Abstract
Fibroblast growth factor 23 (FGF23) regulates phosphorus metabolism and is a strong predictor of mortality in dialysis patients. FGF23 has been proposed as an early biomarker of disordered phosphorus metabolism in earlier stages of chronic kidney disease (CKD), but data from large, well-characterized CKD cohorts are lacking. We measured FGF23 in baseline samples from 3,879 participants in the Chronic Renal Insufficiency Cohort study, a nationally representative, diverse CKD cohort with mean (± sd) estimated glomerular filtration rate (eGFR) of 42.8 ± 13.5 ml/min/1.73m2. Serum phosphate (3.7 ± 0.7 mg/dl) and parathyroid hormone (PTH; median 54, interquartile range [IQR] 35 – 89 pg/ml) levels were in the normal range, but FGF23 (median 145, IQR 96 – 239 RU/ml) was markedly greater than in healthy populations and increased significantly with decreasing eGFR. FGF23 excess, defined as ≥ 100 RU/ml, was more common than secondary hyperparathyroidism (≥ 65 pg/ml) and hyperphosphatemia (≥ 4.6 mg/dl) in all strata of eGFR, and the eGFR threshold at which the slope of FGF23 increased (57.8; 95%CI: 55.4 – 60.8 ml/min/1.73m2) was higher than the corresponding threshold for PTH (46.9; 95%CI: 45.5 – 51.4 ml/min/1.73m2). Thus, increased FGF23 is a common manifestation of CKD that develops earlier than increases in phosphate or PTH. These findings provide additional support for use of FGF23 as a sensitive early screening test to identify disordered phosphorus metabolism in CKD patients with normal serum phosphate levels.
Introduction
Fibroblast growth factor 23 (FGF23) regulates phosphorus and vitamin D metabolism. In healthy individuals, FGF23 is secreted by osteocytes in response to dietary phosphorus loading or increases in 1,25-dihydroxyvitamin D (1,25D) levels, and it stimulates phosphaturia, and reduces 1,25D and PTH levels.1, 2 FGF23 achieves its cellular specificity in the kidney and parathyroid glands by binding the co-receptor klotho, which increases the affinity of FGF23 for ubiquitously expressed FGF receptors.3 In patients with chronic kidney disease (CKD), FGF23 levels are thought to increase as a compensatory response to maintain normal phosphate balance as the capacity for renal phosphorus excretion declines.4-6
Hyperphosphatemia is a risk factor for cardiovascular disease and mortality and thus a potential target for interventions to improve clinical outcomes in CKD.7 However, serum phosphate levels are normal in the vast majority of patients with early and intermediate stages of CKD due to compensatory increases in FGF23 and PTH.5, 8 While this limits the use of serum phosphate as an early indicator of disordered phosphorus metabolism, it has been proposed that FGF23 screening could identify which patients with early-stage CKD and normal serum phosphate levels might benefit from intervention.7 In support of this view, high FGF23 levels are more strongly associated with kidney disease progression, left ventricular hypertrophy, vascular disease, and mortality than serum phosphate levels, and were most predictive of adverse events in patients with normal serum phosphate.9-13
Although these results are promising, critical questions about the role of FGF23 in mineral metabolism remain unresolved.14 For example, whether FGF23 or PTH increases first in patients with CKD is not clear, because prior human studies were limited by small sample size or a narrow distribution of glomerular filtration rate (GFR).4-6 As a result, few if any studies directly compared the prevalence of FGF23 excess versus secondary hyperparathyroidism within fine strata across the spectrum of GFR. Furthermore, no large cohort studies of CKD patients examined the impact of dietary phosphorus intake, race, ethnicity and socioeconomic status on FGF23 levels. We measured FGF23 in baseline samples from 3,879 participants in the prospective Chronic Renal Insufficiency Cohort (CRIC) study. The purpose of our study was to characterize FGF23 levels in this national multi-center, racially and ethnically diverse population of CKD patients not yet on dialysis; to compare the relationships of FGF23 versus PTH with declining GFR; and to describe the relationship between FGF23, dietary phosphorus intake and other measures of phosphorus metabolism across the spectrum of GFR.
Results
Description of the Study Population
Characteristics of the 3,879 CRIC participants are presented in Table 1. The mean eGFR at the baseline visit was 42.8 ± 13.5 ml/min/1.73 m2 and the range was 7.0 – 114.0 ml/min/1.73m2; 10% of patients had CKD stage 2, 70% stage 3, and 19% stage 4. Median estimated daily consumption of phosphorus was 1050 mg (interquartile range [IQR] 745 – 1417), median 24-hour urinary phosphate excretion was 713 mg (IQR 512 – 953), and median fractional excretion of phosphate (FEPi) calculated from the 24-hour specimens was 25% (IQR 19 – 35). In the overall population, mean serum phosphate (3.7 ± 0.7 mg/dl), mean serum calcium (9.2 ± 0.5 mg/dl) and median plasma PTH (54; IQR: 35 – 89 pg/ml) were within the normal range. Among the 333 participants with available measurements, mean 25-hydroxyvitamin D (25D) was 20 ± 11 ng/ml, 39% of patients had insufficient vitamin D levels (15 – 30 ng/ml), an additional 39% were deficient (<15 ng/ml), and 25D levels correlated inversely with PTH (r = −0.35, P <0.001).
Table 1. Characteristics of the study population.
| All Participants (N=3879) | |
|---|---|
| Age | 58 ± 11 |
| Female (%) | 44.8 |
| Black (%) | 41.8 |
| Hispanic (%) | 12.8 |
| Current smoker (%) | 13.1 |
| Body Mass Index, kg/m2 | 32 ± 8 |
| Systolic blood pressure, mm Hg | 129 ± 22 |
| Hypertension (%) | 86.1 |
| Diabetes (%) | 48.4 |
| Phosphate binder use (%) | 7.0 |
| Calcium-based binder use (%) | 6.6 |
| Non-calcium-based binder use (%) | 0.4 |
| Active vitamin D use (%) | 3.2 |
| Vitamin D supplement use (%) | 10.2 |
| Renal Function | |
| Creatinine, mg/dl | 1.7 ± 0.6 |
| eGFR, ml/min/1.73 m2 | 42.8 ± 13.5 |
| stage 2, % | 10.3 |
| stage 3, % | 70.1 |
| stage 4, % | 19.4 |
| Etiology of CKD | |
| Diabetes (%) | 26.1 |
| Hypertension (%) | 16.4 |
| Other (%) | 13.9 |
| Unknown (%) | 43.6 |
| Laboratory Results (serum) | |
| Albumin, g/dl | 3.9 ± 0.5 |
| Calcium, mg/dl | 9.2 ± 0.5 |
| Phosphate, mg/dl | 3.7 ± 0.7 |
| 25D, ng/ml* | 20 ± 11 |
| 1,25D, ng/ml* | 28 ± 11 |
| PTH, pg/ml | 54 (35 – 89) |
| FGF23, RU/ml | 145 (96 – 239) |
| Laboratory Results (Urine)† | |
| 24-hr urinary phosphate, mg/day | 713 (512 – 953) |
| Fractional excretion of phosphate, % | 25 (19 – 35) |
| 24-hr urinary calcium, mg/day | 39 (17 – 86) |
| Fractional excretion of calcium, % | 0.61 (0.30 – 1.15) |
| Dietary Data‡ | |
| Dietary phosphorus intake, mg/d | 1050 (745 – 1417) |
| Dietary calcium intake, mg/d | 615 (422 – 870) |
| Total caloric intake, kcal/day | 1626 (1214 – 2267) |
Values are %, means ± standard deviation, medians (interquartile range).
25-hydroxyvitamin D and 1,25-hydroxyvitamin D levels were available in 333 and 332 participants, respectively.
Twenty four-hour urinary data were available for 3,659 participants.
Dietary data were available for 2,797 participants.
The median FGF23 level was 145 RU/ml (IQR 96 – 239), which is markedly higher than levels observed in healthy patients.15, 16 When stricter criteria for CKD were applied (eGFR < 60 ml/min/1.73 m2, age < 70 years, and urine albumin to creatinine ratio > 30 mg/g), the median FGF23 rose to 175 RU/ml (IQR: 114 – 289). Seventy-two percent of participants met the criterion for FGF23 excess, which we defined as ≥ 100 RU/ml. This threshold is 2.5–fold higher than the median FGF23, and it is in the 90th percentile of the FGF23 distribution observed in previous studies of populations in which approximately 80% of patients had normal kidney function (eGFR >60 ml/min/1.73m2).15, 16 This threshold has also been associated with CKD progression, cardiovascular disease and mortality in previous prospective studies of CKD patients.9, 13 Using an even more conservative threshold of ≥125 RU/ml to define FGF23 excess, 59% of participants qualified. Consistent with prior reports,4, 5 the strongest univariate correlate of FGF23 was eGFR (r= −0.52, P<0.0001). FGF23 also correlated directly with PTH (r=0.37; P< 0.0001) and serum phosphate (r=0.35; P<0.0001), and inversely with 1,25D, (r= −0.23; P< 0.0001) in the 332 participants for whom these levels were available. With the exception of 1,25D, each of these associations remained significant after adjusting for eGFR. While there was no significant relationship with 24-hour urinary phosphate when adjusted for eGFR, higher FGF23 was linearly associated with FEPi (r=0.25; P<0.0001). Additional factors associated with a higher FGF23 adjusted for eGFR included younger age, female gender, black race and lower annual income (P<0.008 for each).
Mineral Metabolites in Relation to GFR
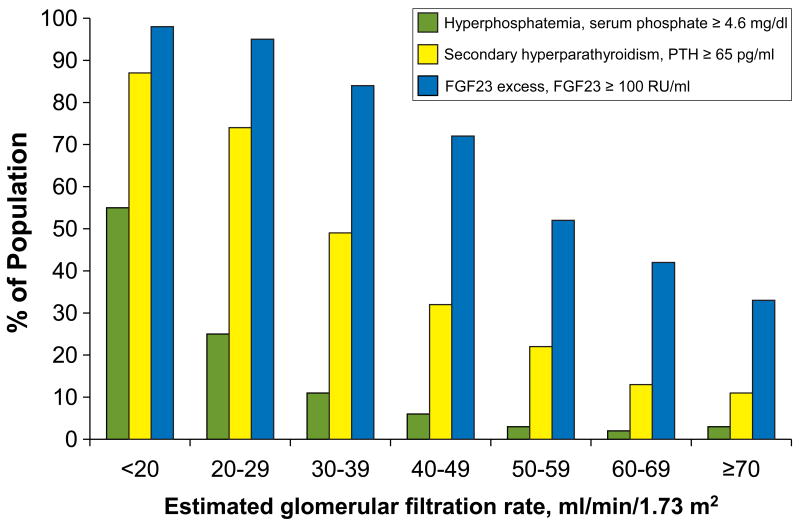
Table 2 presents the interrelationships between mineral metabolites and renal function after dividing the cohort into 7 fine strata of descending eGFR. Beginning at an eGFR < 60 ml/min/1.73m2, serum phosphate, PTH and FGF23 levels increased in each consecutive stratum of descending eGFR. Similarly, the prevalence of hyperphosphatemia (phosphate ≥ 4.6 mg/dl), secondary hyperparathyroidism (PTH ≥ 65 pg/ml) and FGF23 excess (FGF23 ≥ 100 RU/ml) increased progressively with declining eGFR (Figure 1). Consistent with prior reports,8 hyperphosphatemia was a late manifestation of CKD, as the prevalence first exceeded 10% in patients with eGFR < 39 ml/min/1.73m2. In contrast, abnormalities in FGF23 levels were an early finding. At an eGFR of 50 – 59 ml/min/1.73m2 > 50% of patients had an FGF23 ≥ 100 RU/ml when only 3% were hyperphosphatemic and only 22% manifested secondary hyperparathyroidism. Among participants with eGFR ≥ 70 ml/min/1.73m2, 33% had FGF23 levels ≥ 100 RU/ml. Given the imprecision of eGFR, especially among individuals with mostly preserved renal function,17 we reevaluated the above relationships in the subset of participants (N=1434) who underwent direct GFR measurements with 125-I iothalamate clearance (iGFR). Estimated GFR and iGFR were highly correlated (r = 0.82; P <0.0001), hence the results conveyed in Table 2 and Figure 1 were qualitatively unchanged when analyzed according to iGFR.
Table 2. Mineral metabolites according to cut points of eGFR.
| eGFR <20 N=87 |
eGFR 20-29 N=671 |
eGFR 30-39 N=955 |
eGFR 40-49 N=1024 |
eGFR 50-59 N=742 |
eGFR 60-69 N=287 |
eGFR ≥70 N=113 |
|
|---|---|---|---|---|---|---|---|
| eGFR, ml/min/1.73m2 | 18 ± 2 | 26 ± 3 | 35 ± 3 | 45 ± 3 | 54 ± 3 | 64 ± 2 | 77 ± 7 |
| Serum phosphate, mg/dl | 4.7 ± 1.0❋ | 4.1 ± 0.7❋ | 3.8 ± 0.6❋ | 3.6 ± 0.6❋ | 3.5 ± 0.6 | 3.4 ± 0.5 | 3.5 ± 0.6 |
| Serum calcium, mg/dl | 8.8 ± 0.7❋ | 9.1 ± 0.6 | 9.2 ± 0.5 | 9.2 ± 0.5 | 9.2 ± 0.5 | 9.2 ± 0.4 | 9.1 ± 0.4 |
| FGF23, RU/ml | 348❋ (234 – 486) |
247❋ (169 – 378) |
173❋ (118 – 270) |
135❋ (94 – 188) |
102❋ (77 – 152) |
94 (67 – 121) |
82 (62 – 114) |
| PTH, pg/ml | 175❋ (101 – 234) |
103❋ (63 – 163) |
64❋ (41 – 99) |
48❋ (32 – 73) |
41❋ (30 – 59) |
36 (28 – 51) |
33 (25 – 48) |
| 1,25D, ng/ml* | 23 ± 5 | 23 ± 8❋ | 25 ± 11❋ | 28 ± 11 | 29 ± 10 | 30 ± 11 | 33 ± 17 |
| 25D, ng/ml* | 11 ± 3 | 17 ± 12 | 18 ± 10 | 21 ± 12 | 22 ± 11 | 23 ± 12❋ | 17 ± 8 |
| FEPi, % | 41❋ (32 – 50) |
35❋ (26 – 46) |
28❋ (21 – 36) |
24❋ (18 – 32) |
22❋ (17–28) |
20❋ (14 – 24) |
17 (13 – 22) |
| 24-hr urinary phosphate, mg/day† | 589❋ (409 – 793) |
632❋ (465 – 845) |
680❋ (492 – 895) |
723❋ (530 – 952) |
799 (568 – 1049) |
812 (598 – 1103) |
836 (566 – 1106) |
| Dietary phosphorus intake, mg/day‡ | 1250 (827 – 1574) |
1002❋ (702 – 1387) |
1006❋ (690 – 1346) |
1050 (762 – 1432) |
1083 (776 – 1471) |
1085 (786 – 1409) |
1131 (841 – 1544) |
| 24-hr urinary phosphate/dietary phosphorus intake§ | 0.53❋ (0.34 – 0.86) |
0.65 (0.44 – 0.94) |
0.69 (0.45 – 0.99) |
0.70 (0.48 – 0.99) |
0.72 (0.52 – 1.0) |
0.74 (0.54 – 1.04) |
0.72 (0.52 – 0.96) |
| Total caloric intake, kcal/day‡ | 1942 (1382 – 2715) |
1530❋ (1163 – 2210) |
1592❋ (1126 – 2198) |
1636 (1244 – 2273) |
1704 (1243 – 2310) |
1644 (1297 – 2363) |
1852 (1388 – 2348) |
Values are means ± standard deviation, medians (interquartile range).
P <0.05 compared with eGFR >70 ml/min/1.73m2. eGFR was not tested.
25-hydroxyvitamin D and 1,25-hydroxyvitamin D levels were available in 333 and 332 participants, respectively.
Twenty four-hour urinary data were available for 3,659 participants.
Dietary data were available for 2,797 participants.
24-hr urinary phosphate/dietary phosphorus intake was available for 2702 participants.
The CRIC central laboratory's reference ranges were 2.4 – 4.7 mg/dl for serum phosphate, 8.9 – 10.3 mg/dl for serum calcium, and 14 – 66 pg/ml for PTH.
Figure 1. Prevalence of hyperphosphatemia, secondary hyperparathyroidism and elevated FGF23 in relation to eGFR.
Hyperphosphatemia was defined as serum phosphate ≥ 4.6 mg/dl, secondary hyperparathyroidism as PTH ≥ 65 pg/ml and FGF23 excess as FGF23 ≥ 100 RU/ml.
Estimated daily dietary phosphorus intake was qualitatively similar across the spectrum of eGFR and did not correlate significantly with FGF23, phosphate, or PTH levels. FEPi increased but total 24-hour urinary phosphate excretion decreased with decreasing strata of eGFR (Table 2). Similarly, the ratio of 24-hour urinary phosphate excretion to estimated daily dietary phosphorus intake decreased from >70% in participants with eGFR > 39 ml/min/1.73m2 to 53% in those with eGFR < 20 m/min/1.73m2. This decrease coincided with the gradual increase in serum phosphate levels.
Relationships of FGF23 and PTH with eGFR
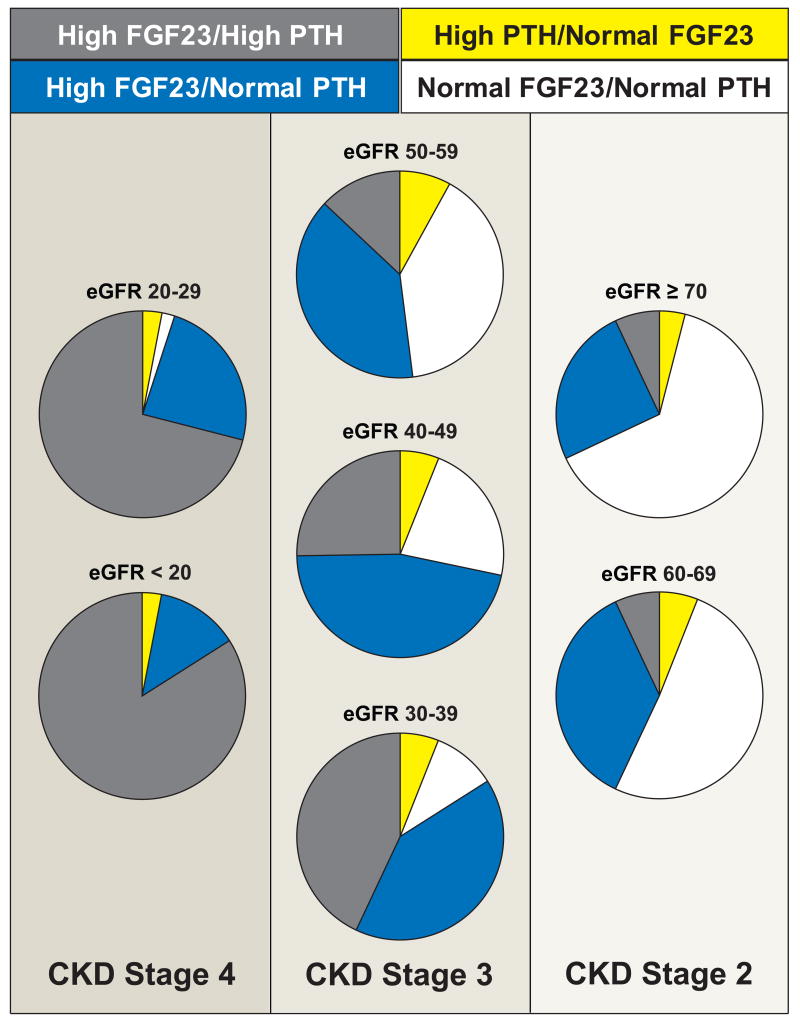
To further compare the relative prevalence of abnormally high FGF23 versus PTH levels across the spectrum of eGFR, we calculated the proportions of participants with normal or high FGF23 and PTH levels within each eGFR category (Figure 2). As expected, the most common pattern in the strata of more preserved eGFR was normal FGF23/normal PTH, whereas high FGF23/high PTH was predominant in the lower eGFR strata. In the intermediate eGFR range of 30 – 60 ml/min/1.73m2, an isolated increase in FGF23 was the most common pattern and was far more likely than an isolated increase in PTH, which was the rarest combination in all eGFR categories. For example, among participants with eGFR 30 – 60 ml/min/1.73m2 and an FGF23 ≥100 RU/ml, only 40% met criteria for secondary hyperparathyroidism, whereas among those with established secondary hyperparathyroidism, FGF23 was ≥100 RU/ml in 85% of participants. Indeed, in the overall population the combination of high FGF23/normal PTH was 6.4–fold more prevalent than normal FGF23/high PTH (P<0.0001). Using a more conservative cut-point of ≥125 RU/ml to define FGF23 excess did not qualitatively alter the results: the combination of high FGF23/normal PTH remained 2.8 –fold more prevalent than normal FGF23/high PTH (P<0.0001). The results were also unchanged when defining secondary hyperparathyroidism based on the 1-84 PTH assay and its upper limit of 36 pg/ml.18 Excluding participants treated with phosphate binders, active vitamin D, or vitamin D supplements did not alter these findings. The relationships between eGFR and mineral metabolites did not vary by sex or race.
Figure 2. Proportions of participants with normal or high FGF23 and PTH levels within each eGFR category.
Sections of pie charts indicate proportions of individuals with FGF23 and/or PTH abnormalities by eGFR category: white – normal PTH and FGF23 levels; yellow – high PTH/normal FGF23; gray – high FGF23/high PTH; and blue – high FGF23/normal PTH.
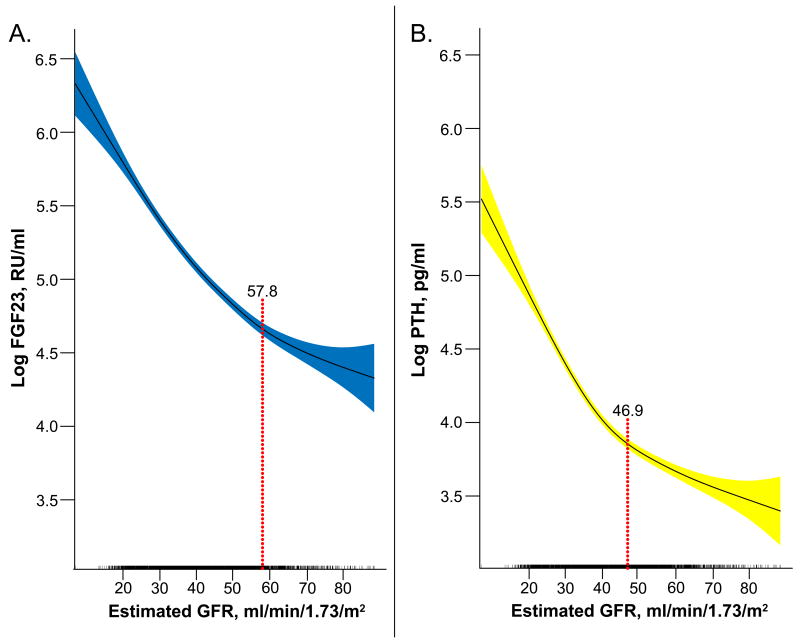
Finally, we plotted FGF23 and PTH versus eGFR expressed on a continuous scale using cubic splines, and estimated the eGFR threshold at which there was a statistically significant increase in the slope of each hormone. As shown in Figures 3A and 3B, the slope of the FGF23 curve increased at an eGFR threshold of 57.8 ml/min/1.73m2 (95%CI: 55.4 – 60.8), which was significantly higher (based on non-overlapping 95% confidence intervals) than the comparable threshold for PTH of 46.9 (95%CI: 45.5 – 51.4) ml/min/1.73m2. Adjustment for age and fasting status did not change the results. In aggregate, these data suggest that FGF23 increases earlier in the course of CKD than PTH.
Figure 3. Cubic spline functions of the associations of eGFR with logFGF23 and with logPTH.
The shadowed areas represent 95% confidence intervals for the fitted splines. The red dotted lines represents the statistically significantly thresholds of eGFR at which the slopes increased. Tick marks on the x axis indicate individual observations at each level of eGFR. (A) logFGF23 versus eGFR; (B) logPTH versus eGFR.
Serum and Urinary Phosphate in Relation to eGFR
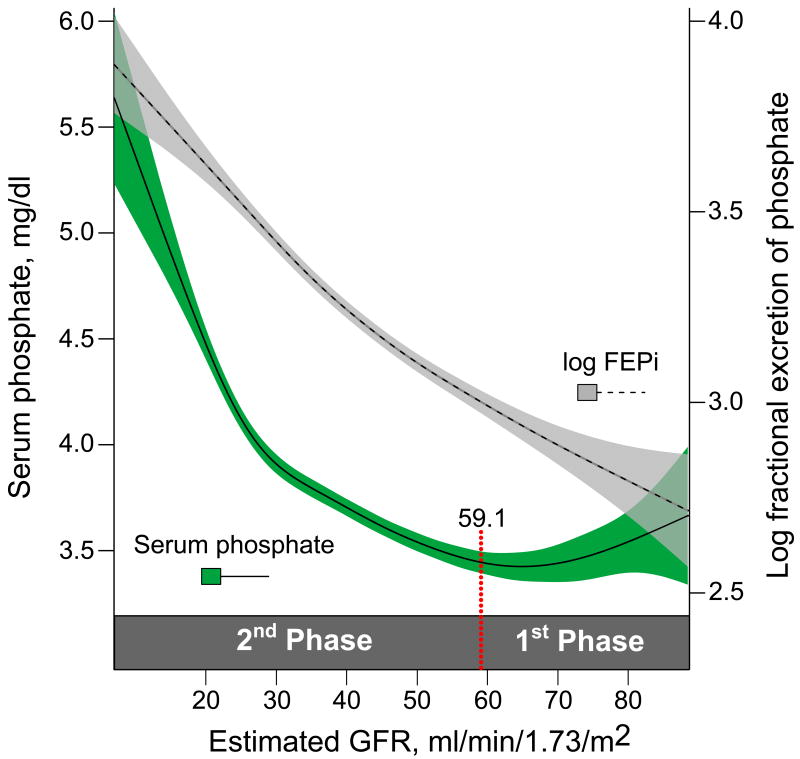
Next, we plotted serum phosphate and urinary FEPi in relation to eGFR using fitted splines and tested whether there were thresholds of eGFR at which there was a statistically significant change in their slopes (Figure 4). LogFEPi increased uniformly across the spectrum of decreasing eGFR without a detectable threshold. In contrast, serum phosphate demonstrated a J-shaped curve: levels declined (slope = −0.017 ± 0.002) between the upper range of eGFR through an eGFR of 59.1 ml/min/1.73m2 (95%CI: 48.6 – 63.9), whereas below an eGFR of 59.1, serum phosphate increased (slope = +0.067 ± 0.003). The results remained unchanged following adjustment for age and fasting status. These data are consistent with the subtle decrease in serum phosphate levels in the stratum with eGFR of 60 – 69 ml/min/1.73m2 compared to the adjacent higher and lower strata (Table 2). Furthermore, when juxtaposed with data in Table 2, these results suggest two phases in the serum phosphate curve (Figure 4). In the proposed 1st phase (eGFR > 59.1 ml/min/1.73 m2), the subtle reduction in serum phosphate levels was observed in association with increased FGF23 and FEPi and decreased 1,25D levels. In the proposed 2nd phase (eGFR < 59.1 ml/min/1.73 m2), serum phosphate levels gradually increased from their nadir while FGF23 increased more sharply, FEPi continued to increase, and 1,25D decreased further.
Figure 4. Cubic spline functions of the associations between serum phosphate and logFEPi with eGFR.
The shadowed areas represent 95% confidence intervals for the fitted splines. The red dotted line represents the statistically significantly thresholds of eGFR at which the slope of serum phosphate changed. Based on this change, two phases of the phosphate curve are proposed.
Discussion
In this study of nearly 4000 CKD patients, the largest human study of FGF23 to date, we validate that FGF23 is elevated in CKD patients, increases as eGFR falls, and that both FGF23 and PTH increase before hyperphosphatemia first appears.4-6 Despite being limited by its cross-sectional design and incomplete vitamin D data, our study suggests that FGF23 excess may be the earliest bellwether of disordered mineral metabolism in CKD.
Although levels of FGF23 and PTH are each markedly increased in advanced renal failure,5, 8 the results of this study suggest that the increase in FGF23 precedes that of PTH. As expected, both FGF23 and PTH were elevated in most patients with extremely low eGFR, and many patients with the most preserved eGFR had normal levels of each. However, an increased FGF23 in the setting of a normal PTH was >6 times more likely than an isolated increase in PTH. Furthermore, the slope of FGF23 versus eGFR turned upwards at a significantly higher eGFR than the comparable threshold for PTH. Importantly, vitamin D deficiency, which is known to raise PTH, was present in 78% of participants and was inversely correlated with PTH. The finding that PTH rises after FGF23 despite the high frequency of vitamin D deficiency further supports the temporal sequence we propose. Nonetheless, 5.8% of participants had isolated PTH elevations, suggesting that additional FGF23-independent factors drive an initial increase in PTH in certain CKD patients.
Although the weight of evidence favors FGF23 elevation as the upstream pathophysiological event that lowers 1,25D and thus increases PTH, as was recently shown in elegant experiments using an animal model of CKD and anti-FGF23 antibodies,19 the primary stimulus for enhanced FGF23 secretion in early CKD is less clear. Based on scant human and experimental data, it has been proposed that decreased expression of klotho may be one step further upstream from FGF23 excess.20 Indeed, parathyroid resistance to the inhibitory effects of FGF23 is mediated, in part, by down regulation of klotho.21-23 However, if klotho deficiency is the primary factor that causes renal resistance to FGF23 and drives secondary, appropriate increases in FGF23 secretion, CKD patients should manifest at least subtle signs of klotho deficiency early in the course of their disease: modest increases in serum phosphate, 1,25D and calcium levels. On the contrary, increases in 1,25D and serum calcium are not observed in early CKD, and we and others observed a modest reduction in serum phosphate during early CKD.24, 25 This decrement was accompanied by elevated FGF23 and FEPi and lower 1,25D levels, a constellation of metabolic changes that characterize syndromes of primary FGF23 excess, such as X-linked hypophosphatemia,26 but not syndromes of primary klotho deficiency.27 An alternative, unifying explanation is that the initial increase in FGF23 in early CKD causes secondary down regulation of klotho, perhaps in response to relative hypophosphatemia or reduced 1,25D, which is known to regulate klotho expression.28 Indeed, in transgenic mice engineered to overproduce FGF23, klotho was the most significantly inhibited transcript.29 Although we were not able to measure klotho levels, the pattern of metabolic changes that we observed suggests that increased FGF23 precedes klotho deficiency.
It has also been postulated that FGF23 increases in CKD as an appropriate response to minimize “phosphate retention”, but our data suggest other possible factors. The early reduction in serum phosphate levels in association with already elevated FGF23 levels (proposed phase 1) suggests that perhaps renal injury itself may be an initial stimulus for FGF23 secretion in early CKD by causing increased production of a factor that stimulates FGF23 secretion or decreased expression of a tonic inhibitor. Animal studies in which FGF23 rose immediately after the kidney was injured but before any increase in serum phosphate support this possibility.19 as do human data that reported an upwards inflection in FGF23 levels as early as an eGFR of 90 ml/min/1.73m2,30 and our finding that 33% of participants with an eGFR ≥ 70 ml/min/1.73m2 had FGF23 levels ≥ 100 RU/ml. Furthermore, patients with autosomal dominant polycystic kidney disease and preserved eGFR exhibited elevated FGF23 levels and subtly reduced serum phosphate levels,31 which is consistent with our results.
Below an eGFR of 60 ml/min/1.73m2, serum phosphate rose gradually (proposed phase 2) concurrent with a decrease in the ratio of 24-hour phosphate excretion to estimated daily phosphorus intake. Though we only had data from a single 24-hour urine collection and dietary assessment, and we did not have 1,25D measurements in the entire cohort, several possibilities may explain this finding. First, serum phosphate could have risen with declining eGFR as a simple adaptation to increase the filtered load of phosphate to overcome the partially reduced capacity for urinary excretion, as has been previously described.32 This construct implies the maintenance of even phosphate balance at the cost of a modest increase in serum levels. Second, the eGFR-dependent, progressive reduction in the ratio of phosphorus excretion/intake could reflect a gradual reduction in the percent of ingested phosphorus that was absorbed, as has been reported previously.33-35 This would also imply even phosphate balance and could occur through direct FGF23-dependent inhibition of sodium-phosphate transporters in the gut;36 FGF23-mediated reductions in 1,25D levels that decrease vitamin D-dependent phosphorus absorption; or reduced vitamin D-dependent calcium absorption that leads to an increase in luminal calcium that acts as an endogenous phosphate binder to limit absorption.37 Third, if the percent of dietary phosphorus intake that was absorbed remained constant as eGFR declined, then the lower 24-hour excretion would signify positive phosphate balance that was reflected in the modest increase in serum phosphate. This paradigm would entail progressive total body phosphate overload, beginning in late stage 3 – stage 4, as has been commonly assumed but contradicted by classic balance studies.38, 39 Deciphering these possibilities will require detailed balance studies across a range of eGFR.
Not all of the CKD patients had elevated FGF23 levels and there was significant variability across the spectrum of eGFR. Some of this heterogeneity may be due to unknown physiologic or genetic factors,40 but our finding that lower income was associated with higher FGF23 independent of eGFR suggests important environmental factors. We previously reported that poverty was independently associated with higher serum phosphate levels in CKD and non-CKD patients and speculated that this may be due to impoverished individuals disproportionately consuming excessive amounts of cheap, processed foods laden with highly bioavailable phosphorus-rich additives.41, 42 The independent association between poverty and increased FGF23 supports this hypothesis. Based on these data and the published literature, we propose that the heterogeneous distribution of FGF23 levels across CKD patients likely results from a combination of fixed genetic determinants, the severity of kidney disease, and modifiable components, such as dietary phosphorus intake.
The recent observation that even small increments in serum phosphate are independently associated with adverse renal and cardiovascular events 43, 44 has sparked intense interest in initiating treatment of disordered phosphorus metabolism earlier in the course of CKD. While additional work is needed to establish physiologically-based normal ranges of FGF23 and threshold levels that predict clinically meaningful outcomes, FGF23 has the potential to be a novel, easily measurable biomarker to help guide the efficient design of randomized controlled trials to determine whether earlier initiation of therapy with phosphate binders and dietary phosphorus restriction will improve clinical outcomes in CKD.7 In support of this approach, previous data demonstrated that FGF23 is a powerful prognostic predictor of adverse clinical outcomes in CKD9-13 and that dietary phosphate binders can lower FGF23 in this population.45 The current study offers additional important insight into the design of a clinical outcomes trial targeting phosphorus metabolism in early CKD. Based on the results of this study, we propose that patients with the triad of eGFR < 60 ml/min/1.73m2, FGF23 ≥ 100 RU/ml and serum phosphate ≥ 3.5 mg/dl would be ideal candidates most likely to benefit interventions targeting phosphorus metabolism.
Methods
Description of Cohort
CRIC is a multi-center prospective cohort study of risk factors for cardiovascular disease and progression of CKD.46 Additional Hispanic participants were enrolled in the ancillary Hispanic CRIC (HCRIC) study to distinguish CRIC as the largest, most racially and ethnically diverse CKD cohort available to date. Adult patients aged 21 to 74 years with mild to moderate CKD, defined as an age-stratified eGFR at the screening visit between 20 – 70 ml/min/1.73m2, were enrolled. Individuals who were eligible based on their screening eGFR but whose eGFR at the first study visit was found to be outside the range of 20 – 70 ml/min/1.73m2 were retained in the study. Patients were excluded for pregnancy, New York Heart Association class III – IV heart failure, HIV, cirrhosis, myeloma, renal cancer, recent chemotherapy or immunosuppressive therapy, polycystic kidney disease, organ transplantation, or prior treatment with dialysis for > 1 month. The CRIC protocol was approved by the institutional review boards at each of the 7 primary sites. All participants provided written informed consent.
Study Population
We evaluated 3,879 of the 3,939 total CRIC and HCRIC participants who had baseline plasma FGF23 measurements. The mean eGFR of the 60 participants for whom FGF23 measurements were not available did not differ significantly from levels of those included in this analysis (45.0 ± 13.5 vs. 42.8 ± 13.5 ml/min/1.73m2). Dietary data were available for 2,797 participants. The remainder either did not complete the food frequency questionnaire or their data were excluded by the CRIC data coordinating center because of implausible values for total energy intake. Twenty four-hour urinary data were available for 3,659 participants. Serum 25D and 1,25D levels were available in a subset of 333 and 332 participants, respectively. A subset of 1434 participants, had GFR measured directly using 125-I iothalamate clearance (iGFR).
Data Collection and Measurements
We analyzed the following data that were collected at the baseline visit: demographics, medical history, use of phosphate binders, vitamin D supplements and active vitamin D analogs, blood pressure, anthropometry, fasting blood samples, 24-hour urine specimens, and estimated dietary phosphorus, calcium, and caloric intake from the National Cancer Institute's Diet History Questionnaire (DHQ).47 Annual household income was self-reported and categorized as <$20,000 per year, $20,001 to $50,000 per year, or >$50,000 per year.
Comprehensive metabolic panels and urinary biochemical panels were measured using standard assays. In addition to total 24-hour urinary phosphate excretion (mg/day), we analyzed urinary FEPi ([urine phosphate × serum creatinine] / [serum phosphate × urine creatinine] × 100%). The latter corrects for over- or under-collections of 24-hour urine samples and standardizes urinary phosphate excretion to the degree of renal dysfunction, which is important in CKD cohorts. Plasma FGF23 was measured using the second generation C-terminal assay that detects two epitopes in the C-terminus of FGF23 (Immutopics, San Clemente, CA; coefficient of variation (CV) <5%). C-terminal and intact FGF23 assays are highly correlated in CKD,10 and a recent report of peritoneal dialysis patients that used an in vitro reporter assay suggested that virtually all circulating FGF23 was intact, biologically active, and accurately measured by either assay.48 Plasma PTH was measured using a total PTH assay, which includes the 1-84 PTH molecule and 7-84 fragments (Scantibodies, Santee, CA; CV <5%). Results from the 1-84 PTH assay were used in secondary analyses. Serum 25D was quantified by radioimmunoassay (Diasorin, Stillwater, MN; CV < 3%) and serum 1,25D was measured by competitive chemiluminescent immunoassay (Heartland Assays, Ames, IA; CV < 12%).
Statistical Analysis
We evaluated the univariate and eGFR-adjusted correlations between FGF23 and other mineral metabolites using Spearman correlation and linear regression. We performed descriptive analyses of mineral metabolites in the overall population and after categorizing participants into 7 fine categories of eGFR (<20, 20 – 29, 30 – 39, 40 – 49, 50 – 59, 60 – 69, and ≥ 70 ml/min/1.73m2, etc). We compared mean (median) values within each group to those in the eGFR > 70 ml/min/1.73m2 stratum with use of t tests or Wilcoxon tests, as appropriate. We defined hyperphosphatemia as serum phosphate ≥ 4.6 mg/dl,8 secondary hyperparathyroidism as PTH ≥ 65 pg/ml8 and FGF23 excess as FGF23 ≥ 100 RU/ml. Because normal reference values for FGF23 in healthy subjects with sufficient vitamin D levels are not available, we used the FGF23 threshold that was 2.5–fold higher than the median FGF23 and in the 90th percentile of the FGF23 distribution in previous studies of populations in which approximately 80% of patients had normal kidney function (eGFR >60 ml/min/1.73m2).15, 16 We estimated the prevalence of abnormally high values of FGF23, PTH and serum phosphate and calculated the proportions of participants with either isolated increases in FGF23 or PTH, abnormally high values of both hormones, or normal values of both within each of the 7 eGFR categories. We calculated the probability of an elevated FGF23 when PTH levels were < 65 pg/ml and, conversely, the probability of an elevated PTH when FGF23 levels were < 100 RU/ml. We compared proportions using Chi square tests. In additional sensitivity analyses, we repeated the probability calculations while excluding individuals treated with vitamin D supplements, phosphate binders and active vitamin D, after substituting FGF23 ≥125 RU/ml as the FGF23 cutpoint, and PTH >36 pg/ml as the cutpoint for the 1-84 PTH assay.18 We also repeated the main analyses in the subcohort of participants with iGFR.
We used cubic spline regression to fit separate curves for the relationships of log-transformed FGF23 and PTH with eGFR as the independent variable and used a bootstrapping procedure to determine the eGFR thresholds at which the slope of FGF23 and PTH became steeper, as described elsewhere.49 We estimated the value for this threshold as the minimizer of the profile residual sum of squares, as has been done previously.50 To explore the relationship of serum phosphate and FEPi with eGFR, we used similar methods to test for and estimate the threshold of eGFR at which serum phosphate and FEPi change. Because the fitted spline for serum phosphate displayed a J-shaped pattern, we tested if there was an eGFR threshold at which the direction of the slope changed. Because eGFR criteria for inclusion were age-stratified, and fasting status can affect levels of mineral metabolites, we performed additional sensitivity analyses that adjusted the spline models for age and fasting status. P values < 0.05 were considered statistically significant. We performed analyses using SAS, version 9.2 (SAS Institute, Cary, NC, USA) and R, Version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).
Acknowledgments
TI was supported by NIH grant K23DK087858. MW was supported by NIH grants R01DK076116 and R01DK081374. The CRIC Study is supported by cooperative agreement project grants 5U01DK060990, 5U01DK060984, 5U01DK06102, 5U01DK061021, 5U01DK061028, 5U01DK60980, 5U01DK060963, and 5U01DK060902 from the National Institute of Diabetes and Digestive and Kidney Diseases and by grants UL1RR024134, UL1RR025005, M01RR16500, UL1RR024989, M01RR000042, UL1RR024986, UL1RR029879, RR05096, and UL1RR024131 from the National Institutes of Health. We thank all the investigators and participants of the CRIC Study for their contributions.
Footnotes
Disclosures: Dr. Wolf has served as a consultant or received honoraria from Abbott Laboratories, Amgen, Ardelyx, Baxter, Cytochroma, Davita, Genzyme, Lutipold, Novartis, Mitsubishi and Shire. Dr. Isakova has served as a consultant and received honoraria from Shire.
References
- 1.Prie D, Urena Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882–9. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 2.Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–8. doi: 10.1038/ki.2009.466. [DOI] [PubMed] [Google Scholar]
- 3.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 4.Shigematsu T, Kazama J, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, et al. Possible Involvement of Circulating Fibroblast Growth Factor 23 in the Development of Secondary Hyperparathyroidism Associated with Renal Insufficiency. Am J Kidney Dis. 2004;44:250–56. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 6.Evenepoel P, Meijers B, Viaene L, Bammens B, Claes K, Kuypers D, et al. Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010;5:1268–76. doi: 10.2215/CJN.08241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isakova T, Gutierrez OM, Wolf M. A blueprint for randomized trials targeting phosphorus metabolism in chronic kidney disease. Kidney Int. 2009;76:705–16. doi: 10.1038/ki.2009.246. [DOI] [PubMed] [Google Scholar]
- 8.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–8. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 9.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–31. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 13.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq309. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–35. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- 15.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–90. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–8. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–57. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 18.Gao P, Scheibel S, D'Amour P, John MR, Rao SD, Schmidt-Gayk H, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16:605–14. doi: 10.1359/jbmr.2001.16.4.605. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–80. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 20.Kuro-o M. Klotho in chronic kidney disease--what's new? Nephrol Dial Transplant. 2009;24:1705–8. doi: 10.1093/ndt/gfp069. [DOI] [PubMed] [Google Scholar]
- 21.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–8. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 22.Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–8. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 23.Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, et al. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010;21:1125–35. doi: 10.1681/ASN.2009040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craver L, Marco MP, Martinez I, Rue M, Borras M, Martin ML, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1-5--achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–6. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 25.Martinez I, Saracho R, Montenegro J, Llach F. A deficit of calcitriol synthesis may not be the initial factor in the pathogenesis of secondary hyperparathyroidism. Nephrol Dial Transplant. 1996;11 3:22–8. doi: 10.1093/ndt/11.supp3.22. [DOI] [PubMed] [Google Scholar]
- 26.Bastepe M, Juppner H. Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Rev Endocr Metab Disord. 2008;9:171–80. doi: 10.1007/s11154-008-9075-3. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–91. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 29.Marsell R, Krajisnik T, Goransson H, Ohlsson C, Ljunggren O, Larsson TE, et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol Dial Transplant. 2008;23:827–33. doi: 10.1093/ndt/gfm672. [DOI] [PubMed] [Google Scholar]
- 30.Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant. 2010;25:993–7. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavik I, Jaeger P, Kistler AD, Poster D, Krauer F, Cavelti-Weder C, et al. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 2010 doi: 10.1038/ki.2010.375. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Slatopolsky E, Gradowska L, Kashemsant C, Keltner R, Manley C, Bricker NS. The control of phosphate excretion in uremia. J Clin Invest. 1966;45:672–7. doi: 10.1172/JCI105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrington K, Varghese Z, Newman SP, Ahmed KY, Fernando ON, Moorhead JF. Dissociation of absorptions of calcium and phosphate after successful cadaveric renal transplantation. Br Med J. 1979;1:712–4. doi: 10.1136/bmj.1.6165.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrington K, Mohammed MN, Newman SP, Varghese Z, Moorhead JF. Comparison of radioisotope methods for the measurement of phosphate absorption in normal subjects and in patients with chronic renal failure. Clin Sci (Lond) 1981;60:55–63. doi: 10.1042/cs0600055. [DOI] [PubMed] [Google Scholar]
- 35.Wiegmann TB, Kaye M. Malabsorption of calcium and phosphate in chronic renal failure: 32P and 45Ca studies in dialysis patients. Clin Nephrol. 1990;34:35–41. [PubMed] [Google Scholar]
- 36.Miyamoto K, Ito M, Kuwahata M, Kato S, Segawa H. Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial. 2005;9:331–5. doi: 10.1111/j.1744-9987.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 37.Heaney RP, Nordin BE. Calcium effects on phosphorus absorption: implications for the prevention and co-therapy of osteoporosis. J Am Coll Nutr. 2002;21:239–44. doi: 10.1080/07315724.2002.10719216. [DOI] [PubMed] [Google Scholar]
- 38.Liu SH, Chu HI. Studies on calcium and phosphorus metabolism with special reference to pathogenesis and effect of dihydrotachysterol (A.T. 10) and iron. Medicine. 1943;22:103–61. [Google Scholar]
- 39.Litzow JR, Lemann J, Jr, Lennon EJ. The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J Clin Invest. 1967;46:280–6. doi: 10.1172/JCI105530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kestenbaum B, Glazer NL, Kottgen A, Felix JF, Hwang SJ, Liu Y, et al. Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol. 2010;21:1223–32. doi: 10.1681/ASN.2009111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez OM, Isakova T, Enfield G, Wolf M. Impact of poverty on serum phosphate concentrations in the third National Health and Nutrition Examination Survey. J Ren Nutr. 2010 doi: 10.1053/j.jrn.2010.03.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez OM, Anderson C, Isakova T, Scialla J, Negrea L, Anderson AH, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. JASN. 2010 doi: 10.1681/ASN.2010020221. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–8. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 44.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17:2928–36. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, et al. Early Control of PTH and FGF23 in Normophosphatemic CKD Patients: A New Target in CKD-MBD Therapy? Clin J Am Soc Nephrol. 2010;5:286–91. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–53. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 47.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 48.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–85. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji M, Xiong C, Grundman M. Hypothesis testing of a change point during cognitive decline among Alzheimer's disease patients. J Alzheimers Dis. 2003;5:375–82. doi: 10.3233/jad-2003-5504. [DOI] [PubMed] [Google Scholar]
- 50.Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Stat Med. 2000;19:1555–66. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]