Abstract
Methods for translating the findings of controlled trials, such as the Diabetes Prevention Program, into real-world community application have not been clearly defined. A standardized research methodology for making and evaluating such a transition is needed. We introduce the multisite translational community trial (mTCT) as the research analog to the multisite randomized controlled trial. The mTCT is adapted to incorporate the principles and practices of community-based participatory research and the increased relevance and generalizability gained from diverse community settings. The mTCT is a tool designed to bridge the gap between what a clinical trial demonstrates can work in principle and what is needed to make it workable and effective in real-world settings. Its utility could be put to the test, in particular with practice-based research networks such as the Prevention Research Centers.
The generally robust methods of multisite randomized controlled trials (RCTs)1,2 purchase high standards of internal validity and statistical power with the currency of external validity. The very discipline applied to such trials that makes them trustworthy engines for the generation of insights about efficacy seriously compromises their utility for the demonstration of real-world effectiveness.1 What happens in an RCT may, alas, stay in an RCT.2 Most multisite RCTs add diversity of geography, populations, and settings, but they generally insist on standardization of the interventions being tested, which means that they do not allow for adaptation of intervention to the local circumstances and populations.
LIMITATIONS OF RANDOMIZED CONTROLLED TRIALS
The RCT as a construct has a number of important limitations intrinsic to the salient aspects of its methods; what makes it strong also makes it weak, at least when it comes to bridging the gulf from efficacy to effectiveness. The standard requirement for a placebo or nonintervention control arm may limit acceptance and distort sampling. Randomization of individuals may also constrain sampling, affect differential dropout rates, and limit external validity. Blinding obviates the active participation of study subjects, if not their proxies, in the development and implementation of an intervention.3–5
Because advancing the human condition is the ultimate purpose of biomedical research, important clinical trials will increasingly need to emphasize lifestyle and social change in addition to, if not instead of, more conventionally medical interventions such as surgery and pharmacotherapy. This emphasis is needed because a compelling and consistent body of literature indicates that potentially modifiable lifestyle factors (encompassing both the risk behaviors and their social milieu) are the principal determinants of medical destiny, both in the length of life and its quality.6–10 Lifestyle change (with all its behavioral-environmental reciprocal determinism) can do for health what nothing else in all of medicine can do. This finding is particularly relevant for type 2 diabetes, and the enormous public health toll it represents.11–14
Emerging as a major public health problem during the late 20th century in the United States and elsewhere, diabetes impacts the health of an estimated 21 million people in the United States (7% of the entire population), a third of whom are unaware they even have the disease. Over the last several decades, diabetes prevalence has increased 5- to 7-fold. For persons born in the year 2000, the lifetime risk of developing diabetes is approximately 40% among women and 30% among men. Each year, approximately 1.5 million new cases are diagnosed in the United States. Diabetes is a major cause of blindness, kidney failure, cardiovascular disease, reductions in quality of life, and premature death. In addition to causing much human suffering, it imparts a major economic burden, costing an estimated $132 billion in the United States annually.15
The prevention and control of diabetes is thus an urgent public health priority. One response to this urgency has been costly efficacy trials, such as the Diabetes Prevention Program (DPP), which have compellingly demonstrated the preventability of diabetes in high-risk individuals.16 Results achieved in the DPP, however, owe much to the rigorous controls and labor-intensive methods of an RCT, and have to date exerted little influence under real-world conditions.17
RANDOMIZED CONTROLLED TRIALS AND COMMUNITY-BASED PARTICIPATORY RESEARCH
It is substantially in response to such exigencies—the grave toll of preventable chronic disease, the need to cover the distance from efficacy to effectiveness, and the ethical and procedural transgressions of traditional research methods in vulnerable communities—that the principles of community-based participatory research (CBPR) have emerged.18–20 These principles, which require the active collaboration of researchers and the members of the communities in which the research will play out and for whom its ultimate benefits are intended, directly subtend the goal of translation to real-world settings, culturally sensitive tailoring, and sustainability.
CBPR is a subcategory of community-based research in which communities are not only the focus of the research but also active participants in its design and implementation. CBPR in public health is defined as:
a collaborative approach to research that equitably involves community members, organizational representatives, and researchers in all aspects of the [applied] research process; including identifying the health focus of the research, disseminating results, and devising methods of sustainability.21(p174)
The partners contribute unique strengths and shared responsibility and have an equal and participatory role.22 This is especially important in populations that have historically been disenfranchised or oppressed.23 In CBPR, along with specific measures of health promotion, social action for change is a desired research goal.24 This method has been the foundation of many important projects that aim to reduce health disparities, such as Racial and Ethnic Approaches to Community Health (REACH 2010), a national demonstration program supported by the US Department of Health and Human Services.25 The success of CBPR projects depends on collaboration between the research team and community stakeholders in the planning, design, and execution of the project. A major challenge for CBPR researchers is to balance the needs of the community with the demands of scientific research without making unacceptable sacrifices on either end.26 The necessary tradeoffs between the rigor of the RCT and the participation in, and potential biasing of, community residents to the selection and development of interventions involves some sacrifice of internal validity for the net gains in relevance, utility, applicability, feasibility, and other aspects of translation and external validity that have limited the implementation of knowledge from past research.27,28
Examples of the effective blending of CBPR and RCT methods certainly exist.29–33 Such exceptions, however, highlight the far more commonly prevailing rule that these are generally separate enterprises. To our knowledge, no construct has been specifically and explicitly developed to hybridize the approach of CBPR to the key design elements of the multisite RCT for the express purpose of taking the insights from RCT efficacy trials to the final stage of real-world testing and general use in diverse community settings. The closest counterpart is found in “empowerment evaluation,” whereby communities of intended end-users of the knowledge are actively engaged in the evaluation of programs,34 but such evaluation generally starts with an existing, evolved community program rather than importing an efficacy-tested intervention to be tested in the community.
The lack of such methodology comes at high cost, as illustrated by the failure to disseminate the success of the $174 million DPP.16 Despite a clearly articulated program of lifestyle change shown to reduce the incidence of type 2 diabetes in high-risk adults by a stunning 58%, the DPP—fundamentally a conventional, multisite RCT—has largely failed to translate to widely applied interventions in real-world settings.35 In other words, efficacy has not traversed the chasm of community application to effectiveness. This liability pertains especially to trials that address, as the DPP did, disease prevention and health promotion through the medium of lifestyle change, in which no easy prescription or stand-alone clinical intervention exists, and community supports to achieve the desired ends are essential. Achieving the salutary effects of lifestyle change requires the mobilization of communities and the articulation of multilevel and integrated services.
The DPP serves as just one particularly good illustration—there are many others—of the potency of the RCT to clearly elucidate the influence of lifestyle factors on health outcomes and its relative impotency to reliably convert such insights into population benefit. This gap grows ever more disquieting as chronic disease burdens steadily rise36 and the potential capacity for lifestyle change to arrest this trend receives ever-increasing recognition.6–8,37
One infrastructure ideally suited to serve as a proving ground for research methodology explicitly designed to meld CBPR and multisite RCT features is the Prevention Research Center (PRC) Program of the Centers for Disease Control and Prevention (CDC). The program now comprises 32 comprehensive and 5 developmental centers nationwide.38,39 Situated in widely diverse communities, ranging from inner cities to rural Appalachia and from adolescents to the elderly, the PRCs represent academic–community partnerships dedicated to CBPR. The PRCs have attributes perfectly aligned with the goals of translational research: a commitment to disease prevention and health promotion, methodologically robust research, and the practice of CBPR; strong academic–community relationships; and a policy of functioning in networks.
TRANSLATING RESEARCH THROUGH COMMUNITY PORTALS
As indicated by the fate of the DPP, RCTs often fail to apply what is needed for routine and widespread benefit. This is especially true for lifestyle change, over which clinicians exercise far less control than they do over drugs and procedures. The challenges involved in translating advances in our understanding of lifestyle effects into generalized, real-world benefit have been described elsewhere in some detail.38,40–49 Because lifestyle, with its essential family and community context, is more complex and less easily prescribed than is a discrete clinical treatment such as a drug, lifestyle interventions require the understanding and commitment of patient, household, and community. The “power center,” as it were, is the patient, not the clinician, and the community settings are homes, workplaces, schools, churches, and recreational areas rather than clinical settings. We identify these settings as community portals to account for their role as channels—ideally bidirectional—of communication and influence, rather than places to assess needs and outcomes, as in the usual usage of “settings” in public health.50
With the challenge of converting what we know about the power of lifestyle change to routine action and the promise of such interventions as the DPP lifestyle arm tantalizingly just out of reach, neither RCT methodology nor CBPR is perfectly suited to do the job of translation alone.51,52 A melding of the two is warranted. In particular, to demonstrate the effectiveness of an intervention in diverse communities, a method is needed that honors the approach of CBPR so that community action is customized, while also honoring key methodological features of the multisite RCT to achieve suitable standardization across sites. The case has been made—and elegantly demonstrated on multiple occasions—that robust research methods and participatory principles can be combined.53 But a standard method and organizational roles have not been defined for trials that engage multiple, diverse communities in the pursuit of common and uniformly measured outcomes.
THE ADDED VALUE OF THE MULTISITE TRANSLATIONAL COMMUNITY TRIAL
The multisite translational community trial (mTCT) that we introduce offers that RCT–CBPR meld with acknowledgment of some necessary tradeoffs between the purist ideals of each. The mTCT is analogous to the multisite RCT in crucial ways; it borrows from the RCT's methodological rigor but, in accordance with the principles of CBPR, is modified for the job of effectiveness testing with appropriately adapted interventions. The principles of CBPR might be considered tools of the translational trade and RCT methods as tools for generating the highest quality research evidence. To date, however, no tool kit exists that accommodates and adapts these tools for combined transport to the ultimate worksite: real-world populations. The mTCT is an effort to provide that kit: a methodological construct carrying the complementary strengths of the RCT (particularly cluster-randomized trial methods) and CBPR. Examples of that marriage exist, but, to date, no standard and replicable method for achieving it does. In particular, the best examples of RCT–CBPR unions have been site-specific with a given community exerting considerable influence on study methods and measures and, sometimes, on the adaptation of the intervention. A single trial intended to demonstrate translation from efficacy to effectiveness in diverse communities all at once offers potentially far greater efficiency and economy than a community-by-community, trial-by-trial approach; however, it requires that community customization be balanced against crucial uniformities across sites. Others have suggested the need for just such an approach, indicating that essential functions might need to be standardized across sites while form in different contexts could vary.54
We introduce the mTCT model in response to that need. The model is not intended to dilute or replace CBPR where its full expression is currently suitable, any more than it is intended to replace the multisite RCT. Rather, the mTCT is a hybrid method intended for a very specific use: concurrent evaluation of translation from efficacy into effectiveness in diverse communities in a single trial.
There is, however, a precautionary note to sound about an unintended but nonetheless plausible consequence of a CBPR-based methodology that is not purely CBPR: namely, a dilution of the important principles of community-based and fully participatory research. The mTCT approach seeks to accommodate both RCT and CBPR demands, but may force some tradeoffs between them. Research funding–driven requirements for problem selection and for RCT design preferences may limit the degrees of local freedom. The RCT design tradeoffs are no different than those inevitably faced in community studies requiring cluster sampling and the cooperation and effort of local practitioners and other local policymakers or program planners.55 As for the CBPR tradeoffs, we acknowledge the preference for a place-based model in which those most affected by the problems to be addressed set the priorities for the health, risk-factor, or social determinants to be treated as dependent variables. When those priorities can be matched with available funding and with available evidence of efficacy-tested interventions, there is no compromise of that most cherished CBPR principle of local determination.
Federal and foundation funding, however, will often drive priorities for research and field testing toward problems that are widely felt in other communities. Similarly, the efficacy-tested interventions that federal funding wants to see field tested may not be what a given community sees as the form of the intervention it believes will work in its place, population, and circumstances. The local adaptation of efficacy-tested interventions remains contested territory between the advocates of “fidelity” and those who believe that efficacy-tested interventions will be improved with local adaptation.56 Resolving this set of tradeoffs awaits a more extensive review of participatory research in general,57 and a continuing development of participatory research and practice-based evidence funding as a complement to research driven by the linear developmental needs of science.58,59
That said, we offer the mTCT as a model to expand the purview and accelerate the contributions of CBPR, not dilute its principles. The role of this model is illustrated by its potential application to DPP translational research, using a network of PRCs and their communities as collaborators and sites, analogous to practice-based research networks in primary care.
DESCRIPTION OF THE PROPOSED MULTISITE TRANSLATIONAL COMMUNITY TRIAL
Whereas RCT study design and CBPR have proven valuable tools individually, effective uptake of relevant research findings at the community level warrants a methodology that combines the 2 approaches.60 RCTs have the benefit of strict oversight and rigorous controls, but real-world application of treatments emerging from such trials is hindered by the imposition of these very controls.61,62 In CBPR, treatments must be translated to fit into the established community environment, using the community infrastructure so that they can be understood, relayed, practiced, and retained.63 The mTCT applies CBPR principles to the traditional multisite RCT model. A comparison of the salient features of the RCT and mTCT is provided in Table 1.
TABLE 1.
Comparison of Salient Features of the Multisite Randomized Controlled Trial (RCT) and the Multisite Translational Community Trial (mTCT)
| Feature | Multisite RCT | mTCT |
| General | A standardized intervention with identical content, practice, and measures across all sites; designed to establish efficacy | A standardized intervention across multiple and diverse community sites, but with considerable variability among sites in delivery methods; designed to establish real-world effectiveness |
| Random assignment | Required | Cluster randomization preferred, not required |
| Control group | Required | Required, but may be simulated through multiple baseline measures or interrupted time series analyses in one population |
| Blinding | Required | Preferred, not required; may not be feasible |
| Standardized outcome measures | Required | Required |
| Standardized evaluation or analytical methods | Required | Required |
| Design and oversight responsibilities | Research methodologists | Research methodologists and community leaders |
Note. The mTCT blends elements from multisite randomized controlled clinical trials with principles of community-based participatory research. The result is a methodology that is fixed across sites with regard to core intervention components, outcome measures, and methods of evaluation, but free to vary widely and in accord with local priorities with regard to the best means of administering the intervention to the community in question. Primary responsibility for evidence-based intervention and practice components would reside with the researchers on the study team trained in methodology; primary responsibility for locally tailored delivery methods would reside with the community partners, with the expectation that final decisions in both cases would represent the consensus of both partners. Thus, the operational foundation for the construct is a partnership in which the labor of protocol development is divided in accord with relevant expertise.
Overview of the Model
The mTCT is based partly on the Social Ecological Model that has been used to explain systems change in health promotion programming64–67; it is a direct response to prior arguments made by Hawe et al. that in “complex interventions,” essential functions should be uniform across sites whereas forms should vary by context.54,68 It illustrates the interactions between the individual and the various systems that influence behavior and the potential in such interactions for individuals and groups to influence environments and, reciprocally, for the environments to influence behavior changes in diverse settings.69–72 The mTCT approach aims to achieve communitywide change in health status by using a culturally tailored health message, community portals, and trusted community health administrators.73 It is a replicable means to test the function of promising and usually complex interventions across diverse community settings, while preserving core intervention elements and allowing for local tailoring of the form of those interventions.54
Design Features of the Model
As shown in Table 1, the mTCT is a multisite research design with both fixed and variable components. As with the RCT, the mTCT requires at all sites (1) standard intervention components, (2) outcome measures, (3) data capture methods, and (4) evaluation. The means of delivering the intervention and reaching the population of interest, however, should be specific to a given community and its form (e.g., language, illustrations, tone) devised locally to suit it. This variability is quite distinct from RCT methods, and essential to the principles of CBPR.
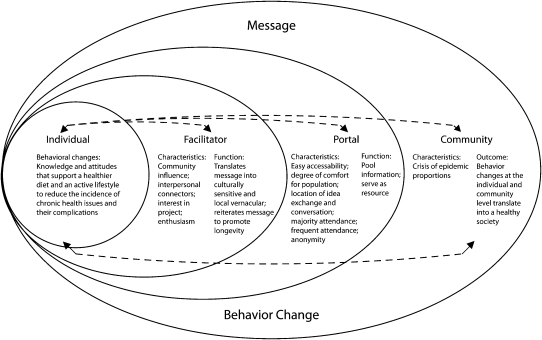
The mTCT map (Figure 1) is a schematic demonstrating the key components of a multisite translational community trial. It illustrates the flow of information and influence between individuals and other entities in the community. As indicated in Figure 1, a culturally sensitive health message is passed downstream from the community (those partners who have participated in its development) through portals, facilitators, and, ultimately, to individual community members. Message propagation, employment, contextualization, and retention are the basic functions of the system, although each level of the system has a distinct role in the operation. Certain characteristics must be present at each level to ensure that the message is transmitted and retained with an adequate degree of fidelity to the function (not the form) of the intervention, as previously tested in one or more efficacy trials.
FIGURE 1.
Schematic model of the multisite translational community trial.
Note. The community serves as the beginning and endpoint of a feedback loop targeted to the individual who will receive the health message, or intervention. The message is delivered through portals and delivered by facilitators in a culturally relevant way. The behavior changes feed back to the community, resulting in improved health measures at the community level.
Community Portals
Whereas a setting is circumscribed so that effects of an intervention are measured there, portals are means to a greater end, a way of reaching the community at large. Interventions passing through a portal should have effects measured in the general population of interest. If, for example, a wellness program is designed for a school setting, the intervention would be delivered in schools and the relevant outcomes measured in schools, with the goal of influencing the health of schoolchildren. If used as a portal, schools would be a means of delivering the wellness programming to families via their children; although the intervention might largely be confined to schools, outcomes would be measured in the community at large to determine the utility of schools as a conduit for the delivery of health promotion interventions to the targeted population.
Potential community portals should offer the following: reliable access to a significant portion of the general population, fixed infrastructure in the form of both facilities and personnel, an authority structure so that ownership of any health promotion enterprise may be clearly defined, and, ideally, the capacity to institutionalize the tested health promotion programming to ensure its sustainability and to generalize its experience to similar (preferably typical, not unique) institutions in other communities.
The community portal is a critical component of the model. Like any doorway, a community portal represents a means of gaining reliable access to what lies across the threshold as well as transporting materials across—ideally, in both directions. The term “community portal” is used to describe an element in community structure that provides both consistent access to a defined population and definable infrastructure and resources amenable to institutionalizing and sustaining interventions. The concept of the community portal is a natural outgrowth of McQueen's “settings”74–81 that takes into account additional considerations related to CBPR methods, as characterized in Figure 1. Although the concept is specifically developed here as an alternative to a “setting” as a means of reaching a community with an intervention or technology, the portal concept has other potential implications, such as the flow of ideas and insights from either partner to the other. A portal is potentially any community setting that provides for reliable interaction with a population of significant size, and thus some degree of access to the community population at large; examples include—but are by no means limited to—schools, churches, health centers, supermarkets, libraries, pharmacies, hair or beauty salons, and worksites.
Whereas settings are the places where interventions are delivered to specific groups (e.g., students in schools, patients in hospitals), portals are entryways through which researchers access a population representing the community at large. An intervention delivered through a portal is expected to reach individuals beyond the portal, and usually in various settings. For example, a school may be used as a portal, with the expectation that the intervention will reach not only students but also parents and other family members who do not directly receive the intervention.82 Portals have clearly defined leadership structures so that ownership, responsibility, and accountability needs can be met. The portal is not only a gateway providing access but also an entryway into a setting for which rules, roles, and resources are clearly defined and readily available.
The community portal concept does not intend to supplant the primacy of community in CBPR. Rather, this approach maps diverse routes to key members of a community, thereby suggesting means by which intervention components can be delivered to community members, and means by which specific populations within a community may influence intervention components and their delivery. When enough such routes are used, the entire community can be accessed and engaged. However, by establishing access via key portals, each intervention along the way is supported by stable infrastructure, a defined governance structure, and the opportunity to “curricularize” elements that should be sustained. An ideal portal offers ease, reliability, consistency, and universality of access—generally the result of dedicated infrastructure (e.g., buildings, staff), a dedicated constituency or population, and a program, curriculum, or mission that encompasses, or at least does not preclude, a focus on health.
In health promotion research, the “materials” to be brought across these portals include a relevant health message to predispose people to see the value to them of the actions recommended, resources or training to enable them to act, and environmental arrangements to enable and reinforce their actions. Education in the lifestyle arm of the DPP is one example; others are enabling resources or policies and reinforcing messages or rewards. In the mTCT model, the message is passed from medical experts and researchers to facilitators, who translate the message into a culturally and contextually sensitive one targeted to individuals within the community. In this way, the mTCT promotes individual uptake, employment, and propagation of the beneficial messages and resources or policies to effect change at the community level. The dissemination of messages, resources, policies, and reinforcements involves the stages of innovation diffusion, including adoption, implementation, and sustainability.83 Although the message itself is important, it is essential that the message be packaged appropriately (i.e., in a culturally relevant manner and with the contextually necessary supporting resources, policies, and reinforcements) to ensure progression through these 3 stages, promote better understanding, and encourage the adoption of healthful behaviors and environmental changes.
Intermediaries to Translate or Adapt Interventions
An example of a facilitator in the mTCT model is the community health advisor (CHA). CHAs (or promotoras in Hispanic communities) have participated in a variety of activities to reduce the impact of diabetes in underserved and minority communities, including individual counseling, group support, outreach, walking clubs, diabetes management classes, and education on depression.84,85 The CHA model is based in part on the empowerment advocacy of the 1950s and social network theory.86 As the name suggests, empowerment advocacy is a social movement calling for equitable distribution of authority and decision-making, particularly with those most likely to be influenced by the decisions in question. Social network theory emphasizes that the relationships among people exert influences beyond the specific roles played by individuals and, like diffusion theory, the homophily (likeness) of workers inserted into such networks is seen to be a determinant of their influence.41 CHAs facilitate discussion among people in the community about health problems, thereby empowering them to identify the root causes of those problems and find solutions.86
CHAs live and work in the targeted communities, often sharing the same dietary and physical activity lifestyles and health risk factors as their constituents.87 These lay health workers are instrumental in developing and managing relationships between individuals and sustaining the level of individual and group engagement. A growing body of evidence supports the effectiveness of CHAs and their malleable role in culturally and situationally tailoring and delivering health messages.84–86,88 CHAs are uniquely qualified intermediaries who share distinguished bonds with population members and possess the ability to recognize cultural health practices and communicate in the language of the people.84 They represent individuals native to their neighborhood, who share similar socioeconomic, ethnic, and cultural characteristics and are embedded in the social networks of the community.86
Application of the Model to Diabetes Prevention
The landmark DPP study, a multisite RCT, was a large-scale research initiative involving 27 clinical centers around the United States.16 More than 3000 individuals participated in the study, all of whom were classified as “prediabetic.”89,90 Participants were randomly assigned to 1 of 3 groups: metformin (Glucophage), an intensive lifestyle intervention, or placebo. At the conclusion of the trial, a 58% reduction in the rate of progression to diabetes was observed among individuals in the intensive lifestyle intervention group, compared with 31% in the metformin assignment.16 Overall, 5% of individuals in the lifestyle intervention group developed diabetes, compared with 7.8% and 11% in the metformin and placebo groups, respectively.16,91
Given the prevalence of obesity and diabetes in the United States and their disproportionate toll in African American and Hispanic communities, the DPP represents an ideal intervention with which to test this new model. Potential barriers, such as poor diffusion of knowledge, behavioral impediments, health care organizations’ focus on acute and episodic care rather than lifestyle, lack of reimbursement for prevention efforts, and ineffective public policies, hinder the translation of diabetes clinical research to practice. Successful translational efforts require a multifactorial approach based on an ecological perspective. Even under the most optimistic scenarios for health care reform, clinical settings will not be able to deliver all that the lifestyle interventions in the DPP would demand. The mTCT takes into account the specific barriers to translational research in each setting or population in which interventions must be identified and tailored with input from recipients.92 Effective prevention and self-care programs cannot be based solely on medical solutions, but must include “social support, outreach, consistent follow-up, preventive care, community and family education and community mobilization.”93 The mTCT meets these requirements and can be applied to translate clinical trial–tested methodologies for effective diabetes prevention in real-world clinical and community settings. Although previous work suggests the potential to apply CBPR principles to translate an intervention such as the DPP into one community at a time, the mTCT indicates how this effort might be made substantially more efficient and economical, consolidating the related efforts of many communities into a single initiative.
Role of Prevention Research Centers
The PRCs39 constitute an established network available to test the mTCT model in diverse community settings simultaneously and then to use it for the full range of translational research needed in public health.38 Congressionally mandated, originally in 1986, the PRC program now comprises 32 comprehensive and 5 developmental centers distributed throughout the United States.39 Located in widely diverse communities, ranging from inner cities to rural Appalachia, from adolescents to the elderly, the PRCs all represent academic–community partnerships dedicated to CBPR. The centers are committed to disease prevention, health promotion, and methodologically robust research. In allocating funding, the CDC judges and peer reviews each center for the strength of its academic–community relationships.
PRCs have the ability to tap into their local infrastructure, identifying portals through which a health message can be delivered in a manner most acceptable to the community in question. PRCs are particularly well positioned for this task. Developing a strategy for promoting community health requires understanding the places where people live, work, and play, and taking advantage of community assets.94 In this sense, portals must be selected on the basis of accessibility and frequency of interaction with the population.
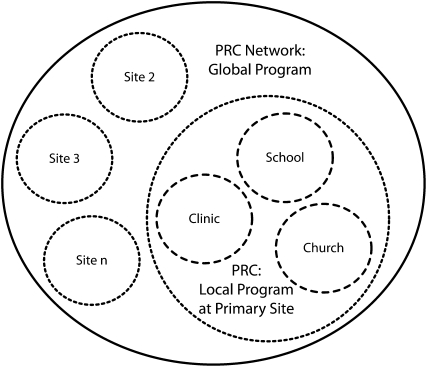
The application of mTCT to diabetes prevention by a group of PRCs in diverse communities (Figure 2) is predicated on a time-honored adage often invoked in public health practice: “Think globally, act locally.” For purposes of illustration, the Yale University Prevention Research Center is used as the hypothetical primary site. Local action at the primary PRC involves 3 community portals—churches, clinics, and schools.17,95–97 The combination of these 3 intervention portals locally is intended to demonstrate their synergistic effects on alleviating the burden of diabetes in underserved and minority neighborhoods. Within the local program or focal site of the primary PRC, community members (church leaders, educators, community volunteers, etc.) are identified and trained as CHAs.
FIGURE 2.
Multisite translational community trial construct and the Prevention Research Center network.
Note. n = up to any number of sites; PRC = Prevention Research Center Program of the Centers for Disease Control and Prevention (CDC). The circles with dashed lines represent discrete diabetes–obesity prevention and control interventions at a specific primary site PRC. The circles with dotted lines represent comparable interventions at the primary site as well other sites, all tailored to local communities. The large black circle denotes the collective, global effort of a group of PRCs to establish a shared methodology for translating Diabetes Prevention Program components into sustainable community programming and to engage in rigorous, shared evaluation.
The local intervention at the primary site would occur in tandem with comparable interventions at other sites. Each site would capture and evaluate the same primary outcome measures, such as body mass index, diet quality, physical activity level, and incident diabetes. Each site would also deliver the same lifestyle education program based on the DPP; however, the means of doing so would vary with community characteristics. The national network of PRCs encompasses communities as diverse as predominantly African American or Hispanic neighborhoods in inner cities, rural Appalachia, and large Native American tribal reservations. Of note, the support of PRCs for such an effort is not entirely hypothetical. Such an effort has been proposed, and it garnered support from the PRC Program Office at the CDC and the enrollment of more than a dozen PRCs representing such diverse communities (Yale University PRC, unpublished data). Relevant portals, suitable CHAs, and acceptable message framing would naturally vary across this expanse. The mTCT construct would allow for this variability while preserving consistency in the functional integrity of the intervention and evaluation across sites.
In this way, multiple and varied sites would identify community portals appropriate for the infrastructure of their targeted population, translate the message of diabetes prevention in a culturally sensitive manner, and identify community members (clinicians, educators, community health advisors, pastors, tribal leaders, etc.) who can best convey the health message to at-risk community members. The multiplicity and variability of sites also provide a test of the capability of the intervention to be taken to scale in a national program.
DISCUSSION
Widespread consensus exists among experts in disease prevention and health promotion that application of the knowledge we already possess could reduce heart disease rates in the United States by 80%, diabetes by 90%, and cancer by more than 30%.10 The DPP provides explicit evidence that lifestyle change can prevent diabetes in high-risk adults almost twice as effectively as state-of-the-art pharmacotherapy. Unlike strictly clinical interventions, however, lifestyle change requires environmental supports in real-world settings and a socioecological perspective. Clinical trial results related to lifestyle interventions do not necessarily translate readily to application in community populations. This translational impasse has encumbered the promise of the DPP and is germane to the potential of lifestyle interventions for chronic disease prevention and control in general. A means to overcome this translational impasse is an urgent priority.
CBPR may be the ideal solution for translating a public health advance into a single community, but the community tailoring required for CBPR to honor fully its principles largely precludes the uniformity across sites expected in a multisite RCT. The fixed elements of a multisite RCT, in turn, largely preclude site-by-site customization. Tensions in efforts to reconcile CBPR and RCT methods are inevitable although not insurmountable.27 These tensions have been effectively overcome in single-site interventions, but site-by-site customization is a slow, costly, inefficient means of achieving external validity and advancing urgent public health goals related to lifestyle and chronic disease prevention in the population at large. The mTCT does raise a concern about a potential dilution of the core principle of CBPR requiring the full participation of community partners in all aspects of research, including the identification of the research priority area, intervention methods, and measures. The mTCT model, which calls for standardization of certain methods and measures across sites, admittedly limits participation or control in at least one aspect of the research—the essential functional characteristics of the intervention to be tested. However, the mTCT is not intended to replace CBPR where it works. Rather, it is intended to introduce opportunities for the application of CBPR approaches into multisite trials that previously excluded them. Used as intended, the mTCT should expand the purview of CBPR, not dilute its principles.
Hawe et al. have suggested the need for a model that stipulates what is fixed, and what may vary, across sites when complex interventions are being conducted.54,68 We have introduced a research model directly responsive to that suggestion. The mTCT is intended to preserve and apply salient principles of CBPR to customizing intervention delivery (form) while imposing sufficient site-by-site standardization of essential functions (e.g., key intervention components, outcome assessments) so that a single intervention trial may efficiently explore translation into diverse communities.
Where CBPR as currently established would work without assuming any generalizability of effects to other communities, there is no need for the mTCT. It is not intended to replace or dilute community-specific CBPR. Similarly, if a standard multisite RCT would work, there is no need for the mTCT methodology. The model is offered specifically and exclusively for those situations in which the next logical step is to test translation of a clinical advance into diverse communities—for which neither CBPR nor RCT methodology is quite right. CBPR invokes a degree of community customization that precludes conducting the same trial at multiple, diverse sites, whereas a conventional RCT precludes the necessary community customization required to make the intervention real-world relevant and sustainable. The mTCT splits the difference—allowing for considerable community customization and respecting the shared ownership of academic and community partners while imposing enough restrictions to allow for testing of the same intervention across diverse sites. Hawe et al. suggested such an approach54,68; we define it as a trial methodology in its own right, and cite its specific utility.
The importance of community-based research is increasingly evident. Previous health interventions focusing solely on individual risk factors have failed to produce widespread change in all population segments leading to movement toward interventions aimed at health determinants beyond the individual.98 The sociocultural environment has been recognized as a key feature of community interventions.98 Whitelaw et al. suggest that although the core health problem lies within the individual the solution is encompassed within the characteristics of the larger system, which is controllable, independent, and capable of influencing the behaviors of community members.99 The mTCT design recognizes the importance of the sociocultural environment, and uses its infrastructure as a conduit both to learn about, and to deliver interventions to, individuals, families, and groups in a community. Because relevant systems changes are part of the form that may vary by site, the mTCT defines systems change as a process (or, as expressed by Hawe et al., “context evaluation”)68 while defining the specific health changes of interest in individuals as an outcome. By examining characteristics of the physical, social, cultural, and economic environment, researchers can determine optimal means of locally tailored intervention delivery. Strategically selecting portals through which to deliver a program should greatly increase overall effectiveness.
The Social Ecological Model, which provides an overarching set of theoretical principles for understanding the interrelations among diverse personal and environmental factors in health and illness, supports a focus on communities.65–67,100 A general consensus exists among experts in public health practice that the amelioration of the epidemiology of lifestyle-related chronic diseases will “take a village.”101 In other words, changing the complex interplay of biopsychosocial inputs that influence chronic disease will require altering many features, programs, policies, and resources in the typical community. The Stanford Five-City Project is one example of successful community-focused research. The project investigated whether community-wide health education could reduce stroke and coronary heart disease. After 30 to 64 months of education, significant net reductions in community averages favoring treatment occurred in plasma cholesterol levels (2%) and blood pressure (4%) and resulted in important decreases in composite total mortality risk scores and coronary heart disease risk scores.102 Because of efforts like the Stanford Five-City Project, community-wide, multidisciplinary interventions have a strong advocacy.103
Developing relationships is one of the formidable challenges of CBPR. As noted, the success of CBPR projects depends on the collaboration between the research team and community stakeholders at every stage in the planning, design, and execution of the project. The mTCT requires that researchers work across disciplines and levels of political and social organization to respect the principles of CBPR while preserving key elements of methodology across potentially diverse community sites. Developing reciprocal, trusting, and equitable relationships with communities is a strategy that should enhance the effectiveness of interventions delivered within those communities. The PRC program is used in the illustration of this model because it offers a nationwide infrastructure of research centers committed to CBPR with well-established community partnerships. We acknowledge that such relationships are found only in select communities.
The mTCT model provides a standardized construct for blending the best methods of multisite RCTs with the important principles of CBPR. The model can be tested by applying the same intervention—diabetes prevention based on the lifestyle arm of the DPP, for example—across very heterogeneous sites, as offered by a representative group of PRCs with shared commitment to diabetes prevention and control. All sites would commit to the same core intervention elements and the same outcome measures as a basis for evaluation, but sites could deliver the program by diverse means, through diverse portals. A significant plurality of PRCs has indicated its readiness, willingness, and ability to participate in just such a trial (PRC Directors, Yale University communication, 2007-2008). Once applied successfully to one intervention, the method could be adapted to many others.
Limitations
The mTCT construct has important limitations the most obvious of which is that the concept we have introduced has not yet benefited from attempted use in diverse hands. Should this occur, as hoped, it will doubtless result in an incremental accumulation of modifications, variations, and improvements in the model. In addition, as we have initially presented, the portal concept places an emphasis on the multiple means of reaching community members with interventions; the potential use of portals as conduits for information flow from community members to researchers is not precluded but has not been clearly elaborated. Certainly, only some communities will be receptive to the mTCT construct, and only a subsample of these will enjoy the well-established academic–community relationships conducive to this work. Communities that are home to PRCs are likely candidates for proving the utility of the mTCT construct, but generalizability to other communities is less certain. The many requirements for conducting robust trials and for fidelity to all of the principles of CBPR represent potential barriers to the implementation of the mTCT in any given site that is not well prepared.
Finally, we concede that our specific focus here is on the translation of very promising clinical trials to real-world application in which well-defined intervention elements and outcome measures would be established with a high degree of statistical certainty. In this context, the role of CBPR is somewhat limited to the determination of how best to deliver such interventions and capture such outcomes in diverse communities. A previously tested intervention might be what some communities seek, rather than inventing homegrown interventions from scratch. Contextual factors with implications for preferred intervention methods are also likely to vary greatly across communities104; although the mTCT requires some concessions in this area, the broader implications for CBPR are not to be ignored.
Implications
Systems-based change can be an important element of sustainable health promotion at the community level.105 Because the focus is on translating clinical or other controlled trial results—such as those seen in the DPP—into community health benefit, the emphasis has been placed on measures of health status in individuals as the outcomes of interest. This focus does not ignore the importance of systemic changes but rather views them as facilitators of desired health outcomes. The distinction is important in the context of this article because the “means to the ends” are the very thing the mTCT design would allow to vary as warranted across sites, whereas the essential elements of RCT-tested interventions and their established health outcomes in individuals would be fixed.
The mTCT should have utility in translating seminal clinical trial results into community context, but it is certainly not a one-size-fits-all solution to the challenges of community health promotion research. The mTCT concept addresses one particular aspect of CBPR—namely, its application to methodologically robust trial designs that seek to establish a degree of consistency or generalizability of core functions of an intervention across sites. By its very nature CBPR is in potential conflict with the uniformity across sites expected in multisite RCTs as it would focus on methods custom-developed by each community and thus introduce considerable site-by-site variability. This variability is certainly not a bad thing, nor even a limitation of CBPR, but it would tend to preclude a multisite collaboration in which essential intervention elements are standardized. The mTCT is a “middle path” method that allows for considerable site-by-site autonomy, discretion, and scope in varying the form of interventions so that CBPR principles are honored while stipulating in advance those aspects of the study and the intervention not subject to site-by-site customizing so that requisite homogeneity and functionality are established. These characteristics, which should constitute strength for specific applications, could produce weaknesses for others.
The ultimate goal of the mTCT is to add to current research methods a standard construct to facilitate translating what can be learned from well-controlled efficacy trials into what actually works in diverse, real-world communities. The hybrid vigor of the mTCT concept has been achieved episodically to date, but the key elements of a replicable construct have not previously been defined. The utility of the construct should be judged by putting it to the test and by the degree to which it fulfills its promise to advance the cause of real-world health promotion and disease prevention.
Acknowledgments
Funding for this article was provided by Centers for Disease Control and Prevention (CDC; grant 5U48DP000053-05).
Thanks to Eduardo Simoes, MD, MSc, MPH, director of the Prevention Research Center Program office at the CDC, for discussions of the model as it evolved, and to Michelle Pinto-Evans for technical support.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention.
Human Participant Protection
Protocol approval was not required because no human study participants were involved.
References
- 1.Rosenberger WF. Randomization in Clinical Trials. London, England: Henry Stewart Talks; 2007 [Google Scholar]
- 2.Bauman KE. Research Methods for Community Health and Welfare: An Introduction. New York, NY: Oxford University Press; 1980 [Google Scholar]
- 3.Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv. 2008;1(3):211–217 [DOI] [PubMed] [Google Scholar]
- 4.Schensul JJ, Trickett E. Introduction to multi-level community based culturally situated interventions. Am J Community Psychol. 2009;43(3–4):232–240 [DOI] [PubMed] [Google Scholar]
- 5.Rapkin B, Trickett E. Comprehensive dynamic trial designs for behavioral prevention research with communities: overcoming inadequacies of the randomized controlled trial paradigm. Trickett E, Pequegnat W, Community Interventions and AIDS. New York, NY: Oxford University Press; 2005:249–277 [Google Scholar]
- 6.McGinnis J, Foege W. Actual causes of death in the United States. JAMA. 1993;270(18):2207–2212 [PubMed] [Google Scholar]
- 7.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245 [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009;169(15):1355–1362 [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: The Cardiovascular Health Study. Arch Intern Med. 2009;169(8):798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz DL. Life and death, knowledge and power: why knowing what matters is not what's the matter. Arch Intern Med. 2009;169(15):1362–1363 [DOI] [PubMed] [Google Scholar]
- 11.Milstein B, Jones A, Homer JB, Murphy D, Essien J, Seville D. Charting plausible futures for diabetes prevalence in the United States: a role for system dynamics simulation modeling. Prev Chronic Dis. 2007;4(3):A52. [PMC free article] [PubMed] [Google Scholar]
- 12.Dall TM, Zhang Y, Chen YJ, Quick WW, Yang WG, Fogli J. The economic burden of diabetes. Health Aff (Millwood). 2010;29(2):297–303 [DOI] [PubMed] [Google Scholar]
- 13.National Institute of Diabetes and Digestive and Kidney Diseases Diabetes overview. NIH publication 09–3873. Available at: http://diabetes.niddk.nih.gov/dm/pubs/overview/index.htm. Accessed March 17, 2011
- 14.Centers for Disease Control and Prevention National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed March 17, 2011
- 15.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890 [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faridi Z, Shuval K, Yanchou Njike V, et al. Partners reducing effects of diabetes (PREDICT): a diabetes prevention physical activity and dietary intervention through African-American churches. Health Educ Res. 2010;25(2):306–315 [DOI] [PubMed] [Google Scholar]
- 18.Liburd LC, Vinicor G. Rethinking diabetes prevention and control in racial and ethnic communities. J Public Health Manag Pract. 2003;(suppl):S74–S79 [DOI] [PubMed] [Google Scholar]
- 19.Israel B, Schultz A, Parker E, Becker A. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202 [DOI] [PubMed] [Google Scholar]
- 20.Jones L, Wells K. Strategies for academic and clinician engagement in community-participatory partnered research. JAMA. 2007;297(4):407–410 [DOI] [PubMed] [Google Scholar]
- 21.George MA, Daniel M, Green LW. Appraising and funding participatory research in health promotion. 1998–1999. Int Q Community Health Educ. 2006-2007;26:171–187 [DOI] [PubMed] [Google Scholar]
- 22.Green LW, George MA, Daniel M, et al. Study of Participatory Research in Health Promotion: Review and Recommendations for the Development of Participatory Research in Health Promotion in Canada. Vancouver, British Columbia: Royal Society of Canada; 1995 [Google Scholar]
- 23.Reinharz S. Feminist Methods in Social Research. New York, NY: Oxford University Press; 1992 [Google Scholar]
- 24.Minkler M, Wallerstein N. Community-Based Participatory Research for Health. San Francisco, CA: Jossey-Bass; 2003 [Google Scholar]
- 25.Jenkins C, McNary S, Carlson BA, et al. Reducing disparities for African Americans with diabetes: progress made by REACH 2010 Charlestown and Georgetown Diabetes Coalition. Public Health Rep. 2004;119(3):322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horowitz CR, Robinson M, Seifer S. Community-based participatory research from the margin to the mainstream: are researchers prepared? Circulation. 2009;119(19):2633–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchanan DR, Miller FG, Wallerstein N. Ethical issues in community-based participatory research: balancing rigorous research with community participation in community intervention studies. Prog Community Health Partnersh. 2007;1(2):153–160 [DOI] [PubMed] [Google Scholar]
- 28.Jones L, Koegel P, Wells K. Bringing experimental design to community-participatory research. Minkler M, Wallerstein N, Community-Based Participatory Research for Health. New York, NY: Jossey-Bass/John Wiley &Sons; 2008:67–84 [Google Scholar]
- 29.Horn K, McCracken L, Dino G, Brayboy M. Applying community-based participatory research principles to the development of a smoking-cessation program for American Indian teens: “telling our story.” Health Educ Behav. 2008;35(1):44–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvatore AL, Chevrier J, Bradman A, et al. A community-based participatory worksite intervention to reduce pesticide exposures to farmworkers and their families. Am J Public Health. 2009;99(suppl 3):S578–S581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger J, Takaro TK, Song L, Beaudet N, Edwards K. A randomized controlled trial of asthma self-management support comparing clinic-based nurses and in-home community health workers: the Seattle-King County Healthy Homes II Project. Arch Pediatr Adolesc Med. 2009;163(2):141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker EA, Israel BA, Robins TG, et al. Evaluation of Community Action Against Asthma: a community health worker intervention to improve children's asthma-related health by reducing household environmental triggers for asthma. Health Educ Behav. 2008;35(3):376–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fetterman DM, Wandersman A, Empowerment Evaluation Principles in Practice. New York, NY: Guilford; 2005 [Google Scholar]
- 35.National Institute of Diabetes and Digestive and Kidney Diseases From Clinical Trials to Community: The Science of Translating Diabetes and Obesity Research. Bethesda, MD: US Dept of Health and Human Services; 2004 [Google Scholar]
- 36.Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the US health care workforce do the job? Health Aff (Millwood). 2009;28(1):64–74 [DOI] [PubMed] [Google Scholar]
- 37.Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: The United Kingdom Health and Lifestyle Survey. Arch Intern Med. 2010;170(8):711–718 [DOI] [PubMed] [Google Scholar]
- 38.Green LW. The prevention research centers as models of practice-based evidence two decades on. Am J Prev Med. 2007;33(1 suppl):S6–S8 [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Prevention research centers: center descriptions. Available at: http://www.cdc.gov/prc/center-descriptions/index.htm. Accessed July 1, 2010
- 40.Green LW, Glasgow RE, Atkins D, Stange K. Making evidence from research more relevant, useful, and actionable in policy, program planning, and practice slips “twixt cup and lip.” Am J Prev Med. 2009;37(6 suppl. 1):S187–S191. [DOI] [PubMed] [Google Scholar]
- 41.Green LW, Ottoson JM, Garcia C, Hiatt RA. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–174 [DOI] [PubMed] [Google Scholar]
- 42.Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Eval Health Prof. 2006;29(1):126–153 [DOI] [PubMed] [Google Scholar]
- 43.Green LW. Public health asks of systems science: to advance our evidence-based practice, can you help us get more practice-based evidence? Am J Public Health. 2006;96(3):406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glasgow RE, Green LW, Klesges LM, et al. External validity: we need to do more. Ann Behav Med. 2006;31(2):105–108 [DOI] [PubMed] [Google Scholar]
- 45.Glasgow RE, Green LW, Ammerman A. A focus on external validity. Eval Health Prof. 2007;30(2):115–117 [Google Scholar]
- 46.Green LW. Translation 2 research: the roadmap less traveled. Am J Prev Med. 2007;33(2):137–138 [Google Scholar]
- 47.Green LW. Public health asks of community psychology. Am J Community Psychol. 2008;41(3–4):404–406 [DOI] [PubMed] [Google Scholar]
- 48.Green LW. From Alma Ata to prescription for health: correcting 30 years of drift in primary care prevention and behavioral interventions. Am J Prev Med. 2008;35(5 suppl):S434–S436 [DOI] [PubMed] [Google Scholar]
- 49.Green LW. From research to “best practices” in other settings and populations. Am J Health Behav. 2001;25(3):165–178 [DOI] [PubMed] [Google Scholar]
- 50.Poland B, Green L, Rootman I, Settings for Health Promotion: Linking Theory and Practice. Newbury Park, CA: Sage Publishers Co; 2000 [Google Scholar]
- 51.Cornwall A. Towards Participatory Practice: Participatory Rural Appraisal (PRA) and the Participatory Process. London, England: Zed Books; 1996 [Google Scholar]
- 52.Ugarte CA, Duarte P, Wilson KM. PATCH as a model for development of a Hispanic health needs assessment: the El Paso experience. J Health Educ. 1992;23:153–156 [Google Scholar]
- 53.Katz D, Nawaz H, Jennings G, et al. Community health promotion and the randomized, controlled trial: approaches to finding common ground. J Public Health Manag Pract. 2001;7(2):33–40 [DOI] [PubMed] [Google Scholar]
- 54.Hawe P, Shiell A, Riley T. Complex interventions: how “out of control” can a randomised controlled trial be? BMJ. 2004;328(7455):1561–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mercer SL, Devinney BJ, Fine LJ, Green LW, Dougherty D. Study designs for effectiveness and translation research: identifying trade-offs. Am J Prev Med. 2007;33(2):139–154 [DOI] [PubMed] [Google Scholar]
- 56.Cohen DJ, Crabtree BF, Etz RS, et al. Fidelity versus flexibility: translating evidence-based research into practice. Am J Prev Med. 2008;35(5 suppl):S381–S389 [DOI] [PubMed] [Google Scholar]
- 57.Jagosh JJ, Pluye P, Macaulay AC, et al. Assessing the outcomes of participatory research: protocol for identifying, selecting, appraising and synthesizing the literature for realist review. Implementation Sci 2011;6(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercer SL, Green LW. Federal funding and support for participatory research in public health and health care. Minkler M, Wallerstein N, Community-Based Participatory Research in Health. San Francisco, CA: Jossey-Bass; 2008:399–406 [Google Scholar]
- 59.Mercer SL, MacDonald G, Green LW. Participatory research and evaluation: from best practices for all states to achievable practices within each state in the context of the Master Settlement Agreement. Health Promot Pract. 2004;5(3 suppl):167S–178S [DOI] [PubMed] [Google Scholar]
- 60.Faridi Z, Grunbaum JA, Gray BS, Franks A, Simoes E. Community-based participatory research: necessary next steps. Prev Chronic Dis. 2007;4(3):A70. [PMC free article] [PubMed] [Google Scholar]
- 61.Merriam PA, Tellez TL, Rosal MC, et al. Methodology of a diabetes prevention translational research project utilizing a community-academic partnership for implementation in an underserved Latino community. BMC Med Res Methodol. 2009;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Community Intervention Trial for Smoking Cessation (COMMIT): I . cohort results from a four-year community intervention. Am J Public Health. 1995;85(2):183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Community Health Scholars Program Goals and Competencies. Ann Arbor: University of Michigan School of Public Health; 2007 [Google Scholar]
- 64.Davis SM, Clay T, Smyth M, et al. Pathways curriculum and family interventions to promote healthy eating and physical activity in American Indian school children. Prev Med. 2003;37(6 pt 2):S24–S34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–377 [DOI] [PubMed] [Google Scholar]
- 66.Kok G, Gottlieb NH, Commers M, Smerecnik C. The ecological approach in health promotion programs: a decade later. Am J Health Promot. 2008;22(6):437–442 [DOI] [PubMed] [Google Scholar]
- 67.Green LW, Richard L, Potvin L. Ecological foundations of health promotion. Am J Health Promot. 1996;10(4):270–281 [DOI] [PubMed] [Google Scholar]
- 68.Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. J Epidemiol Community Health. 2004;58(9):788–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fleury J, Lee SM. The social ecological model and physical activity in African American women. Am J Community Psychol. 2006;37(1–2):129–140 [DOI] [PubMed] [Google Scholar]
- 70.Suarez-Balcazar Y, Redmond L, Kouba J, et al. Introducing systems change in the schools: the case of school luncheons and vending machines. Am J Community Psychol. 2007;39(3–4):335–345 [DOI] [PubMed] [Google Scholar]
- 71.Elder JP, Lytle L, Sallis JF, et al. A description of the social-ecological framework used in the trial of activity for adolescent girls (TAAG). Health Educ Res. 2007;22(2):155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sallis JF, Cervero RB, Ascher W, Henderson KA, Kraft MK, Kerr J. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322 [DOI] [PubMed] [Google Scholar]
- 73.Gary TL, Hill-Briggs F, Batts-Turner M, Brancati FL. Translational research principles of an effectiveness trial for diabetes care in an urban African American population. Diabetes Educ. 2005;31(6):880–889 [DOI] [PubMed] [Google Scholar]
- 74.Campostrini S, McQueen DV. Institutionalization of social and behavioral risk factor surveillance as a learning system. Soz Praventivmed. 2005;50(suppl 1):S9–S15 [DOI] [PubMed] [Google Scholar]
- 75.Jones C, McQueen DV. The European Region's contribution to the Global Programme on Health Promotion Effectiveness (GPHPE). Promot Educ. 2005(1):9–10, 44–45, 54–55, passim. [DOI] [PubMed] [Google Scholar]
- 76.McQueen DV, Anderson LM. Using evidence to assess the effectiveness of health promotion programs: a few fundamental issues. Promot Educ. 2004;(spec no. 1):11–16, 49. [PubMed] [Google Scholar]
- 77.McQueen DV. Judging the success of the Global Programme on Health Promotion Effectiveness. Promot Educ. 2003;10(3):117–118, 136 [PubMed] [Google Scholar]
- 78.Tang KC, Ehsani JP, McQueen DV. Evidence based health promotion: recollections, reflections, and reconsiderations. J Epidemiol Community Health. 2003;57(11):841–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McQueen DV. Strengthening the evidence base for health promotion. Health Promot Int. 2001;16(3):261–268 [DOI] [PubMed] [Google Scholar]
- 80.McQueen DV. A research programme in lifestyle and health: methodological and theoretical considerations. Rev Epidemiol Sante Publique. 1987;35(1):28–35 [PubMed] [Google Scholar]
- 81.McQueen DV. Shaping the future of health promotion: priorities for action. Promot Educ. 2007;14(4):268. [DOI] [PubMed] [Google Scholar]
- 82.Katz DL, Michael J, Treu J, et al. Teaching healthful food choices to elementary school students and their parents: the Nutrition Detectives™ program. J Sch Health. 2011;81(1):21–28 [DOI] [PubMed] [Google Scholar]
- 83.Mendel P, Meredith LS, Schoenbaum M, Sherbourne CD, Wells KB. Interventions in organizational and community context: a framework for building evidence on dissemination and implementation in health services research. Adm Policy Ment Health. 2008;35(1–2):21–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plescia M, Groblewski M, Chavis L. A lay health advisor program to promote community capacity and change among change agents. Health Promot Pract. 2008;9(4):434–439 [DOI] [PubMed] [Google Scholar]
- 85.Thompson JR, Horon C, Flores C. Advancing diabetes self-management in the Mexican American population: a community health worker model in a primary care setting. Diabetes Educ. 2007;33(suppl 6):159S–165S [DOI] [PubMed] [Google Scholar]
- 86.Hinton A, Downey J, Lisovicz N, Mayfield-Johnson S, White-Johnson F. The community health advisor program and the deep South network for cancer control: health promotion programs for volunteer community health advisors. Fam Community Health. 2005;28(1):20–27 [DOI] [PubMed] [Google Scholar]
- 87.Deitrick LM, Paxton HD, Rivera A, et al. Understanding the role of the promotora in a Latino diabetes education program. Qual Health Res. 2010;20(3):386–399 [DOI] [PubMed] [Google Scholar]
- 88.Kim S, Koniak-Griffin D, Flaskerud JH, Guarnero PA. The impact of lay health advisors on cardiovascular health promotion: using a community-based participatory approach. J Cardiovasc Nurs. 2004;19(3):192–199 [DOI] [PubMed] [Google Scholar]
- 89.Aroda VR, Ratner R. Approach to the patient with prediabetes. J Clin Endocrinol Metab. 2008;93(9):3259–3265 [DOI] [PubMed] [Google Scholar]
- 90.Irons BK, Mazzolini TA, Greene RS. Delaying the onset of type 2 diabetes mellitus in patients with prediabetes. Pharmacotherapy. 2004;24(3):362–371 [DOI] [PubMed] [Google Scholar]
- 91.Fisher EB, Walker EA, Bostrom A, Fischhoff B, Haire-Joshu D, Johnson SB. Behavioral science research in the prevention of diabetes: status and opportunities. Diabetes Care. 2002;25(3):599–606 [DOI] [PubMed] [Google Scholar]
- 92.Garfield SA, Malozowski S, Chin MH, et al. Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26(9):2670–2674 [DOI] [PubMed] [Google Scholar]
- 93.Centers for Disease Control and Prevention (CDC) CDC's Division of Diabetes Translation Community Health Workers/Promotores de Salud: Critical Connections in Communities. Available at: http://www.cdc.gov/diabetes/about/index.htm. Accessed March 17, 2011
- 94.Flores LM, Davis R, Culross P. Community health: a critical approach to addressing chronic diseases. Prev Chronic Dis. 2007;4(4):A108. [PMC free article] [PubMed] [Google Scholar]
- 95.Katz DL. School-based interventions for health promotion and weight control: not just waiting on the world to change. Annu Rev Public Health. 2009;30:253–272 [DOI] [PubMed] [Google Scholar]
- 96.Katz DL, O'Connell M, Njike VY, Yeh MC, Nawaz H. Strategies for the prevention and control of obesity in the school setting: systematic review and meta-analysis. Int J Obes (Lond). 2008;32(12):1780–1789 [DOI] [PubMed] [Google Scholar]
- 97.Katz DL, Shuval K, Comerford BP, Faridi Z, Njike VY. Impact of an educational intervention on internal medicine residents’ physical activity counselling: the Pressure System Model. J Eval Clin Pract. 2008;14(2):294–299 [DOI] [PubMed] [Google Scholar]
- 98.Poland BD, Green LW, Rootman I. Settings for Health Promotion: Linking Theory and Practice. London, England: Sage Publications; 2000 [Google Scholar]
- 99.Whitelaw S, Baxendale A, Bryce C, MacHardy L, Young I, Witney E. “Settings” based health promotion: a review. Health Promot Int. 2001;16(4):339–353 [DOI] [PubMed] [Google Scholar]
- 100.Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot. 1996;10(4):282–298 [DOI] [PubMed] [Google Scholar]
- 101.Clinton H. It Takes a Village: And Other Lessons Children Teach Us. New York, NY: Simon & Schuster; 1996 [Google Scholar]
- 102.Farquhar J, Fortmann S, Flora J, et al. Effect of community wide education on cardiovascular disease risk factors: the Stanford Five-City Project. JAMA. 1990;264(3):359–365 [PubMed] [Google Scholar]
- 103.Meininger JC. School-based interventions for primary prevention of cardiovascular disease: evidence of effects for minority populations. Annu Rev Nurs Res. 2000;18:219–244 [PubMed] [Google Scholar]
- 104.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Economos CD, Curtatone JA. Shaping up Somerville: a community initiative in Massachusetts. Prev Med. 2010;50(suppl 1):S97–S98 [DOI] [PubMed] [Google Scholar]