Abstract
A complex and diverse vascular system is requisite for the survival of higher organisms. The process of vascular development is highly regulated involving the de novo formation of vessels (vasculogenesis), followed by expansion and remodeling of the primitive vasculature (angiogenesis), culminating in differentiation of endothelial phenotypes as found in the mature vascular system. Over the last decade significant advances have been accomplished in understanding the molecular regulation of endothelial cell development and differentiation. Endothelial development, in particular the mechanisms in play during vasculogenesis and angiogenesis, is discussed in a sister review to this article. This review highlights the key pathways governing in endothelial differentiation with a focus on the major molecular mechanisms of endothelial specification and heterogeneity.
Keywords: Vascular biology, endothelium, angiogenesis, developmental biology, endothelial differentiation
INTRODUCTION
In order to distribute oxygen, nutrients, and paracrine factors to far corners of a multicellular organism, a closed-loop circulatory system must be formed and connected to a pump very early in development. The afferent loop– the arterial system– must be able to endure high pressure, pulsatile blood flow and accomplish tissue-specific delivery of circulating materials. Uninterrupted return of blood to the pumping chamber must be maintained under the low pressure, low shear stress, high-capacitance conditions of the venous system. The diverse functions of a continuous system requires specialization of components of the system, and the heterogeneity of endothelial cells (ECs) lining the lumen of the system play a large part in creating this specialization.
Until the late 1990’s the initial driving force in creation of heterogeneous EC phenotypes was thought to be the exposure of those cells to flowing blood. We now have data demonstrating that EC heterogeneity, while retaining the plasticity to alter under a changing environment, has a genetic component that comes into play perhaps even before hemangioblasts differentiate into ECs and hematopoietic cells. Elegant experiments in zebrafish and mouse embryos have provided the majority of the molecular data that drive our current understanding of EC heterogeneity. Human studies are largely limited to in vitro experiments with human-derived cells/tissues or to correlations with human vascular disease. Thus, the data reviewed below is generally from fish or rodent experiments. Unless otherwise noted, support of the molecular mechanisms described below was accomplished through classic genetic analyses with over-expression or knockdown of the molecule of interest and subsequent assessment of vascular consequences. Absolute concordance between the fish, rodent, and human systems has not been demonstrated. The aim of this review is to synthesize the available data in order to provide a general overview of the (vertebrate) pathway(s) to endothelial heterogeneity. For those wishing detailed experimental and system-specific discussions, several excellent recent reviews are available1–6.
MOLECULAR MECHANISMS OF SPECIFICATION
Vascular progenitor cells (angioblasts) originate in the lateral plate mesoderm and migrate to a midline position just ventral to the notochord, forming the inner cell mass (ICM). The ICM gives rise to both blood and ECs. Cell-tracing experiments in zebrafish suggest that the arterial-venous cell fate decision of angioblasts is made even prior to migration7. The major embryonic vessels are formed by the coalescence of angioblasts to form the dorsal aorta (DA), which lies ventral to the notochord, and the posterior cardinal vein (PCV), located just below and parallel to the dorsal aorta3, 8. In addition to a genetic predestination of the angioblasts, the response to a VEGF gradient initiates a hierarchy of signaling events that culminates in arterial vs. venous EC differentiation (illustrated in Figure 1).
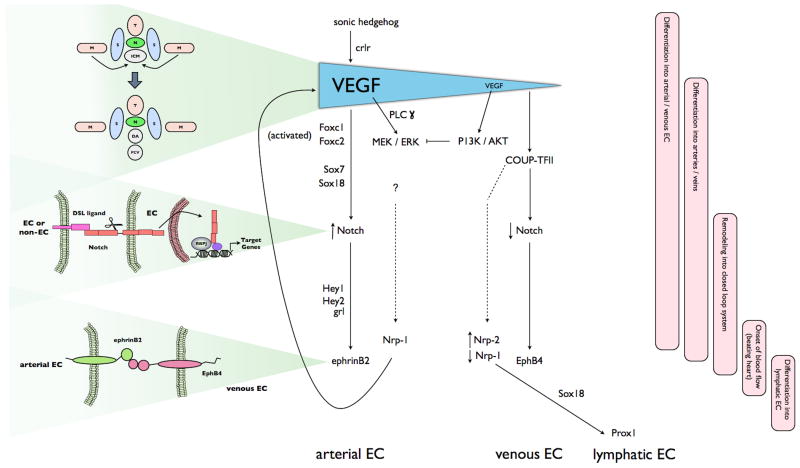
Figure 1.
A model for the proposed molecular pathways in arterial, venous and lymphatic specification in a developing embryo. Schematics on the left portion of the figure illustrate anatomic correlates of the molecular pathways described in the central flow diagram. The bars on the right portion of the figure highlight developmental correlates to the molecular pathways. Shh expression in the notchochord triggers VEGF expression by the somites and creation of a VEGF gradient. High levels of VEGF activate PLCγ and induce arterial specification by activating the MEK/ERK pathway through the VEGFR1-NRP1 receptor complex, and the PI3K/AKT pathway in cells residing further ventral. Arterial specification proceeds with activation of Foxc1/2 and Notch signaling, inducing ephrinB2 and Nrp-1expression. Nrp-1 creates a positive feedback loop by acting as co-receptor for VEGF. Venous specification occurs when ERK is suppressed, and COUP-TFII induced, by AKT. COUP-TFII inhibits arterial specification by down-regulating Notch and Nrp-1, allowing expression of venous-specific markers including Nrp-2. A subpopulation of venous EC subsequently express Sox18 and Prox1, resulting in lymphatic EC specification. The upper schematic highlights the migration of angioblasts and subsequent development, directed by VEGF concentrations, of the DA and PCV; the middle schematic the importance of activation of the Notch pathway by cleavage and nuclear translocation of the cytoplasmic domain of the receptor; and the lower schematic the requirement of cell-to-cell communication via ephrin-Eph receptor kinases in maintaining arterial/venous discrimination. T, neural tube; M, lateral mesoderm; S, somite; N, notochord; ICM, inner cell mass. Full details are found in the text.
Arterial Specification
At the top of the hierarchy is the secreted ligand sonic hedgehog (Shh). Shh is a pleiotropic molecule, with diverse roles in embryogenesis and patterning. It is expressed in the notochord and results in expression of VEGF by somites bordering the developing vessels. The exact mechanism of mediation of the Shh signal in this instance is unknown; classically, it acts through its transmembrane receptor, patched-1, to increase VEGF activity and thus arterial specification4, 9, 10. However, there is evidence that Shh also increases VEGF by regulating expression of the calcitonin receptor-like receptor (crlr) expressed in somites and arterial progenitors11, 12. Diffusion of VEGF from the somites to the developing vessels creates a gradient with higher levels of VEGF near the DA and lower levels at the PCV. In vascular plexuses, increased VEGF expression leads to an increase in the arterial:venous ratio. VEGF acts through one of several VEGF receptor tyrosine kinases to activate, amongst other targets, the PLCγ1-MEK/ERK signaling pathway – solely in the dorsal angioblasts of zebrafish 2, 13, 14. In vitro experiments using mouse-derived EC suggest that ERK can contribute to activation of the Foxc1 and Foxc2 transcription receptors that subsequently upregulate expression of members of the Notch pathway in vivo 15, 16. Activation of the Notch pathway is a defining characteristic of arterial EC. The Notch family is composed of four receptors (Notch1 to Notch4) and 5 ligands (Jagged-1 and -2 and Delta-like ligands (Dll) 1 to 4). In mice, Notch1, Notch4, Jag1, Jag2, and Dll4 are all expressed in arterial but not venous ECs 3. When Notch is activated, the intracellular domain is cleaved and translocates to the nucleus where it acts as a cofactor to upregulate transcription. Integrated expression of Notch genes during vessel development is required for appropriate vessel identity. Hemizygous deficiency of Dll4 is embryonic lethal in mice due to abnormal arterial development 17. While Dll4 is essential for initiation of the arterial development program, Dll1 is required for maintenance 5, 18. Alterations in levels of downstream targets of Notch lead to loss of arterial markers and arterial/venous fusion (compound Hey1/Hey2 mutant in mice;19, 20) and localized defects in the dorsal aorta (gridlock (grl) deficiency in zebrafish 21). The Sox7 and Sox18 gene products may regulate arterial-venous specification by acting upstream of grl22–24. In fact, arteriovenous malformations develop when Notch signaling is either reduced or constitutively active.
The ephrinB2 ligand and its cognate receptor, EphB4, are differentially expressed in the arterial and venous ECs, respectively, of the mouse primary vascular plexus prior to the onset of circulation 25, 26. This seminal discovery, in 1998, provided the first evidence that arterial-venous identity is genetically predetermined. Although this ligand-receptor pair have distinct cellular locations, interactions between the two are required for proper vascular development/remodeling. The ephrin-Eph subclass of receptor tyrosine kinases can participate in an unusual bidirectional signaling process. Forward signaling (ephrin ligand to Eph receptor) is initiated by ephrin ligand engagement and activates the receptor kinase domain. Reverse signaling (Eph receptor to ephrin subclass B ligand) leads to phosphorylation of tyrosine residues in the cytoplasmic domain of the ligand. This large subfamily of signaling molecules regulates a variety of morphogenetic processes in different tissues (reviewed in 1). The lack of ephrin B2 or EphB4 does not impair the initial specification of arteries and veins, but ephrinB2-EphB4 signaling is required for maintenance of the arterial-venous interaction. Mouse mutants defective for the pair lose the differentiation of blood vessels into morphologically distinguishable arteries and veins 25–27. This limited role lead to the subsequent identification of upstream factors, such as Notch, in the process of arterial/venous EC differentiation.
Expression of the neuropilins (nrp) is temporally related to the expression of the ephrins and also displays a restricted pattern of expression. Nrp-1 is found exclusively in cells fated to be arterial, and Nrp-2 in venous 28. In mice with a CD1 background, Nrp-1 deficiency leads to abnormal vascular network formation and embryonic lethality at E13.5. In the C57Bl/6 background, the vascular defects are not apparent until after the onset of blood flow. In these mice, deficiency abrogates the normal vascular remodeling that occurs coincident with the initiation of flowing blood (E10.5) 29–31. The neuropilins were previously identified as cell surface receptors for semaphorins. In this system, Nrp-1 functions at least in part by acting as a co-receptor for VEGF and facilitating VEGF signaling in concert with VEGF receptor-2 32.
Venous Specification
Unlike arterial specification, details of the molecular mechanisms controlling venous specification are still largely unknown. Exposure to lower VEGF concentrations is likely to be important as a negative regulator of arterial specification. In vitro experiments have shown that higher concentrations of VEGF (50ng/ml) induces expression of arterial markers such as ephrinB2 and Nrp-1 in embryonic stem cells whereas low VEGF concentrations (2ng/ml) led to expression of venous endothelial markers 33. In zebrafish, loss of Shh signaling leads to loss of the arterial marker Ephrin-B2a and greater amounts of the venous marker Flt434. Thus, the greater distance of the PCV from the VEGF-spewing somites (as compared to the DA) may contribute to its differentiation into a venous structure (reviewed in 3). In vitro studies have shown that VEGF activates a plethora of signaling pathways including PI3K/Akt pathway, which can act in concert with VEGF-activated ERK; however, in regard to arterial EC specification, in vitro and in vivo experiments suggest that the MEK/ERK and PI3K/AKT pathways may have competing roles 35. Specifically, activation of AKT can inhibit the activity of MEK/ERK in zebrafish, thus steering EC away from arterial specification 14. (Replication of Notch inhibition by PI3K in in vitro experiments has not been accomplished; loss of the complexities of the in vivo context is considered a potentially important factor 36, 37.) AKT is also hypothesized to induce chicken ovalbumin upstream promoter-transcription factor (COUP-TFII) expression 2. COUP-TFII is specifically expressed in venous ECs and is a genetic determinant of venous specification. While compound homozygous mutants for Foxc1 and Foxc2 lack arterial specific genes, they do express the venous marker COUP-TFII16. As a nuclear orphan receptor, the natural ligand for COUP-TFII is retinoic acid. Activation of COUP-TFII by retinoic acid suppresses Notch signaling, potentially at the level of NRP-1 expression, thus releasing factors such as EphB4 from Notch-mediated repression, and conferring a venous EC phenotype 38. COUP-TFII directly regulates expression of Nrp-2, leading both to its restricted expression in venous (vs. arterial) ECs and preparing cells for differentiation into lymphatic EC 39.
Lymphatic Specification
Lymphatic EC (LEC) are derived from lymphatic vessel hyaluronan-1 receptor-1 (LYVE-1) positive subpopulations of cells in the cardinal vein which bud off to form primary lymphatic sacs. LYVE-1 is a specific marker for cells capable of becoming LEC, but is not required for normal lymphatic development40, 41. By molecular mechanisms not yet elucidated, the transcription factor SRY box 18 (Sox18) is induced in murine LYVE-1 positive venous cells that will commit to the LEC lineage. Sox18 directly induces expression of Prox1, the master regulator of LEC identity 42. Recent data suggests that Prox1 is required for both initiation and maintenance of the LEC phenotype. Prox1 deficient EC can bud from the cardinal vein, but they display abnormal VEGF-induced migration and do not express LEC markers 43, 44. Recent data shows that lymphatic sprouting, specifically (versus angiogenic), is regulated in zebrafish by the PDZ domain-containing scaffold protein synectin; in vitro experiments with human dermal LECs suggest that synectin functions, in part, by regulating Sox18-mediated induction of Prox145. Pedrioli and colleagues used microarray analysis to identify human blood EC- or LEC-specific microRNAs, and found that miR31 suppresses lymphatic differentiation46. Mechanistic analysis demonstrated that the inhibitory effect is partially mediated via direct repression of Prox1.
Prox1 mediates upregulation of important regulators/markers of lymphatic differentiation, including the VEGF receptor, VEGFR-3. VEGFR-3 is expressed in all EC during early development; however, its expression is enhanced in cells committed to the LEC lineage, and as the lymphatic system develops, becomes largely restricted to LECs47. Activation of VEGFR-3, in particular, by VEGF-C is required for lymphatic development 48. It is proposed that the VEGFR-3 co-receptor Nrp-2 increases the response of LEC to VEGF-C by a mechanism analogous to the interaction of Nrp-1 and VEGFR-2 in arterial EC (as reviewed in6).
The presence of COUP-TFII in EC may well be a determinant factor for the venous origin of cells destined to become LEC. Sox18 cooperates with COUP-TFII to promote expression of Prox-142. Prox1 appears to act in concert with COUP-TFII in enhancing expression of VEGFR-3, and by upregulating the VEGFR-3 co-receptor Nrp-2, COUP-TFII enhances VEGFR-3 activity 39.
Plasticity of Vascular Specification
While the initial molecular identity of ECs is genetically predetermined, there is significant plasticity in subsequent arteriovenous differentiation. Physiological requirements and hemodynamic influences can reverse the phenotype of an apparently committed cell. ECs in transplanted arterial and venous vessel grafts can change identity, completely switching their expression profile to match the host vessel 27, 49. Forced over-expression or loss-of-function of critical molecular determinants of specification can also reprogram a differentiated EC. For example, Notch over-expression in the venous compartment results in arterialization with upregulation of arterial EC markers while inhibition of Notch signaling in the arterial compartment results in loss of arterial fate and upregulation of venous markers50. Ablation of EC COUP-TFII allows veins to acquire arterial characteristics and express arterial markers38. The mature LEC phenotype is highly plastic51, 52. As discussed above, constant expression of Prox1 maintains the mature differentiated LEC state. Conditional embryonic, post-natal, or adult deletion of Prox1 in mice results in the appearance of blood-filled lymphatic vessels and down regulation of LEC markers with the concomitant ectopic expression of blood EC markers53. Conversely, ectopic over-expression of Prox1 in cultured blood ECs leads to the acquisition of LEC identity54, 55. An exquisite feedback regulatory equilibrium exists among the three major EC fate regulators (Notch, COUP-TFII, and Prox1) that directs the plasticity in arteriovenous-lymphatic cell fate56.
MOLECULAR MECHANISMS OF HETEROGENEITY
Beyond the specification to arterial, venous, or lymphatic fate, it is currently recognized that ECs undergo further differentiation specific to the vascular bed or organ in which they reside. This phenotypic heterogeneity is the primary mechanism by which the endothelium carries out myriad vital functions including: control of microvascular permeability, vessel wall tone, coagulation and anticoagulation, inflammation, and angiogenesis1, 57, 58. Endothelial heterogeneity is also responsible for the varied and diverse responses across differing vascular beds to pathologic stimuli and disease states59–61.
Structural Classifications
Based on structural content, the endothelium has been classically characterized into three main structural types: continuous, which is further subdivided into fenestrated or non-fenestrated, and discontinuous (or sinusoidal)57, 62 (illustrated in Figure 2A).
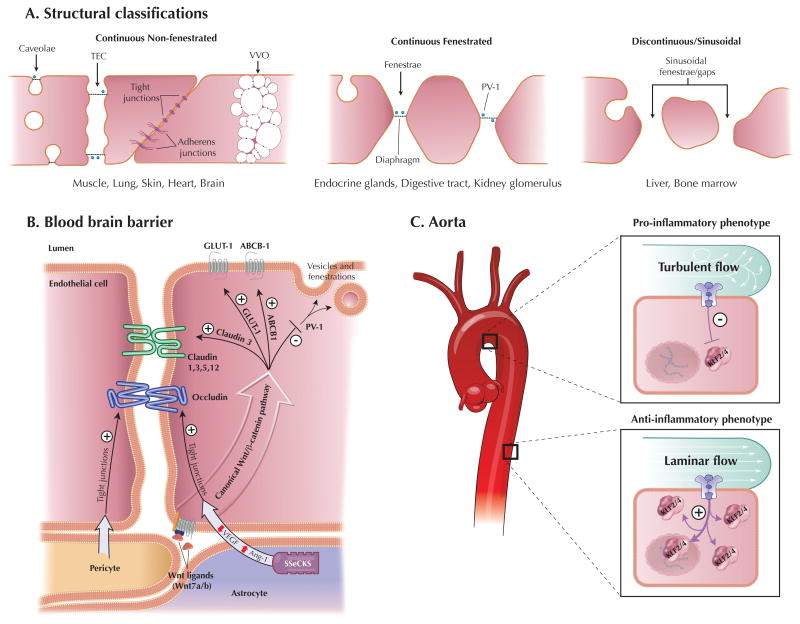
Figure 2.
Endothelial Cell Heterogeneity. A, Schematic diagram of the three main endothelial structural classifications (modified from 57). TEC= transendothelial channel, VVO=vesiculo-vacuolar organelles. B, Schematic diagram of the molecular mechanisms of blood brain barriergenesis (modified from 100). C, Heterogeneity of the aortic endothelium. Turbulent flow occurring in the lesser curvature of the aortic arch or at branch points in the vascular tree inhibits KLF2/4 expression resulting in a more pro-inflammatory endothelial phenotype. Whereas laminar flow occurring in linear segments upregulates endothelial KLF2/4 expression resulting in a more anti-inflammatory phenotype.
Continuous Non-fenestrated
Continuous non-fenestrated endothelium is found predominantly in arteries, veins, and capillaries of the brain, skin, muscle, heart, and lung. Tight junctions and adherens junctions are the two main types of barrier forming intracellular junctions found in this type of endothelium63, 64. Their expression is variable across the endothelial tree with higher expression in the continuous endothelium of arterioles when compared to capillaries and venules. Molecules cross this endothelium by the active process of transcytosis, which is mediated by specialized structures including caveolae and vesiculo-vacuolar organelles (VVOs). Caveolae, flask-shaped membrane bound vesicles (~70nm in diameter) that usually open to the luminal or abluminal side65, 66, are present in all types of endothelium but are highest in capillaries that contain continuous non-fenestrated endothelium67. Caveolin-1 is the main structural component of caveolae and is regulated by distinct transcriptional mechanisms in ECs68. VVOs also contain caveolin-1 and are focal collections of membrane bound vesicles of variable size that span the cytoplasm of the ECs69 and are mostly found in venules with continuous non-fenestrated endothelium70.
Continuous Fenestrated
Fenestrae are transcellular pores (~70nm in diameter) that extend through the full thickness of the EC and are thought to allow rapid exchange of molecules between the circulation and the surrounding tissue71. The majority of fenestrae contain a thin diaphragm across their opening which acts as a molecular filter. The type II membrane glycoprotein plasmalemmal vesicle associated protein-1 (PV-1) is currently the only molecular protein localized to the diaphragm72 and it has been discovered to be both necessary and sufficient for diaphragm formation in cultured ECs73, 74. Other diaphragm containing components of continuous endothelium include caveolae and transendothelial channels (TECs), which are patent pores spanning the EC from the luminal to abluminal side 75. Compared to non-fenestrated endothelium, continuous fenestrated endothelium is more permeable to water and small solutes. This endothelium typically occurs in locations that are characterized by increased filtration or increased transendothelial transport and is found in capillaries of all exocrine and endocrine glands, digestive tract mucosa, and kidney (e.g. glomeruli and a subpopulation of renal tubules).
Discontinuous Fenestrated
Discontinuous fenestrated endothelium is characterized by large heterogeneous fenestrae (100–200nm in diameter) without diaphragms76. It has few caveolae and contains clathrin-coated pits and vesicles, which play an important role in receptor-mediated endocytosis. This endothelium is found in certain sinusoidal vascular beds, most notably the liver and bone marrow which lack a well formed basement membrane.
The phenotypic heterogeneity of the endothelium of the various organ and vascular beds has been highly studied1, 57, 58. Remarkably, despite these detailed observations, the molecular mechanisms of endothelial heterogeneity remain largely unknown. In recent years, the study of ECs in several vascular beds have made significant strides in elucidating some of these molecular mechanisms.
Kidney Endothelium
The kidney is a highly vascular organ that contains a large degree of endothelial heterogeneity. Blood from the renal arteries reaches the kidney and branches into the afferent arterioles that enter into the glomerular tufts. The glomerular endothelium in conjunction with the basement membrane and underlying podocytes helps to form the glomerular filtration barrier which serves as both a size and charge selective filter77, 78. The glomerular endothelium actively synthesizes a 60–300nm thick gelatinous surface coat called glycocalyx (composed of proteoglycans, glycosaminoglycans, glycoproteins, glycolipids, and associated plasma proteins) that covers the luminal side and facilitates charge selectivity77, 79. The glomerular capillaries consist of a continuous fenestrated endothelium with large fenestrae that cover 20–50% of the entire endothelial surface 80. A unique feature of the glomerular endothelium is that most fenestrae do not contain diaphragms. The majority of evidence suggests that the glomerular ECs lose their expression of PV-1 and fenestral diaphragms during the development and maturation of the glomerulus81–83. Importantly, recent studies of the glomerular endothelium have confirmed the initial observations that VEGF plays a role in the formation of EC fenestrations84–86. Cross talk between podocytes, expressing VEGF-A, and the glomerular endothelium, expressing VEGF receptors, has been shown to be important for the development and maintenance of fenestrae and barrier function87–89. Studies are ongoing investigating the detailed molecular pathways, but initial findings implicate activation of the small GTPases (i.e. Rho/Rac) and rearrangement of the actin cytoskeleton83.
Efferent arterioles exit the renal glomeruli and terminate in the vasa recta. Endothelial heterogeneity of the vasa recta is critical for the countercurrent exchange that occurs in the medulla of the kidney90. The descending vasa recta (i.e. an arteriole entering the medulla) is lined by continuous non-fenestrated endothelium and contains large concentrations of urea transporters and aquaporin-1 water channels91. In contrast the ascending vasa recta (i.e. a vein exiting the medulla) is lined with fenestrated endothelium. This hetereogeneity allows for shuttling of osmotically active solutes between descending and ascending capillaries thereby maintaining the hypertonicity of the medulla that is essential for gradient mediated filtration in the kidneys. In addition this process helps to deliver nutrients and oxygen to the medullary tissue 91.
Brain Endothelium
The brain endothelium has an extremely specialized characteristic that allows it to selectively control permeability between blood and the central nervous system, thereby forming the blood brain barrier (BBB)92, 93. This barrier function is primarily mediated by both a physical barrier due to tight interendothelial junctions and a highly selective transporter system. The hallmark feature of the endothelium of the BBB is a continuous non-fenestrated endothelium with few caveolae and high expression of tight junction proteins, namely occludin and members of the claudin family (claudin 1, 3, 5, and 12). As tight junctions form, the brain ECs also begin to express selective membrane transporter proteins such as glucose transporter type 1 (Glut-1) and members of the ATP binding cassette (ABC) transporter family (ABCB1/P-glycoprotein/MDR1 and BCRP/ABCG2). This differentiation and maturation process of acquiring the unique properties of the BBB is frequently called barriergenesis94, 95.
The brain endothelium is surrounded by several other cell types including pericytes, astrocytes, and neurons, forming the neurovascular unit (NVU)96, and interaction between multiple components of the NVU have been found to be necessary for proper formation and function of the BBB. Recent studies demonstrate that pericyte-EC interactions are necessary for BBB development by regulating the formation of tight junctions as well as controlling pinocytic transport vesicles in ECs97, 98. Additionally, the astrocyte-derived factor Src-suppressed C kinase substrate (SSeCKS) strengthens the BBB by decreasing VEGF expression and inducing Angiopoietin-1 (Ang-1), resulting in increased tight junction expression in brain ECs 99 (illustrated in Figure 2B).
Recently the canonical Wnt pathway has been discovered to play a major role in barriergenesis100. In these studies, the expression of Glut1 was induced by Wnt7a and Wnt7b101, 102. Additionally, Wnt signalling in ECs was necessary and sufficient for the induction and maintenance of BBB characteristics by increasing claudin 3 expression and inhibiting PV-1103 (illustrated in Figure 2B). These recent findings in barriergenesis predict that the Wnt signaling pathway, which has been shown to play important roles in vascular morphogenesis, is likely to be involved in the mechanism of endothelial heterogeneity in other vascular beds and organs104.
Aortic Endothelium
Endothelial heterogeneity exists not only between various organs and vascular beds but also within a single vascular bed. One of the most striking examples of this type of heterogeneity is the aortic endothelium, where variable blood flow dynamics results in the nonuniform distribution of atherosclerosis105, 106. Laminar blood flow in straight segments of the aorta induces factors such as endothelial nitric oxide synthase (eNOS) and thrombomodulin (TM), thereby conferring potent anti-thrombotic, anti-adhesive, and anti-inflammatory properties to the endothelium107, 108. Conversely, non-laminar or turbulent blood flow at areas where arteries branch or turn sharply reduces eNOS expression and induces adhesion molecules such as vascular cell adhesion molecule [VCAM]-1, resulting in an inflammatory endothelial phenotype106, 109, 110. Two members of the Kruppel-like factor transcription family (KLF2 and KLF4) are strongly induced by laminar flow, via the MEK/ERK5/MEF2 pathway, and have been shown to be key molecular mediators of flow-mediated endothelial heterogeneity111 (illustrated in Figure 2C).
SUMMARY AND FUTURE DIRECTIONS
As we continue to unravel the molecular and physiological mechanisms that create the diversity of ECs, we hope to become increasingly able to harness the plasticity of the EC phenotype in order to modulate the pathophysiological events specific to various vascular beds. Such molecular insights may allow one to manipulate EC phenotype in the treatment of vasculo-centric disease states that are major sources of morbidity and mortality including atherothrombosis, sepsis, or tumorigenesis. Finally, advances in this area of biology may be facilitated through establishment of improved models of vascular development and plasticity. While much of the work to date has relied on studies in zebrafish and mice, a recent study found that the mechanism of cell commitment in early embryos differs significantly between mice and cows112. In this regard, use of human iPS technology may allow for novel insights into human endothelial differentiation, specification, and heterogeneity.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH Grants HL72952, HL75427, HL76754, HL086548, HL084154, and P01 HL048743 (to M.K.J.); HL088740 (to G.B.A.) and HL083090 (to A.H.); a Dominic Visconsi Scholar Award (G.B.A., A.H.). We acknowledge the creative assistance of Kevin P. Montgomery for Figure 1.
Footnotes
DISCLOSURES
None.
References
- 1.Aitsebaomo J, Portbury AL, Schisler JC, Patterson C. Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circ Res. 2008;103:929–939. doi: 10.1161/CIRCRESAHA.108.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong CC, Kume T, Peterson RT. Role of crosstalk between phosphatidylinositol 3-kinase and extracellular signal-regulated kinase/mitogen-activated protein kinase pathways in artery-vein specification. Circ Res. 2008;103:573–579. doi: 10.1161/CIRCRESAHA.108.180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 4.Le Bras A, Vijayaraj P, Oettgen P. Molecular mechanisms of endothelial differentiation. Vasc Med. 2010;15:321–331. doi: 10.1177/1358863X10371685. [DOI] [PubMed] [Google Scholar]
- 5.Kume T. Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol. 2010;25:637–646. doi: 10.14670/hh-25.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 7.Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- 8.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 9.Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, Krieg PA. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- 10.Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–1050. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- 11.Nicoli S, Tobia C, Gualandi L, De Sena G, Presta M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood. 2008;111:4965–4972. doi: 10.1182/blood-2007-10-118166. [DOI] [PubMed] [Google Scholar]
- 12.Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E, Ara T, Nagasawa T, Just U, Nakao K, Nishikawa S, Yamashita JK. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol. 2006;26:1977–1984. doi: 10.1161/01.ATV.0000234978.10658.41. [DOI] [PubMed] [Google Scholar]
- 13.Lawson ND, Mugford JW, Diamond BA, Weinstein BM. phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–1351. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi H, Kume T. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One. 2008;3:e2401. doi: 10.1371/journal.pone.0002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–5688. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 19.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol. 2005;278:301–309. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein BM, Stemple DL, Driever W, Fishman MC. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med. 1995;1:1143–1147. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- 22.Cermenati S, Moleri S, Cimbro S, Corti P, Del Giacco L, Amodeo R, Dejana E, Koopman P, Cotelli F, Beltrame M. Sox18 and Sox7 play redundant roles in vascular development. Blood. 2008;111:2657–2666. doi: 10.1182/blood-2007-07-100412. [DOI] [PubMed] [Google Scholar]
- 23.Herpers R, van de Kamp E, Duckers HJ, Schulte-Merker S. Redundant roles for sox7 and sox18 in arteriovenous specification in zebrafish. Circ Res. 2008;102:12–15. doi: 10.1161/CIRCRESAHA.107.166066. [DOI] [PubMed] [Google Scholar]
- 24.Pendeville H, Winandy M, Manfroid I, Nivelles O, Motte P, Pasque V, Peers B, Struman I, Martial JA, Voz ML. Zebrafish Sox7 and Sox18 function together to control arterial-venous identity. Dev Biol. 2008;317:405–416. doi: 10.1016/j.ydbio.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 26.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- 28.Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- 29.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 31.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 32.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 33.Lanner F, Sohl M, Farnebo F. Functional arterial and venous fate is determined by graded VEGF signaling and notch status during embryonic stem cell differentiation. Arterioscler Thromb Vasc Biol. 2007;27:487–493. doi: 10.1161/01.ATV.0000255990.91805.6d. [DOI] [PubMed] [Google Scholar]
- 34.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 35.Blum S, Issbruker K, Willuweit A, Hehlgans S, Lucerna M, Mechtcheriakova D, Walsh K, von der Ahe D, Hofer E, Clauss M. An inhibitory role of the phosphatidylinositol 3-kinase-signaling pathway in vascular endothelial growth factor-induced tissue factor expression. J Biol Chem. 2001;276:33428–33434. doi: 10.1074/jbc.M105474200. [DOI] [PubMed] [Google Scholar]
- 36.Liu ZJ, Xiao M, Balint K, Soma A, Pinnix CC, Capobianco AJ, Velazquez OC, Herlyn M. Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J. 2006;20:1009–1011. doi: 10.1096/fj.05-4880fje. [DOI] [PubMed] [Google Scholar]
- 37.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 39.Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010;120:1694–1707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 43.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 44.Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4:35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 45.Hermans K, Claes F, Vandevelde W, Zheng W, Geudens I, Orsenigo F, De Smet F, Gjini E, Anthonis K, Ren B, Kerjaschki D, Autiero M, Ny A, Simons M, Dewerchin M, Schulte-Merker S, Dejana E, Alitalo K, Carmeliet P. Role of synectin in lymphatic development in zebrafish and frogs. Blood. 2010;116:3356–3366. doi: 10.1182/blood-2009-11-254557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedrioli DM, Karpanen T, Dabouras V, Jurisic G, van de Hoek G, Shin JW, Marino D, Kalin RE, Leidel S, Cinelli P, Schulte-Merker S, Brandli AW, Detmar M. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol. 2010;30:3620–3634. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 49.Othman-Hassan K, Patel K, Papoutsi M, Rodriguez-Niedenfuhr M, Christ B, Wilting J. Arterial identity of endothelial cells is controlled by local cues. Dev Biol. 2001;237:398–409. doi: 10.1006/dbio.2001.0383. [DOI] [PubMed] [Google Scholar]
- 50.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Choi I, Hong YK. Heterogeneity and plasticity of lymphatic endothelial cells. Semin Thromb Hemost. 2010;36:352–361. doi: 10.1055/s-0030-1253457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliver G, Srinivasan RS. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development. 2010;137:363–372. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 55.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang J, Yoo J, Lee S, Tang W, Aguilar B, Ramu S, Choi I, Otu HH, Shin JW, Dotto GP, Koh CJ, Detmar M, Hong YK. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116:140–150. doi: 10.1182/blood-2009-11-252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 58.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 59.Molema G. Heterogeneity in endothelial responsiveness to cytokines, molecular causes, and pharmacological consequences. Semin Thromb Hemost. 2010;36:246–264. doi: 10.1055/s-0030-1253448. [DOI] [PubMed] [Google Scholar]
- 60.Davies PF, Civelek M, Fang Y, Guerraty MA, Passerini AG. Endothelial heterogeneity associated with regional athero-susceptibility and adaptation to disturbed blood flow in vivo. Semin Thromb Hemost. 2010;36:265–275. doi: 10.1055/s-0030-1253449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwaan HC, Samama MM. The significance of endothelial heterogeneity in thrombosis and hemostasis. Semin Thromb Hemost. 2010;36:286–300. doi: 10.1055/s-0030-1253451. [DOI] [PubMed] [Google Scholar]
- 62.Tse D, Stan RV. Morphological heterogeneity of endothelium. Semin Thromb Hemost. 2010;36:236–245. doi: 10.1055/s-0030-1253447. [DOI] [PubMed] [Google Scholar]
- 63.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 64.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 65.Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 67.Bendayan M. Morphological and cytochemical aspects of capillary permeability. Microsc Res Tech. 2002;57:327–349. doi: 10.1002/jemt.10088. [DOI] [PubMed] [Google Scholar]
- 68.Kathuria H, Cao YX, Ramirez MI, Williams MC. Transcription of the caveolin-1 gene is differentially regulated in lung type I epithelial and endothelial cell lines. A role for ETS proteins in epithelial cell expression. J Biol Chem. 2004;279:30028–30036. doi: 10.1074/jbc.M402236200. [DOI] [PubMed] [Google Scholar]
- 69.Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol. 1996;59:100–115. [PubMed] [Google Scholar]
- 70.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49:419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 71.Simionescu M, Simionescu N, Palade GE. Morphometric data on the endothelium of blood capillaries. J Cell Biol. 1974;60:128–152. doi: 10.1083/jcb.60.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A. 1999;96:13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stan RV, Tkachenko E, Niesman IR. PV1 is a key structural component for the formation of the stomatal and fenestral diaphragms. Mol Biol Cell. 2004;15:3615–3630. doi: 10.1091/mbc.E03-08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ioannidou S, Deinhardt K, Miotla J, Bradley J, Cheung E, Samuelsson S, Ng YS, Shima DT. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc Natl Acad Sci U S A. 2006;103:16770–16775. doi: 10.1073/pnas.0603501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stan RV. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J Cell Mol Med. 2007;11:621–643. doi: 10.1111/j.1582-4934.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haraldsson B, Jeansson M. Glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:331–335. doi: 10.1097/MNH.0b013e32832c9dba. [DOI] [PubMed] [Google Scholar]
- 78.Levidiotis V, Power DA. New insights into the molecular biology of the glomerular filtration barrier and associated disease. Nephrology (Carlton) 2005;10:157–166. doi: 10.1111/j.1440-1797.2005.00385.x. [DOI] [PubMed] [Google Scholar]
- 79.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 80.Bulger RE, Eknoyan G, Purcell DJ, 2nd, Dobyan DC. Endothelial characteristics of glomerular capillaries in normal, mercuric chloride-induced, and gentamicin-induced acute renal failure in the rat. J Clin Invest. 1983;72:128–141. doi: 10.1172/JCI110950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kriz W. Fenestrated glomerular capillaries are unique. J Am Soc Nephrol. 2008;19:1439–1440. doi: 10.1681/ASN.2008060583. [DOI] [PubMed] [Google Scholar]
- 82.Ichimura K, Stan RV, Kurihara H, Sakai T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J Am Soc Nephrol. 2008;19:1463–1471. doi: 10.1681/ASN.2007101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296:F947–956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 85.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108 (Pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 86.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- 87.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF--a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:p32–37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 89.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1153–1175. doi: 10.1152/ajpregu.00657.2002. [DOI] [PubMed] [Google Scholar]
- 91.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253–266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- 92.Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 93.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 94.Paolinelli R, Corada M, Orsenigo F, Dejana E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol Res. 2011;63:165–171. doi: 10.1016/j.phrs.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 95.Lee HS, Han J, Bai HJ, Kim KW. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. Febs J. 2009;276:4622–4635. doi: 10.1111/j.1742-4658.2009.07174.x. [DOI] [PubMed] [Google Scholar]
- 96.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 97.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 98.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 100.Liebner S, Plate KH. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenes Res. 2010;2:1. doi: 10.1186/2040-2384-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 103.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev. 2009;19:476–483. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Gimbrone MA., Jr Vascular endothelium, hemodynamic forces, and atherogenesis. Am J Pathol. 1999;155:1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- 107.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takada Y, Shinkai F, Kondo S, Yamamoto S, Tsuboi H, Korenaga R, Ando J. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun. 1994;205:1345–1352. doi: 10.1006/bbrc.1994.2813. [DOI] [PubMed] [Google Scholar]
- 109.Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med. 1992;176:1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 112.Berg DK, Smith CS, Pearton DJ, Wells DN, Broadhurst R, Donnison M, Pfeffer PL. Trophectoderm lineage determination in cattle. Dev Cell. 2011;20:244–255. doi: 10.1016/j.devcel.2011.01.003. [DOI] [PubMed] [Google Scholar]