Abstract
The possible bioterrorism threat using the variola virus, the causative agent of smallpox, has promoted us to further investigate the immunogenicity profiles of existing vaccines. Here, we study for the first time the immunogenicity profile of a replication-competent smallpox vaccine (vaccinia Tiantan, VTT strain) for inducing neutralizing antibodies (Nabs) through mucosal vaccination, which is noninvasive and has a critical implication for massive vaccination programs. Four different routes of vaccination were tested in parallel including intramuscular (i.m.), intranasal (i.n.), oral (i.o.), and subcutaneous (s.c.) inoculations in mice. We found that one time vaccination with an optimal dose of VTT was able to induce anti-VTT Nabs via each of the four routes. Higher levels of antiviral Nabs, however, were induced via the i.n. and i.o. inoculations when compared with the i.m. and s.c. routes. Moreover, the i.n. and i.o. vaccinations also induced higher sustained levels of Nabs overtime, which conferred better protections against homologous or alternating mucosal routes of viral challenges six months post vaccination. The VTT-induced immunity via all four routes, however, was partially effective against the intramuscular viral challenge. Our data have implications for understanding the potential application of mucosal smallpox vaccination and for developing VTT-based vaccines to overcome preexisting antivaccinia immunity.
1. Introduction
Smallpox, which was caused by infectionwith the variola virus, was one of the most deadly diseasesin human history with a mortality rate of up to 50% [1, 2]. Fortunately, this disease was completely eradicated throughout the world by 1980 after the introduction of a global smallpox vaccination campaign [3]. During this process, vaccinia virus, which shares broad antigenic properties with the variola virus, played an essential role as an effective vaccine in inducing protective immunity against smallpox [3–5]. It is well accepted that vaccine-induced neutralizing antibodies are critical to protection [6–8]. The fear of variola virus being deliberately released in potential bioterrorism attacks and the increasing use of vaccinia as vaccine vectors for other diseases such as AIDS have led to more recent studies aimed at understanding the protective immune responses induced by the smallpox vaccine [9, 10]. It is, therefore, necessary to investigate the neutralizing antibody responses induced by historically used smallpox vaccines via different route of vaccination.
The most extensively used smallpox vaccine in China was the vaccinia virus Tiantan (VTT) strain [11]. Accordingly, the original VTT was isolated from the skin lesion of a smallpox patient in China around 1926 followed by extensive passages of 3 times in monkeys, 5 in rabbits, 3 in bovines, then twice in rabbits and 3 times in bovines, repeatedly [12, 13]. Genetic analysis of VTT genome, however, suggested that it is a vaccinia strain instead of a variola viral variant [14]. The clinical safety of this vaccine has not been clearly documented although VTT was historically used for millions of people. These issues are essential for a safe smallpox vaccine [15]. The biological characteristics of VTT have been described in our recent studies [16, 17]. It was reported that VTT caused larger lesions after intradermal vaccination and was likely more virulent than other widely used smallpox vaccines such as Lister or Wyeth [12, 18]. To date, whether or not VTT can induce protective neutralizing antibody responses through noninvasive mucosal vaccination remains less understood [19, 20].
Here, we study VTT to investigate its immunogenicity in terms of inducing neutralizing antibodies through four different routes of vaccination in a mouse model, which has not been previously studied. Moreover, by conducting homologous and heterologous routes of viral challenges, we aimed to determine the efficacy of VTT for protection and to identify a strategy to overcome preexisting immunity to VTT-based vaccines. This study involved a safe, nonpathogenic viral challenge model using a high dose of modified VTT, namely, MVTT-S, which expresses the spike (S) glycoprotein of SARS-CoV. Since S is not expressed on the surface of vaccinia virus, we aimed to determine the role of anti-VTT neutralizing antibody (Nab) responses in achieving protection by evaluating the seroconversion to S. Our results have implications for understanding an aspect of vaccinia-induced protective immunity and for developing vaccinia-based vaccines.
2. Materials and Methods
2.1. Virus Stock and Cell Line
The background and biological properties of the smallpox vaccine vaccinia Tiantan (VTT) have been described previously [16]. VTT stocks were propagated in Vero cells and then purified by repeated freezing and thawing and centrifugation through a 36% sucrose cushion. The viral pellet was subjected to a sucrose gradient centrifugation. Purified viruses were collected and studied [21]. The plaque forming unit (PFU) of viral stocks was titrated on Vero cells by a plaque-forming assay using crystal violet staining. The construction and characterization of the challenge virus MVTT-S have been described previously [17].
2.2. Immunization of Mice
Five groups of female BALB/c mice were included in the study. Each group of nine mice was inoculated with an optimal dose of 106 PFU VTT through one of four different routes including intramuscular (i.m.), intranasal (i.n.), oral (i.o.), and subcutaneous (s.c.) inoculations, respectively, [17]. Another group of nine mice received a placebo as controls. To measure lasting Nab responses, mice were kept for six months for observation and testing.
2.3. Viral Challenge of VTT-Immunized Mice
After six months of observation, each group of mice was further divided into three subgroups. Each subgroup of three mice was challenged with a dose of 2 × 106 PFU MVTT-S through one of three different routes including i.m., i.n., and i.o., respectively. It is the highest dose possible for mucosal challenge based on the concentration of our viral stocks and the size of animals. After a three-week interval, all animals were challenged again with the same dose of 2 × 106 PFU MVTT-S. Blood samples were collected on week 3, week 26, week 29, and week 32 for measuring Nab responses after MVTT-S challenge. Our animal protocols were approved by the Institutional Committee on Laboratory Animal Use of Wuhan University. Since MVTT-S is an attenuated VTT variant which is not lethal in mice, we define the full protection by the lack of detectable anti-S Nab response induced by the challenge virus MVTT-S in this study.
2.4. Anti-S Neutralizing Assay
A pseudovirus-based neutralization assay was applied to determine the humoral immune responses against the S glycoprotein of SARS-CoV [22]. The pseudotype virus was generated by cotransfecting 293T cells with two plasmids pcDNA-Sopt9 and pNL4-3Luc+Env−Vpr− carrying the optimized S gene and a human immunodeficiency virus type 1 backbone, respectively. For each experiment, pooled sera, which contain an equal amount of serum of each vaccinated mouse in each group, were subjected to the assay as described by others [23]. Luciferase activity was measured by the PerkinElmer Victor Microplate Reader according to manufacturer's instruction. In comparison to serum samples of unvaccinated mice in the same experiments, we score seropositive when an IC50 value reaches a serum dilution factor of 1 : 30. An IC50 value is a serum dilution factor that achieves 50% of viral inhibition.
2.5. Anti-VTT Neutralizing Assay
To test the serum neutralization activity against VTT, we used a flow-cytometry-based assay similar to a previously published method [24]. For this assay, a modified VTT that contains a GFP (MVTT-GFP) expression cassette was constructed [14]. Briefly, the neutralizing activity of heat-inactivated sera (56°C, 30 min) was determined by mixing 1.25 × 106 PFU/mL of VTT-GFP virus in 20 μL with an equal amount of diluted serum at 37°C, for 1 h. After the incubation, 60 μL of the 293T cells were added into each well of the plate and incubated at 37°C. 293T cells were diluted into 2.5 × 106/mL using culture medium containing 10% FBS, 1% streptomycin and penicillin, and 44 μg of cytosine arabinoside per mL. After 24 hr incubation, the infected cells were washed with PBS twice and then trypsinized (50 μL/well trypsin). The cells were pelleted, washed, and then fixed with 200 μL of 2% paraformaldehyde, and subsequently subjected to the FACS analysis. The titer of viral neutralization was determined by calculating the percent reduction of infected cells showing GFP positive expression. Similarly, an IC50 value is a serum dilution factor that achieves 50% of viral inhibition.
2.6. Statistical Analyses
The Student's t-test was used to evaluate the statistical significance of Nab titers among various groups of animals. The Origin 8 computer software was used for this analysis.
3. Results
3.1. Higher Levels of Anti-VTT Nabs Were Induced via the i.n. and i.o. Inoculations When Compared with the i.m. and s.c. Routes
In this study, we sought to determine whether VTT would induce different levels of Nabs via various routes of vaccination. This is a critical question because VTT was historically used as a smallpox vaccine for millions of people yet its potential use as a noninvasive vaccine remains elusive. To address this question, four groups of mice were inoculated with an optimal dose of 106 PFU VTT via s.c, i.m., i.n., and i.o. routes, respectively. Another group of mice received a placebo as controls. Three weeks after inoculation, the animals were tested for the production of anti-VTT Nabs using a newly developed FACS-based MVTT-GFP neutralization assay (Figure 1(a)) [24]. Since the sample quantity is small, pooled sera were tested for each experimental group. The pooled sera contain an equal amount of serum of individualmicein eachgroup. We found that each route of vaccination was able to induce anti-VTT Nab responses after one-time vaccination (Figure 1(b)). The levels of anti-VTT Nabs, however, varied depending on the route of vaccination. Apparently, higher levels of Nabs were induced via the i.n. and i.o. inoculations when compared with the i.m. and s.c. routes. The serum dilution factors based on the IC50 value via the different routes (i.n., i.o., i.m., and s.c.) were 1 : 711, 1 : 2370, 1 : 428, and 1 : 32, respectively.
Figure 1.
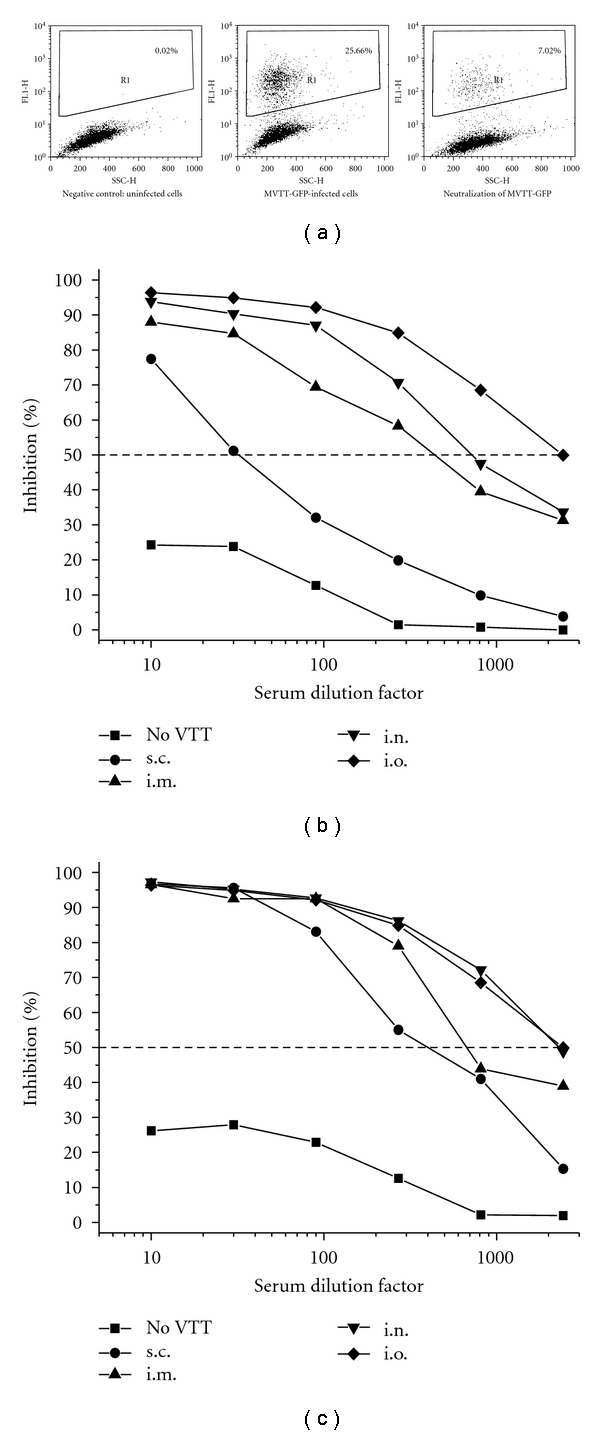
Anti-VTT neutralizing antibody responses after the VTT vaccination. (a) A FACS-based neutralization assay was newly developed to measure the anti-VTT neutralizing antibody responses. (b) Serum samples were tested three weeks after the VTT vaccination. (c) Serum samples were tested six months after the VTT vaccination. Due to a limited amount of serum collected from each mouse, pooled sera, which contain an equal amount of serum of each vaccinated mouse in each group, were subjected to each experiment. The X-axis represents the serum dilution factor whereas the Y-axis indicates the percentage of viral inhibition. An IC50 value is a serum dilution factor which achieves 50% of viral inhibition as indicated by the dashed horizontal line. The experiment was repeated twice with similar results obtained. Placebo (no VTT) mice were tested as well.
3.2. Mucosal Vaccination Also Induced Higher Sustained Levels of Nabs over time
In order to measure the long-lasting levels of Nabs generated, we kept separate groups of the vaccinated animals for six months. Despite the virulent effects of VTT (e.g., body weight changes) during the acute phase of vaccination, all mice survived for continuous observation. After six months of the vaccination, serum samples were collected and subsequently subjected to the same neutralization assay. Interestingly, although the IC50 was not significantly improved for the i.o. group, the rest of three groups of mice, especially the i.n. group, developed higher levels of lasting antivaccinia Nabs when compared to the early time point (Figures 1(b) and 1(c)), suggesting a prolonged VTT-antigen stimulation to immune system. The serum dilution factors based on the IC50 value via the different routes (i.n. and i.o. versus i.m. and s.c.) were 1 : 2265 and 1 : 2397 (average 1 : 2331) versus 1 : 671 and 1 : 387 (average 1 : 529), respectively (Table 1). Furthermore, higher levels of long-lasting antivaccinia Nabs were consistently found in the i.n. and i.o. groups when compared with the i.m. and s.c. groups (Figure 1(c)). The average IC50 value of mucosal groups (i.n. and i.o.) is about 4.4-fold higher than that of i.m. and s.c routes (1 : 2331 versus 1 : 529, P < .01). Randomly selected individualmiceof each group were tested with consistent results obtained.
Table 1.
Neutralizing antibody titer from different time points after VTT vaccination and MVTT-S Challenges.
| Groups | i.m. | i.n. | i.o. | s.c. | placebo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination | ||||||||||
| VTT (3 weeks) VTT (6 months) |
NA NA |
428 671 |
NA NA |
711 2265 |
NA NA |
2370 2397 |
NA NA |
32 387 |
NA NA |
ND ND |
| MVTT-S Challenge | ||||||||||
| i.m. (1st) i.m. (2nd) i.n. (1st) i.n. (2nd) i.o. (1st) i.o. (2nd) |
ND 2732 ND ND ND ND |
3288 6280 2065 1587 4555 2362 |
ND 9369 ND ND ND ND |
5249 5210 5141 2944 4937 5148 |
ND 936 ND ND ND ND |
4742 4721 6838 6205 2601 4901 |
ND 1598 ND 1026 ND 2710 |
333 1386 520 350 35 221 |
ND 14261 ND 8251 ND 21523 |
46 718 349 1004 101 1014 |
The values of anti-S IC50 are in bold whereas the values of anti-VTT IC50 are not. NA: not applied; ND: not detected.
3.3. Protection against Homologous Routes of Mucosal MVTT-S Challenges
To determine the long-lasting efficacy of the four routes of VTT vaccination in parallel, ten groups of vaccinated mice were challenged with MVTT-S six months after the vaccination. Each mouse received 2 × 106 PFU of MVTT-S, the highest possible dose based on our viral stocks, via the i.n. or i.o. routes, respectively, which mimic the natural transmission of the variola virus. Since no anti-S antibodies were detected three weeks after challenge in all groups, we subsequently gave another MVTT-S challenge at the same dose in the same way. We found that i.o. and i.n. VTT vaccinations protected mice significantly against the homologous i.o. and i.n. MVTT-S challenges, respectively, without anti-S antibodies detected after the second challenge (Figure 2(a) and Table 1). The lack of seroconversion to S serves as a biomarker of the protection against MVTT-S infection.
Figure 2.
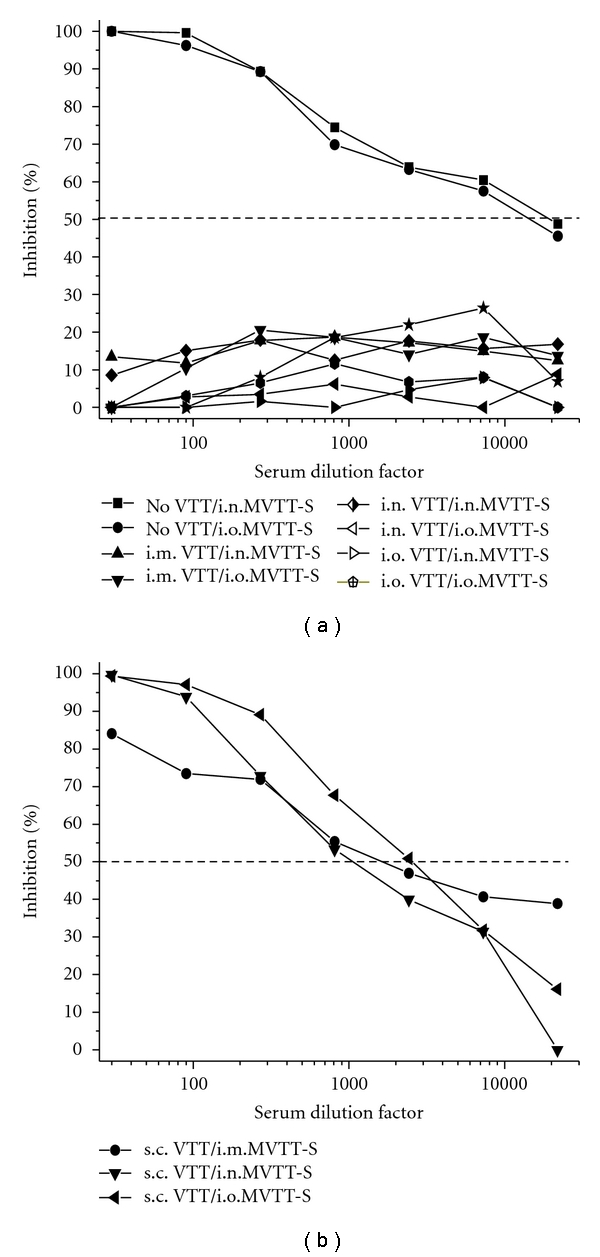
Anti-S neutralizing antibody responses in VTT-vaccinated mice after two MVTT-S challenges. (a) Serum samples of i.m., i.n., and i.o. VTT-vaccinated as well as of placebo-inoculated (no VTT) mice were tested. (b) Serum samples of s.c. VTT-vaccinated mice were tested. Pooled sera, which contain an equal amount of serum of each vaccinated mouse in each group, were subjected to each experiment. The X-axis represents the serum dilution factor whereas the Y-axis indicated the percentage of viral inhibition. The dashed horizontal line indicates IC50 values. The experiment was repeated twice with similar results obtained.
3.4. Protection against Alternating Routes of Mucosal MVTT-S Challenges
We then analyzed other groups of animals. We found that i.o. and i.n. VTT vaccinations protected mice completely against heterologous routes of i.n. and i.o. MVTT-S challenges, respectively, without any detectable anti-S Nabs (Figure 2(a)). In contrast, MVTT-S challenges resulted in high levels of anti-S Nabs in VTT-naïve or nonvaccinated mice after the second challenge (Figure 2(a)). The IC50 values reached over 1 : 10,000 serum dilution titer. Moreover, i.m. VTT vaccination also protected mice completely against i.n. and i.o. MVTT-S challenges, respectively (Figure 2(a)). Here, the lack of detectable anti-S Nabs after two consecutive MVTT-S challenges likely suggests a possible complete protection (Figure 2(a)). Notably, the s.c. VTT vaccination did not completely protect mice against i.n. or i.o. MVTT-S challenges as anti-S antibodies were readily detected three weeks after the second challenge (Figure 2(b)). Since the mean anti-S IC50 titer of all s.c. vaccinated mice was significantly lower than that of all nonvaccinated controls (1 : 1778 versus 1 : 14678, P = .03) (Figures 2(a) and 2(b)), we speculated that at least partial significant protection was achieved by the s.c. VTT vaccination. This P = .03 value was determined by comparing the anti-S mean value of the s.c. vaccinated group (1 : 1598, 1 : 1026, and 1 : 2710, with an average value of 1 : 1778) versus control group (1 : 14261, 1 : 8251, and 1 : 21523, with an average value of 1 : 14678). It is possible that the less protection of s.c. vaccinated mice is related to lower levels of anti-VTT neutralizing antibodies induced (Figure 1(a) and Table 1). Since MVTT-S causes transient viremia and infection in mice as we recently described [19], our efforts to detect live challenge virus around three weeks after each challenge in all groups were unsuccessful.
3.5. Lack of Complete Protection against Intramuscular MVTT-S Challenges
Since mucosal VTT vaccination induced likely full protection against homologous and heterologous routes of mucosal MVTT-S challenges (Figure 2(a)), we sought to determine whether the protection would apply to a systemic route of viral challenge. For this purpose, another three groups of VTT-vaccinated mice were challenged with MVTT-S via the i.m. route also at six months postvaccination. Similarly, no detectable levels of anti-S Nabs were found three weeks after the first MVTT-S challenge, suggesting a protective role of VTT-induced immunity. However, we found that the second MVTT-S challenge was able to generate substantial levels of anti-S Nabs in all three groups (Figure 3). Since the levels of anti-S Nabs (both IC90 and IC50 values) were lower in three vaccinated groups compared to the placebo nonvaccinated group, it is likely that i.m., i.n., and i.o. vaccination conferred partial significant protection against MVTT-S challenges. For example, the serum dilution factors based on the IC50 value via different routes of prevaccination (placebo > i.n., i.o., or i.m.) were 1 : 14261 > 1 : 9369, 1 : 936, or 1 : 2732, respectively (Table 1 and Figure 3).
Figure 3.
Anti-S neutralizing antibody responses in VTT-vaccinated mice after two i.m. MVTT-S challenges. Serum samples collected three weeks after the second MVTT-S challenge were tested in this experiment. Pooled sera, which contain an equal amount of serum of each vaccinated mouse in each group, were subjected to each experiment. The X-axis represents the serum dilution factor whereas the Y-axis indicated the percentage of viral inhibition. The dashed horizontal line indicates IC50 values. The experiment was repeated twice with similar results obtained. As controls, no VTT represents mice, who did not receive VTT-vaccination.
Since substantial levels of anti-VTT Nab responses were induced by a one time VTT vaccination via four different routes (Figure 1(b)), it was puzzling why these Nabs were not able to block i.m. MVTT-S infections completely. For this reason, we speculated that there might be a difference in the recalled anti-VTT Nab responses after the MVTT-S challenges. Therefore, we measured the levels of anti-VTT Nab responses after each of the two MVTT-S challenges. We found that the first MVTT-S challenge boosted the levels of anti-VTT Nabs significantly, confirming its shared neutralizing determinants with VTT (Figure 4(a)). The average serum dilution factor of antivaccinia Nabs among i.m., i.n., and i.o. VTT-vaccinated mice had reached comparable levels with IC50 values of 1 : 3303, 1 : 5109, and 1 : 4727, respectively, which is not statistically significant (Figure 4(a)). This P value was calculated from the average values of anti-VTT Nabs after the first challenge from three groups: i.m. (1 : 3288, 1 : 2065, and 1 : 4555, with an average of 1 : 3303), i.n. (1 : 5249, 1 : 5141, and 1 : 4937, with an average of 1 : 5109), and i.o. (1 : 4742, 1 : 6838, 1 : 2601, with an average of 1 : 4727). In addition, we determined the anti-VTT Nab response after the second challenge of MVTT-S. We found that the average serum dilution titer of anti-VTT Nabs among i.m., i.n., and i.o. pre-VTT-vaccinated mice had likely saturated at comparable levels with the IC50 values of 1 : 3410, 1 : 4434, and 1 : 5276, respectively, which is not statistically significant (Figure 4(b)). This P value was calculated from the average values of anti-VTT Nabs after the second challenge from three groups: i.m. (1 : 6280, 1 : 1587, and 1 : 2362, with an average of 1 : 3410), i.n. (1 : 5210, 1 : 2944, and 1 : 5148, with an average of 1 : 4434), and i.o. (1 : 4721, 1 : 6205, and 1 : 4901, with an average of 1 : 5276). Apparently, these levels of the recalled anti-VTT Nabs were sufficient to block completely the mucosal (i.n. and i.o.) but not the systemic (i.m.) MVTT-S challenges, suggesting a likely difference between mucosal and systemic immunity.
Figure 4.
Anti-VTT neutralizing antibody responses after the first (a) and the second (b) MVTT-S challenges. Serum samples collected three weeks after each of the two MVTT-S challenges were included in this experiment. Pooled sera, which contain an equal amount of serum of each vaccinated mouse in each group, were subjected to each experiment. The X-axis represents the serum dilution factor whereas the Y-axis indicates the percentage of viral inhibition. The dashed horizontal line indicates IC50 values. The experiment was repeated twice with similar results obtained. As controls, no VTT represents mice, who did not receive VTT-vaccination.
4. Discussion
The smallpox vaccination, which involves live vaccinia virus, remains a gold standard of vaccines because it has led to the complete eradication of a deadly human viral disease. To our knowledge, this study is the first to provide a parallel experimental comparison of four different routes of VTT vaccination for inducing protective efficacy in a mouse model. We provide evidence that one-time vaccination with VTT through each of four different routes was able to induce long-lasting antivaccinia Nab responses in mice. Apparently, higher levels (4.4-fold) of the Nab responses were induced via the mucosal i.n. and i.o. inoculations when compared with the i.m. and s.c. routes. Moreover, the i.n. and i.o. VTT vaccination likely protected animals completely from two consecutive mucosal challenges of the MVTT-S strain via either homologous (i.n. and i.o.) or heterologous (i.o. and i.n.) routes, respectively, (Figure 2(a)). As controls, s.c. VTT vaccination did not achieve full protection against i.m., i.n., or i.o. MVTT-S challenges, respectively, (Figure 2(b)). While i.m. VTT vaccination fully protected animals from i.n. and i.o. MVTT-S challenges, i.m., i.n., and i.o. VTT-vaccinated animals were not fully protected from i.m. challenges (Figures 2(a) and 3). Our results, therefore, suggested that the route of VTT vaccination is critical for inducing protection in mice. In the meantime, the finding that i.m. MVTT-S challenge overcomes preexisting antivaccinia immunity induced via mucosal routes has implications for MVTT-based mucosal vaccine development.
The route of VTT vaccination determines the level of long-lasting antivaccinia Nabs induced. It has been demonstrated that Nabs induced by vaccinia vaccine play an essential role in providing sterile immunity against smallpox infections [25, 26]. Here we show that VTT displays an advantage for inducing higher levels of antivaccinia Nabs through the noninvasive mucosal route of vaccination. The underlying mechanism of this finding remains largely unknown, although we have recently provided evidence that the tissue tropism may play a role in affecting the immunogenicity of a vaccinia-based vaccine [19]. Since VTT and its variants unlikely establish long-term persistent infection in mice, mechanisms for the maintenance of immunological memory for lasting Nabs remain to be further investigated [8]. One study showed that antigen can be retained in the draining lymph nodes for extended periods of time [27]. Whether this finding would have implications for the continuous maturation of VTT-induced Nabs observed in our study will require future studies. We acknowledge that different route of vaccination may have different protective effects. We did not conduct a direct comparison between intradermal (i.d.) vaccination with other routes because i.d. vaccination did not seem to induce high levels of Nabs in our previous studies [17]. We, however, will compare the skin scarification vaccination with other routes in our future studies as it conferred sterile protection in humans against smallpox infection historically.
Traditional VV challenge models involved the use of highly pathogenic and lethal dose viruses for challenges, which require special biosafety containments. In this study, we developed a convenient model for animal challenge based on testing of the antibody response to the highly immunogenic S protein. Through our protocol, the preparation of vaccinia virus was mostly intracellular mature virion (IMV) particles. Since the S spike protein was not located on the IMV by Western blot analysis (data not shown), the MVTT-S particles themselves cannot induce S-specific antibodies unless they enter the cells and initiate infection. This design provides anti-S antibody as a valuable biological indicator for protection of MVTT-S challenge in mice and eliminates the biosafety issues related to the restricted use of pathogenic vaccinia WR by laboratory regulations [28], as well as helps to reduce study costs. Nonpathogenic viral challenge model has previously been used to study the neutralizing antibody-mediated protection against immunodeficiency viral infection [29]. We, however, recognize the limitation of our experimental system because there might be major differences between lethal and nonpathogenic vaccinia challenge systems. For example, NKT-cells, CD8+ CTLs together with Nabs may be involved in protection against a highly pathogenic virus [30]. This issue should be further studied in future studies.
Mucosal VTT vaccination elicited protection against both homologous and heterologous routes of mucosal MVTT-S challenges. Our results have provided scientific evidence that mucosal VTT vaccination (e.g., one time i.n. or i.o. VTT vaccination in this study) likely confers complete protection against mucosal routes of viral challenges (Figure 2(a)), which is consistent to previous findings [14, 31]. These findings are relevant to smallpox prevention because the vaccination likely provided a protective barrier at the dominant portal of variola viral entry, in the mucosa. This is concluded by the fact that the transmission of smallpox occurs mainly through inhalation of airborne variola virus, usually droplets expressed from the oral, nasal, or pharyngeal mucosa of an infected person [32]. Critically, the role of mucosal immunization with a smallpox vaccine has been demonstrated in previous studies [33, 34]. Since four groups of mice all developed systemic antivaccinia Nabs before MVTT-S challenges (Figure 1(c)), we speculated that higher levels (4.4-fold) of the Nab responses induced via the mucosal i.n. and i.o. inoculations compared with the s.c. route might have played a role in blocking MVTT-S challenge completely. To this end, the rapidly recalled high levels of anti-VTT Nabs after the MVTT-S challenges might also contribute to this protection (Figure 4(a)). In spite of these findings, extra attentions should be paid when VTT is used as a human smallpox vaccine for mucosal vaccination due to (1) its in vivo toxicity or virulence [16] as well as (2) its complicated quasispecies containing a group of viral variants [14]. As a substitute, it is evident to use a safely attenuated and homogenous MVTT strain to conduct mucosal smallpox vaccinations [14].
One obstacle of the application of vaccinia-based vaccine is the preexisting antivector immunity [35]. This is often an issue for the clinical development of a vaccinia-based vaccine. Previous studies indicated that mucosal vaccination overcomes preexisting antivaccinia immunity generated through skin vaccination [35, 36]. Consistently, we showed that i.n. or i.o. MVTT-S challenges overcame preexisting antivaccinia immunity generated through s.c. VTT vaccination for inducing anti-S Nabs (Figure 2(b)) [17]. Moreover, we now provide a new finding that i.m. MVTT-S challenges overcome preexisting antivaccinia immunity generated through s.c., i.n., or i.o. route of VTT vaccination (Figures 2(a) and 3). Our findings have implications either for overcoming the preexisting antivaccinia immunity or for improving the immunogenicity of a vaccinia-based vaccine. Since vaccinia-based vaccines have been developed for inducing protective immune responses at the mucosal sites of viral transmission for HIV and other pathogens [37–39], our findings provide evidence to support a new strategy of heterologous routes of prime and boost vaccination using the same vaccine. In comparison to heterologous prime and boost regimens using different viral vectors [40, 41], this is an area of interest for further investigations.
One limitation of our study is the lack of measurements of T-cell-mediated immunity. Besides Nabs, it is possible that the observed VTT-induced protection was partially contributed by cell-mediated immunity. To this end, the mucosal route of vaccination may also be crucial for the generation of highly protective T-cell-mediated immunity. A recent study demonstrated that protection against lethal respiratory vaccinia challenge requires both respiratory mucosal TEM cells and central memory T cells [42]. A future study is, therefore, necessary to address the protective effects of specific cell-mediated immunity under the similar experimental conditions.
Acknowledgments
This work was supported by the Chinese 973 project 2006CB504208, the 11th 5-year project 2008ZX10001-011 and 2008ZX10001-015, Hong Kong research grant council (HK-RGC762209 to ZC), and the UDF/LKS grants of the University of Hong Kong to its AIDS Institute. We thank Jenny Ng for editorial inputs. B. Lu, W. Yu, and X. Huang contributed equally to this work.
References
- 1.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clinical Infectious Diseases. 2003;37(2):251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 2.Nafziger SD. Smallpox. Critical Care Clinics. 2005;21(4):739–746. doi: 10.1016/j.ccc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Henderson DA. The eradication of smallpox. Scientific American. 1976;235(4):25–33. doi: 10.1038/scientificamerican1076-25. [DOI] [PubMed] [Google Scholar]
- 4.Sauri MA, Frelinger JA, Garba ML, Belshe RB, Frey SE. Responses to smallpox vaccine. The New England Journal of Medicine. 2002;347(9):689–690. [PubMed] [Google Scholar]
- 5.Heymann DL. Smallpox containment updated: considerations for the 21st century. International Journal of Infectious Diseases. 2004;8(supplement 2):S15–S20. doi: 10.1016/j.ijid.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: how is it defined? Human Vaccines. 2008;4(4):316–319. doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. The New England Journal of Medicine. 2007;357(19):1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 8.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunological Reviews. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 9.Mahalingam S, Damon IK, Lidbury BA. 25 years since the eradication of smallpox: why poxvirus research is still relevant. Trends in Immunology. 2004;25(12):636–639. doi: 10.1016/j.it.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Weltzin R, Liu J, Pugachev KV, et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nature Medicine. 2003;9(9):1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 11.Tang X, Chen Z. The development of an AIDS mucosal vaccine. Viruses. 2010;1(2):283–297. doi: 10.3390/v2010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and Its Eradication. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 13.Dong, Q. C. SL. The founder of vaccinia virus Tiantan strain. Weishengwuxue Mianyixue Jinzhan. 2009;37(3):1–3. [Google Scholar]
- 14.Yu W, Fang Q, Zhu W, et al. One time intranasal vaccination with a modified vaccinia Tiantan strain MVTT(ZCI) protects animals against pathogenic viral challenge. Vaccine. 2010;28(9):2088–2096. doi: 10.1016/j.vaccine.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kretzschmar M, Wallinga J, Teunis P, Xing S, Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Medicine. 2006;3(8, article e272) doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Q, Yang L, Zhu W, et al. Host range, growth property, and virulence of the smallpox vaccine: vaccinia virus Tian Tan strain. Virology. 2005;335(2):242–251. doi: 10.1016/j.virol.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Lu B, Yu W, et al. A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS One. 2009;4(1) doi: 10.1371/journal.pone.0004180. Article ID e4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tscharke DC, Reading PC, Smith GL. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. Journal of General Virology. 2002;83, part 8:1977–1986. doi: 10.1099/0022-1317-83-8-1977. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Yu W, Tang X, et al. The route of inoculation determines the tissue tropism of modified vaccinia Tiantan expressing the spike glycoprotein of SARS-CoV in mice. Journal of Medical Virology. 2010;82(5):727–734. doi: 10.1002/jmv.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Liu L, Ren L, Qiu C, Wan Y, Xu J. Mucosal priming with replicative Tiantan vaccinia and systemic boosting with DNA vaccine raised strong mucosal and systemic HIV-specific immune responses. Vaccine. 2007;25(52):8874–8884. doi: 10.1016/j.vaccine.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 21.Joklik WK. The purification of four strains of poxvirus. Virology. 1962;18(1):9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Zhang L, Qin C, et al. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. Journal of Virology. 2005;79(5):2678–2688. doi: 10.1128/JVI.79.5.2678-2688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai C, Caillet C, Hu H, et al. Measurement of neutralizing antibody responses against H5N1 clades in immunized mice and ferrets using pseudotypes expressing influenza hemagglutinin and neuraminidase. Vaccine. 2009;27(48):6777–6790. doi: 10.1016/j.vaccine.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earl PL, Americo JL, Moss B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. Journal of Virology. 2003;77(19):10684–10688. doi: 10.1128/JVI.77.19.10684-10688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benhnia MRE, McCausland MM, Su HP, et al. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. Journal of Virology. 2008;82(7):3751–3768. doi: 10.1128/JVI.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nature Medicine. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 27.Fazilleau N, Eisenbraun MD, Malherbe L, et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nature Immunology. 2007;8(7):753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 28.Neff JM, Michael LJ, Fulginiti VA, Henderson DA. Contact vaccinia—transmission of vaccinia from smallpox vaccination. Journal of the American Medical Association. 2002;288(15):1901–1905. doi: 10.1001/jama.288.15.1901. [DOI] [PubMed] [Google Scholar]
- 29.Robinson HL, Montefiori DC, Johnson RP, et al. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nature Medicine. 1999;5(5):526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 30.Snyder JT, Belyakov IM, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. Journal of Virology. 2004;78(13):7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastenmuller W, Gasteiger G, Stross L, Busch DH, Drexler I. Cutting edge: mucosal application of a lyophilized viral vector vaccine confers systemic and protective immunity toward intracellular pathogens. Journal of Immunology. 2009;182(5):2573–2577. doi: 10.4049/jimmunol.0803871. [DOI] [PubMed] [Google Scholar]
- 32. Elst E. The transmission mechanism of smallpox. Ärztliche Forschung. 1955;9(1):13–32. [PubMed] [Google Scholar]
- 33.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Klinman D, Berzofsky JA. Enhancement of CD8+ T cell immunity in the lung by CpG oligodeoxynucleotides increases protective efficacy of a modified vaccinia Ankara vaccine against lethal poxvirus infection even in a CD4-deficient host. Journal of Immunology. 2006;177(9):6336–6343. doi: 10.4049/jimmunol.177.9.6336. [DOI] [PubMed] [Google Scholar]
- 34.Belyakov IM, Earl P, Dzutsev A, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belyakov IM, Moss B, Strober W, Berzofsky JA. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4512–4517. doi: 10.1073/pnas.96.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naito T, Kaneko Y, Kozbor D. Oral vaccination with modified vaccinia virus Ankara attached covalently to TMPEG-modified cationic liposomes overcomes pre-existing poxvirus immunity from recombinant vaccinia immunization. Journal of General Virology. 2007;88, part 1:61–70. doi: 10.1099/vir.0.82216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranasinghe C, Medveczky JC, Woltring D, et al. Evaluation of fowlpox-vaccinia virus prime-boost vaccine strategies for high-level mucosal and systemic immunity against HIV-1. Vaccine. 2006;24(31-32):5881–5895. doi: 10.1016/j.vaccine.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Martinon F, Brochard P, Ripaux M, et al. Improved protection against simian immunodeficiency virus mucosal challenge in macaques primed with a DNA vaccine and boosted with the recombinant modified vaccinia virus Ankara and recombinant Semliki Forest virus. Vaccine. 2008;26(4):532–545. doi: 10.1016/j.vaccine.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AVS. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. Journal of Immunology. 2003;171(3):1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 40.Lu S. Heterologous prime-boost vaccination. Current Opinion in Immunology. 2009;21(3):346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ba L, Yi CE, Zhang L, Ho DD, Chen Z. Heterologous MVA-S prime Ad5-S boost regimen induces high and persistent levels of neutralizing antibody response against SARS coronavirus. Applied Microbiology and Biotechnology. 2007;76(5):1131–1136. doi: 10.1007/s00253-007-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nature Medicine. 2010;16(2):224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]


