Abstract
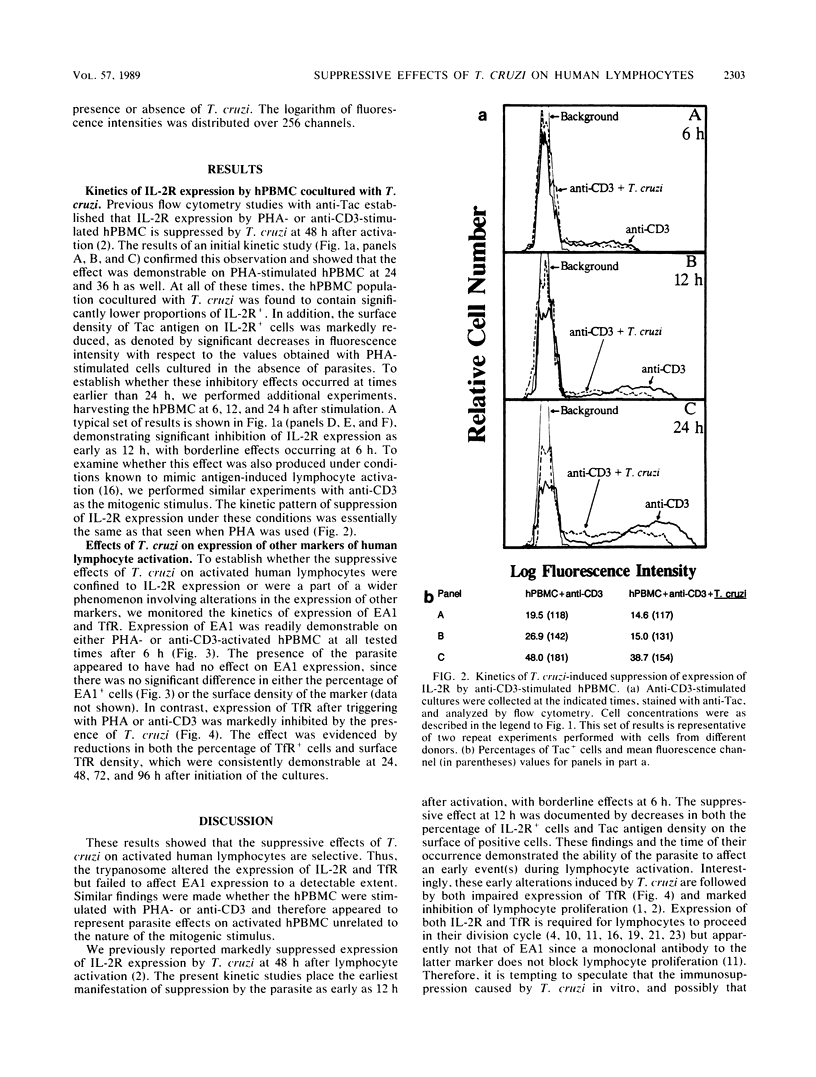
The acute phase of Chagas' disease is accompanied by immunosuppression. To explore the underlying mechanism(s), we used an in vitro culture system in which the capacities of activated human peripheral blood mononuclear cells to express interleukin-2 receptors (IL-2R) and proliferate are markedly inhibited in the presence of Trypanosoma cruzi, the etiologic agent. The present work was designed to define the earliest time at which T. cruzi-induced suppression is manifested in terms of IL-2R expression on the cell surface and establish whether expression of other lymphocyte activation markers is also suppressed by the parasite. We found that expression of IL-2R by human peripheral blood mononuclear cells cocultured with T. cruzi and stimulated with either phytohemagglutinin or anti-CD3 (a monoclonal antibody specific for an epitope of the T cell receptor complex T3-Ti) was significantly suppressed as early as 12 h after culture initiation. Both the percentage of IL-2R+ cells and the surface density of IL-2R, measured by flow cytometry, were affected. However, expression of EA1, a human lymphocyte activation antigen known to be expressed 4 to 6 h after stimulation, was not altered by T. cruzi whether phytohemagglutinin or anti-CD3 was used. On the other hand, expression of transferrin receptors (TfR), which first occurs between 20 and 24 h after lymphocyte activation, was markedly suppressed by T. cruzi. This effect was denoted by significant reductions in both the percentage of TfR+ cells and the cell surface density of TfR whether phytohemagglutinin or anti-CD3 was used as the mitogen and was observed at all test times, i.e., at 24, 48, 72, and 96 h. Because expression of IL-2R and TfR is required for lymphoproliferation but that of the EA1 lymphocyte activation marker is apparently not, these results are consistent with the possibility that T. cruzi, at a relatively early stage during lymphocyte activation, selectively affects certain key events on which clonal expansion is dependent. Inhibition of IL-2R and TfR expression by the parasite might play a role in causing the suppressive effects associated with acute Chagas' disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltz L. A., Kierszenbaum F. Suppression of human lymphocyte responses by Trypanosoma cruzi. Immunology. 1987 Feb;60(2):309–315. [PMC free article] [PubMed] [Google Scholar]
- Beltz L. A., Sztein M. B., Kierszenbaum F. Novel mechanism for Trypanosoma cruzi-induced suppression of human lymphocytes. Inhibition of IL-2 receptor expression. J Immunol. 1988 Jul 1;141(1):289–294. [PubMed] [Google Scholar]
- Budzko D. B., Kierszenbaum F. Isolation of Trypanosoma cruzi from blood. J Parasitol. 1974 Dec;60(6):1037–1038. [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Interleukin 2 enhances specific and nonspecific immune responses in experimental Chagas' disease. Infect Immun. 1985 Nov;50(2):354–357. doi: 10.1128/iai.50.2.354-357.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton B. A., Ortiz-Ortiz L., Garcia W., Martinez T., Capin R. Trypanosoma cruzi: early immune responses in infected mice. Exp Parasitol. 1975 Jun;37(3):417–425. doi: 10.1016/0014-4894(75)90012-0. [DOI] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Lymphoblast transformation as a measure of immune competence during experimental Chagas' disease. J Parasitol. 1980 Jun;66(3):390–398. [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E. Trypanosoma cruzi-induced suppression of the primary immune response in murine cell cultures to T-cell-dependent and -independent antigens. J Parasitol. 1980 Feb;66(1):16–27. [PubMed] [Google Scholar]
- Farrar W. L., Ruscetti F. W. Association of protein kinase C activation with IL 2 receptor expression. J Immunol. 1986 Feb 15;136(4):1266–1273. [PubMed] [Google Scholar]
- Hammarström M. L., Axelsson B., Ivansen M., Hammarström S., Perlmann P. Transferrin receptors on mitogen-stimulated human thymus-derived lymphocytes. Scand J Immunol. 1982 Oct;16(4):355–360. doi: 10.1111/j.1365-3083.1982.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Hara T., Jung L. K., Bjorndahl J. M., Fu S. M. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986 Dec 1;164(6):1988–2005. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. M., Kierszenbaum F. Experimental Chagas' disease: kinetics of lymphocyte responses and immunological control of the transition from acute to chronic Trypanosoma cruzi infection. Infect Immun. 1981 Mar;31(3):1117–1124. doi: 10.1128/iai.31.3.1117-1124.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F. Immunologic deficiency during experimental Chagas' disease (Trypanosoma cruzi infection): role of adherent, nonspecific esterase-positive splenic cells. J Immunol. 1982 Nov;129(5):2202–2205. [PubMed] [Google Scholar]
- Kierszenbaum F. On evasion of Trypanosoma cruzi from the host immune response. Lymphoproliferative responses to trypanosomal antigens during acute and chronic experimental Chagas' disease. Immunology. 1981 Nov;44(3):641–648. [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Krönke M., Peffer N. J., Depper J. M., Greene W. C. Interleukin 2 receptor gene expression in normal human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6281–6285. doi: 10.1073/pnas.82.18.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleckar J. R., Kierszenbaum F. Inhibition of mitogen-induced proliferation of mouse T and B lymphocytes by bloodstream forms of Trypanosoma cruzi. J Immunol. 1983 Feb;130(2):908–911. [PubMed] [Google Scholar]
- Mercado T. I., Katusha K. Isolation of Trypanosoma cruzi from the blood of infected mice by column chromatography. Prep Biochem. 1979;9(1):97–106. doi: 10.1080/00327487908061675. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin T. R., Roberto R. R., Juranek D. D., Limpakarnjanarat K., Mortenson E. W., Clover J. R., Yescott R. E., Taclindo C., Steurer F., Allain D. Human and sylvatic Trypanosoma cruzi infection in California. Am J Public Health. 1985 Apr;75(4):366–369. doi: 10.2105/ajph.75.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos C., Schädtler-Siwon I., Ortiz-Ortiz L. Suppressor cells present in the spleens of Trypanosoma cruzi-infected mice. J Immunol. 1979 Apr;122(4):1243–1247. [PubMed] [Google Scholar]
- Redelman D., Wormsley S. The induction of the human T-cell growth factor receptor precedes the production of RNA and occurs in the presence of inhibitors of RNA synthesis. Cytometry. 1986 Sep;7(5):453–462. doi: 10.1002/cyto.990070511. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Larson C. L., Speer C. A. Suppression of cell-mediated immunity in experimental Chagas' disease. Z Parasitenkd. 1977 Jun 3;52(1):11–17. doi: 10.1007/BF00380553. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Restoration of in vitro immune responses of spleen cells from mice infected with Trypanosoma cruzi by supernatants containing interleukin 2. J Immunol. 1984 Sep;133(3):1570–1575. [PubMed] [Google Scholar]
- Tarleton R. L. Trypanosoma cruzi-induced suppression of IL-2 production. II. Evidence for a role for suppressor cells. J Immunol. 1988 Apr 15;140(8):2769–2773. [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira G., Macêdo V., Prata A. Acquired cell-mediated immunodepression in acute Chagas' disease. J Clin Invest. 1978 Dec;62(6):1132–1141. doi: 10.1172/JCI109232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Voltarelli J. C., Donadi E. A., Falcao R. P. Immunosuppression in human acute Chagas disease. Trans R Soc Trop Med Hyg. 1987;81(1):169–170. doi: 10.1016/0035-9203(87)90324-5. [DOI] [PubMed] [Google Scholar]
- WOODY N. C., WOODY H. B. American trypanosomiasis (Chagas' disease); first indigenous case in the United States. J Am Med Assoc. 1955 Oct 15;159(7):676–677. doi: 10.1001/jama.1955.02960240042010a. [DOI] [PubMed] [Google Scholar]



