Abstract
β-catenin/TCF signaling regulates a varied set of cellular functions including development and remodeling. Fibronectin is a TCF-regulated gene that is highly expressed in arterial endothelium during atherosclerosis development and contributes to the pathophysiology of the disease. However, the activation of endothelial β-catenin/TCF signaling and its role in fibronectin expression in atherosclerosis are not currently known.
Objective
To assess the activity of β-catenin/TCF signaling in atherosclerosis development and its regulation of fibronectin in vascular endothelium.
Methods and Results
Histological staining identified preferential nuclear localization of β-catenin in the endothelium of atheroprone aorta prior to and during lesion development. Transgenic reporter studies revealed that increased levels of TCF transcriptional activity in endothelium correlated anatomically with β-catenin nuclear localization and fibronectin deposition. Exposure of endothelial cells to human-derived atheroprone shear stress induced nuclear localization of β-catenin, transcriptional activation of TCF, and expression of fibronectin. Activation of fibronectin expression required β-catenin, TCF and the transcriptional co-activator CBP. Finally, we identified PECAM-1as a critical regulator of constitutive β-catenin and GSK-3β activities.
Conclusions
This data uncovers novel constitutive activation of the endothelial β-catenin/TCF signaling pathway in atherosclerosis and regulation of fibronectin through hemodynamic shear stress.
Keywords: β-catenin, TCF/LEF, endothelium, atherosclerosis, hemodynamics, fibronectin
Introduction
β-catenin (βcat) is a highly conserved, multifunctional member of the armadillo family whose nuclear translocation and co-activation of the TCF/LEF family of transcription factors represents a critical step in a variety of cell processes including development, epithelial-mesenchymal transition, angiogenesis, and differentiation1. Studies have identified a role for TCF/LEF activity in several pathological features of advanced atherosclerotic lesions including vascular calcification2-4 and smooth muscle cell proliferation5. However, the involvement of this signaling pathway in the endothelium during early atherosclerosis development is poorly understood.
Cytosolic βcat is constitutively targeted for ubiquitination-mediated degradation via glycogen synthase kinase 3-β (GSK-3β)-dependent phosphorylation. Upon stimulation by various factors (including canonical Wnts and growth factors) GSK-3β activity is decreased, leading to nuclear accumulation of βcat, followed by binding and activation of the TCF/LEF family of transcription factors. One target of TCF/LEF-dependent transcription is the extracellular matrix protein fibronectin6. TCF-dependent fibronectin expression has been identified to play important roles in several cell contexts including fibroblast differentiation7, lung branching morphogenesis8 and epithelial-mesenchymal transition6. Fibronectin is also highly regulated in atherosclerotic tissue and is involved in atherosclerosis development through promotion of inflammation and endothelial permeability9-12. However, the role of endothelial βcat/TCF in this process remains unknown.
One prominent feature of the atherosclerotic environment is hemodynamic shear stress, which regulates the phenotype of endothelial cells13-14 (ECs) and largely explains the regional bias of atherosclerosis development15-16. Specifically, low in magnitude, reversing shear stress, such as that which occurs in branching and curved vessels17-18, induces a chronic inflammatory phenotype in pre-atherosclerotic endothelium. GSK-3β inactivation occurs in response to onset of shear stress in a PECAM-1-dependent manner19, although the role of PECAM-1 in βcat/TCF transcriptional regulation is not known. In this paper, we describe the preferential activation of βcat/TCF signaling in the atherosclerotic environment by hemodynamic shear stress as well as its contribution to expression of the pro-atherogenic protein fibronectin. The overall goal of this work was to identify novel pathways involved in atherosclerosis development, inflammation and shear stress that may provide valuable insight into the heterogeneous anatomic distribution of the disease as well as provide new preventative or interventional opportunities.
Methods
Cell culture
Primary human umbilical vein endothelial cells (HUVEC) were isolated as previously described20 and maintained in M199 (Lonza) with 10% fetal bovine serum (GIBCO), 5ug/ml endothelial cell growth supplement (Biomedical Technologies), 10ug/ml heparin (Sigma), 2mM L-glutamine (Gibco), and 100U penicillin/streptomycin (Invitrogen). Bovine aortic endothelial cells (BAEC) were maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum, 2mM L-glutamine, and 100U penicillin/streptomycin. β-cat null murine endothelial cells (MEC) (described here21) were maintained in MCDB-131 (Gibco) supplemented with 20% fetal bovine serum, 5ug/ml endothelial cell growth supplement, 10ug/ml heparin, 2mM L-glutamine, and 100U penicillin/streptomycin.
Hemodynamic shear stress application
Shear stress profiles measured from healthy human subjects17 were applied to cells using a cone and plate viscometer as previously described20, 22-23. Shear stress was applied for 24hr except where indicated to assess the role of TCF/LEF signaling in a shear stress-adapted phenotype22-24.
Mice
Mouse studies were conducted with the approval of the University of Virginia Animal Care and Use Committee (ACUC#3597), and in accordance with the NIH recommendation outlined in “Guide for the Care and Use of Laboratory Animals”.
Male Wild type, ApoE-/- and PECAM-1-/-ApoE-/- mice on a C57BL/6 (B6) background were maintained on low fat chow diet except where indicated. Studies were performed with a minimum of 3 mice in each condition. Heterozygous TOPGAL transgenic mice on a CD1 background (Jackson Laboratories) were crossed with B6 mice for three generations. To study TCF/LEF activity in atherosclerotic lesions, TOPGAL (CD1:B6) mice were crossed with ApoE-/- mice such that a minimum of five B6 backcrosses were completed. Mice were maintained on a low fat chow diet and tissue was harvested between 12 and 20 weeks of age.
Statistics
Significance was determined through 2-tailed unpaired student's t-test. P values less than 0.05 were considered statistically significant. All images and blots are representative of at least 3 independent experiments, and data are expressed as average ± SEM.
Results
Regional distribution and activation of βcat/TCF in murine endothelium in vivo
The aortic arch is comprised of geometrically opposed regions of relatively high and low shear stress. The greater curvature and descending aorta experience high, unidirectional shear stress and are resistant to atherosclerosis development (atheroprotective). Conversely, the lesser curvature of the aortic arch is exposed to lower, reversing shear stress (atheroprone) and in hypercholesteremic animal models, tends to develop atherosclerosis16. To assess the distribution of βcat in environments of different atherosclerosis predisposition, tissue sections from wild type B6 mice were examined (Figure 1A). In atheroprotective regions βcat was primarily localized to the cell-cell border, with modest cytosolic staining present in the greater curvature. In contrast, the atheroprone lesser curvature exhibited a strong nuclear fraction of βcat.
Figure 1.

Endothelial subcellular localization of βcat depends on atherosclerotic susceptibility of the aortic region. (A) En face staining of endothelial βcat in different regions of the C57BL/6 mouse aorta. Images were acquired from the greater and lesser curvatures of the aortic arch and descending abdominal aorta. Images from greater curvature, lesser curvature and descending aorta are representative of 3, 6 and 6 mice respectively. (B) En face staining of βcat in atheroprotective (descending) and atheroprone (lesser curvature) regions of 8-13wk ApoE-/- aorta. Arrows refer to exclusion of βcat from the nuclei of ECs of descending aorta. (C) βcat nuclear localization in endothelium of early atherosclerotic lesions in the greater (i-iii) and lesser (iv-vi) curvature of chow diet fed 8-13wk ApoE-/- aorta. To detect nuclear accumulation, aortic cross-sections were fluorescently stained and imaged for βcat (red), nuclei (blue) and tissue autofluorescence (green) (i,iv). Single channel images of nuclei (ii,v) and βcat (iii,vi) reveal unique staining patterns. Arrows denote individual endothelial nuclei. (*) denotes a nascent atherosclerotic lesion. (D) Quantitative analysis of endothelial nuclear βcat in 22wk Western diet-fed ApoE-/-. Individual endothelial nuclei from three different mice (×,□,Δ) were separated into either lesion-free (healthy) and lesion-burdened (lesion) groups and then assessed for relative nuclear βcat expression. Horizontal dashed lines denote geometric mean of all nuclei for a given group. Error bars represent ± SD, *p<10-5.
To assess the distribution of βcat in endothelium during atherosclerosis development, en face aortic sections from ApoE-/- mice were examined (Figure 1B). In atheroprotected regions, βcat appears to be excluded from the nucleus (arrows). Atheroprone regions of ApoE-/- displayed higher βcat present in the cytosol, though cells lacked clear nuclear definition. In cross sections of early lesions (8-13wk of age), both nuclear βcat and total levels of βcat were elevated compared to lesion-free areas (Figure 1C). Additionally, nuclear βcat correlated well with nuclear NF-κB – a hallmark of endothelial dysfunction (Supplemental Figure I)25. Nuclear βcat was also elevated in advanced atherosclerotic plaques, where endothelium overlying lesions exhibited 36% more nuclear βcat than atherosclerosis-free regions in 22wk Western diet-fed ApoE-/- mice (p<0.05; Figure 1D).
Nuclear translocation of βcat classically leads to TCF/LEF-dependent transcriptional activity. To directly assess the activity of TCF/LEF transcription factors in the murine aorta, transgene expression in reporter mice (TOPGAL) was measured. The expression of lacZ was greater in the atheroprone lesser curvature compared to the greater curvature (Figure 2A), suggesting the atheroprone environment contributes to activation of TCF/LEF transcriptional activation. The activity of βcat/TCF within developing atherosclerotic lesions was assessed in TOPGAL+/-/ApoE-/- mice (Figure 2B). LacZ expression was observed in endothelium superficial and adjacent to lesion development, which suggests that TCF/LEF-dependent transcription precedes lesion growth. Consistent with previous reports, fibronectin was strongly expressed in early atherosclerotic lesions (Figure 2C). Fibronectin expression and TCF/LEF activation exhibited parallel region-specific staining patterns. Cumulatively, these findings indicate that βcat nuclear signaling preferentially occurs in regions predisposed to atherosclerosis.
Figure 2.

Preferential TCF/LEF activation in the atherosclerotic prone aorta. (A) En face analysis of TCF/LEF reporter TOPGAL transgenic mice. Mixed background CD1:B6 heterzygous transgenic reporter mice were assessed for the relative expression of lacZ within the atheroprotected greater and atheroprone lesser curvature of the aortic arch. Aortic rings were fluorescently stained for lacZ expression and staining intensity quantified. (n=3, *p<0.05). (B) TCF/LEF activation in the aorta during early atherosclerosis. LacZ expression was assessed in early atherosclerotic lesions of chow diet fed 12-20wk TOPGAL+/-/ApoE-/- mice using immunohistochestry in paraffin embedded cross sections. (i) Representative aortic cross section of an early atherosclerotic lesion. Higher magnification images of lacZ expression (ii,iii). (iv) No primary antibody staining control. (v) TOPGAL null, ApoE-/- littermate stained for lacZ expression as a non-specific control. (C) Fibronectin expression correlates with TCF/LEF activity in early atherosclerosis. Sections adjacent to those presented in (B) were stained for fibronectin expression. Higher magnification images of fibronectin expression (ii,iii). (iv) Isotype staining control.
Atheroprone hemodynamics promote βcat/TCF activation and expression of fibronectin
Because constitutive nuclear βcat and TCF/LEF activities were observed in arterial regions that constantly experience low, reversing shear stress, we next tested relative activity of this pathway in response to hemodynamic forces. Cultured EC monolayers were subjected to human-derived atheroprone and atheroprotective shear stresses via a shear stress cell culture system (Figure 3A, Supplemental Figure II). Following shear stress exposure, levels of nuclear accumulation of βcat were significantly increased by atheroprone compared to atheroprotective shear stress (Figure 3B). Further, cells transfected with a TCF-responsive luciferase reporter plasmid exhibited significantly increased TCF activity when exposed to the atheroprone flow compared to atheroprotective (Figure 3C).
Figure 3.

Shear stress differentially regulates βcat nuclear localization and TCF/LEF transcriptional activation in endothelial cells. (A) Schematic of human-derived shear stress profiles from the common carotid artery (protected) and internal carotid sinus (prone) measured previously17. ECs were exposed to these hemodnynamic shear stress profiles for indicated times by a cone and plate cell culture device described in the Methods section. (B) Nuclear lysates from HUVEC exposed to either protected and prone shear stress for 24hr were assessed relative βcat levels by Western blot. TBP (TATA-Binding Protein) was used to ensure equal loading. *p<0.05, n=3 (C) BAEC transfected with the TOP-Flash were used to assess TCF/LEF transcriptional activity under different shear stress profiles. Luciferase activity was measured after 16hr of exposure to either protected or prone shear stress. *p<0.05, n=4-6.
To directly test the transcriptional capacity of βcat/TCF signaling in response to arterial hemodynamic simulation, we focused on a small cohort of known TCF-dependent, atherosclerosis-related genes. To identify the requirement of βcat/TCF signaling to gene expression, we analyzed mRNA transcripts from ECs treated with adenovirus containing either TCF-4 lacking the N-terminal binding26 (Ad-DN-TCF4) or an empty CMV promoter control (Ad-Empty) and exposed to shear stress. Three candidate genes (CyclinD1, IL-8 and fibronectin) were identified as being both upregulated under atheroprone hemodyanmics and inhibited by Ad-DN-TCF4 treatment (Figure 4A, Supplemental Figure III). Because of the critical role of fibronectin in endothelial biology, we focused on this target. Application of atheroprone hemodynamics increased expression of fibronectin mRNA by approximately 2-fold compared to atheroprotective (Figure 4A). Pre-treatment of ECs with Ad-DN-TCF4 significantly reduced expression of fibronectin under atheroprone flow. Protein analysis confirmed both the atheroprone hemodynamic-induced increase as well as the reduction in fibronectin protein levels by Ad-DN-TCF treatment (Figure 4B). This suggests a novel role for βcat/TCF regulation of EC fibronectin in response to hemodynamic stimulation. To determine whether βcat binds to the fibronectin promoter, we performed a ChIP assay. We observed a significant level of enrichment of the proximal fibronectin promoter when lysates were precipitated with a βcat antibody (Figure 4C, Supplemental Figure IV) compared to mock control and compared to a more upstream genomic region. This finding confirms the specific interaction between βcat and the fibronectin transcriptional regulatory region.
Figure 4.

Fibronectin gene expression in ECs exposed to prone shear stress depends on TCF/LEF transcription. (A) HUVEC treated with either empty adenovirus (Ad-Empty) or adenovirus containing dominant negative N-terminal deleted TCF-4 (Ad-DN-TCF4) were exposed to atheroprotective or atheroprone shear stress for 24hr. mRNA levels of fibronectin was assessed by RT-PCR. Expression was normalized to β-2-microglobulin. *p<0.05, †p<0.01, n=4-6. (B) HUVEC exposed to 24hr of protected or prone shear stress in the presence of either Ad-Empty or Ad-DN-TCF4 were analyzed for fibronectin protein expression by Western blot. *p<0.05 †p<0.01, n=3-4. (C) βcat binds directly to the human fibronectin promoter in ECs. PCR amplification of the proximal human fibronectin promoter was performed following pull-down with a anti-βcat antibody or rabbit IgG (mock) control. Upstream control refers to PCR amplification of a region 2kb upstream of the proximal fibronectin promoter. *p<0.05, n=6. (D) β-cat staining localizes to the cell-cell border in wildtype MECs (left), but is absent in knockout cells (middle). Knockout cells reconstituted with Xβ-cat-Eng and stained with anti-Engrailed antibody show localization to the cell-cell border (arrows), demonstrating proper incorporation to the adherens junction. (E) MECs were exposed to 24hrs of atheroprone hemodynamics and fibronectin protein levels were assessed. *p<0.05 n=3. (F) During shear stress application, HUVEC were treated with DMSO or 5μM of compounds ICG-001 or IQ-1 to inhibit β-cat/CBP and β-cat p300 interactions respectively. Fibronectin mRNA and protein levels were assessed by PCR and Western blot. *p<0.05, n=3-7. (G) HUVEC were pretreated with either Ad-Empty or Ad-DN-TCF4 in combination with an NF-κB reporter and exposed to atheroprone shear stress for 24hr. Following shear stress exposure, lysates were assessed for luciferase activity. †p<0.01, n=4.
In order to confirm the requirement of β-cat in fibronectin expression, we next interrogated the expression of fibronectin in β-cat knockout MECs. Cells were reconstituted with Xenopus β-cat in the presence or absence of a drosophila-derived engrailed repressor domain fused to the C-terminus (Xβ-cat-Eng) (Figure 4D). This construct has been shown to effectively inhibit β-catenin/TCF transcriptional activity27. Atheroprone hemodynamic-induced expression of fibronectin was rescued in knockout cells reconstituted with Xβ-cat, but not in cells expressing Xβ-cat-Eng (Figure 4E), providing direct evidence for the transcriptional requirement of β-cat in fibronectin expression. Thus, the requirement of βcat/TCF signaling in shear stress-induced fibronectin expression was substantiated by loss- and gain-of-function experiments.
To achieve maximal activation, β-cat must recruit histone acetyl transferases (HATs) including CBP28 and p30029 to the transcription complex. Human ECs were exposed to specific inhibitors of β-cat/CBP and β-cat/p300 interactions (ICG-00130-33 and IQ-134 respectively). Inhibition of β-cat/CBP interactions inhibited both fibronectin gene and protein expression (Figure 4F). Pharmacological inhibition of β-cat/p300 had no statistically significant effect, though we cannot eliminate the possiblility of βcat/p300 regulation of fibronectin expression. This suggests that recruitment of CBP to the β-cat/TCF complex is required for expression of fibronectin in ECs induced by atheroprone hemodynamics.
DN-TCF4 inhibits shear stress-induced EC inflammation
The transcription factor complex NF-κB represents a major inflammatory mediator in ECs and atherosclerosis. Atheroprone shear stress enhances NF-κB activity in vitro to prime ECs towards a pro-inflammatory phenotype13, 25. Here we wanted to test the hypothesis that downstream activation of βcat/TCF contributes the “primed” NF-kB activation in response to atheroprone flow. Infection with Ad-DN-TCF4 reduced atheroprone flow-stimulated NF-κB activity by 70% (Figure 4G), supporting the physiological importance of βcat/TCF signaling in the atheroprone environment.
PECAM-1 is required for constitutive βcat nuclear localization
Having established that activation of βcat/TCF signaling occurs in response to hemodynamics, we next interrogated the importance of a critical shear stress-sensing protein PECAM-1. Immunostained aortic sections from young (8wk) PECAM-1-/-ApoE-/- exhibited no nuclear localized βcat compared to age matched ApoE-/- mice (Figure 5A). Additionally, 8-13wk PECAM-1-/-ApoE-/- mice, whose ApoE-/- counterparts exhibited early lesion development, showed no nuclear βcat staining in atheroprone regions (Figure 5B). Together, this points towards a novel regulatory role for PECAM-1 in the constitutive regional activation of βcat nuclear translocation.
Figure 5.
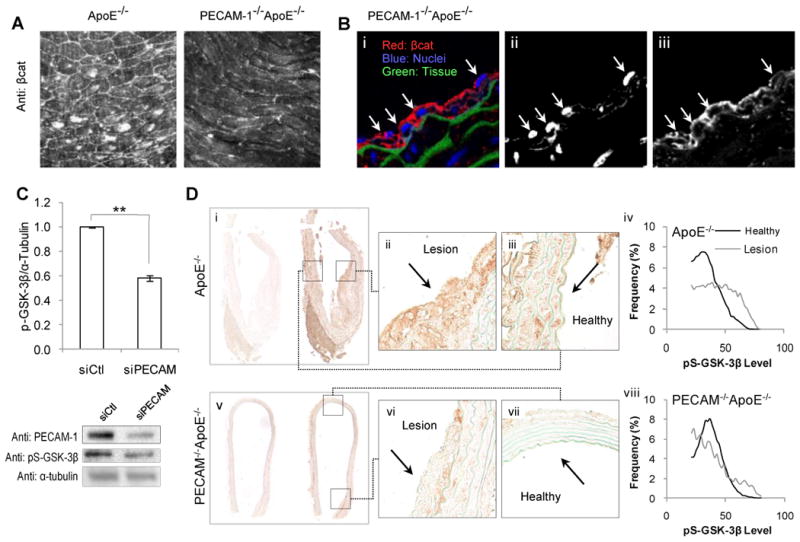
Regulation of nuclear βcat localization and GSK-3 activity by PECAM-1 preceding and during atherosclerosis. (A) En face fluorescent staining of βcat and PECAM-1 in atheroprone regions of chow diet fed ApoE-/- and PECAM-1-/-ApoE-/- aortas prior to atherosclerotic plaque development (8wk). (B) Cross-sections of the lesser curvature of 8-13wk chow diet fed PECAM-1-/-ApoE-/- aortas. Tissue was immune-stained and imaged for βcat (red), nuclei (blue) and tissue autofluorescence (green) (i). Single channel images of nuclei (ii) and βcat (iii) reveal decreased nuclei/βcat colocalization (compare to Figure 1B). Arrows denote individual endothelial cells. (C) HUVEC treated with either scrambled siRNA (siCtl) or siRNA targeted against PECAM-1 (siPECAM-1) were exposed to 24hr of prone shear stress and assessed for relative levels of pS-GSK-3β by Western blot. **p<0.001, n=4-6. (D) Western diet fed 22wk ApoE-/- (i-iii) and PECAM-1-/-ApoE-/- aortas (v-vii) were stained for pS-GSK-3β. (i,v) Low magnification images of IgG control (left) and anti-pS-GSK-3β- (right) stained aortic cross sections. Expression was assessed in lesion (ii, vi) and non-lesion-burdened (i.e. healthy) (iii, vii) endothelium. A histogram of pixel intensities of lesion and healthy endothelial cell pS-GSK-3β expression for ApoE-/- (iv) and PECAM-1-/-ApoE-/- (viii) reveals increased pS-GSK-3β levels in atherosclerotic plaques of ApoE-/-, but not in PECAM-1-/-ApoE-/- plaques (the rightward shift of pS-GSK-3β intensity in lesion-burdened ECs in ApoE-/- is absent in PECAM-1-/-ApoE-/-).
Shear stress-induced inhibition of endothelial GSK-3β depends on PECAM-1
The presence of nuclear βcat and active TCF/LEF in endothelium of atherosclerotic lesions suggests inhibition of the βcat degradation pathway. Following exposure to atheroprone hemodynamics, phospho-serine-GSK-3β (pS-GSK-3β) levels were found to depend on PECAM-1 expression (Figure 5C).
Atherosclerotic lesions exhibit elevated GSK-3β inhibition, which depends on PECAM-1
We next assessed the relative abundance of inactivated GSK-3β in atherosclerotic lesions. pS-GSK-3β levels were elevated at sites of atherosclerotic lesion in ApoE-/- mice (Figure 5D: ii vs. iii). In PECAM-1-/-ApoE-/- aortas, pS-GSK-3β levels were homogenous around the aortic circumference, regardless of lesion burden (Figure 5D: vi vs. vii). Thus, in PECAM-1+/+, the endothelium exists in two distinct populations of pS-GSK-3β expression, whereas genetic deletion of PECAM-1 imparts uniformity along the vessel lumen (Figure 5D: iv vs. viii). pS-GSK-3β levels correlate with both nuclear βcat colocalization (Supplemental Figure II) as well as fibronectin expression. This implicates PECAM-1 as a critical regulator of pS-GSK-3β in atherosclerotic endothelium.
Discussion
This study identifies for the first time the activation of βcat/TCF signaling in the endothelium prior to and during early atherogenesis. Our data suggest that atheroprone shear stress serves as a potent activator of endothelial βcat/TCF signaling through a PECAM-1/GSK-3β-dependent mechanism, and that this in turn drives transcription of fibronectin, a critical regulator of endothelial phenotype.
Activation of βcat/TCF signaling is known to occur in response to a diverse set of cellular cues. Classically, initiation of TCF activity occurs through canonical Wnt signaling, where soluble Wnt factors bind to the Frizzled family receptors leading to repression of the GSK-3β/Axin/APC complex. Nuclear translocation of βcat in ECs by canonical Wnt signaling within the vascular wall is conceivable, given that they express multiple Frizzled receptors35. However, the presence of canonical Wnt ligands in the vessel wall during atherosclerosis is unknown. A recent study points to the presence of Wnt pathway antagonist Dickkopf-1 in atherosclerotic plaques36. Additionally, the presence of the non-canonical Wnt-5a was found to be abundantly expressed in plaques37 and may inhibit canonical Wnt signaling38. In addition to shear stress, other atherogenic stimuli induce activation of βcat/TCF signaling in ECs. IL-1β alone or in combination with tobacco smoke extract activates TCF/LEF through AKT/GSK-3β39. TNF-α drives paracrine Wnt signaling to promote osteogenesis in arterial smooth muscle cells4. In addition to IL-1β, LPS also induced βcat nuclear accumulation (Supplemental Figure V-VI) suggesting activation of this pathway may represent a generic inflammatory response. In the context of the present study, the activation of TCF/LEF likely occurs as a result of a balance between promoting and antagonizing signals.
Our findings support that PECAM-1 is a critical regulator of nuclear accumulation of βcat in ECs within developing lesions. Previous work documents a role for PECAM-1 in regulating βcat localization in the absence of shear stress and atherosclerotic burden. However, divergent responses were reported depending on cell type40-41 suggesting this process depends on cell-specific factors including SHP-2 activity. In the context of atherosclerosis development, PECAM-1 promotes nuclear accumulation of endothelial βcat, consistent with its function in regulating shear stress-dependent endothelial inflammation. This likely occurs via regulation of GSK-3β phosphorylation, which is both shear stress and PECAM-1 dependent19, 42. siRNA-mediated knockdown of PECAM-1 impaired pS-GSK-3β levels, revealing a novel role for PECAM-1 in atheroprone shear stress-adapted ECs and a potential mechanism for increased βcat/TCF activity in atherosclerotic endothelium. Our data support that prolonged exposure to atheroprone hemodynamics induces sustained GSK-3β inactivation. The onset of relatively high levels of shear stress induces an acute inhibition of GSK-3β19 which may represent an early adaptation to shear stress. This is conceptually consistent with activation of NF-κB13, Rac143, ERK42, p3844 and AKT45 signaling in response to acute shear stress exposure which are returned to baseline levels within minutes to hours. Intersetingly, inhibition of GSK-3β is substrate dependent, where RGD-rich matrix promotes GSK-3β phosphorylation compared to collagen substrates42, suggesting the existence of GSK-3β/fibronectin feedback that amplifies βcat/TCF signaling. Indeed βcat, itself a gene target of βcat/TCF, is differentially regulated in relation to shear stress magnitudes in porcine iliac arteries, suggesting a complex role for hemodynamics in regulation of this pathway46.
Prior to this study, the consequences of endothelial βcat/TCF activation on lesion biology were not known. Here we showed that DN-TCF4 inhibits NF-κB in the atheroprone hemodynamic environment suggesting that βcat/TCF activity contributes to endothelial inflammation, thus promoting lesion advancement. This pro-inflammatory function of endothelial βcat/TCF activity is likely related to fibronectin expression. siRNA-mediated knockdown of fibronectin is sufficient to decrease NF-κB activity in the atheroprone hemodynamic environment, which is rescued by treatment with exogenous fibronectin47.
Fibronectin expression, shown here to require CBP/βcat/TCF under atheroprone shear stress, exerts multiple pro-atherogenic effects. Shear stress activation of NF-κB and JNK is enhanced on cells plated on fibronectin matrices11, 48. Fibronectin also contributes to shear stress-induced endothelial barrier dysfunction via activation of p21-activated kinase49. The mechanism by which βcat/TCF signaling drives transcription of fibronectin gene expression is not completely known. A consensus TCF/LEF binding site was identified in the Xenopus fibronectin promoter7; however, our analysis of the human fibronectin promoter revealed no putative proximal TCF/LEF consensus binding site. βcat has been shown to bind to the fibronectin promoter independently of TCF/LEF in SW480 cells50. In this regard, the CBP/βcat/TCF complex may be critical for fibronectin expression independent of TCF/DNA binding. We confirmed that βcat binds to the proximal fibronectin promoter in ECs via ChIP assay. Together, this supports that regulation of fibronectin by βcat may occur via both direct TCF-independent and indirect TCF-dependent transcriptional regulators.
In addition to fibronectin, over 50 genes have been identified as being TCF/LEF dependent51-52. Among these are several atherosclerosis-associated genes including CyclinD153 and IL-854 whose transcript levels were elevated by atheroprone hemodynamics and significantly inhibited by DN-TCF-4 treatment, suggesting that βcat/TCF activity contributes broadly to endothelial gene expression in atheroprone regions. It is appreciated that endothelial turnover at atherogenic sites is significantly elevated. It is conceivable that βcat/TCF-dependent CyclinD1 expression plays a role in this process55. Further, while elevated TCF/LEF reporter activity in atherosclerotic lesions was isolated to the endothelium and subendothelial foam cells, we cannot exclude that activation of this pathway in other cells contributes to lesion development. We observed β-catenin levels in smooth muscle cells as well as several circulating cells that were likely monocytes.
Collectively, this study identifies a novel pathway activated in the early atheroprone environment as well as uncovering the regulation of a key player in arterial remodeling during atherosclerosis development. We demonstrate that βcat nuclear localization and TCF/LEF transcriptional activation in the aortic endothelium occurs in response to atheroprone hemodynamic stimulation and precedes lesion development or advancement. This phenomenon may have diverse effects on atherosclerotic lesion biology in addition to its role in the regulation of the pro-inflammatory molecule fibronectin, and warrants future study to identify other βcat/TCF signaling targets in atherosclerosis.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Ryan Feaver, John Sanders, and Dr. Michael Simmers for technical advice.
Sources of Funding: These studies were performed with support from NIH R01 HL02836 to BRB, BDG supported by: NIH 5T32HL0084.
Footnotes
Disclosures: The authors have no conflict of interest disclosures.
References
- 1.Barker N. The canonical wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- 2.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 2010;107:271–282. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic msx2-wnt calcification cascade is regulated by tnf-alpha-dependent signals in diabetic ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 5.Bedel A, Negre-Salvayre A, Heeneman S, Grazide MH, Thiers JC, Salvayre R, Maupas-Schwalm F. E-cadherin/beta-catenin/t-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ Res. 2008;103:694–701. doi: 10.1161/CIRCRESAHA.107.166405. [DOI] [PubMed] [Google Scholar]
- 6.ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gradl D, Kuhl M, Wedlich D. The wnt/wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (dkk1) reveals that fibronectin is a major target of wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Shekhonin BV, Domogatsky SP, Idelson GL, Koteliansky VE, Rukosuev VS. Relative distribution of fibronectin and type i, iii, iv, v collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987;67:9–16. doi: 10.1016/0021-9150(87)90259-0. [DOI] [PubMed] [Google Scholar]
- 10.Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res. 2010;106:1703–1711. doi: 10.1161/CIRCRESAHA.109.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates jnk activation by flow. Circ Res. 2009;104:995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates nf-kappab activation by flow: A potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand BD, Epstein FH, Blackman BR. Spatial and spectral heterogeneity of time-varying shear stress profiles in the carotid bifurcation by phase-contrast mri. J Magn Reson Imaging. 2006;24:1386–1392. doi: 10.1002/jmri.20765. [DOI] [PubMed] [Google Scholar]
- 18.Kaazempur-Mofrad MR, Isasi AG, Younis HF, Chan RC, Hinton DP, Sukhova G, LaMuraglia GM, Lee RT, Kamm RD. Characterization of the atherosclerotic carotid bifurcation using mri, finite element modeling, and histology. Ann Biomed Eng. 2004;32:932–946. doi: 10.1023/b:abme.0000032456.16097.e0. [DOI] [PubMed] [Google Scholar]
- 19.Biswas P, Canosa S, Schoenfeld D, Schoenfeld J, Li P, Cheas LC, Zhang J, Cordova A, Sumpio B, Madri JA. Pecam-1 affects gsk-3beta-mediated beta-catenin phosphorylation and degradation. Am J Pathol. 2006;169:314–324. doi: 10.2353/ajpath.2006.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackman BR, Garcia-Cardena G, Gimbrone MA., Jr A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J Biomech Eng. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 21.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feaver RE, Hastings NE, Pryor A, Blackman BR. Grp78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1534–1541. doi: 10.1161/ATVBAHA.108.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmers MB, Pryor AW, Blackman BR. Arterial shear stress regulates endothelial cell-directed migration, polarity, and morphology in confluent monolayers. Am J Physiol Heart Circ Physiol. 2007;293:H1937–1946. doi: 10.1152/ajpheart.00534.2007. [DOI] [PubMed] [Google Scholar]
- 24.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 25.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell pecam-1 promotes atherosclerotic lesions in areas of disturbed flow in apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2003–2008. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-myc as a target of the apc pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 27.Montross WT, Ji H, McCrea PD. A beta-catenin/engrailed chimera selectively suppresses wnt signaling. J Cell Sci. 2000;113(Pt 10):1759–1770. doi: 10.1242/jcs.113.10.1759. [DOI] [PubMed] [Google Scholar]
- 28.Takemaru KI, Moon RT. The transcriptional coactivator cbp interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/cbp acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of beta-catenin/creb-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H, Won S, Hwang DY, Lee JS, Kim M, Kim R, Kim W, Cha B, Kim T, Kim D, Costantini F, Jho EH. Downregulation of wnt/beta-catenin signaling causes degeneration of hippocampal neurons in vivo. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators cbp and p300 on tcf/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- 33.Teo JL, Ma H, Nguyen C, Lam C, Kahn M. Specific inhibition of cbp/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc Natl Acad Sci U S A. 2005;102:12171–12176. doi: 10.1073/pnas.0504600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/cbp signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin AM, Sullivan KM, D'Amore PA. Cultured endothelial cells display endogenous activation of the canonical wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of wnt signaling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 36.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 37.Christman MA, 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD, Malgor R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 38.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical wnt pathway by promoting gsk-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1beta to alter beta-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007;21:1831–1843. doi: 10.1096/fj.06-7557com. [DOI] [PubMed] [Google Scholar]
- 40.Biswas P, Canosa S, Schoenfeld J, Schoenfeld D, Tucker A, Madri JA. Pecam-1 promotes beta-catenin accumulation and stimulates endothelial cell proliferation. Biochem Biophys Res Commun. 2003;303:212–218. doi: 10.1016/s0006-291x(03)00313-9. [DOI] [PubMed] [Google Scholar]
- 41.Ilan N, Mahooti S, Rimm DL, Madri JA. Pecam-1 (cd31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci. 1999;Pt 18:112. 3005–3014. doi: 10.1242/jcs.112.18.3005. [DOI] [PubMed] [Google Scholar]
- 42.Chretien ML, Zhang M, Jackson MR, Kapus A, Langille BL. Mechanotransduction by endothelial cells is locally generated, direction-dependent, and ligand-specific. J Cell Physiol. 2010;224:352–361. doi: 10.1002/jcp.22125. [DOI] [PubMed] [Google Scholar]
- 43.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azuma N, Akasaka N, Kito H, Ikeda M, Gahtan V, Sasajima T, Sumpio BE. Role of p38 map kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol. 2001;280:H189–197. doi: 10.1152/ajpheart.2001.280.1.H189. [DOI] [PubMed] [Google Scholar]
- 45.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of akt in human endothelial cells: Involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 46.LaMack JA, Himburg HA, Zhang J, Friedman MH. Endothelial gene expression in regions of defined shear exposure in the porcine iliac arteries. Ann Biomed Eng. 2010;38:2252–2262. doi: 10.1007/s10439-010-0030-6. [DOI] [PubMed] [Google Scholar]
- 47.Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res. 106:1703–1711. doi: 10.1161/CIRCRESAHA.109.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orr AW, Hahn C, Blackman BR, Schwartz MA. P21-activated kinase signaling regulates oxidant-dependent nf-kappa b activation by flow. Circ Res. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solanas G, Porta-de-la-Riva M, Agusti C, Casagolda D, Sanchez-Aguilera F, Larriba MJ, Pons F, Peiro S, Escriva M, Munoz A, Dunach M, de Herreros AG, Baulida J. E-cadherin controls beta-catenin and nf-kappab transcriptional activity in mesenchymal gene expression. J Cell Sci. 2008;121:2224–2234. doi: 10.1242/jcs.021667. [DOI] [PubMed] [Google Scholar]
- 51.Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 53.Gagarin D, Yang Z, Butler J, Wimmer M, Du B, Cahan P, McCaffrey TA. Genomic profiling of acquired resistance to apoptosis in cells derived from human atherosclerotic lesions: Potential role of stats, cyclind1, bad, and bcl-xl. J Mol Cell Cardiol. 2005;39:453–465. doi: 10.1016/j.yjmcc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. Mcp-1 and il-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 55.Foteinos G, Hu Y, Xiao Q, Metzler B, Xu Q. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein e-deficient mice. Circulation. 2008;117:1856–1863. doi: 10.1161/CIRCULATIONAHA.107.746008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


