Abstract
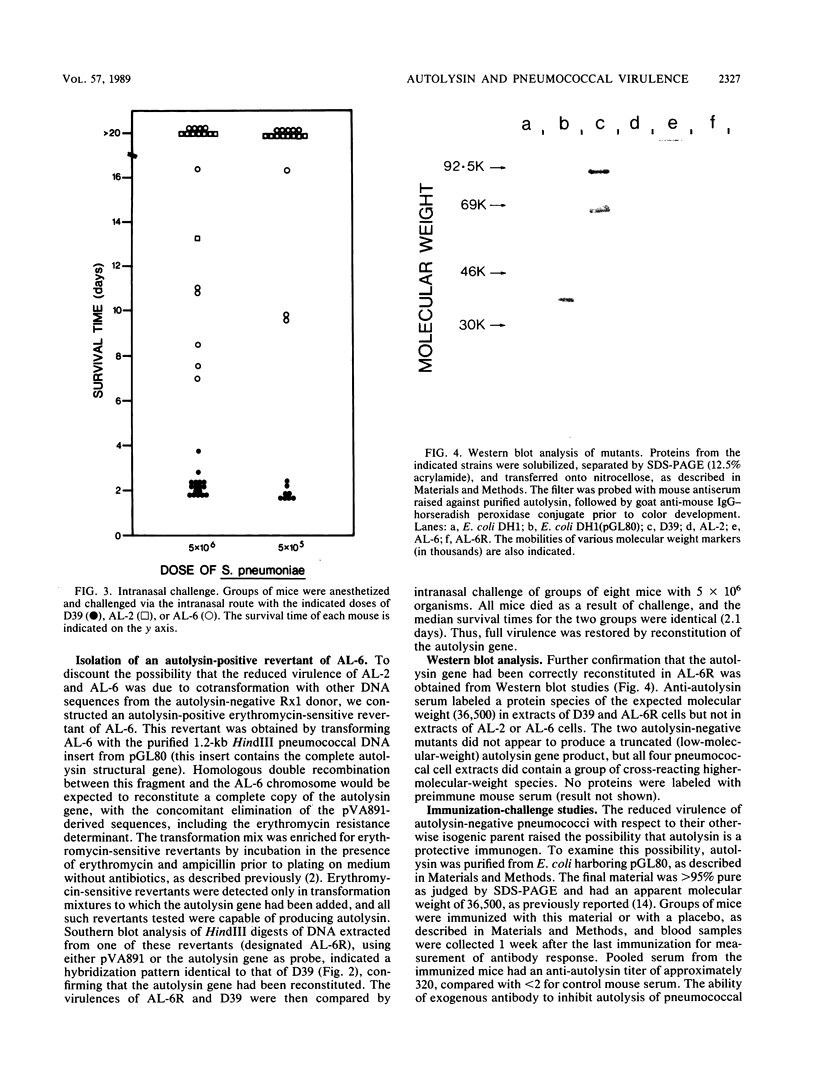
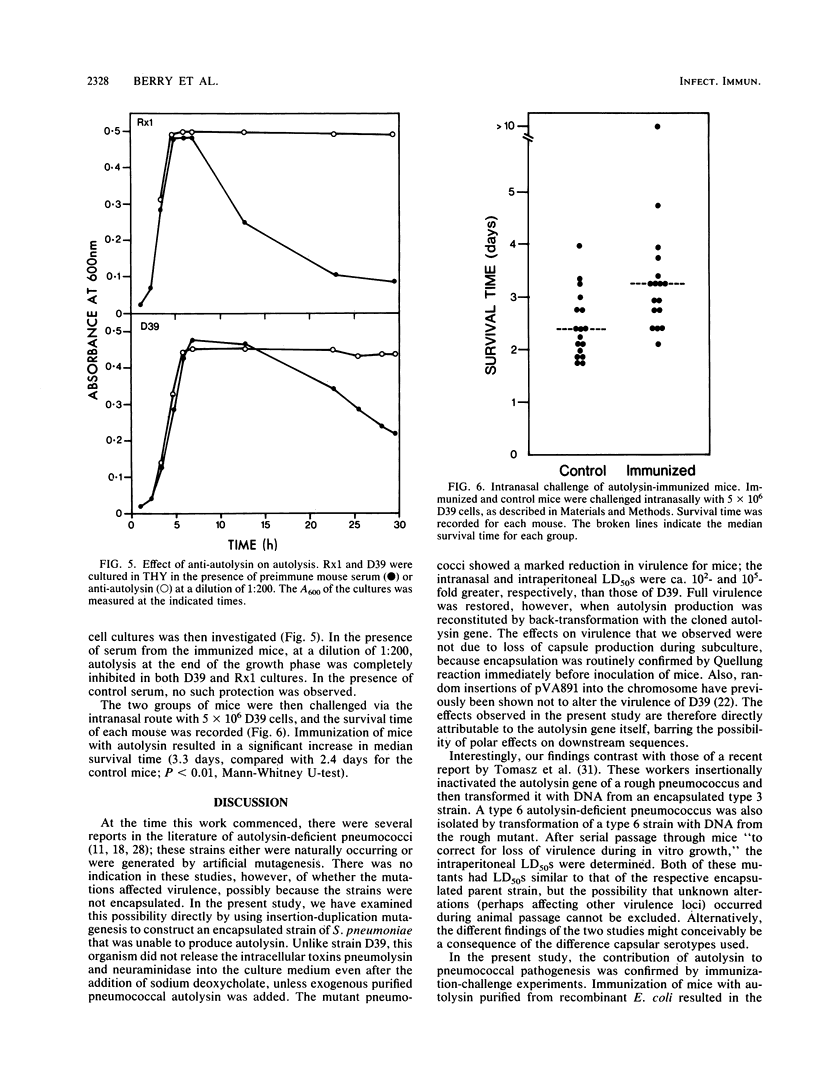
Insertion-duplication mutagenesis was used to construct an autolysin-negative derivative of Streptococcus pneumoniae. This derivative was obtained by first transforming the nonencapsulated strain Rx1 with a derivative of the vector pVA891 carrying a 375-base-pair TaqI DNA fragment from the middle of the autolysin structural gene. DNA was extracted from the resultant erythromycin-resistant, autolysin-negative rough pneumococcus and used to transform S. pneumoniae D39, a virulent type 2 strain. Several erythromycin-resistant transformants were obtained from two independent experiments, and none of these transformants produced autolysin. Southern blot analysis confirmed that the autolysin gene in these transformants had been interrupted by the plasmid-derived sequences. The autolysin-negative mutants showed markedly reduced virulence for mice compared with that of strain D39; intranasal and intraperitoneal 50% lethal doses were increased 10(2)- and 10(5)-fold, respectively. Autolysin production was reinstated in one of the mutants by back-transformation with the cloned autolysin gene, with the concomitant loss of erythromycin resistance; the virulence of this isolate for mice was indistinguishable from that of D39. The importance of autolysin in pathogenesis was confirmed by immunization-challenge studies. Mice immunized with purified autolysin survived significantly longer than did control mice after intranasal challenge with strain D39. This study provides direct evidence that the pneumococcal autolysin contributes to virulence and identifies it as a potential vaccine antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty C., Kreger A. Characterization of pneumococcal purpura-producing principle. Infect Immun. 1980 Jul;29(1):158–164. doi: 10.1128/iai.29.1.158-164.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty C., Kreger A. Role of autolysin in generating the pneumococcal purpura-producing principle. Infect Immun. 1981 Jan;31(1):339–344. doi: 10.1128/iai.31.1.339-344.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Rowan-Kelly B., Paton J. C. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Nov;46(2):585–589. doi: 10.1128/iai.46.2.585-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., García J. L., Ronda C., García P., López R. Cloning and expression of the pneumococcal autolysin gene in Escherichia coli. Mol Gen Genet. 1985;201(2):225–230. doi: 10.1007/BF00425663. [DOI] [PubMed] [Google Scholar]
- García J. L., García E., López R. Overproduction and rapid purification of the amidase of Streptococcus pneumoniae. Arch Microbiol. 1987;149(1):52–56. doi: 10.1007/BF00423136. [DOI] [PubMed] [Google Scholar]
- García J. L., Sánchez-Puelles J. M., García P., López R., Ronda C., García E. Molecular characterization of an autolysin-defective mutant of Streptococcus pneumoniae. Biochem Biophys Res Commun. 1986 Jun 13;137(2):614–619. doi: 10.1016/0006-291x(86)91122-8. [DOI] [PubMed] [Google Scholar]
- García P., García J. L., García E., López R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene. 1986;43(3):265–272. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Purification of the pneumococcal N-acetylmuramyl-L-alanine amidase to biochemical homogeneity. J Biol Chem. 1976 Jul 25;251(14):4199–4207. [PubMed] [Google Scholar]
- Lock R. A., Paton J. C., Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb Pathog. 1988 Dec;5(6):461–467. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- Lock R. A., Paton J. C., Hansman D. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb Pathog. 1988 Jan;4(1):33–43. doi: 10.1016/0882-4010(88)90046-0. [DOI] [PubMed] [Google Scholar]
- López R., Sánchez-Puelles J. M., García E., García J. L., Ronda C., García P. Isolation, characterization and physiological properties of an autolytic-deficient mutant of Streptococcus pneumoniae. Mol Gen Genet. 1986 Aug;204(2):237–242. doi: 10.1007/BF00425504. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Yother J., Vijayakumar M., McGarry L., Guild W. R., Briles D. E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med. 1987 Feb 1;165(2):381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandoskar M., Ferrante A., Bates E. J., Hurst N., Paton J. C. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology. 1986 Dec;59(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Berry A. M., Lock R. A., Hansman D., Manning P. A. Cloning and expression in Escherichia coli of the Streptococcus pneumoniae gene encoding pneumolysin. Infect Immun. 1986 Oct;54(1):50–55. doi: 10.1128/iai.54.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Hansman D. J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983 May;40(2):548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Rowan-Kelly B., Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Mar;43(3):1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Puelles J. M., Ronda C., Garcia J. L., Garcia P., Lopez R., Garcia E. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur J Biochem. 1986 Jul 15;158(2):289–293. doi: 10.1111/j.1432-1033.1986.tb09749.x. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Guild W. R. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol Gen Genet. 1974;128(4):283–290. doi: 10.1007/BF00268516. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Moreillon P., Pozzi G. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J Bacteriol. 1988 Dec;170(12):5931–5934. doi: 10.1128/jb.170.12.5931-5934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Yother J., McDaniel L. S., Briles D. E. Transformation of encapsulated Streptococcus pneumoniae. J Bacteriol. 1986 Dec;168(3):1463–1465. doi: 10.1128/jb.168.3.1463-1465.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]





