Abstract
The Attention Network Test (ANT) assesses alerting, orienting, and executive attention. The current study was designed to achieve three main objectives. First, we determined the reliability, effects, and interactions of attention networks in a relatively large cohort of non-demented older adults (n = 184). Second, in the context of this aged cohort, we examined the effect of chronological age on attention networks. Third, the effect of blood pressure on ANT performance was evaluated. Results revealed high-reliability for the ANT as a whole, and for specific cue and flanker types. We found significant main effects for the three attention networks as well as diminished alerting but enhanced orienting effects during conflict resolution trials. Furthermore, increased chronological age and low blood pressure were both associated with significantly worse performance on the executive attention network. These findings are consistent with executive function decline in older adults and the plausible effect of reduced blood flow to the frontal lobes on individual differences in attention demanding tasks.
Keywords: Aging, Attention, Executive function, Blood pressure, Processing speed
Introduction
Three distinct attentional networks that carry out stimulus recognition and response initiation by maintaining a state of alertness (alerting), orienting to specific-sensory stimuli (orienting), and resolving conflict (executive attention; Fan, Fossella, Sommer, Wu, & Posner, 2003; Fan, McCandliss, Sommer, Raz, & Posner, 2002; Fan, McCandliss, Fossella, Flombaum, & Posner, 2005; Posner & Petersen, 1990; Posner & Rothbart, 2007; Posner, Sheese, Odludas, & Tang, 2006) have been identified. These networks vary in terms of their neuroanatomical substrates (Fan et al., 2002, 2005, 2007; Konrad et al., 2005; Niogi, Mukherjee, Ghajar, & McCandliss, 2010; Posner & Petersen, 1990) and associations with genetic polymorphisms (Fan et al., 2003; Fossella et al., 2002). Alerting, or preparing for a stimulus by establishing and maintaining a state of alertness (Posner & Petersen, 1990), is associated with frontal, parietal and thalamic activity, and with norepinephrine (Coull, Frith, Frackowiak, & Grasby, 1996; Marrocco, Witte, & Davidson, 1994; Posner & Petersen, 1990). Orienting, or the voluntary and involuntary selection of and shifting of attention toward the direction of an incoming stimulus (Posner & Petersen, 1990), is associated with activity in the superior and inferior parietal lobes, frontal eye fields, superior colliculus, pulvinar, and reticular thalamic nuclei (Corbetta & Shulman, 2002), and with acetylcholine (Davidson & Marrocco, 2000). Executive attention involves the detection and resolution of conflict in mental operations between brain regions, as well as the production of accurate behavioral responses (Posner & Petersen, 1990). Executive attention is associated with activity in the anterior cingulate cortex (ACC), medial frontal cortex (MFC), and lateral prefrontal cortex (LPFC), and with dopamine (Bush, Luu, & Posner, 2000; Fan et al., 2005; MacDonald, Cohen, Stenger, & Carter, 2000; Posner et al., 2006).
Age effects on individual attention networks have been examined using several experimental manipulations. Tales, Muir, Bayer, Jones, and Snowden (2002) used a reaction time (RT) task with visual alerting cues and reported comparable benefits of alerting in both young and old groups. Similarly, Nebes and Brady (1993) used a choice RT task with alerting cues and found intact phasic alerting in healthy aging and in Alzheimer's disease (AD) patients. Experiments using peripheral spatial cueing paradigms revealed comparable exogenous orienting in young and old adults (Folk & Hoyer, 1992; Hartley, Kieley, & Slabach, 1990; Lincourt, Folk, & Hoyer, 1997). Using the covert orienting of visual attention task to measure inhibition of return or reorienting, Danckert, Maruff, Crowe, & Currie (1998) and Hartley and Kieley (1995) reported preserved exogenous orienting in aging. Thus, there is evidence to suggest relatively intact alerting and orienting in older adults.
Executive attention is multi-faceted both theoretically and operationally (see Miyake, Friedman, Emerson, Witzki, Howerter, & Wager, 2000). Relevant to the current study is the flanker interference task (Hedge & Marsh, 1975; Simon, Sly, & Vilapakkam, 1981); an executive attention task used to measure conflict resolution or interference effects in older adults. Kramer, Humphrey, Larish, Logan, and Strayer (1994) reported comparable interference effects in young and old adults, while Zeef, Sonke, Kok, Buiten, and Kenemans (1996) found that older adults had larger interference effects than younger adults for target-flanker conditions which were presented in close proximity. In contrast, Madden and Gottlob (1997) revealed smaller interference effects in old as compared to young adults. Kramer and Kray (2006) report that discrepant age-related effects in interference tasks can be explained by stimulus characteristics, age-related differences in perceptual load, processing capacity, and inhibitory processes. It is noteworthy that beyond the flanker interference task, the effect of age on executive attention varies depending on the experimental paradigm used to operationalize it (Verhaeghen & Cerella, 2002).
The Attention Network Test (ANT) simultaneously assesses alerting, orienting, and executive attention, as well as their possible interactions (Fan et al., 2002, 2005; Posner & Rothbart, 2007; Raz & Buhle, 2006). There are three warning cue conditions (no, alert, or orient) that can precede each target stimulus. Alerting cues indicate that the target stimulus is about to appear (i.e., temporal cue). Orienting cues provide both temporal and spatial (i.e., location on the screen) information about the target stimulus. The target stimulus (i.e., central arrow) points either leftward or rightward and is surrounded by two flanker arrows on each side that provide either no, congruent, or incongruent information about the target stimulus. In the incongruent condition, flankers provide conflicting information that causes an interference that typically results in an increase in the time required to respond to the target, as compared to the congruent flanker condition (see Method-ANT section for details). This executive attention task requires the involvement of several brain regions because it requires online monitoring, detection, resolution of conflict, as well as the production of accurate behavioral responses which may be sensitive to aging and blood pressure dysregulation.
The ANT has been used in children ages 10 and younger (Rueda et al., 2004), in children with attention deficit hyperactivity disorder (ADHD) (Johnson et al., 2008), in adults ages 55 and younger (see Fan et al., 2002, 2005; Posner & Rothbart, 2007), and in adults with posttraumatic stress disorder (PTSD) (Leskin & White, 2007) and schizophrenia (Nestor, Kubicki, Spencer, Niznikiewicz, McCarley, & Shenton, 2007). However, investigations of the effects and interactions of these networks in non-demented older adults have been fairly limited and somewhat conflicting. Fernandez-Duque and Black (2006) studied the effects of alerting and orienting on conflict resolution in a small sample of young adults, non-demented older adults, and AD patients. Results from this study revealed an overall slowing of RTs in older as compared to younger adults and comparable attention network effects across all groups. Irrespective of AD, older adults experienced increased difficulty sustaining attention in the absence of alerting cues. Additional results revealed reduced benefit of alerting cues and no beneficial effects of orienting during conflict resolution across groups. Jennings, Dagenbach, Engle, and Funke (2007) found significant attention network effects and cue × flanker interactions for both alerting and orienting. However, these analyses did not adjust for speed of processing or other confounders. Significant reductions in the alerting network in old compared with young adults, with no reliable age differences in orienting or executive attention networks were also reported in a subset of analyses that controlled for speed of processing (Jennings et al., 2007).
The effect of health status on cognitive performance has long been identified (Libow, 1977; Schillerstrom, Horton, & Royall, 2005; Willis, Yeo, Thomas, & Garry, 1988). The need to differentiate between cognitive and biological changes related to aging versus chronic disease is paramount for the welfare and proper management of individuals (Albert, 1981). The Federal Interagency Forum on Aging-Related Statistics (2008) has reported hypertension as the most prevalent chronic disease in the United States for individuals ages 65 and older. Hypertension is a major risk factor for cardiovascular and cerebrovascular diseases, as well as poor cognitive performance and cognitive decline (Elias et al., 1997; Elias, Elias, Robbins, & Budge, 2004; Raz, Rodrigue, Kennedy, & Acker, 2007). However, low blood pressure (BP; i.e., hypotension) is also associated with poor health status (Hakala & Tilvis, 1998), cognitive impairment and decline (Glynn, Beckett, Hebert, Morris, Scherr, & Evans, 1999; Guo, Fratiglioni, Winblad, & Viitanen, 1997; Hestad, Kveberg, & Engedal, 2005; Launer, Masake, Petrovitch, Foley, & Havlik, 1995; Morris et al., 2002; Nilsson, Read, Berg, Johansson, Melander, & Lindblad, 2007), deficits in attention and working memory (Duschek, Matthias, & Schandry, 2005; Duschek & Schandry, 2007), and increased risk of dementia (Verghese, Lipton, Hall, Kuslansky, & Katz, 2003) in older adults.
While associations of systolic (SBP) and diastolic blood pressure (DBP) with cognitive function and dementia in the elderly have been inconsistent, the import of examining the role of BP on cognitive functioning has been recognized (Birns & Kalra, 2009; Hannesdottir, Nitkunan, Charlton, Barrick, MacGregor, & Markus, 2009; Nordahl, Ranganatha, Yonelinas, DeCarli, Reed, & Jagust, 2005; Oosterman, de Vries, & Scherder, 2007; Waldstein, Brown, Maier, & Katzel, 2005; Waldstein, Giggey, Thayer, & Zonderman, 2005; Waldstein & Katzel, 2005). Qiu, Windblad, and Fratiglioni (2005) summarized results from several late-life cross-sectional studies examining the relationship of BP levels with cognitive function into four categories: harmful effects of high BP; harmful effects of low BP; no effect of BP and; and U-shaped associations of BP with cognitive function. The relationship of abnormal BP levels, whether high or low, and cognition has been examined across various neuropsychological domains including response inhibition (Waldstein & Katzel, 2005), verbal memory (Nordahl et al., 2005; Waldstein, Brown, et al., 2005; Waldstein, Giggey, et al., 2005; Waldstein & Katzel, 2005), non-verbal memory (Nordahl et al., 2005; Waldstein, Brown, et al., 2005), executive functioning and attention (Hannesdottir et al., 2009; Oosterman et al., 2007), and psychomotor speed (Hannesdottir et al., 2009).
To date, however, the relationship between BP and attention networks has not been examined. Niogi et al. (2010) report that while distinct alerting, orienting, and executive attention networks exist, variations in white matter tract microstructure could modulate the efficiency of these attentional processes in very specific ways. The potentially differential relationship between BP and attention networks can be optimally determined using the ANT because visual input and motor output are the same across all trials. This is an advantage compared with studies that use separate neuropsychological measures that vary in terms of their input (e.g., visual vs. auditory) and output (oral vs. manual) modalities and demands to assess the relationship between BP and specific cognitive functions. An examination of the association, if any, between BP and the attention networks would provide a preliminary investigation of the possible biological underpinnings of ANT performance in aging.
Results from the Framingham Heart Study and others indicated that SBP was a better predictor of future cardiovascular events than DBP as people age (Franklin et al., 2001; Sever, 2009). Low SBP has been linked to alterations in cerebral white matter (e.g., leuko-araiosis) in older adults with dementia (Räihä, Tarvonen, Kurki, Rajala, & Sourander, 1993). Age, BP dysregulation, and hypertension are risk factors for leuko-araiosis and are associated with ischemic changes in small blood vessels (Pantoni & Garcia, 1997). Nordahl et al. (2005) studied patients diagnosed with mild cognitive impairment (MCI) and found that patients with white matter hyperintensities were severely impaired on tests of verbal and spatial memory, as well as attentional control compared with patients with hippocampal atrophy. These results suggest that small vessel cerebrovascular disease can result in working memory and executive control deficits. Meyer, Rauch, Rauch, and Haque (2000) reported an association between age-related changes in gray and white matter with cortical hypoperfusion, which in turn has been linked to poorly regulated BP. Severe leuko-araiosis and its relation to cognitive decline has been demonstrated in frontal brain regions of adults over age 60 and is associated with frontal cortical atrophy and cerebral hypoperfusion (Kawamura et al., 1993).
Functions mediated by the frontal and prefrontal cortex including executive attention function are among the first cognitive processes to decline with age (Albert & Kaplan, 1980; Dempster, 1992; Fuster, 1989; Raz et al., 1997; West, 1996). Studies assessing functional changes in the aging brain have shown significant reductions in regional cerebral blood flow in anterior relative to posterior cortical regions (i.e., hypofrontality; see Gur, Gur, Obrist, Skolnick, & Reivich, 1987; Melamed, Lavy, Bentin, Cooper, & Rinot, 1980). This age-related decrease in anterior blood flow has been demonstrated during executive tasks (Sorond, Schnyer, Serrador, Milberg, & Lipsitz, 2008), suggesting decreased task-specific efficiency of the frontal lobes. The effects of cerebral microvascular pathology on cognitive impairment (Cabeza, 2001; Farkas & Luiten, 2001; Pugh & Lipsitz, 2002; Sorond et al., 2008), has been linked to frontal cerebral hypoperfusion (Kawamura et al., 1993) and low SBP (Räihä et al., 1993). However, the association of BP with alerting, orienting, and executive attention networks has not been reported.
Current Study
The current study was designed to achieve three main objectives. First, we determined the reliability, effects, and interactions of attention networks, in a relatively large cohort of well-characterized non-demented older adults, while controlling for speed of processing (Salthouse, 1985). We predicted that interactions between cue and flanker types would reveal a diminished alerting and enhanced orienting effect during the incongruent flanker condition. Second, in the context of this aged cohort, we examined the effect of chronological age on attention network performance. Consistent with executive function decline in old age, we hypothesized that increased chronological age would be associated with worse executive attention. Third, in light of findings linking age-related decline in executive function (Sorond et al., 2008) to cerebral hypoperfusion (Kawamura et al., 1993) and low SBP (Räihä et al., 1993) in older adults, we examined the effects of BP on attention network performance. We hypothesized that low SBP would be associated with worse executive attention.
Method
Participants
Participants were enrolled in the Einstein Aging Study (EAS), a longitudinal study of aging and dementia located at the Albert Einstein College of Medicine in Bronx, New York. The study design, recruitment, and follow-up methods have been previously described elsewhere (Lipton et al., 2003; Verghese, Katz, Derby, Kuslansky, Hall, Lipton, 2004). Briefly, eligibility criteria require that participants be 70 years of age and older, reside in Bronx county, and speak English. Exclusion criteria include severe audiovisual disturbances that would interfere with completion of neuropsychological tests, significant loss of vision, inability to ambulate even with a walking aid or in a wheelchair, and institutionalization. Written informed consent was obtained at clinic visits according to study protocols and approved by the Committee on Clinical Investigation (CCI; the institutional review board of the Albert Einstein College of Medicine).
To address the decline in vision inherent in the aging population, we used a variant of the original ANT (Fan et al., 2002) that enhanced the size and luminosity of the stimuli (see the Procedures and Measures section). A total of 184 non-demented participants (see below) whose ANT performance was above 75% accurate, and who did not have a history of stroke were eligible to participate in the current experiment (see Table 1). The 75% accuracy cutoff, which only 4 potentially eligible participants did not meet (accuracy range = 30–69%), was designed to insure that abnormal performance did not influence ANT results.
Table 1. Summary of Participant Characteristics (n=184).
| Continuous Variables | Mean (SD) | Range | |
|---|---|---|---|
| Age, y | 80.41 (4.68) | (70.7 – 93.2) | |
| Education, y | 14.44 (3.18) | (5 – 21) | |
| Blessed Information Memory & Concentration Test | 1.48 (1.61) | (0 – 7) | |
| Global Health Status | 0.99 (0.86) | (0 – 5) | |
| Systolic BP | 134.65 (13.37) | (100 – 180) | |
| Diastolic BP | 77.27 (7.96) | (60 – 98) | |
| Categorical Variables | % [n] | ||
|
| |||
| Gender | |||
| Males | 39 [72] | ||
| Females | 61 [112] | ||
| Ethnicity | |||
| White | 75 [137] | ||
| African American | 21 [39] | ||
| Other | 4 [8] | ||
| Medical Condition | |||
| Angina | 6 [11] | ||
| Arthritis | 3 [6] | ||
| Chronic Heart Failure | 1 [1] | ||
| Chronic Obstructive Pulmonary Disease | 5 [9] | ||
| Depression | 10 [19] | ||
| Diabetes | 12 [22] | ||
| Hypertension | 58 [106] | ||
| Myocardial Infarction | 3 [6] | ||
| Parkinson's disease | 0 [0] | ||
| Prescribed BP Medication | 51 [94] | ||
| Systolic and Diastolic BP by group | Mean (SD) | % [n] | |
|
| |||
| Systolic BP by Group | |||
| SBP ≤ 120 mmHg | 118.06 (4.70) | 17 [32] | |
| SBP >120 mmHg | 138.14 (11.89) | 83 [152] | |
| Diastolic BP by Group | |||
| DBP ≤ 80 mmHg | 73.73 (5.91) | 72[133] | |
| DBP > 80 mmHg | 86.51 (4.41) | 28 [51] | |
| Neuropsychological Test Results | M | S.D. | Z-score |
|
| |||
| WAIS-III - Information | 19.70 | 5.11 | 0.99 |
| WAIS-III - Vocabulary | 47.31 | 10.62 | 0.67 |
| WAIS-III - Digit Span | 15.58 | 3.44 | 0.00 |
| WAIS-III - Digit Symbol | 48.58 | 13.41 | 0.33 |
| WAIS-III - Block Design | 26.01 | 9.23 | 0.00 |
| Free & Cued SRT Free Recall | 33.18 | 5.43 | 0.29 |
| Free & Cued SRT Total Recall | 47.78 | 0.79 | −0.23 |
| Boston Naming Test (15 item) | 12.47 | 1.99 | 0.35 |
| Controlled Oral Word Association Test | 40.60 | 12.67 | 0.46 |
| Category Fluency | 39.12 | 9.34 | 0.56 |
| Trail Making Test - A | 49.31 | 16.75 | 0.72 |
| Trail Making Test - B | 112.27 | 46.96 | 0.55 |
| Stroop Interference (T score) | 52.57 | 6.99 | 0.28 |
Cognitive Status and Function
Participants were determined to be cognitively normal based on established diagnostic case conference procedures (Holtzer, Verghese, Wang, Hall, & Lipton, 2008) that include neuropsychological tests for which robust longitudinal norms have been published (Holtzer, Goldin, Zimmerman, Katz, Buschke, & Lipton, 2008; see Table 1). Tests included in our clinical neuropsychology battery have been validated in previous studies of this aged population (Holtzer, Friedman, Lipton, Katz, Xue, & Verghese, 2007; Holtzer, Verghese, Xue, & Lipton, 2006; Masur, Sliwinski, Lipton, Blau, & Crystal, 1994; Sliwinski, Buschke, Stewart, Masur, & Lipton, 1997). The Blessed Information Memory Concentration Test (BIMCT; best score: 0 errors and worst score: 32 errors; Blessed, Tomlinson, & Roth, 1968), was also used to assess cognitive status. Participants who scored ≥ 8 on the BIMCT were not included in the current study. This test has high test–retest reliability (0.86) and correlates well with the pathology of Alzheimer disease (Fuld, 1978; Grober et al., 1999).
Blood Pressure (BP)
SBP and DBP measurements were recorded by trained registered nurses within the same session as the ANT. We implemented a standardized BP procedure in which trained research staff collected two BP readings in the morning, with a 2-min delay between each reading, from the right forearm after 5 min of rest in a sitting position. Systolic Korotkoff phase I and diastolic Korotkoff phase V were used as cutoff points. The mean of the two readings for both SBP and DBP was used for the current analyses. Based on recent BP level guidelines for the general population (JNC-7th Report; Chobanian et al., 2003) and for ease of interpretation, individuals were divided into two BP groups: low (SBP ≤ 120 mmHg and DBP < 80 mmHg) and high (SBP > 120 mmHg and DBP > 80 mmHg).
Global Disease Status
Trained research assistants used structured clinical interview and the study physician obtained medical history from multiple sources during the neurological examination. Consistent with our previous studies (Holtzer et al., 2006; Holtzer, Verghese, et al., 2008; Verghese, Wang, Lipton, Holtzer, & Xue, 2007) dichotomous rating (presence or absence) of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson's disease, chronic obstructive lung disease, angina, and myocardial infarction was used to calculate a global disease status summary score (range, 0–10).
Procedures and Measures
ANT
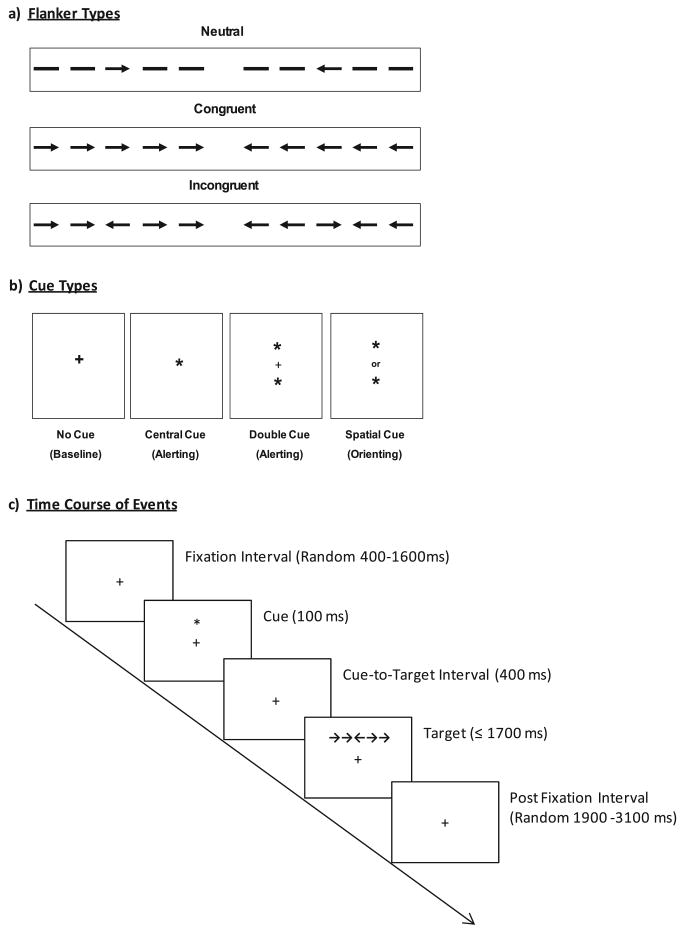
In the ANT, a target stimulus (i.e., central arrow) which points leftward or rightward is surrounded by two flankers on each side. There are three flanker types: neutral (dashes), congruent (arrows that point in the same direction as the central arrow), and incongruent (arrows that point in the opposite direction of the central arrow; see Figure 1a). There are also four types of warning cues that can precede each target: no, center, double, and spatial. The warning cue is an asterisk (*) that is presented in the center of the screen for the center cue, above and below central fixation for the double cue, and either above or below central fixation for the spatial (i.e., orient) cue (see Figure 1b). In the case of the no cue, the central fixation point (+) remains visible until the target is displayed. The no cue condition serves as the control, whereas the double and center cue conditions measure alerting, and the spatial cues measure orienting. Figure 1c depicts the time course of events. All target stimuli were centrally presented, bar the target stimuli that followed the orienting cues which were presented 1.06° above or below central fixation depending upon the location of the spatial cue. This visual angle of 1.06° above and below fixation also corresponded to the placement of the asterisks for the double and spatial cues.
Fig. 1.
Experimental design. A schematic of the three flanker types (a) and four cue types (b) that are used in the Attention Network Test. Notice that the double and center cue conditions measure alerting, while the spatial cue condition measures orienting. Executive attention is measured by comparing congruent and incongruent flanker types. Panel c delineates the time course of events. This figure appears with permission by the Massachusetts Institute of Technology. [Journal of Cognitive Neuroscience] Jin Fan, Bruce D. McCandliss, Tobias Sommer, Amir Raz and Michael I. Posner, “Testing the Efficiency and Independence of Attentional Networks”, 14:3 (April 1, 2002), pp. 340–347. © 2002.
In the current ANT paradigm, the diameter of the cues and the height of the arrows were increased from 0.32 cm to 0.64 cm. The width of the arrows was increased from 2.54 cm to 3.81 cm. In addition, the luminosity of the target and flanker stimuli was modified from 254.45 to 253.99 cd/m2 for the congruent and incongruent flanker conditions, and from 254.50 to 254.09 cd/m2 for the neutral flanker condition.
The participant's task was to report the direction of the central arrow by pressing the left mouse button for leftward arrows and the right mouse button for rightward arrows. Participants were required to fixate a central fixation cross and respond to each stimulus as quickly as possible without making errors. Participants received one practice block of 24 trials and were required to attain 75% accuracy or higher to proceed to three experimental blocks consisting of 96 trials each. Consistent with other studies (e.g., Fan et al., 2002, 2003), only correct responses were included in our statistical analyses.
Statistical Analyses
The Guttman split-half reliability test was used to assess the reliability of the ANT and specific flanker and cue types. Due to concerns of redundancy (see Fan et al., 2005), two-tailed Pearson correlations were used to determine whether the inclusion of neutral and congruent flankers, and double and center cue conditions was extraneous. The mean RTs for the neutral and congruent flankers, averaged across cues, were highly correlated (r = 0.96; p ≤ .01). The mean RTs for the double and center cues, averaged across flanker types, were also highly correlated (r = 0.97; p ≤ .01).
Each attention network is calculated using simple subtractions to determine the influence of alerting cues, orienting cues, and flankers (executive attention) on RTs (see Table 2). Individual median values were averaged across blocks for each condition to avoid the influence of outliers. While larger alerting and orienting network scores are indicative of faster cue-related performance, larger executive attention network scores are indicative of worse performance (i.e., longer RTs required for conflict resolution). Bi-variate correlations were used to examine the statistical independence of the alerting, orienting, and executive attentional networks.
Table 2. Summary of ANT Mean RT (SD) results by Flanker, Cue, and Network Type (n=184).
| Flanker Type | No | Cue Type | Flanker Mean | ||
|---|---|---|---|---|---|
| Center | Orient | Double | |||
| Incongruent | 820.12 (121.18) | 815.49 (133.17) | 758.50 (129.17) | 808.58 (131.47) | 800.67 (123.53) |
| Congruent | 711.55 (103.76) | 676.13 (105.48) | 652.96 (105.94) | 665.57 (107.02) | 676.55 (101.93) |
| Neutral | 696.61 (101.20) | 666.20 (105.66) | 644.67 (105.45) | 655.14 (101.79) | 665.65 (100.35) |
| Cue Mean | 742.76 (103.69) | 719.27 (109.38) | 685.38 (107.43) | 709.76 (107.71) | |
| Network Effect Calculations | |||||
| Network | Cue Type 1 - | Cue Type 2 | = Network Effect | ||
|
| |||||
| Alerting |
No 742.76 (103.69) |
Center 719.27 (109.38) |
Alerting 23.49 (30.50) |
||
| Orienting |
Center 719.27 (109.38) |
Orient 685.38 (107.43) |
Orienting 33.89 (31.53) |
||
| Executive Attention |
Incongruent 800.67 (123.53) |
Congruent 676.55 (101.93) |
Executive 124.12 (57.30) |
||
Neutral flanker and Double cue conditions were not used for Network Effect Calculations.
Linear Mixed Effects Models
The effects of attention networks and their interactions were assessed using two separate linear mixed effects models. Repeated-measures included in the models were a two-level flanker (congruent vs. incongruent) and a three-level cue (no, center, or orient). To address the issue of age-related decline in speed of processing (Salthouse, 1985) the first model controlled for overall RT averaged across all ANT trials. The second model was specifically designed to assess the effects and interactions of chronological age and BP group with attention networks, adjusting for overall RT, sex, education, global disease status, and BP medications.
Results
Demographics
Participants were community dwelling non-demented individuals with a mean age of 80.41(4.68) years who were relatively healthy (see Table 1 for details). The demographic characteristics did not vary based on SBP and DBP grouping (see Table 1) and were comparable to those reported for the source EAS sample at baseline (Holtzer, Verghese, et al., 2008). Of note, 58% of the participants reported a history of hypertension and 51% were currently prescribed antihypertensive medication.
ANT Results
On average, ANT performance accuracy was very high (97%), indicating that the participants understood the instructions and were able to reliably determine the direction of the target stimulus. Split-half reliability, as measured by the Guttman Split-Half Coefficient, revealed high correlations between the first and second half of all ANT trials (0.93), and more specifically, between the first and second half of trials separated by no cue (0.94), alerting cue (0.94), orienting cue (0.95), congruent flanker (0.97), and incongruent flanker (0.97). Table 2 depicts the mean RTs for each cue type, flanker type, and their corresponding overall mean. Calculation of the attention networks yielded effects for alerting 23.49 (SD = 30.50), orienting 33.89 (SD = 31.53), and executive attention 124.12 (SD = 57.30) ms. Two-tailed bi-variate correlations between alerting and executive attention (r = -0.07), and orienting and executive attention (r = -0.05) were not significant. However, the low, but significant correlation between the alerting and orienting networks (r = -0.27; p < .01) can be explained by the incremental effect of spatial as compared to alerting cues and has been recognized as an overlap in orienting and alerting attention networks (see Fan et al., 2002).
Results from the first linear mixed effects model (Table 3), which adjusted for speed of processing showed a main effect of flanker type (p < .0001), and revealed that RTs were significantly longer for incongruent (M = 800.67; SD = 123.53 ms) than for congruent (M = 676.55, SD = 101.93 ms) flankers. There was a main effect of alerting (p < .0001) and orienting (p < .0001) cues. The interaction between alerting × flanker type (p < .0001) was significant and revealed that relative to no cues, alerting cues enhanced performance in congruent (M = 35.42; SD = 40.30 ms) but not in incongruent (M = 4.63; SD = 62.21 ms; see Figure 2) trials. Conversely, the significant interaction of orienting × flanker revealed that relative to the center cues, orienting cues enhanced performance in incongruent (M = 56.99; SD = 56.10 ms) compared with congruent trials (M = 23.16; SD = 42.01 ms; see Figure 2).
Table 3. Model 1. Summary of ANT results using linear mixed effects models adjusted for overall reaction time, (n=184).
| Parameter | Beta | SE | t | 95% CI (lower, upper) | p |
|---|---|---|---|---|---|
| Alerting × Flanker | −30.79 | 6.12 | −5.03 | (−42.80, −18.78) | < 0.001 |
| Orienting × Flanker | −33.83 | 6.42 | −5.27 | (−46.44, −21.23) | < 0.001 |
| Alerting | 35.42 | 3.83 | 9.24 | (27.88, 42.96) | < 0.001 |
| Orienting | −23.16 | 4.06 | −5.70 | (−31.15, −15.17) | < 0.001 |
| Flanker (Executive Attention) | 139.36 | 4.45 | 31.30 | (130.61, 148.12) | < 0.001 |
| Overall RT | 0.98 | 0.01 | 85.35 | (−0.96, 1.00) | < 0.001 |
Fig. 2.
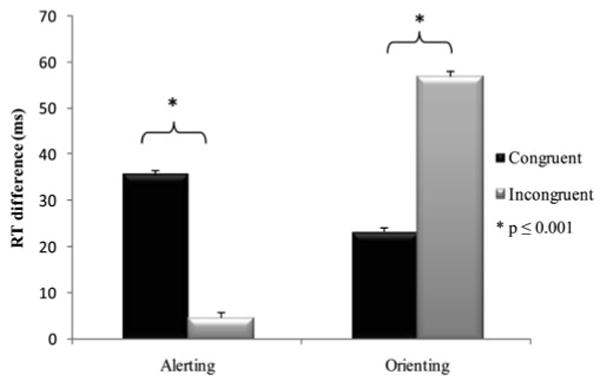
Alerting and orienting effects by flanker type. Mean RT difference (i.e., network effect) of alerting and orienting attention networks for congruent (black bars) and incongruent flankers (gray bars). Clear benefits of alerting and orienting are noticeable for congruent flankers. However, a significantly diminished alerting effect and a significantly enhanced orienting effect during conflict resolution trials (gray bars) is depicted.
A second linear mixed effects model controlling for overall RT, sex, education level, global disease status, and blood pressure medications was conducted specifically to examine the effects and interactions of chronological age and blood pressure with attention networks (see Table 4). Chronological age interacted with flanker type (p = .002), but not with alerting or orienting cues. As depicted in Figure 3, individuals ages 80 and above had a larger executive attention effect (131.17 ms; SD = 65.92) than individuals ages 70–79 (117.37; SD = 46.98).
Table 4. Model 2. Summary of ANT results using linear mixed effects models controlled for age, sex, education level, global health status, overall reaction time, and blood pressure (BP) medication (n=184).
| Parameter | Beta | SE | t | 95% CI (lower, upper) | p |
|---|---|---|---|---|---|
| Alerting × Flanker | −30.79 | 6.09 | −5.06 | (−42.74, −18.84) | < 0.001 |
| Orienting × Flanker | −33.83 | 6.41 | −5.28 | (−46.42, −21.25) | < 0.001 |
| Alerting | 40.38 | 51.55 | 0.78 | (−60.84, 141.60) | 0.434 |
| Orienting | −5.79 | 54.56 | −0.11 | (−112.91, 101.33) | 0.916 |
| Flanker (Executive Attention) | 20.03 | 44.36 | 0.45 | (−67.00, 107.07) | 0.652 |
| Age × Alerting | −0.10 | 0.64 | −0.15 | (−1.35, 1.16) | 0.881 |
| Age × Orienting | −0.12 | 0.67 | −0.17 | (−1.44, 1.21) | 0.862 |
| Age × Flanker | 1.67 | 0.55 | 3.06 | (0.60, 2.75) | 0.002 |
| Systolic BP Group × Alerting | 1.37 | 8.09 | 0.17 | (−14.52, 17.26) | 0.865 |
| Systolic BP Group × Orienting | −11.96 | 8.56 | −1.40 | (−28.77, 4.86) | 0.163 |
| Systolic BP Group × Flanker | −19.86 | 6.95 | −2.86 | (−33.50, −6.23) | 0.004 |
| Systolic BP Group | 14.58 | 6.46 | 2.26 | (1.88, 27.29) | 0.025 |
| Diastolic BP Group | −6.57 | 5.47 | −1.20 | (−17.33, 4.19) | 0.230 |
| Age | −0.67 | 0.52 | −1.29 | (−1.68, 0.35) | 0.197 |
| Sex | −0.86 | 2.67 | −0.32 | (−6.09, 4.38) | 0.749 |
| Education Level | −0.20 | 0.40 | −0.51 | (−0.98, 0.58) | 0.614 |
| Global Disease Status | −0.58 | 1.71 | −0.34 | (−3.94, 2.79) | 0.736 |
| Overall Reaction Time | 0.98 | 0.01 | 80.58 | (0.95, 1.00) | < 0.001 |
| BP Medication | −0.46 | 2.91 | −0.16 | (−6.17, 5.24) | 0.873 |
Note. Diastolic BP Group interactions with flanker and cue were not significant (data not shown but can be provided upon request).
Fig. 3.
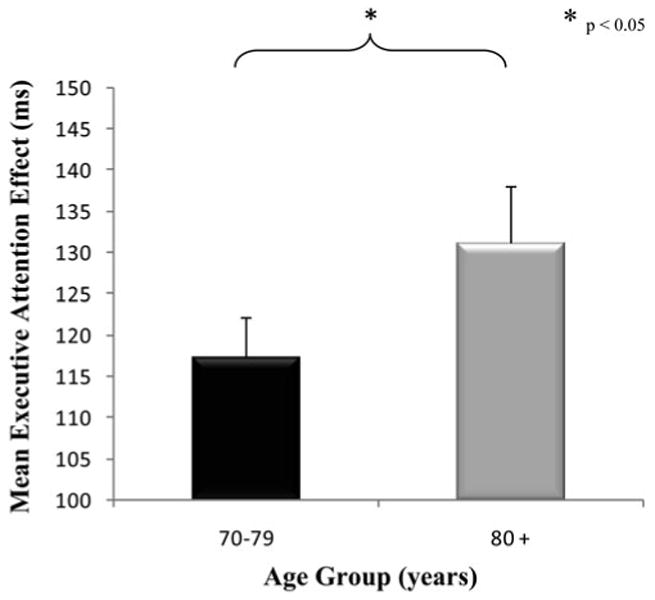
Executive attention by age group. For descriptive purposes, we separated individuals into two age groups: young-old (70–79 years old) and old-old (80+ years old). Mean executive attention network effects (±SE) for both age groups are displayed. Elders in the 70–79 age group performed significantly better on the flanker task, than elders in the 80+ age group. Thus, ability to resolve conflict significantly decreases with chronological age.
The main effect (p = .025) and interaction of SBP group with executive attention (p = .004; see Figure 4) were significant indicating that low SBP was associated with worse conflict resolution. The interactions of SBP with alerting and orienting were not significant. The main effect and interactions of DBP with the attention networks were not significant and are not reported in Table 4 because of space limitations (see the Discussion section). Additionally, the main effects of alerting, orienting, and executive attention networks were not significant; however, these main effects are explained by their interactions in the adjusted model.
Fig. 4.
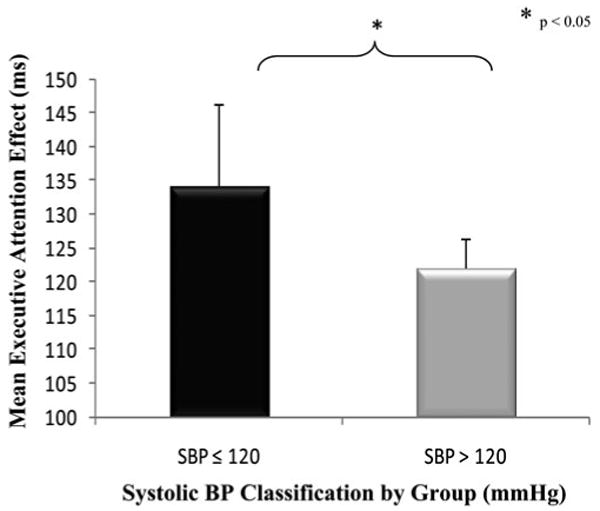
The effect of systolic blood pressure (SBP). Mean executive attention network effects (±SE) for elders in the systolic blood pressure ≤ 120 and >120 groups. Elders in the SBP ≤ 120 group performed worse on the flanker task, than elders in the SBP > 120 group. Ability to resolve conflict was significantly different for elders in these two SBP groups.
Discussion
This study demonstrated high reliability for the ANT and for specific cue and flanker types in older adults. As expected, participants were significantly faster at responding to congruent compared to incongruent flankers. When adjusting for overall speed of processing, significant alerting, orienting, and executive attention, network effects were found. The facilitating effects of cues on performance were influenced by flanker type. Specifically, alerting cues which offer no information regarding target location, facilitated performance only in congruent trials. In contrast, the facilitating effect of orienting cues on performance was enhanced during conflict resolution (Figure 2).
While this diminished alerting effect during conflict resolution trials of the ANT is noteworthy, it has been previously reported in older adults (Fernandez-Duque & Black, 2006; Jennings et al., 2007). Diminished alerting effects for incongruent flankers has also been reported in young adults and described as an enhanced flanker interference effect for alerting cues (Fan et al., 2002, 2009) and an inhibition of the executive attention network due to a highly active alerting network (Callejas, Lupianez, & Tudela, 2004; see also Posner, 1994). Furthermore, recent neuroimaging studies suggest that alerting and executive attention networks share common activation sites in both the ACC and the fronto-parietal network (Fan et al., 2007, 2009), suggesting that diminished alerting may be attributed to competition for attentional resources which are known to be limited in aging (Craik & Byrd, 1982).
In contrast, we found that orienting cues facilitated performance during incongruent trials. This enhanced orienting effect can be explained, in part, by the diminished alerting effect observed in incongruent trials. However, it is noteworthy that the orienting network is linked to the superior parietal regions and temporal parietal junction. These brain areas and networks are distinct from the fronto-parietal circuits implicated in executive attention. The recruitment of additional neural resources to enhance performance in conditions that maximize cognitive demands is consistent with compensatory reallocation (Cabeza, 2002; Cabeza, Anderson, Locantore, & McIntosh, 2002) or neural compensation (Stern et al., 2005) models of aging. The enhancement of orienting during conflict resolution can be attributed to the allocation of additional neural networks which aid in the facilitation of conflict resolution when provided with specific spatial cues.
In the context of this aged cohort, increased chronological age was associated with decreased ability to resolve conflict. This finding is contrasted to previous studies (Fernandez-Duque & Black, 2006; Jennings et al., 2007) and can be attributed to differences in sample size and methodological rigor. However, chronological age did not influence alerting or orienting. The differential effect of age on attention network performance is noteworthy given that visual input and motor output are the same across all ANT trial types. The relationship between chronological age and executive attention is consistent with previous work suggesting executive functions decline disproportionally with increasing age due to deteriorating prefrontal cortex function (see Albert & Kaplan, 1980; Dempster, 1992; Fuster, 1989; Raz et al., 1997; Sorond et al., 2008; West, 1996).
Individuals with low SBP demonstrated worse executive attention than individuals with normal to high SBP. This finding is consistent with previous work linking low SBP to leuko-araiosis and hypoperfusion (Meyer et al., 2000; Pantoni & Garcia, 1997; Räihä et al., 1993) which in turn have been linked to poor performance on executive function tasks (Kawamura et al., 1993; Sorond et al., 2008). Similar to previous studies (Franklin et al., 2001; Sever, 2009) DBP was not significant in the current study. Our findings suggest that the maintenance of optimal SBP levels in older adults may be crucial for optimal cognitive functions that depend on the frontal lobes. Low SBP has been associated with dementia (Guo, Viitanen, Fratiglioni, & Winblad, 1996) and mortality (Molander, Lovheim, Norman, Nordstrom, & Gustafson, 2008; Morris et al., 2000). Specifically, Molander et al. (2008) report that even after controlling for global health status and antihypertensive medicine, the highest mortality was observed in older adults with SBP ≤ 120 mmHg.
The finding that older adults with “normal” SBP levels, as defined by the JNC-7th report (Chobanian et al., 2003), demonstrate worse ability to resolve conflict in the current study may suggest that “normal” SBP range for individuals in their early-to-middle stages of life (<120 mmHg) may not be “normal” for adults in later stages of life. In support, Guo et al. (1997) revealed that SBP of at least 130 mmHg was crucial for the maintenance of cognitive function in the very old. More recently, Qiu et al. (2005) reported that the harmful cognitive effects of low BP in older adults may reveal that an appropriate level of BP is necessary to retain cognitive functioning in late-life by maintaining adequate cerebral perfusion, and furthers that such an optimal level still remains unidentified.
Limitations and Future Directions
A longitudinal design will be necessary to establish low SBP as a predictor of decline in executive attention. When used as a continuous measure SBP was not associated with executive attention. This may be attributed to low representation of more extreme BP values in this relatively healthy sample that did not allow us to assess threshold effects. However, continuous SBP may be associated with executive attention in larger and more heterogeneous samples. In addition, future neuroimaging studies will help validate the role of cerebral hypoperfusion and normal aging on executive attention effects. Visual-acuity was not formally tested in the current experiment. However, average ANT accuracy was at 97% indicating that participants were able to reliably determine the direction of the target stimulus. Results from the current study, as well as others (Fan et al., 2005) have reported a high-correlation between neutral and congruent flanker types, and central and double alerting cues in the ANT. Due to this redundancy, removal of extraneous flankers and alerting cues from the ANT paradigm seems appropriate.
Conclusion
In summary, the current study provided evidence in support of the effects, interactions, and reliability of attention networks in a relatively large cohort of non-demented older adults. We also found that increased chronological age and low blood pressure were both associated with significantly worse performance on the executive attention network.
Acknowledgments
The Einstein Aging Study is supported by the National Institute on Aging program project grant AGO3949. Dr. Holtzer is supported by the National Institute on Aging Paul B. Beeson Award K23 AG030857. The study authors have no financial or personal conflicts of interest related to this manuscript.
References
- Albert MS. Geriatric neuropsychology. Journal of Consulting and Clinical Psychology. 1981;49:835–850. doi: 10.1037//0022-006x.49.6.835. [DOI] [PubMed] [Google Scholar]
- Albert M, Kaplan E. Organic implications of neuropsychological deficits in the elderly. In: Poon LW, Fozard JL, Cermark LS, Arenberg S, Thompson LW, editors. New directions in memory and aging. Hillsdale, NJ: Lawrence Erlbaum Associates; 1980. pp. 403–429. [Google Scholar]
- Birns J, Kalra L. Cognitive function and hypertension. Journal of Human Hypertension. 2009;23:86–96. doi: 10.1038/jhh.2008.80. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: Contributions of functional neuroimaging. Scandinavian Journal of Psychology. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Callejas A, Lupianez J, Tudela P. The three attentional networks: On their independence and interactions. Brain and Cognition. 2004;54:225–227. doi: 10.1016/j.bandc.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Craik F, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Ceaik FIM, Trehub S, editors. Aging and cognitive processes. New York, NY: Plenum; 1982. pp. 191–211. [Google Scholar]
- Danckert J, Maruff P, Crowe S, Currie J. Inhibitory processes in covert orienting in patients with Alzheimer's disease. Neuropsychology. 1998;12:225–241. doi: 10.1037//0894-4105.12.2.225. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. Journal of Neurophysiology. 2000;83:1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Duschek S, Matthias E, Schandry R. Essential hypotension is accompanied by deficits in attention and working memory. Behavioral Medicine. 2005;30:149–158. doi: 10.3200/BMED.30.4.149-160. [DOI] [PubMed] [Google Scholar]
- Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clinical Autonomic Research. 2007;17:69–76. doi: 10.1007/s10286-006-0379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D'Agostino RB, Cupples LA, Wilson PW, Silbershatz H, et al. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: Does age make a difference? Hypertension. 2004;44:631–636. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Gu X, Guise KG, Liu X, Fossella J, Wang H, et al. Testing the behavioral interaction and integration of attentional networks. Brain and Cognition. 2009;70:209–220. doi: 10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, et al. Response anticipation and response conflict: An event-related potential and functional magnetic resonance imaging study. Journal of Neuroscience. 2007;27:2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Progress in Neurobiology. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics. Older Americans 2008: Key Indicators of Well-Being. [accessed September 10, 2009];Federal Interagency Forum on Aging-Related Statistics, National Center for Health Statistics. 2008 Retrieved from http://www.agingstats.gov/
- Fernandez-Duque D, Black SE. Attentional networks in normal aging and Alzheimer's disease. Neuropsychology. 2006;20:133–143. doi: 10.1037/0894-4105.20.2.133. [DOI] [PubMed] [Google Scholar]
- Folk CL, Hoyer WJ. Aging and shifts of visual spatial attention. Psychology and Aging. 1992;7:453–465. doi: 10.1037//0882-7974.7.3.453. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, et al. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- Fuld P. Psychological testing in the differential diagnosis of the dementias. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer's disease: Senile dementia and related disorders. Vol. 7. New York, NY: Raven Press; 1978. pp. 185–193. [Google Scholar]
- Fuster JM. The prefrontal cortex. 2nd. New York, NY: Raven Press; 1989. [Google Scholar]
- Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. The Journal of the American Medical Association. 1999;281:438–445. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, et al. Memory and mental status correlates of modified Braak staging. Neurobiology of Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Guo Z, Fratiglioni L, Winblad B, Viitanen M. Blood pressure and performance on the Mini-Mental State Examination in the very old. Cross-sectional and longitudinal data from the Kungsholmen Project. American Journal of Epidemiology. 1997;145:1106–1113. doi: 10.1093/oxfordjournals.aje.a009073. [DOI] [PubMed] [Google Scholar]
- Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: The Kungsholmen project. British Medical Journal. 1996;312:805–808. doi: 10.1136/bmj.312.7034.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M. Age and regional cerebral blood flow at rest and during cognitive activity. Archives of General Psychiatry. 1987;44:617–621. doi: 10.1001/archpsyc.1987.01800190037006. [DOI] [PubMed] [Google Scholar]
- Hakala SM, Tilvis RS. Determinants and significance of declining blood pressure in old age. A prospective birth cohort study. European Heart Journal. 1998;19:1872–1878. doi: 10.1053/euhj.1998.1232. [DOI] [PubMed] [Google Scholar]
- Hannesdottir K, Nitkunan A, Charlton RA, Barrick TR, MacGregor GA, Markus HS. Cognitive impairment and white matter damage in hypertension: A pilot study. Acta Neurologica Scandinavica. 2009;119:261–268. doi: 10.1111/j.1600-0404.2008.01098.x. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Kieley JM. Adult age differences of inhibition of return of visual attention. Psychology and Aging. 1995;10:670–683. doi: 10.1037//0882-7974.10.4.670. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Kieley JM, Slabach EH. Age differences and similarities in the effects of cues and prompts. Journal of Experimental Psychology. Human Perception and Performance. 1990;16:523–537. doi: 10.1037//0096-1523.16.3.523. [DOI] [PubMed] [Google Scholar]
- Hedge A, Marsh NW. The effect of irrelevant spatial correspondences on two- choice response-time. Acta Psychologica. 1975;39:427–439. doi: 10.1016/0001-6918(75)90041-4. [DOI] [PubMed] [Google Scholar]
- Hestad K, Kveberg B, Engedal K. Low blood pressure is a better predictor of cognitive deficits than the apolipoprotein e4 allele in the oldest old. Acta Neurologica Scandinavica. 2005;111:323–328. doi: 10.1111/j.1600-0404.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21:540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Goldin Y, Zimmerman M, Katz M, Buschke H, Lipton RB. Robust norms for selected neuropsychological tests in older adults. Archives of Clinical Neuropsychology. 2008;23:531–541. doi: 10.1016/j.acn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across- neuropsychological test variability and incident dementia. Journal of the American Medical Association. 2008;300:823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: Results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Dagenbach D, Engle CM, Funke LJ. Age-related changes and the attention network task: An examination of alerting, orienting, and executive function. Neuropsychology, Development, and cognition. Section B, Aging, Neuropsychology and Cognition. 2007;14:353–369. doi: 10.1080/13825580600788837. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Robertson IH, Barry E, Mulligan A, Daibhis A, Daly M, et al. Impaired conflict resolution and alerting in children with ADHD: Evidence from the Attention Network Task (ANT) Journal of Child Psychology and Psychiatry. 2008;49:1339–1347. doi: 10.1111/j.1469-7610.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- Kawamura J, Terayama Y, Takashima S, Obara K, Pavol MA, Meyer JS, et al. Leuko-araiosis and cerebral perfusion in normal aging. Experimental Aging Research. 1993;19:225–240. doi: 10.1080/03610739308253935. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, et al. Development of attentional networks: An fMRI study with children and adults. Neuroimage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: Beyond a unitary view of inhibitory processing in attention. Psychology and Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- Kramer AF, Kray J. Aging and Attention. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. New York, NY: Oxford University Press; 2006. pp. 57–69. [Google Scholar]
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. Journal of the American Medical Association. 1995;274:1846–1851. [PubMed] [Google Scholar]
- Leskin LP, White PM. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology. 2007;21:275–284. doi: 10.1037/0894-4105.21.3.275. [DOI] [PubMed] [Google Scholar]
- Libow LS. Senile dementia and psuedosenility: Clinical diagnosis. In: Eisdorfer C, Friedel RO, editors. Cognitive and emotional disturbance in the elderly. Chicago, IL: Year Book Medical Publishing; 1977. [Google Scholar]
- Lincourt AE, Folk CL, Hoyer WJ. Effects of aging on voluntary and involuntary shifts of attention. Aging, Neuropsychology, and Cognition. 1997;4:290–303. doi: 10.1080/13825589708256654. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, et al. Screening for dementia by telephone using the memory impairment screen. Journal of the American Geriatrics Society. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR. Adult age differences in strategic and dynamic components of focusing visual attention. Aging, Neuropsychology, and Cognition. 1997;4:185–210. [Google Scholar]
- Marrocco RT, Witte EA, Davidson MC. Arousal systems. Current Opinion in Neurobiology. 1994;4:166–170. doi: 10.1016/0959-4388(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- Melamed E, Lavy S, Bentin S, Cooper G, Rinot Y. Reduction in regional cerebral blood flow during normal aging in man. Stroke. 1980;11:31–35. doi: 10.1161/01.str.11.1.31. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rauch G, Rauch RA, Haque A. Risk factors for cerebral hypoperfusion, mild cognitive impairment, and dementia. Neurobiology of Aging. 2000;21:161–169. doi: 10.1016/s0197-4580(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Molander L, Lovheim H, Norman T, Nordstrom P, Gustafson Y. Lower systolic blood pressure is associated with greater mortality in people aged 85 and older. Journal of the American Geriatrics Society. 2008;56:1853–1859. doi: 10.1111/j.1532-5415.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- Morris MC, Scherr PA, Hebert LE, Bennett DA, Wilson RS, Glynn RJ, et al. The cross-sectional association between blood pressure and Alzheimer's disease in a biracial community population of older persons. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2000;55:M130–M136. doi: 10.1093/gerona/55.3.m130. [DOI] [PubMed] [Google Scholar]
- Morris MC, Scherr PA, Hebert LE, Bennett DA, Wilson RS, Glynn RJ, Evans DA. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology. 2002;21:123–130. doi: 10.1159/000054809. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB. Phasic and tonic alertness in Alzheimer's disease. Cortex. 1993;29:77–90. doi: 10.1016/s0010-9452(13)80213-4. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophrenia Research. 2007;90:308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: Longitudinal observations in a population-based sample 80 years and older. Aging Clinical and Experimental Research. 2007;19:41–47. doi: 10.1007/BF03325209. [DOI] [PubMed] [Google Scholar]
- Niogi S, Mukherjee P, Ghajar J, McCandliss BD. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Frontiers in Neuroanatomy. 2010;4:1–12. doi: 10.3389/neuro.05.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Ranganatha C, Yonelinas AP, DeCarli C, Reed BR, Jagust WD. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43:1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman JM, de Vries K, Scherder EJ. Executive ability in relation to blood pressure in residents of homes for the elderly. Archives of Clinical Neuropsychology. 2007;22:731–738. doi: 10.1016/j.acn.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: A review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Posner MI. Attention: The mechanisms of consciousness. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7398–7403. doi: 10.1073/pnas.91.16.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Posner MI, Sheese BE, Odludas Y, Tang Y. Analyzing and shaping human attentional networks. Neural Networks. 2006;19:1422–1429. doi: 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiology of Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Räihä I, Tarvonen S, Kurki T, Rajala T, Sourander L. Relationship between vascular factors and white matter low attenuation of the brain. Acta Neurologica Scandinavica. 1993;87:286–289. doi: 10.1111/j.1600-0404.1993.tb05509.x. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nature Reviews. Neuroscience. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed of behavior and its implication for cognition. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 2nd. Amsterdam: North-Holland; 1985. pp. 400–426. [Google Scholar]
- Schillerstrom JE, Horton MS, Royall DR. The impact of medical illness on executive function. Psychosomatics. 2005;46:508–516. doi: 10.1176/appi.psy.46.6.508. [DOI] [PubMed] [Google Scholar]
- Sever P. Is systolic blood pressure all that matters? Yes. British Medical Journal. 2009;339:b2665. doi: 10.1136/bmj.b2665. [DOI] [PubMed] [Google Scholar]
- Simon JR, Sly PE, Vilapakkam S. Effect of compatibility of S-R mapping on reactions toward the stimulus source. Acta Psychologica. 1981;47:63–81. doi: 10.1016/0001-6918(81)90039-1. [DOI] [PubMed] [Google Scholar]
- Sliwinski M, Buschke H, Stewart WF, Masur D, Lipton RB. The effect of dementia risk factors on comparative and diagnostic selective reminding norms. Journal of the International Neuropsychological Society. 1997;3:317–326. [PubMed] [Google Scholar]
- Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: Effects of healthy aging. Cortex. 2008;44:179–184. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cerebral Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tales A, Muir JL, Bayer A, Jones R, Snowden RJ. Phasic visual alertness in Alzheimer's disease and ageing. Neuroreport. 2002;13:2557–2560. doi: 10.1097/00001756-200212200-00035. [DOI] [PubMed] [Google Scholar]
- Verghese J, Katz MJ, Derby CA, Kuslansky G, Hall CB, Lipton RB. Reliability and validity of a telephone-based mobility assessment questionnaire. Age and Ageing. 2004;33:628–632. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61:1667–1672. doi: 10.1212/01.wnl.0000098934.18300.be. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: A review of meta-analyses. Neuroscience and Biobehavioral Reviews. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Brown JR, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Annals of Behavioral Medicine. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: The Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Katzel LI. Stress-induced blood pressure reactivity and cognitive function. Neurology. 2005;64:1746–1749. doi: 10.1212/01.WNL.0000161851.01243.62. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Willis L, Yeo RA, Thomas P, Garry PJ. Differential declines in cognitive function with aging: The possible role of health status. Developmental Neuropsychology. 1988;4:23–28. [Google Scholar]
- Zeef EJ, Sonke CJ, Kok A, Buiten MM, Kenemans JL. Perceptual factors affecting age-related differences in focused attention: Performance and psychophysiological analyses. Psychophysiology. 1996;33:555–565. doi: 10.1111/j.1469-8986.1996.tb02432.x. [DOI] [PubMed] [Google Scholar]