Abstract
The extent to which approach and avoidance personality trait sensitivities are associated with specific cognitive control abilities as well as with verbal and nonverbal domains remains unclear. In the current study, we investigated whether approach and avoidance trait sensitivities predict performance on verbal and nonverbal versions of the Stroop task, which taps the specific cognitive control ability of inhibiting task-irrelevant information. The findings from the current study indicate that whereas approach (specifically, Extraversion) sensitivity was predictive of verbal Stroop performance, avoidance (specifically, Behavioral Inhibition System) sensitivity was predictive of nonverbal Stroop performance. These results provide novel evidence suggestive of the integration of motivational personality traits and the ability to inhibit task-irrelevant information in a domain-specific fashion.
Keywords: approach, avoidance, personality, motivation, cognitive control, inhibition, individual differences
1. Introduction
Several researchers have proposed that approach and avoidance motivations serve as fundamental dimensions of personality and behavior (e.g. Carver, Sutton, & Scheier, 2000; Elliot & Thrash, 2002). Approach motivation refers to behavior toward desired stimuli, sensitivity to reward cues, and is associated with positive affective states. Avoidance motivation is characterized by behavior away from aversive stimuli, sensitivity to punishment cues, and is associated with negative affective states (see Carver et al., 2000 for a review).
Approach and avoidance have been studied at the level of personality traits, with studies focusing on various dimensions of these motivations. Several studies have employed the behavioral inhibition system/behavioral activation system (BIS/BAS) scales, which were developed to assess punishment and reward sensitivity, respectively (Carver & White, 1994). Other studies have focused on Extraversion and Neuroticism, which have been associated with tendencies to experience positive and negative affect, respectively (e.g. Eysenck & Eysenck, 1964; Watson & Clark, 1984). Although beyond the scope of the current study, approach and avoidance motivations have also been examined through the lens of promotion and prevention regulatory focus systems (see Higgins, 1997). In an attempt to bridge the literatures on different dimensions of personality, it has been proposed that the commonality across these different dimensions lies in the distinction between approach and avoidance motivation (e.g. Carver et al., 2000; Elliot & Thrash, 2002). For example, Carver et al. (2000) suggested that Extraversion can be characterized as the tendency to approach rewards, whereas Neuroticism can be characterized as the tendency to avoid threats.
Approach and avoidance motivations have been suggested to interact with cognitive control abilities, as both motivation and cognitive control are thought to guide goal-directed behavior (see Gray & Braver, 2002 for a review). Furthermore, previous evidence suggests that approach and avoidance motivations may be differentially associated with verbal and nonverbal cognitive control abilities. Gray (2001) demonstrated that the induction of approach motivational states improved verbal working memory performance and impaired spatial working memory performance, whereas avoidance motivational states improved spatial working memory performance and impaired verbal working memory performance (c.f. Shackman et al., 2006). Gray (2001) suggested that these selective influences of motivational states on cognitive control abilities may stem from hemispheric lateralization underlying both approach/avoidance motivation and verbal/nonverbal working memory. Separate studies have associated greater left prefrontal activity with approach state and trait variables as well as with verbal working memory, whereas greater right prefrontal activity has been associated with avoidance state and trait variables as well as with nonverbal working memory (e.g. Harmon-Jones & Gable, 2009; Smith & Jonides, 1999; Spielberg et al., 2011; Sutton & Davidson, 1997; c.f. Coan & Allen, 2003). Although Gray's (2001) findings provide intriguing evidence for selective modulation of cognitive control abilities by different motivational states, the nature of this interaction remains unclear. The n-back task, which was used by Gray (2001) and Shackman et al. (2006), taps several different processing mechanisms, including maintenance, rehearsal, updating, and response selection (Smith & Jonides, 1997). Thus, the locus of impact of approach and avoidance sensitivity on verbal and nonverbal cognitive control abilities requires further investigation.
In the current study, we aimed to determine whether approach and avoidance trait sensitivities are differentially predictive of performance on verbal and nonverbal versions of the Stroop task (Stroop, 1935). This task was chosen because it taps a specific cognitive control ability, inhibition: ignoring task-irrelevant information in order to respond on the basis of task-relevant information. Based on the results of Gray (2001) and Elliot & Thrash (2002), we predicted that both BAS and Extraversion trait sensitivities would predict better verbal Stroop performance (i.e. smaller conflict effects), whereas both BIS and Neuroticism trait sensitivities would predict better nonverbal Stroop performance.
2. Methods
2.1. Participants
Seventy-nine participants (26 men, 53 women, ages 18-35) participated in this study. Data from one additional participant were excluded from all analyses due to at-chance performance on the nonverbal Stroop task. All participants were right-handed, native speakers of English, and were not taking any psychoactive medications. All participants gave informed consent prior to participating in the experiment according to guidelines established by the Institutional Review Board of the University of Pennsylvania.
2.2. Materials
2.2.1. Verbal Stroop Task
Participants indicated the font color of a presented color word via button press on a computer keyboard. Three possible response options were available to the participant: yellow, green, and blue. A colored square corresponding to each color was affixed to a different key on the computer keyboard, and participants were instructed to press the key corresponding to the appropriate color as quickly and as accurately as possible. The verbal Stroop task featured two main types of trials: congruent and incongruent. Two types of incongruent trials were included in which the font color did not match the meaning of the word (see Milham et al., 2001 for further detail), and all reported results collapse across both types of incongruent trials.
For each trial, participants were first presented with a fixation cross for 1000 ms. The stimulus was then presented for 1500 ms, followed by a 500 ms inter-trial interval (blank screen). Participants completed four blocks of each task, where each block comprised 72 trials: 36 congruent and 36 incongruent trials. Trials were presented in a fixed pseudo-randomized order across all participants. Participants also performed 24 practice trials (12 congruent and 12 incongruent trials) prior to the task in order to familiarize them with the stimuli and task procedures. Stimuli were presented and responses were collected with E-prime software (Schneider, Eschman, & Zuccolotto, 2002).
2.2.2. Nonverbal Stroop Task
A modified variant of the nonverbal Stroop task that was originally developed by Pomerantz (1983) was used in the current study (see Figure A.1 in Appendix). Participants viewed a moving square window (global motion) containing moving dots (local motion) and indicated the direction of (local) motion of the dots, by pressing either the left or right key of two designated keys on a computer keyboard. As in the verbal Stroop task, participants were presented with two types of trials: congruent and incongruent. For congruent trials, local and global motion occurred in the same direction (e.g. window moving to the right containing dots moving to the right). Two types of incongruent trials were included in which global and local motion did not occur in the same direction. As with the verbal Stroop task, all reported results collapse across both types of incongruent trials for the nonverbal Stroop task.
Trial timing parameters and the number of trials per condition were the same as for the verbal Stroop task described above (Section 2.2.1). Stimuli were presented and responses were collected with MATLAB® software (2007a, The Mathworks).
2.2.3. Behavioral Inhibition/Behavioral Activation System Scales (BIS/BAS)
Participants completed the BIS/BAS scales (Carver & White, 1994). The BIS scale consists of 7 items, each of which is designed to assess individuals' sensitivity to punishment cues (e.g. “If I think something unpleasant is going to happen, I get pretty worked up”). The BAS scale consists of a total of 13 items, each of which assesses individuals' sensitivity to cues of reward. The BAS scale comprises three sub-scales: BAS-Drive (4 items; e.g. “When I want something, I usually go all-out to get it”), BAS-Fun Seeking (4 items; e.g. “I'm always willing to try something new if I think it will be fun”), and BAS-Reward Responsiveness (5 items; e.g. “When good things happen to me, it affects me strongly”). Participants responded using a scale ranging from 1 (strongly disagree) to 4 (strongly agree). The sums of responses to items from each scale were used as BIS and BAS (collapsed across the three subscales) scores in further analyses.
2.2.4. Eysenck Personality Inventory (Form A)
Participants completed Form A of the Eysenck Personality Inventory (Eysenck & Eysenck, 1964), which comprises the following dimensions: Extraversion (24 items, e.g. “Do other people think of you as being very lively?”), Neuroticism (24 items, e.g. “Would you call yourself tense or highly strung?”), and Lie (9 items, e.g. “Are all your habits good and desirable ones?”). For the purposes of the current study, we focus on the Extraversion and Neuroticism scales. The Extraversion scale was designed to assess participants' sociability, impulsivity, and activity levels, whereas the Neuroticism scale was designed to measure participants' tendency to experience negative affect. Participants responded by pressing either “1” (yes) or “2” (no). “No” responses were later recoded as “0” for scoring purposes.
2.3. Procedure
Participants completed the tasks and questionnaires in the following order: Verbal Stroop, Nonverbal Stroop, BIS/BAS scales, and the Eysenck Personality Inventory (Form A). Tasks and questionnaires were administered to all participants in the same order in order to minimize measurement error due to participant × task order interactions (e.g. Friedman et al., 2008; Miyake et al., 2000).
2.3.1. Statistical Procedures
Conflict effects were more robust in the first two blocks compared with all four blocks for the nonverbal Stroop task (t[78] = 3.06, p < 0.01; Cohen's d = 0.34). This is likely due to participants becoming more practiced over the course of the nonverbal Stroop task, resulting in smaller conflict effects across all four blocks of the task. Thus, in order to better assess the relationship between personality variables and conflict effects, all reported results include data from only the first two blocks for both the verbal and nonverbal Stroop tasks.
For both verbal and nonverbal Stroop tasks, a within-participant trimming procedure recommended by Wilcox & Keselman (2003) was applied to each participant's reaction time (RT) data. For each participant and each condition, RTs whose deviation from the median was greater than 3.32 times the median absolute deviation were excluded prior to calculating mean RTs. This procedure resulted in no more than 9.4 % of observations excluded in each condition. Mean RTs and percent error rates for verbal and nonverbal Stroop tasks are presented in Table 1. RT conflict effects expressed as difference scores (incongruent RT – congruent RT) and as percentage RT conflict effects [(incongruent RT – congruent RT)/congruent RT] are presented in Table 2. Only correct trials were included in all RT analyses.
Table 1.
Performance summary for verbal and nonverbal Stroop tasks.
| Condition | Mean RT | SD | Mean % Error | SD |
|---|---|---|---|---|
| Verbal Stroop | ||||
| Congruent | 556 | 81 | 2 | 2 |
| Incongruent | 611 | 96 | 4 | 4 |
| Nonverbal Stroop | ||||
| Congruent | 546 | 104 | 1 | 2 |
| Incongruent | 584 | 142 | 5 | 6 |
Note. RTs are given in milliseconds. N = 79 for verbal and nonverbal Stroop tasks.
Table 2.
Conflict effects for verbal and nonverbal Stroop tasks.
| Conflict Condition | RT Conflict | SD | % RT Conflict | SD | Reliability | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|
| Verbal Stroop | 55 | 36 | 9.9 | 6 | 0.68 | -0.10 | -0.05 |
| Nonverbal Stroop | 36 | 51 | 6.1 | 8.2 | 0.88 | 1.4 | 1.8 |
Note. Conflict condition, incongruent trials compared to the congruent condition; RT Conflict, mean reaction time difference scores in milliseconds; % RT Conflict, mean percentage RT conflict ([incongruent-congruent]/congruent); Reliability, split-half (odd-even) correlations for percentage RT conflict effects adjusted with the Spearman-Brown prophecy formula; Skewness and Kurtosis, skewness and kurtosis statistics for percentage RT conflict effects. N = 79 for verbal and nonverbal Stroop tasks.
Due to low error rates for both verbal and nonverbal Stroop tasks, multiple regression analyses were performed on the RT data, which revealed more reliable conflict effects. In order to ensure that observed relationships between personality measures and conflict effects did not merely reflect effects of personality variables on overall speed, multiple regression analyses were performed using percentage RT conflict effects. In order to improve normality and reduce the influence of extreme values, an additional between-participant trimming procedure was employed. Observations greater than three standard deviations from the group mean were replaced with observations three standard deviations from the mean for each variable included in multiple regression analyses (see Friedman et al., 2008). No more than 1.3 % of the observations for each variable were affected by this additional trimming procedure.
3. Results
3.1. BIS/BAS Scales & Eysenck Personality Inventory (Form A)
Means, standard deviations, ranges, reliabilities, and zero-order correlations for the BIS, BAS, Neuroticism, and Extraversion scales are presented in Table 3. As our sample included a greater number of female participants, we investigated sex differences for all of the variables assessed in the current study. Significant sex differences were found for participants' BIS scores, where female participants (M = 21.8, SD = 3.5) had higher BIS scores compared with male participants (M = 19.2, SD = 3.0; t[77] = 3.30, p < 0.01; Cohen's d = 0.79). Additionally, we found significantly lower Extraversion scores for female (M = 11.3, SD = 4.2) compared with male participants (M = 14.0, SD = 3.1; t[77] = -2.92, p < 0.01; Cohen's d = 0.70). No sex differences were found for BAS or Neuroticism scores, or for verbal and nonverbal conflict effects (p's > 0.25). Multiple regression analyses were performed to investigate whether the interactions of sex with BIS and Extraversion predicted participants' conflict effects on the verbal and nonverbal Stroop tasks. As these interaction terms were not significant predictors of verbal and nonverbal Stroop conflict (p's > 0.51), we collapsed across both sexes for all reported analyses.
Table 3.
Descriptive statistics for self-report personality trait measures.
| Trait measure | Range | M | SD | Reliability | Zero-order correlations (r) | ||
|---|---|---|---|---|---|---|---|
| BIS | BAS(Total) | Extraversion | |||||
| BIS | 12-28 | 20.94 | 3.57 | 0.81 | - | - | |
| BAS (Total) | 28-50 | 40.23 | 4.6 | 0.8 | -0.14 | - | - |
| Extraversion | 3-22 | 12.23 | 4.05 | 0.74 | -0.32 ** | 0.33 ** | - |
| Neuroticism | 2-23 | 10.82 | 4.92 | 0.82 | 0.59 ** | -0.09 | -0.17 |
Note. BIS = Behavioral Inhibition System; BAS (Total) = Behavioral Activation System (sum of the three BAS subscale scores). Reliability was calculated using Cronbach's alpha. N = 79 for verbal and nonverbal Stroop tasks.
p < 0.05.
In order to ensure independent contributions of personality variables to Stroop performance, multiple regression analyses (see Section 3.3) were performed including both personality variables in each scale as predictors of z-scored verbal and nonverbal Stroop conflict (e.g. multiple regression analysis for verbal Stroop conflict and BIS/BAS scales include both BIS and BAS as predictors to control for both personality variables simultaneously).
3.2. Stroop Performance
For the verbal Stroop task, participants demonstrated significantly longer reaction times (t[78] = 13.68, p < 0.001; Cohen's d = 1.52) and higher error rates (t[78] = 4.14, p < 0.001; Cohen's d = 0.47) for incongruent compared to congruent trials. Similarly, for the nonverbal Stroop task, participants demonstrated significantly longer reaction times (t[78] = 5.60, p < 0.001; Cohen's d = 0.63) and higher error rates (t[78] = 5.27, p < 0.001; Cohen's d = 0.59) for incongruent compared to congruent trials.
3.3. Personality Traits & Verbal Stroop Conflict
Participants' BAS total scores were found to be a marginally significant predictor of their verbal Stroop percentage RT conflict effects (β = -0.20, p = 0.08). However, participants' BIS scores did not predict their verbal Stroop conflict effects (β = -0.08, p = 0.47). A separate multiple regression analysis revealed that Extraversion (β = -0.37, p < 0.01), but not Neuroticism (β = - 0.08, p = 0.45), was a significant predictor of verbal Stroop percentage RT conflict effects.
In sum, these results suggest that those participants who had higher BAS and Extraversion scores tended to demonstrate smaller verbal Stroop conflict effects.
3.4. Personality Traits & Nonverbal Stroop Conflict
Participants' BIS scores (β = 0.25, p < 0.05), but not BAS total scores (β = - 0.01, p < 0.05), were found to be a significant predictor of nonverbal Stroop percentage RT conflict effects. A separate multiple regression analysis for Extraversion/Neuroticism personality traits revealed that although the relationship between Neuroticism and nonverbal Stroop conflict was also positive (similar to BIS scores), this relationship did not reach significance (β = 0.16, p = 0.16). Extraversion scores were not a significant predictor of nonverbal Stroop conflict effects (β = -0.09, p = 0.45).
In sum, these results suggest that BIS scores predicted larger nonverbal Stroop conflict effects.
3.5. Personality Traits & Stroop Conflict: Hierarchical Regression Analyses
The results of the multiple regression analyses reported in Sections 3.3 and 3.4 suggest that verbal conflict was associated with Extraversion trait sensitivity, whereas nonverbal conflict was associated with BIS trait sensitivity. In order to confirm the domain-specificity of these relationships, we performed hierarchical regression analyses for Extraversion and BIS scores using z-scored verbal and nonverbal percentage conflict effects as predictors. For Extraversion scores, nonverbal Stroop conflict was not a significant predictor (F < 1.03; Step 1); however, verbal Stroop conflict was a significant predictor of Extraversion trait sensitivity after accounting for the variance explained by nonverbal Stroop conflict (ΔR2 = 0.13, ΔF[1,76] = 11.86, p < 0.01; Step 2). For BIS scores, verbal Stroop conflict was not a significant predictor (F < 1; Step 1). However, nonverbal Stroop conflict explained unique variance in BIS scores after accounting for the variance explained by verbal Stroop conflict (ΔR2 = 0.06, ΔF[1,76] = 5.12, p < 0.05; Step 2). In sum, these results are suggestive of a domain-specific relationship between Extraversion sensitivity and verbal conflict resolution abilities, and between BIS sensitivity and nonverbal conflict resolution abilities.
4. Discussion
The evidence presented here demonstrates that approach and avoidance personality traits are linked to domain-specific cognitive control abilities. Specifically, the results of the current study provide novel evidence indicating that whereas Extraversion trait sensitivity was predictive of verbal (and not nonverbal) Stroop performance, BIS trait sensitivity was predictive of nonverbal (and not verbal) Stroop performance. Below, we discuss the implications of these results in terms of the nature of the relationship between personality traits and cognitive control abilities, implications for hemispheric asymmetry, and methodological comparisons with previous work as well as limitations of the current study.
4.1. Personality Traits & Cognitive Control Abilities
Our finding that Extraversion predicts verbal, but not nonverbal, conflict effects provides novel evidence suggesting that the relationship between Extraversion trait sensitivity and the ability to inhibit task-irrelevant information is domain-specific in nature. It is interesting to note that Gray (2001) found that BAS trait sensitivity was associated with a greater impact of an approach state induction on verbal n-back performance compared to Extraversion trait sensitivity. Although we found evidence of a marginally significant relationship between BAS trait sensitivity and reduced verbal Stroop conflict, Extraversion was a significant predictor of verbal conflict effects. These results may reflect a stronger association between Extraversion and inhibition ability, rather than the ability to actively maintain verbal representations in working memory. Nonetheless, in that our results demonstrate that an aspect of approach trait sensitivity predicts reduced conflict in the verbal domain, these findings are consistent with Gray (2001).
Our finding that BIS scores predict cognitive control abilities in the nonverbal Stroop task is consistent with previous studies that have suggested an association between the BIS and conflict monitoring (e.g. Amodio et al., 2008). We have extended these findings to show that not only are BIS scores associated with conflict effects, but also that this relationship appears specific to the nonverbal domain. However, based on Gray (2001)'s findings, we had predicted that BIS trait sensitivity would be associated with smaller nonverbal Stroop conflict effects. That is, if the right hemisphere supports nonverbal processing and an avoidant motivational style, one might expect that individuals higher in BIS sensitivity might be facilitated on the nonverbal Stroop task. However, our results indicate that higher BIS trait sensitivity resulted in larger nonverbal Stroop conflict effects. What could explain this pattern of results? Previous research suggests that whereas the left hemisphere is specialized for local processing, the right hemisphere is specialized for global processing (e.g. van Kleeck, 1989; Volberg & Hübner, 2004). In light of these results, it is important to note that for the nonverbal Stroop task used in the current study, participants were instructed to report the direction of local motion (i.e. moving dots) in the face of interference from global motion (i.e. moving window). Thus, a more global processing style may lead to larger nonverbal Stroop conflict effects due to increased attention to the global, irrelevant dimension of the stimulus. Although this explanation seems plausible, it is post-hoc in nature and other explanations for this pattern of results are certainly possible.
4.2. Implications for Hemispheric Asymmetry
The association between Extraversion and verbal cognitive control ability, on the one hand, and the BIS and nonverbal cognitive control ability, on the other hand, is consistent with a large body of literature on hemispheric differences in affective and cognitive processing (e.g. Harmon-Jones & Gable, 2009; Morimoto et al., 2008; Smith & Jonides, 1999; Sutton & Davidson, 1997). As noted by Gray (2001), previous research suggests that hemispheric specialization for cognitive and affective processing may stem from differential distribution of neurotransmitter pathways across the right and left hemispheres. The greater activity of dopamine pathways in the left hemisphere (Glick, Ross, & Hough, 1982) has been associated with more tonic forms of attention as well as with Extraversion and the BAS (Depue & Collins, 1999; see Tucker & Williamson, 1984 for a review). The greater activity of norepinephrine and serotonin pathways in the right hemisphere (Gottfries, Perris, & Roos, 1974; Oke, Keller, Mefford, & Adams, 1978) has been associated with a global, receptive mode of information processing, as well as with Neuroticism and the BIS (Gray & McNaughton, 2000; Tucker & Williamson, 1984).
Based on these previous results, it is possible that greater dopamine activity in the left hemisphere underlies approach sensitivity and increased attention to task-relevant information, resulting in smaller conflict effects in the domain supported by the left hemisphere (i.e. verbal). In contrast, greater activity of the norepinephrine and serotonergic systems in the right hemisphere may underlie avoidance sensitivity and a more global mode of control, resulting in larger conflict effects (for the present task) in the domain supported by the right hemisphere (i.e. nonverbal). Although the current study was not designed to explicitly test this hemispheric hypothesis, it appears to be a plausible explanation for the present results and should be investigated in further research.
4.3. Methodological Comparisons with Previous Studies and Limitations
It is important to note that the majority of prior work on emotion-cognition interactions (e.g. Gray, 2001; Shackman et al., 2006) has employed tasks that tap several processing mechanisms. Our use of the Stroop task, which has been shown to load highly on an inhibition latent variable (e.g. Friedman et al., 2008; Miyake et al., 2000), in the current study allows for more specific conclusions to be drawn regarding the locus of impact of motivation on cognitive control ability. In terms of the personality assessments employed in the current study, self-reports have been suggested to serve as indirect measures of the constructs that they intend to assess (see Eisenberger, Lieberman, & Satpute, 2005). Although we acknowledge the limitations inherent in the use of self-report measures, it is important to note that the personality assessments used in the current study have been well validated.
Another potential criticism of the current study concerns psychometric matching of the verbal and nonverbal tasks. As evident in Table 2, the verbal Stroop task was characterized by a larger percentage RT conflict effect and lower reliability compared with the nonverbal Stroop task. Thus, despite our efforts to match the verbal and nonverbal Stroop tasks in terms of task demands, one potential limitation of the current study concerns the differential sensitivity of the two tasks. However, we feel that this would be cause for greater concern if it had been the case that all of our personality trait measures had been associated with only one task. Given that associations between different personality measures were found with both tasks, this suggests that the verbal and nonverbal Stroop tasks each possessed sufficient sensitivity.
5. Conclusions
In sum, we found that higher Extraversion trait sensitivity was associated with increased ability to ignore task-irrelevant information in the verbal domain. Higher BIS trait sensitivity was associated with decreased ability to ignore task-irrelevant information in the nonverbal domain. Thus, we have shown that personality traits are associated with individual differences in cognitive control abilities. These results serve to refine our understanding of the nature of the relationship between personality and cognition.
Acknowledgments
This research was supported by NIH Grants DC009209 and MH67008, and a Ruth L. Kirschstein predoctoral National Research Service Award (National Institute of Mental Health). We thank Clement Richard for developing the nonverbal Stroop script in Matlab, and we thank Jennifer DeSantis, Eric Mhyre, and Philip Cawkwell for assistance with data collection. We also thank Jennifer Faerber for assistance with statistical analyses.
Appendix
Figure A.1.
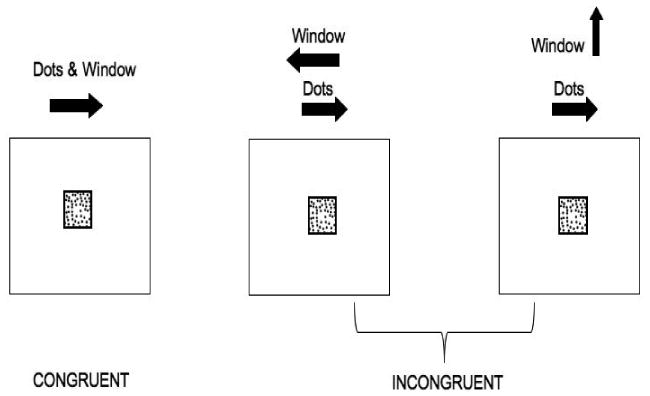
Sample congruent and incongruent trials for nonverbal Stroop task. For half of the incongruent trials, the window moved either up or down. Arrows and words are shown only to aid the reader, and were not presented to the participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Carver CS, Sutton SK, Scheier MF. Action, emotion, and personality: Emerging conceptual integration. Personality and Social Psychology Bulletin. 2000;26:741–751. [Google Scholar]
- Carver CS, White TL. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Thrash TM. Approach-Avoidance Motivation in Personality: Approach and Avoidance Temperaments and Goals. Journal of Personality and Social Psychology. 2002;82:804–818. doi: 10.1037//0022-3514.82.5.804. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Inventory. London: University of London Press; 1964. [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual Differences in Executive Function Are Almost Entirely Genetic in Origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Ross DA, Hough LB. Lateral asymmetry of neurotransmitters in human brain. Brain Research. 1982;234:53–63. doi: 10.1016/0006-8993(82)90472-3. [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Perris C, Roos BE. Visual averaged evoked responses (AER) and monoamine metabolites in cerebrospinal fluid (CSF) Acta Psychiatrica Scandanavia. 1974;255:135–142. doi: 10.1111/j.1600-0447.1974.tb08902.x. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. London: Oxford University Press; 2000. [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach-withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS. Integration of emotion and cognitive control: A neurocomputational hypothesis of dynamic goal regulation. In: Moore SC, Oaksford M, editors. Emotional cognition: From brain to behavior Advances in Conciousness Research. Vol. 44. Amsterdam: John Benjamins Publishing Company; 2002. pp. 289–316. [Google Scholar]
- Harmon-Jones E, Gable PA. Neural Activity Underlying the Effect of Approach-Motivated Positive Affect on Narrowed Attention. Psychological Science. 2009;20:406–409. doi: 10.1111/j.1467-9280.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morimoto HM, Hirose S, Chikazoe J, Jimura K, Asari T, Yamashita K, Miyashita Y, Konishi S. On Verbal/Nonverbal Modality Dependence of Left and Right Inferior Prefrontal Activation during Performance of Flanker Interference Task. Journal of Cognitive Neuroscience. 2008;20:2006–2014. doi: 10.1162/jocn.2008.20138. [DOI] [PubMed] [Google Scholar]
- Oke A, Keller R, Mefford I, Adams R. Lateralization of norepinephrine in human thalamus. Science. 1978;200:1411–1413. doi: 10.1126/science.663623. [DOI] [PubMed] [Google Scholar]
- Pomerantz JR. Global and Local Precedence: Selective Attention in Form and Motion Perception. Journal of Experimental Psychology: General. 1983;112:516–540. doi: 10.1037//0096-3445.112.4.516. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User's Guide. Pittsburgh: Psychology Software Tools Inc; 2002. [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working Memory: A View from Neuroimaging. Cognitive Psychology. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and Executive Processes in the Frontal Lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Miller GM, Engels AS, Herrington JD, Sutton BP, Banich MT, Heller W. Trait approach and avoidance motivation: Lateralized neural activity associated with executive function. NeuroImage. 2011;54:661–670. doi: 10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]
- Tucker DM, Williamson PA. Asymmetric Neural Control Systems in Human Self-Regulation. Psychological Review. 1984;91:185–215. [PubMed] [Google Scholar]
- Van Kleeck MH. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: new data and a meta-analysis of previous studies. Neuropsychologia. 1989;27:1165–1178. doi: 10.1016/0028-3932(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Volberg G, Hübner R. On the role of response conflicts and stimulus position for hemispheric differences in global/local processing: an ERP study. Neuropsychologia. 2004;42:1805–1813. doi: 10.1016/j.neuropsychologia.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative Affectivity: The Disposition to Experience Aversive Emotional States. Psychological Bulletin. 1984;96:465–490. [PubMed] [Google Scholar]
- Wilcox RR, Keselman HJ. Modern Robust Data Analysis Methods: Measures of Central Tendency. Psychological Methods. 2003;8:254–274. doi: 10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]


