Abstract
Cyclin D1 is a component of the core cell cycle machinery1. Abnormally high levels of cyclin D1 are detected in many human cancer types2. To elucidate the molecular functions of cyclin D1 in human cancers, here we performed a proteomic screen for cyclin D1 protein partners in several types of human tumors. Analyses of cyclin D1-interactors revealed a network of DNA repair proteins, including RAD51, a recombinase that drives the homologous recombination process3. We found that cyclin D1 directly binds RAD51, and that cyclin D1-RAD51 interaction is induced by radiation. Like RAD51, cyclin D1 is recruited to DNA damage sites in a BRCA2-dependent fashion. Reduction of cyclin D1 levels in human cancer cells impaired recruitment of RAD51 to damaged DNA, impeded the homologous recombination-mediated DNA repair, and increased sensitivity of cells to radiation in vitro and in vivo. This effect was seen in cancer cells lacking the retinoblastoma protein, which do not require D-cyclins for proliferation4, 5. These findings reveal an unexpected function of a core cell cycle protein in DNA repair and suggest that targeting cyclin D1 may be beneficial also in retinoblastoma-negative cancers which are currently thought to be oblivious to cyclin D1 inhibition.
In order to elucidate the molecular functions of cyclin D1 in human cancer cells, we set to identify cyclin D1-interacting proteins in four types of human tumors: mantle cell lymphoma (Granta 519 cells), breast cancer (MCF7 and ZR-75-1), squamous cell carcinoma (UMSCC-2), and colorectal cancer (HT-29). These cell lines overexpress cyclin D1 due to amplification or rearrangements within the cyclin D1 gene, or mutations within the cyclin D1 degradation machinery6–9. Cyclin D1-containing complexes were purified using double immunoaffinity purification10 (Supplementary Fig. 1), and the identity of cyclin D1-interactors was determined by rounds of liquid chromatography and high-throughput mass spectrometry (LC-MS/MS). A total of 132 proteins were identified with high confidence (Fig. 1a, Supplementary Fig. 2, Supplementary Tables 1–5).
Figure 1. Analyses of cyclin D1-interactors identified in human cancers.
a, A diagram depicting cyclin D1-interactors, grouped by cell line in which they were detected. Line thickness corresponds to the abundance of peptides for each protein detected in MS.
b, Biological process/molecular function enrichment heatmap of cyclin D1-interactors. Each column corresponds to the indicated cell line, rows denote distinct biological process/molecular function. Red color depicts enriched functions, green color – no enrichment. Left panel shows a complete map, right panel an enlarged map of molecular functions/biological processes enriched across all 5 cancer cell lines analyzed.
We constructed a biological process/molecular function enrichment heatmap of cyclin D1-interactors (Fig. 1b, Supplementary Table 6), and searched for common functions. Unexpectedly, apart from cell cycle control proteins, we observed DNA repair category amongst the most enriched functions (Fig. 1b Supplementary Fig. 3a and Supplementary Table 6). Using published databases of physical and functional interactions, we constructed a protein interaction network of all cyclin D1-interactors encountered by us, and observed a cluster of DNA repair proteins centered on RAD51, a key DNA recombinase mediating DNA repair via homologous recombination (HR)3 (Supplementary Fig. 3b). These observations suggested that cyclin D1 may play a role in DNA damage repair.
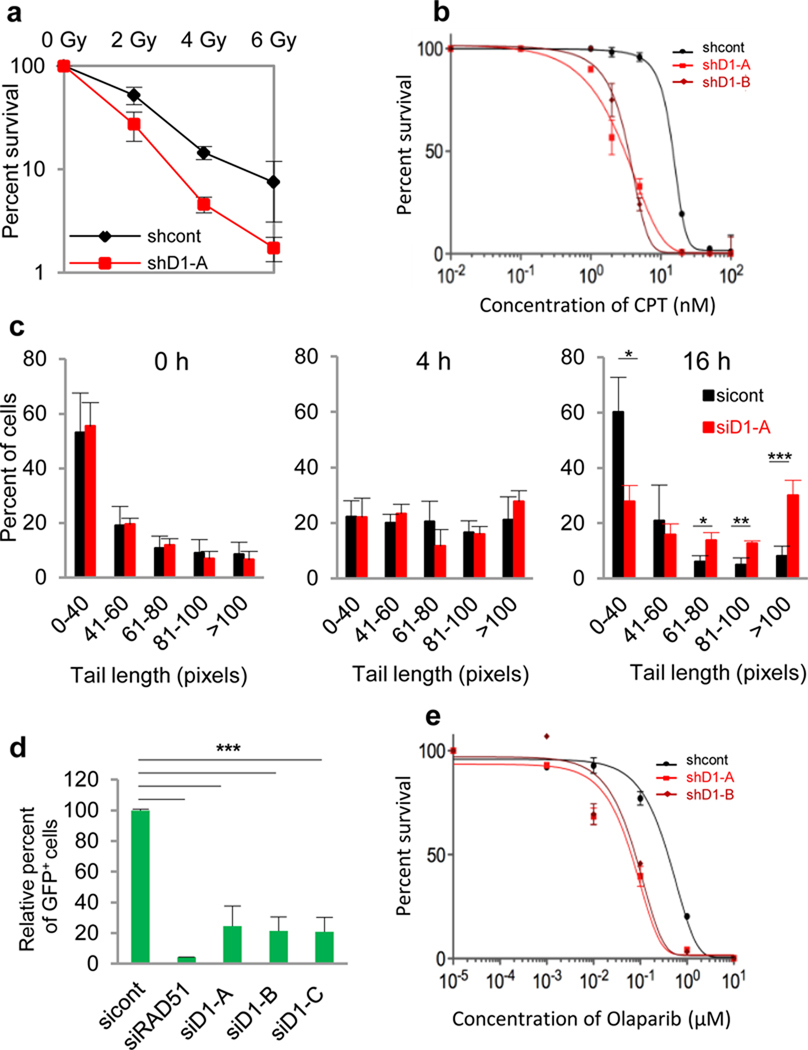
Consistent with this possibility, we found that depletion of cyclin D1 by short hairpin RNA (shRNA) in cervical carcinoma HeLa and lung cancer H2009 cells, significantly increased the sensitivity of cancer cells to ionizing radiation (IR) (Fig. 2a, Supplementary Fig. 4a, c). We utilized these two retinoblastoma protein (pRB)-negative cell lines to rule out cell cycle effects of cyclin D1 knock-down, as pRB-negative cancer cells do not require D-cyclins for proliferation4, 5 (Supplementary Fig. 5). Reduction of cyclin D1 levels also sensitized cancer cells to DNA-damaging drugs camptothecin and etoposide (Fig. 2b, Supplementary Fig. 4d). Moreover, fibroblasts lacking D-cyclins (D1−/−D2−/−D3−/−)11, which have normal cell cycle progression, showed increased sensitivity to ionizing radiation; re-expression of cyclin D1 in these cells restored radiation sensitivity (Supplementary Figs. 4e–f, 6).
Figure 2. Impaired DNA repair upon reduction of cyclin D1 levels.
a, Colony survival assay of HeLa cells expressing anti-cyclin D1 shRNA (shD1-A) or control shRNA (shcont), after the indicated doses of IR. SF50= 2.15 Gy (shcont), 1.07 Gy (shD1).
b, e, Colony survival assays of H2009 cells expressing anti-cyclin D1 shRNAs (shD1-A or shD1-B), or control shRNA (shcont), after exposure to indicated doses of camptothecin (CPT) or AZD2281 (Olaparib). SF50 for camptothecin: 15.1 nM (shcont), 2.8 nM (shD1-A), 3.4 nM (shD1-B); Olaparib: 0.39 µM (shcont), 0.083 µM (shD1-A), 0.065 µM (shD1-B).
c, Results of comet assays to measure DNA repair in cyclin D1-depleted HeLa cells (siD1-A) and control cells (sicont). Shown are percentages of cells containing various comet tail lengths at the indicated time-points after IR.
d, HR assay in HeLa cells using DR-eGFP-reporter system. sicont, control siRNA; siRAD51, anti-RAD51 siRNA; siD1-A, -B,-C, anti-cyclin D1 siRNAs.
*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.005; Error bars, standard deviation; n = 3–5.
Cyclin D1 activates cyclin-dependent kinases CDK4 and CDK61. However, treatment of HeLa cells with PD0332991, a specific inhibitor of cyclin D-CDK4/6 kinase12, had no effect on sensitivity of cancer cells to IR (Supplementary Fig. 4b). Moreover, expression of cyclin D1K112E point-mutant which is unable to activate the CDKs13, 14, restored radiation susceptibility in cyclin D-null MEFs (Supplementary Fig. 6). Collectively, these observations suggested that cyclin D1 might play a kinase-independent function in DNA repair.
We used comet assay15 to directly assess the efficiency of DNA repair in cyclin D1-depleted cells. We found that radiation induced comparable levels of DNA damage in control and in cyclin D1-depleted cells (Fig. 2c, 4hrs, Supplementary Fig. 7a). However, at 16 hours post-radiation, more unrepaired DNA persisted in cyclin D1-depleted cells (Fig. 2c, Supplementary Fig. 7). We verified that this impaired rate of DNA repair was not a result of grossly compromised DNA damage-induced signaling (Supplementary Fig. 8a, b).
Since among cyclin D1-interactors we observed a network of DNA repair proteins including RAD51, a key DNA recombinase that drives the HR process3, 16 (Supplementary Fig. 1), we investigated a possible functional connection between cyclin D1 and HR-dependent DNA repair. Using a HR repair reporter, the DR-eGFP system17, we found that depletion of cyclin D1 in HeLa and H2009 cells significantly reduced the HR rate (Fig. 2d, Supplementary Fig. 9). As expected, re-expression of siRNA-resistant cyclin D1 rescued this effect (Supplementary Fig. 10a–c). These results suggested that cyclin D1 is required for an efficient HR DNA repair process. Consistent with this notion, cyclin D1-depletion sensitized cancer cells to treatment with poly (ADP-ribose) polymerase (PARP)-inhibitor (Fig. 2e), in concordance with the reports that deficiency in HR renders cells hypersensitive to these agents18.
To investigate a function of cyclin D1 in HR, we focused on cyclin D1 interaction with RAD51, detected in our screen. We established using purified, recombinant proteins that cyclin D1 directly binds to RAD51, and that N-terminus of cyclin D1 and C-terminus of RAD51 mediate the interaction (Fig. 3a, Supplementary Fig. 11). Expression of cyclin D1 mutant which is deficient in RAD51 binding failed to restore normal HR rate in cyclin D1-depleted cells (Supplementary Fig. 10d). We also verified physical binding of the endogenous cyclin D1 and RAD51 proteins in several human cancer cell lines (Fig. 3b, Supplementary Fig. 12a). The interaction between cyclin D1 and RAD51 was induced by radiation and it intensified with the increased IR dose (Fig 3c, upper panel, Supplementary Fig. 12b, c). As reported, radiation resulted in a decrease of total levels of cyclin D1, without major changes in RAD51 levels19, 20 (Fig. 3c, lower panel, Supplementary Fig. 12c). The strong induction of cyclin D1-RAD51 interaction after radiation, in the face of reduced total cyclin D1 levels, indicates that an increased number of remaining cyclin D1 molecules is recruited to RAD51 complex to facilitate a DNA repair process. In pRB-positive cancer cells, decrease of total cyclin D1 levels upon radiation contributes to cell cycle arrest of these cells19. Conversely, forced overexpression of cyclin D1 may overcome cell cycle checkpoints, and may lead to unscheduled DNA synthesis and apoptosis20,21. Our results reveal that after radiation the remaining pool of cyclin D1 plays a positive role in DNA repair.
Figure 3. Functional interaction between cyclin D1 and RAD51.
a, Direct binding between GST-cyclin D1 and recombinant RAD51 protein. GST-Synapsin1, GST-Wave1 and GST served as negative controls for RAD51 binding. Upper panel: RAD51 was detected by immunoblotting. Lower panel: input GST-proteins visualized by Ponceau staining.
b, Interaction between endogenous cyclin D1 and RAD51, detected by cyclin D1 immunoprecipitation and anti-RAD51 immunoblotting, in indicated cell lines.
c, Cyclin D1-RAD51 interaction at different time-points after irradiating HeLa cells. Top panel: cyclin D1 immunoprecipitation - RAD51 immunoblotting. Lower panel: levels of cyclin D1 and RAD51 after IR, determined by western blotting.
d, Left panel: Recruitment of RAD51 to I-SceI-induced double-stranded DNA break, gauged by anti-RAD51 ChIP followed by PCR with primers adjacent to DNA damage site. Bars show enrichment around DNA damage site before and after induction of a DNA break. For control, we used anti-E2F4 and non-immune IgG (IgG) ChIP. Right panel: same assay in cells expressing anti-cyclin D1 siRNA.
e, Recruitment of cyclin D1 to I-SceI-induced double-stranded DNA break, tested as in d using anti-cyclin D1 ChIP. Bars show enrichment around DNA damage site.
f, Co-localization of cyclin D1 and RAD51 at the DNA damage site. Anti-cyclin D1 ChIP was followed by anti-RAD51 re-ChIP and PCR with primers adjacent to double-stranded DNA break.
g, Reduction of RAD51 recruitment to DNA damage foci in HeLa cells depleted of cyclin D1 (siD1-A, -C). Percentage of cells displaying a given number of RAD51 foci per nucleus was determined before (0 h) and 8 hours after irradiation.
h, In vitro binding assays using the indicated GST-BRCA2 fragments26 and recombinant cyclin D1. Cyclin D1 was detected by anti-cyclin D1 immunoblotting. Amounts of GST-BRCA2 fragments were verified by Ponceau staining (lower panel).
i, Interaction between endogenous cyclin D1 and BRCA2 (B2) in H2009 cells, detected by immunoprecipitation–immunoblotting.
j, Recruitment of cyclin D1 to an I-SceI-induced double-stranded DNA break, tested as in e, using cells transfected with independent anti-BRCA2 siRNAs (siBRCA2-A, and -B), or with control siRNA (sicont), Bars show enrichment around DNA damage site.
IP, immunoprecipitation; WCL, whole cell lysate; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.005; Error bars, standard deviation; n=3–5.
To investigate whether cyclin D1 is recruited to the sites of damaged DNA, where RAD51 localizes after radiation, we utilized a system in which a unique I-SceI recognition site has been integrated into the genome of HeLa cells17. Using this system, one can monitor recruitment of repair proteins to the site of DNA damage using targeted chromatin immunoprecipitation (ChIP), followed by PCR with primers adjacent to DNA damage site22–24. As expected, we observed recruitment of RAD51 to the DNA damage site (Fig. 3d and Supplementary Fig. 13a, d, e). Importantly, targeted anti-cyclin D1 ChIP revealed that cyclin D1 was also recruited to the site of DNA damage (Fig. 3e, Supplementary Fig. 13f); this recruitment disappeared once cyclin D1 had been knocked-down (Supplementary Fig. 13b,g). Moreover, ChIP of cyclin D1 followed by re-ChIP with anti-RAD51 antibody revealed that cyclin D1 and RAD51 co-localize on the sites of double-stranded DNA breaks (Fig. 3f, Supplementary Fig. 13c). Cyclin D1 recruitment to the damaged DNA was also detected by co-immunofluorescent staining with γ-H2AX at laser-induced DNA-damage stripes (Supplementary Fig. 14).
To investigate how cyclin D1 facilitates the HR process, we studied recruitment of RAD51 to damaged DNA 25. We found that knock-down of cyclin D1 reduced recruitment of RAD51 to I-SceI-induced DNA breaks (Fig. 3d, Supplementary Fig. 13a), to laser-induced DNA-damage stripes (Supplementary Fig. 15), and to radiation-induced DNA damage foci (Fig. 3g, Supplementary Figs 16, 17). We verified that the overall levels of RAD51 and of several other DNA damage-response proteins were not affected by cyclin D1 knock-down (Supplementary Fig. 8c). Collectively, these findings indicate that cyclin D1 helps to recruit RAD51 to DNA damage sites through a direct cyclin D1-RAD51 physical interaction.
We next asked how cyclin D1 is recruited to sites of DNA damage. Among cyclin D1-interactors we observed BRCA2 (Fig. 1a, Supplementary Fig. 3, Supplementary Tables 1–5), an essential HR protein which is recruited to DNA damage sites prior to RAD5125. We established using purified recombinant cyclin D1 protein and BRCA2 protein fragments 26 that cyclin D1 directly interacts with BRCA2 (Fig. 3h), and we verified physical interaction of the endogenous proteins (Fig. 3i). Importantly, we found that knock-down of BRCA2 reduced recruitment of cyclin D1 to DNA damage sites (Fig. 3j, Supplementary Fig. 18a). Depletion of cyclin D1 had no observable effect on BRCA2 recruitment (Supplementary Fig. 19a), consistent with the notion that BRCA2 acts upstream of cyclin D1. Likewise, depletion of cyclin D1 had no detectable impact on other upstream events, such as DNA end-resection (Supplementary Fig. 20a–d) and loading of a single-stranded DNA binding protein RPA34 (Supplementary Fig. 20e, f). In contrast, depletion of cyclin D1 inhibited downstream events, namely recruitment of RAD51, and led to decreased co-localization of RAD51 on BRCA2 at DNA damage sites (Supplementary Fig. 18b).
Collectively, these results are consistent with the model that cyclin D1 is recruited to DNA damage sites through BRCA2; cyclin D1 then helps to recruit RAD51 through a direct cyclin D1-RAD51 interaction. Depletion of cyclin D1 reduces RAD51 recruitment and reduces RAD51-BRCA2 co-localization at DNA damage sites, leading to impaired HR rate. Our findings are in agreement with a recent report that forced targeting of cyclin D1 to DNA resulted in increased co-recruitment of RAD51 27.
We verified that cyclin D1 is not required for recruitment of other DNA damage proteins detected as cyclin D1-interactors in our screen such as FANCD2, FANCI, PCNA and MSH6, as well as BRCA1 and MRE11 (Supplementary Figs. 19,21). We also found that depletion of cyclin D1 has no detectable impact on DNA mismatch repair rate (Supplementary Fig. 22).
Our observations suggested that depletion of cyclin D1 could sensitize human cancers to radiation, by limiting DNA repair. To test this, we knocked-down cyclin D1 in three pRB-negative cancer cell lines: H2009, HeLa and in prostate cancer DU145. Cells were then injected subcutaneously into nude mice, which resulted in formation of tumors. As expected, depletion of cyclin D1 did not affect proliferation of cancer cells in vitro (Supplementary Figs 5d and 23a–c, 24a–c), and had no impact on the rate of tumor growth in vivo (Fig. 4a, b, Supplementary Figs. 24d–g, 25a). In contrast, upon irradiation, cyclin D1-depleted tumors displayed retarded growth as compared to control tumors, revealing increased sensitivity to ionizing radiation (Fig. 4a, b, Supplementary Figs 23d, e, 24d–g, 25b–e, 26–28 and data not shown). Hence, while cyclin D1 is dispensable for proliferation of pRB-negative tumor cells, it plays an important role in DNA repair, which becomes evident once cells undergo DNA damage.
Figure 4. Increased radiation-sensitivity of tumors with reduced cyclin D1 levels.
a, H2009 cells expressing control or anti-cyclin D1 shRNA were injected into nude mice and tumor growth was monitored on the indicated days. Left panel, mice received no radiation. Middle and right panels: at days 5 and 10, tumors were exposed to 2 Gy or 4 Gy of IR. Error bars, standard deviation; **, p ≤ 0.01; ***, p ≤ 0.005.
b, Weight of tumors at the end of observation period. Each circle represents an individual tumor, horizontal bars - mean values. N, number of mice analyzed
The cyclin D1 gene corresponds to the second most frequently amplified region within the human cancer genome 28. Our proteomic screen revealed that cyclin D1 plays a function in human cancer cells in DNA repair via the HR. Thus, targeting cyclin D1 in combination with radiation treatment may have potential therapeutic value in a large pool of pRB-negative cancers.
METHODS SUMMARY
Affinity immunopurification and mass-spectrometry were as described 29. Comet assays were according to manufacturer’s protocol (Trevigen). RAD51 and cyclin D1 ChIP were performed using anti-RAD51 (H-92) or anti-cyclin D1 antibody (H-295, Santa Cruz). For ChIP-re-ChIP, HA-tagged cyclin D1 was ChIP with anti-HA antibody (12CA5, Covance), eluted with HA peptide, and re-ChIP with anti-RAD51 antibody. GST-proteins were pGEX-5x-3 based (GE Healthcare). Cancer cells used for in vivo tumor study were transduced with lentiviruses expressing anti-cyclin D1 or control shRNAs. 107 tumor cells were injected subcutaneously into nu/nu mice. Tumors were target-irradiated using Cs137 as a source. Tumor size was assessed on indicated days, tumor weight at the end of experiments. Statistical significance of the differences was evaluated using paired two-tailed Student’s t-test.
Supplementary Material
Acknowledgements
We thank the members of the Sicinski lab for help and advice, N. Li for help with initial experiments, S Panyim and Y. Geng for discussions and reading the manuscript, P. Nakatani for pOZ-F-HA construct, M. Jasin for DR-eGFP system, D. Bulavin and E. Apella for anti-phospho-CDC25A antibodies, A. Smogorzewska for anti-FANCI antibody, A. Venkitaraman and M. Lee for GST-BRCA2 fragments, and DFCI Confocal and Light Microscopy Core Facility for assisting with confocal microscopy. This work was supported by R01 CA083688, P01 CA080111 and P01 CA109901 grants from NIH (to P.S.). S.J. is supported by The Thailand Research Fund MRG5280248. W.M. by Foundation for Polish Science, Y.W. through the CCCB and the Dana-Farber Strategic Plan Initiative, T.A.K. and A.B.C. by Project Z01 ES065089 (to T.A.K.) from the Division of Intramural Research of the NIH, NIEHS.
Footnotes
Author Contributions
S.J. and P.S. designed the study, analyzed the data and wrote the manuscript. S.J. performed most of the experiments with help from collaborators. Y.H. and D.M.L. contributed to DNA repair analyses. W.M. contributed in vitro protein binding analyses J.E.E. and T.G. performed mass spectrometry; J.E.E. analyzed mass spec data with S.P.G. L.B., F.B., A.Z., T.v.H helped with experiments. Y.W. M.C. and J.Q. performed computational analyses of interactors. A.B.C and T.A.K. contributed mismatch DNA repair analyses. B.X. helped with BRCA2 analyses.
References
- 1.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- 3.Baumann P, West SC. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 4.Bates S, et al. Absence of cyclin D/cdk complexes in cells lacking functional retinoblastoma protein. Oncogene. 1994;9:1633–1640. [PubMed] [Google Scholar]
- 5.Lukas J, et al. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 6.Ek S, Ortega E, Borrebaeck CA. Transcriptional profiling and assessment of cell lines as in vitro models for mantle cell lymphoma. Leuk Res. 2005;29:205–213. doi: 10.1016/j.leukres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Lin DI, et al. Phosphorylation-Dependent Ubiquitination of Cyclin D1 by the SCF EBX4-aB Crystallin Complex. Molecular Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosokawa Y, Arnold A. Mechanism of cyclin D1 (CCND1, PRAD1) overexpression in human cancer cells: analysis of allele-specific expression. Genes Chromosomes Cancer. 1998;22:66–71. doi: 10.1002/(sici)1098-2264(199805)22:1<66::aid-gcc9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Bartkova J, et al. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 1995;55:949–956. [PubMed] [Google Scholar]
- 10.Nakatani Y, Ogryzko V. Immunoaffinity purification of mammalian protein complexes. Methods Enzymol. 2003;370:430–444. doi: 10.1016/S0076-6879(03)70037-8. [DOI] [PubMed] [Google Scholar]
- 11.Kozar K, et al. Mouse development and cell proliferation in the absence of Dcyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Toogood PL, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48:2388–2406. doi: 10.1021/jm049354h. [DOI] [PubMed] [Google Scholar]
- 13.Hinds PW, Dowdy SF, Eaton EN, Arnold A, Weinberg RA. Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci U S A 91. 1994:709–713. doi: 10.1073/pnas.91.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22. doi: 10.1016/j.ccr.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123:291–298. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- 16.Baumann P, Benson FE, West SC. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell. 1996;87:757–766. doi: 10.1016/s0092-8674(00)81394-x. [DOI] [PubMed] [Google Scholar]
- 17.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol. 2008;8:363–369. doi: 10.1016/j.coph.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102:55–66. doi: 10.1016/s0092-8674(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 20.Pontano LL, et al. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol Cell Biol. 2008;28:7245–7258. doi: 10.1128/MCB.01085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coco Martin JM, Balkenende A, Verschoor T, Lallemand F, Michalides R. Cyclin D1 overexpression enhances radiation-induced apoptosis and radiosensitivity in a breast tumor cell line. Cancer Res. 1999;59:1134–1140. [PubMed] [Google Scholar]
- 22.Sugawara N, Wang X, Haber JE. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol Cell. 2003;12:209–219. doi: 10.1016/s1097-2765(03)00269-7. [DOI] [PubMed] [Google Scholar]
- 23.Wolner B, van Komen S, Sung P, Peterson CL. Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol Cell. 2003;12:221–232. doi: 10.1016/s1097-2765(03)00242-9. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigue A, et al. Interplay between human DNA repair proteins at a unique double-strand break in vivo. Embo J. 2006;25:222–231. doi: 10.1038/sj.emboj.7600914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West SC. Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol. 2003;4:435–445. doi: 10.1038/nrm1127. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Daniels MJ, Venkitaraman AR. Phosphorylation of BRCA2 by the Polo-like kinase Plk1 is regulated by DNA damage and mitotic progression. Oncogene. 2004;23:865–872. doi: 10.1038/sj.onc.1207223. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, et al. Alternative cyclin D1 splice forms differentially regulate the DNA damage response. Cancer Res. 2010;70:8802–8811. doi: 10.1158/0008-5472.CAN-10-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bienvenu F, et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature. 2010;463:374–378. doi: 10.1038/nature08684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.