Abstract
Objectives. We assessed whether state Medicaid preferred drug lists are concordant with the World Health Organization's 2009 16th Essential Medicines List and with each other. We also characterized listed medicines by generic availability and appearance on treatment guidelines.
Methods. We derived generic availability and first-line treatment status from the US Food and Drug Administration's Orange Book and the 2004–2009 National Health Service National Institute for Clinical Excellence guidelines. We report characteristics of Essential Medicines List and preferred drug list (PDL)-only medicines and describe differences between medicines that are frequently and infrequently listed on PDLs.
Results. Only 6 of 120 Essential Medicines List medicines appeared on fewer than 50% of PDLs. PDL-only medicines (n = 249) were less likely than were Essential Medicines List medicines (n = 120) to have generic versions available (56% vs 76%) and to be first-line treatments (21% vs 41%). The content of PDLs was variable: 33% of medicines appeared on 80% to 100% of PDLs.
Conclusions. Application of the essential medicines concept to Medicaid PDLs could reduce costs and provide more equitable and evidence-based health care to low-income patients in the United States.
The essential medicines concept is designed to promote the availability, accessibility, affordability, quality, and rational use of medicines that meet public health needs.1 A key element of the concept is the World Health Organization (WHO) Essential Medicines List, which serves as a model for public supply and reimbursement. The first WHO Essential Medicines List was drafted in 1977 to address the gap in medication access between citizens of high- and low-income countries. The list aimed to highlight the most critical medicines for the needs of the national population.1 Since 2002, biannual revisions of the list have adhered to rigorous standards of evidence and consider disease prevalence and the safety and efficacy of medicines.2 Relative cost may be evaluated as well, but no medicine is excluded from consideration because of high cost.3 Additional features of the essential medicines concept are the development of evidence-based clinical guidelines and a national medicines policy.
WHO advises countries to adapt the Essential Medicines List according to their priority health care needs.1 In 2007, WHO found that 131 of 151 countries surveyed (87%) had a national essential medicines list. However, discrepancies exist between the WHO Essential Medicines List and these national lists, for a variety of reasons. For example, an Essential Medicines List medicine may not be a recommended therapy on a national guideline, or a medicine deemed as essential by the WHO may not be licensed in a country. An essential medicines list can serve as a model for procurement, local licensing and manufacturing, and the rational use of high-quality essential medicines. It also helps in allocating limited resources effectively and can be used as an advocacy tool to ensure that essential medicines are available and affordable for the population. The United States does not officially consult the Essential Medicines List, nor does it have a national medicines list.1
In the United States, the Medicaid program was enacted in 1965 to provide health care services to eligible low-income individuals, including families with children, the elderly, and the disabled.4 Currently, about 20% of the US population—60 million people—are enrolled in Medicaid,4 a number expected to increase with recent health care reforms. Medicaid preferred drug lists (PDLs) enumerate medicines that are fully reimbursed by Medicaid without prior authorization.5 Each state's Medicaid agency maintains a single PDL for its fee-for-service patients.
No standardized method for PDL development across states currently exists.6 As of May 2010, 11 states make financial contributions to the Drug Effectiveness Review Project,7 which offers evidence-based drug class reviews. However, many states offer no information on their PDL development process. One review of 18 studies of restrictive Medicaid formularies noted that none of the studies provided details as to how the formularies were constructed.8 One small study suggests that PDLs vary widely, but neither a large-scale comparison of PDLs nor a characterization of the most variable or most consistent medicines currently exists.6
Low-income countries use the WHO Essential Medicines List to target their scarce resources for the procurement of the most-needed medicines.3 Medicaid drug reimbursements are linked to PDLs that are developed by each state. Thus, Medicaid recipients, who are typically low-income people, are likely to receive medicines from these preferred drug lists.6
Our objectives were (1) to determine whether Medicaid patients have access to all Essential Medicines List medicines, (2) to assess the concordance between the Essential Medicines List and PDLs, and (3) to evaluate the consistency of PDLs. To better understand sources of variability among PDLs and the Essential Medicines List, we describe whether listed medicines had generic versions available and whether they are recommended as first-line treatments in evidence-based clinical guidelines. We expected that PDLs would have more brand name–only medicines and fewer first-line treatments than would the Essential Medicines List.
METHODS
Our 2-part analysis evaluated the concordance between the 2009 16th WHO Model List of Essential Medicines9 and PDLs and among state PDLs. We refer to medicines that are on PDLs but absent from the Essential Medicines List as “PDL-only medicines.”
Selection of Therapeutic Categories for Comparison
We identified 11 therapeutic classes with the highest annual Medicaid reimbursement for dual or nondual eligible patients, according to the most recent published Medicaid Chartbook (2005).5 These 11 classes (antipsychotic, antiasthma, anticonvulsant, antidepressant, antiulcer, narcotic, antidiabetic, antihyperlipidemic, antihypertensive, antiretroviral, and analeptic medicines) represent over 50% of annual Medicaid spending and are among the most highly used medicines.5 Thus, we assumed that these medicines are used to treat priority diseases in the Medicaid population.
We excluded 2 of the 11 classes, analeptic and antiretroviral medicines, from analysis. Analeptics are used to treat conditions such as obesity and attention deficit hyperactivity disorder, which are not priority health conditions in low-income countries and thus were not listed on the 2009 Essential Medicines List. Many states require that antiretrovirals be available to Medicaid patients and therefore exempt this class from PDL requirements.6
Obtaining State Preferred Drug Lists
We focused only on fee-for-service PDLs because they are publicly available from state Medicaid Web sites and limited to 1 per state. Medicaid patients who are enrolled in managed care organizations have access to medicines on the organization's formulary, but these lists are not publicly available. The most recent Medicaid fee-for-service PDLs were collected from October through November 2009 for 40 states and the District of Columbia. We excluded Tennessee because it does not have a fee-for-service Medicaid population. We excluded 9 additional states (Arizona, Hawaii, Missouri, New Jersey, North Carolina, North Dakota, New Mexico, Oklahoma, and South Dakota) because their PDLs were not available online at the time of the search.
Data Collection
We recorded international nonproprietary names of medicines if they were listed on the 2009 16th WHO Model List of Essential Medicines9 or on any state PDL. We considered a medicine to be on a PDL if it was listed there without restrictions. Medicines that did not appear on a PDL were considered absent from that PDL because all of these medicines require prior authorization for reimbursement. In some cases, an entire pharmacological class had not been evaluated for PDL listing. Because reimbursement policies for these medicines vary from state to state, we excluded unevaluated medicines from analysis.
We also indicated whether each medicine appeared on the Essential Medicines List. A medicine that appeared on the Essential Medicines List with a “square box” annotation served as a representative medicine from a clinically equivalent pharmacological class (e.g., diazepam represents the class of benzodiazepines) and was intended to be the least costly medicine in its pharmacological class. However, some countries may be able to obtain other medicines in the class for lower cost and thus should use these other medicines on their national medicine lists. For example, a 36-country survey of availability and pricing of 15 medicines found that public sector procurement prices for lowest-priced generics varied widely. In low-income countries, the median price ratios of generics (defined as the median local unit price divided by the international reference unit price) ranged from 0.09 in Sudan to 5.37 in Nigeria; in lower-middle-income countries, price ratios ranged from 0.33 in Jordan to 2.94 in the Philippines.10 For this study, if a medicine was listed on the Essential Medicines List with a square box, all medicines in the same pharmacological class were also considered to be listed on the Essential Medicines List.
We consulted the US Food and Drug Administration's Orange Book11 to determine whether each medicine has a licensed generic version available in the United States. The actual cost of a medicine is difficult to measure and varies widely from state to state. We therefore considered generic availability to be a proxy for low cost.
To determine whether a medicine was a recommended first-line treatment, we consulted the most recent evidence-based treatment guidelines published by the National Institute for Clinical Excellence (NICE, a division of the British National Health Service).12 The NICE guidelines were selected because they are evidence based and the United States does not publish national, standardized, evidence-based treatment guidelines. A medicine was considered to be a first-line treatment if it was recommended for previously untreated patients with no compelling comorbidities. Guidelines did not exist for antiasthmatics or analgesics, so we excluded medicines in these classes from this part of the analysis.
We also describe the population of PDLs in October and November 2009. No statistical tests were used since sampling was not performed.
Comparison of Essential Medicines List and Preferred Drug Lists
We first separated medicines into 2 groups: Essential Medicines List medicines and PDL-only medicines. We calculated the percentage of medicines in each group with available generic formulations and the percentage of medicines indicated as first-line treatments on NICE guidelines. Next, we ranked Essential Medicines List medicines by the percentage of PDLs that listed each medicine to identify any medicines that did not appear on PDLs. Finally, we sorted medicines by pharmacological class to identify the classes with the highest percentage of PDL-only medicines that did not appear on the Essential Medicines List. We examined medicines in these PDL-only classes for common characteristics.
Comparison of Preferred Drug Lists
We sorted medicines into quintiles by frequency of appearance on PDLs to identify those that were listed frequently (on 81%–100% of PDLs) and infrequently (on 0%–20% of PDLs). To identify medicine characteristics that influence PDL listing, we sorted PDL medicines by generic availability, presence on Essential Medicines List, and indication as first-line treatment. We then calculated the mean PDL coverage rate for generics versus nongenerics, Essential Medicines List versus PDL-only medicines, and first-line versus non–first-line treatments. We compared frequently and infrequently listed medicines on the basis of percentage of medicines with generics available, percentage on the Essential Medicines List, and percentage indicated as first-line treatments. Finally, we identified the most infrequently listed PDL medicines and examined them for common characteristics.
RESULTS
We examined 369 medicines in 9 therapeutic classes. PDL-only medicine classes were composed entirely of medicines that are either nongeneric, not first-line treatments, or combination therapies (Table 1). Of the 249 PDL-only medicines, 56% were available as generics, whereas 76% (93 of 120) of Essential Medicines List medicines were generics. PDL-only medicines were less likely to be indicated as first-line treatments (21%, 52 of 249) than were Essential Medicines List medicines (41%, 49 of 120). There are very few Essential Medicines List medicines that were not listed on PDLs; only 6 Essential Medicines List medicines appeared on fewer than 50% of PDLs (Table 2). There are reasonable explanations for why 2 of these Essential Medicines List medicines—magnesium sulfate injection and prescription omeprazole powder—did not appear on any PDLs. Because patients in the United States generally receive injectable medicines such as magnesium sulfate in inpatient settings, most PDLs do not evaluate injectable medicines. Although prescription omeprazole powder was not listed on any PDLs, omeprazole is readily available to patients as a generic, nonprescription capsule.
TABLE 1.
Characteristics of Pharmacological Classes That Appear on Preferred Drug Lists (PDLs) but Not on the Essential Medicines List: 40 US States and the District of Columbia, 2009
| Medicine Characteristics |
||||||
| Therapeutic Class | Pharmacological Class | No. of Medicines | % of PDLs Listing Medicine (n = 41) | Not Generic | Not First-Line | Combination Drug |
| Antidepressants | Other | 7 | 73 | X | ||
| Antihypertensives | α-blockers | 4 | 63 | X | ||
| Antihyperlipidemics | Fibrates | 4 | 57 | X | ||
| Antihyperlipidemics | BAS | 5 | 56 | X | ||
| Antipsychoticsa | Atypicals | 13 | 55 | X | ||
| Antidiabetics | Combinations | 8 | 53 | X | X | |
| Antihypertensives | ARBs | 7 | 53 | X | ||
| Analgesics | Combinations | 17 | 50 | NA | X | |
| Antidepressants | MAOIs | 4 | 50 | X | ||
| Antihypertensives | Combinations | 29 | 49 | X | X | |
| Analgesics | Long acting | 8 | 47 | NA | ||
| Antidepressants | SNRIs | 4 | 44 | X | ||
| Antiasthmatics | Combinations | 3 | 37 | NA | X | |
| Antihyperlipidemics | Combinations | 5 | 36 | X | X | |
| Antihyperlipidemics | Other | 3 | 24 | X | ||
| Antiasthmatics | LTRAs | 4 | 22 | X | NA | |
Note. ARBs = angiotensin II receptor blockers; BAS = bile acid sequestrants; LTRAs = leukotriene receptor antagonists; MAOIs = monoamine oxidase inhibitors; NA = not available; SNRIs = serotonin norepinephrine reuptake inhibitors. X indicates that the specified characteristic was met. Excluded Tennessee because it does not have a fee-for-service Medicaid population and 9 additional states (Arizona, Hawaii, Missouri, New Jersey, North Carolina, North Dakota, New Mexico, Oklahoma, and South Dakota) because their PDLs were not available online at the time of the search.
One of 13 antipsychotics (risperidone) is available as a generic.
TABLE 2.
Essential Medicines List Medicines That Appear on Fewer Than 70% of Preferred Drug Lists (PDLs): 40 US States and the District of Columbia, 2009
| Medicine | Therapeutic Class | % of PDLs Listing Medicine (n = 41) |
| Magnesium sulfate injection | Anticonvulsants | 0 |
| Omeprazole powder | Gastrointestinal | 0 |
| Clorazepate | Anticonvulsants | 6 |
| Prochlorperazine | Antipsychotics | 25 |
| Phenobarbital | Anticonvulsants | 38 |
| Omeprazolea | Gastrointestinal | 38 |
| Budesonide | Antiasthmatics | 59 |
| Loxapine | Antipsychotics | 67 |
| Lorazepam | Anticonvulsants | 69 |
Note. Excluded Tennessee because it does not have a fee-for-service Medicaid population and 9 additional states (Arizona, Hawaii, Missouri, New Jersey, North Carolina, North Dakota, New Mexico, Oklahoma, and South Dakota) because their PDLs were not available online at the time of the search.
Prescription pill form.
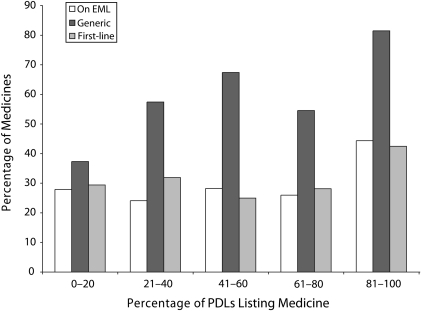
Of the 369 medicines evaluated, only one third (n = 124) appeared on 80% to 100% of PDLs and were thus the least variable medicines listed across PDLs. As shown in Figure 1, compared with the least frequently listed or most variable medicines, the medicines listed most frequently among PDLs were more likely to have generic versions available, to be listed on the Essential Medicines List, and to be indicated as first-line treatments. Overall, however, there does not appear to be a clear association between the frequency with which a medicine was listed on the PDLs and its presence on the Essential Medicines List or indication of its use as a first-line treatment (Figure 1). Eight medicines were listed on only 1 PDL; none of these medicines were available as a generic formulation or recommended as a first-line treatment (Table 3).
FIGURE 1.
Characteristics of 369 medicines by frequency of appearance on 41 preferred drug lists (PDLs): 40 US States and the District of Columbia, 2009.
Note. EML = Essential Medicines List. Excluded Tennessee because it does not have a fee-for-service Medicaid population and 9 additional states (Arizona, Hawaii, Missouri, New Jersey, North Carolina, North Dakota, New Mexico, Oklahoma, and South Dakota) because their PDLs were not available online at the time of the search.
TABLE 3.
Medicines Appearing on Only 1 Preferred Drug List: 40 US States and the District of Columbia, 2009
| Medicine | Therapeutic Class | State |
| Zileuton CR | Antiasthmatic | Pennsylvania |
| Arformoterol | Antiasthmatic | Utah |
| Eprosartan | Antihypertensive | South Carolina |
| Eprosartan + HCTZ | Antihypertensive | South Carolina |
| Saxagliptin | Antidiabetic | Montana |
| Aliskiren + valsartan | Antihypertensive | District of Columbia |
| Paliperidone | Antipsychotic | Washington |
| Tapentadol | Analgesic | Alabama |
Note. CR = controlled release; HCTZ = hydrochlorothiazide. Excluded Tennessee because it does not have a fee-for-service Medicaid population and 9 additional states (Arizona, Hawaii, Missouri, New Jersey, North Carolina, North Dakota, New Mexico, Oklahoma, and South Dakota) because their PDLs were not available online at the time of the search.
DISCUSSION
Because Essential Medicines List medicines appeared on most PDLs, the Essential Medicines List could function as a core list for PDL development as it does for the development of national essential medicines lists.1 The high degree of variation among PDLs suggests that state committees charged with developing PDLs do not have a consistent methodology for listing medicines, and it points to possible disparities in access to medication for Medicaid patients in different states. The weak association between a medicine's presence on NICE guidelines and PDL listing may indicate that clinical effectiveness is not a primary motivator of state PDL decisions. Almost half of the medicines that appear on PDLs but not on the Essential Medicines List are not generics. Thus, the relatively low cost of generic products compared with brand-name products also does not appear to drive PDL listing. Furthermore, in contrast with the transparent Essential Medicines List selection process,2 methods for PDL development are not readily available.8 Applications for the Essential Medicines List, the evidence provided in support of the application, and the reviews of the Essential Medicines List Selection Committee are all publicly available. It is unclear how evidence of efficacy and safety are reviewed and whether relative cost is considered for listing on state PDLs. Several newer drugs with unproven effectiveness appeared on only 1 or 2 PDLs, suggesting that no strong evidence base exists to support their listing.
Limitations
Our conclusions about the cost-effectiveness of medicines are limited because we were unable to determine the true cost of medicines listed on PDLs, and we did not conduct systematic drug class reviews to assess effectiveness. Because generic medicines are usually less expensive than are brand-name medicines, especially within a pharmacological class, we used generic availability as an indicator of relatively low cost. In reality, complex rebate arrangements and bundling agreements exist between state Medicaid agencies and pharmaceutical manufacturers, and prices paid by Medicaid for medicines can be vastly different from average wholesale prices (Lisa Ashton, Medi-Cal, oral communication, November 4, 2009).
Additional data are needed to describe the PDL development processes of each state and to assess the real costs paid for medicines by state Medicaid agencies. Another limitation of our study was the use of NICE first-line treatment recommendations as a surrogate marker of effectiveness. Although the NICE guidelines adhere to rigorous standards of evidence, they may not always reflect clinical practice in the United States.
Conclusions
The goal of health care reform in the United States is to provide more efficient, coordinated, and effective care.13 In addition, health care reform has expanded coverage for the uninsured, about half of whom will be covered by Medicaid.14 Medicaid prescription drug benefits remain the second largest spending category for Medicaid, even after a shift of some prescription drug costs from Medicaid to Medicare Part D.15 Thus, providing effective and efficient medicines for Medicaid patients is essential to the success of health care reform. Our findings suggest that it is unclear how the effectiveness of a medicine influences Medicaid preferred drug listing. Application of the essential medicines concept would promote more equitable access to medicines for Medicaid recipients in all states by requiring that listing of a medicine be based on public health need, efficacy, and safety.
As health care reform evolves, state Medicaid agencies could use the Essential Medicines List as a core PDL and apply the WHO process for medicines selection to add other medicines to the list. Agencies could also consider creating a national list of safe and effective medicines for prevalent diseases. Although this process could reduce the number of medicines available to Medicaid patients in some states, patients could have more confidence that the medicines they receive are effective and safe. Application of the essential medicines concept to the development of lists of medicines that are available for Medicaid patients could reduce costs and provide more equitable and evidence-based health care to low-income patients in the United States.
Acknowledgments
We thank Elisa Ashton, PharmD, University of California San Francisco (UCSF), for information on Medicaid drug benefits and Jim Lightwood, PhD, UCSF, for statistical advice. We also thank Hans Hogerzeil, MD, PhD, FRCP, World Health Organization (WHO); Suzanne Hill, PhD, WHO; Bruce Lambert, PhD, University of Illinois Chicago; and Gordon Schiff, MD, Brigham and Women's Hospital, for critical comments.
Human Participant Protection
Institutional review board approval was not needed for this study because no human participants were enrolled in the study and all data were obtained from public sources.
References
- 1.Laing R, Waning B, Gray A, et al. 25 Years of the WHO essential medicines lists: progress and challenges. Lancet. 2003;361(9370):1723–1729 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Definition and concept of essential medicines. Available at: http://www.who.int/selection_medicines/committees/en/index.html. Accessed January 7, 2010
- 3.Hogerzeil HV. The concept of essential medicines: lessons for the rich countries. BMJ. 2004;329(7475):1169–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiser Family Foundation Medicaid Facts. Available at: http://www.kff.org. Accessed November 13, 2009.
- 5.Mathematica Policy Research Chartbook: Medicaid pharmacy benefit use and reimbursement in 2005. June 2009. Available at: http://www.mathematica-mpr.com/publications/PDFs/health/chartbook2005.pdf. Accessed November 11, 2009
- 6.Ketcham J, Ngai J. How similar are states' Medicaid preferred drug lists? Am J Managed Care. 2008;14(11 spec no.):SP46–SP52 [PubMed] [Google Scholar]
- 7.Oregon Health & Science University Drug Effectiveness Review Project (DERP) Available at: http://www.ohsu.edu/ohsuedu/research/policycenter/DERP. Accessed November 13, 2009.
- 8.Lexchin J. Effects of restrictive formularies in the ambulatory care setting. Am J Manag Care. 2002;8(1):69–76 [PubMed] [Google Scholar]
- 9.World Health Organization Model List of Essential Medicines. 16th list. March 2009. Available at: http://www.who.int/medicines/publications/essentialmedicines/Updated_sixteenth_adult_list_en.pdf. Accessed April 24, 2011.
- 10.Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240–249 [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration Orange Book. Available at: http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed January 3, 2010.
- 12.British National Health Service National Institute for Clinical Excellence Web site. Available at: http://www.nice.org.uk/guidance. Accessed January 5, 2010.
- 13.Guterman S, Davis K, Stremikas K, Drake H. Innovation in Medicare and Medicaid will be central to health reform's success. Health Aff. 2010;29(6):1188–1193 [DOI] [PubMed] [Google Scholar]
- 14.Doherty RB. The certitudes and uncertainties of health care reform. Ann Intern Med. 2010;152(10):679–682 [DOI] [PubMed] [Google Scholar]
- 15.Peters CP. Medicaid payment for generic drugs. National Health Policy Forum, Issue Brief 839. September 30, 2010. Available at: http://www.nhpf.org/library/details.cfm/2826. Accessed October 6, 2010 [PubMed]