SUMMARY
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the brain. GABAA receptors are heteropentamers formed by assembly of multiple subunits that generate a wide array of receptors with particular distribution and pharmacological profiles. Malfunction of these receptors has been associated with the pathophysiology of epilepsy and contribute to an imbalance of excitatory and inhibitory neurotransmission. The process of epilepsy development (epileptogenesis) is associated with changes in the expression and function of a large number of gene products. One of the major challenges is to effectively determine which changes directly contribute to epilepsy development versus those that are compensatory or not involved in the pathology. Substantial evidence suggests that changes in the expression and function of GABAA receptors are involved in the pathogenesis of epilepsy. Identification of the mechanisms involved in GABAA receptor malfunction during epileptogenesis and the ability to reverse this malfunction are crucial steps towards definitively answering this question and developing specific and effective therapies.
EPILEPSY
Epilepsy is one of the oldest conditions known to mankind and is still the most common neurological condition affecting individuals of all ages. At any given time approximately 50 million people world wide have epilepsy [2]. Epilepsies are characterized by spontaneous recurrent unprovoked seizures (2 or more) caused by focal or generalized paroxysomal changes in neurological function triggered by abnormal synchronized electrical activity [2, 15, 19, 23, 47]. Temporal lobe epilepsy (TLE) is the most common form of epilepsy and is often associated with a pathology termed mesial temporal sclerosis characterized by neuronal loss and synaptic reorganization. The most common risk factors for epilepsy are cerebrovascular disease, brain tumors, alcohol, traumatic head injuries, malformations of cortical development, genetic inheritance and infections of the central nervous system (CNS)[47].
GABA RECEPTORS AND EPILEPSY
Most fast synaptic inhibition in the mature brain is mediated by pentameric anion-selective GABAA receptors assembled from multiple subunit subtypes (α1–2, β1–3, γ1–3, δ, ε, π, θ, and σ1–3). GABAA receptors subunit composition dictates the intrinsic properties of each channel in terms of GABA affinity, kinetics, conductance, allosteric modulation, probability of channel opening and interaction with modulatory proteins and subcellular distribution [12, 19, 26]. Synaptic GABAA receptors contain γ subunits whereas those located at perisynaptic or extrasynaptic sites contain predominantly δ subunits, electrophysiologically these receptors are responsible for phasic and tonic inhibition, respectively [19, 26]. Alterations in the expression and function of GABAA receptor subunits have been documented in animal models and human cases of TLE [25, 31, 48]. The general observation from these studies is that GABAA receptor function is augmented, but despite increased inhibition there is significant alterations in the subunit composition and function of GABAA receptors [5, 8, 18, 31, 37–39, 54]. In dentate gyrus neurons (DGN) from the hippocampus, an increase in GABAA receptor current density, zinc blockade and clonazepam augmentation is observed, while neurons from the CA1 region have reduced GABA currents and clonazepam augmentation [20, 30]. These changes appear to be directly correlated with changes in the gene expression and subcellular distribution of receptors within individual pyramidal neurons that result in increased somatic but reduced dendritic inhibition [8, 13].
EPILEPTOGENESIS
The term “epileptogenesis” describes the process by which a brain develops spontaneous seizures or epilepsy, and is often divided into three stages: acute injury, latent period, and spontaneous seizures [7, 40, 42, 49]. In humans, the development of spontaneous seizures is preceded by a latent period lasting months or years after an initial precipitating event like head injury, prolonged febrile seizures, stroke, or status epilepticus (SE) [7, 40, 42]. Studies in animal models have shown that the latent period is characterized by a diverse array of cellular and molecular changes. Continuous monitoring of animal models has been used to define the time needed for the development of spontaneous seizures, and some studies have found that spontaneous seizures may appear in as few as 3 days after SE [9, 27, 43, 55]. Despite intense investigation, it is still unclear whether all epileptogenic events are restricted to the latent period, or whether the end of the latent period is not a terminal milestone but just another step in a continuous chain of events that extends beyond the first spontaneous seizure [49, 55]. Several laboratories, including our own, have focused on the study of diverse mechanisms involved in gene regulation and plasticity of GABA receptors during this period. Clarification of the cause-effect relationship of the changes in ion channel expression during the epileptogenic period is a major goal in this area of research.
STATUS EPILEPTICUS
Status epilepticus was initially defined by Gastaut as ‘a term used whenever a seizure persists for a sufficient length of time or is repeated frequently enough to produce a fixed or enduring epileptic condition’ [10]. There is clinical and experimental evidence that status epilepticus becomes self-sustaining after 30 min of continuous seizures promoting evident tissue damage and pharmacoresistance [10]. Clinically, this period of time has been limited to 5–15 min mostly to avoid a delay in therapeutic intervention. The fundamental pathophysiology of SE involves a failure of mechanisms that would normally abort an isolated seizure resulting in neuronal hyperexcitability and compromised GABAergic neurotransmission [17, 32, 50]. Mechanistic information suggests that reduced presence of GABAA receptors at the plasma membrane may be responsible for the loss of GABAergic responses and the resistance to benzodiazepines [21, 28, 35]. In support of this, pharmacological blockade of GABA receptors with allosteric modulators is only effective shortly after SE initiation because at later time points (>1 h) pharmacoresistance develops and GABAergic drugs become ineffective [21, 28, 52].
RECEPTOR REGULATION DURING SE
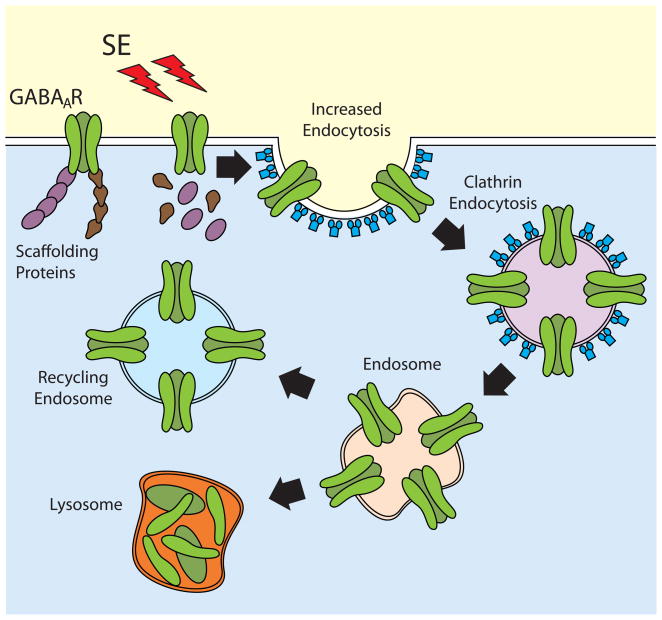
During status epilepticus there is increased neuronal hyperexcitability and inhibitory GABAergic synaptic transmission becomes compromised, miniature inhibitory post-synaptic currents (mIPSCs) are reduced and the number of active GABAA receptors per dentate granule cell is also decreased [10, 22, 35]. Enhanced clathrin-dependent GABAA receptor internalization has emerged as one of the rapid mechanisms to explain the self-sustaining nature of seizures during SE [22, 26]. In vitro studies using hippocampal neurons stimulated with low-magnesium to promote spontaneous recurrent epileptiform discharges showed a large decrease in GABA-gated chloride currents that correlates with reduced cell surface expression and intracellular accumulation of GABAA receptors [4, 22]. In vivo studies using chemoconvulsants have shown that SE promotes a rapid reduction in the number of physiologically active GABA receptors in granule cells that correlates with a reduction in the level of β2/β3 and γ2 immunoreactivity present in the vicinity of a presynaptic marker [35]. In fact, SE appears to trigger subunit specific events to regulate the trafficking of GABAA receptors by promoting the dephosphorylation of β3 subunits [21, 52]. Decreased phosphorylation of β3 increases the interaction of GABA receptors with the clathrin-adaptor protein 2 (AP2), facilitating the recruitment of GABA receptors into clathrin-coated pits and promoting its removal from the plasma membrane [21, 52] (Fig. 1). Increased GABA receptor phosphorylation or the blockade AP2 normal function results in GABA receptor accumulation at the plasma membrane and improved synaptic inhibition in hippocampal slices obtained from mice after SE [52]. Although internalization and intracellular accumulation of different GABA receptor subunits has been documented immediately after SE induction, the fate of internalized receptors has not been detailed. Internalized receptors can be recycled back to the plasma membrane or transported to the lysosomes for degradation [10], but the details of this step of the regulation remain to be investigated.
Figure 1. Regulation of GABAA receptor trafficking during SE.
After SE induction GABAA receptors are internalized via a clathrin-dependent mechanism. Following endocytosis, receptors can be recycled back to the plasma membrane or sorted to the lysosomes for degradation.
EPILEPTOGENIC PERIOD
Epileptogenesis is accompanied by many changes in synaptic plasticity and in passive and active membrane properties of neurons [3, 29, 34]. Hippocampal networks are hyperexcitable during the latent period and EEG measurements display interictal-like activity [16]. Hippocampal pyramidal neurons change from a normal regular firing pattern to burst firing in response to depolarization or even spontaneously a few days after SE [46]. This abnormal activity acts as a pacemaker synchronizing entire cell populations [46]. During this period, there appears to be an overall reorganization of glutamatergic and GABAergic networks. A loss of interneurons and mossy cells reduces the number of GABAergic and glutamatergic synapses [6, 53].
GENE REGULATION DURING THE LATENT PERIOD
Repeated or prolonged seizures produce a broad and complex cascade of pathophysiological and biochemical changes in the brain. In the minutes to hours after SE, activation of plasma membrane receptors result in changes in the intracellular signal transduction pathways involved in the maintenance of vital cellular functions [10, 34]. In the following hours and days, long-term changes in gene expression result from the combined effects of repeated seizures, seizure-induced cell death, and subsequent neuronal reorganization [10]. In DGC of adult rodents, pilocarpine-induced SE reduces the expression of the α1 [8] and γ [37] subunits of GABAA receptors and increases the expression of α4 subunits [8, 37]. This is associated with an increased abundance of α4γ2 containing receptors, a reduction in α1γ2 containing receptors in dentate gyrus [33] and shift of γ2-containing receptors from synaptic to perisynaptic locations, likely as part of α4βxγ2 receptors [57]. In contrast, when neonatal rodents (at postnatal day 10) are subject to SE, mRNA of the α1 subunit increases over-time [56]. Adult rats uniformly develop the recurrent spontaneous seizures that define epilepsy, but neonatal rats do not [56]. The changes in GABAA receptor subunit expression observed in adult animals precede the development of spontaneous seizures, suggesting a possible correlation between the changes in GABAA receptor expression and the epileptogenic process.
More direct evidence for a role of GABA receptor subunit expression in epileptogenesis was obtained by over-expression of α1 subunits using an adeno-associated virus for gene transfer [43]. Expression of a bicistronic RNA containing the coding information for the α1 subunit and the yellow enhanced fluorescent protein was placed under control of the α4 subunit promoter, a promoter that is markedly activated in the dentate gyrus after SE [43]. Rats injected with the virus showed increased expression of α1 subunits in the dentate gyrus two weeks after SE and had a 3-fold increase in the mean time to the first spontaneous seizure. More importantly, in the first 4 weeks after SE, only ~40% of the virus injected rats develop spontaneous seizures while a 100% of the rats receiving sham-injections became epileptic, providing direct evidence that increasing the levels of a single GABA receptor subunit in DG can inhibit the development of spontaneous seizures after SE [43].
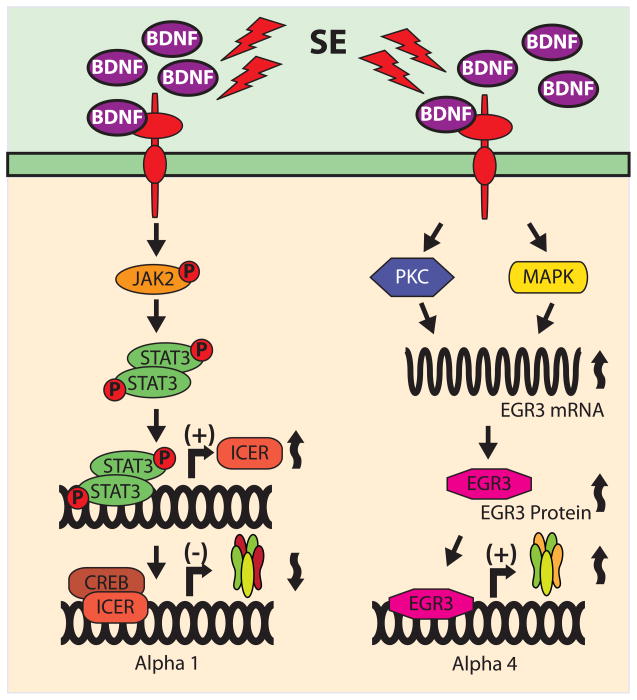
SE is associated with excessive neuronal activity that stimulates many different signaling pathways. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is activated during seizures and ischemia as well as by stress and inflammation [11, 14, 24, 41, 51]. Typically, cytokine receptors activate JAK/STAT by first promoting JAK phosphorylation that in turn phosphorylates STATs [14, 24, 58, 59]. Tyrosine phosphorylation of STATs promotes its homo- or hetero-dimerization followed by translocation to the nucleus where they bind to specific DNA elements (STAT-recognition sites) to regulate gene expression [1, 58]. One of the transcriptional elements regulated by STAT binding is found in the promoter of the inducible cAMP early repressor (ICER). In vitro studies using hippocampal neurons show that brain derived neurotrophic factor (BDNF) differentially regulates the expression of α1 and α4 GABAA receptor subunits [44]. In cultured neurons, BDNF increases binding of phosphorylated STAT3 to the ICER promoter and up-regulates ICER mRNA and protein expression. Blockade of JAK/STAT signaling with pyridone 6 or by STAT3 siRNA-knockdown inhibits the effects of BDNF on ICER expression [33]. Most importantly, in vivo experiments show that administration of the JAK inhibitor pyridone 6 into the dentate gyrus blocks the induction of ICER and the subsequent decrease in the expression of mRNA for the α1 subunit [33]. These findings suggest a key interplay among signaling pathways involving BDNF, JAK/STAT, and ICER that are critical for the regulation of GABAA receptor subunits in response to SE (Fig. 2). The potential for these signaling pathways as novel therapeutic targets for prevention and/or treatment of epilepsy are currently under investigation.
Figure 2. BDNF-stimulated signaling pathways differentially regulate GABAA receptor expression.
BDNF may regulate the final composition of GABAA receptors by differentially altering the expression of α1 and α4 subunits. Both in vivo and in vitro evidence suggest that increased levels of BDNF following status epilepticus (SE) activate at least two different signaling pathways: JAK/STAT and PKC/MAPK, resulting in the down-regulation of α1 subunits and the up-regulation of α4 subunits, respectively.
Shortly after SE induction, animal models of epilepsy show increased expression of the α4 subunit [45] and increased abundance of α4γ2 containing receptors with a concomitant reduction in α1γ2 containing receptors [33]. Strong evidence suggests that, BDNF is also responsible for triggering the endogenous signals involved in the regulation of α4 subunit expression. In vitro experiments showed that activation of PKC and MAPK by BDNF (or phorbol ester) application results in increased expression of the early growth response factor 3 (Egr3) (Fig. 2) [44]. Egr3 belongs to a family of transcription factors composed of four proteins (Egr1, 2, 3 and 4) that share nearly identical zinc finger DNA binding domains and bind to a common Egr response element (ERE) consensus sequence [36]. In vivo experiments showed that SE triggered increases in mRNA and protein levels of Egr3 and enhanced binding of Egr3 to the promoter of the α4 GABAA receptor subunit gene in cells from the dentate gyrus 24 hours after pilocarpine-induced SE [45]. In addition, mice devoid of Egr3 have significant lower levels of α4 mRNA strongly suggesting that Egr3 may be a critical regulator of endogenous GABAA receptors containing α4 subunits [45].
CONCLUSION
Temporal information concerning the cascade of molecular events underlying the pathophysiology of epilepsy is starting to emerge, and the time during which epilepsy slowly develops is now been more carefully studied [49, 55]. At this point, there is no doubt that the epileptogenic period includes a diverse array of molecular events that contribute to increased excitability in the immediate days following SE and continue as epilepsy develops [16, 27, 30]. It will be necessary to identify many additional transcriptional and posttranscriptional events triggered by SE in order to build an integrated view of the myriad of molecular events taking place during epileptogenesis. A detailed characterization of the regulation of GABAA receptors as well as that of many other individual proteins during this period is a necessary step in the process of establishing true correlations between molecules and function in order to tailor new therapeutic strategies for the prevention and treatment of epilepsy.
Acknowledgments
We would like to acknowledge Dr. Shelley J. Russek (S.J.R.) for her collaboration, which was essential for all of our studies regarding the transcriptional regulation of GABAA receptors. NINDS R01-NS051710 (A.B.-K.), R01-NS038595 (A.B.-K. and S.J.R.), R01-NS050393 (S.J.R. and A.B.-K.), and K01-NS069583 (M.I.G) support our research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy-a review. Epilepsy Res. 2009;85:31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- 4.Blair RE, Sombati S, Lawrence DC, McCay BD, DeLorenzo RJ. Epileptogenesis causes acute and chronic increases in GABA(A) receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J Pharmacol Exp Ther. 2004;310:871–880. doi: 10.1124/jpet.104.068478. [DOI] [PubMed] [Google Scholar]
- 5.Bouilleret LF, Kiener V, Marescaux TC, Fritschy J-M. Early Loss of Interneurons and Delayed Subunit-Specific Changes in GABA(A)-Receptor Expression in a Mouse Model of Mesial Temporal Epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Boulland JL, Ferhat L, Tallak Solbu T, Ferrand N, Chaudhry FA, Storm-Mathisen J, Esclapez M. Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J Comp Neurol. 2007;503:466–485. doi: 10.1002/cne.21384. [DOI] [PubMed] [Google Scholar]
- 7.Brooks-Kayal AR, Raol YH, Russek SJ. Alteration of epileptogenesis genes. Neurotherapeutics. 2009;6:312–318. doi: 10.1016/j.nurt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 9.Bumanglag AV, Sloviter RS. Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats. J Comp Neurol. 2008;510:561–580. doi: 10.1002/cne.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. 2007;186:7–15. [PubMed] [Google Scholar]
- 11.Choi JS, Kim SY, Park HJ, Cha JH, Choi YS, Kang JE, Chung JW, Chun MH, Lee MY. Upregulation of gp130 and differential activation of STAT and p42/44 MAPK in the rat hippocampus following kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;119:10–18. doi: 10.1016/j.molbrainres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 14.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 15.Dichter MA. Emerging insights into mechanisms of epilepsy: implications for new antiepileptic drug development. Epilepsia. 1994;35(Suppl 4):S51–57. doi: 10.1111/j.1528-1157.1994.tb05956.x. [DOI] [PubMed] [Google Scholar]
- 16.El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng HJ, Mathews GC, Kao C, Macdonald RL. Alterations of GABA(A)-receptor function and allosteric modulation during development of status epilepticus. J Neurophysiol. 2008;99:1285–1293. doi: 10.1152/jn.01180.2007. [DOI] [PubMed] [Google Scholar]
- 18.Fritschy KT, Bouilleret J-MV, Loup F. GABAergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–445. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 19.Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbs J, Shumate M, Coulter D. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. Journal of Neurophysiology. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- 21.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABA(A) receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia. 1975;16:1–66. doi: 10.1111/j.1528-1157.1975.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 24.Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 25.Isokawa M. Decrement of GABAA receptor-mediated inhibitory postsynaptic currents in dentate granule cells in epileptic hippocampus. J Neurophysiol. 1996;75:1901–1908. doi: 10.1152/jn.1996.75.5.1901. [DOI] [PubMed] [Google Scholar]
- 26.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate graule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol. 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective Alterations in GABA(A) receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20:5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 33.Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 35.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- 37.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perlin JB, Gerwin CM, Panchision DM, Vick RS, Jakoi ER, DeLorenzo RJ. Kindling produces long-lasting and selective changes in gene expression of hippocampal neurons. Proc Natl Acad Sci U S A. 1993;90:1741–1745. doi: 10.1073/pnas.90.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirker SC, Czech T, Baumgartner C, Pockberger H, Maier H, Hauer B, Sieghart W, Furtinger S, Sperk G. Increased expression of GABA(A) receptor beta-subunits in the hippocampus of patients with temporal lobe epilepsy. J Neuropathol Exp Neurol. 2003;62:820–834. doi: 10.1093/jnen/62.8.820. [DOI] [PubMed] [Google Scholar]
- 40.Pitkanen A, Immonen RJ, Grohn OH, Kharatishvili I. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia. 2009;50(Suppl 2):21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 41.Planas AM, Soriano MA, Berruezo M, Justicia C, Estrada A, Pitarch S, Ferrer I. Induction of Stat3, a signal transducer and transcription factor, in reactive microglia following transient focal cerebral ischaemia. Eur J Neurosci. 1996;8:2612–2618. doi: 10.1111/j.1460-9568.1996.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 42.Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- 45.Roberts DS, Raol YH, Bandyopadhyay S, Lund IV, Budreck EC, Passini MA, Wolfe JH, Brooks-Kayal AR, Russek SJ. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc Natl Acad Sci U S A. 2005;102:11894–11899. doi: 10.1073/pnas.0501434102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanabria ER, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol. 2001;532:205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scorza FA, Arida RM, Naffah-Mazzacoratti Mda G, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc. 2009;81:345–365. doi: 10.1590/s0001-37652009000300003. [DOI] [PubMed] [Google Scholar]
- 48.Shumate M, Lin D, JG, Holloway K, Coulter D. GABAA Receptor Function in Epileptic Human Dentate Granule Cells: Comparison to Epileptic and Control Rat. Epilepsy Res. 1998;32:114–128. doi: 10.1016/s0920-1211(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 49.Sloviter RS. Hippocampal epileptogenesis in animal models of mesial temporal lobe epilepsy with hippocampal sclerosis: the importance of the “latent period” and other concepts. Epilepsia. 2008;49(Suppl 9):85–92. doi: 10.1111/j.1528-1167.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 50.Staley KJ. Role of the depolarizing GABA response in epilepsy. Adv Exp Med Biol. 2004;548:104–109. doi: 10.1007/978-1-4757-6376-8_8. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki S, Tanaka K, Nogawa S, Dembo T, Kosakai A, Fukuuchi Y. Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol. 2001;170:63–71. doi: 10.1006/exnr.2001.7701. [DOI] [PubMed] [Google Scholar]
- 52.Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol. 518:647–667. doi: 10.1002/cne.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 55.Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang G, Raol YH, Hsu FC, Coulter DA, Brooks-Kayal AR. Effects of status epilepticus on hippocampal GABAA receptors are age-dependent. Neuroscience. 2004;125:299–303. doi: 10.1016/j.neuroscience.2004.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong Z, Wen Z, Darnell JE. STAT3 and STAT4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci USA. 1994;91:4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong Z, Wen Z, Darnell JE. STAT3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]