Abstract
Food fortification is an effective public health tool for addressing micronutrient deficiencies. The mandatory fortification of enriched cereal grains (e.g., wheat flour) with folic acid, which began in the United States in 1998, is an example of a successful intervention that significantly reduced the rate of neural tube defects (NTDs).
However, despite the drop in NTD rates across all racial/ethnic groups after fortification, Hispanics continue to have the highest rates of this condition. One possible way to reduce this disparity is to fortify corn masa flour to increase the overall intake of folic acid in Hispanic women.
We present the available evidence in favor of this approach, address possible safety issues, and outline next steps in the fortification of corn masa flour with folic acid in the United States.
Neural tube defects (NTDs), one of the more common birth defects, are estimated to affect approximately 3000 US pregnancies each year.1 NTDs are also one of the most serious birth defects. The 2 most common types of NTDs are spina bifida and anencephaly, which are caused by the incomplete development of the neural tube, a sheath that closes during early embryonic development to form the brain and spinal cord. NTDs are significant contributors to infant mortality. Infants born with anencephaly generally die within days or weeks; infants with spina bifida who survive usually have serious long-term morbidity and medical problems, including hydrocephalus, which is associated with the Arnold-Chiari type II malformation of the spinal cord that accompanies spina bifida.2
The prevalence of NTDs varies by race/ethnicity, with Hispanic women having a higher prevalence rate of births with NTDs compared to non-Hispanic White women and non-Hispanic Black women.3 The development of the neural tube is normally completed within 28 days after conception4; thus, in light of the fact that half the pregnancies in the United States are unplanned,5 NTDs can develop before women know they are pregnant. Randomized controlled trials in the early 1990s showed that up to 70% of NTDs could be prevented if women consumed adequate folic acid around conception and in early pregnancy.6,7 This discovery led the US Public Health Service and the Institute of Medicine to recommend that women capable of becoming pregnant should consume 400 micrograms of folic acid per day.8,9 To further improve folic acid intake in women of childbearing age, the US Food and Drug Administration (FDA) mandated in 1996 that by January 1, 1998, all enriched cereal-grain products (e.g., wheat flour and related products, corn meal, and rice) would be fortified with folic acid at 140 micrograms per 100 grams of product.10
The mandatory folic acid fortification of enriched cereal grains in the United States has proven to be a successful public health intervention to reduce the rate of NTDs. Various comparisons of NTD prevalence before and after fortification reveal a significant decrease ranging from approximately 19% to 26%, with even larger decreases found by studies using data from state birth defects surveillance systems that use prenatal ascertainment.1,11–13 Another measure of the effectiveness of fortification is the blood folate status of the US population. The National Health and Nutrition Examination Survey (NHANES), designed to assess the health and nutritional status of the US population, documents red blood cell folate (which measures long-term folate intake) and serum folate (which measures short-term intake).14 Both the red blood cell folate and serum folate concentrations in the overall US population increased after fortification.14,15 Among women of childbearing age (15–45 years), median red blood cell folate levels increased 65% between the pre- and postfortification periods, and the prevalence of low red blood cell folate (< 140 ng/mL) declined from 37.6% in 1988–1994 to 5.1% in 1999–2000 and to 4.5% in 2005–2006.14 The median serum folate levels among women of childbearing age followed the same pattern. Levels increased from 4.8 nanograms per milliliter in 1988–1994 to 13.0 nanograms per milliliter in 1999–2000, and the prevalence of low serum folate (< 3 ng/mL) improved from 20.6% in 1988–1994 to 0.8% in 1999–2000.14 Prevalance of low serum folate stayed low from that time through 2005–2006.14
RACIAL/ETHNIC DISPARITIES IN NEURAL TUBE DEFECT RATES
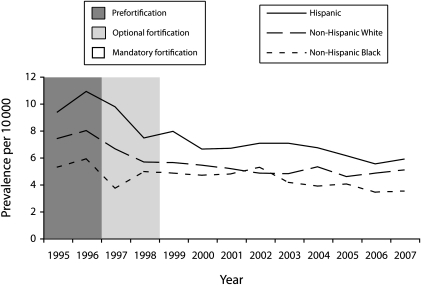
Despite the initial success of folic acid fortification in the United States, it is not known whether all NTDs that could be prevented by folic acid are being prevented.16 A study evaluating the relationship between NTDs and maternal folic acid consumption in US women who conceived at least six months after mandatory folic acid fortification began found no association between maternal folic acid supplement use or dietary intake and NTD occurrence.17 This finding may indicate that fortified foods are most likely providing enough folic acid to prevent folate-related defects, but Hispanic women have consistently had a higher prevalence rate for NTDs than have non-Hispanic White women and non-Hispanic Black women, both before and after fortification, despite the overall decline in prevalence rates across all racial/ethnic groups.3 An analysis of data from 21 population-based birth defects surveillance systems in the United States showed that although there was a significant decline in the prevalence rate for both spina bifida and anencephaly in Hispanic births after fortification, the rate of NTDs was higher in Hispanic births than it was in any other ethnic group.3 According to recent data from the National Birth Defects Prevention Network (2005–2007), Hispanic women were 1.21 times more likely to have an NTD than were non-Hispanic White women (95% confidence interval [CI] = 1.11, 1.31; Figure 1).18
FIGURE 1.
Neural tube defect prevalence by race/ethnicity for 25 surveillance programs: National Birth Defects Prevention Network, United States, 1995–2007.
Note. Fortification refers to the 1996 US Food and Drug Administration mandate that by January 1, 1998, all enriched cereal-grain products (e.g., wheat flour and related products, corn meal, and rice) would be fortified with folic acid at 140 μg per 100 g of product.10Source. Centers for Disease Control and Prevention.18
Acculturation may also play a role in the rate of NTDs within the Hispanic population, with less acculturated Hispanic women being at highest risk of NTDs. For example, one study found that Hispanic mothers whose predominant language at home was Spanish were 1.87 times (95% CI = 1.48, 2.35) more likely to have a baby with spina bifida, whereas those whose predominant language at home was English were only slightly more at risk than was the reference group.19 Blood folate levels also vary by race/ethnicity, and although the median serum folate levels of non-Hispanic White women were significantly higher than those of Mexican American women, the median red blood cell folate levels of non-Hispanic White women were no different from those of Mexican American women.14 The reasons for this disparity are unknown; there may be a combination of genetic20 and environmental factors such as racial/ethnic differences in folate metabolism, maternal diabetes, and obesity.3
These nonfolate factors may help to explain the racial/ethnic disparity in NTD rates and the discrepancy between the high rates of NTDs among Hispanics and their seemingly sufficient red blood cell folate levels. This phenomenon merits further study. The Hispanic population in the United States has grown significantly over the last 30 years and is projected to be more than 24% of the total population by 2050.21 Hispanic women have consistently had the highest fertility rate among all racial/ethnic groups, and they account for about 24% of live births in the United States.22 These data underscore the importance of addressing the higher rates of NTDs in the Hispanic population, as this population will continue to grow rapidly.
Although serum folate levels have significantly increased in women of childbearing age since fortification, many nonpregnant women of childbearing age in the United States are still not consuming the recommended usual daily intake (400 μg) of folic acid through diet and supplements.23 Despite educational campaigns to increase women's consumption of supplements containing folic acid, including campaigns targeted toward specific racial/ethnic groups,24 evidence shows that women of childbearing age are still not consuming enough. One study using NHANES data estimated that from 2003 through 2006, approximately 75% of nonpregnant US women of childbearing age did not consume the recommended usual daily intake of folic acid.25 Supplement use was the strongest predictor of adequate intake; women who used a supplement that contained folic acid had a prevalence of recommended usual intake that was 10 times that of women who did not.25 Unfortunately, the prevalence of self-reported daily use of supplements that contained folic acid was less than 40% from 1995 through 2008 according to a survey of a national sample of US women aged 18 to 45 years.26
Furthermore, Hispanic women have lower intake of folic acid overall than do non-Hispanic White women. Studies have revealed that a smaller percentage of Hispanic women consumed at least 400 micrograms of folic acid from supplements and fortified foods than did non-Hispanic White women, and in general Hispanic women used supplements that contained folic acid less than did non-Hispanic White women.23,27
Because of the existing racial/ethnic disparity of higher NTD rates in Hispanic women, and because consumer education alone has not been sufficient to increase consumption of folic acid in all women of childbearing age, additional targeted fortification is needed to increase folic acid consumption in the Hispanic population. A key ingredient in most Latin American diets is corn masa flour, a flour made from specially treated corn used to make corn tortillas, tamales, and other dishes common in Mexico and Central America. Fortification of corn masa flour has the potential to reduce the disparity in NTD incidence in the Hispanic population by increasing the overall intake of folic acid in Hispanic women.
CORN MASA FLOUR FORTIFICATION IN THE UNITED STATES
Some countries, such as Mexico, and several countries in Central America (e.g., Costa Rica), have voluntary or mandatory fortification of corn masa flour and related products (e.g., corn tortillas) with folic acid.28 Although implementation has been successful in these countries, there are currently no population-based data to extrapolate the possible effects that fortification of corn masa flour would have on Hispanic women in the United States. For example, Costa Rica has seen a reduction of NTDs at birth since fortification of both wheat and corn flour, but the data on Costa Rica do not show the independent effect of corn flour fortification alone.29
Therefore, Hamner et al. developed a mathematical model to test the hypothesis that folic acid fortification of corn masa flour in the United States would increase folic acid intake in Hispanic women, specifically Mexican American women, without substantially increasing folic acid intake in the rest of the population.30 They used NHANES data to calculate the potential effects of fortifying corn masa flour with folic acid in the United States. In the population they analyzed, non-Hispanic White women of childbearing age (15–44 years) were more likely to report consuming a supplement containing folic acid than were both Mexican American women and non-Hispanic Black women. More than a quarter (28%) of women of childbearing age (15—44 years) reported consuming corn masa flour on day 1 or day 2 of the survey. More Mexican American women (60%) reported consuming corn masa flour compared to non-Hispanic White women (23%) or non-Hispanic Black women (27%), confirming that corn masa flour constituted a more significant part of the daily diet for Mexican American women in this study population than for women of other races/ethnicities.
The analysis found that corn masa flour fortification in the United States would increase the intake of total usual daily folic acid in Mexican American women of childbearing age (15–44 years) by approximately 20%, compared with approximately 4% to 5% in the other two groups. In this model, Mexican American women would have median usual daily intakes much closer to those reported by non-Hispanic White women, despite the higher percentage of supplement use among non-Hispanic White women. In addition, an estimated 7% more Mexican American women would consume 400 micrograms of folic acid per day. This analysis predicted that the median usual daily folic acid intake for Mexican American women in the United States would still be below the recommended intake of 400 micrograms per day, but fewer Mexican American women would be below the daily recommended intake. According to this model, the fortification of corn masa flour would successfully target Mexican American women and would likely decrease the incidence of NTDs in this population.
In the past, the process of fortifying corn masa flour presented some unique challenges. During the production of corn masa, dried corn is cooked in hot water with lime (a process called nixtamalization), which affects the pH of the masa and decreases the stability of added vitamins. Modern production techniques add the premixed vitamins to dry corn flour after milling, resulting in consistent and significant levels of folic acid in the finished product.31–33
SAFETY AND POSSIBLE RISKS
Because food fortification is a public health intervention that affects a large number of people who consume a particular food, there is always concern about any possible adverse effects. Multiple sources of folic acid exist, including supplements and enriched foods, so it is possible to exceed the tolerable upper intake level set by the Institute of Medicine.9 Fewer than 3% of US adults exceeded the upper intake level for folic acid (1000 μg for adults aged ≥ 19 years) from 2003 through 2006, and almost all of these adults consumed an average of more than 400 micrograms of folic acid per day from supplements.34
A number of possible adverse effects of folic acid have been hypothesized, including cancer and tumor promotion, epigenetic hypermethylation, interference with antifolate treatment, miscarriages, and multiple births. Two reviews of relevant human studies in each of these areas concluded that no available evidence supported the conclusion that folic acid intake, either through fortified foods or through supplements, causes adverse effects.35,36 A meta-analysis of eight large randomized studies to examine the effect of high-dose folic acid supplementation on preventing cardiovascular disease found no harmful effects from taking folic acid, including no significant adverse effects on vascular events, cancer incidence, cancer mortality, and overall mortality.37 One Australian study found an association between maternal supplemental folic acid in late pregnancy and childhood asthma, but this association is unconfirmed, and no association was found with supplementation before pregnancy or in early pregnancy.38
Another potential adverse effect of folic acid is the masking of vitamin B12 deficiency, particularly in the elderly. Megaloblastic (abnormally large red blood cell) anemia caused by vitamin B12 deficiency is clinically identical to megaloblastic anemia caused by folic acid, so diagnosis of vitamin B12 deficiency may be delayed in individuals with increased folic acid intake. In addition, the neurological complications of vitamin B12 deficiency do not respond to folic acid, so masking vitamin B12 megaloblastic anemia with folic acid may result in a delayed diagnosis and treatment of B12 deficiency and its neurological symptoms.39 One study has specifically addressed this concern in the postfortification era.40 Folic acid exposure has increased, but there is no evidence of an increase in low vitamin B12 levels without anemia (i.e., masking of the neurological consequences of vitamin B12 deficiency).40
Considerations related to the risk of cancer, particularly colorectal cancer, merit further discussion. Several recent studies examining a possible relationship between colorectal cancer and folic acid have received some attention. One double-blind randomized controlled trial found that high-dose folic acid supplements (1000 μg/d) with or without aspirin did not reduce the risk of colorectal adenomas in people with a recent history of colorectal adenomas, and there was some evidence of increased risk of multiple adenomas and advanced lesions in the folic acid group.41 Another epidemiological study revealed that there was a brief increase in the incidence of diagnosed colorectal cancer from 1996 to 1999 associated with the initiation of folic acid fortification in the United States and Canada.42 However, the improvement in colorectal cancer screening was considered as a possible explanation for this increase. On the other hand, two randomized controlled trials investigating whether folic acid could prevent recurrence of colorectal adenomas found no increase in the incidence of these tumors attributed to folic acid supplementation.43,44 In summary, at the time of this writing evidence from human studies does not show increased cancer risk related to folic acid supplementation, but continued research in this area is certainly warranted.
NEXT STEPS TOWARD FORTIFICATION IN THE UNITED STATES
In the United States, the FDA regulates food fortification with micronutrients such as folic acid.45–47 There are currently no federal regulations to permit folic acid fortification of corn masa flour. In general, there are two ways to proceed, both of which require a petition to the FDA. One approach is to develop a new standard of identity45 for “enriched” corn masa flour products (corn masa flour and any food products made from it, such as tortillas) that would require manufacturers selling these products as “enriched” to fortify with them with folic acid. These mandatory standards define a given food product and any specific nutrients and amounts required in the manufacture of the food.47 The second way is to amend the current FDA food additive regulation for folic acid48 to permit a manufacturer to voluntarily add folic acid to corn masa flour products. Some issues that will need to be addressed by a petition in this regulatory review process include establishing a documented need, addressing safety (showing reasonable certainty that fortification would result in no harm to the general public), and food technology and manufacturing issues (e.g., amount to be added, manufacturing processes, stability of the additive during processing, and issues of taste, color, or texture that result from fortification).
CONCLUSIONS
Despite success in reducing NTD rates in the United States through folic acid fortification of enriched cereal grains, racial/ethnic disparities in the prevalence of NTDs persist. Hispanic women continue to have the highest rates of this serious birth defect. Although the precise reason for this disparity is not well understood, it is imperative to address this serious public health issue as we continue to investigate the other nonfolate risk factors that may contribute to the disparity. Fortification of corn masa flour with folic acid in the United States presents an opportunity to affect this specific population significantly.
There are no population-based data from other countries that have successfully fortified corn masa flour with folic acid to show a direct correlation between corn masa flour fortification and decreased NTD rates. However, we do have concrete evidence that fortification of enriched cereal grains in the United States has decreased NTD rates and improved the blood folate status among women of childbearing age. Fortifying corn masa flour and related corn products should have the same effect on those women whose diet is primarily based on corn products, and a theoretical model has shown that folic acid consumption would be increased among the target Hispanic population as a result of corn masa flour fortification. Although fortified corn masa flour might cause a small increase in the overall median usual folic acid intake of the US population, the model has shown that this approach will not significantly increase intake in non-Hispanic populations and can reduce the disparity in intake between Mexican American women and non-Hispanic White women. In addition, it is highly unlikely that corn masa flour fortification would cause more people in the United States to exceed the recommended upper limit of daily folic acid intake.
The process of fortifying corn masa flour has been accomplished safely in other countries, and there is sufficient evidence to support the proposition that the fortification of corn masa flour with folic acid in the United States can reduce the racial/ethnic disparity in folic acid status and decrease the incidence of NTDs in the Hispanic population without adversely affecting the health of the general population. We call upon key stakeholders, including health care professionals, food industry leaders, and consumer advocacy groups, to work together to help prevent this serious birth defect.
References
- 1.Centers for Disease Control and Prevention Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. MMWR Morb Mortal Wkly Rep. 2004;53(17):362–365 [PubMed] [Google Scholar]
- 2.Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341(20):1509–1519 [DOI] [PubMed] [Google Scholar]
- 3.Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005;116(3):580–586 [DOI] [PubMed] [Google Scholar]
- 4.Sadler TW. Embryology of neural tube development. Am J Med Genet C Semin Med Genet. 2005;135C(1):2–8 [DOI] [PubMed] [Google Scholar]
- 5.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38(2):90–96 [DOI] [PubMed] [Google Scholar]
- 6.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137 [PubMed] [Google Scholar]
- 7.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832–1835 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14):1–7 [PubMed] [Google Scholar]
- 9.Institute of Medicine Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press; 1998 [PubMed] [Google Scholar]
- 10.US Food and Drug Administration Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid, final rule. 21 CFR parts 136, 137 and 139. Fed Reg. 1996:8781–8797 [Google Scholar]
- 11.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986 [DOI] [PubMed] [Google Scholar]
- 12.Mathews TJ. NCHS Health E-Stats 2009. Trends in spina bifida and anencephalus in the United States, 1991–2006. Available at: http://www.cdc.gov/nchs/data/hestat/spine_anen/spine_anen.pdf. Published April 2009. Accessed April 4, 2011
- 13.Williams LJ, Mai CT, Edmonds LD, et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66(1):33–39 [DOI] [PubMed] [Google Scholar]
- 14.McDowell MA, Lacher DA, Pfeiffer CM, et al. Blood Folate Levels: The Latest NHANES Results. Hyattsville, MD: National Center for Health Statistics; 2008. NCHS Data Brief no. 6 [PubMed] [Google Scholar]
- 15.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86(3):718–727 [DOI] [PubMed] [Google Scholar]
- 16.Heseker HB, Mason JB, Selhub J, Rosenberg IH, Jacques PF. Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. Br J Nutr. 2009;102(2):173–180 [DOI] [PubMed] [Google Scholar]
- 17.Mosley BS, Cleves MA, Siega-Riz AM, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol. 2009;169(1):9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention CDC grand rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010;59(31):980–984 [PubMed] [Google Scholar]
- 19.Canfield MA, Ramadhani TA, Shaw GM, et al. Anencephaly and spina bifida among Hispanics: maternal, sociodemographic, and acculturation factors in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2009;85(7):637–646 [DOI] [PubMed] [Google Scholar]
- 20.Volcik KA, Blanton SH, Tyerman GH, et al. Methylenetetrahydrofolate reductase and spina bifida: evaluation of level of defect and maternal genotypic risk in Hispanics. Am J Med Genet. 2000;95(1):21–27 [PubMed] [Google Scholar]
- 21. US Census Bureau Hispanic population in the United States: 1970 to 2050. Available at: http://www.census.gov/population/www/socdemo/hispanic/hispanic_pop_presentation.html. Accessed June 22, 2010
- 22.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2006. Natl Vital Stat Rep. 2009;57(7):1–102 [PubMed] [Google Scholar]
- 23.Yang QH, Carter HK, Mulinare J, Berry RJ, Friedman JM, Erickson JD. Race-ethnicity differences in folic acid intake in women of childbearing age in the United States after folic acid fortification: findings from the National Health and Nutrition Examination Survey, 2001–2002. Am J Clin Nutr. 2007;85(5):1409–1416 [DOI] [PubMed] [Google Scholar]
- 24.Prue CE, Hamner HC, Flores AL. Effects of folic acid awareness on knowledge and consumption for the prevention of birth defects among Hispanic women in several US communities. J Womens Health (Larchmt). 2010;19(4):689–698 [DOI] [PubMed] [Google Scholar]
- 25.Tinker SC, Cogswell ME, Devine O, Berry RJ. Folic acid intake among US women aged 15–44 years, National Health and Nutrition Examination Survey, 2003–2006. Am J Prev Med. 2010;38(5):534–542 [DOI] [PubMed] [Google Scholar]
- 26.Gallup Organization and March of Dimes Foundation. Improving Preconception Health: Women's Knowledge and Use of Folic Acid. White Plains, NY: March of Dimes Foundation; 2008 [Google Scholar]
- 27.Centers for Disease Control and Prevention Trends in folic acid supplement intake among women of reproductive age—California, 2002–2006. MMWR Morb Mortal Wkly Rep. 2007;56(42):1106–1109 [PubMed] [Google Scholar]
- 28.Mandatory food enrichment. Basel, Switzerland: Roche Vitamins Europe; 2003. NutriView special issue 2003 [Google Scholar]
- 29.Chen LT, Rivera MA. The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr Rev. 2004;62(6 pt 2):S40–S43 [DOI] [PubMed] [Google Scholar]
- 30.Hamner HC, Mulinare J, Cogswell ME, et al. Predicted contribution of folic acid fortification of corn masa flour to the usual folic acid intake for the US population: National Health and Nutrition Examination Survey 2001–2004. Am J Clin Nutr. 2009;89(1):305–315 [DOI] [PubMed] [Google Scholar]
- 31.Burton KE, Steele FM, Jefferies L, Pike OA, Dunn ML. Effect of micronutrient fortification on nutritional and other properties of nixtamal tortillas. Cereal Chem. 2008;85(1):70–75 [Google Scholar]
- 32.Dunn ML, Serna-Sandivar SO, Sanchez-Hernandez D, Griffin RW. Commercial evaluation of a continuous micronutrient fortification process for nixtamal tortillas. Cereal Chem. 2008;85(6):746–752 [Google Scholar]
- 33.Dunn ML, Serna-Sandivar SO, Turner EH. Industrial approaches to micronutrient fortification of traditional nixtamal tortillas. Cereal Foods World. 2007;52(5):240–248 [Google Scholar]
- 34.Yang Q, Cogswell ME, Hamner HC, et al. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am J Clin Nutr. 2010;91(1):64–72 [DOI] [PubMed] [Google Scholar]
- 35.European Food Safety Authority Scientific Cooperation Working Group on Analysis of Risks and Benefits of Fortification of Food With Folic Acid Appendix 2: EFSA meeting summary report. Folic acid: an update on scientific developments. : ECSO Report on Analysis of Risks and Benefits of Fortification of Food with Folic Acid. Parma, Italy: European Food Safety Authority; 2009:94–115 Available at: http://www.efsa.europa.eu/en/supporting/doc/3e.pdf. Accessed June 22, 2010 [Google Scholar]
- 36.Johnston RB., Jr Will increasing folic acid in fortified grain products further reduce neural tube defects without causing harm? Consideration of the evidence. Pediatr Res. 2008;63(1):2–8 [DOI] [PubMed] [Google Scholar]
- 37.Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170(18):1622–1631 [DOI] [PubMed] [Google Scholar]
- 38.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170(12):1486–1493 [DOI] [PubMed] [Google Scholar]
- 39.Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part I—folate, vitamin B12, vitamin B6. J Matern Fetal Neonatal Med. 2010;23(12):1323–1343 [DOI] [PubMed] [Google Scholar]
- 40.Mills JL, Von Kohorn I, Conley MR, et al. Low vitamin B-12 concentrations in patients without anemia: the effect of folic acid fortification of grain. Am J Clin Nutr. 2003;77(6):1474–1477 [DOI] [PubMed] [Google Scholar]
- 41.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359 [DOI] [PubMed] [Google Scholar]
- 42.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1325–1329 [DOI] [PubMed] [Google Scholar]
- 43.Jaszewski R, Misra S, Tobi M, et al. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World J Gastroenterol. 2008;14(28):4492–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38 [DOI] [PubMed] [Google Scholar]
- 45. Federal Food, Drug and Cosmetic Act, Definitions and Standards for Food, 21 USC §341.
- 46. Food and drugs. Statement of purpose. 21 CFR §104.20.
- 47.Committee on Use of Dietary Reference Intakes in Nutrition Labeling. Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification. Washington, DC: National Academies Press; 2003 [PubMed] [Google Scholar]
- 48. Food and drugs. Folic acid (folacin). 21 CFR §172.345.