Fig. 3A). No significant difference between the two varieties, in terms of leaf nitrogen content, was apparent at the tillering and panicle formation stages. The leaf nitrogen content of Takanari became significantly higher than that of Koshihikari after heading. The rate of leaf photosynthesis in Takanari was higher than that in Koshihikari from the panicle formation stage through the ripening stage after no difference in the rate at the tillering stage (Fig. 3B).
Fig. 3.
Changes in leaf nitrogen content (A) and the rate of leaf photosynthesis at an ambient CO2 concentration of 370 μmol mol−1 (B) of the uppermost fully expanded leaf in Takanari (filled squares) and Koshihikari (open squares) grown in 12.0 l pots. Measurements at heading were taken when a panicle of a main stem emerged completely. Vertical bars represent the standard deviation (n=5). For explanations of I, II, III, IV, V, and asterisks, see the legend of Fig. 1.
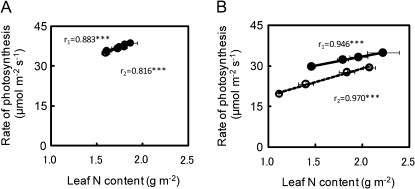
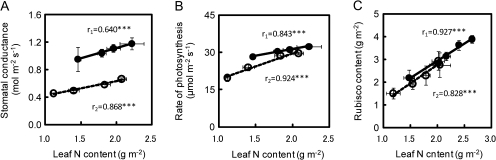
The relationship between the rates of leaf photosynthesis and leaf nitrogen content was examined. The rates of leaf photosynthesis at an ambient CO2 concentration of 370 μmol mol−1 were closely correlated to leaf nitrogen content for each variety at the tillering and full-heading stages (Fig. 4). At the tillering stage, there was no varietal difference in the relationship between Takanari and Koshihikari (Fig. 4A). However, the rate of leaf photosynthesis at the heading (Fig. 4B) and ripening stages (data not shown) was higher in Takanari than in Koshihikari over all the leaf nitrogen contents examined. The stomatal conductance at an ambient CO2 concentration of 370 μmol mol−1 in Takanari was also greater than that in Koshihikari at an identical nitrogen content (Fig. 5A). In the two varieties, the rate of photosynthesis at an intercellular CO2 concentration of 280±2 μmol mol−1 in terms of leaf nitrogen content was very similar both at the heading (Fig. 5B) and at the ripening stage (data not shown). This means that the leaf photosynthetic activity was very similar at an identical nitrogen content of a leaf in the two varieties. The level of leaf Rubisco in terms of leaf nitrogen content was also very similar in the two varieties (Fig. 5C). These results indicate that the higher rates of photosynthesis after heading might have been due to the higher stomatal conductance as well as the higher nitrogen content of a leaf.
Fig. 4.
Relationships between leaf nitrogen content and rates of photosynthesis at an ambient CO2 concentration of 370 μmol mol−1 at the tillering stage (A) and at the heading stage (B) in Takanari (filled circles) and Koshihikari (open circles) grown in 12.0 l pots. Measurements at heading were taken when a panicle of a main stem emerged completely. Vertical and horizontal bars represent the standard deviation (n=5). Values of r1 and r2 represent correlation coefficients for Takanari and Koshihikari, respectively, and asterisks *** represent significance at the 0.1% level.
Fig. 5.
Relationships between (A) stomatal conductance at an ambient CO2 concentration of 370 μmol mol−1, (B) rates of photosynthesis at an intercellular CO2 concentration of 280±2 μmol mol−1, and (C) Rubisco content and the leaf nitrogen content of the flag leaf at the heading stage in Takanari (filled circles) and Koshihikari (open circles) grown in 12.0 l pots. Measurements were taken when a panicle of a main stem emerged completely. Vertical and horizontal bars represent the standard deviation (n=5). Values of r1 and r2 represent correlation coefficients for Takanari and Koshihikari, respectively, and asterisks *** represent significance at the 0.1% level.
Leaf water potential, root surface area, hydraulic conductance, and hydraulic conductivity
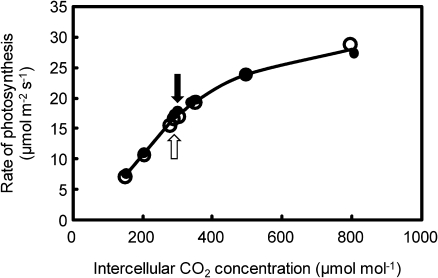
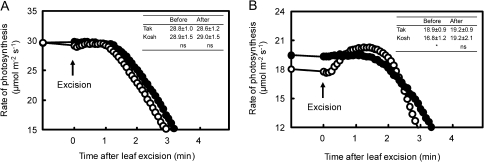
The rate of leaf photosynthesis, leaf water potential, and hydraulic conductance was compared between Takanari and Koshihikari by using leaves with almost the same nitrogen content at the full-heading and tillering stages. When compared at the heading stage, the rate of leaf photosynthesis was still higher in Takanari than in Koshihikari (Table 1). The water potential of the flag leaf in Koshihikari decreased significantly compared with that in Takanari despite the fact that plants of both cultivars were growing under submerged soil conditions and the leaf–air vapour pressure difference was as low as 1.5 kPa. There was no difference between Koshihikari and Takanari in terms of the relationship between the intercellular CO2 concentration and the rate of photosynthesis (Fig. 6). This indicates that the higher rate of photosynthesis in Takanari at an ambient CO2 concentration of 370 μmol mol−1 was due to the larger stomatal conductance of Takanari. After leaves had been covered with transparent polyethylene bags for 20 min under an irradiance of ∼1000 μmol photons m−2 s−1, the rate of leaf photosynthesis in Koshihikari increased to the level of that in Takanari at the heading stage as a result of significant increases in stomatal conductance because of high humidity and a low CO2 concentration inside the bag, while increases in the rate of leaf photosynthesis in Takanari were not observed (Table 1). After excision of a leaf at its base and release of the hydrostatic pressure in the xylem, after leaf gas exchange had reached a steady state, the rate of photosynthesis in Koshihikari increased to that in Takanari within a few minutes at the heading stage, a phenomenon known as the ‘Ivanov effect’ (Slavik, 1974), while there was little increase in Takanari (Fig. 7B). The root surface area was ∼200% larger in Takanari than in Koshihikari at the heading stage (Table 2). The hydraulic conductance from roots to leaves (Cpp) was far higher in Takanari than in Koshihikari (Table 2), while there is no difference in Lp between Koshihikari and Takanari. The larger root surface area might be responsible for the higher hydraulic conductance of Takanari.
Table 1.
The rate of leaf photosynthesis (Pn), stomatal conductance (gs), leaf nitrogen content (N content), leaf water potential (Ψ1), and effects of the polyethylene bag treatment of leaves on the rate of leaf photosynthesis [Poly(Pn)] and stomatal conductance [Poly(gs)] in Takanari and Koshihikari grown in 3.0 l pots at the tillering and heading stages
| Growth stage | Variety | Pn (μmol m−2 s−1) | gs (mol m−2 s−1) | Poly(Pn) (μmol m−2 s−1) | Poly(gs) (mol m−2 s−1) | N content (g m−2) | Ψ1 |
| Tillering | Takanari (A) | 30.4±1.0 | 0.97±0.10 | 30.1±2.4 | 0.97±0.19 | 1.60±0.07 | –0.25±0.02 |
| Koshihikari (B) | 30.8±0.5 | 0.79±0.04 | 29.6±3.5 | 1.08±0.40 | 1.77±0.03 | –0.26±0.02 | |
| A/B | 0.99 | 1.23 | 1.02 | 0.90 | 0.91 | 0.95 | |
| NS | * | NS | NS | * | NS | ||
| Heading | Takanari (A) | 19.1±0.7 | 0.78±0.16 | 19.1±1.3 | 0.89±0.07 | 1.26±0.07 | –0.18±0.03 |
| Koshihikari (B) | 16.5±0.7 | 0.44±0.04 | 18.0±0.7 | 0.73±0.08 | 1.31±0.03 | –0.42±0.04 | |
| A/B | 1.15 | 1.78 | 1.06 | 1.22 | 0.96 | 0.43 | |
| *** | ** | NS | * | NS | *** |
The uppermost fully expanded leaves were used for measurements. Measurements at the heading were taken when a panicle of a main stem emerged completely. *, **, and *** indicate asignificant difference at the 5, 1, and 0.1 % level, respectively, between two varieties. NS, non-significant.
Fig. 6.
Rates of photosynthesis plotted against the intercellular CO2 concentration of the flag leaf with a similar nitrogen content at the heading stage in Takanari (filled circles) and Koshihikari (open circles) grown in 3.0 l pots. Black (Takanari) and white (Koshihikari) arrows indicate the rates at an ambient CO2 concentration of 370 μmol mol−1. Measurements were taken when a panicle of a main stem emerged completely.
Fig. 7.
Changes in rates of photosynthesis after excision of leaves of Takanari (filled circles) and Koshihikari (open circles) that had been grown in 3.0 l pots as determined at the tillering (A) and the heading (B) stages. Each leaf was excised at the base of the leaf blade after leaf gas exchange had reached a steady state. An ambient CO2 concentration and the leaf–air vapour pressure difference were kept at 370 μmol mol−1 and ∼1.5 kPa, respectively, before leaf excision, but they were not controlled after excision. Measurements at heading were taken when a panicle of a main stem emerged completely. Inserted tables show the rates of leaf photosynthesis of Takanari (Tak) and Koshihikari (Kosh) before and after excision (mean ±SD, n=3). An asterisk * indicates a significant difference at the 5% level between two varieties. ns: no significant difference.
Table 2.
Leaf area, root length, root surface area, hydraulic conductance (Cpp), and hydraulic conductivity (Lp) of Takanari and Koshihikari grown in 3.0 l pots at the tillering and heading stages
| Growth stage | Variety | Per hill |
A/C (m2 m−2) | Per stem |
|||
| Leaf area (A) (m2) | Root length (B) (km) | Root surface area (C) (m2) | Cpp (10−8 m3 MPa−1 s−1) | Lp (10−8 m MPa−1 s−1) | |||
| Tillering | Takanari (A) | 0.13±0.01 | 0.64±0.05 | 0.47±0.02 | 0.27±0.03 | 0.12±0.01 | 5.62±1.03 |
| Koshihikari (B) | 0.11±0.02 | 0.72±0.09 | 0.46±0.08 | 0.25±0.01 | 0.10±0.01 | 5.26±0.54 | |
| A/B | 1.12 | 0.89 | 1.03 | 1.09 | 1.24 | 1.07 | |
| NS | NS | NS | NS | * | NS | ||
| Heading | Takanari (A) | 0.15±0.02 | 2.56±0.44 | 1.80±0.29 | 0.08±0.01 | 0.33±0.02 | 2.95±0.51 |
| Koshihikari (B) | 0.19±0.03 | 1.34±0.12 | 0.89±0.04 | 0.18±0.01 | 0.13±0.01 | 3.28±0.10 | |
| A/B | 0.81 | 1.92 | 2.04 | 0.44 | 2.50 | 0.90 | |
| NS | * | * | ** | *** | NS | ||
*, **, and *** indicate a significant difference at the 5, 1, and 0.1% level, respectively, between two varieties. NS, non- significant.
In contrast, no differences were observed in the rate of leaf photosynthesis before and after leaves had been covered with transparent polyethylene bags and in leaf water potential between Koshihikari and Takanari at the tillering stage (Table 1). Also no clear increases in the rate of leaf photosynthesis after leaf excision were observed in Koshihikari or Takanari at the tillering stage (Fig. 7A). The Cpp in Takanari was large compared with that in Koshihikari, but the difference was small at the tillering stage and there was also no difference in root surface area between the varieties (Table 2).
Comparisons of the rate of leaf photosynthesis in terms of leaf nitrogen content and hydraulic conductance with other Japanese varieties
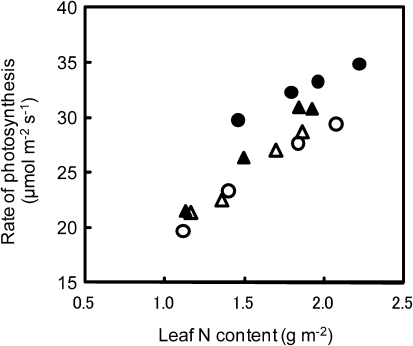
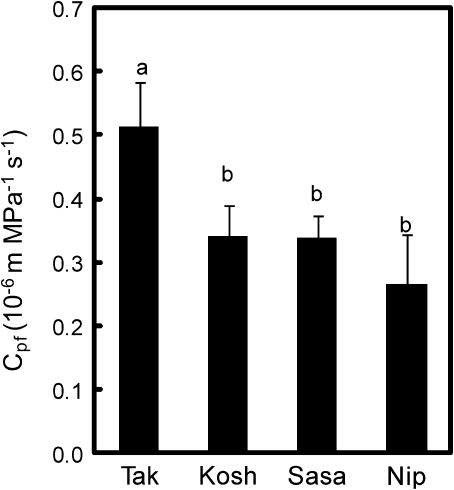
The rate of leaf photosynthesis was compared in terms of leaf nitrogen and the hydraulic conductance of Takanari with other Japanese commercial varieties at the heading to ripening stages. The rate of leaf photosynthesis was high in Takanari compared with Nipponbare and Asanohikari, as well as Koshihikari, even at identical nitrogen contents of a leaf (Fig. 8). The hydraulic conductance was also large in Takanari compared with Nipponbare and Sasanishiki, as well as Koshihikari (Fig. 9).
Fig. 8.
Relationships between leaf nitrogen content and the rate of flag leaf photosynthesis at the ambient CO2 concentration of 370 μmol mol−1 in Takanari (filled circles) and common Japanese varieties, Asanohikari (filled triangles) and Nipponbare (open triangles), as well as Koshihikari (open circles) grown in 12.0 l pots at the heading stage. Measurements were taken when a panicle of a main stem emerged completely.
Fig. 9.
Hydraulic conductance (Cpf) of Takanari (Tak) and common Japanese varieties, Sasanishiki (Sasa), Nipponbare (Nip), and Koshihikari (Kosh) grown in the paddy field at the early ripening stage. Different letters represent a significant difference at the 5% level.
Discussion
The superiority of Takanari in terms of the rate of leaf photosynthesis was apparent after the panicle formation stage and, especially, after heading (Fig. 3B). The production of heavier dry matter after heading in Takanari (Fig. 1A) might be attributable to the maintenance of a higher rate of leaf photosynthesis after heading (Fig. 3B). The greater production of dry matter by Takanari was supported by a higher NAR after heading (Fig. 2B), which was supported mainly by the elevated rate of photosynthesis of individual leaves since each pot was isolated from others and mutual shading was minimal.
The rate of leaf photosynthesis is closely correlated with leaf nitrogen content (Ishihara et al., 1979; Makino et al., 1987). The level of leaf nitrogen was always larger in Takanari after heading than in Koshihikari (Fig. 3A). It can be concluded that the higher level of leaf nitrogen in Takanari was one of the causes of the higher rates of leaf photosynthesis after heading. However, after heading, the rate of leaf photosynthesis was higher in Takanari than in Koshihikari even at the same level of leaf nitrogen (Fig. 4B; Hirasawa et al., 2010). This phenomenon also reflects the generally higher rate of leaf photosynthesis in Takanari.
Takanari plants accumulated larger amounts of nitrogen, but relative partitioning of nitrogen to leaves tended to be lower than in Koshihikari (Fig. 1D, E). These observations suggest that the enhanced accumulation of nitrogen might have been responsible for the higher leaf nitrogen content and, as a consequence, the higher rates of leaf photosynthesis in Takanari. The accumulation of nitrogen in plants is affected not only by the capacity of roots for nitrogen assimilation but also, in trees, by the transportation of nitrogen to shoots via the transpirational stream (Cernusak et al., 2009). The transpirational uptake of water should be higher in plants with larger root systems (Hirasawa et al., 1992b) and provides a plausible explanation for the greater accumulation of nitrogen by Takanari.
Takanari accumulated significantly more nitrogen from the panicle formation stage through the ripening stage and, in particular, after heading than Koshihikari (Fig. 1D). The roots of Takanari were already significantly heavier at the panicle formation stage than those of Koshihikari (Fig. 1C). The marked varietal difference in nitrogen accumulation reflected the marked varietal difference in root weight (Fig. 1C, D). No effects of top-dressing with nitrogen on nitrogen accumulation were evident at the tillering stage when, moreover, there was no difference in root mass between Takanari and Koshihikari (Fig. 1C, Supplementary Table S1 at JXB online). However, the effects of top-dressing with nitrogen on nitrogen accumulation were apparent and significantly greater in Takanari than in Koshihikari at heading and at the ripening stage when there was also a significant difference in root mass between the two varieties (Fig. 1C, Supplementary Table S1). From these results, the larger root mass in Takanari than in Koshihikari might be attributable to the greater accumulation of nitrogen by the former than the latter (Ookawa et al., 2004; San-oh et al., 2006; Taylaran et al., 2009).
In rice, rates of photosynthesis are closely associated with the level of Rubisco during ripening (Makino et al., 1985; Ookawa et al., 2004). Makino et al. (1985) also reported a close correlation between the level of Rubisco and leaf nitrogen. Not only the level of Rubisco but also the leaf nitrogen content can be limiting factors in the maintenance of a higher rate of photosynthesis (Makino et al., 1983). Although there are several reports of comparisons of rates of photosynthesis among rice varieties (e.g. Murata, 1961; Cook and Evans, 1983; Sasaki and Ishii, 1992; Ishii, 1995; Peng et al, 1995), none of the cited studies included a comparison of rates of photosynthesis at the same leaf nitrogen content. At the heading stage, the rate of photosynthesis and the stomatal conductance were higher in Takanari than in Koshihikari, even at the same leaf nitrogen content (Figs 4B, 5A). A very small varietal difference was observed in the relationship between leaf nitrogen content and the rate of photosynthesis at an intercellular CO2 concentration of 280±2 μmol mol−1 (Fig. 5B) and no difference in the relationship between leaf nitrogen content and Rubisco content (Fig. 5C). The results suggest that the higher rate of photosynthesis in Takanari might have resulted from the greater stomatal conductance at the same level of leaf nitrogen, as reported previously by Hirasawa et al. (2010). A similar result was also obtained at the ripening stage (data not shown). However, there was no difference between the two varieties in the relationship between leaf nitrogen content and the rate of leaf photosynthesis at the tillering stage (Fig. 4A).
The response of rice leaf stomata is very sensitive to reductions in air humidity and leaf water potential. In an earlier study, stomatal conductance of a japonica variety decreased when the vapour pressure difference between the leaf and the atmosphere increased from ∼1 kPa to 1.5 kPa (Hirasawa et al., 1988). The vapour pressure deficit of the atmosphere usually increases to ∼1.5 kPa at ∼9:00 h on a clear day. Under such conditions, the stomata of rice leaves should start to close. Water balance in a plant is greatly affected by the plant hydraulic conductance. That is, with the increase in transpiration, leaf water potential decreases in plants with small hydraulic conductance compared with plants with large hydraulic conductance. The critical water potential for stomatal closure is very high in rice compared with other crop plants (Hirasawa et al., 1988; Hirasawa, 1999). This might be a reason why the hydraulic conductance affects stomatal conductance significantly.
The increase in the rate of leaf photosynthesis was large in Koshihikari leaves after the polyethylene bag (Table 1) and leaf excision (Fig. 7B) treatments at the heading stage. When leaves were subected to treatment with a polyethylene bag, stomatal conductance increased significantly in Koshihikari due to the conditions of high humidity and, probably, of decreased CO2 concentration, and any difference in the rate of photosynthesis between the varieties was not observed at the same level of leaf nitrogen (Table 1). Results of the polyethylene bag treatment indicate that the rate of leaf photosynthesis of Koshihikari could increase to that of Takanari when stomatal conductance increases. When the hydrostatic pressure in leaf xylem was released with the leaf excision treatment, the rate of leaf photosynthesis of Koshihikari increased to that of Takanari (Fig. 7B). In contrast, any significant increase in the rate of leaf photosynthesis was not observed in Takanari. Results of the leaf excision treatment indicate that the rate of leaf photosynthesis of Koshihikari could increase to that of Takanari when the hydrostatic pressure in xylem decreases, or leaf water potential increases. Leaf water potential decreased significantly in Koshihikari with a low hydraulic conductance compared with Takanari which has a higher hydraulic conductance (Tables 1, 2). In contrast, no increase in the rate of leaf photosynthesis after the polyethylene bag and leaf excision treatments was observed in Koshihikari or in Takanari at the tillering stage (Fig. 7A, Table 1). Leaf water potential was kept high in both varieties (Table 1).
These observations indicate that stomata had already started to close in Koshihikari at the heading stage as a result of water stress, even under the mild condition of a vapour pressure deficit of ∼1.5 kPa, while those of Takanari remained open. In contrast, at the tillering stage leaf water potential and, therefore, stomatal conductance were kept high in Koshihikari as well as Takanari, and there was no significant difference in the rate of leaf photosynthesis between them (Tables 1, 2).
The rate of leaf photosynthesis was also larger in Takanari with larger hydraulic conductance than that of other japonica varieties with smaller hydraulic conductance (Figs 8, 9). Brodribb et al. (2007) found that, in an analysis of 43 plant species, the maximum rate of photosynthesis was correlated both with the distance between veins and the leaf surface and with the hydraulic conductance of the leaf. The present results confirm, in rice, the importance of hydraulic conductance in defining photosynthetic capacity (Brodribb et al., 2005).
The hydraulic conductance in entire rice plants is determined mainly by the conductance of the roots (Hirasawa et al., 1992a). Since the axial conductance of roots is very large compared with the radial conductance, root hydraulic conductance can be considered to be derived from two components: root hydraulic conductivity (Lpr, conductance per root surface area) and root surface area (Steudle and Peterson, 1998). It might be possible to use Lp, calculated in this research, in comparing Lpr. Since there was no difference in Lp between Koshihikari and Takanari, root surface area might be attributable to the difference in hydraulic conductance between them at the full-heading stage. Differences in root mass might explain differences in rates of leaf photosynthesis via the involvement of plant hydraulic conductance. The regulation mechanisms and genetic mechanisms will be the focus of future studies.
Conclusion
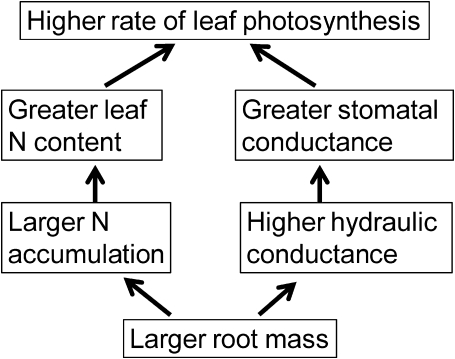
A high-yielding variety of indica rice, Takanari, accumulates larger amounts of nitrogen and has a higher rate of leaf photosynthesis from the panicle formation through the ripening stages, subsequent to formation of a large root system at the panicle formation stage, than a japonica variety, Koshihikari. These properties of Takanari explain its higher grain yield, dry matter production, and harvest index. As illustrated in Fig. 10, the higher rate of photosynthesis in Takanari appeared to result from both the greater leaf nitrogen content and the greater stomatal conductance than those in Koshihikari even under the same application of nitrogen and at the same level of leaf nitrogen, respectively. The characteristics of the increased nitrogen uptake and hydraulic conductance might be caused by the larger root surface area and this might be a cause of the higher rate of leaf photosynthesis in Takanari. The larger root surface area of Takanari might be a target trait in future rice breeding for increasing dry matter production through the improvement of the rate of leaf photosynthesis.
Fig. 10.
A schematic to illustrate how the high-yielding indica variety, Takanari, achieves a higher rate of leaf photosynthesis than other japonica varieties.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Effect of nitrogen top-dressing on dry weight (DW) and accumulated nitrogen (AN) at various growth stages in rice plants grown in 12.0 l pots.
Acknowledgments
This work was supported, in part, by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 19380009), from the Ministry of Agriculture, Forestry and Fisheries, Japan (Genomics for Agricultural Innovation, QTL-1002), and from the Japan Society for the Promotion of Science (AA Science Platform Program).
References
- Beadle CL. Growth analysis. In: Hall DO, Scurlock GMO, Bolhar-Nordenkampf MR, Leegood RC, Long SP, editors. Photosynthesis and production in a changing environment. A field and laboratory manual. London: Chapman and Hall; 1993. pp. 36–46. [Google Scholar]
- Brodribb TJ, Field TS, Jordan GJ. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology. 2007;144:1890–1898. doi: 10.1104/pp.107.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Zwieniecki MA, Palma B. Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytologist. 2005;165:839–846. doi: 10.1111/j.1469-8137.2004.01259.x. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Resistance to water transport in soybean, bean, and sunflower. Crop Science. 1971;11:403–407. [Google Scholar]
- Cernusak LA, Winter K, Turner BL. Plant δ15N correlates with the transpiration efficiency of nitrogen acquisition in tropical trees. Plant Physiology. 2009;151:1667–1676. doi: 10.1104/pp.109.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MG, Evans LT. Some physiological aspects of the domestication and improvement of rice. Field Crops Research. 1983;6:219–238. [Google Scholar]
- Hayami K. Studies on the physiological and ecological characteristics of high-yielding rice varieties with a high fertilizer response. 1. The effect of a nitrogen supply on the photosynthetic characteristics of high-yielding rice varieties with a high fertilizer response. Bulletin of Tohoku National Agricultural Experimental Station. 1982;68:21–43. [Google Scholar]
- Hirasawa T. Physiological characterization of the rice plant for tolerance of water deficit. In: Ito O, O'Tool J, Hardy B, editors. Genetic improvement of rice for water-limited environments. Los Baños, Philippines: International Rice Research Institute; 1999. pp. 89–98. [Google Scholar]
- Hirasawa T, Gotou T, Ishihara K. On resistance to water transport from roots to the leaves at the different positions on a stem in rice plants. Japanese Journal of Crop Science. 1992a;61:153–158. [Google Scholar]
- Hirasawa T, Iida Y, Ishihara K. Effect of leaf water potential and air humidity on photosynthetic rate and diffusive conductance in rice plants. Japanese Journal of Crop Science. 1988;57:112–118. [Google Scholar]
- Hirasawa T, Ishihara K. On resistance to water transport in crop plants for estimating water uptake ability under intense transpiration. Japanese Journal of Crop Science. 1991;60:174–183. [Google Scholar]
- Hirasawa T, Ozawa S, Taylaran RD, Ookawa T. Varietal differences in photosynthetic rates in rice plants, with special reference to the nitrogen content of leaves. Plant Production Science. 2010;13:53–57. [Google Scholar]
- Hirasawa T, Tsuchida M, Ishihara K. Relationship between resistance to water transport and exudation rate and the effect of the resistance on the midday depression of stomatal aperture in rice plants. Japanese Journal of Crop Science. 1992b;61:143–152. [Google Scholar]
- Hubbart S, Peng S, Horton P, Chen Y, Murchie EH. Trends in leaf photosynthesis in historical rice varieties developed in the Philippines since 1966. Journal of Experimental Botany. 2007;58:3429–3438. doi: 10.1093/jxb/erm192. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Iida O, Hirasawa T, Ogura T. Relationship between nitrogen content in leaf blades and photosynthetic rate of rice plants with reference to stomatal aperture and conductance. Japanese Journal of Crop Science. 1979;48:543–550. [Google Scholar]
- Ishihara K, Saito K. Diurnal courses of photosynthesis, transpiration, and diffusive conductance in the single-leaf of the rice plants grown in the paddy field under submerged condition. Japanese Journal of Crop Science. 1987;56:8–17. [Google Scholar]
- Ishii R. Roles of photosynthesis and respiration in the yield-determining process. In: Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H, editors. Science of the rice plant. Vol. 2. Physiology. Tokyo: Food and Agriculture Policy Research Center; 1995. pp. 691–696. [Google Scholar]
- Juliano BO. Rice in human nutrition. Manila, Philippines: International Rice Research Institute; 1993. pp. 1–162. [Google Scholar]
- Kramer PJ. Water relations of plants. New York: Academic Press; 1983. pp. 1–489. [Google Scholar]
- Kumura A. Physiology of high-yielding rice plants from the viewpoint of dry matter production and its partitioning. In: Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H, editors. Science of the rice plant. Vol. 2. Physiology. Tokyo: Food and Agriculture Policy Research Center; 1995. pp. 691–696. [Google Scholar]
- Kuroda E, Kumura A. Difference in single leaf photosynthesis and its dependence on stomatal conductance. Japanese Journal of Crop Science. 1990;59:283–292. [Google Scholar]
- Makino A, Mae T, Ohira K. Photosynthesis and ribulose-1,5-bisphosphate carboxylase in rice leaves. Plant Physiology. 1983;73:1002–1007. doi: 10.1104/pp.73.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. Photosynthesis and ribulose-1,5-bisphosphate carboxylase/oxygenase in rice leaves from emergence through senescence. Quantitative analysis by carboxylation/oxygenation and regeneration of ribulose-1,5-bisphosphate. Planta. 1985;166:414–420. doi: 10.1007/BF00401181. [DOI] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. Variations in the contents and kinetic properties of ribulose-1,5-bisphosphate carboxylases among rice species. Plant and Cell Physiology. 1987;28:799–804. [Google Scholar]
- Murata Y. Studies on the photosynthesis of rice plants and its culture significance. Bulletin of the National Institute of Agricutural Science. 1961;D9:1–169. [Google Scholar]
- Ohsumi A, Hamasaki A, Nakagawa H, Homma K, Horie T, Shiraiwa T. Response of leaf photosynthesis to vapor pressure difference in rice (Oryza sativa L.) varieties in relation to stomatal and leaf internal conductance. Plant Production Science. 2008;11:184–191. [Google Scholar]
- Ookawa T, Naruoka Y, Sayama A, Hirasawa T. Cytokinin effects on ribulose-1,5-bisphosphate carboxylase/oxygenase and nitrogen partitioning in rice during ripening. Crop Science. 2004;44:2107–2115. [Google Scholar]
- Ookawa T, Naruoka Y, Yamazaki T, Suga J, Hirasawa T. A comparison of the accumulation and partitioning of nitrogen in plants between two rice cultivars, Akenohoshi and Nipponbare, at the ripening stage. Plant Production Science. 2003;6:172–178. [Google Scholar]
- Osada A. Photosynthesis and respiration in relation to nitrogen responsiveness. In: Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H, editors. Science of the rice plant. Vol. 2. Physiology. Tokyo: Food and Agriculture Policy Research Center; 1995. pp. 696–703. [Google Scholar]
- Peng S, Cassman KG, Kropff MJ. Relationship between leaf photosynthesis and nitrogen content of field grown rice in the tropics. Crop Science. 1995;35:1627–1630. [Google Scholar]
- San-oh Y, Mano Y, Ookawa T, Hirasawa T. Comparison of dry matter production and associated characteristics between direct-sown and transplanted rice plants in a submerged paddy field and relationships to planting patterns. Field Crops Research. 2004;87:43–58. [Google Scholar]
- San-oh Y, Sugiyama T, Yoshita D, Ookawa T, Hirasawa T. The effect of planting pattern on the rate of photosynthesis and related processes during ripening in rice plants. Field Crops Research. 2006;96:113–124. [Google Scholar]
- Sasaki H, Ishii R. Cultivar differences in leaf photosynthesis of rice bred in Japan. Photosynthesis Research. 1992;32:139–146. doi: 10.1007/BF00035948. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H, Yamagishi T, Ishii R. Leaf nitrogen distribution to maximize the canopy photosynthesis in rice. Field Crops Research. 2006;95:291–304. [Google Scholar]
- Slavik B. Methods of studying plant water relations. Prague, Czechoslovakia: Czechoslovak Academy of Science; 1974. Transpiration; pp. 252–292. [Google Scholar]
- Steudle E, Peterson CA. How does water get through roots? Journal of Experimental Botany. 1998;49:775–788. [Google Scholar]
- Sugiyama T, Hirayama Y. Correlation of the activities of phosphoenolpyruvate carboxylase and pyruvate, orthophosphate dikinase with biomass in maize seedling. Plant and Cell Physiology. 1983;24:783–787. [Google Scholar]
- Takeda T, Oka M, Agata W. Studies on the dry matter production between old and new types of rice cultivars. Japanese Journal of Crop Science. 1983;52:299–306. [Google Scholar]
- Tanaka A, Yamaguchi J, Shimazaki Y, Shibata K. Historical changes in the plant type of rice cultivars in Hokkaido. Journal of the Science of Soil and Manure, Japan. 1968;39:526–534. [Google Scholar]
- Taylaran RD, Ozawa S, Miyamoto N, Ookawa T, Motobayashi T, Hirasawa T. Performance of a high-yielding modern rice cultivar Takanari and several old and new cultivars grown with and without chemical fertilizer in a submerged paddy field. Plant Production Science. 2009;12:365–380. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Xu YF, Ookawa T, Ishihara K. Analysis of the photosynthetic characteristics of the high-yielding rice cultivar Takanari. Japanese Journal of Crop Science. 1997;66:616–623. [Google Scholar]
- Yamamoto T, Yonemaru J, Yano M. Towards the understanding of complex traits in rice: substantially or superficially? DNA Research. 2009;16:141–154. doi: 10.1093/dnares/dsp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Fundamentals of rice crop science. Manila, Philippines: The International Rice Research Institute; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.