Abstract
Venezuelan equine encephalitis (VEE) is an emerging infectious disease in Latin America. Outbreaks have been recorded for decades in countries with enzootic circulation, and the recent implementation of surveillance systems has allowed the detection of additional human cases in countries and areas with previously unknown VEE activity. Clinically, VEE is indistinguishable from dengue and other arboviral diseases and confirmatory diagnosis requires the use of specialized laboratory tests that are difficult to afford in resource-limited regions. Thus, the disease burden of endemic VEE in developing countries remains largely unknown, but recent surveillance suggests that it may represent up to 10% of the dengue burden in neotropical cities, or tens-of-thousands of cases per year throughout Latin America. The potential emergence of epizootic viruses from enzootic progenitors further highlights the need to strengthen surveillance activities, identify mosquito vectors and reservoirs and develop effective strategies to control the disease. In this article, we provide an overview of the current status of endemic VEE that results from spillover of the enzootic cycles, and we discuss public health measures for disease control as well as future avenues for VEE research.
Keywords: alphavirus, encephalitis, endemic, epizootic, mosquito, surveillance
Venezuelan equine encephalitis virus (VEEV) is a member of the family Togaviridae, genus Alphavirus, and is one of the virus species in the VEE complex. This complex also includes Mosso das Pedras, Cabassou, Everglades, Mucambo, Pixuna and Rio Negro species (Table 1). Each of these subtypes demonstrates unique characteristics with respect to ecology, epidemiology and virulence in both humans and equids, and most are known to cause both animal and/or human disease ranging from febrile illness to encephalitis.
Table 1.
Venezuelan equine encephalitis antigenic complex alphaviruses
| Species | Antigenic subtype | Antigenic variety | Clinical syndrome | Distribution | Vector |
|---|---|---|---|---|---|
| VEEV | VEE-I | AB | Febrile illness, encephalitis | North, Central and South America | Mammalophilic mosquitoes |
| C | Febrile illness, encephalitis | South America | Mammalophilic mosquitoes | ||
| D | Febrile illness, encephalitis | South America, Panama | Culex (Melanoconion) ocossa, panocossa, vomerifer, pedroi, adamesi | ||
| E | Febrile illness, encephalitis | Central America, Mexico | Culex (Melanoconion) taeniopus, Deinocerites pseudes, Aedes (Ochlerotatus) taeniorhynchus | ||
| Mosso das Pedras | F | None recognized | Brazil | Unknown | |
| Everglades | VEE-II | Febrile illness, encephalitis | Florida (USA) | Culex (Melanoconion) cedecei | |
| Mucambo | VEE-III | A | Febrile illness, myalgia | South America, Trinidad | Culex (Melanoconion) portesi |
| C (strain 71D1252) | Unknown | Peru | Unknown | ||
| D | Febrile illness | Peru | Culex (Melanoconion) gnomatos | ||
| Tonate | VEE-IIIB | Febrile illness, encephalitis | Brazil, Colorado (USA) | Unknown (Brazil), Oeciacus vicarius (Colorado) | |
| Pixuna | VEE-IV | Febrile illness, myalgia | Brazil | Aedes (Ochlerotatus) hastatus, Aedes aegypti | |
| Cabassou | VEE-V | None recognized | French Guiana | Unknown | |
| Rio Negro | VEE-VI | Febrile illness, myalgia | Argentina | Mammalophilic mosquitoes |
VEE: Venezuelan equine encephalitis; VEEV: Venezuelan equine encephalitis virus.
Venezuelan equine encephalitis virus was first isolated in 1938 from the brain of a horse that died of encephalitis [1,2]. However, clinical disease in equids, referred as ‘peste loca’, was documented in South America in the 1920s [3,4]. In 1950, the virus was isolated from human cases during an outbreak of febrile illness in Espinal, Colombia. Occasional neurological complications and associated mortality were also first reported in humans during this time period [5].
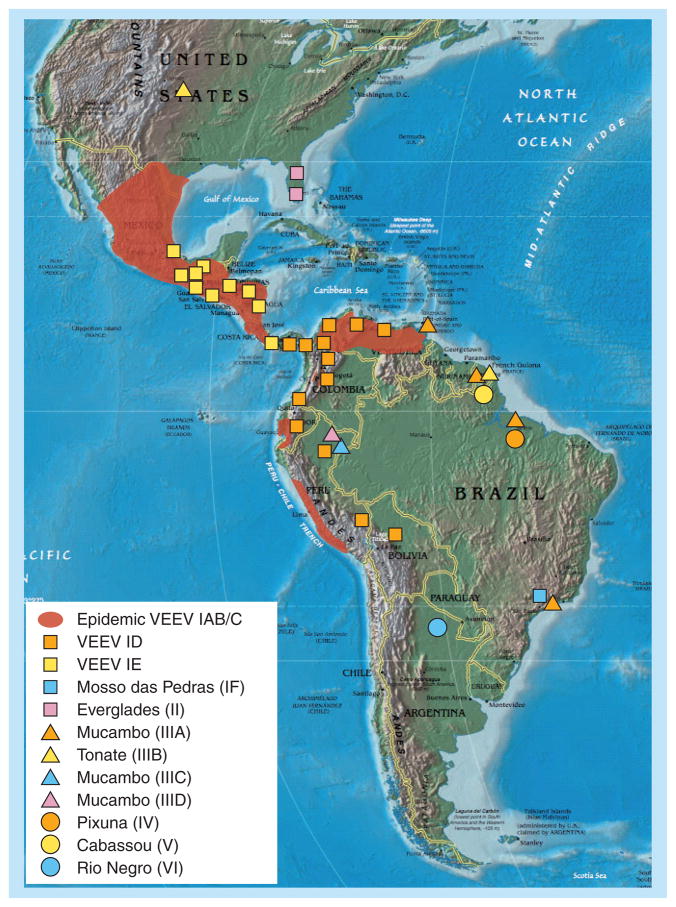
Venezuelan equine encephalitis virus is widely distributed throughout the Americas. Outbreaks of VEE in humans and equids have been reported in at least 12 countries, including Venezuela, Colombia, Peru, Ecuador, Costa Rica, Nicaragua, Honduras, El Salvador, Guatemala, Panama, Mexico and the USA (Figure 1).
Figure 1. Map showing the known distribution of Venezuelan equine encephalitis complex alphaviruses in the Americas.
VEEV: Venezuelan equine encephalitis virus.
Reproduced with permission from [116] © Elsevier.
VEE complex
Viruses
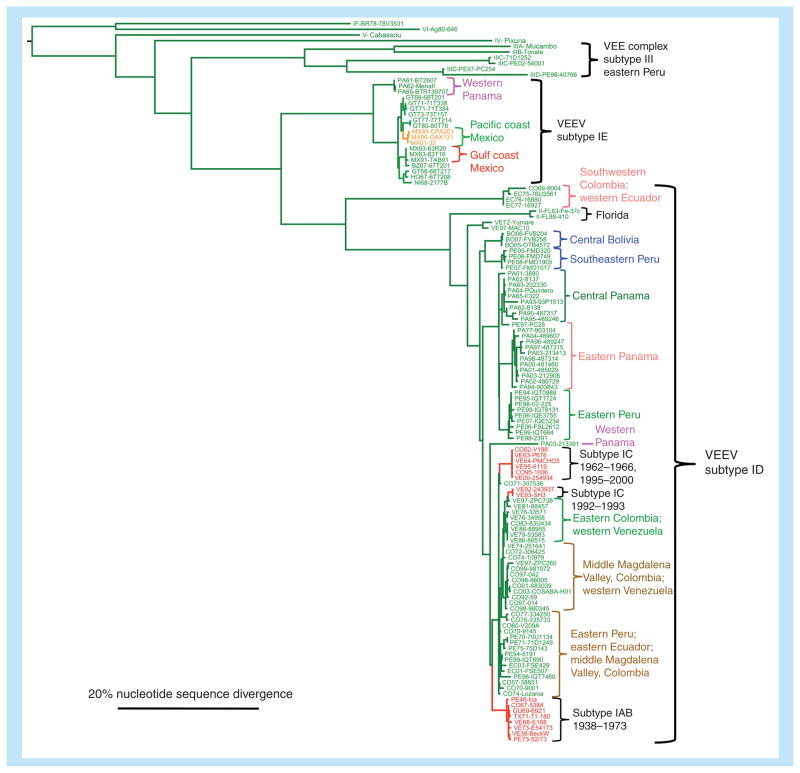
At least 14 subtypes and varieties within the VEE complex have been described (Table 1 & Figure 2) [6]. Only subtype I varieties AB and C have been associated with major equine epizootics and epidemics involving up to hundreds of thousands of equids and humans. In this article, we refer to these subtype IAB/C, equid-amplified strains as epizootic. They exist in the epizootic cycle for periods of only months to years, and are thought to become extinct between outbreaks because the supply of naive horses becomes exhausted through mortality and immunity in survivors. By contrast, the enzootic strains in subtype I varieties D–F, and subtypes II–VI, are typically avirulent for equids, although they cause clinical disease in humans that is indistinguishable from that caused by epizootic strains. These enzootic strains generally circulate among rodents in forest or swamp habitats in continuous cycles. Interestingly, VEEV IE strains from recent Mexican epizootics appear to be equine neurovirulent but are not known to produce high-titer viremia in equids. It is therefore unlikely that equids serve as important amplification hosts for this variety of VEEV [7].
Figure 2. Phylogenetic tree showing relationships of epizootic and enzootic/endemic Venezuelan equine encephalitis virus strains.
Clades including isolates from outbreaks discussed in the text are color-coded to correspond to the shading of the maps shown in Figures 4–7. The tree was generated using 817-nucleotide sequences from the PE2 envelope glycoprotein gene using maximum likelihood methods. Scale shows 20% nucleotide sequence divergence.
VEE: Venezuelan equine encephalitis; VEEV: Venezuelan equine encephalitis virus.
Epizootic VEEV
Vectors & hosts
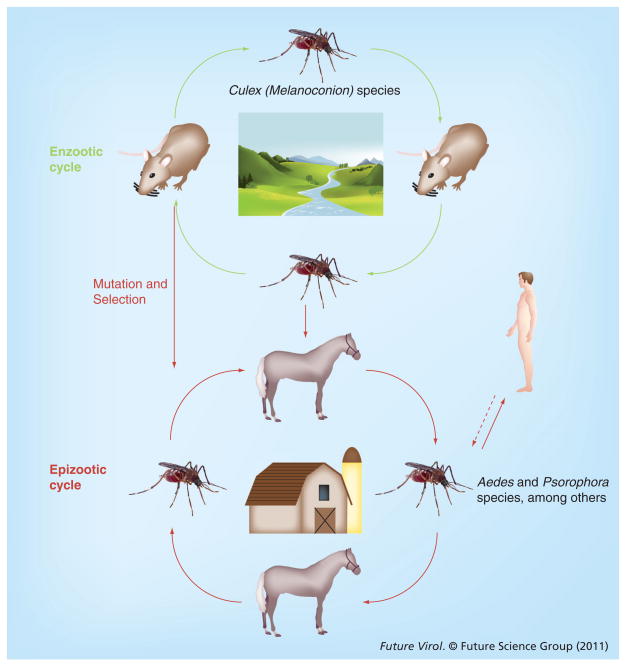
By contrast to other members within the VEE complex, epizootic VEEV strains are efficiently amplified in an equids–mosquito cycle that typically occurs in agricultural settings (Figure 3). Through a combination of observations made during field studies and through experimental infections, the infection of horses, donkeys and mules with epizootic VEEV varieties are known to typically result in high rates of overt illness, with animals developing viremia levels that are sufficient for infection of susceptible mosquitoes [4,8]. Several species have been incriminated as vectors of epizootic VEEV IAB and IC, including Psorophora columbiae, Psorophora confinnis, Aedes sollicitans, Aedes taeniorhynchus, Mansonia indubitans and Deinocerites pseudes [9–12].
Figure 3. The enzootic and epizootic transmission cycles of Venezuelan equine encephalitis virus.
The vertical red arrow indicates host range changes involving adaptive E2 envelope glycoprotein amino acid substitutions that adapt epizootic strains for equine amplification or transmission by epizootic vectors.
Epizootics & epidemics
The first well-documented VEE outbreak involving equids occurred in the central river valleys of Colombia in 1935 and spread to Venezuela in the following year. By 1943, the outbreak had spread to Trinidad [13]. Additional epizootics were reported from coastal Peru from 1942 through to 1946 [14].
One of the largest VEE outbreaks began in La Guajira, Colombia, in 1962. It initially involved approximately 3000 human cases, of which 20 were fatal. This outbreak then spread into Venezuela, where it caused 23,283 human cases, including 960 neurologic cases and 156 deaths, reported over a 26-month period. Data regarding the number of equine cases in this outbreak are scarce. During 1967 and 1968, epizootics were observed in Colombia, but the exact numbers of equine and human cases were not documented. In early 1969, a large outbreak was reported in Ecuador involving approximately 31,000 human cases with 310 fatalities and approximately 20,000 equine deaths. Late in 1969, epizootics were reported in El Salvador and Guatemala; these outbreaks eventually spread to most of Central America and Mexico [15,16]. During this outbreak, an estimated 50,000 horses died, in addition to an estimated 52,000 human cases, of which 93 were fatal in Mexico alone [13,17,18]. Initially, equine deaths in Mexico were reported in Chiapas state near the Guatemalan border in the summer of 1969, but by 1970, approximately 10,000 equine deaths had occurred in the Pacific states of Chiapas and Oaxaca. This outbreak then spread northward into 17 Mexican states, following the path of the susceptible equids, to the Gulf coast and eventually into southern Texas [18,19]. The outbreak was finally contained when more than 8 million doses of TC-83 vaccine were administered to equids and vector control was implemented [19]. The last Mexican equine cases were recorded in September 1972 on the Islas Marias, Nayarit [19]. In Texas, between June and August of 1971, almost 2000 infected horses were reported, including 1426 associated deaths. During the same time period, 110 human cases were confirmed.
After 19 years of inactivity, outbreaks of VEE were again reported in the Americas. In 1992, an initial outbreak was reported in Venezuela, followed by additional outbreaks in 1993 in both Venezuela and Mexico [20]. In 1995, both Venezuela and Colombia reported outbreaks [21–23] involving an estimated 100,000 human cases, 3000 of which experienced neurologic complications, with 300 associated deaths [21]. There were also at least 4000 equine deaths associated with this outbreak. Small equine epizootics were reported in Mexico in 1993 and 1996 [20].
Evolution of epizootic subtype IAB & IC strains
Due to the sporadic nature of VEEV epizootics, identification of the origin and factors that contribute to the emergence of the etiologic viruses is critical for controling and preventing disease. At least five hypotheses have been formulated [4] to explain the origin of epizootic viruses:
Their existence in cryptic transmission cycles;
Their maintenance in equine populations or other animal populations;
Incomplete inactivation of vaccine preparations that, upon administration, lead to viremia and wild-type VEEV circulation;
Their existence as minority populations within enzootic VEEV populations;
Their emergence from an enzootic VEEV progenitor population.
Evidence supporting the incomplete inactivation of vaccine preparations as the origin of epizootic viruses was obtained from genetic studies of subtype IAB viruses [24,25]. In addition, evidence for the emergence of epizootic VEEV from enzootic progenitors was first obtained from antigenic and RNA fingerprinting studies. Later, genetic studies described remarkable similarities between epizootic IC and ID viruses (Figure 2) [25–28]. Critical evidence was gained from the IC epidemic of western Venezuela in 1992–1993, when closely related subtype ID isolates were almost simultaneously isolated in the Venezuelan region affected by the outbreak. A total of 15 amino acid differences were identified between the sympatric ID and IC strains, and two of these were within the E2 glycoprotein, which is suspected to be the major determinant of equine virulence and amplification potential [29]. Strikingly, a single amino acid substitution from Thr to Lys at position 213 of the E2 glycoprotein was sufficient to transform the equine viremia phenotype of a subtype ID virus from enzootic to epizootic, providing strong evidence for the emergence of epizootic virus from enzootic progenitors [30]. Additional studies are required to investigate whether other enzootic strains can become epizootic via the introduction of similar mutations in the E2 glycoprotein.
Enzootic & endemic VEEV
Vectors & hosts
Enzootic VEE complex viruses are frequently isolated in specific ecological habitats, where they circulate in transmission cycles involving rodents and mosquitoes (Figure 3). The principal rodent reservoirs are sylvatic species in the genera Sigmodon, Oryzomys, Zigodontomys, Heteromys, Peromyscus and Proechimys. These animals are frequently found to be infected in nature and develop viremia that is sufficient for vector infection [4,31,32]. Enzootic transmission cycles have been described for the VEEV varieties ID, IE, Everglades, Mucambo and Tonate (Bijou Bridge); all of which, with the exception of Bijou Bridge, are transmitted and maintained in a cycle involving rodents and mosquitoes in the subgenus Culex (Melanoconion) [31,33–36]. Bijou Bridge virus is transmitted by the cliff swallow bug, Oeciacus vicarius, to birds in western North America [37]. Enzootic cycles have also been inferred for Pixuna and Rio Negro viruses and involve Culex (Melanoconion), Aedes, Psorophora and other mosquitoes [38,39]. Thus, Culex (Melanoconion) species are believed to be the primary vectors of most or all enzootic VEE complex strains [4,31].
Endemic VEE in the Americas
Evidence for human infections via spillover from the enzootic cycles began to emerge in the 1960s. Today, there is growing evidence that this form of endemic VEE is widespread in the neotropics. In the following sections, we summarize endemic VEE in several countries of the Americas.
Bolivia & Ecuador
The circulation of VEEV subtype ID in Bolivia was first reported in 2005, when the virus was isolated from febrile patients residing in Eterazama, Cochabamba [40,41]. Since this initial report, VEEV has been almost continuously isolated in Bolivia from patients with no history of travel within 30 days of their illness, suggesting that VEE is endemic in Bolivia. Surveillance conducted in Cochabamba revealed that VEEV causes a similar number of febrile cases to dengue virus (7 vs 6.6%, respectively, of dengue-like illness) [41], providing evidence that VEE represents a serious public health concern in the area and that its impact has been greatly underestimated. Moreover, an adult Bolivian patient developed neurological disease after infection with this Bolivia/Peru VEEV subtype ID genotype, confirming the severe disease potential of enzootic VEEV from this region [40]. Ecological studies are still needed to identify reservoir hosts and mosquito vectors involved in enzootic transmission in Bolivia, as well as to determine whether VEEV circulates in other areas of the country that are not sampled by current surveillance activities.
In Ecuador, VEE was first confirmed in 1944 when VEEV was isolated from the blood of a horse suffering from encephalitis [42]. Additional equine and human cases compatible with VEEV infection have been reported since 1944.
Limited surveillance conducted at two health centers in Pastaza, Ecuador, yielded the isolation of VEEV in 2001 and again in 2003. VEE accounted for approximately 0.3% of the febrile cases seeking medical attention, whereas dengue cases represented approximately 5.3% of the undifferentiated febrile illness in this region [43].
In summary, surveillance activities for febrile illness have detected enzootic VEEV in Bolivia, a country with no prior history of VEEV activity. In addition, the presence of VEE in a new area of Ecuador has also been recorded.
Phylogenetic relationships
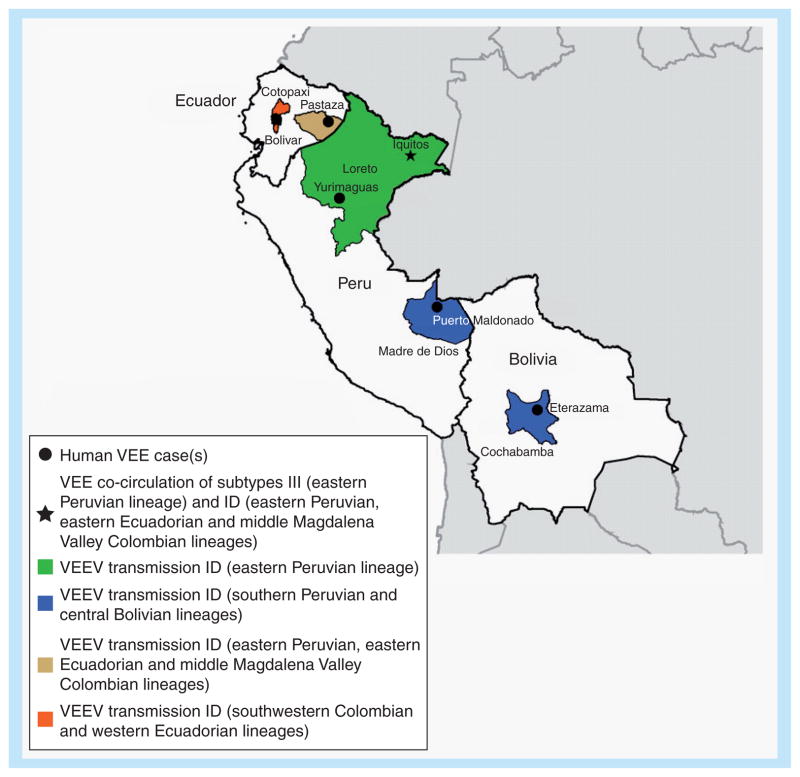
The VEEV strains from Bolivia differ genetically from those found in Panama, Colombia, Venezuela and Ecuador, and are closely related to strains from Madre de Dios in southern Peru (Figure 4). Genetic analyses of the VEEV strains from Pastaza, Ecuador, demonstrate that these isolates cluster within the Colombia/Venezuela ID genotype, which includes the progenitors of epizootic IAB and IC viruses (Figure 4) [40]. Field studies conducted in the coastal region from 1975 to 1977 detected the presence of VEEV subtype ID of the southwestern Colombia/Ecuador genotype [44,45].
Figure 4. Map of Peru, Ecuador and Bolivia showing regions of endemic Venezuelan equine encephalitis.
Shading of individual states/departments corresponds to the lineages found in Figure 2, and black circles indicate exact locations of virus isolation.
VEE; Venezuelan equine encephalitis; VEEV: Venezuelan equine encephalitis virus.
Colombia
The first documented VEE epidemic in Colombia began in March 1952 in the village of Espinal (Tolima) and involved approximately 0.7% of the population (70/9000). A medical assistance team arrived in June, near the end of the epidemic, and VEEV was isolated from a mother (38 years old) and her child (3 years old) during the acute phase of a febrile illness, and additional cases were diagnosed based on serological assays. The epidemic, which affected all age groups, was tentatively diagnosed as dengue. The true extent of the epidemic was never determined because residents rarely sought medical attention for febrile illnesses and laboratory diagnostics were not locally available. The ‘Espinal virus’ was determined antigenically to be similar but not identical to the original 1938 VEEV strain isolated from a horse in Venezuela. Entomological surveillance revealed Aedes aegypti in 687 dwellings and Culex fatigans (now quinquefasciatus) in 214 of the 2295 village homes; however, the role of these mosquito species during this outbreak, if any, remain unknown.
The second isolate of VEEV in Colombia came in 1955 from a patient with high fever residing in La Albania, a town within the municipality of San Vicente de Chucurí in the middle Magdalena Valley. In 1957, in the same location, additional human infections were detected by virus isolation and serological analyses. In 1958, another human case was confirmed in a forested region within the nearby municipality of Puerto Boyacá [46].
Enzootic VEEV transmission was demonstrated in La Albania by the detection of antibodies in birds, monkeys and other mammals [47]. In the city of Barrancabermeja, VEEV was isolated in 1958–1959 from pools of Culex species, and a mixture of Psorophora ferox and Psorophora albipes mosquitoes and from sentinel mice in the forest [46]. A high VEE seroprevalence rate of 58% was also detected at the same time in indigenous populations near the town of Acandi, department of Chocó, Golfo de Urabá [47].
In 1971, human VEE cases were again detected in the middle Magdalena Valley in the municipality of Puerto Boyacá. A total of 30% of the residents had antibodies against VEEV. In 1970–1972, VEEV was isolated from sentinel hamsters and wild mosquitoes in the area. Antibodies were found in 21% of spiny rats (Proechimys species) and in 60% of equids. In another sector within the same municipality, VEEV was isolated in a boy who developed high fever and intense headache after entering the forest. A total of 11% (1/9) of the individuals sampled in the vicinity were seropositive for VEEV. VEEV was also isolated from Culex (Melanoconion) species mosquitoes and sentinel hamsters in the area, and antibodies were detected in wild animals, indicating enzootic activity [47].
Groot et al. described VEE epidemics from November 1973 through to February 1974 in Lozanía, Department of Tolima, a small village of 346 inhabitants located in the vicinity of the Prado dam, and the small forest of Cerro Corrales [48]. A total of 84% (26/31) of febrile patients had antibodies against VEEV, and VEEV was isolated from four persons with acute fever. Only 5% (2/44) of persons without a history of recent febrile illness were seropositive, suggesting a high rate of apparent infection. No evidence of equine disease was observed and, although many horses had been immunized, two out of seven unvaccinated equids had serological evidence of recent VEEV infection, and 65% (15/23) of dogs were seropositive. Entomological studies indicated that Culex (Melanoconion) aikenii was the most abundant mosquito present. Studies in 1974 in the secondary forest of Cerro Corrales found no evidence of transmission to sentinel hamsters; therefore, it was concluded that VEEV may have been introduced into Lozanía from other areas [48]. Importantly, the absence of affected equids suggested that this outbreak resulted from either direct spillover of an enzootic cycle or possibly human–mosquito–human transmission rather than the presence of a typical epizootic cycle. The role of dogs as amplification hosts remains to be determined.
In 2003, human VEE cases continued to be detected in the middle Magdalena Valley, in a periurban area of the city of Barrancabermeja. The identification of five human cases of febrile illness similar to VEE prompted field studies that isolated VEEV from sentinel hamsters and from three known vectors (Culex [Melanoconion] pedroi, Culex [Melanoconion] vomerifer and Culex [Melanoconion] adamesi). The latter two species were also collected inside houses. No rodents were captured, and Didelphis marsupialis (opossum) was the only mammal trapped in the city. It was concluded that the periurban area of Barrancabermeja, which has since been completed deforested, was an enzootic/endemic focus of VEEV [49,50].
In April 2005, a 3-year-old girl developed a febrile illness and convulsions compatible with VEE in the small village of Chingalé. The village of Puerto Wilches, department of Santander, also reported eight confirmed VEE cases based on IgM detection in patients presenting with febrile illness. Entomological surveillance 2 months later revealed the presence of four mosquito species of the Spissipes group of the subgenus Culex (Melanoconion), including C. pedroi, C. occossa, C. spissipes and C. crybda. The first two species, although collected in lower numbers than M. indubitans, were found inside houses [51].
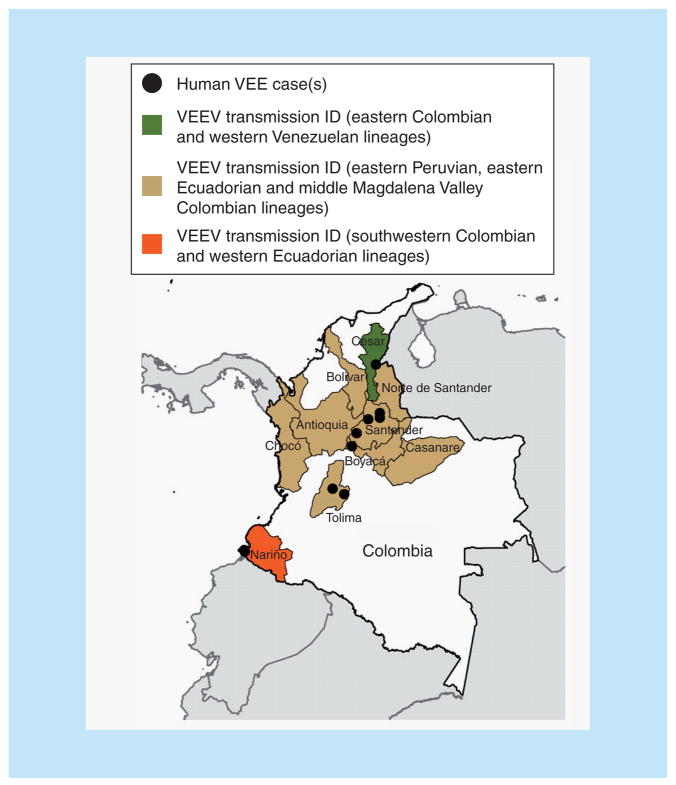
The most recent Colombian VEE epidemic occurred in the department of Chocó in February–March 2008. Approximately 13% (250/2000) of the village residents suffered from febrile illness compatible with VEE, and two died; however, no virus isolates were obtained. The incubation period was approximately 1–7 days and the most frequent signs and symptoms were fever, malaise, severe headache, nausea, vomiting, abdominal pain, asthenia, adynamia, arthralgia in wrists and ankles, macular rash and conjunctival injection. The illness was self-limiting and typically resolved in 7–10 days. The only sequelae reported were pain in wrists and ankles [52]. These recent outbreaks in the Magdalena Valley (Figure 5) suggest direct spillover of local enzootic VEEV. However, the presence of enzootic vectors within human habitations indicates that contact with the enzootic cycle may not require direct entry into forest habitats, but that enzootic transmission can occur in peridomestic habitats.
Figure 5. Map of Colombia showing regions of endemic Venezuelan equine encephalitis.
Shading of individual departments corresponds to the lineages found in Figure 2, and black dots indicate exact locations of virus isolation.
VEE: Venezuelan equine encephalitis; VEEV: Venezuelan equine encephalitis virus.
Serological studies among residents of the lowlands along the Pacific coast who did not present clinical evidence of VEE infection also indicate endemic transmission. In 1969, VEEV activity in this heavily forested area of high rainfall was confirmed by virus isolation from sentinel hamsters in Tumaco, close to the Ecuadorian border [53].
Overall, endemic Colombian VEE activity has been almost continuously documented, mainly in the middle Magdalena Valley. Importantly, transmission within peridomestic habitats highlights the risk for potential human VEE epidemics.
Phylogenetic relationships
With the exception of the southwestern Colombian ID strains, all enzootic/endemic Colombian isolates fall into the major ID lineage that also occurs in eastern Ecuador and Peru, as well as Western Venezuela (Figures 2 & 5). This lineage has given rise to all known subtype IAB/C strains, suggesting the potential for epizootic emergence throughout this range.
Mexico
The human impact of VEE in Mexico was first recognized in the 1960s, when serosurveys conducted in the village of Tlacotalpan, Veracruz state, detected antibodies in seven individuals aged 12–51 years [54]. VEE illness was later associated with VEEV seropositivity in 13 patients from Champoton, Campeche state, in southern Mexico: five died and three developed neurological sequelae [55].
A more complete serosurvey was conducted in four Mexican states on the Gulf coast involving 770 individuals and demonstrated a 3% seroprevalence [54]. In 1963, the first VEEV isolates from Mexico were obtained from sentinel hamsters and mosquitoes in the Sontecomapan region in southeastern Veracruz [56]. In 1965, VEEV was associated with a fatal human case in the village of Jaltipan, in Veracruz state [57]. Almost simultaneously in 1966 in the southern areas of Tamaulipas and northern Veracruz states, an equine outbreak affecting more than 1000 horses with 300 deaths was reported [58]. No virus isolates were made, but a VEEV etiology was determined serologically. The lack of vaccination in Mexico at this time suggested that this outbreak was caused by a local, enzootic subtype IE strain.
After almost 20 years of inactivity, renewed endemic VEE was reported in Paraiso, in the state of Tabasco, in 1991. Children aged 5 and 7 years were hospitalized with high fever and severe headache. Serological findings indicated VEEV infection with no evidence of dengue. Entomological studies isolated VEEV subtype IE, and serosurveys showed VEEV antibodies in 19% (76/394) of the individuals sampled in the village [59]. Subsequently, in 1993 and 1996, equine epizootics also due to VEEV subtype IE occurred on the Pacific coast of the states of Chiapas and Oaxaca. This was the first evidence of a VEE epizootic associated with subtype IE, suggesting that an equine-virulent strain emerged or was recently introduced into southern Mexico [20,60]. Experimental infections carried out with isolates from both outbreaks indicated that, unlike the major 1971 epizootic, the 1990s outbreaks probably did not spread beyond southern Mexico because the strains involved did not amplify efficiently in equids [7]. Serosurveys and VEEV isolations indicated that VEEV had been endemic in southern Mexico for decades [61]. It was also demonstrated that A. taeniorhynchus, an abundant epizootic vector in coastal areas of Chiapas and Oaxaca, was more susceptible to isolates obtained during the 1993 and 1996 epizootics compared with closely related enzootic IE strains isolated previously in Guatemala [62]. A mechanism of VEEV emergence was proposed by reverse genetic studies demonstrating that a single Ser → Asn amino acid substitution at position 218 of the E2 envelope glycoprotein was the major determinant of the increased A. taeniorhynchus infectivity. Thus, viral adaptation to a vector that prefers to bite large mammals was suggested as the emergence mechanism in the 1990s outbreaks of southern Mexico [62].
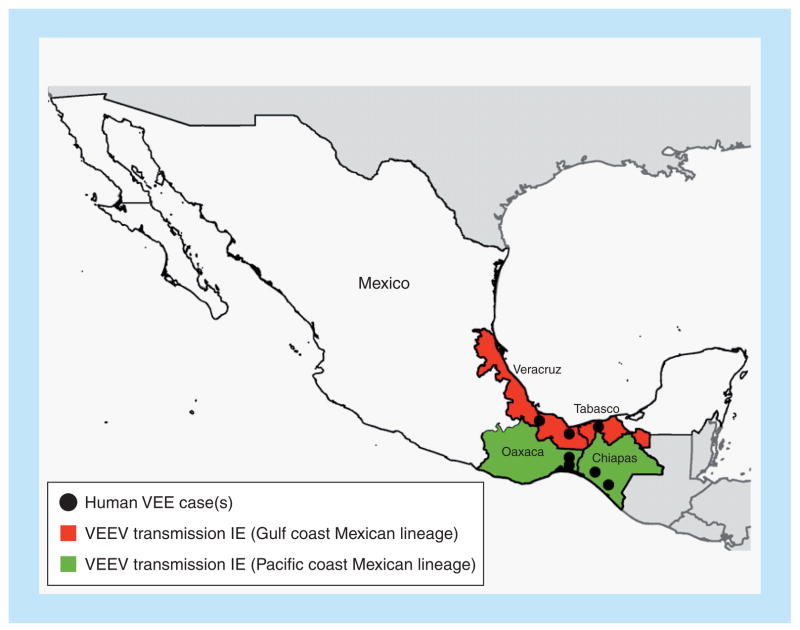
Recent studies conducted with the established enzootic vector Culex (Melanoconion) taeniopus, found in estuarine areas of Chiapas, demonstrated a high degree of susceptibility to subtype IE VEEV isolates from both Mexican outbreaks and to a 2001 sentinel hamster isolate from the Chiapas coast [63]. C. taeniopus feeds on a wide variety of hosts [64] and appears to maintain continuous subtype IE VEEV circulation in the region. These data suggest that C. taeniopus maintains VEEV subtype IE circulation adjacent to the Pacific coast, while A. taeniorhynchus may be responsible for infecting equids and possibly humans at more inland locations. The ability of equine-virulent VEEV subtype IE from Chiapas to be efficiently transmitted by both enzootic and epizootic vectors increases the risks of further outbreaks in this region. Data from serosurveys, sporadic equine cases and viral isolates indicate that the coastal Pacific plains from Guatemala to the Oaxaca isthmus represents an enzootic and endemic zone of VEE. This zone also extends east from the Papaloapan basin to the Gulf coast, including lowland coastal regions of Tabasco (from the municipalities of Balancan and Tenosique in the Guatemalan border), Veracruz and southern Tamaulipas in the municipality of Aldama [Estrada-Franco J & Weaver SC Unpublished Data]. Histopathology studies of encephalitic equids from Oaxaca, Veracruz and Chiapas states from 1996 to 2010 showed brain lesions typical of VEE, including polymorphic lymphocytic infiltration (100% of cases), necrotic foci, gliosis (72% of the cases) and edema in the blood vessel walls (50% of the cases) [Navarro-Lopez R, Unpublished Data]. Presumptive equine VEE disease (without viral isolates) was also reported in coastal Chiapas in the early summers of 1998, 1999, 2000, 2006, 2009 and 2010. During the fall of 2009, VEEV subtype IE was isolated from two diseased horses in coastal regions of Veracruz. In both Mexican regions (Chiapas and Veracruz), subtype IE isolates have also been obtained from sentinel hamsters and mosquitoes (Figure 6).
Figure 6. Map of Mexico showing regions of endemic and epizootic Venezuelan equine encephalitis.
Shading of individual states corresponds to the lineages found in Figure 2, and black dots indicate exact locations of virus isolation.
VEE: Venezuelan equine encephalitis; VEEV: Venezuelan equine encephalitis virus.
The results of field studies conducted in five states of Mexico (Chiapas, Oaxaca, Tabasco Veracruz and Tamaulipas), including high rates of seroprevalence, detection of IgM-positive humans initially diagnosed with dengue and subtype IE VEEV isolations from mosquitoes and sentinel hamsters [65] indicate endemic transmission in these regions, which are also endemic for other arboviral diseases [54,56,66,67]. Although human VEE seroprevalence is high in these regions, VEE is seldom recognized by the medical community because clinical signs and symptoms overlap extensively with those of dengue and other arboviral diseases (Table 2). During 2007–2009, extensive human serosurveys were conducted on suspected dengue patients (both dengue-seropositive and -seronegative) along coastal Pacific regions of Chiapas and Veracruz states. On average, 10–14% of febrile patients had VEEV-specific IgM antibodies, suggesting recent infection [Estrada-Franco J & Weaver SC Unpublished Data].
Table 2.
Clinical signs and symptoms in cases of Venezuelan equine encephalitis evaluated in Iquitos, Peru, from 1995 to 2001, with comparative data for dengue
| Sign/symptom | Percentage of dengue patients (n = 967) exhibiting sign/symptom | Percentage of Venezuelan equine encephalitis patients (n = 164) exhibiting sign/symptom |
|---|---|---|
| Fever | 98 | 99 |
| Headache | 98 | 98 |
| Myalgia | 93 | 95 |
| Chills | 91 | 85 |
| Arthralgia | 89 | 88 |
| Retro-orbital pain | 85 | 84 |
| Rash | 25 | 9 |
| Nausea/vomiting | 55 | 60 |
| Diarrhea | 22 | 23 |
| Cough | 31 | 27 |
| Sore throat | 19 | 16 |
| Nasal congestion | 15 | 10 |
Data taken with permission from [Watts D, Unpublished Data].
In summary, VEEV subtype IE is endemic in large coastal strips, both in the Pacific and in the Gulf coasts of Mexico (Figure 6). However, the extent of human VEE in most regions of Mexico remains unknown due to the lack of surveillance and laboratory diagnostics.
Phylogenetic relationships
The subtype IE strains fall into three main lineages: Pacific coast of Mexico and Central America, Gulf/Atlantic coasts of the same regions, and western Panama (Figures 2 & 6). These lineages have been independently maintained at least since the 1960s. Equine virulence has been known for the Pacific Mexican strains only since 1993, but there is no evidence that any of the IE strains have the potential to become equine-amplification competent.
Panama
The first reported human VEE in Panama was detected in April 1961. The patient, a 14-year-old boy, lived in the small village of Cañito on the shore of Lake Gatun, 20 miles from Panama City [68]. Initial signs and symptoms included headache, fever, myalgia, nausea and vomiting, with progression to delirium, disorientation, restlessness and coma, followed by patient death. Subsequent antigenic studies identified the etiologic VEEV strain as subtype ID, typically found in enzootic foci [26]. The boy’s older brother had died following a similar course of disease progression 2 months prior.
After 6 years, seven of 24 US soldiers training within a secondary growth forest on the western shores of Gatun lake, within the Canal Zone, became infected with VEEV [69]. Although these cases were not fatal, a similar set of signs and symptoms was documented, including fever, intense headache, myalgia, arthralgia and leukopenia. The isolation of VEEV from two of these soldiers, as well as the subsequent isolation from sentinel hamsters at the site of human infection, suggested that this area was an enzootic focus of transmission. Later studies detected VEEV circulation even during the dry season, indicating that central Panama was a stable enzootic VEEV focus [70,71]. None of these outbreaks was accompanied by disease in equids, and indeed equine VEE has never been documented in Panama, presumably because the enzootic subtype ID and IE strains there are equine avirulent and do not amplify efficiently in these animals [72].
The discoveries of enzootic and endemic VEE in Panama led to extensive ecological studies during the 1960s and 1970s, culminating in the identification of a subtype IE focus in western Panama [73] and the incrimination of C. aikenii (now ocossa and panocossa) as an enzootic vector in the Canal Zone in 1971 [74].
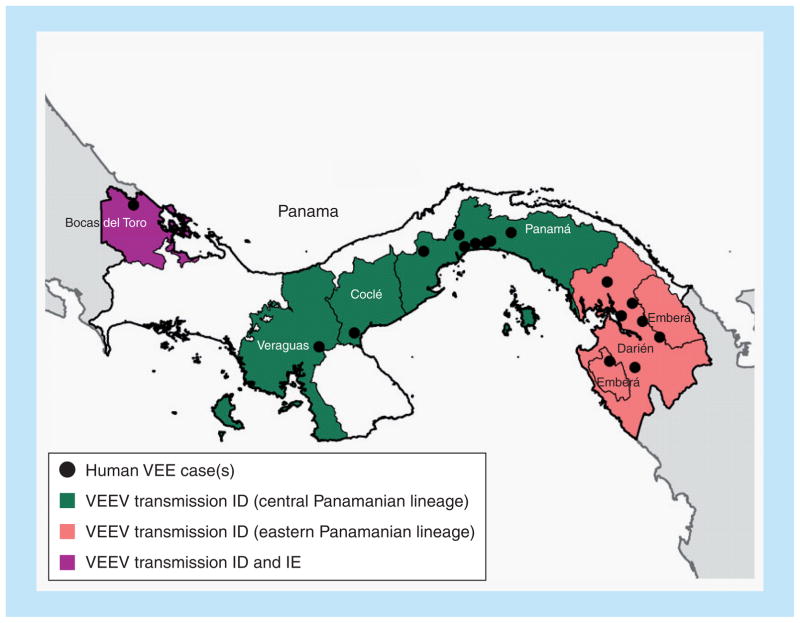
A comprehensive genetic analysis of all available VEEV strains isolated in Panama since 1961 revealed patterns of disease, virus isolation and genetic VEEV partitioning [75]. More recent isolates come from the provinces of Darién, Panamá, Coclé, Veraguas and Bocas del Toro, with clusters of human isolates from Darién and Panamá provinces (Figure 7). Darién is a relatively remote region near the Colombian border, characterized by abundant swamps and forests, habitats that support large populations of competent rodent reservoir hosts and Culex (Melanoconion) species vectors. The second cluster occurred in Panama province, between Gatun and Bayano lakes, a region known since the 1960s to be enzootic/endemic for VEE. Overall, human cases were documented in patients ranging from 10 months to 48 years of age, with the most severe cases characterized by neurologic complications in children less than 15 years of age, as reported previously [76]. The vast majority of human cases were accompanied by fever (94%), headache (55%) with retro-orbital pain (27%) and tremors (27%), signs and symptoms that are also characteristic of dengue, malaria and other acute tropical infectious diseases that are also common to this region [75]. Overall, the case fatality rate was 5%, which is higher than that reported during epidemics caused by VEEV subtype IAB and IC strains that undergo equine amplification [4].
Figure 7. Map of Panama showing regions of endemic Venezuelan equine encephalitis.
Shading of individual states corresponds to the lineages found in Figure 2, and black dots indicate exact locations of virus isolation.
VEE: Venezuelan equine encephalitis; VEEV: Venezuelan equine encephalitis virus.
In conclusion, extensive endemic VEE has been documented in Panama, with the vast majority of human cases undoubtedly being unrecognized or misdiagnosed as dengue, malaria or other common acute tropical infectious diseases in the absence of laboratory testing. Panama has never experienced an equine-amplified VEE epidemic, which may also have limited the attention paid to endemic VEE.
Phylogenetic relationships
PE2 envelope glycoprotein precursor gene analyses identified four main lineages of Panamanian VEEV strains [75]. Subtype IE strains isolated in western Panama grouped with others in this serotype from Central America and Mexico (Figure 2). A new Panamanian ID lineage was also identified in western Panama, suggesting geographic overlap in the distribution of the two enzootic VEEV subtypes in this region. Another major Panamanian subtype ID lineage represented a sister group to ID strains from eastern Peru. Within the Panamanian clade of this lineage, virus strains were partitioned geographically regardless of the year of isolation into central and eastern Panama. As discussed previously [77], these genetic relationships based primarily on lineages with restricted geographic ranges may reflect the limited dispersal potential of the rodent reservoir hosts and mosquito vectors.
Peru
Venezuelan equine encephalitis virus was first isolated in Peru during the 1940s, when subtype IAB caused epizootics along the Pacific coast [4,31]. A second epizootic was reported in 1969, which resulted in an estimated 550 equine deaths [13]. Following this outbreak, field investigations were initiated to determine the cause of the Peruvian VEE epizootics, which were hypothesized to originate in the Amazon region. The studies revealed the presence of subtype ID and IIIC viruses, but failed to isolate subtype IAB strains [78–80].
The first direct evidence of human VEE in Peru came in the 1990s. From 1993 to 1995, VEEV subtype ID was isolated from febrile patients residing in Pantoja and later in patients living in and around Iquitos [81,82]. Since then, VEEV subtype ID has been isolated annually from patients presenting with ‘dengue-like illness’. Within clinically diagnosed patients in Iquitos, there has been no reported neurological disease, although at least 60 cases have occurred in children <15 years of age [41,83]. However, severe and fatal disease associated with VEEV infection was recently documented in 2006 in Yurimaguas, Loreto, a rural community in the Amazon basin (Figure 4) [84,85]. Two out of nine documented VEE cases were fatal. Genetic studies based on partial PE2 envelope glycoprotein sequences confirmed the placement of the Yurimaguas strains within subtype ID genotype of Panama/Peru, which also includes the Iquitos strains. These reports suggest that fatal human disease associated with enzootic VEEV does occur, but that it is usually misdiagnosed and/or under-reported.
The most recent VEEV subtype ID outbreaks in Peru were detected in Iquitos from 2005 to 2006, and resulted in clinical disease in approximately 100 patients [86]. Since 2004, there has been an increase in VEE incidence rates in Iquitos, where extensive surveillance for dengue-like illness is conducted [41,86]. VEEV seroprevalence exceeds 23%, and the detection of C. occossa, Psorophora, Mansonia and Coquilletidia species within the city suggests the possibility of urban transmission of the enzootic subtype ID strain. Since 2005, VEEV subtype ID has also been isolated from febrile patients residing in Puerto Maldonado, Madre de Dios (Figure 4). The nearly continuous isolation of VEEV in this region suggests its endemic circulation [40].
In the Amazon basin of Peru, VEEV has been frequently isolated from Culex (Melanoconion) species mosquitoes, and they are very efficient laboratory vectors of subtype ID strains [87]. Subtype IIID was isolated from spiny rats (Proechimys species) and Culex (Melanoconion) species, and Culex (Melanoconion) gnomatos was identified as a natural vector of subtype III strains [88,89].
In summary, epidemiologic data suggest that VEE is endemic throughout the Amazon basin of Peru, causing an average of ten to 15 confirmed cases per year, despite very limited surveillance and laboratory diagnostics. It is the second most prevalent arboviral illness in the country, accounting for approximately 2.1% of dengue-like illness there and in neighboring countries, or approximately a tenth as much disease as dengue (dengue comprises only ~26% of dengue-like disease) [41]. Thus, the exact public health and economic impact of VEE in Peru remains largely unknown. If the estimate of VEE accounting for approximately 10% of the disease burden attributed to dengue [90] can be extended to other parts of Latin America, VEE could account for as many as 50,000 cases of acute febrile illness per year, with hundreds to thousands of cases of neurologic disease.
Phylogenetic relationships
Analyses of the VEE isolates from Peru have revealed the circulation of at least three lineages within subtype ID and, more importantly, have confirmed the human virulence potential of VEE subtype III [83]. In 2000, a new subtype IIID VEE complex alphavirus was isolated from a febrile patient living in Iquitos [40]. Interestingly, the subtype ID Colombia/Venezuela VEEV genotype, which is believed to give rise to epizootic strains, was detected in Peru during the 1970s and in 1998; however, genetic analyses of recent strains have failed to detect this genotype in Peru [40]. A new subtype ID genotype including strains from Madre de Dios and Bolivia was recently identified [40], and detailed reverse genetic studies are needed to determine whether this newly described genotype could be a precursor of epizootic viruses.
Clinical overview
Human disease
Based on results from the surveillance studies described earlier, a large proportion (0.1–7%) of dengue-like illness in Latin America is caused by VEEV [41,43]. Therefore, endemic VEE represents a serious public health concern, highlighting the need to strengthen laboratory diagnostics and surveillance in other areas of Latin America.
Clinically, human VEE is usually misdiagnosed as dengue or other arboviral diseases such as oropouche, group C bunyaviruses and/or guaroa viruses, making it difficult to estimate the exact extent of its public health and economic impact. No differences in disease presentation have been observed in humans upon infection with epizootic versus enzootic VEEV strains. The most common signs and symptoms include fever, headache, retro-orbital pain, tremors, prostration, nausea and vomiting (Table 2) [75]. These nonspecific signs and symptoms that are characteristic of VEE cases that do not progress to neurologic disease, overlap extensively with those produced by dengue virus infection without hemorrhagic manifestations. Acute VEE typically lasts 3–4 days. A small proportion of patients develops more serious neurological disease, characterized by convulsions, disorientation, drowsiness, mental depression and, in some cases, death [22]. Viremia levels detected in infected individuals typically range from 2.5 to PFU/ml among patients infected with 5.7 log10 VEEV subtype ID [75,85]. However, titers as high suckling mouse intracranial 50% as 8.2 log10 lethal dose/ml have been reported in patients infected with VEEV subtype IAB and IC [91]. These viremia levels are sufficient to infect susceptible mosquito vectors, and therefore the possibility for human–mosquito–human transmission likely exists. During the 1962–1964 and 1995 epidemics in Venezuela, a case–fatality rate of 0.7% was reported [13,22], and in the 1969 Ecuador outbreak, a case–fatality rate of up to 1% was observed [13]. However, these numbers are most likely an underestimate of the precise number of fatalities associated with VEEV infection due to the limitation of reporting and laboratory systems in rural areas of South American countries. An inapparent infection ratio of 1:11 was reported during a study conducted in Texas, USA [91].
All VEEV subtype ID genotypes that have been characterized to date are apparently equally capable of causing neurological/fatal disease, which is principally observed in children. Neurological and/or fatal disease after VEEV subtype ID infection has been documented in Bolivia, Colombia, Panama and Peru [68,75,85]. Hemorrhagic manifestations have also been described in patients infected with subtype ID viruses in Panama and Peru [68,84].
During the 1995 VEE outbreak in Colombia and Venezuela, VEEV was recovered from the brains of ten aborted or stillborn term fetuses, and an increase in the number of spontaneous abortions was detected compared with prior years [21,22]. However, the exact extent of abortion associated with VEE infection during the outbreak remains largely unknown. A pregnant woman exposed to the TC-83 VEEV vaccine strain experienced the loss of her fetus, further supporting the abortigenic potential of VEEV [92]. Neurological sequelae, including recurrent seizures, motor impairment, psychomotor retardation and behavioral disorders, are more commonly observed in children [93].
The misdiagnosis of VEE as dengue is primarily due to the overlapping complement of signs and symptoms associated with typical infections (Table 2). However, endemic VEE cases are much more likely to be misdiagnosed than epidemic cases that occur during ongoing equine outbreaks because the latter alert public health officials of the possibility of VEEV involvement. Endemic VEE has therefore received little attention and only rarely are control measures instituted to target the Culex (Melanoconion) species vectors that transmit from rodents to humans.
Public health measures: control & prevention
Mosquito control
The control of mosquito vectors that are responsible for the maintenance and transmission of VEEV represents a serious challenge. During equine-amplified outbreaks, vectors are typically floodwater Aedes and/or Psorophora species. These mosquitoes are present in high numbers during periods of high rainfall. By contrast, the enzootic/endemic vectors are typically Culex (Melanoconion) species, which transmit the virus among rodent reservoir hosts associated with forested environments. However, surveillance in the endemic regions described earlier indicates that the adult females of some enzootic vectors are found in urban areas, sometimes within human habitations. Larval habitats of enzootic vectors are poorly known and, in some cases, have never been described. Therefore, the control of adult mosquitoes using aerosol insecticide applications near and within human habitations is probably the most realistic control method for the foreseeable future.
VEE vaccines
The vaccination of equids has been recognized since the 1930s to be the best method to control epizootics and thereby to reduce human disease during equid-amplified epidemics. Early vaccines were made by inactivating wild-type VEEV produced in mouse brains, but incomplete inactivation likely resulted in several epidemics [24]. The TC-83 live-attenuated vaccine strain was developed from a wild-type IAB strain to immunize at-risk humans in laboratories [94], and was later used for vaccinating equids [72,95]. TC-83 is highly effective in equids and may have contributed to halting the 1971 Texas epidemic/epizootic. However, it has only two point- attenuating mutations [96] and has been shown to be naturally transmitted by mosquito vectors from immunized horses [97]. This suggests the possibility of reversion to wild-type virulence and transmission to initiate an epidemic. As a human vaccine, TC-83 suffers from poor immunogenicity and reactogenicity, which has limited its use to at-risk laboratory workers [98].
Several approaches have been used to develop improved VEE vaccines for equids and humans. Formalin-inactivated TC-83 is used in the USA for vaccination of equids and has also been given to laboratory workers who do not respond to initial immunization with the live version [98]. However, as with most inactivated viral vaccines, immunity is short lived, with frequent boosters needed to maintain protective immunity.
For use in endemic locations with limited financial resources, as well as to combat an epidemic, an ideal VEE vaccine would induce rapid and long-lived immunity after a single dose and have a low risk of reactogenicity and reversion to virulence and a low risk of transmission via mosquitoes. The live-attenuated yellow fever 17D vaccine is an example of such a vaccine that has been highly successful in preventing disease in developing countries of the tropics, although adverse outcomes have been described with increased frequency in recent years [99]. Several genetically engineered approaches to VEEV attenuation have been developed recently (reviewed in [100]) and offer promise for the immunization of humans and equids in endemic regions of Latin America.
Conclusion
Surveillance activities continue to detect enzootic VEE activity in many Latin American countries (most recently in 2010) and thus there is a constant threat of emergence of epizootic strains from enzootic progenitors. Continued monitoring of VEE activity in endemic regions will likely result in the prevention of large epizootics, as early detection will allow for the implementation of effective control measures such as vaccination and mosquito control. Vaccination of equids should transition toward a safer, live-attenuated product, such as V3526 [101], for administration to equids in order to prevent disease in equids and to prevent amplification in mosquito species leading to future epidemics/epizootics.
Venezuelan equine encephalitis is commonly presumptively diagnosed as ‘dengue fever’ [43,75,102]. Despite the existence of confirmatory VEE diagnostic tests, including virus isolation, PCR and serological analyses, many cases are only diagnosed clinically and are thus mistaken for dengue or another febrile illnesses. The lack of a confirmatory diagnosis is primarily due to the costs associated with diagnostics in resource-poor regions. The recent detection of human VEE cases in Bolivia, which occurred after the implementation of febrile surveillance activities in Eterazama, Cochabamba, highlights the need to strengthen these activities in other regions of South America.
Estimating the public health impact of VEEV infection in developing countries remains challenging. Despite the fact that VEE is endemic in many parts of Latin America, reports of neurological sequelae and mortality associated with VEEV infection are rare, most likely due to the limitations of current surveillance systems that do not conduct follow-up studies on patients who are seen during the acute phase of disease before neurologic signs develop and who do not follow-up with healthcare providers.
Future perspective
Prospects for future VEE epizootics/epidemics
Recent studies elucidating the enzootic origins of subtype IAB and IC VEEV strains, as well as the dramatic effects of single mutations on the equine amplification- [30] and mosquito infection- competent [62] phenotypes, indicate that epizootic strains will continue to arise. The frequency of major outbreaks depends primarily on the equine herd immunity induced by past outbreaks and vaccination, and weather patterns that result in unusually rainy seasons accompanied by large mosquito vector populations [103]. Equine vaccination with live TC-83, which is manufactured in Colombia and Mexico, could in theory prevent most or all major epidemics by eliminating equine amplification. However, continuous, broad vaccine coverage, especially in northern South America where subtype IAB and IC strains appear to originate, would probably require government subsidies. With other major public health challenges such as dengue, HIV, malaria and other parasitic diseases, it is difficult to prioritize the vaccination of domesticated animals to protect human health, especially for a disease like VEE that has been absent in its epidemic form for decades. Human vaccination against VEE could also prevent epidemics even in the face of equine amplification, as well as endemic VEE, as discussed in the following section.
Prospects for future endemic VEE
It is also clear from the epidemiologic data presented earlier that direct exposure of people to VEEV infection from the enzootic cycles will continue for the foreseeable future. The limited studies performed to date indicate that this endemic VEE represents a large burden of disease, perhaps 10% of that caused by dengue in at least some Latin American regions, which can be extrapolated to tens of thousands of cases per year. The continuing destruction of neotropical forests that are enzootic VEEV foci may increase the exposure of humans and equids in these areas. Fragmentation of these forests increases the length of ecotone habitats that are species-rich for mosquitoes, including enzootic VEEV vectors [104]. Spatial analyses using remote sensing suggest that VEEV vectors can reach 82–97% of the total land area in an enzootic region of western Venezuela by flying only 1–3 km from forests [105].
Climate change could also affect the distribution of enzootic VEEV by increasing the northward distribution of the tropical mosquito vectors. For example, Culex (Melanoconion) cedecei, which transmits Everglades virus (VEE complex subtype II) in southern Florida [35,106], could expand its range northward. Because the major rodent amplification/reservoir hosts extend further north into southeastern USA [107], the northward expansion of C. cedecei might expand the range of Everglades virus.
Perhaps the greatest risk for an increased range and transmission of endemic VEE is the possibility that endemic or epizootic strains could initiate an urban transmission cycle by exploiting the highly efficient vector of dengue virus, A. aegypti. This species, which has reinfested most of the neotropics, is a competent vector of a variety of VEEV strains [108]. Furthermore, Aedes albopictus, which is present in both tropical and temperate regions, is also an efficient laboratory vector [109]. Thus, human viremia levels after infection with both endemic [75] and epizootic VEEV strains [22,110], combined with urban vector susceptibility, could potentially lead to a stable, endemic, urban VEEV cycle that could have devastating public health implications throughout Latin America.
This major disease burden caused by enzootic and endemic VEE, as well as the risk of urbanization described earlier, could be greatly reduced or eliminated by an effective VEE vaccine. The limited data on the burden of human endemic VEE suggest that vaccine development for human use could be cost–effective if safe, live-attenuated product(s) could be developed to cover antigenic ID and IE variants [100]. Because most regions are endemic for only one or a few VEE complex serotypes, and no immune enhancement has been shown to affect VEE pathogenesis, the development of a few effective VEE vaccines should be feasible. TC-83 is clearly too reactogenic and insufficiently immunogenic for large-scale human use. Recent advances in alphavirus genetics have led to several approaches for the development of more rationally attenuated vaccines that could provide rapid and long-lived immunity after a single dose at low cost. These include the genetically engineered V3526 vaccine strain that combines attenuating defects in proteolytic cleavages with a suppressor mutation that is also attenuating [101]. However, V3526 proved reactogenic in Phase I human trials [111]. Chimeric alphaviruses that combine the genetic backbone of the relatively benign Sindbis virus with wild-type VEEV structural protein genes have been shown to be safe and efficacious in murine models [112,113], as well as more stably attenuated than the point mutation-based TC-83 [114]. DNA vaccines, which offer greater safety and lower cost, could also prove effective if robust and long-lived immunity could be achieved [115]. However, even if any of these new vaccines prove promising in further preclinical evaluation, the high cost of human trials would probably require government sponsorship for biodefense purposes to proceed toward licensure.
Executive summary.
The Venezuelan equine encephalitis (VEE) complex of alphaviruses is widely distributed throughout the Americas, and includes epizootic strains (VEE virus [VEEV] subtypes IAB and IC) that are virulent for equids and amplify efficiently in these hosts via mosquito transmission.
Enzootic strains (VEEV subtypes ID and IE, as well as other species comprising VEE complex subtypes IF and II–VI) circulate among rodents in sylvatic or swamp habitats but are equid amplification-incompetent.
Epidemics caused by epizootic VEEV strains were first recognized during the 1920s and have occurred periodically in northern South America, Central America, Mexico and Texas, USA, involving hundreds of thousands of people and equids.
Epizootic, equid amplification-competent IAB and IC strains appear to evolve from enzootic ID strains that circulate in northern South America.
Spillover of VEEV and other viruses in the VEE complex regularly occurs when humans contact the rodent–mosquito cycles, resulting in endemic disease.
Endemic VEE was recently discovered in Bolivia and southern Peru, with a similar burden of disease compared with dengue and with neurologic disease.
Endemic VEE was also recently detected in eastern Ecuador.
Southern Peruvian and Bolivian VEEV strains belong to a subtype ID lineage found also in Colombia and western Venezuela, but comprise a distinct ID sublineage.
Endemic VEE was first documented in Colombia during the 1950s and has caused small outbreaks ever since, mainly documented in the central Magdalena Valley. High seroprevalence in this region suggests a large burden of endemic disease.
Recent outbreak investigations in central Colombia have documented the presence of proven enzootic subtype ID vectors within human habitations in urban areas, suggesting that enzootic circulation occurs outside of forest and swamp habitats.
The subtype ID Colombian VEEV strains from the central Magdalena Valley belong to the lineage that is responsible for generating all known epizootic subtype IAB and IC strains.
In Mexico, evidence of endemic VEE has been known since the 1960s, when several human cases, including one fatal case, were documented near the Gulf coast.
Recent Mexican VEE activity has included equine epizootics on the Pacific coast of Chiapas and Oaxaca states, where an equine-virulent subtype IE strain emerged in the early 1990s.
Follow-up surveillance on the Pacific coast of Mexico, as well as near the Gulf coast, has yielded evidence of widespread endemic VEE that is usually misdiagnosed as dengue.
In both regions of endemic Mexican VEE, Culex (Melanoconion) taeniopus appears to serve as the principal enzootic vector, but Pacific coastal subtype IE strains have acquired an increased infectivity for Aedes (Ochlerotatus) taeniorhynchus, a vector with increased dispersal and a preference for large mammals.
Endemic VEE, including fatal disease, has been recognized in Panama since 1960, and recent outbreaks document its continued occurrence almost throughout the country.
Panamanian VEEV strains in subtypes ID and IE are not associated with the emergence of the epizootic subtype IAB and IC strains, which is consistent with a historic lack of equine VEE in Panama.
There has been nearly continuous detection of endemic VEE cases in eastern Peru since 1995, almost all of which are presumptively diagnosed as dengue. Estimates from the Iquitos area indicate that the incidence of VEE is approximately a tenth that of dengue, suggesting a large burden of human disease in the Amazon basin.
The typical signs and symptoms of VEE overlap extensively with those of dengue, except when the former results in neurologic disease (typically in less than 10% of cases) or hemorrhagic disease in the latter (a small minority of dengue virus infections).
Endemic VEE is difficult to control due to the forested enzootic habitats and the widespread, continuous circulation of enzootic VEEV strains in the neotropics.
Continued deforestation of the neotropics could result in increased human VEE if continued fragmentation of enzootic habitats enhances contact with humans and/or adaptation of the enzootic viruses to urban circulation occurs.
Although recent advances in alphavirus vaccine designs offer promise for the control of human disease, a licensed vaccine is unlikely to be financially feasible unless development costs are funded by the US government for biodefense purposes, by a Latin American government or by philanthropy.
Acknowledgments
The authors would like to thank Rubing Chen for assistance with the phylogenetic tree in Figure 2.
Footnotes
Financial & competing interests disclosure
SC Weaver is an applicant for the patent of a vaccine designed to protect against Venezuelan equine encephalitis. The authors’ research is supported by NIH grants AI48807 and AI057156. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Kubes V, Ríos FA. The causative agent of infectious equine encephalomyelitis in Venezuela. Science. 1939;90(2323):20–21. doi: 10.1126/science.90.2323.20. [DOI] [PubMed] [Google Scholar]
- 2.Beck CE, Wyckoff RW. Venezuelan equine encephalomyelitis. Science. 1938;88(2292):521–530. doi: 10.1126/science.88.2292.530. [DOI] [PubMed] [Google Scholar]
- 3.Albornoz JE. Mad fever of the beasts (Borna disease) Colombia. Bol Agric (Suppl) Min Agr Com Bogota. 1935;26:1–8. [Google Scholar]
- 4▪▪.Johnson KM, Martin DH. Venezuelan equine encephalitis. Adv Vet Sci Comp Med. 1974;18(0):79–116. Classic review providing excellent descriptions of the first documentation of endemic Venezuelan equine encephalitis (VEE), as well as studies of equine virulence and amplification. [PubMed] [Google Scholar]
- 5.Sanmartin-Barberi C, Groot H, Osorno-Mesa E. Human epidemic in Colombia caused by the Venezuelan equine encephalomyelitis virus. Am J Trop Med Hyg. 1954;3(2):283–293. doi: 10.4269/ajtmh.1954.3.283. [DOI] [PubMed] [Google Scholar]
- 6.Weaver S, Dalgarno L, Frey T, Huang H, Kinney R. Family Togaviridae. In: van Regenmortel MHV, Fauquet CM, Bishop DHL, et al., editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press; CA, USA: 2000. pp. 879–889. [Google Scholar]
- 7.Gonzalez-Salazar D, Estrada-Franco JG, Carrara AS, Aronson JF, Weaver SC. Equine amplification and virulence of subtype IE Venezuelan equine encephalitis viruses isolated during the 1993 and 1996 Mexican epizootics. Emerg Infect Dis. 2003;9(2):161–168. doi: 10.3201/eid0902.020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang E, Bowen RA, Medina G, et al. Virulence and viremia characteristics of 1992 epizootic subtype IC Venezuelan equine encephalitis viruses and closely related enzootic subtype ID strains. Am J Trop Med Hyg. 2001;65(1):64–69. doi: 10.4269/ajtmh.2001.65.64. [DOI] [PubMed] [Google Scholar]
- 9.Sudia WD, Newhouse VF, Beadle ID, et al. Epidemic Venezuelan equine encephalitis in North America in 1971: vector studies. Am J Epidemiol. 1975;101(1):17–35. doi: 10.1093/oxfordjournals.aje.a112068. [DOI] [PubMed] [Google Scholar]
- 10.Turell MJ, Ludwig GV, Beaman JR. Transmission of Venezuelan equine encephalomyelitis virus by Aedes sollicitans and Aedes taeniorhynchus (Diptera: Culicidae) J Med Entomol. 1992;29(1):62–65. doi: 10.1093/jmedent/29.1.62. [DOI] [PubMed] [Google Scholar]
- 11.Kramer LD, Scherer WF. Vector competence of mosquitoes as a marker to distinguish Central American and Mexican epizootic from enzootic strains of Venezuelan encephalitis virus. Am J Trop Med Hyg. 1976;25(2):336–346. doi: 10.4269/ajtmh.1976.25.336. [DOI] [PubMed] [Google Scholar]
- 12.Grayson MA, Galindo P. Experimental transmission of Venezuelan equine encephalitis virus by Deinocerites pseudes Dyar and Knab, 1909. J Med Entomol. 1972;9(3):196–200. doi: 10.1093/jmedent/9.3.196. [DOI] [PubMed] [Google Scholar]
- 13.Groot H. Venezuelan Encephalitis. Pan American Health Organization; Washington, DC, USA: 1972. The health and economic impact of Venezuelan equine encephalitis (VEE) pp. 7–16. [Google Scholar]
- 14.Lord RD. History and geographic distribution of Venezuelan equine encephalitis. Bull Pan Am Health Organ. 1974;8(2):100–110. [PubMed] [Google Scholar]
- 15.Hinman AR, McGowan JE, Henderson BE. Venezuelan equine encephalomyelitis: surveys of human illness during an epizootic in Guatemala and El Salvador. Am J Epidemiol. 1971;93(2):130–136. doi: 10.1093/oxfordjournals.aje.a121233. [DOI] [PubMed] [Google Scholar]
- 16.Sudia WD, Lord RD, Newhouse VF, Miller DL, Kissling RE. Vector–host studies of an epizootic of Venezuelan equine encephalomyelitis in Guatemala, 1969. Am J Epidemiol. 1971;93(2):137–143. doi: 10.1093/oxfordjournals.aje.a121234. [DOI] [PubMed] [Google Scholar]
- 17.Reta-Pettersson G. International Round Table on VEE. Mexican Ministry of Agriculture and Livestock and the Pan American Health Organization; Mexico: 1970. The problem of Venezuelan equine encephalitis in Mexico; pp. 1–22. [Google Scholar]
- 18.Zarate M. Arbovirus and arbovirosis in Mexico. In: Chang RE, editor. Veterinary Sciences (Spanish version) Veterinary School UNAM; Mexico: 1978. pp. 157–180. [Google Scholar]
- 19.Morilla-Gonzales A. Venezuelan equine encephalitis. In: Chang RE, editor. Veterinary Sciences (Spanish version) Veterinary School UNAM; Mexico: 1976. pp. 163–194. [Google Scholar]
- 20.Oberste MS, Fraire M, Navarro R, et al. Association of Venezuelan equine encephalitis virus subtype IE with two equine epizootics in Mexico. Am J Trop Med Hyg. 1998;59(1):100–107. doi: 10.4269/ajtmh.1998.59.100. [DOI] [PubMed] [Google Scholar]
- 21▪.Rivas F, Diaz LA, Cardenas VM, et al. Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. J Infect Dis. 1997;175(4):828–832. doi: 10.1086/513978. Excellent epidemiological study of the last major VEE epizootic/epidemic in the La Guajira peninsula of Colombia. [DOI] [PubMed] [Google Scholar]
- 22▪.Weaver SC, Salas R, Rico-Hesse R, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet. 1996;348(9025):436–440. doi: 10.1016/s0140-6736(96)02275-1. Description of the major 1995 Venezuelan/Colombian epizootic/epidemic documenting the re-emergence of a subtype IC strain that first circulated in the same regions during the 1960s. [DOI] [PubMed] [Google Scholar]
- 23.CDC. Venezuelan equine encephalitis – Colombia, 1995. MMWR Morb Mortal Wkly Rep. 1995;44(39):721–724. [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver SC, Pfeffer M, Marriott K, Kang W, Kinney RM. Genetic evidence for the origins of Venezuelan equine encephalitis virus subtype IAB outbreaks. Am J Trop Med Hyg. 1999;60(3):441–448. doi: 10.4269/ajtmh.1999.60.441. [DOI] [PubMed] [Google Scholar]
- 25.Kinney RM, Tsuchiya KR, Sneider JM, Trent DW. Molecular evidence for the origin of the widespread Venezuelan equine encephalitis epizootic of 1969 to 1972. J Gen Virol. 1992;73(Pt 12):3301–3305. doi: 10.1099/0022-1317-73-12-3301. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Young NA, Johnson KM. Antigenic variants of Venezuelan equine encephalitis virus: their geographic distribution and epidemiologic significance. Am J Epidemiol. 1969;89(3):286–307. doi: 10.1093/oxfordjournals.aje.a120942. Landmark publication providing the first framework for the organization of the VEE complex, as well as the first antigenic evidence that epizootic VEE virus (VEEV) strains may be derived from enzootic subtype ID strains. [DOI] [PubMed] [Google Scholar]
- 27.Kinney RM, Tsuchiya KR, Sneider JM, Trent DW. Genetic evidence that epizootic Venezuelan equine encephalitis (VEE) viruses may have evolved from enzootic VEE subtype I-D virus. Virology. 1992;191(2):569–580. doi: 10.1016/0042-6822(92)90232-e. [DOI] [PubMed] [Google Scholar]
- 28.Rico-Hesse R, Roehrig JT, Trent DW, Dickerman RW. Genetic variation of Venezuelan equine encephalitis virus strains of the ID variety in Colombia. Am J Trop Med Hyg. 1988;38(1):195–204. doi: 10.4269/ajtmh.1988.38.195. [DOI] [PubMed] [Google Scholar]
- 29.Greene IP, Paessler S, Austgen L, et al. Envelope glycoprotein mutations mediate equine amplification and virulence of epizootic Venezuelan equine encephalitis virus. J Virol. 2005;79(14):9128–9133. doi: 10.1128/JVI.79.14.9128-9133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪▪.Anishchenko M, Bowen RA, Paessler S, Austgen L, Greene IP, Weaver SC. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc Natl Acad Sci USA. 2006;103(13):4994–4999. doi: 10.1073/pnas.0509961103. Using reverse genetics, this seminal study demonstrated that an epizootic subtype IC strain can result from a single nucleotide mutation in a subtype ID enzootic VEEV strain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walton T, Grayson M. Venezuelan equine encephalomyelitis. In: Monath T, editor. The Arboviruses: Epidemiology and Ecology. CRC Press; FL, USA: 1988. pp. 203–231. [Google Scholar]
- 32.Young NA, Johnson KM, Gauld LW. Viruses of the Venezuelan equine encephalomyelitis complex Experimental infection of Panamanian rodents. Am J Trop Med Hyg. 1969;18(2):290–296. [PubMed] [Google Scholar]
- 33.Grayson MA, Galindo P. Ecology of Venezuelan equine encephalitis virus in Panama. J Am Vet Med Assoc. 1969;155(12):2141–2145. [PubMed] [Google Scholar]
- 34.Barrera R, Ferro C, Navarro JC, et al. Contrasting sylvatic foci of Venezuelan equine encephalitis virus in northern South America. Am J Trop Med Hyg. 2002;67(3):324–334. doi: 10.4269/ajtmh.2002.67.324. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain RW, Sudia WD, Work TH, Coleman PH, Newhouse VF, Johnston JG. Arbovirus studies in south Florida, with emphasis on Venezuelan equine encephalomyelitis virus. Am J Epidemiol. 1969;89(2):197–210. doi: 10.1093/oxfordjournals.aje.a120929. [DOI] [PubMed] [Google Scholar]
- 36.Lord RD, Calisher CH, Sudia WD, Work TH. Ecological investigation of vertebrate hosts of Venezuelan equine encephalomyelitis virus in south Florida. Am J Trop Med Hyg. 1973;22(1):116–123. doi: 10.4269/ajtmh.1973.22.116. [DOI] [PubMed] [Google Scholar]
- 37.Monath TP, Lazuick JS, Cropp CB, et al. Recovery of Tonate virus (‘Bijou Bridge’ strain), a member of the Venezuelan equine encephalomyelitis virus complex, from cliff swallow nest bugs (Oeciacus vicarius) and nestling birds in North America. Am J Trop Med Hyg. 1980;29(5):969–983. doi: 10.4269/ajtmh.1980.29.969. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell CJ, Monath TP, Sabattini MS, et al. Arbovirus investigations in Argentina, 1977–1980 II Arthropod collections and virus isolations from Argentine mosquitoes. Am J Trop Med Hyg. 1985;34(5):945–955. [PubMed] [Google Scholar]
- 39.Pisano MB, Ré VE, Díaz LA, et al. Enzootic activity of pixuna and Rio Negro viruses (Venezuelan equine encephalitis complex) in a neotropical region of Argentina. Vector Borne Zoonotic Dis. 2010;10(2):199–201. doi: 10.1089/vbz.2008.0156. [DOI] [PubMed] [Google Scholar]
- 40▪.Aguilar PV, Adams AP, Suárez V, et al. Genetic characterization of Venezuelan equine encephalitis virus from Bolivia, Ecuador and Peru: identification of a new subtype ID lineage. PLoS Negl Trop Dis. 2009;3(9):E514. doi: 10.1371/journal.pntd.0000514. Describes the recent identification of endemic VEE in southern Peru and Bolivia, the southernmost location known to date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Forshey BM, Guevara C, Laguna-Torres VA, et al. Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Negl Trop Dis. 2010;4(8):E787. doi: 10.1371/journal.pntd.0000787. Documents the regular occurrence of endemic VEE in the Amazon basin of Peru, with signs and symptoms that are typically confused with those of dengue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalo Sotomayor C. A study of the virus of equine encephalomyelitis in Ecuador. J Am Vet Med Assoc. 1946;109(837):478–480. [PubMed] [Google Scholar]
- 43.Manock SR, Jacobsen KH, de Bravo NB, et al. Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg. 2009;81(1):146–151. [PubMed] [Google Scholar]
- 44.Powers AM, Oberste MS, Brault AC, et al. Repeated emergence of epidemic/epizootic Venezuelan equine encephalitis from a single genotype of enzootic subtype ID virus. J Virol. 1997;71(9):6697–6705. doi: 10.1128/jvi.71.9.6697-6705.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrev E, Monath TP, Alava A, Uriguen D, Arzube M, Chamberlain RW. Epidemiologic investigations of the 1969 epidemic of Venezuelan encephalitis in Ecuador. Am J Epidemiol. 1975;102(5):400–413. doi: 10.1093/oxfordjournals.aje.a112179. [DOI] [PubMed] [Google Scholar]
- 46.Groot H, Kerr JA, Sanmartin C, Vidales H. Antibodies to yellow fever and other arthropod-borne viruses in human residents of San Vicente de Chucuri, Santander, Colombia. Am J Trop Med Hyg. 1959;8(2 Pt 1):175–189. doi: 10.4269/ajtmh.1959.8.175. [DOI] [PubMed] [Google Scholar]
- 47.Groot H. Studies on arthropod-borne viruses in Colombia. Rev Acad Colomb Cien Exact Nat. 1964;12:197–217. [Google Scholar]
- 48.Groot H, Morales A, Romero M, et al. Studies on arboviruses in Colombia in the 1970s. Biomedica. 1996;16:331–344. [Google Scholar]
- 49.Ferro C, Boshell J, Moncayo AC, et al. Natural enzootic vectors of Venezuelan equine encephalitis virus, Magdalena Valley, Colombia. Emerg Infect Dis. 2003;9(1):49–54. doi: 10.3201/eid0901.020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferro C, Ramirez M, Gonzales M, Ahumada M, Boshell J, Weaver SC. A periurban, enzootic transmission cycle of Venezuelan equine encephalitis virus subtype ID in the Magdalena Valley of central Colombia. Presented at: 52nd Annual Meeting of the American Society of Tropical Medicine and Hygiene; Philadelphia, PA, USA. 3–7 December 2003. [Google Scholar]
- 51.Ferro C, Olano VA, Ahumada M, Weaver S. Mosquitos (Diptera: Culicidae) in the small village where a human case of Venezuelan equine encephalitis was recorded. Biomedica. 2008;28(2):234–244. [PubMed] [Google Scholar]
- 52.Sanchez-Seco M, Casanova C, Ory F, Gallego-Gomez C, Tenorio A. Outbreak of febrile illness in Colombia (2008) by VEEV. Presented at: International Meeting on Emerging Diseases and Surveillance; Vienna, Austria. 4–7 February 2011.2011. [Google Scholar]
- 53.Sanmartín C, Trapido H, Barreto P, Lesmes CI. Isolations of Venezuelan and Eastern equine encephalomyelitis viruses from sentinel hamsters exposed in the Pacific lowlands of Colombia. Am J Trop Med Hyg. 1971;20(3):469–473. doi: 10.4269/ajtmh.1971.20.469. [DOI] [PubMed] [Google Scholar]
- 54.De Mucha-Macías J, Sanchez-Spindola I, Campillo-Sainz C. Venezuelan equine encephalomyelitis antibodies in human beings of southeastern Mexico. Am J Trop Med Hyg. 1966;15(3):364–368. doi: 10.4269/ajtmh.1966.15.364. [DOI] [PubMed] [Google Scholar]
- 55.De Mucha Macias J. Arbovirus infections. Studies made at the Instituto Nacional de Virologia de la Secretaria de Salubridad y Asistencia. Gac Med Mex. 1963;93:415–420. [PubMed] [Google Scholar]
- 56▪.Scherer WF, Dickerman RW, Chia CW, Ventura A, Moorhouse A, Geiger R. Venezuelan equine encephalitis virus in Veracruz, Mexico, and the use of hamsters as sentinels. Science. 1964;145:274–275. doi: 10.1126/science.145.3629.274. Landmark study that first detected VEEV circulation in Mexico and described the use of sentinel hamsters, which is still the most sensitive method to detect enzootic circulation. [DOI] [PubMed] [Google Scholar]
- 57.Zárate ML, Scherer WF, Dickerman RW. A probable case of Venezuelan equine encephalitis occurring in Jáltipan, Veracruz, Mexico, 1965. Salud Publica Mex. 1971;13(1):97–99. [PubMed] [Google Scholar]
- 58.Morilla-González A, de Mucha-Macías J. Occurrence of Venezuelan epizootic equine encephalitis in Tamaulipas, Mex. Rev Invest Salud Publica. 1969;29(1):3–20. [PubMed] [Google Scholar]
- 59.Zarate M, Morilla-Gonzalez D. Circulation of Venezuelan equine encephalitis virus after 20 years of silence in Tabasco, Mexico 1991. In: Zarate-Aquino M, Morilla-Gonzalez A, Batalla-Campero D, editors. Encefalitis Equinas por Arbovirus. Instituto Nacional de Investigaciones Forestales Agricolas y Pecuarias, Instituto Interamericano de Cooperacion para la Agricultura and the Pan American Health Organization; Mexico: 1999. pp. 175–187. [Google Scholar]
- 60.Oberste MS, Schmura SM, Weaver SC, Smith JF. Geographic distribution of Venezuelan equine encephalitis virus subtype IE genotypes in Central America and Mexico. Am J Trop Med Hyg. 1999;60(4):630–634. doi: 10.4269/ajtmh.1999.60.630. [DOI] [PubMed] [Google Scholar]
- 61.Estrada-Franco JG, Navarro-Lopez R, Freier JE, et al. Venezuelan equine encephalitis virus, southern Mexico. Emerg Infect Dis. 2004;10(12):2113–2121. doi: 10.3201/eid1012.040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪.Brault AC, Powers AM, Ortiz D, Estrada-Franco JG, Navarro-Lopez R, Weaver SC. Venezuelan equine encephalitis emergence: enhanced vector infection from a single amino acid substitution in the envelope glycoprotein. Proc Natl Acad Sci USA. 2004;101(31):11344–11349. doi: 10.1073/pnas.0402905101. Reverse genetic study demonstrating the first association of adaptation of an alphavirus to a moquito vector associated with epizootic emergence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deardorff ER, Weaver SC. Vector competence of Culex (Melanoconion) taeniopus for equine-virulent subtype IE strains of Venezuelan equine encephalitis virus. Am J Trop Med Hyg. 2010;82(6):1047–1052. doi: 10.4269/ajtmh.2010.09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cupp EW, Scherer WF, Lok JB, Brenner RJ, Dziem GM, Ordonez JV. Entomological studies at an enzootic Venezuelan equine encephalitis virus focus in Guatemala, 1977–1980. Am J Trop Med Hyg. 1986;35(4):851–859. doi: 10.4269/ajtmh.1986.35.851. [DOI] [PubMed] [Google Scholar]
- 65.Deardorff ER, Forrester NL, Travassos da Rosa AP, et al. Experimental infections of Oryzomys couesi with sympatric arboviruses from Mexico. Am J Trop Med Hyg. 2010;82(2):350–353. doi: 10.4269/ajtmh.2010.09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scherer WF, Campillo-Sainz C, Dickerman RW, Diaz-Najera A, Madalengoitia J. Isolation of Tlacotalpan virus, a new Bunyamwera-group virus from Mexican mosquitoes. Am J Trop Med Hyg. 1967;16(1):79–91. doi: 10.4269/ajtmh.1967.16.79. [DOI] [PubMed] [Google Scholar]
- 67.Scherer WF, Zarate ML, Dickerman RW. Discovery and identification of group C, Nepuyo arbovirus in Mexico. Bol Oficina Sanit Panam. 1969;66(4):325–338. [PubMed] [Google Scholar]
- 68▪.Johnson KM, Shelokov A, Peralta PH, Dammin GJ, Young NA. Recovery of Venezuelan equine encephalomyelitis virus in Panama. A fatal case in man. Am J Trop Med Hyg. 1968;17(3):432–440. doi: 10.4269/ajtmh.1968.17.432. Landmark paper describing the first documented fatal human endemic VEE case that resulted from spillover of an enzootic strain. [DOI] [PubMed] [Google Scholar]
- 69.Franck PT, Johnson KM. An outbreak of Venezuelan encephalitis in man in the Panama Canal Zone. Am J Trop Med Hyg. 1970;19(5):860–865. doi: 10.4269/ajtmh.1970.19.860. [DOI] [PubMed] [Google Scholar]
- 70.Srihongse S, Scherer WF, Galindo P. Detection of arboviruses by sentinel hamsters during the low period of transmission. Am J Trop Med Hyg. 1967;16(4):519–524. doi: 10.4269/ajtmh.1967.16.519. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez JL, Takafuji ET, Lednar WM, et al. Venezuelan equine encephalomyelitis: report of an outbreak associated with jungle exposure. Mil Med. 1984;149(11):618–621. [PubMed] [Google Scholar]
- 72▪▪.Walton TE, Alvarez O, Jr, Buckwalter RM, Johnson KM. Experimental infection of horses with enzootic and epizootic strains of Venezuelan equine encephalomyelitis virus. J Infect Dis. 1973;128(3):271–282. doi: 10.1093/infdis/128.3.271. Seminal study demonstrating that epizootic subtype IAB and IC VEEV strains are virulent for equids and generate viremia that is sufficient for transmission by mosquitoes, by contrast to the equid amplification-incompetent enzootic strains. [DOI] [PubMed] [Google Scholar]
- 73.Grayson MA, Galindo P. Epidemiologic studies of Venezuelan equine encephalitis virus in Almirante, Panama. Am J Epidemiol. 1968;88(1):80–96. doi: 10.1093/oxfordjournals.aje.a120870. [DOI] [PubMed] [Google Scholar]
- 74.Galindo P, Grayson MA. Culex (Melanoconion) aikenii: natural vector in Panama of endemic Venezuelan encephalitis. Science. 1971;172(983):594–595. doi: 10.1126/science.172.3983.594. [DOI] [PubMed] [Google Scholar]
- 75.Quiroz E, Aguilar PV, Cisneros J, Tesh RB, Weaver SC. Venezuelan equine encephalitis in Panama: fatal endemic disease and genetic diversity of etiologic viral strains. PLoS Negl Trop Dis. 2009;3(6):E472. doi: 10.1371/journal.pntd.0000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Briceno Rossi AL. Rural epidemic encephalitis in Venezuela caused by a group A arbovirus (VEE) Progr Med Virol. 1967;9:176–203. [PubMed] [Google Scholar]
- 77.Weaver SC, Ferro C, Barrera R, Boshell J, Navarro JC. Venezuelan equine encephalitis. Annu Rev Entomol. 2004;49:141–174. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]
- 78.Scherer WF, Chin J. An unusual strain of Venezuelan encephalitis virus existing sympatrically with subtype I-D strains in a Peruvian rain forest. Am J Trop Med Hyg. 1983;32(4):871–876. doi: 10.4269/ajtmh.1983.32.871. [DOI] [PubMed] [Google Scholar]
- 79.Scherer WF, Anderson K. Antigenic and biologic characteristics of Venezuelan encephalitis virus strains including a possible new subtype, isolated from the Amazon region of Peru in 1971. Am J Epidemiol. 1975;101(4):356–361. doi: 10.1093/oxfordjournals.aje.a112104. [DOI] [PubMed] [Google Scholar]
- 80.Scherer WF, Madalengoitia J, Flores W, Acosta M. Ecologic studies of Venezuelan encephalitis virus in Peru during 1970–1971. Am J Epidemiol. 1975;101(4):347–355. doi: 10.1093/oxfordjournals.aje.a112103. [DOI] [PubMed] [Google Scholar]
- 81.Watts DM, Lavera V, Callahan J, et al. Venezuelan equine encephalitis and Oropouche virus infections among Peruvian army troops in the Amazon region of Peru. Am J Trop Med Hyg. 1997;56(6):661–667. doi: 10.4269/ajtmh.1997.56.661. [DOI] [PubMed] [Google Scholar]
- 82.Watts DM, Callahan J, Rossi C, et al. Venezuelan equine encephalitis febrile cases among humans in the Peruvian Amazon river region. Am J Trop Med Hyg. 1998;58(1):35–40. doi: 10.4269/ajtmh.1998.58.35. [DOI] [PubMed] [Google Scholar]
- 83.Aguilar PV, Greene IP, Coffey LL, et al. Endemic Venezuelan equine encephalitis in northern Peru. Emerg Infect Dis. 2004;10(5):880–888. doi: 10.3201/eid1005.030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vilcarromero S, Laguna-Torres VA, Fernández C, et al. Venezuelan equine encephalitis and upper gastrointestinal bleeding in child. Emerg Infect Dis. 2009;15(2):323–325. doi: 10.3201/eid1502.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85▪.Vilcarromero S, Aguilar PV, Halsey ES, et al. Venezuelan equine encephalitis and 2 human deaths, Peru. Emerg Infect Dis. 2010;16(3):553–556. doi: 10.3201/eid1603.090970. Reports human neurological disease associated with VEEV infection in the Amazon region of Peru. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86▪.Morrison AC, Forshey BM, Notyce D, et al. Venezuelan equine encephalitis virus in iquitos, Peru: urban transmission of a sylvatic strain. PLoS Negl Trop Dis. 2008;2(12):E349. doi: 10.1371/journal.pntd.0000349. Describes a VEEV outbreak among residents who did not report recent travel outside the city of Iquitos, Peru, and the detection of Culex (Melanoconion) species of mosquitoes throughout the city. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turell MJ, Jones JW, Sardelis MR, et al. Vector competence of Peruvian mosquitoes (Diptera: Culicidae) for epizootic and enzootic strains of Venezuelan equine encephalomyelitis virus. J Med Entomol. 2000;37(6):835–839. doi: 10.1603/0022-2585-37.6.835. [DOI] [PubMed] [Google Scholar]
- 88.Turell MJ, Dohm DJ, Fernandez R, Calampa C, O’Guinn ML. Vector competence of Peruvian mosquitoes (Diptera: Culicidae) for a subtype IIIC virus in the Venezuelan equine encephalomyelitis complex isolated from mosquitoes captured in Peru. J Am Mosq Control Assoc. 2006;22(1):70–75. doi: 10.2987/8756-971X(2006)22[70:VCOPMD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 89.Yanoviak SP, Aguilar PV, Lounibos LP, Weaver SC. Transmission of a Venezuelan equine encephalitis complex alphavirus by Culex (Melanoconion) gnomatos (Diptera: Culicidae) in northeastern Peru. J Med Entomol. 2005;42(3):404–408. doi: 10.1093/jmedent/42.3.404. [DOI] [PubMed] [Google Scholar]
- 90.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2(9):E300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bowen GS, Calisher CH. Virological and serological studies of Venezuelan equine encephalomyelitis in humans. J Clin Microbiol. 1976;4(1):22–27. doi: 10.1128/jcm.4.1.22-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casamassima AC, Hess LW, Marty A. TC-83 Venezuelan equine encephalitis vaccine exposure during pregnancy. Teratology. 1987;36(3):287–289. doi: 10.1002/tera.1420360303. [DOI] [PubMed] [Google Scholar]
- 93.Bowen GS. Venezuelan Equine Encephalitis. Pan American Health Organization; Washington, DC, USA: 1972. Human disease: USA; pp. 231–234. [Google Scholar]
- 94▪.Berge TO, Banks IS, Tigertt WD. Attenuation of Venezuelan equine encephalomyelitis virus by in vitro cultivation in guinea pig heart cells. Am J Hyg. 1961;73:209–218. Describes the generation of the live-attenuated TC-83 vaccine strain that has been used to protect equids since 1971 and has been adminstered to humans who are at risk from laboratory exposure. [Google Scholar]
- 95.Walton TE, Johnson KM. Persistence of neutralizing antibody in Equidae vaccinated with Venezuelan equine encephalomyelitis vaccine strain TC-83. J Am Vet Med Assoc. 1972;161(8):916–918. [PubMed] [Google Scholar]
- 96.Kinney RM, Chang GJ, Tsuchiya KR, et al. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5′-noncoding region and the E2 envelope glycoprotein. J Virol. 1993;67(3):1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pedersen CE, Jr, Robinson DM, Cole FE., Jr Isolation of the vaccine strain of Venezuelan equine encephalomyelitis virus from mosquitoes in Louisiana. Am J Epidemiol. 1972;95(5):490–496. doi: 10.1093/oxfordjournals.aje.a121416. [DOI] [PubMed] [Google Scholar]
- 98▪.Pittman PR, Makuch RS, Mangiafico JA, Cannon TL, Gibbs PH, Peters CJ. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14(4):337–343. doi: 10.1016/0264-410x(95)00168-z. Describes the use of the TC-83 VEEV vaccine strain in humans, including documentation of its reactogenicity and limited immunogenicity. [DOI] [PubMed] [Google Scholar]
- 99.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9(10):741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 100.Paessler S, Weaver SC. Vaccines for Venezuelan equine encephalitis. Vaccine. 2009;27(Suppl 4):D80–D85. doi: 10.1016/j.vaccine.2009.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis NL, Brown KW, Greenwald GF, et al. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212(1):102–110. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- 102.Cabezas C. Etiological profile of acute febrile syndrome in high risk areas of infectious diseases transmission in Peru 2000–2001. Rev Peru Med Exp Salud Publica. 2005;22(3):165–174. [Google Scholar]
- 103.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2009;85(2):328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mendez W, Liria J, Navarro JC, et al. Spatial dispersion of adult mosquitoes (Diptera: Culicidae) in a sylvatic focus of Venezuelan equine encephalitis virus. J Med Entomol. 2001;38(6):813–821. doi: 10.1603/0022-2585-38.6.813. [DOI] [PubMed] [Google Scholar]
- 105.Barrera R, Torres N, Freier JE, et al. Characterization of enzootic foci of Venezuelan equine encephalitis virus in western Venezuela. Vector Borne Zoonotic Dis. 2001;1(3):219–230. doi: 10.1089/153036601753552585. [DOI] [PubMed] [Google Scholar]
- 106.Weaver SC, Scherer WF, Taylor CA, Castello DA, Cupp EW. Laboratory vector competence of Culex (Melanoconion) cedecei for sympatric and allopatric Venezuelan equine encephalomyelitis viruses. Am J Trop Med Hyg. 1986;35(3):619–623. doi: 10.4269/ajtmh.1986.35.619. [DOI] [PubMed] [Google Scholar]
- 107.Coffey LL, Carrara AS, Paessler S, et al. Experimental Everglades virus infection of cotton rats (Sigmodon hispidus) Emerg Infect Dis. 2004;10(12):2182–2188. doi: 10.3201/eid1012.040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ortiz DI, Kang W, Weaver SC. Susceptibility of Ae aegypti (Diptera: Culicidae) to infection with epidemic (subtype IC) and enzootic (subtypes ID, IIIC, IIID) Venezuelan equine encephalitis complex alphaviruses. J Med Entomol. 2008;45(6):1117–1125. doi: 10.1603/0022-2585(2008)45[1117:soaadc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 109.Turell MJ, Beaman JR. Experimental transmission of Venezuelan equine encephalomyelitis virus by a strain of Aedes albopictus (Diptera: Culicidae) from New Orleans, Louisiana. J Med Entomol. 1992;29(5):802–805. doi: 10.1093/jmedent/29.5.802. [DOI] [PubMed] [Google Scholar]
- 110▪.Suarez OM, Bergold GH. Investigations of an outbreak of Venezuelan equine encephalitis in towns of eastern Venezuela. Am J Trop Med Hyg. 1968;17(6):875–880. doi: 10.4269/ajtmh.1968.17.875. Landmark description of the 1962–1966 Venezuelan VEE epizootic that includes the earliest and most extensively documented descriptions of human disease. [DOI] [PubMed] [Google Scholar]
- 111.Martin SS, Bakken RR, Lind CM, et al. Evaluation of formalin inactivated V3526 virus with adjuvant as a next generation vaccine candidate for Venezuelan equine encephalitis virus. Vaccine. 2010;28(18):3143–3151. doi: 10.1016/j.vaccine.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paessler S, Ni H, Petrakova O, et al. Replication and clearance of Venezuelan equine encephalitis virus from the brains of animals vaccinated with chimeric SIN/VEE viruses. J Virol. 2006;80(6):2784–2796. doi: 10.1128/JVI.80.6.2784-2796.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, Frolov I. Recombinant Sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol. 2003;77(17):9278–9286. doi: 10.1128/JVI.77.17.9278-9286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kenney JL, Volk SM, Pandya J, Wang E, Liang X, Weaver SC. Stability of RNA virus attenuation approaches. Vaccine. 2011;29(12):2230–2234. doi: 10.1016/j.vaccine.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dupuy LC, Locher CP, Paidhungat M, et al. Directed molecular evolution improves the immunogenicity and protective efficacy of a Venezuelan equine encephalitis virus DNA vaccine. Vaccine. 2009;27(31):4152–4160. doi: 10.1016/j.vaccine.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 116.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2009;85(2):328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]