Abstract
Objectives
This study was designed to investigate and characterize the ability of platelet-activating factor (PAF) to induce the expression of platelet-activating factor acetylhydrolase (PAF-AH).
Methods
Ribonuclease protection assays and quantitative real-time PCR were used to investigate the ability of lipopolysaccharide (LPS) and PAF to regulate PAF-AH mRNA expression in human monocyte–macrophage 6 (MM6) cells. Pharmacological inhibitors of mitogen activated protein kinases (MAPK) and PAF receptor antagonists were used to investigate the mechanism of regulation of PAF-AH.
Results
PAF-AH mRNA levels were increased upon exposure to LPS or PAF in a dose-dependent manner. LPS elicited a more potent and rapid increase in PAF-AH expression than the PAF-stimulated response. However, when administered concomitantly, PAF augmented the LPS-stimulated response. LPS-stimulated PAF-AH expression was susceptible to partial inhibition by a p38 MAPK inhibitor and PAF receptor antagonists. PAF-induced up-regulation of PAF-AH levels was solely mediated via the PAF receptor and was p38 MAPK-independent.
Conclusion
The proinflammatory mediators, LPS and PAF, increased levels of PAF-AH mRNA via distinct signaling pathways.
Keywords: PAF acetylhydrolase, Regulation, Lipopolysaccharide, Mitogen activated protein kinase, PAF receptor
Introduction
Activation of an inflammatory response on encountering a pathogen or tissue injury is a complex biological response that balances the need to remove the offending organism and necrotic tissue while limiting damage to the surrounding tissue [1]. Monocytes/macrophages are part of the non-specific innate immune system and are integral to all stages of inflammation. These cells rapidly respond to conserved microbial structures through pattern recognition receptors (PRRs). A family of more than ten Toll-like receptors (TLRs), prototypical PRRs, initiates signaling cascades which lead to the release of both proinflammatory and anti-inflammatory mediators [2].
One such proinflammatory mediator is the phospholipid platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine). PAF is produced primarily by cells of the hematopoietic cell lineage including macrophages and monocytes [3]. PAF, acting via its G protein-coupled receptor, stimulates numerous complex signaling pathways producing diverse biological actions. Of crucial importance, PAF receptor stimulation results in the activation of cytosolic phospholipase A2 and the generation of lyso-PAF, the immediate precursor for PAF production. An autocrine cycle of new PAF synthesis and PAF receptor activation is thus established. PAF synthesis is also linked to the formation of various eicosanoids via the release of arachidonic acid from the esterified fatty acid at the sn-2 position of membrane phospholipids [4]. These lipids, working together, can augment and prolong the inflammatory response [1].
PAF is rendered biologically inactive via the actions of a specific phospholipase A2 called PAF acetylhydrolase (PAF-AH), also known as lipoprotein-associated phospholipase A2 (Lp-PLA2). Thus, PAF-AH plays a crucial role in terminating inflammatory responses elicited by both PAF and PAF-like phospholipids. There are two categories of PAF-AHs: intracellular and secreted [5]. The plasma PAF-AH (referred to solely as PAF-AH) was isolated in 1985 by Farr et al. [6, 7] and the cDNA was subsequently cloned in 1995 by Tjoelker et al. [6, 7]. Circulating PAF-AH arises exclusively from cells of the hematopoietic lineage [8]. Increased PAF-AH activity has been associated with numerous disease states such as vascular disease, ischemic stroke, and diabetes mellitus [9, 10]. PAF-AH is strongly up-regulated in resident tissue macrophages exposed to LPS in vivo [11, 12]; however, contradictory data describing regulation of PAF-AH in human inflammatory states and in several human monocyte/macrophage cell lines have also been published [11, 13, 14]. This study was conducted to investigate the ability of LPS and PAF to induce the expression of PAF-AH in a non-adherent human monocyte/macrophage cell line and to explore the utilization of signal transduction pathways to regulate PAF-AH expression.
Methods
Reagents
Unless otherwise stated, all laboratory reagents were purchased from Fisher Scientific (Pittsburgh, PA, USA) or Sigma-Aldrich (St. Louis, MO, USA) and were of the highest biological grade available. We obtained fetal calf serum (FCS, low endotoxin) from Atlanta Biological (Lawrenceville, GA, USA), and penicillin/streptomycin, non-essential amino acids, and RPMI media from Hyclone (Logan, UT, USA). E. coli lipopolysaccharide (LPS), serotype 0111:B4, was purchased from Sigma-Aldrich. PAF and lysoPAF were obtained from Cayman Chemical (Ann Arbor, MI, USA). Applied Biosystems (Foster City, CA, USA) supplied all reagents for cDNA synthesis and real-time PCR. The PAF receptor antagonists, WEB2170 and BN50739, were a generous gift provided by Merle S. Olson, University of Texas Health Sciences Center at San Antonio.
Culture of human monocyte–macrophage 6 cells
Human monocyte–macrophage 6 (MM6) cells, grown in suspension, were cultured in RPMI media supplemented with FCS (10% v/v), penicillin (100 U/ml), streptomycin (100 μg/ml), oxaloacetate (1 mM), pyruvate (0.45 mM), insulin (0.2 U/ml), and 1× non-essential amino acids and maintained at 37°C and 5% CO2. Prior to use, MM6 cells were seeded at an initial density of 2 × 105 cells/mL in 24-well tissue-culture plates (2 ml/well). These cells were allowed to recover for 24 h prior to performing experiments. For experiments conducted in serum-free conditions, the cells were harvested by centrifugation, washed 2 times in 1× PBS, and resuspended in supplemented RPMI lacking serum. For all experiments, the cells did not exceed sixteen passages. During routine culture, cell viability was assessed by trypan blue exclusion and remained above 95% at all times.
Stimulation of MM6 cells with LPS, PAF, and lyso-PAF
All experimental protocols throughout this study were performed following stimulation of MM6 cells with LPS, PAF, lyso-PAF, or LPS plus PAF. Relevant controls (vehicle alone) were performed in parallel. E. coli LPS 0111:B4, PAF (1-O-hexadecyl-2-O-acetyl-sn-glycero-3-phosphocholine) and lyso-PAF were used to simulate 2 × 105 cells/well in 24-well tissues culture plates (2 mL/ well) for the times indicated in individual experiments. LPS dissolved in endotoxin-free 1× PBS was administered at concentrations ranging from 0 to 500 ng/ml. PAF and lyso-PAF were administered at concentrations ranging from 0 to 500 nM. Treated cells were compared with vehicle-administered control cells cultured for the same period of time. To obtain sufficient RNA for analyses, two duplicate wells were pooled and harvested by brief centrifugation (300g for 3 min) and immediately lysed in Trizol Reagent for the purification of RNA.
Administration of inhibitors to MM6 cells
Experiments were performed to ascertain the degree of involvement of various signaling pathways in PAF-AH regulation. MM6 cells were seeded at an initial density of 2 × 105 cells/mL in 2 mL of complete media and cultured for 24 h. The cells were treated with either 15 μM SB203580 (p38 MAPK inhibitor), 15 μM PD980058 (ERK1/2 inhibitor), 20 μM SP600125 (JNK inhibitor), and/or 50 μM PAF receptor antagonists (WEB 2170 or BN50739). MM6 cells were treated with the specific inhibitors 1 h prior to addition of either LPS (200 ng/mL) or PAF (500 nM). Cells were harvested at 24 h following exposure by brief centrifugation and lysed in Trizol (Invitrogen, Grand Island, NY, USA) for RNA isolation.
Isolation and quantitation of RNA
All RNA isolation procedures were based on the method of Chomczynski and Sacchi [15]. Briefly, MM6 cells were lysed in 1 mL Trizol Reagent by repetitive pipetting and RNA was isolated according to the manufacturer’s instructions. The RNA concentration was obtained by reading the optical density at 260 nm in a microplate reader (Spectra Max Plus, Molecular Devices).
Analyses of PAF-AH and PAF receptor expression levels
PAF-AH mRNA levels in experimental samples were assayed by ribonuclease protection assays (RPA) and/or quantitative real-time reverse-transcription PCR (qRT-PCR) according to the following protocols.
Ribonuclease protection assay
For the ribonuclease protection assay (RPA), a human PAF-AH cDNA clone (Homo sapiens phospholipase A2 group VII, I.M.A.G.E. clone #5203018) obtained from Invitrogen was used to create an appropriate antisense RNA probe as follows: a 524-bp EcoRI fragment corresponding to nucleotides 599–1123 of the human PAF-AH cDNA was excised and ligated into the unique EcoRI site of the multiple cloning region of pBluescript II phagemid vector. After determining the orientation of the insert, the plasmid was linearized with PstI and T3 RNA polymerase was used to create a 388-bp [α-32P]UTP-labeled antisense RNA probe (MaxiScript, Ambion, Austin, TX, USA). As an internal control, a 281-bp β-actin [α-32P]UTP-labeled antisense RNA probe was generated from pTRI-β-actin-human (PNAM7424, Ambion). Because of the extreme difference in mRNA abundance between actin and PAF-AH, the specific activity of the actin antisense probe was reduced more than 1,000-fold by the addition of 250 μM UTP in the in-vitro transcription reaction. Eighty micrograms of MM6 RNA isolated from the experimental samples was hybridized in solution with both antisense RNA probes (RPAII Kit, Ambion). After ribonuclease digestion, the samples were separated on a denaturing 5% polyacrylamide 8 M urea gel. Differences in the amount of the PAF-AH (338 bp) and actin (247 bp) protected fragments were visualized and quantitated using a Storm PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA). The integrity of the antisense RNA probes was assayed by running a 1-μl aliquot in parallel with the experimental samples. Yeast tRNA was always included as a negative control.
Real-time reverse-transcription PCR (qRT-PCR)
Five micrograms of sample RNA was reverse transcribed in 60-μL cDNA reactions according to the manufacturer’s instructions (High Capacity cDNA synthesis kit, Applied Biosystems). Random primers were used to initiate the cDNA synthesis and the reverse transcription reaction (Multiscribe, 50 U/μL) was performed at 25°C for 10 min followed by incubation at 37°C for 2 h using the GeneAmp 2400 PCR system (Applied Biosystems). Thirty microlitre qPCR reactions were performed using 5 μL of cDNA created as described above. TaqMan™ primers specific for the human PAF-AH or the human PAF receptor and 18S ribosomal RNA or cyclophilin A (for internal controls) and 2× Universal PCR Master Mix were obtained from Applied Biosystems. Real-time PCRs were performed on an Applied Biosystem Prism 7000 Sequence Detection/ Quantitation instrument using amplification conditions recommended for the TaqMan primers. When 18S was used as the internal control, the cDNA was diluted 1/500 and the PAF-AH and 18S were performed in separate reactions. When cyclophilin A was used as the internal control, PAF-AH or PAF-receptor and cyclophilin A reactions were multiplexed. Standard curves for PAF-AH or PAF receptor and 18S or cyclophilin A were generated by amplifying four fivefold serial dilutions of cDNA prepared from reverse transcription reactions utilizing 6 μg of RNA isolated from LPS-treated MM6 cells (to insure the standard curve encompassed the range of all experimental samples). The standard curves were run simultaneously with the experimentally derived cDNAs. Each standard curve dilution was assigned an arbitrary numerical value that was indicative of a fivefold serial dilution. Quantitative numerical values were obtained for each sample from the standard curves, the samples were normalized to 18S or cyclophilin A content, and the fold-induction in PAF-AH levels or PAF receptor levels over control levels were calculated. All standard curve samples and all experimental samples were amplified in triplicate.
Statistical analyses
Statistical analyses were performed as detailed in each of the figure legends. In general, unpaired Student’s t tests were used to assess statistical differences between groups and repeated measures were used to assess differences across time. Analysis of variance (ANOVA) with subsequent Bonferroni post-hoc tests were used to assess differences between groups. ANOVA was considered significant with a p value<0.05. All experimental values were expressed as means ± SD and are representative of ≥3 independent experimental samples. For qRT-PCR analyses, all experimental samples were assayed in triplicate. A p value <0.05 for the post-hoc tests was accepted as statistically significant. All statistical analyses were performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
Results
LPS and PAF stimulate plasma PAF-AH mRNA in a dose-dependent manner
In the current study, we chose to explore PAF-AH expression in non-adherent human monocyte-macrophage 6 cells (MM6) and to investigate the effect of proinflammatory mediators LPS and PAF on PAF-AH mRNA levels. To determine if LPS and PAF are capable of up-regulating PAF-AH expression in MM6 cells, dose–response experiments were performed. After initial seeding of the MM6 cells, the cells were treated with LPS (0, 100 or 200 ng/ml) and PAF (50, 250, and 500 nM) for 24 h as this was the time-frame of maximal induction of PAF-AH detected in vivo [11]. Total RNA was isolated from the cells and PAF-AH mRNA levels were evaluated by ribonuclease protection assay (RPA). Figure 1a depicts the results of a representative RPA examining PAF-AH mRNA levels. MM6 cells contain constitutively low levels of PAF-AH mRNA (control lane) and both LPS and PAF administration resulted in substantial increases in the levels of PAF-AH mRNA. Densitometric quantitation of three independent RPA experiments revealed that PAF-AH mRNA levels increased 10- to 12-fold above control levels when stimulated with LPS (Fig. 1b). The increase was dose-dependent for the concentrations of LPS examined (100 and 200 ng/ml). Likewise, increasing concentrations of PAF (50, 250, and 500 nM) resulted in increasing levels of PAF-AH mRNA, albeit the magnitude of the increase (two- to fivefold) was less than that detected for LPS (Fig. 1a, b).
Fig. 1.
Ribonuclease protection analysis of PAF-AH mRNA in MM6 cells following LPS or PAF exposure. Aliquots (80 μg) of total RNA isolated from saline-treated (control), LPS-treated (100 and 200 ng/ml) or PAF-treated (50–500 nM) MM6 cells cultured in serum-containing media (24 h) were hybridized with 32P-labeled antisense RNA probes for human PAF-AH (524 bp) and human actin (281 bp). The RNA–RNA hybrids were digested with RNaseA/T1 and separated on a 5% polyacrylamide 8 M urea gel (a). Probe alone, undigested probes; tRNA, digested negative control. The ribonuclease protection shown is representative of three independent experiments. b. Graphical representation of the densitometric quantitation of the PAF-AH fold-induction over control after normalization to actin levels. Statistical significance was determined by Student’s t tests (n = 3; *p < 0.001 LPS vs. saline-treated control; **p < 0.01 PAF vs. saline-treated control)
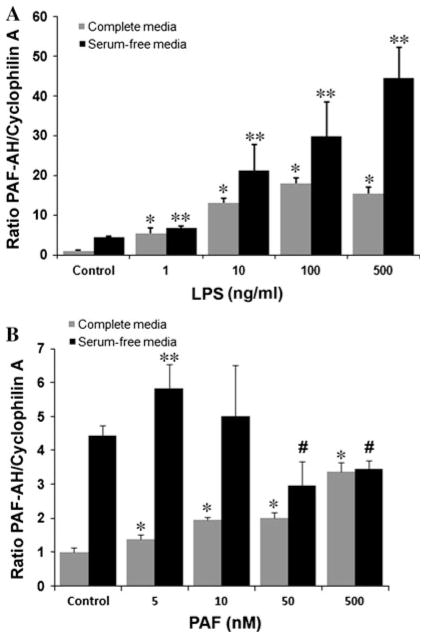
To confirm the dose-dependent nature of the responses elicited by LPS and PAF, additional experiments were performed at lower LPS and PAF concentrations and in both serum-containing and serum-free conditions. PAF-AH mRNA expression levels were examined by qRT-PCR 24 h after exposure of the cells to mediators. PAF-AH mRNA levels increased with increasing concentrations of administered LPS in both complete and serum-free media (Fig. 2a). In complete media, a fivefold increase in the PAF-AH expression level above control levels was detected with as little as 1 ng/ml LPS. Peak PAF-AH expression levels resulted from the administration of 100 ng/ml LPS in complete media. When MM6 cells were cultured in serum-free conditions, the level of PAF-AH in untreated cells was four- to fivefold higher than in cells cultured in complete media (Fig. 2a). Administration of increasing amounts of LPS (1–500 ng/ml) resulted in significantly increased PAF-AF expression. PAF-AH levels were approximately tenfold higher than the PAF-AH level detected in the serum-free media control cells at the highest LPS doses examined. Administration of PAF resulted in significantly increased expression of PAF-AH at all doses examined (5–500 nM) in the MM6 cells cultured in complete media (Fig. 2b). In the serum-free environment, PAF-AH expression levels had greater variability, with a significant increase detected in response to 5 nM PAF. At higher PAF concentrations, PAF-AH expression levels were lower than that detected in the serum-free vehicle-treated cells (control).
Fig. 2.
PAF-AH mRNA expression in response to different LPS and PAF concentrations. Total RNA (5 μg) isolated from (a) LPS-(0–500 ng/ml), and (b) PAF- (0–500 nM) treated MM6 cells at 24 h cultured in either complete media (grey) or serum-free media (black) were analyzed by qRT-PCR using TaqMan primers specific to the human PAF-AH and human cyclophilin A as detailed in the Methods section. Data reflect the mean ± SD of at least three independent experiments assayed in triplicate. Statistical significance was determined by ANOVA (p < 0.05) with subsequent Bonferroni post hoc tests. Post-hoc tests: (a) *p < 0.0001 vs. complete media saline control. **p < 0.001 vs. serum-free saline control. (b) *p < 0.003 vs. complete media saline control, **p < 0.03 vs. serum-free media saline control. #p < 0.004 vs. serum-free media saline control
Time course of stimulated PAF-AH mRNA levels
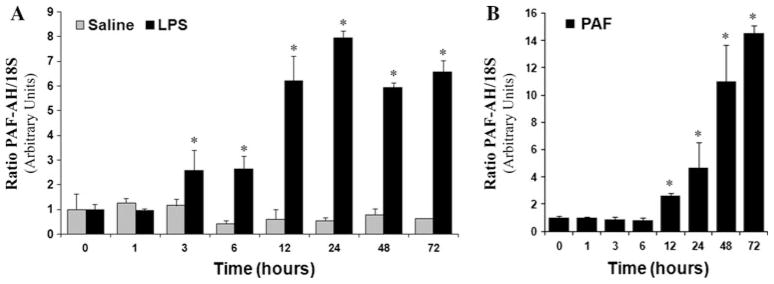
To ascertain the temporal pattern of the LPS-induced PAF-AH up-regulation, MM6 cells were cultured either in the presence of LPS (200 ng/ml) or vehicle control over a 72-h period in serum-containing RPMI media. At the indicated times, PAF-AH mRNA levels were ascertained by qRT-PCR using TaqMan primers specific for the human PAF-AH. 18S levels were simultaneously determined to correct for variations in initial RNA content. As illustrated in Fig. 3a, PAF-AH mRNA levels increased in response to LPS administration. Time course experiments revealed that PAF-AH mRNA levels began to increase as early as 3 h after LPS administration. Subsequent to this, PAF-AH mRNA levels rose steadily, reaching a maximal eightfold induction over control levels (0 h) at 24 h. Elevated levels of PAF-AH mRNA persisted at 48 and 72 h post-LPS administration. MM6 cells receiving vehicle alone were incubated for the entire time course and near-constitutive levels of PAF-AH mRNA were detected in all samples (Fig. 3a). Seventy-two-hour time course experiments were also performed in the presence of 500 nM PAF. In response to PAF administration, PAF-AH mRNA levels increased 2- to 15-fold over control levels (0 h). PAF stimulation did not result in any significant increase in PAF-AH mRNA until 12 h after exposure and PAF-AH levels continued to increase throughout the time course (Fig. 3b).
Fig. 3.
Time course of MM6 cell PAF-AH expression in response to saline, LPS, or PAF administration. Total RNA (5 μg) isolated from (a) saline- and LPS-treated cells or (b) PAF-treated cells at 0, 1, 3, 6, 12, 24, 48, and 72 h in complete media were analyzed by qRT-PCR using TaqMan primers specific to the human PAF-AH and human 18S ribosomal RNA as detailed in the Methods section. Data reflect the mean ± SD of at least three independent experiments assayed in triplicate. No significant differences over time were observed for the saline treated samples (grey shading, a). Independent t tests were used to compare 0 h LPS to LPS treatment at various times or 0 h PAF to PAF treatment at individual time points. (a) *p <0.001 vs. 0 h LPS-treated, (b) *p < 0.001 vs. 0 h PAF-treated)
Additive effects of LPS and PAF on PAF-AH stimulation
Either LPS or PAF administration induced PAF-AH mRNA expression in MM6 cells. Additional experiments were performed to investigate whether these two mediators, if given concomitantly, could result in enhanced PAF-AH expression. LPS (10 ng/ml), PAF (10 and 50 nM), or LPS plus PAF were added to MM6 cell cultures and the levels of PAF-AH mRNA at 24 h was determined by qRT-PCR and compared to vehicle-administered control or LPS alone. When PAF (10 and 50 nM) was added with LPS (10 ng), PAF-AH levels were significantly higher than that detected with LPS alone (Fig. 4). These results showed an additive effect in PAF-AH mRNA up-regulation over LPS or PAF alone. To insure that the effect of PAF on PAF-AH expression was not the result of lyso-PAF being generated by the hydrolysis of PAF, MM6 cells were stimulated with 100 and 500 nM of lyso-PAF, which had no effect on PAF-AH expression levels (data not shown).
Fig. 4.
Additive effect of LPS plus PAF on stimulation of PAF-AH expression. Total RNA (5 μg) isolated from control (saline), LPS-(10 ng/ml), PAF- (10 and 50 nM), and LPS plus PAF- (10 ng/ml LPS plus 10 nM PAF or 50 nM PAF) treated MM6 cells cultured for 24 h in serum-free media were analyzed by qRT-PCR using TaqMan primers specific to the human PAF-AH and human cyclophilin A as detailed in the Methods section. Data reflect the mean ± SD of at least three independent experiments assayed in triplicate. Statistical significance was determined by ANOVA (p < 0.05) with subsequent Bonferroni post-hoc tests. *p < 0.001 vs. LPS alone
LPS stimulates PAF receptor expression
In order to ascertain if the MM6 cells become more responsive to PAF exposure after stimulation, PAF receptor expression levels were examined by qRT-PCR. MM6 cells were treated with 200 ng/ml LPS and monitored for PAF receptor expression over a 72-h time frame. Administration of LPS produced a six- to eightfold increase in PAF receptor expression detected between 6 and 48 h. By 72 h, PAF receptor expression levels were declining but were still significantly higher than the 0 h control levels (Fig. 5).
Fig. 5.
LPS-stimulated PAF receptor expression. Total RNA (5 μg) isolated from LPS- (200 ng/ml) treated MM6 cells cultured in serum-containing media at times ranging from 0 to 72 h was analyzed by qRT-PCR using TaqMan primers specific to the human PAF receptor and human cyclophilin A as detailed in the Methods section. Data reflect the mean ± SD of at least three independent experiments assayed in triplicate. Statistical significance was determined by ANOVA (p < 0.05) with subsequent Bonferroni post-hoc tests. *p < 0.001 vs. 0 h LPS-treated. **p < 0.004 vs. 0 h LPS-treated
Determination of essential MAPK signaling pathways for LPS-stimulated up-regulation of PAF-AH
LPS is able to induce proinflammatory responses via activation of numerous complex intracellular signaling pathways, as reviewed in [16]. These pathways include the MAPK cascades—ERK1/2, JNK, and p38—as well as the NF-κB/IκB intracellular signaling pathway. To determine the involvement of various MAPK pathways essential for up-regulation of PAF-AH mRNA in response to LPS exposure, various MAPK inhibitors were employed in additional experiments. The inhibitors, PD98059, SP600125, and SB203580, were used to inhibit the ERK1/2, JNK, and p38 MAPK portions of the MAPK pathways, respectively. MM6 cells, cultured in complete media, were exposed to the respective inhibitors 1 h prior to administration of LPS (200 ng/ml). At 24 h after LPS exposure, total RNA was isolated and PAF-AH mRNA levels were assayed by RPA experiments. Figure 6a illustrates a representative RPA of three independent experiments. The administration of 15 μM SB203580 induced an approximately 60% decrease in PAF-AH levels compared to MM6 cells treated with 200 ng/mL LPS alone (Fig. 6b). Paradoxically, inhibition of the ERK1/2 portion of the MAPK pathway with 15 μM PD98059 consistently increased PAF-AH expression relative to administration of LPS alone. Although the administration of SP600125 (20 μM), an inhibitor of the JNK pathway, showed a slight reduction in PAF-AH levels, this was not significantly different across the independent samples.
Fig. 6.
Ribonuclease protection analysis of PAF-AH mRNA after exposure to MAPK inhibitors. Aliquots (80 μg) of total RNA isolated from control-, LPS-, and LPS plus MAPK inhibitor-treated MM6 cells cultured in serum-containg media for 24 h were hybridized with 32P-labeled antisense RNA probes for human PAF-AH (524 bp) and human actin (281 bp). The RNA–RNA hybrids were digested with RNaseA/T1 and separated on a 5% polyacrylamide 8 M urea gel (a). The ribonuclease protection shown is representative of three independent experiments. b Graphical representation of the densitometric quantitation of the PAF-AH fold-induction over control after normalization to actin levels (n = 3; *p < 0.02 vs. LPS-stimulated; **p < 0.001 vs. LPS-stimulated)
Determination of whether LPS and PAF induce PAF-AH mRNA via identical signaling pathways
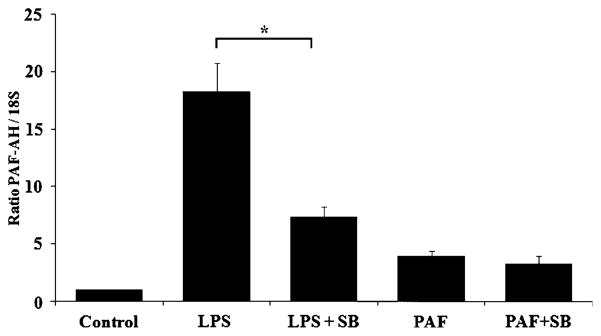
Our initial findings that LPS-stimulated up-regulation of PAF-AH is partially dependent upon activation of p38 is supported by previous studies [17]. However, the previous study reported p38 inhibitors completely abolished LPS-stimulated responses in RAW264.7 macrophage cells and produced almost complete inhibition in human THP-1 cells overexpressing surface CD14. Our next goal was to elucidate the involvement of p38 MAPK in the PAF-mediated up-regulation of PAF-AH expression. MM6 cells were exposed to SB203580 (15 μM) prior to stimulation with PAF or LPS in serum-containing media for 24 h. Total RNA was isolated and PAF-AH levels were quantitated by qRT-PCR. Both LPS and PAF in the absence of SB203580 significantly increased expression of PAF-AH over control levels (Fig. 7). The qRT-PCR results also reproduced and independently verified the results we obtained in the RPA experiments, namely that administration of SB203580 inhibited the induction of LPS-stimulated increases in PAF-AH mRNA levels by 60% (Figs. 6, 7). However, the administration of the p38 MAPK inhibitor to PAF-stimulated MM6 cells demonstrated no significant inhibition of the induced PAF-AH mRNA levels. The levels of PAF-AH mRNA after the addition of SB203580 alone were not statistically different from control (data not shown). Our results suggested that both p38 MAPK-dependent and p38 MAPK-independent signaling pathways are utilized for LPS- and PAF-stimulated increases in PAF-AH mRNA levels. Further exploring the mechanism of PAF-AH up-regulation, we performed experiments with either singly or dually administered WEB2170 (50 μM), a PAF receptor antagonist, and/or the p38 MAPK inhibitor. The results illustrated in Fig. 8a conclusively demonstrated that the LPS-stimulated increase in PAF-AH mRNA at 24 h is sensitive to antagonism by both SB203580 and the PAF receptor antagonist WEB2170 (60 and 80% inhibition, respectively). As would be expected, the PAF receptor antagonist completely inhibited the PAF-stimulated up-regulation of the PAF-AH expression levels. Concomitant administration of both SB203580 and WEB2170 completely abolished any up-regulation of LPS-stimulated PAF expression above control levels, implying that LPS-stimulated production of PAF contributes to the up-regulation of its own degradative enzyme. Additional experiments were performed to confirm the inhibitory effects of PAF receptor antagonism on PAF-AH expression levels. RPA experiments were performed evaluating the levels of LPS-stimulated PAF-AH expression in response to the administration of a pharmacologically distinct PAF receptor antagonist, BN50739. The results of the RPA are illustrated in Fig. 8b. Prior incubation with the PAF receptor antagonist significantly reduced the LPS-stimulated expression of PAF-AH by approximately 60% (Fig. 8c, p < 0.007). These results independently verified that inhibition of PAF receptor signaling significantly inhibits the up-regulation of LPS-stimulated PAF-AH expression.
Fig. 7.
Effect of the p38 MAPK inhibitor SB203580 on LPS- and PAF-stimulated PAF-AH expression. Total RNA (5 μg) isolated from control-, LPS-, LPS plus SB203580-, PAF-, and PAF plus SB203580-treated MM6 cells cultured for 24 h in complete media were analyzed by qRT-PCR using TaqMan primers specific to human PAF-AH and human 18S ribosomal RNA as detailed in the Methods section. Data reflect the mean ± SD of at least three independent experiments assayed in triplicate. Statistical significance was determined by ANOVA (p < 0.05) with subsequent Bonferroni post-hoc tests. All treatment groups were found to be statistically different from the control. *p < 0.003 LPS plus SB203580 vs. LPS alone
Fig. 8.
Effect of the p38 MAPK inhibitor SB203580 and the PAF receptor antagonists WEB2170 and BN50739 on LPS- and PAF-stimulated PAF-AH expression. Pharmacological inhibitors were administered 1 h prior to administration of LPS or PAF as detailed in the Methods section. Total RNA (5 μg) isolated from control or treated MM6 cells cultured for 24 h in complete media were analyzed by qRT-PCR using TaqMan primers specific to human PAF-AH and human 18S ribosomal RNA as detailed in the Methods section (a) or by ribonuclease protection assay (b; representative example). c Densitometric quantitation of the ribonuclease protection assays. Data reflect the mean ± SD of at least three independent experiments assayed in triplicate. Statistical significance was determined by ANOVA (p <0.05) with subsequent Bonferroni post-hoc tests. (a) *p <0.003 vs. LPS-treated cells; **p < 0.001 vs. PAF-treated cells. (c) p < 0.007 vs. LPS-treated cells
Discussion
We previously demonstrated that LPS acts as a potent inducer of PAF-AH expression in resident liver macrophages in vivo [11, 12]; however, other studies in cultured monocyte/macrophage cells have reported conflicting results. For instance, Narahara et al. [14] demonstrated LPS-induced down-regulation of PAF-AH activity in TPA-differentiated HL-60 cells. Likewise, Cao et al. [13] reported that PAF-AH mRNA levels are reduced in response to LPS stimulation in the murine macrophage cell line, RAW264.7. However, in a subsequent publication, LPS was shown to increase PAF-AH expression in both RAW264.7 cells and in human THP-1 cells overexpressing CD14 [17]. Isolated Kupffer cells, in the absence of stimuli, increased expression of PAF-AH when cultured and the subsequent administration of LPS down-regulated the expression of PAF-AH [11]. In addition to LPS, PAF has also been implicated in regulating PAF-AH levels [12, 13]; however, the mechanism of regulation has not been extensively explored. Because of the seemingly contradictory results for PAF-AH expression in response to LPS administration obtained in human versus murine cells and adherent versus non-adherent macrophages, we elected to examine the expression of PAF-AH in human non-adherent monocyte–macrophage cells (Mono–Mac 6; MM6). The goal of this study was to investigate PAF-AH expression in response to LPS, PAF, and LPS plus PAF and to elucidate the mechanism(s) of regulation.
In this study, we found that both LPS and PAF up-regulate the expression of PAF-AH in a dose-dependent manner, with LPS more potent than PAF in stimulating increases in PAF-AH mRNA. LPS elicited increased PAF-AH expression both in the presence and absence of serum, indicating that LPS-binding protein (LBP) is not required for signaling. LBP-independent LPS stimulation has been demonstrated in numerous macrophage cells and has been previously demonstrated in MM6 cells [18, 19]. Published reports indicate that a majority of the physiological actions of PAF are achieved at very low concentrations (1–0.001 nM) [1]. In our studies, 5 nM PAF was able to elicit increases in PAF-AH mRNA. LPS and PAF likely function independently to promote the up-regulation of PAF-AH, as the increase in PAF-AH expression was approximately additive in response to both LPS and PAF administration.
Our data investigating the temporal pattern of PAF-AH expression in MM6 cells are in excellent agreement with our results in vivo that demonstrated maximal induction of PAF-AH mRNA and protein in Kupffer cells at 24 h in LPS-treated rats [11, 12]. Furthermore, we established that PAF is also capable of inducing PAF-AH expression in MM6 cells, and this likely represents a physiologically relevant response. The delayed responsiveness in PAF-AH induction could reflect the need to control and limit PAF and PAF-like mediator production at this later stage of inflammation and limit the autocrine cycle of PAF and eicosanoid production to limit tissue damage.
LPS in combination with various accessory proteins (LBP and CD14) activates the PPR, TLR-4. The activation of TLR-4 initiates signaling through the MAPK cascades [16]. Examination of the MAPK pathways that were important in the LPS-mediated up-regulation of PAF-AH showed predominant signaling through the p38 MAPK pathway, marked by 60% inhibition by SB203580. In contrast, PAF-induced induction of PAF-AH was refractory to inhibition by the p38 MAPK inhibitor. In our studies, the effect of PAF was mediated specifically through its receptor, since the stimulation was completely blocked by pretreatment of the cells with PAF receptor antagonists. These data demonstrate a significant difference between LPS signaling and PAF signaling in regulating PAF-AH expression.
Stafforini et al. have shown that LPS-induced PAF-AH induction is solely mediated through the p38 portion of the MAPK pathway in THP1/CD14 cells [17]. Our work in MM6 cells showed 60% inhibition of PAF-AH mRNA induction upon exposure to SB203580—the p38 MAPK inhibitor. Interestingly, Howard et al. 12demonstrated that the PAF receptor antagonist, WEB2170, was able to down-regulate the in-vivo LPS-induced up-regulation of PAF-AH in rats by 50%[12]. In our current study, the PAF receptor antagonist, WEB2170, was administered prior to stimulation with LPS or PAF. As expected, WEB2170 was an effective PAF receptor antagonist and was able to inhibit the PAF-induced response. LPS-induced up-regulation of PAF-AH mRNA was inhibited by approximately 50% by the PAF receptor antagonist. Importantly, dual administration of SB203580 in conjunction with WEB2170 completely abolished PAF-AH induction. Our data strongly suggest that the LPS-induced PAF-AH mRNA levels present after SB203580 administration are the result of autocrine activation of the PAF receptor.
Conclusions
This research further expands our understanding of the regulation of an important anti-inflammatory enzyme, PAF-AH. We have demonstrated that, in addition to LPS, PAF is able to up-regulate the expression of its own degradative enzyme. Though the LPS-induced response is partly p38 MAPK-dependent, the induction observed from PAF administration is not. The LPS-stimulated up-regulation of PAF-AH is a result of activation of p38 MAPK as well as PAF receptor activation, possibly via LPS-stimulated production of PAF. The increase in PAF-AH levels, in response to these mediators, likely represents the physiological requirement to curb the progression of the inflammatory response and maintain the viability of the host.
Acknowledgments
We would like to express our sincere gratitude to Merle S. Olson, University of Texas Health Science Center, who donated much of the equipment to our laboratory to conduct this research. We would like to thank Ziming Cheng for technical help with the RPA assays and Drs. Gillian Galbraith and Barbara St Pierre Schneider for critically reading the manuscript. This work was supported by grants from the National Institutes of Health (HL66130) and the American Heart Association (0465061) to KMH.
Footnotes
Conflict of interest The authors declare that they have no competing interests.
Contributor Information
Katherine M. Howard, Email: Katherine.howard@unlv.edu, Department of Biomedical Sciences, University of Nevada Las Vegas School of Dental Medicine, 1001 Shadow Lane, Las Vegas, Nevada 89106, USA
Mohammed Abdel-al, Email: delal@bu.edu, Department of Chemistry, University of Nevada Las Vegas School of Dental Medicine, 1001 Shadow Lane, Las Vegas, Nevada 89106, USA.
Marcia Ditmyer, Email: marcia.ditmyer@unlv.edu, Department of Biomedical Sciences, University of Nevada Las Vegas School of Dental Medicine, 1001 Shadow Lane, Las Vegas, Nevada 89106, USA.
Nipa Patel, Email: Nipa.Patel@sdm.unlv.edu, Department of Biomedical Sciences, University of Nevada Las Vegas School of Dental Medicine, 1001 Shadow Lane, Las Vegas, Nevada 89106, USA.
References
- 1.McManus LM, Pinckard RN. PAF, a putative mediator of oral inflammation. Crit Rev Oral Biol Med. 2000;11:240–58. doi: 10.1177/10454411000110020701. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol. 2005;25:503–10. doi: 10.1007/s10875-005-8065-4. [DOI] [PubMed] [Google Scholar]
- 3.Castro Faria Neto HC, Stafforini DM, Prescott SM, Zimmerman GA. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):83–91. doi: 10.1590/s0074-02762005000900014. [DOI] [PubMed] [Google Scholar]
- 4.Snyder F, Blank M, Lee TC, Robinson M, Woodard D. Measurement of key enzyme activities involved in the metabolism of platelet activating factor. Methods Enzymol. 1987;141:379–96. doi: 10.1016/0076-6879(87)41085-9. [DOI] [PubMed] [Google Scholar]
- 5.Stafforini DM, Prescott SM, Zimmerman GA, McIntyre TM. Mammalian platelet-activating factor acetylhydrolases. Biochim Biophys Acta. 1996;1301:161–73. doi: 10.1016/0005-2760(96)00040-9. [DOI] [PubMed] [Google Scholar]
- 6.Farr RS, Cox CP, Wardlow ML, Jorgensen R. Preliminary studies of an acid-labile factor (ALF) in human sera that inactivates platelet-activating factor (PAF) Clin Immunol Immunopathol. 1980;15:318–30. doi: 10.1016/0090-1229(80)90044-6. [DOI] [PubMed] [Google Scholar]
- 7.Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, Schimpf B, et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase (see comments) Nature. 1995;374:549–53. doi: 10.1038/374549a0. [DOI] [PubMed] [Google Scholar]
- 8.Asano K, Okamoto S, Fukunaga K, Shiomi T, Mori T, Iwata M, et al. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem Biophys Res Commun. 1999;261:511–4. doi: 10.1006/bbrc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 9.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor acetylhydrolases. J Biol Chem. 1997;272:17895–8. doi: 10.1074/jbc.272.29.17895. [DOI] [PubMed] [Google Scholar]
- 10.Imaizumi TA, Stafforini DM, Yamada Y, McIntyre TM, Prescott SM, Zimmerman GA. Platelet-activating factor: a mediator for clinicians. J Intern Med. 1995;238:5–20. doi: 10.1111/j.1365-2796.1995.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 11.Howard KM, Miller JE, Miwa M, Olson MS. Cell-specific regulation of expression of plasma-type platelet-activating factor acetylhydrolase in the liver. J Biol Chem. 1997;272:27543–8. doi: 10.1074/jbc.272.44.27543. [DOI] [PubMed] [Google Scholar]
- 12.Howard KM, Olson MS. The expression and localization of plasma platelet-activating factor acetylhydrolase in endotoxemic rats. J Biol Chem. 2000;275:19891–6. doi: 10.1074/jbc.M001462200. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Stafforini DM, Zimmerman GA, McIntyre TM, Prescott SM. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J Biol Chem. 1998;273:4012–20. doi: 10.1074/jbc.273.7.4012. [DOI] [PubMed] [Google Scholar]
- 14.Narahara H, Johnston JM. Effects of endotoxins and cytokines on the secretion of platelet-activating factor-acetylhydrolase by human decidual macrophages. Am J Obstet Gynecol. 1993;169:531–7. doi: 10.1016/0002-9378(93)90614-o. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Zimmerman GA, Prescott SM, Stafforini DM. The p38 MAPK pathway mediates transcriptional activation of the plasma platelet-activating factor acetylhydrolase gene in macrophages stimulated with lipopolysaccharide. J Biol Chem. 2004;279:36158–65. doi: 10.1074/jbc.M402454200. [DOI] [PubMed] [Google Scholar]
- 18.Jungi TW, Brcic M, Eperon S. Human macrophages respond to LPS in a serum-independent, CD14-dependent manner. Immunol Lett. 1996;54:37–43. doi: 10.1016/s0165-2478(96)02645-4. [DOI] [PubMed] [Google Scholar]
- 19.Zipfel A, Schenk M, Metzdorf B, Bode C, Viebahn R. Release of TNF-alpha from lipopolysaccharide (LPS)-stimulated Kupffer cells in serum- and nutrient-free medium. Inflammation. 2001;25:287–92. doi: 10.1023/a:1012856408531. [DOI] [PubMed] [Google Scholar]