Weight loss surgery (WLS) has had a tumultuous history. The initial operations performed 5 decades ago had questionable weight loss, carried unacceptably high risks, and had unknown long-term health benefits [1]. For many years, WLS was the option of last resort and only for the most extremely debilitated patients. But things have changed, and dramatically so. Unbridled growth in severe obesity has been matched by advances in surgical techniques and technologies available for its treatment. Soon it may not be unreasonable to consider WLS as the first line treatment for obesity and weight-related comorbidities like diabetes and sleep apnea.
Indeed WLS offers much more than significant and sustained weight loss. Recent studies demonstrate its ability to cure diabetes [2], to improve outcomes from cardiovascular disease (CVD) in a large, matched cohort of patients [3], and to reduce the risk of death by approximately 35% over time [4,5]. Findings from the Longitudinal Assessment of Bariatric Surgery I (LABS-1) trial, a prospective, multicenter observational study in 4776 WLS patients, reported a 30-day overall death rate of 0.3%, with serious complications in 4.1% of patients—figures similar to those seen in other major operations [1,6–9] (Fig 1). Between 1998 and 2004, the number of weight loss procedures performed in the United States soared by 800% to 121,500 [10]. That number reached 171,000 in 2005 [1]. Despite this exponential growth, WLS still has the perception of being a risky procedure among the general public, insurance companies, and even other health care providers. The sheer number of cases performed annually has raised concerns among third party payers and government agencies about provider qualifications and patient safety. For its part, the obesity health care providers have gone through great lengths to ensure that the quality of WLS has kept pace with quantity. Fellowships devoted solely to bariatric surgery have been established [1], and more importantly, evidence-based standards for the care of WLS patients have been published [11].
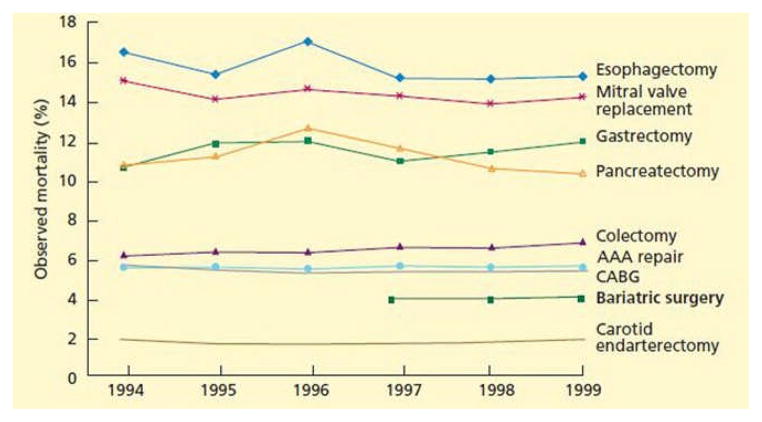
Fig 1.
Mortality after bariatric and other surgery after age 65. AAA, abdominal aortic aneurysm; CABG, coronary artery bypass grafting. (Reprinted with permission from Flum et al [84]. Adapted with permission from original in Goodney PP, Siewers AE, Stukel TA, Lucas FL, Wennberg DE, Birkmeyer JD. Is surgery getting safer? National trends in operative mortality. J Am Coll Surg 2002;195:219–27.) (Color version of figure is available online.)
The first such report came in the wake of a massive chemotherapy overdose that killed Boston Globe journalist Betsy Lehman [12] and led to the subsequent creation of the Betsy Lehman Center for Patient Safety and Medical Error Reduction (Lehman Center). This organization’s mission is to improve patient safety by developing evidence-based, best practice standards of care.
In 2004, the Lehman Center and the Massachusetts Department of Public Health convened an Expert Panel [13] to assess weight loss procedures, identify issues related to patient safety, and develop evidence-based best practice recommendations. The Panel worked with more than 100 specialists in 9 separate task groups to examine every facet of care—from psychological evaluation and anesthetic perioperative procedures to multidisciplinary treatment and data collection (Table 1).
Table 1.
| Surgical care |
| Criteria for patient selection and multidisciplinary evaluation and treatment |
| Patient education/informed consent |
| Anesthetic perioperative care and pain management |
| Nursing perioperative care |
| Pediatric/adolescent care |
| Facility resources |
| Coding and reimbursement |
| Data collection/future considerations |
| Endoscopic intervention |
| Policy and access to care |
The resulting document, published as a supplement to Obesity in 2005 [13], set the standard for WLS across the state and well beyond it. The Agency for Healthcare Research and Quality (AHRQ) abstracted the report for broad use and the American College of Surgeons (ACS) used it as the blueprint for its Bariatric Surgery Network Center accreditation program. Its recommendations influenced health care policy and medical practice in the United States and abroad [14].
Much has happened since 2005, including rapid growth in the literature, development of new procedures, shifting patient demographics, shorter lengths of hospital stay, and widespread use of laparoscopy. To address the impact of these changes on patient safety, the Lehman Center reconvened the Expert Panel in 2007 to update the earlier systematic literature review and evidence-based recommendations.
The new report, published in Obesity 2009 [15], is even more comprehensive than the first. It covers every practice area in the original publication as well as 2 new topics: endoscopic interventions and policy and access. Recommendations were developed using an established evidence-based model. This approach was used to optimize patient safety in a high-risk specialty that continues to grow at a breakneck pace, fueled, in part, by the high failure rate of alternative therapies (eg, improved nutrition, behavior modification, increased exercise, and medications) [9,16].
In 2006, the number of weight loss procedures performed in the United States topped 200,000 [17]; in 2008, that figure reached an estimated 220,000 [18,19]. Weight loss operations will continue to grow at an accelerating pace as evidence on their safety and efficacy mounts and more insurers provide coverage [20]. Today, there are approximately 15 million people in the United States with a body mass index (BMI) greater than 40 kg/m2, but only 1% of the clinically eligible population receives surgical treatment for their obesity [18].
This situation is not limited to the United States. The World Health Organization (WHO) estimates that more than 400 million adults currently have class I obesity (BMI >30). By the year 2015, that number is expected to reach an estimated 700 million [21]. Without intervention, only 1 in 7 obese patients will reach their full life expectancy [22,23].
Bariatric surgery has taken on many forms since the first operations in the 1950s. Five decades later, no single “best” operation has emerged from the available options. But a lot of effort has been placed on determining the best way to treat obesity and to avoid the complications of WLS. Care starts with a multidisciplinary approach to surgical weight loss and ends with life-long follow-up for those who undergo WLS.
Multidisciplinary Teams
WLS patients suffer from a multifactorial disease that makes them a uniquely vulnerable population in need of specialized resources and ongoing multidisciplinary care [15,24]. The Lehman Center report stressed the need to use dedicated teams to provide best practice treatment (Table 2). These should include surgeons, nurses, anesthesiologists, psychologists, dietitians, and others who are specially trained to deliver pre-, peri-, and postoperative care. Use of such teams can identify obesity-related conditions that may put patients at increased operative risk for complications, morbidity, and mortality [25]. Dedicated, multidisciplinary treatment teams and support groups for long-term follow-up have improved the efficacy and safety of WLS. So, too, have accredited “Centers of Excellence” that implement evidence-based, best practice standards.
Table 2.
Assets of multidisciplinary surgical weight loss team [14]
| Assets to multidisciplinary surgical weight loss program (required) |
| Bariatric surgeons (2) |
| Bariatrician |
| Pediatric obesity specialist* Program coordinator Dietitian/nutritionist |
| Psychiatrist/psychologist/social worker |
| Exercise physiotherapist |
| Surgical floor nurses |
| Operating room nurses |
| Operating room technicians |
| Receptionist |
| Additional assets to multidisciplinary surgical weight loss program (preferred) |
| Anesthesiologist |
| Gastroenterologist |
| Radiologist |
| Cardiologist |
| Pulmonologist |
| Endocrinologist |
For pediatric weight loss surgery only.
Minimally Invasive WLS
Since 2004, WLS has been mainstreamed into accredited training programs in the United States [26]. This change has helped shorten the learning curve for laparoscopic operations. In recent years, minimally invasive WLS has increased from 9.4% of procedures to 71.0%. In large part, this shift accounts for the growing popularity of WLS and the rapid increase in the number operations performed [27].
Minimally invasive techniques have reduced some of the complications in readmissions rates, and a 2.3-day decrease in length of stay [27] (Table 3). Laparoscopy has not only led to greater acceptance of WLS as a treatment for obesity, but it has also paved the way for new kinds of procedures. Overall, laparoscopy has been a notable advance. A comparison between patients who had WLS in the 2001 to 2002 period and those who had it between 2005 and 2006 shows a 21% decrease in total complications, 37% decrease in inpatient complications, 31% decrease in readmissions rates, and a 2.3-day decrease in length of stay [27] (Table 3). Laparoscopy has not only led to greater acceptance of WLS as a treatment for obesity, but it has also paved the way for new kinds of procedures.
Table 3.
Risk-adjusted changes in bariatric outcomes and utilization over time
| Unadjusted | Risk adjusted | |||
|---|---|---|---|---|
| 2001–2002 | 2005–2006 | 2001–2002 | 2005–2006 | |
| Inpatient complication rate | 21.93% | 15.31%* | 23.60% | 14.81%* |
| 30-day overall complication rate | 32.39% | 26.31%* | 33.68% | 25.45%* |
| 180-day overall complication rate | 39.57% | 33.64%* | 41.69% | 32.81%* |
| Specific 180-day complications | ||||
| Anastomosis complications | 12.29% | 9.48%* | 13.01% | 9.26%* |
| Marginal ulcer | 0.99% | 2.05%* | 1.81% | 2.05% |
| Abdominal hernia | 7.10% | 4.83%* | 7.19% | 4.81%* |
| Dumping, vomiting, diarrhea, etc. | 19.59% | 19.34% | 21.44% | 18.63% |
| Hemorrhage | 1.67% | 2.06% | 1.96% | 1.94% |
| Wound dehiscence | 1.78% | 2.32% | 2.19% | 2.15% |
| Infection | 5.59% | 3.32%* | 7.16% | 3.03%* |
| Deep vein thrombosis/pulmonary embolism | 2.34% | 2.47% | 2.50% | 2.40% |
| Respiratory failure | 3.05% | 2.42%† | 4.27% | 2.15%* |
| Pneumonia | 4.08% | 3.26%† | 5.02% | 3.01%‡ |
| Postoperative acute myocardial infarction | 0.32% | 0.40% | 0.46% | 0.37% |
| Postoperative stroke | 0.00% | 0.08% | — | 0.10% |
| Readmission with complication | 7.18% | 7.59% | 9.78% | 6.79%* |
| Emergency room visit with complication | 1.31% | 1.86%† | 1.44% | 1.79% |
| Outpatient hospital visit with complication 14.23% | 13.48% | 14.78% | 13.26%* | |
| Office visit with complication | 11.22% | 11.11% | 12.61% | 10.60% |
| 180-daytotal hospital days (days) | 6.0 | 4.0* | 6.1 | 3.7* |
| 180-day total hospital payments ($) | 31,016 | 27,591‡ | 29,563 | 27,905* |
| 180-day inpatient physician payments ($) | 3308 | 3151 | 3383 | 3128 |
Adapted with permission from Encinosa and colleagues [27].
Significantly different from the 2001–2002 complication rate at the 99% level.
Significantly different from the 2001–2002 complication rate at the 90% level.
Significantly different from the 2001–2002 complication rate at the 95% level.
Types of WLS
Primary operations for WLS are either restrictive, malabsorptive, or a combination of both (Table 4). Most malabsorptive procedures fall into the latter category. The 2 most common operations in the United States today are the adjustable gastric band (AGB) and Roux-en-Y gastric bypass (RYGB) [30]. Other approaches include sleeve gastrectomy (SG), biliopancreatic diversion (BPD) with or without a duodenal switch (DS), vertical banded gastroplasty (VBG), and jejunoilial bypass (JIB). Very few VBGs are performed due to numerous complications. JIB has been abandoned altogether for the same reason [31].
Table 4.
Types of weight loss surgery operations
| Types of operation | % excess weight loss | |
|---|---|---|
| Restrictive | Adjustable gastric band (AGB) | 50–60% [165] |
| Sleeve gastrectomy (SG) | 33–83% [187,190] | |
| Vertical banded gastroplasty (VBG) | 63–70% [196] | |
| Malabsorptive | Jejunal-ileal bypass (JIB) | |
| Combined | Roux-en-Y gastric bypass (RYGB) | 70–80% [53] |
| Biliopancreatic diversion/duodenal switch (BPD-DS) | 77–88% [276,386] | |
| Endoscopic | Intragastric balloon | 27–48% [297] |
| Endoluminal sleeve | NA | |
| Endoluminal plication | NA | |
| Others | Gastric pacing | 40% [303] |
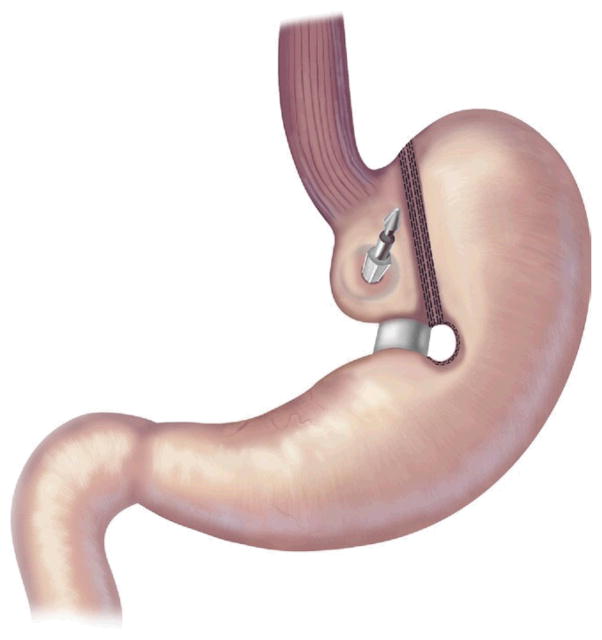
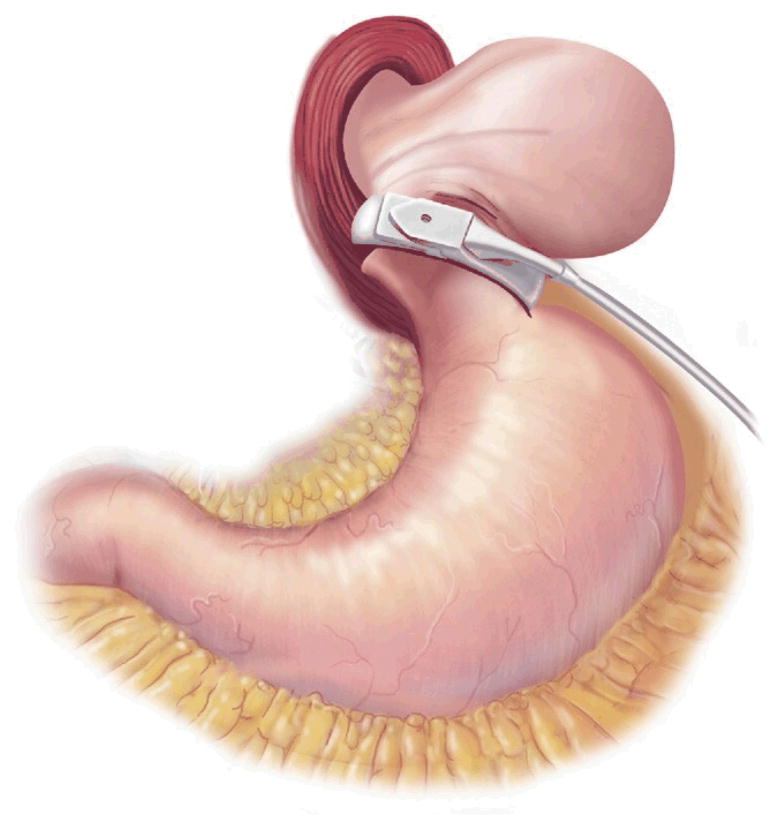
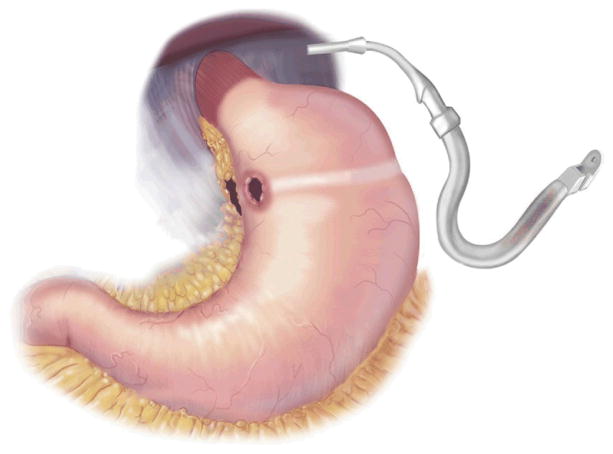
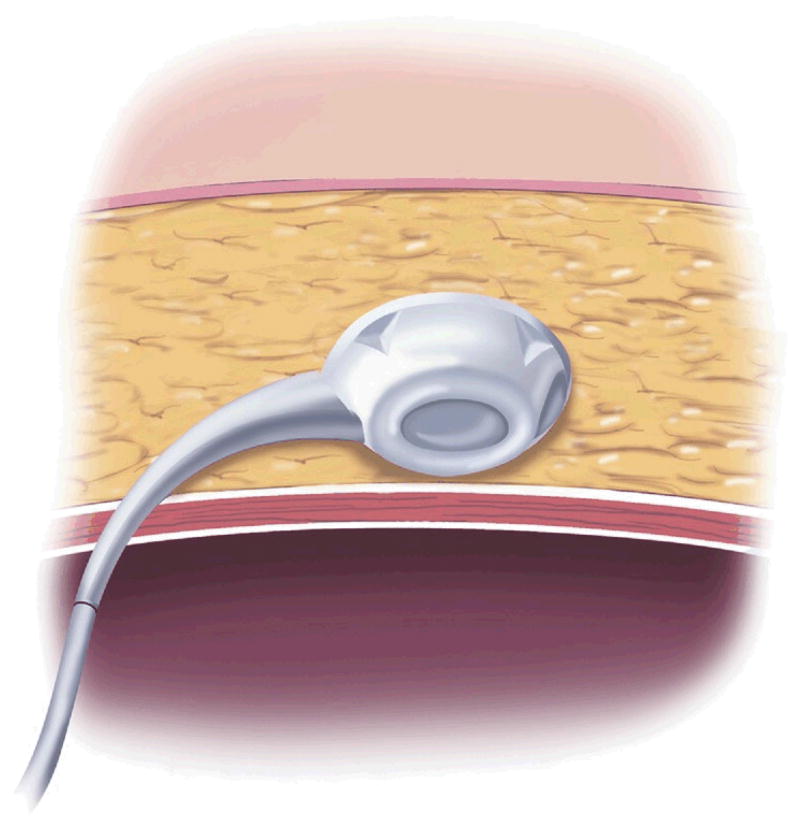
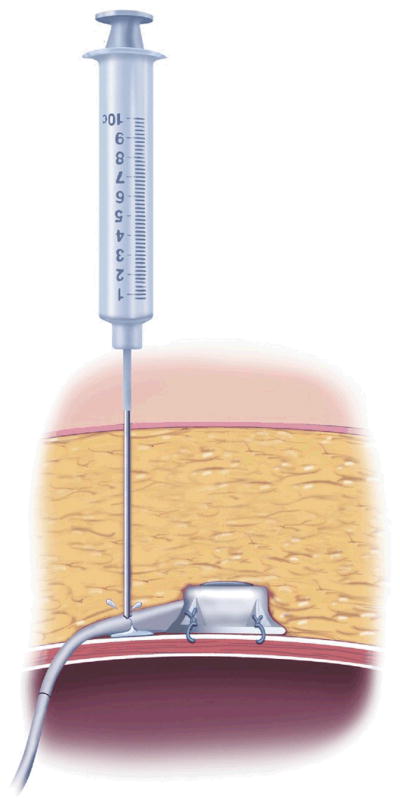
AGB surgery is purely restrictive. It involves placing an inflatable Silastic band around the proximal aspect of the stomach to create a 30-mL gastric pouch [32] (Fig 2). The band attaches via tubing to a port in the subcutaneous tissue, which can be accessed with a Huber needle, much like a chemotherapy port. The port is used by surgeons to inject or withdraw fluid from the band to further restrict or loosen it [33].
Fig 2.
Adjustable gastric band. (Adapted with permission from Jones and colleagues[33], c2007 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
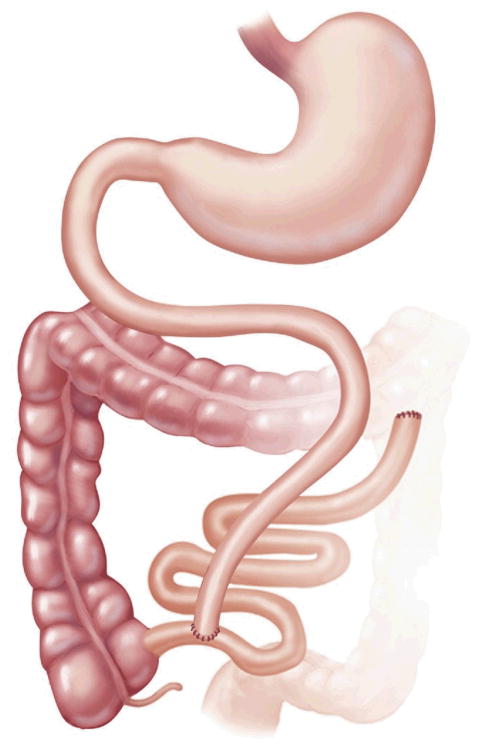
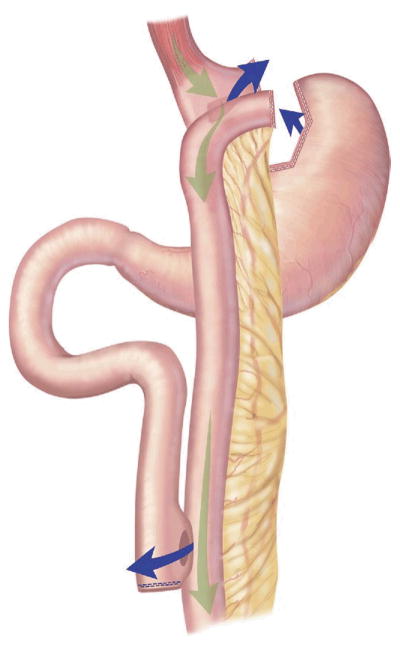
RYGB is a combined restrictive and malabsorptive procedure that entails creating a small 15- to 30-mL stomach pouch and a Roux limb 75 to 150 cm in length that reroutes a portion of the alimentary tract to bypass the distal stomach and proximal small bowel [15]. Connecting the Roux limb to the biliopancreatic limb forms the common channel [34] (Fig 3). Many variations of RYGB are available. The Roux limb can be passed through the transverse mesocolon (retrocolic) or in front of the colon (antecolic). It can also be passed in front of the stomach (antegastric) or behind it (retrogastric). So the procedure can be described as an antegastric, retrocolic RYGB, for instance. Additionally, there have been groups that perform a resectional gastric bypass, where the remnant stomach is removed [35]. No single technique has proven superior in weight loss or overall complications.
Fig 3.
Roux-en-Y gastric bypass.(Adapted with permission from Jones and colleagues [33], c2007 Cine-Med Publishing, Inc., www.cine-med.com.)(Color version of figure is available online.)
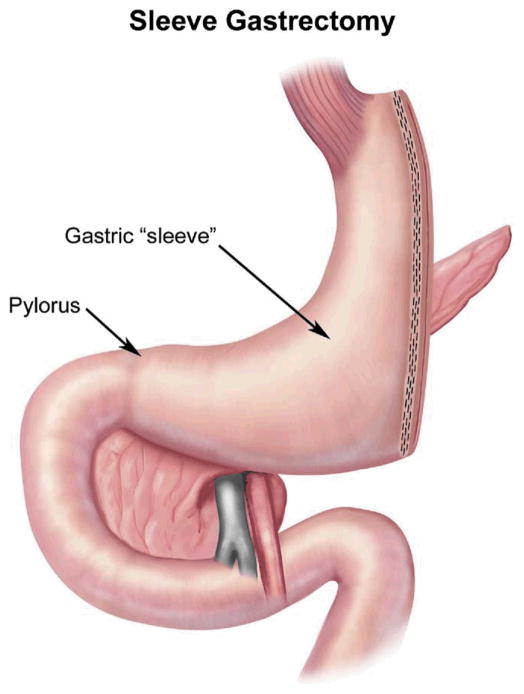
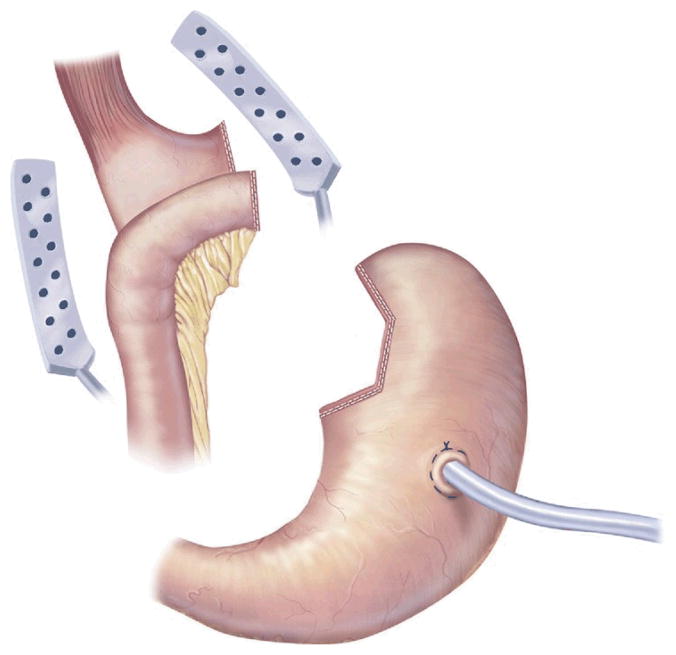
The SG is a restrictive procedure that creates a 100- to 150-mL stomach by performing a partial gastrectomy of the greater curvature side of the stomach [36] (Fig 4). The last 6 to 8 cm of antrum remains intact, and thus, the pylorus is preserved to help prevent gastric emptying problems. At times, an intraoperative decision is made to perform an SG in lieu of a more technically challenging malabsorptive procedure, if the latter is felt to be too dangerous. This choice is usually prompted by adhesions or a body habitus that compromises adequate visualization. After significant weight loss has occurred, the SG can be revised to a BPD-DS or a RYGB to treat the remaining obesity [37]. Unlike AGB, research suggests that hormonal changes occur with SG. Serum levels of ghrelin, a hormone that stimulates appetite, decrease after SG but increase after AGB [38]. For this reason, some surgeons believe that SG may be superior to AGB. However, long-term data on the differences between the 2 approaches are not yet available.
Fig 4.
Sleevegastrectomy. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
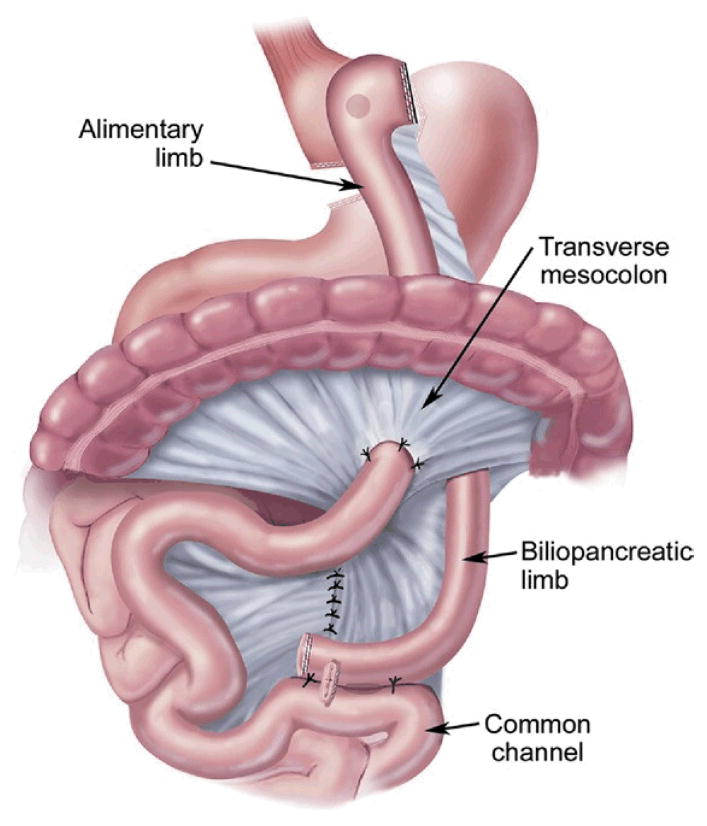
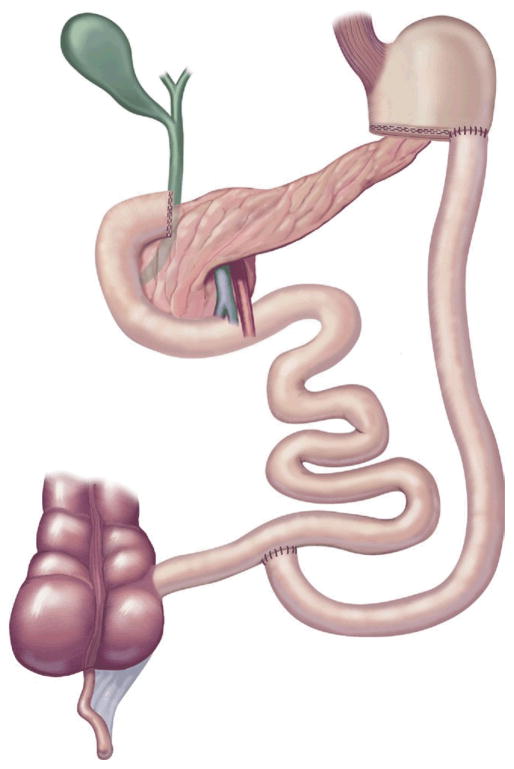
The original BPD or Scopinaro procedure was designed to eliminate the complications from bacterial overgrowth seen with the JIB [39]. The classic Scopinaro procedure was introduced in 1979 and involved: 1) partial gastrectomy, 2) dividing the small bowel halfway between the ligament of Treitz and the ileocecal valve, 3) a Roux-en-Y gastroenterostomy between the stomach pouch and the distal small bowel to create an alimentary limb, and 4) a biliopancreatic limb that joins the alimentary limb to create a common channel [40] (Fig 5).
Fig 5.
Biliopancreatic diversion. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
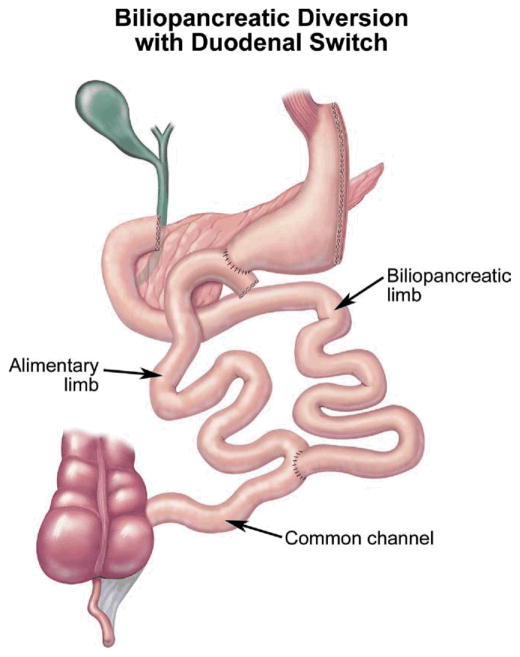
The DS is a modification of the BPD created to control some of the complications of the original Scopinaro procedure [41] (Fig 6). A 150- to 200-mL SG is performed with preservation of the lesser curvature, antrum, pylorus, first portion of the duodenum, and vagal innervation to decrease dumping and marginal ulceration [42]. The duodenum is divided between its first and second portions, and the jejunem is divided halfway between the ligament of Treitz and the ileocecal valve. The alimentary limb is created by anastomosing the distal small bowel limb to the proximal duodenum. The distal duodenum with the remainder of the small bowel is the biliopancreatic limb. It is anastomosed to the distal small bowel to create the common channel [43].
Fig 6.
Biliopancreatic diversion-duodenal switch.(Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
A common channel length of at least 100 cm is generally preferred to decrease metabolic disturbances and the need for revision due to malnutrition [44]. In general, the complications and outcomes after BPD and DS are similar.
VBG involves creating a small stomach pouch by first fashioning a gastrotomy with an end-to-end anastomosis (EEA) stapler in the proximal stomach. A linear stapler is then fired from the gastrotomy to the angle of His to create the lateral edge of the neo-stomach. A ring is placed around the stomach from the lesser curvature to the initial gastrotomy to form the distal aspect of the neo-stomach [39]. The remainder of the stomach remains intact (Fig 7). The use of VBG has fallen out of favor among most weight loss surgeons due to higher rates of complication and inferior long-term weight loss compared with the easier-to-perform AGB [39].
Fig 7.
Vertical banded gastroplasty. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
JIB is a purely malabsorptive procedure that connects the proximal jejunum to the distal ileum so that the length of the common channel (or absorptive capacity) is about 100 cm (Fig 8). As mentioned earlier, this approach has been abandoned due to numerous complications related to bacterial overgrowth in the blind limb [39].
Fig 8.
Jejunal-ileal bypass. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
Staged Procedures
With the increasing incidence of extreme obesity (BMI >50 kg/m2), more patients are undergoing staged procedures using restrictive operations (AGB or SG) as a bridge to more technically complex malabsorptive techniques, such as a BPD or DS [45]. The latter procedures produce more weight loss than purely restrictive operations. After a few months of significant weight loss, more complex operations are less technically challenging to perform.
Indications for Surgery
In addition to the many different operations available, there is an increasing list of indications for WLS. The original National Institutes of Health (NIH) criteria limited WLS to those with a BMI greater than 40 kg/m2 or a BMI greater than 35 kg/m2 with comorbidities of obesity [46].
Today, more and more adolescents are undergoing WLS as the incidence of obesity increases in this population [47].
A growing body of data also suggests that WLS may be used successfully to treat diabetes in overweight and even normal-weight individuals, and that WLS performed before other kinds of surgeries can improve outcomes. These new indications mean that general surgeons will see more and more WLS patients, increasing the need to be familiar with surgical anatomies, results, and complications. Early recognition of complications is critical. After initial stabilization, post-WLS patients should be referred to their operating surgeon or a full-service WLS program.
Complications
Complications can be divided into 3 major categories: intraoperative, early postoperative, and late postoperative (Table 5). Some apply to all WLS surgeries, whereas others are procedure specific. Complications that are germane to all WLS operations are: DVT/PE, pulmonary/cardiovascular complications, gallstone formation, malnutrition, psychiatric sequelae, failure to lose weight, and death.
Table 5.
General complications of weight loss surgery
| Intraoperative | Splenic injury |
| Trocar injury | |
| Bowel ischemia | |
| Early | Leak DVT/PE |
| Cardiovascular | |
| Pulmonary | |
| Death | |
| Late | Gallstone formation |
| Nutritional deficiencies | |
| Neurologic | |
| Psychiatric | |
| Inadequate weight loss |
DVT, deep venous thrombosis; PE, pulmonary embolism.
According to national registries, significant progress has been made in the care of WLS patients, with better prevention and control of adverse events [16,48,49]. Efforts to promote continuous improvement are being performed by groups like the Lehman Center [50] and the ACS. The National Surgery Quality Improvement Program (NSQIP), which provides risk-adjusted outcomes, allows centers to compare their results [51]. A meta-analysis of more than 80,000 patients who underwent WLS procedures since 1990 shows an overall perioperative mortality rate of 0.28%, with 0.35% mortality in the first 2 years [48].
Registries track resolution of such obesity-related comorbidities as diabetes, hypertension, hyperlipidemia, and obstructive sleep apnea. As mentioned earlier, WLS also decreases overall mortality in obese patients compared with controls [49] (Table 6). However, more work must be done to ensure the best possible outcomes for obese patients. Indeed, future treatment algorithms may differ from those in use today, but for now, WLS remains the most successful and cost-effective way to treat severe obesity.
Table 6.
Benefits of weight loss surgery
| Increased life expectancy |
| Decreased risk of cardiovascular event |
| Resolution of diabetes |
| Resolution of hypertension |
| Resolution of hyperlipidemia |
| Resolution of sleep apnea |
| Resolution of GERD* |
| Resolution of polycystic ovarian syndrome |
| Resolution of stress urinary incontinence |
| Improvement of degenerative joint disease |
| Improvement of venous stasis disease Improvement of nonalcoholic hepatitic steatosis Improvement of pseudotumor cerebri |
| Increased fertility |
| Decreased complications from pregnancy and childbirth |
| Improved quality of life |
| Improved outcomes from nonbariatric operations |
| Decreased cancer risk |
GERD, gastroesophageal reflux disease.
Roux-en-Y gastric bypass specifically.
Intraoperative Complications
WLS in obese patients is technically challenging. Thick subcutaneous tissue increases the torque on the parts, making fine movements more difficult. Extensive intra-abdominal and visceral fat can obscure visualization and limit exposure. Despite safeguards, intraoperative complications may occur even among highly skilled surgical teams.
Splenic Injury
Minor splenic injury usually consists of a capsule tear, and splenectomy is rarely required. In 1 series of open WLS that included revision procedures, the rate of splenic injury was 3%; only 1 splenectomy (0.5%) was required [52]. Splenic injuries typically occur from excess traction on short gastric vessels used to identify the greater curve side of the stomach’s cardia. In RYGB and SG, the short gastric vessels are commonly divided using electrocautery, the harmonic scalpel, the ligature device, or clips. Retracting downward on the stomach can tear the capsule of the spleen and lead to bleeding.
If bleeding is minimal, direct pressure can be applied laparoscopically with gauze pads. Products like Surgicel promote hemostasis. Any question of continued bleeding should lead to conversion to open surgery. If bleeding cannot be controlled after conversion to the open approach, use of instruments like the argon beam, followed by splenectomy, may be required. Surgeons should not hesitate to convert to an open procedure when there is brisk hemorrhaging from a splenic source.
Bowel Ischemia
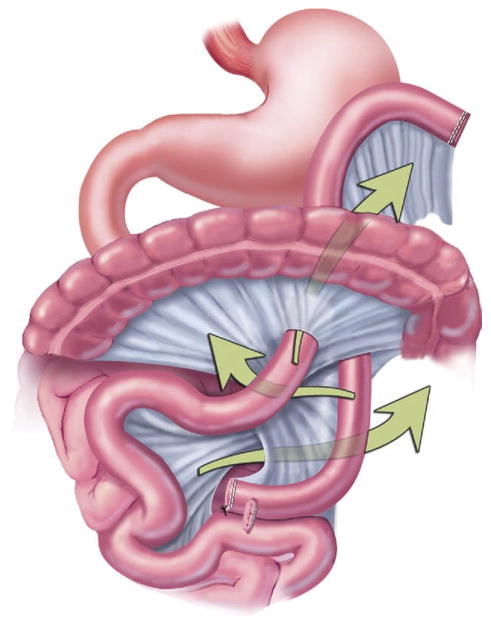
Intraoperative bowel ischemia can occur from several different maneuvers during malabsorptive WLS procedures. When the small bowel is divided, intestinal ischemia can result from a division of the mesentery that compromises the mesenteric root. Too much tension on the Roux limb can affect the vascular supply. In addition, the Roux limb can twist if not properly oriented, causing interrupted blood flow. Internal herniation between bypassed segments can lead to ischemia (Fig 9). During repair of mesenteric defects to prevent herniation, an injury to the mesenteric vessels can also result in ischemia.
Fig 9.
Possible internal hernia sites after RYGB. (Adapted with permission from Jones and colleagues [33], c2007 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
If a prior WLS surgery patient presents with signs of severe abdominal pain, hematochezia, and an acute abdomen [53], the diagnosis of intestinal ischemia should be considered. In the long term, mesenteric ischemia may contribute to leaks [54] and stenoses. The stable patient can be transferred to a bariatric team familiar with the surgical anatomy. For the unstable patient, prompt surgical intervention should include examination for ischemia, internal hernias, and adhesions.
Trocar Injury
It can be very difficult during laparoscopic procedures to obtain a pneumoperitoneum, and injuries can occur during placement of the Veress needle or the ports. Damage to the aorta and iliac vessels can be life threatening, but the overall incidence of such injuries is quite low, ranging from 0% to 0.16% [55,56]. Schwartz and colleagues described the use of a Veress needle placed in the left upper quadrant near the costal margin in the midclavicular line to obtain the pneumoperitoneum before placing the trocar. Of 600 severely obese patients, they reported only 1 incident, a colonic serosal injury, where the mucosa was not violated. Sub- and intraomental air occurred, but was not of clinical significane [55]. Others using an optical view bladeless trocar without a pneumoperitoneum report no mortality [56]. A Cochrane review of 17 randomized controlled trials comparing pneumoperitoneum techniques in 3040 patients concluded that there was no statistical difference in complication rates between closed, open, or optical view insertion techniques for establishing a pneumoperitoneum [57]. Obese patients, though, were not analyzed separately, and there are no randomized controlled trials comparing the different techniques in patients with a BMI greater than 35 kg/m2.
Transabdominal ultrasound has also been used in patients with previous abdominal operations who are undergoing RYGB. Kothari and colleagues found that preincision outcomes from radiology correlated with the surgeon’s intraoperative findings [58]. However, this technique has never been compared with other approaches for avoiding trocar associated injury. When performing nonbariatric procedures in obese patients, Jones and colleagues favor use of the left upper quadrant Veress needle placement to gain the pneumoperitoneum and optical view for trocar entry [33]. However access is achieved, the surgeon should routinely inspect the area underneath the insertion site for bleeding or intestinal injury. Uncontrolled bleeding requires prompt conversion to an open procedure to treat the injury.
Early Postoperative Complications
Leak
The overall leak rate after WLS is 0.0% to 5.6% [53]. Leaks are the most dreaded complication due to their association with life-threatening sequelae. Gonzalez and colleagues reported a mortality of 15% in patients with leaks versus 1.7% in those without them [54]. Interestingly, randomized controlled trials comparing open RYGB to laparoscopic RYGB do not show a difference in leak rates [28]. Leaks are more apt to occur with either technique if the patients are male and over the age of 50 [59]. In laparoscopic BPD-DS, the leak rate ranges from 0.4% to 0.9% [41]. No randomized trials have compared leak rates in open versus laparoscopic BPD-DS. SG leak rates range from approximately 0% to 1.4% [60]. In purely restrictive procedures (eg, AGB and VBG), they vary from 0.0% to 0.05% [49,61].
It is important to note that leak rates increase greatly in all revision operations. Revision surgery is the most important predictor of leaks, with a rate of 18% to 35% [62,65]. Leaks may occur as a result of technical factors such as ischemia, tension [54], or stapler misfire. Surgeons who have performed less than 75 cases seem to have a higher likelihood of developing a leak complication during laparoscopic procedures [28].
Several studies have evaluated techniques used to prevent leaks. Oversewing of the staple line using a continuous suture may reduce gastrogastric fistula formation [66]. Bovine pericardial strips may reduce the incidence of staple line bleeding, but their effect on leak rate is questionable [67]. Fibrin sealant has yet to prove a decrease in the incidence of leaks [54]. Although some subclinical leaks have been managed nonoperatively [68], prompt recognition of the clinical signs, evaluation, diagnosis, and management can be life saving. Leaks are discussed in more detail in the RYGB section.
Deep Venous Thrombosis/Pulmonary Embolism
Pulmonary embolism (PE) is the most common cause of death in the perioperative period, accounting for 50% of all such deaths [69]. The rates of deep venous thrombosis (DVT) after WLS are similar between open and laparoscopic techniques: up to 1.3% and 0.4%, respectively [70]. The rate of PE after open WLS ranges from 0.25% to 3%; after laparoscopic procedures, it ranges from 0.7% to 2.4% [70]. In AGB surgery, PE is also the most common cause of death [71]. Melinek and colleagues found microscopic evidence of DVT in 80% of gastric bypass patients, although only 20% were diagnosed clinically [72]. In that obesity induces a prothrombotic state, WLS candidates should be considered at high risk for the development of venous thromboemboli [73].
Unfortunately there are no quality data identifying the best forms of DVT/PE prophylaxis, and the practice of experienced weight loss surgeons varies widely [74]. Unless there are other clinical concerns, the Lehman Center report recommends the use of anticoagulants with sequential compression devices [13]. For patients at increased risk for DVT/PE, extended prophylaxis should also be considered [13].
Another approach is the use of inferior vena cava (IVC) filters in patients who are at the highest risk for a DVT or PE (ie, those with a history of a venous thrombolic event or venous stasis, poor ambulation, pulmonary hypertension, severe sleep apnea, a BMI >60 kg/m2, or central obesity) [75]. In a series of 330 such patients, those who had IVC filters placed preoperatively were less apt to develop a PE than those who did not (0.63% vs. 2.94%) [76]. It is important to note that for patients who have undergone WLS, their risk of DVT/PE is likely to remain elevated despite substantial weight loss, especially if their preoperative BMI was greater than 50 kg/m2. Unless there are contraindications, postoperative WLS patients who remain severely obese should receive anticoagulants and sequential compression devices during general anesthesia. Consultation with a hematologist or vascular surgeon may be helpful when treating high-risk patients.
Cardiovascular Complications
The incidence of an ischemic event after WLS is less than 1% [53]. Fatal cardiovascular events can range from 12.5% to 17.6% of all perioperative deaths [77], making them the second most common cause of postoperative mortality [71]. Between 1 and 6 months after WLS, cardiovascular complications cause 33% of all such deaths [22]. Cardiovascular risk decreases over time [78], but remains high immediately after operation.
Pulmonary Complications
Atelectasis occurs at a rate of 8.4% after laparoscopic WLS [79], but the incidence of other pulmonary complications is approximately 4.5% [80]. Of all perioperative deaths, respiratory failure accounts for an estimated 11.8%, making it the third most common cause of mortality [77]. Persistent vomiting or reflux after WLS can be due to stomal obstruction or stenosis, and such patients are at long-term risk for aspiration pneumonia [81]. Those on Medicare, with chronic lung disease, men, and patients over age 50 have the highest incidence of postoperative pulmonary complications [82].
Mortality
Even among well-selected patients, the rate of mortality after the most commonly performed WLS procedures ranges from 0% to 2.5%. One meta-analysis of RYGB, AGB, VBG, and BPD found no statistically significant difference in mortality rate [49]. However, another study indicated that AGB had the lowest overall death rate, at 0.01%, and BPD the highest, at 0.8% [77]. Several recognized risk factors for death after WLS include male gender, age greater than 65, and surgeon inexperience.
Data show that low-volume hospitals with fewer than 50 cases per year have the highest rate of adverse outcomes [83]. Odds of death at 90 days are 1.6 times higher for patients whose surgeons perform less than themedian volume of bariatric procedures [84]. Flum and colleagues found that within the first 30 days, patients older than 65 years had a 4.8% death rate, with older men having a higher risk of 3.7% [84]. The most common causes of death after WLS are PE, followed by myocardial infarction, leak, and respiratory failure [77].
Late Postoperative Complications
Complications can occur from several weeks to several years after WLS. Physicians should be wary of abdominal pain, extremity weaknesses, rashes, psychiatric complaints, or inability to tolerate a diet. WLS patients require lifelong follow-up and multidisciplinary care with a team that includes a nutritionist, psychiatrist, and other consultants [15].
Gallstone Formation
The incidence of gallstone formation is 27% to 38% after RYGB [53]. Several factors, including decreased gallbladder emptying, contribute to development of stones. Surgical disruption of hepatic branches of the vagus nerve and altered enteric stimulation can also result in biliary dyskinesia and bile stasis [85]. Gallstones also tend to form as a result of postoperative changes in gallbladder mucin production, calcium concentration, and the bile salt/cholesterol ratio [86,87]. For these reasons, many weight loss surgeons perform a concomitant cholecystectomy, especially if gallstones are present during an RYGB. In those without preoperative gallstones, use of ursodiol for 6 months after RYGB can reduce the incidence of gallstone formation from 32% to 2% [88].
The use of prophylactic cholecystectomy remains controversial and may not be reimbursed by third party payers. If prophylactic cholecystectomy has not been performed, patients with right upper quadrant or epigastric pain should be evaluated for gallstones and common bile duct stones. Choledocholithiasis, in particular, poses a problem with the divided pouch and resultant inability to rely on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and treatment.
The incidence of gallstone formation in AGB and VBG is similar to that in RYGB. One study shows that 21.4% of patients develop gallstones after laparoscopic AGB [85]. These patients lost more than 1.7% of their weight per week [85]. One single-institution, randomized, double-blind, prospective trial compared the incidence of gallstone formation with and without the use of ursodiol for 6 months after AGB and VBG. With ursodiol, the rate of gallstone formation fell from 22% to 3%. The authors concluded that it should be used for gastric restrictive as well as malabsorptive or combination procedures [89].
Unfortunately, most insurance companies do not cover expenses associated with routine use of ursodiol after purely restrictive procedures. Alternatively, a cholecystectomy can be performed a few weeks before WLS in patients with preoperative gallstones. In theory, doing so will reduce the odds that spilled bile will infect the band. Data show the incidence of cholecystectomy after SG ranges from 0.007% to 14% [90–92].
However, it is unknown whether patients were taking postoperative ursodiol. Recommendations call for SG patients to do so for 6 months after their operations [36].
In cases of choledocholithiasis in RYGB or BPD-DS patients, magnetic resonance cholangiopancreatography (MRCP) should be used to evaluate the common bile duct. If stones are detected and the duodenum cannot be accessed via ERCP or transgastric ERCP, open common bile exploration is usually necessary. Anecdotally, we have seen several post-RYGB patients with ampullary stenosis and right upper quadrant discomfort, dilated hepatic ducts, and elevated liver enzymes, and no stones with ultrasound or MCRP; yet choledochojejunostomy produced significant improvement. Ultimately, any WLS patient with postoperative right-sided abdominal or epigastric pain should be evaluated for gallstone disease and appropriately treated.
Nutritional Deficiencies
Many obese persons undergoing WLS have preoperative nutritional deficiencies [93] that can be exacerbated by malabsorptive procedures. Even patients undergoing purely restrictive procedures are at risk for nutritional deficiencies due to poor eating habits as well as food intolerances and eating restrictions [94,95]. This heightened risk underscores the importance of lifelong follow-up of WLS patients, and the need for clinicians to have a high index of suspicion for nutritional-related abnormalities. Nutritional deficiencies can occur in up to 44% of patients several years after operation [93]. The incidence of anemia can be as high as 74%, especially among women of childbearing age. Premenopausal women are also likely to have poor iron status due to menstruation [96].
Vitamin B12/Folate
After WLS, vitamin B12 deficiency results from the body’s inability to separate the vitamin from protein foodstuffs, and failure to absorb free vitamin B12 [93,97,98]. Patients may present with weakness and fatigue from megaloblastic anemia, parasthesias, peripheral neuropathy, and demyelination of the corticospinal tract and dorsal columns [99]. After RYGB and BPD, this can occur in 12% to 33% of patients [100]. The deficiency can be corrected with 350 J.g/day of vitamin B12. Some patients do very well with monthly subcutaneous injections [101]. Very few require parenteral administration (2000 J.g/mo) [101].
Folate deficiency has been reported in 38% of patients after RYGB [102]. Treatment is critical for those who intend to become pregnant. Folate deficiency may lead to neural tube defects in infants [103]. Supplementation with 400 J.g/day, an amount contained in most multivitamins [101], is generally recommended. Vitamin B12 and folate deficiencies rarely manifest clinically when patients are compliant with multivitamin use and nutrition follow-up.
Maintaining vitamin B12 and folate levels helps suppress rising homocysteine levels in WLS patients who already have, or are at risk for, metabolic syndrome. Homocysteine is an amino acid with direct toxic effects on vascular endothelium [104], and is recognized as an independent risk factor for CVD and thromboembolic events [105]. Several studies have demonstrated a rise of homocysteine above 10 J.mol/L in patients after WLS, and have attributed it to a decrease in vitamin B12 and folate [106–109].
Higher folate and vitamin B12 concentrations are needed to maintain normal homocysteine levels [97]. To keep them at less than 10 J.mol/L [106], Dixon and colleagues recommend a serum folate level of approximately 15 ng/mL and a vitamin B12 serum level greater than 600 pg/mL.
Iron
Iron deficiency results from 2 main mechanisms. With purely restrictive procedures, there is less gastric acid secretion to reduce dietary iron into the ferrous state required for absorption [93,110]. In malabsorptive procedures, bypassing the duodenum and proximal jejunem eliminates the 2 main areas of iron absorption [93]. Iron deficiency can be seen in up to 32% [111] of patients who undergo restrictive procedures, and in 14% to 52% of those who have malabsorptive WLS [100].
Patients typically present with diminished exercise and work tolerance, impaired thermoregulation, immune dysfunction, gastrointestinal disturbances, and cognitive impairment. They can also present with pica. Kushner and colleagues published a report of 2 RYGB patients who developed pica so severe, they routinely awoke in the middle of the night to satisfy their ice cravings [112]. This symptom resolved with iron replenishment [112].
Iron deficiency is usually treated with 650 mg of daily oral ferrous sulfate tablets [110]. Vitamin C helps promote iron absorption [110]. Additionally, pro-phylactic oral iron supplements are recommended for premenopausal women who undergo RYGB, especially if they are already anemic[97].
Thiamine (B1)
Thiamine deficiency (beriberi) can be due to decreased duodenal absorption, but more often, it is a consequence of persistent vomiting [113].
In WLS patients, it can occur early in the postoperative period, when weight loss is most rapid, or after prolonged vomiting caused by a variety of factors [114]. Bacterial overgrowth seen after some BPD and JIB procedures is also associated with thiamine deficiency [115]. Because thiamine is involved with carbohydrate metabolism, its reserves can be depleted in WLS patients on high carbohydrate diets [116].
Wernicke-Korsakoff syndrome (WKS)—a neurologic derangement characterized by ataxia, ophthalmoplegia, nystagmus, and mental confusion—is an increasingly recognized complication of thiamine deficiency. When brought on by dietary deficiency [114], it is most commonly seen in alcoholics. It has also been reported in patients suffering from hyperemesis gravidarum, AIDS, Crohn’s disease, and those receiving total parenteral nutrition (TPN) [114].
In WLS patients, WKS is associated with polyneuropathy and encephalopathic manifestations [117]. Given the lack of biochemical, radiologic, and histologic evidence, this complication can be difficult to diagnose [114]. However, rapid recognition and intervention are critical. Delay in thiamine replenishment increases the risk of long-term and even irreversible problems [118].
When thiamine deficiency is suspected, replacement should be quick and continuous until the rapidity of weight loss subsides or the cause of the prolonged vomiting is treated. In general, oral repletion with 50 to 100 mg of thiamine up to 3 times per day should correct the deficiency, but parenteral or intramuscular administration may be necessary in patients with hyperemesis [119]. Such therapy should last 7 to 14 days, then be continued orally [116] WLS patients should continue to receive a multivitamin. Most of these contain amounts of thiamine that exceed the recommended 1 mg/day for men and 0.8 mg/day for women [101].
Protein
Protein deficiency is defined as serum albumin below 3.5 g/dL [93]. It can occur in 18% of BPD patients [120], and 13% of those who have RYGB [121]. However, it is rare in those with Roux limbs shorter than 150 cm [122]. Hospitalization for severe protein deficiency can occur in 3.7% of BPD patients, and revision surgery in 6% [123]. Those with a common channel only 50 cm long have the highest rates of protein deficiency [121].
Patients with protein malnutrition can present with excessive weight loss, severe diarrhea, hyperphagia, muscle wasting (marasmus), and edema [124]. Approximately 3 weeks of TPN can correct the acute problems [125], although dietary counseling to increase protein intake can help prevent recurrences [126]. In general, WLS patients should have 1.2 g of protein/kg/day postoperatively [127].
Calcium and Vitamin D
Several studies show reduced bone mineral density in patients years after WLS [128–132]. Calcium is absorbed in the duodenum and proximal jejunum with malabsorptive procedures, making patients prone to calcium deficiency [93]. Conversely, vitamin D is absorbed in the jejunum and ileum. The condition is exacerbated by defective absorption of fat and fat-soluble vitamins [93]. Low serum calcium levels prompt increased parathyroid hormone production to induce release of calcium from bone, thereby increasing the long-term risk of osteoporosis [93].
Patients present with myalgias, arthralgias, muscle weakness, and fatigue—symptoms that may occur up to 12 years after gastric bypass [133].
Coates and colleagues found a reduction of bone mineral density 9 months after laparoscopic RYGB despite increased dietary calcium and vitamin D, and normal levels of parathyroid hormone and serum 25-hydroxyvitamin D [128]. The incidence of calcium deficiency and secondary hyperparathyroidism can be as high as 69% 4 years after BPD, with the prevalence of clinically significant hyperparathyroidism up to 27% [134].
Calcium, phosphorus, alkaline phosphatase, parathyroid hormone, and 25-hydroxyvitamin D should be monitored regularly in WLS patients. Calcium supplementation of 1.2 to 1.5 g/day and ergocalciferol dosing of 400 IU daily are recommended [135]. Calcium levels are maintained at the expense of mobilization from bone. Thus, it is important to note that secondary hyperparathyroidism manifests as a late consequence of calcium deficiency [136]. Treatment may require daily calcium dosages in excess of recommended amounts to prevent it [137,138]. In that calcium carbonate requires bioavailability of stomach acid, calcium citrate should be used to help correct the deficiency [93].
Other Fat-Soluble Vitamins: A, E,K
Shorter common channels delay mixing of fat with pancreatic enzymes and bile salts, decreasing fat absorption and increasing the risk of fat-soluble vitamin deficiencies after malabsorptive procedures [93]. As little as 32% of dietary fat is absorbed after BPD [139]. Slater and colleagues reported respective deficiencies in vitamins A, D, and K of 69%, 4%, and 68% 4 years after malabsorptive WLS [134]. Brolin and colleagues found a 10% incidence of vitamin A deficiency after RYGB [122].
A few case studies note vitamin A deficiency leading to night blindness [140]. In 1 report, it caused xerophthalmia, nyctalopia, and eventually, visual deterioration to legal blindness after RYGB [141]. Deficiencies in vitamins E and K have no significant clinical effect [125].
Zinc
Zinc deficiency is seen mainly after BPD [134], but can also occur after purely restrictive procedures due to poor dietary intake [142]. It can cause alopecia, but in general, clinical manifestations are uncommon [93] (Table 7).
Table 7.
Nutritional deficiencies
| Deficiency | Symptoms | Incidence | Prophylactic treatment | Deficiency treatment |
|---|---|---|---|---|
| Vitamin B12/Folate [101] | Megaloblastic anemia, parasthesia, peripheral neuropathy, demyelination of the corticospinal tract and dorsal columns | 12–38% | 350–400 J.g/day orally | 2000 J.g/mo IM or IV |
| Vitamin B1 thiamine [119] | Hyperemesis, Wernicke- Korsakoff syndrome, peripheral polyneuropathy | 0.8–1.0 mg/day | 50–100 mg three times per day for 7 to 14 days | |
| Vitamin A [97,134] | Night blindness, xerophthalmia, nyctalopia, blindness | 10–69% | Most MVI | |
| Vitamin D/Calcium [133] | Myalgias, arthralgias, muscle weakness, fatigue, decreased bone mineral density, hyperparathyroidism | 48–69% | 1.2–1.5 g/day calcium citrate, 400 IU/day ergocalciferol | |
| Vitamin E [134] | 4% | Most MVI | ||
| Vitamin K [134] | 68% | Most MVI | ||
| Iron [110] | Microcytic anemia, decreased exercise tolerance, immune dysfunction, impaired thermoregulation, GI disturbances, cognitive impairment, pica | 14–52% | 650 mg/day of vitamin C containing iron supplement | |
| Protein [124] | Excessive weight loss, diarrhea, marasmus, edema, hair loss | 13–18% | 1.2 g/kg/day | TPN |
| Zinc [134] | Alopecia | Rare | MVI with zinc |
MVI, multivitamin; GI, gastrointestinal; TPN, total parenteral nutrition.
Neurologic Complications
The prevalence of neurologic complications after WLS ranges from 5% to 16% [143]. Many symptoms result from nutrient and vitamin deficiencies, but not all can be directly attributed to malnutrition. Problems often start to manifest years after WLS, and are often misdiagnosed. Over a 10-year period in 1 institution, Juhasz-Pocsine and colleagues found 26 patients whose neurologic conditions could be related to WLS [144]. The average time to onset of symptoms was 6.6 years. Conditions were grouped into 5 major categories: encephalopathy, optic neuropathy, posterolateral myelopathy, acute polyradiculopathy, and polyneuropathy. Several patients fell into more than one neurologic category; all were treated for nutritional deficiencies.
One patient had a disabling posterolateral myelopathy that failed to respond to nutrition supplementation. Full recovery followed RYGB revision that shortened the bypassed limb of jejunem by 70 cm. In all, 42.6% of patients had a persistent neurologic deficit 10 years after surgery. This suggests that their neuropathies were either not caused by malnutrition, or that the deficiency led to irreparable damage [144].
Thaisetthawatkul and colleagues compared 435 WLS patients to 126 open cholecystectomy patients and found that the rate of peripheral neuropathy was higher in those who had WLS [145]. This group had an abnormal amount of inflammation on nerve biopsy. The authors concluded that no specific nutritional deficiency could account for the neuropathies. However, some patients had an altered immune response after WLS [145]. Multivitamin supplementation, close follow-up, and ongoing patient education are essential to prevent, identify, and treat nutritional and neurologic complications. WLS patients should see a nutritionist once per year.
Psychiatric
Most overweight patients do not have a psychological illness. However, 27.3% to 41.8% [146] of severely obese patients have axis I disorders, and up to 25% have axis II disorders [147]. Depression is the most common axis I disorder, prevalent in approximately 66% of WLS candidates with an axis I disorder [148]. Anxiety disorders [147], binge eating [149], and substance abuse [150] may also be present. Severely obese patients may suffer from somatization, negative body attitude, and low self-esteem [151]. The most common axis II disorders are passive-aggressive, schizotypal, histrionic, and borderline personality disorders [150]. Psychiatric issues must be addressed before and after surgery, and are part of the lifelong multidisciplinary approach to obesity management.
Mental illness is not an absolute contraindication to WLS; no evidence shows that it is a negative predictor of weight loss [6]. However, active psychosis and severe mental retardation (IQ <50) are typically contraindications if the patient cannot demonstrate understanding of the procedure or comply with postoperative diet, exercise, and follow-up instructions [152].
In addition to weight loss and control of comorbidities, one of the goals of WLS is to improve quality of life (QOL). Patients suffering from postsurgical depression lose less weight and have a lower QOL [153]. They may find that their lives do not dramatically improve once their obesity is treated. For some, underlying emotional problems were not all due to their obesity [154]. Data show that the psychosocial benefits of WLS may decline after several years, returning patients to their preoperative state [154]. A thorough assessment is needed before WLS, and those with psychopathologies are encouraged to participate in a support group or follow-up with their therapists after the WLS.
Depression
Depression and anxiety disorders are the most common psychiatric illnesses in the severely obese [155]. Patients with a BMI greater than 40 kg/m2 are 5 times more likely to have had a major depressive episode within the past year than their less obese counterparts [156]. During assessment, the mental health expert needs to distinguish between endogenous and obesity-related depression and determine the relationship, if any, between the 2 diseases. Although most depression is relieved with weight loss, a subgroup of patients remains depressed after operation [157]. Indeed, the suicide rate is higher than expected after WLS [158,159]. Primary care providers and other members of the multidisciplinary treatment team need to be aware of psychiatric illnesses, and make certain that appropriate referrals for treatment are arranged before and after WLS.
Eating Disorders/Binge Eating
Binge eating is present in up to 30% of WLS candidates [160], and continues in up to 46% of these patients after operation [149]. Preoperative binge eating is not a negative predictor for adequate weight loss, but postoperative binging undermines it [150]. Grazing is a risk factor for binge eating in the postoperative setting [161]. After WLS, approximately 23% of patients will have some form of eating disorder that may reduce weight loss or lead to weight regain [162]. In general, studies show that patients have better and more flexible control over their eating after WLS [163].
However, all patients are encouraged to participate in a bariatric program that includes nutritional education and counseling to help combat eating disorders. In summary, obesity-related psychological issues may improve after weight loss, but patients often face new psychological challenges or return to preoperative symptoms as body image changes. The Lehman Center report recommends that mental health assessment should be a standard part of multidisciplinary care. Inquiring about psychiatric problems and making appropriate referrals to mental health professionals is important to the overall health and well-being of patients.
Procedure-Specific Complications
Restrictive Procedures
The following sections will focus on procedure-specific complications. Restrictive operations (eg, AGB, VBG, SG) work by limiting the amount of food patients can ingest at any one time. Restrictive WLS tends to be better tolerated in the immediate postoperative period with less mortality than malabsorptive procedures, but overall long-term morbidity may be higher.
Adjustable Gastric Band
AGB is the second most commonly performed WLS procedure in the United States today and the most common worldwide [164]. In general, patient satisfaction with gastric banding is high, and the early postoperative complication rate is low. Patients can expect to lose 50% to 60% of excess weight and resolve 60% to 80% of comorbid illnesses [165]. Total short-term morbidity is approximately 1.2%, with a 0.05% mortality rate [95]. In a review of 9682 AGB patients, PE caused most deaths that occurred in the first 30 days after operation [71]. Late complications can range from as low as 10% to as high as 40%, with 21.7% to 35.5% of patients needing revision or removal of either the band or the port [165,166] (Table 8).
Table 8.
Complications of adjustable gastric band
| Rate of occurrence (%) | |
|---|---|
| Gastric prolapse: anterior | 1–22 [169] |
| Gastric prolapse: posterior | 2 [172] |
| Esophageal/pouch dilation | — |
| Gastric erosion | 1 [173] |
| Band leakage | 4.4 [174] |
| Tubing/port leakage | 0.4 [174] |
| Acute stomal obstruction | 14 [167] |
| Port flip | — |
| Port infection | 0.3–9 [178] |
| Inadequate weight loss | 40 [165] |
Three technical advances can reduce the rate of complications associated with AGB placement. First, use of the pars flaccida technique can prevent band slippage and prolapse by limiting retrogastric dissection and adding a gastrogastric imbrication [33]. Second, removal of the esophageal fat pad may reduce the incidence of acute stomal obstruction [167]. Third, concomitant repair of a hiatal hernia is known to reduce the incidence of band slippage, pouch dilation, and the need for reoperation [168].
Acute Stomal Obstruction
Acute stomal obstruction has been reported to occur in as many as 14% of patients and is due to an overly tight band [169]. Causes include tissue edema, hematoma, or excess tissue incorporated under the band as it is placed around the proximal stomach [169]. Patients present in the first few days after surgery with oral (PO) intolerance that includes secretions, along with nausea, vomiting, and epigastric pain [169]. Diagnosis is confirmed by an upper gastrointestinal series of X-rays with no passage of contrast past the band [169]. Removal of the esophageal fat pad can reduce the rate of obstruction from 8% to 0% [167]. Acute stomal obstruction may be managed by waiting 3 to 6 days for the edema to resolve, or by prompt surgical intervention [169].
AGB patients frequently complain of an inability to tolerate food and liquids in the morning—a sensation that dissipates over the course of the day. This is likely due to edema that may occur when patients are supine. Over time, the swelling resolves and passage through the band becomes easier. This is normal after AGB placement and should not be confused with acute stomal obstruction [170]. As patients lose weight, the band may become looser and the sensation may cease.
Those who undergo a recent band fill may also experience symptoms similar to acute stomal obstruction. An upper gastrointestinal series is not needed; the history of a fill within the last 72 hours associated with acute onset of nausea, vomiting, and PO intolerance should be sufficient to make the diagnosis. Since many WLS patients may live a distance away from their bariatric surgeon, patients will sometimes present at the nearest emergency room for relief of their symptoms. If possible, they should be transferred immediately to a bariatric center or admitted for intravenous hydration while arranging follow-up with their weight loss surgeon or the nearest WLS center. If transfer is not possible, or treatment delay will cause undue distress, the port may be accessed to unfill the band.
Port access should be performed under sterile conditions using only a Huber needle. The ports used in most band systems are not unlike those used to access long-term central venous catheters, such as port-a-caths. Use of a typical hollow core needle will damage the port and cause a leak in the band system. If the physician cannot feel the port or is unsure of its location, access can be performed under fluoroscopy. It is reasonable to remove all of the fluid from the port when patients present with acute symptoms. They can then follow up with their weight loss surgeon for a subsequent refill. Once the fluid is removed, patients should be given a glass of water to drink. They will note right away if they are able to swallow or if they are still overly restricted. Failure to immediately improve after an unfill should signal a more serious problem, such as a prolapse.
Band Slip/Anterior Gastric Prolapse
The incidence of band slippage varies widely and occurs anywhere from 1% to 22% of cases [169]. The band moves cephalad and creates an acute angle with the stomach pouch and the esophagus [169], causing an obstruction (Fig 10). Patients complain of poor tolerance to oral intake and of reflux symptoms that are worse when supine. Ironically, they may actually gain weight because they can only tolerate soft and liquid foods (eg, candy and ice cream) that can easily slide past the acute angle created by the band. This complication usually requires replacement of the band or conversion to another weight loss operation.
Fig 10.
Gastric prolapse. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
Band Slip/Posterior Gastric Prolapse
With posterior gastric prolapse, the stomach body migrates upward, displacing the band caudally and creating a large new pouch [169]. Posterior gastric prolapse is more common than anterior prolapse [171]. Patients present with symptoms of obstruction: reflux, food intolerance, and epigastric pain. They may also have weight gain. Diagnosis is made by upper gastrointestinal series that show the posterior displacement of the body of the stomach above the band, perhaps best seen on a lateral view [169]. The band will angle downward.
Use of the pars flaccida technique has reduced the incidence of this complication from 24% to 2% [172]. Although generally not an emergency, it requires surgical removal of the band. Transfer to a weight loss surgeon should be expedited. Left untreated, patients are at risk of gastric strangulation (patients present with acute severe epigastric pain and vomiting). It is important for the emergency room physician and general surgeon to distinguish between gastric prolapse and gastric strangulation.
Band Erosion
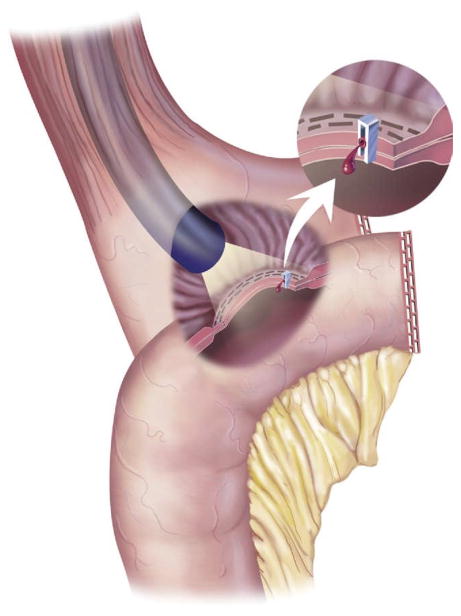
Band erosion occurs in approximately 1% of cases [173]. It may be caused by gastric wall ischemia, pressure necrosis, or possibly an infection that allows the band to erode through the stomach wall [173] (Fig 11). This complication can cause loss of band restriction and weight gain, peritonitis, abscess formation, port-cutaneous fistula, and most commonly, a port-site infection [173]. Diagnosis of the erosion can be made by upper gastrointestinal series or endoscopy [173]. Treatment is removal of the band and primary closure of the stomach ulcer [173].
Fig 11.
Gastric erosion. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
Band and Balloon Leakage
A band that leaks saline provides no restriction. Patients complain of the lack of restriction despite good results with a prior fill [174]. The band can be tested by emptying it with a syringe to find out whether significantly less or no fluid returns on aspiration. The leak can be confirmed radiographically by attempting port fill under fluoroscopy and noting if the contrast leaks out of the tubing or balloon [175].
Disruption of the inflatable portion of the band is rare; the highest incidence rate is 4.4% [174]. When leakage occurs, the band should be removed.
Pouch/Esophageal Dilation
The pouch and esophagus stretch when food is consumed faster than it can empty from the pouch [169]. Patients present with food and saliva intolerance, reflux, and a sensation of fullness in their chests [169]. This complication may be due to behavioral problems more than mechanical ones. Data show that patients with pouch/esophageal dilation are much more likely to have eating and mood disorders [176]. The diagnosis can be confirmed with upper gastrointestinal series radiographs. The initial treatment is behavioral diet modifications, along with removal of all the fluid in the band for a few months [169]. If that fails, the band should be removed [177]. Patients should be referred to a WLS program for appropriate management.
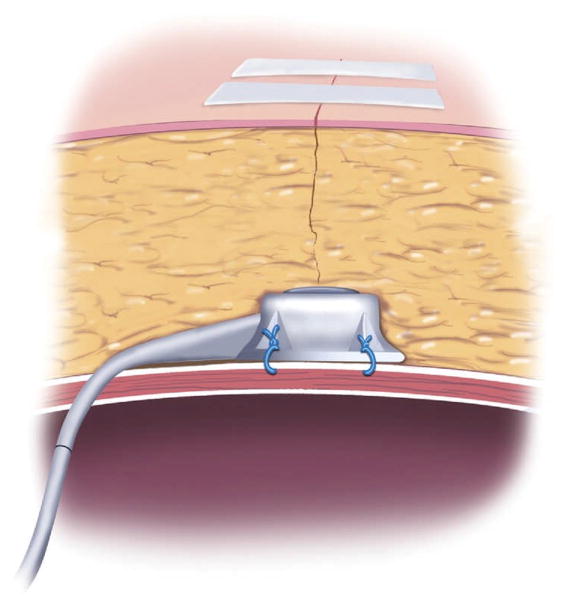
Port Complications
The location of the port may not be obvious to surgeons unfamiliar with AGB placement. Typically, the device is just inferior to the largest abdominal incision. Attempts to access it without knowing exactly where it is may damage the port or the tubing (Fig 12). A plain film of the abdomen may be helpful. When the patient performs a straight leg raise using both legs, the port is easier to palpate. Overall port complications occur in 7.1% to 14.5% of band placements [178,179]. They can be divided into 2 types: infectious and port malfunctions, including port flip and port/tube leakage.
Fig 12.
Position of AGB port. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
Port Flip
A port flip can occur from poorly tied knots or excessive physical movement by the patient (Fig 13). Sutures are typically tacked to the abdominal wall fascia with a large permanent suture, such as 0-prolene [33]. Breakage of the suture allows the port to flip. There are some reports of ports flipping due to excessive abdominal movement.178 Flipped ports must be repositioned surgically. This can be performed on an outpatient surgery basis, without replacing the band or tubing portion of the system.
Fig 13.
Port flip. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
Port/Tubing Leak
Port or tubing leaks occur 0.4% of the time, typically after multiple attempts to fill the band [178] (Fig 14). The complication is more common when the band is difficult to palpate due to abdominal girth [178]. Patients with no fluid in their bands usually have a leak. Uncertain diagnosis can be confirmed by accessing the band under fluoroscopy and noting the extravasation of fluid with the injection of contrast. If the leak is in the port or tubing, the tubing can be replaced without removing the band itself
Fig 14.
Cause of port/tubing leak in AGB. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
Port/Band Infection
Infections occur 0.3% to 9% of the time [178]. At the level of the band, these can be associated with gastric erosion. In the event of an intra-abdominal infection from another source (eg, gangrenous cholecystitis, diverticulitis, or ruptured appendicitis), it may be prudent to remove the band at the time of surgical care for the primary source of infection to prevent potential spread to the band.
If only superficial cellulitis is present, port-level infections can be treated initially with intravenous antibiotics. Failure to respond to antibiotics is an ominous sign and usually requires removal of the entire band system. If the patient presents with purulence, the entire system should generally be removed. It is important to remember that port site infection may result from band erosion or another intra-abdominal source that tracks to the port device.
Inadequate Weight Loss
The data are inconsistent as to the amount of weight loss one can expect after AGB placement. Up to 40% of patients lose less than 25% of excess weight [165]; after 10 years, as few as 28% maintain 20% excess weight loss (EWL) [165]. In cases of inadequate weight loss, it is important to distinguish between band complications that cause weight gain or dietary behaviors that limit weight loss. Reoperation should not be rushed if the problem is not due to a technical complication [180].
Rather, patients should be encouraged to continue follow-up with the appropriate members of the multidisciplinary treatment team. The nutritionist should be consulted to reinforce the importance of an appropriate solid diet. The weight loss surgeon can explain that band tightening will not counteract the effects of such foods as ice cream, sweets, and chips. Psychiatric therapy may address problems adjusting to a postsurgical diet or other emotional issues that undermine success. All patients should be encouraged to actively participate in a support group to help them adjust to their post-WLS lifestyle.
Single Incision Laparoscopic Band Placement
Single incision laparoscopic surgery is an investigational procedure; no published data are available about this approach. The technique involves placement of multiple ports, or 1 port with multiple channels, through a single, slightly larger skin incision. The procedure is performed in similar fashion to AGB placement. One of the goals is to make the incision in or near the umbilicus to help “hide” it. Cosmetic benefit is the only advantage associated with this approach.
Although experience is limited, this technique has some limitations. It requires special laparoscopic equipment. Since range of motion is limited, it is best to use a camera with a flexible tip and a light source that does not project off the side of the laparoscope. When used properly, the scope tip will perform most lateral and vertical movements. The flexible tip allows visualization of almost the entire surgical field with very little motion. Flexible tip cameras, which are not easy to use even by experienced laparoscope operators, require practice in a simulated surgical environment. Flexible tip instruments and very low profile ports can also add to the range of motion and triangulation of the surgical field within the limited space.
Even with these tools, adequate visualization is not always possible. Patient safety should not be compromised when attempting any single incision procedure. The threshold for placing enough ports for proper visualization and instrument positioning should be low; additional ports should be added as needed. Poor visualization and improper angulation of instruments may lead to more complications after AGB placement.
Single incision approaches tend to cause port complications. Because the area proximal to the umbilicus typically has more subcutaneous adipose tissue, access may be more difficult and fluoroscopic guidance required for initial band adjustments. In addition, when patients sit up, extra force on ports placed near the umbilicus may lead to port flips. Although early in its development, single incision laparoscopic access is likely to increase in popularity, especially as instrumentation improves. As patient demand for single incision laparoscopic surgery increases, more and more surgeons will likely provide this option. Surgeons early in their learning curve should discuss their experience with the patient as part of the informed consent process.
Sleeve Gastrectomy
Data on the efficacy and safety of SG as a staged or primary procedure are just now being collected. A few short-term series with more than 100 patients have been conducted, but findings are insufficient to conclude that the approach offers greater perioperative safety than any other WLS procedure. However, collective retrospective data suggest that it is at least as safe as RYGB, with an overall complication rate of approximately 24% and a mortality rate of 0.37% [36] (Table 9). Evidence suggests that SG is as effective as RYGB at treating obesity and its comorbidities [181].
Table 9.
Complications of sleeve gastrectomy
| Occurrence (%) | |
|---|---|
| Bleeding | 0–6.4 [90,187] |
| Leaks | 1.4 [60] |
| Narrowing/stenosis | 0.7 [90] |
| Gastric emptyingabnormality | Unknown |
Like malabsorptive procedures, SG produces a marked and sustained reduction in ghrelin levels up to a year after the procedure [38,182]; an outcome that may reduce desire for food [182]. Gumbs and colleagues suggest that SG is the best restrictive operation for extremely obese patients [183]. It may also be an ideal procedure for those who require anti-inflammatory medication or have inflammatory bowel disease [184].
In a randomized prospective study comparing SG to AGB, the former showed higher % EWL at 1 and 3 years (57.7% vs. 41.4%, P = 0.0004) and (66% vs. 48%, P = 0.0025), respectively [185]. Ultimately, the amount of weight loss maintained may be secondary to the remaining stomach size and antral remnant, but the optimum parameters for these have yet to be determined [186]. It also remains to be seen what happens to stomach size and weight loss after more than 5 years of follow-up.
Bleeding
The incidence of bleeding in SG ranges from 0% to 6.4% [90,187]. It occurs mostly from the staple line after the gastrectomy, due in part to the use of larger staples to help seal the thicker tissue of the distal stomach [188]. Many authors advocate routine reinforcement of the staple line by oversewing, applying fibrin glue, or using buttress materials. Although the latter 2 decrease bleeding, oversewing may lead to some narrowing of the gastric tube [37].
Leaks
Leaks after SG occur in up to 1.4% [60] of primary procedures and as many as 6.25% of revision or second staged operations [90]. Clinical studies have yet to prove that the use of fibrin glue, staple line buttressing materials, or oversewing decreases the chance of leaks. Evidence suggests that a switch to smaller stapler height near the angle of His, where the stomach tissue is thinner and most leaks occur, may help prevent leaks [187,190]. Many surgeons favor leaving a small “dog ear” at the angle of His, and not hugging the gastroesophageal junction.
SG can generate higher gastric pressures, and leaks may consequently be slower to close [191]. Gastric decompression and good drainage are the mainstays for controlling leaks, but reoperation may be required if patients are not hemodynamically stable or develop a chronic leak. Those with leaks may present with tachycardia, respiratory distress, fever, and perhaps a “feeling of doom.”
Narrowing/Stenosis
Narrowing creates gastric outlet obstruction that prevents adequate oral intake. It occurs in approximately 0.7% of patients following SG [90]. It may result from use of a gastric tube that is too small to create the sleeve [192], or oversewing the staple line used to create the sleeve [37]. To avoid this complication, some favor the use of fibrin glue or staple line buttressing materials to prevent bleeding. Bougie sizes along the staple line have ranged from 32 to 60 French, but the ideal size has yet to be determined. Creating the sleeve with a tube that is too large can lead to weight gain or reduced weight loss [186]. Many surgeons use a 36 French bougie.
Narrowing occurs most commonly at the gastroesophageal junction and the incisura angularis [193]. Corkscrewing of the gastric tube may also cause narrowing symptoms [193]. Patients with this complication will present with dysphagia, vomiting, dehydration, reflux, and poor PO tolerance [193,194]. The diagnosis can be made by upper gastrointestinal series [193]. Patients with stenosis will require admission for intravenous hydration. Definitive treatment consists of endoscopic dilation, but if the segment of narrowing is too long, surgical intervention is necessary. Most treatment consists of conversion to another WLS, such as RYGB, but there are also some data about successful laparoscopic seromyotomy (division of the long area of stenosis) to relieve the symptoms of narrowing [193]. Once patients are stabilized, they should be transferred to a weight loss surgeon familiar with SG.
Increased or Decreased Gastric Emptying
Controversy over delayed gastric emptying leading to reflux disease and pouch dilation centers around the amount of antrum to leave behind [186].
Unlike the fundus, the antrum lacks storage capacity and may contribute to feelings of satiety and fullness after meals [186]. Too large an antrum may result in delayed gastric emptying, whereas complete removal may lead to dumping and increased gastric emptying [186].
The incidence of dilation does not directly correlate with weight regain [195]. Many surgeons begin resection 5 to 7 cm from the pylorus. However, Melissas and colleagues determined that gastric emptying time is reduced in SG patients with a 7-cm antrum [91]. This may result in less restriction and lead to weight regain over time [185,186].
Treatment of stenosis consists of sleeve revision to reduce volume, adding a BPD, or converting to a RYGB. SG technique is an area that warrants further examination as the exact mechanism(s) of action remain unknown.
Nutrition
Nutritional recommendations after SG follow those of other restrictive procedures. Data on specific nutritional changes after SG are not available; evidence of more hormonal, nutrient, and caloric concerns after this operation is scant [183]. Although ghrelin levels decline after SG, the effects of that or other changes on morbidity are unknown and require further investigation.
Vertical Banded Gastroplasty
VBG was once a very popular form of WLS. As a purely restrictive weight loss procedure, patients could lose up to 70% of their excess weight [196]. Unfortunately they could also experience an 18% perioperative complication rate [196], and a failure rate as high as 43% [197]. In a comparison of AGB and VGB reoperation rates within 5 years of WLS, Miller and colleagues found a 32.4% difference (7.5% vs. 39.9%, respectively) [61].
The main causes of failure in VBG were stomal stenosis, staple line dehiscence leading to fistula and weight gain, reflux disease, and band erosion [62]. Due to unsatisfactory long-term weight loss, most bariatric surgeons agree that VBG should not be used as a primary treatment for obesity [39] (Table 10).
Table 10.
Complications of vertical banded gastroplasty
| Occurrence (%) | |
|---|---|
| Staple line dehiscence | 48 [200] |
| Obstruction/gastric restriction | 40 [199] |
| Band erosion | 1–7 [197,202] |
| Inadequate weight loss | 58 [111] |
Staple Line Dehiscence
This complication occurs when the vertical staple line separates, leading to a fistula in the fundus [64,198,199]. The result can be a lack of restriction in the VBG pouch, with resulting weight gain. Using routine endoscopy after VBG, MacLean and colleagues found staple line rupture in 48% of patients [200]. The treatment for staple line dehiscence is conversion to a RYGB [201]. Revisions have a high complication rate. Therefore, patients should be referred to a WLS center with experience in revisional surgery.
Obstruction/Gastric Restriction
Obstruction from VBG can be caused by fibrosis in the stomach or by the band itself. In either case, outlet obstruction of the VBG can cause staple line dehiscence, fistulous connection between the VBG pouch and the occluded stomach, reflux disease, and esophageal dilation [199]. This can occur in up to 40% of cases [199]. Patients present with symptoms of reflux disease, pain, and poor PO intake. They may gain weight from poor food choices (eg, ice cream) that can pass the stenosis [199].
VBG Band Erosion
The VBG outlet is typically wrapped with polypropylene mesh strip or a Silastic band to prevent dilation of the stoma [202]. The incidence of band erosion has been reported to be 1% to 7%, and usually occurs 1 to 3 years after operation [197,202] Patients may present with gastrointestinal bleeding, vomiting, abdominal discomfort, and at times, an acute abdomen [199]. Diagnosis is made by upper endoscopy [199]. If weight loss is still desired, the anterior strip of mesh or band must be removed before revision to a RYGB.
Unsatisfactory Weight Loss
Although VBG has good initial weight loss, long-term outcomes can be as low as 31% within 4 years [111]. This may be due to a technical complication that requires revisional surgery. A multidisciplinary team approach is the first step to address behavioral factors that may be present.
Malabsorptive Procedures
Purely malabsorptive procedures are fraught with nutritional concerns. These are minimized by a longer common channel combined with a restrictive component.
Jejunal-Ileal Bypass
This operation was formally abandoned in the 1980s after complications emerged years later [31]. Most of the them were due to bacterial overgrowth in the bypassed limb, leading to diarrhea, malnutrition, arthritis, dermatitis, and liver failure [203] (Table 11).
Table 11.
Complications of jejunal-ileal bypass
| Occurrence (%) | |
|---|---|
| Malnutrition | 29 [204] |
| Diarrhea | 63 [115] |
| Arthritis/dermatitis | 28 [211] |
| Cirrhosis | 40 [215] |
| Liver failure | 7–17 [204] |
| Oxalate stones | 29 [204] |
| Renal failure | 18 reported cases [218] |
Malnutrition
Malnutrition from diarrhea and electrolyte imbalance occurs in up to 29% of patients [204]. Diarrhea itself, defined as more than 3 stools per day, occurs in up to 63% of patients after JIB.115 Chronic diarrhea and concomitant malabsorption may not manifest in any other way clinically for 3 decades [205,206] Protein, essential fatty acids, vitamin B12, folate, magnesium, sodium chloride, and potassium deficiency have all been reported after JIB [115,207,208,209]. Vitamin D deficiency can manifest as delayed fracture healing [210].
Arthritis/Dermatitis
Arthritis occurs approximately 29% of the time after JIB due to the release of bacterial antigens from bacterial overgrowth [211]. The subsequent immune response causes immune complex deposition into areas like the joint spaces and skin, resulting in arthritis [212] and dermatitis, respectively [213]. The arthritic pain is unresponsive to anti-inflammatory medication [213]. Evidence shows that surgical removal of the blind loop where bacterial overgrowth occurs effectively resolves this complication [214].
Liver Failure
Evidence of cirrhosis can occur in up to 40% of patients [215], with complete liver failure in 7% to 17% of them after JIB [204]. The exact etiology is unknown, but it is believed to result from either inadequate enteric protein absorption or anaerobic bacterial overgrowth in the excluded limb [215]. The latter is associated with toxin release that can cause parenchymal liver damage [215] decades after the original procedure [216]. The recommended treatment is reversal of the JIB, but this is not always effective and occasionally liver transplantation is required [217].
Oxalate Stones and Renal Failure
Another complication of JIB is the formation of oxalate stones. This is caused not by bacterial overgrowth, but rather by increased absorption of calcium oxalate [218] that leads to its deposition in the renal parenchyma. The result can be intrinsic damage as well as postobstruction nephropathy seen in up to 19% of JIB patients [204]. Progression of oxalate nephrosis can cause renal failure [218]. The diagnosis can be made by measuring the mean oxalate excretion in the urine [219]. The treatment is reversal of the bypass [220]. Conversion to another WLS procedure can be considered if there is still a need for weight management [221].
For the general surgeon, it is important to note that possible complications from JIB may occur several decades after the initial surgery.
Combined Restrictive/Malabsorption Procedures
Complications from weight loss procedures that combine restrictive and malabsorptive components include leak, hernia, stenosis, and bowel obstruction. As reviewed earlier, nutritional complications tend to be more severe with malabsorption techniques.
Roux-en-Y Gastric Bypass
The basic tenet of this operation is to create a small gastric pouch and anastomose it to a Roux limb that bypasses 75 to 150 cm of the small bowel, thereby restricting food intake and limiting absorption [39]. In the United States, the number of RYGBs performed annually outpaces that of any other weight loss procedure [222, 223] (Table 12).
Table 12.
Complications of Roux-en-Y gastric bypass
| Occurrence (%) | |
|---|---|
| Gastrointestinal leak | 0.7–5.1 [53] |
| Bleeding | 0.8–4.4 [53] |
| Stenosis | 8–19 [230–233] |
| Marginal ulcers | 0.7–5.1[230,235,236] |
| Ulcers in remnant stomach/duodenum | Unknown |
| Bowel obstruction | 0.2–4.5 [232,236,250] |
| Intussusception | Unknown |
| Dumping syndrome | 50 [257] |
| Fistula | Unknown |
| Wound infection (open technique) | 13 [270] |
| Incisional hernias (open technique) | 35 [271] |
Gastrointestinal Leak
Anastomotic leak is the most dreaded and potentially litigious complication after RYGB. Most leaks occur within 7 days of the operation, and the rest within the first 28 days [224]. They may be asymptomatic, but can still lead to fatal complications. The mortality rate can be as high as 30% [225]. The incidence rate of leaks is 0.7% to 5.1%, regardless of technique (eg, open vs. laparoscopic) [53]. In revisional WLS, the rate can be as high as 35% [63]. Tissue ischemia is the most prevalent risk factor [54].
Some believe that the antecolic approach increases risk of leaks although this is controversial [226]. Hamilton and colleagues found that the most specific physical signs of a leak are sustained tachycardia above 120 and respiratory compromise. In their series, laparotomy showed positive findings in all patients with these symptoms [227].
Gonzalez and Murr reported an overall morbidity rate of 55% in patients with leaks compared with 25% in those without them [54]. The incidence of gastrogastric fistula, gastrointestinal bleeding, thrombolic events, wound infection, respiratory failure, and mortality were each at least 4 times more likely to happen in those patients who had a leak postoperatively [54].
If the diagnosis is in question, radiologic evaluation can be considered, typically an upper gastrointestinal series. Often times these are done initially with gastrograffin to identify larger leaks, then with thin barium to find smaller leaks [54]. However, Hamilton and colleagues concluded that upper gastrointestinal series may miss leaks in 78% of patients [227].
Computed tomographic (CT) scans are equally unreliable in detecting leaks, but may rule out fluid collections, internal hernias, and abscesses [227]. When performing a CT to rule out a leak, oral contrast should be given just before the study to opacify the gastric pouch [54]. Neither an upper gastrointestinal series nor CT scans are reliable for detection of a leak at the jejuno-jejunostomy (Fig 15).
Fig 15.
Potential leak sites after retrocolic RYGB. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlasof Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com .) (Color version of figure is available online.)
In clinically stable patients, it is reasonable to do an evaluation for postoperative tachycardia. Cardiac ischemia should be ruled out by electrocardiogram (ECG) and serial cardiac enzymes. Patients should be on telemetry monitoring, with transfer to an intensive care unit (ICU) if needed for closer continued assessment and one-on-one nursing care. Serial hematocrits will determine if bleeding is the cause of tachycardia. PE may be ruled out with CT. Anticoagulation therapy should be started if the clinical suspicion of a PE is high. If hypovolemia is causing the tachycardia, it should respond to a fluid bolus. Finally, anxiety, pain, and rebound tachycardia should be considered as possible etiologies of sustained tachycardia. Most of the evaluation can be accomplished within hours. If the tachycardia persists despite a negative evaluation, surgical exploration should be considered.
If the patient clinically deteriorates, or their tachycardia is accompanied by respiratory distress, the appropriate therapy is usually urgent exploration. If the leak is found, primary repair should be assessed, along with an abdominal washout and wide drainage. The use of sealants is popular, but has not proven beneficial in this application. If the leak cannot be found by insufflation or methylene blue, then it is reasonable to wash out and widely drain the area in an effort to contain the leak (Fig 16). Many surgeons will place the nasogastric tube past the gastrojejunostomy anastomosis and place a gastric tube in the remnant stomach for delivery of postoperative nutrition and medication. Since systemic inflammatory response syndrome (SIRS) is expected after a leak, the patient will most likely need ICU care.
Fig 16.
Wide drainage of gastrointestinal leak and G-tube placement. (Adapted with permission from Jones DB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
Thodiyil and colleagues made a distinction between contained and diffuse leaks on radiographic examination [68]. They concluded that the majority of contained leaks could be treated conservatively, with management consisting of nasogastric decompression, “nothing by mouth” (NPO) status, and drainage of the leak [68]. For the general surgeon who does not manage WLS patients often, if tachycardia greater than 120 beats per minute persists despite a normal evaluation, it is usually prudent to proceed with surgical exploration despite an otherwise “good” clinical picture.
Bleeding
Bleeding after RYGB occurs 0.8% to 4.4% of the time and usually results from staple line bleeding of the gastric remnant, gastrojejunostomy, or jejuno-jejunostomy [53]. A distinction should be made between intraluminal and extraluminal bleeding as well as timing of the bleeding. Bleeding that occurs within several hours after the operation is more likely to require operative intervention than bleeding that occurs several days later [228]. Bleeding that occurs intraluminally will present with melena, tachycardia, hematemesis, hematochezia, and a drop in hematocrit [228] (Fig 17). Endoscopic repair may play a role in management of bleeding at the gastrojejunostomy [228]. Extraluminal bleeding may show increased bloody output if drains are used. Otherwise, patients may present with more insidious anemia or an ileus.
Fig 17.
Potential site of intraluminal bleeding after gastric bypass. (Adapted with permission from JonesDB, Olbers T, Schneider B, Atlas of Metabolic and Bariatric Surgery, c2010 Cine-Med Publishing, Inc., www.cine-med.com.) (Color version of figure is available online.)
When bleeding occurs, 85% of patients can be managed successfully nonoperatively [228]. Initial management includes fluid resuscitation, discontinuation of anticoagulation, correction of an abnormal coagulation profile, and possibly, red blood cell transfusion. Hypotension, tachycardia, and a decreasing hematocrit despite therapy require endoscopic and/or operative intervention. In general, the operation to localize the source of ongoing bleeding should not be delayed.
Use of preoperative heparin is controversial, and there are many differing opinions on its use. Ultimately, the benefit of using it must be weighed against the risk of DVT/PE events. No reports have directly compared use versus withholding of preoperative heparin to reduce the risk of DVT/PE development and surgical bleeding.
Stenosis
Stenosis or an anastomotic stricture is marked by vomiting and the inability to tolerate oral intake. Its etiology is uncertain but stenosis may be caused by ischemia at the anastomotic site or tension on the Roux limb, or be associated with marginal ulcers [225]. It typically occurs in the first few months after operation [229]. Early on, patients may complain of nausea, pain, and regurgitation of saliva [225]. They may require hospitalization, intravenous fluid, resuscitation, and correction of nutritional deficiencies, and, in particular, thiamine. The incidence of stenosis ranges from 6% to 19% regardless of surgical technique [230–232]. Fisher and colleagues prospectively studied 200 patients and randomized them to either 21-mm or 25-mm EEA staplers for their gastrojejunostomy. The stenosis rate was 19% in the 21-mm group and 8% in the 25-mm group. Both groups had more than 80% EWL after 2 years [233].
Endoscopic balloon dilation is the initial treatment for stenoses. Nguyen and colleagues found this approach to be 100% successful in patients with stenosis; only 17% required more than 1 procedure [229]. Carrodeguas and colleagues reported a perforation rate of 2% in a retrospective analysis of 94 patients requiring dilation (in some cases, up to 4 of them) [234]. This procedure is best performed in facilities with experienced endoscopists, and, if possible, patients should be transferred to such a site. Repeat dilation may result in swelling at the anastomotic site, making further dilation more difficult and operative intervention more likely.
Marginal Ulcers
Marginal ulcers occur in 0.72% to 5.1% of cases [230,235,236]. Those that are distal to the gastrojejunostomy result from acid irritating the mucosa of the transposed jejunum [235]; 19% are associated with a gastrogastric fistula [237]. Other risk factors for ulcers are nonsteroidal anti-inflammatory medications (NSAIDS), smoking, and foreign bodies (eg, staples or nonabsorbable sutures) [225]. A large stomach pouch may contain enough parietal cells to promote an acidic environment near the gastrojejunal anastomosis [238]. Patients with ulcers present with pain, nausea, bleeding, and perforation [235]. In Dallal and Bailey, 14% of those who developed a marginal ulcer required reoperation; the rest were managed successfully with proton pump inhibitors [235].
If an operation is required, revision of the gastrojejunal anastomosis is preferred as long as the patient is hemodynamically stable. The surgeon should look for a gastrogastric fistula, and may downsize a large pouch. Most of the time, the patient will present with an acute perforation and no obvious source for the ulcer. In such cases, a Graham patch is performed.
Ulcers in the Remnant Stomach and Duodenum
Ulcers can also appear in the remnant stomach and duodenum independent of Helicobacter pylori status [239]. The remnant stomach has a mean pH of less than 2 to 3 [240,241] and can still respond to vagal and hormonal stimuli [242]. As such, RYGB patients can have ulcer bleeding and perforation years after their operation [243]. They may present with abdominal pain, melena, and, if perforation is present, an acute abdomen. Endoscopic evaluation will be difficult because of the divided pouch, although there have been reports of endoscopic examination using a laparoscopic assisted transgastric approach with the endoscope placed via the remnant stomach [244]. If stable, the patient may benefit from transfer to a facility where transgastric endoscopy can be done.
Unstable patients with ulcers require early surgical exploration. If a perforated ulcer is found in the remnant stomach or duodenum, a Graham patch repair should be followed by postoperative proton pump inhibitor therapy. Resection can be considered if the ulcer is limited to the remnant stomach. Helicobacter eradication therapy should also be considered postoperatively [245].
Bowel Obstruction
The majority of bowel obstructions occur between 6 and 24 months after operation [246]. Patients may present with nausea, vomiting, and colicky abdominal pain [247]. Accurate diagnosis can be made with CT scans and/or an upper gastrointestinal series [232]. Some patients present with chronic abdominal pain [248]. This may be from intermittent obstruction through an internal hernia. Diagnostic laparoscopy should be considered part of the evaluation to rule out an internal hernia [225].
The incidence of bowel obstruction after laparoscopic RYGB is 0.2% to 4.5% [230,232,236,249,250]. Some authors make a distinction between early (within 6 weeks of operation) and late bowel obstruction. Early bowel obstructions were most likely due to technical issues [232,236,250]. Nguyen and colleagues recommended closing mesenteric defects in the jejuno-jejunostomy, the transverse mesocolon, and the Petersen space (that created between the Roux limb, the transverse mesocolon, and the jejunal mesentery as the Roux limb passes through the transverse mesocolon [33]); placing an antiobstruction stitch; and closing the common jejuno- jejunostomy with suture. This approach decreased their small bowel obstruction rate from 6% to 3% [250].
In a series of 1715 patients, Hwang and colleagues reported a difference in occurrence between retrocolic (7%) and antecolic (2%) bowel obstruction [247]. They noted that small bowel resection was more likely to be required in obstructions that occurred early (79.1%) rather than late (19.1%) [247].
Early obstruction caused by adhesive disease rather than technical error tends to resolve with nasogastric decompression. Bowel obstructions that occur several months after RYGB can be treated like any other small bowel obstruction, with an initial trial of nasogastric tube decompression.
However, nasogastric tube placement should be performed carefully and may best be performed under fluoroscopic guidance.
Intussusception
Intussusception takes place when the bowel telescopes into itself. The common channel is most often affected, but it can also occur in the Roux-en-Y and biliopancreatic limbs [251], and at the gastrojejunal anastomosis [252]. The presentation of both retrograde and antegrade intussusception is similar to a bowel obstruction, with abdominal pain, nausea and vomiting [253]. The exact cause of intussusception remains unknown [254].
This complication can develop several years after RYGB [255]. Patients who have lost more than 90% of their excess weight are at the highest risk of developing intussusception. Computed tomography has an accuracy rate of approximately 80% in identifying this complication. A normal scan does not rule it out [255].
Exploration is warranted in patients who present acutely or have chronic pain that cannot be diagnosed. Evaluation for internal hernias as well as other causes of epigastric pain (eg, gallstone disease, ulcers in the remnant stomach or biliopancreatic limb) should be considered. Treatment of intussusception is somewhat controversial. Revision of the anastomosis is best if it occurs at the jejuno-jejunostomy. If this does not resolve the condition, reduction and pexy may be sufficient [256]. Resection should be performed if there is evidence of necrosis [231,255].
Dumping Syndrome
Dumping syndrome occurs in up to 50% of RYGB patients [257]. There are no reports of dumping after AGB, SG, and JIB. There are 2 distinct types of dumping: early and late. The early kind happens within 15 to 30 minutes of a meal, and is characterized by crampy abdominal pain, voluminous diarrhea, bloating, dizziness, nausea, flushing, and tachycardia [258]. Data suggest that it results from rapid entry of hyperosmotic foods into the jejunem, which causes jejunal distention and increased intestinal contractility. Fluid shifts from the plasma into the intestinal lumen due to the hyperosmolar content, resulting in hypovolemia and consequent sympathetic response [258]. Evidence indicates that serotonin, vasoactive intestinal peptide, neurotensin, and PYY3-36 also play a role [257].
Treatment usually calls for adjusting intake to avoid foods that are sweet (simple sugars) or acidic (citric-based, tomatoes), and nutrient-rich drinks (e.g., Gatorade, Powerade). These should be replaced with complex carbohydrates and high-fiber, protein-rich foods [257]. Patients should supplement lost vitamins and minerals, especially iron and calcium, and may benefit from behavior modification to help them eat small, frequent, and dry meals; lie down after eating; and avoid very hot and very cold foods. This problem is self-limiting in the majority of patients and resolves in 7 to 12 weeks [257].
Late dumping occurs 2 to 3 hours after a meal. Rapid glucose absorption causes hyperglycemia [257] and release of GLP-1 and GIP. An exaggerated insulin response [257] leads to hypoglycemia and hypokalemia. Patients with this type of dumping present with diaphoresis, weakness, fatigue, and dizziness [257]. Most benefit from the same dietary and behavioral changes described for early dumping.
Dumping after RYGB is sometimes seen as a “desirable” complication that reinforces the need to avoid foods high in simple sugars and the importance of eating smaller portions. For patients whose symptoms do not resolve despite dietary and behavior modifications, medications like acarbose alone or in combination with verapamil and octreotide can improve symptoms [257,259].
Hyperinsulinemic hypoglycemia or nesidioblastosis is another dumping-related complication that can occur after gastric bypass. An asymptomatic form of hypoglycemia can occur after AGB [260]. Studies suggest a pathophysiology of pancreatic beta-cell hypertrophy resulting in elevated insulin release [259]. This can occur 1.5 to 8 years after operation, with dumping symptoms as well as hypoglycemia, weakness, and even syncope [261–263].
Suspected nesidioblastosis can be treated by dietary, medical, and surgical interventions. The goal of management is to control serum glucose levels. Diet should be addressed first. Patients should follow a pattern of 3 meals and up to 3 snacks a day with avoidance of simple sugars (eg, juice, soda, candy) and continuous use of high-fiber and protein-rich meals [264]. Medical management includes the use of a-glucosidase inhibitors, such as acarbose and miglitol, which inhibit glucose absorption in the intestine. Diazoxide, somatostatin, and steroids have also been used with modest success [264]. Surgical management includes subtotal pancreatectomy to remove the hypertrophied beta cells [265,266], restoration of gastric pouch restriction with a Silastic band to limit glucose intake, or revision of the gastrojejunostomy to slow emptying [266].
Fistula
Gastrointestinal leaks may erode into another surface and create a fistula. The most common type is a fistulous connection between the gastric pouch and the gastric remnant [267]. Some 19% of patients with a marginal ulcer also have a fistula [237]. Large fistulas may result in weight regain because patients no longer feel restriction. The diagnosis can be made using an upper gastrointestinal series or CT scan that shows contrast in the excluded stomach. Fistulas should be confirmed with endoscopy [232] and repaired surgically. Attempts to close fistulas endoscopically have had disappointing results [60,268]. Since treatment is not a surgical emergency, patients should be referred electively to a bariatric center.
Laparoscopic versus Open WLS
The advent of laparoscopy has greatly enhanced WLS outcomes by decreasing pain, expediting return to work, and reducing hospital stay [269].
Laparoscopic RYGB (LRYGB) has decreased respiratory complications, incisional hernias, and wound infections from as much as 13.1% to 0% [270,271]. Podnos and colleagues reported that LRYGB has a lower death rate than open RYGB [69]. Conversely, open RYGB costs less, causes fewer internal hernias, and reduces reoperation rates from 4% to 1% [49]. Open RYGB also reduces leak and stenosis rates despite operations in higher- risk patients [29].
The laparoscopic approach is technically challenging; most authors report a decrease in complications only after performance of 75 to 100 procedures [84]. Laparoscopic and open RYGB have comparable leak, DVT, and death rates from PE [70]. Weight loss is also similar. The rate of conversion from laparoscopic to open RYGB is approximately 1.7% [269].
The general surgeon seeing RYGB patients for the first time in the emergency room or in consultation must distinguish between laparoscopic and open complications. Laparoscopy is associated with gastric stenoses requiring prompt consultation with a gastroenterologist for endoscopic dilation; leaks that may require exploratory operations; and despite negative imaging studies, small bowel obstructions from internal hernias. The open approach is associated with incisional hernias, adhesive disease with small bowel obstruction, and larger incisions more likely to have wound infection. Malnutrition, marginal ulceration, and dumping syndrome from the 2 approaches have never been directly compared, but there is no reason to believe they differ.
Biliopancreatic Diversion/Duodenal Switch
Devised to help decrease some of the complications from purely malabsorptive procedures like the JIB, BPD is highly effective in achieving long-term weight loss. DS is another variant designed to prevent marginal ulceration seen in BPD. Mortality from BPD and DS can range up to 1.1%, with overall morbidity higher than most WLS, at 37.7% [16,77,272]. Weight regain over time can also occur in significant numbers of patients. According to Biron and colleagues, the failure rate doubles every 5 years [273] (Table 13).
Table 13.
Complications of biliopancreatic diversion/duodenal switch
| Occurrence (%) | |
|---|---|
| Marginal ulcers | 15 [281] |
| Steatorrhea | 39 [284] |
| Protein malnutrition272 | Unknown |
| Weight regain | 10–26 [273] |
Several authors report excellent results with BPD-DS of 75% EWL after 10 years, with 94% of patients achieving more than 50% EWL [274]. Laparoscopic BPD-DS achieves similar outcomes in extremely obese patients [275,276]. BPD/DS provides more effective control of diabetes, hyperlipidemia, hypertension, and obstructive sleep apnea than RYGB [16], but RYGB is probably better for patients with severe gastroesophageal reflux disease (GERD) [277]. Using the Bariatric Analysis Reporting Outcome System (BAROS), Marinari and colleagues found that after 15 years, 83% to 92% of patients had a good to excellent outcome after BPD [123]. This may be due to the fact that BPD-DS allows them to eat meals that are closer to the social norm than RYGB [278].
However, BPD is associated with more severe nutritional and vitamin deficiencies than those seen after RYGB [279]. Protein malnutrition is more common and severe, most likely from profound dumping [272]. Stomal ulceration is also common. DS was added to prevent some of the side effects from BPD [280]. Lower glucose intake reduces insulin response and dumping episodes. Decreased acid in the distal ileum may abate marginal ulceration. However, stomal ulceration, severe protein malnutrition, and steatorrhea have led to limited acceptance of this procedure.
Anastomotic Ulcers
Ulcers at the gastrojejunostomy anastomosis can occur in up to 15% of BPD patients [281], and are more prevalent in men, female smokers, and those with a history of peptic ulcer disease [41]. The use of a DS with gastric resection can help reduce the acid load to the distal intestines, but, in one series, the rate of ileal ulceration was still 29% [282].
Steatorrhea
Steatorrhea is defined as fatty diarrhea with 3 or more bowel movements per day for at least 3 weeks [283]. This type of diarrhea can be particularly foul smelling. Diagnosis is confirmed by using the Sudan III fecal stain to examine the stool for fat content [283]. Steatorrhea occurs in 39% of post-BPD patients [284]. Oral antibiotics and dietary enzymes help control this problem. Approximately 5% of patients will require surgical lengthening of the common channel to increase absorption of fat, especially if the steatorrhea is associated with a nutritional deficiency that cannot be controlled with supplementation alone [285].
Operations in the Extremely Obese
Extreme obesity (BMI >50 kg/m2) is associated with a higher rate of complications [48]. Evidence suggests that Roux limb lengths up to 150 cm result in greater weight loss, but common channels of less than 100 cm are associated with significant nutritional deficiencies [285]. A longer Roux limb or shorter common channel may result in superior outcomes in this population [122].
Some surgeons support the use of a staged approach—performing a less technically challenging operation, such as AGB or SG, followed with a longer lasting, more effective one after the patient’s BMI is below 50 kg/m2. However, many patients lose a significant amount of weight with the first operation and opt out of the second. An estimated average of 1 in 4 to 5 patients has the second operation [37].
Revision Operations
Patients who fail to achieve greater than 50% EWL from their initial operation should be referred to a WLS center with a multidisciplinary approach to weight loss. Before revision surgery, other issues contributing to weight regain or suboptimal weight loss should be addressed.
The main reasons for revision are complications from the initial procedure, failure to lose weight, or weight regain. Reoperations take many forms and there is no consensus on how best to treat patients with failed initial operations. In general, restrictive operations that result in inadequate weight loss should be converted to combined restrictive/malabsorptive procedures.
Weber and colleagues found better sustained weight loss after conversion to RYGB versus repeat AGB, especially in cases with esophageal dysmotility [286]. AGB can be converted to RYGB [201], BPD [287], or SG [288]. Overall morbidity ranges from 9% to 20%, with low mortality [63,289,290]. Subsequent weight loss is typically good, as is resolution of obesity- related comorbidities [201,291,292].
One study found that revision of a RYGB to another RYGB had higher morbidity, particularly from leaks that occur in 35% of patients [63]. Reoperations for leaks, fistulas, staple line dehiscence, and other such complications, are associated with a large amount of inflammation around the site of the leak; this probably contributes to the high leak rate after reoperation. Unless a patient is unstable, he or she should be referred to a weight loss surgeon with experience in reoperations.
Investigational Methods of Weight Management
Intragastric Balloon
Intragastric balloon (IGB) placement works by restriction. A balloon is placed in the stomach endoscopically and inflated to restrict intake [293].
Patients are then placed on a low calorie diet. The balloons are not meant to stay in the stomach for a long period of time [294]. As a result, some practitioners advocate their use as a bridge to more definitive WLS operations, or as preparation for another surgical intervention, such as a ventral hernia repair [295,296].
Complications associated with IGB include nausea, vomiting, gastric rupture, Mallory-Weiss tear, esophagitis, gastric obstruction, gastric ulcer, and balloon rupture [293]. Balloon rupture can lead to migration and subsequent bowel obstruction [294]. The largest IGB series reported a complication rate of 2.8%, with mortality in 2 of 2515 patients (0.0007%); both incidents were due to gastric rupture. Since the patients had prior gastric surgery, the authors concluded that prior gastric surgery is a relative contraindication for IGB. Comorbidity resolved in 44.3% of patients and improved in 44.8% of them; the % EWL was 34% in 6 months [293]. One-year studies report weight loss of 27% to 48% [297]. Still, Gerrits and colleagues found that nearly 40% of patients were “very unsatisfied” with the procedure, an outcome that was unrelated to the amount of weight lost [298].
Removal of an IGB requires an endoscopic approach with puncture to deflate the balloon. As such, it can introduce new complications, such as gastric perforation [294]. One anecdotal report noted gastric perforation resulting in death 2 days after placement of an IGB [299]. In addition, aspiration pneumonitis can occur if there is a lot of retained food in the stomach. IGB removal can be difficult if the balloon is stuck behind one of the esophageal sphincters; forceful extraction can result in esophageal perforation [300]. The procedure may require general anesthesia. One-year data on what happens to comorbidities and weight loss after balloon removal are lacking.
Other Endoluminal Techniques
Endoscopic techniques for weight loss are currently undergoing investigation. In one approach, an endoluminal sleeve that connects the esophagus to the duodenum is deployed in the stomach to restrict the amount of food intake. To date, no trials on complications or outcomes from this technique have been published. A second approach involves the transgastric creation of a small gastric pouch. Sutures are passed to imbricate the stomach and create a smaller stomach, thereby restricting food intake [301]. Again, data are scarce. However, both techniques seem to be well tolerated by patients.
Implantable Gastric Pacing
Implantable gastric pacing (IGP) involves placing a pacer into the stomach wall to stimulate gastric peristalsis, but other mechanisms may affect weight loss [302]. The exact mechanism of action of gastric pacing is unknown. Pacing wires are surgically implanted into the stomach wall and then attached to a generator that lies in the subcutaneous tissue of the abdominal wall. It communicates with a handheld programmer via radio frequency. Electrical stimulation may increase gastric motility, change fundic tone, inhibit vagal stimulation, or alter secretion of gut hormones. Initial experiences involve only a few patients. Data show EWL of up to 40%, with little morbidity and no mortality [303].
Other Areas of Clinical Research
WLS in Patients with a BMI < 35 kg/m2
RYGB and BPD-DS are under investigation as treatments for diabetes in patients with a BMI less than 35 kg/m2. Data suggest that bypassing the duodenum and jejunum to enhance nutrient delivery to the ileum helps regulate glucose homeostasis [304]. Several small studies show that malabsorption operations are highly successful in treating diabetes [305]. Some WLS has even been done in patients with BMIs as low as 23 kg/m2 [306].
These patients are less susceptible to perioperative risks associated with obesity, and thus may have lower risk of perioperative complications (eg, DVT/PE, CVD) than their severely obese peers. However, normal-weight patients with diabetes may also have some of the same complications as their obese counterparts (eg, leak, internal herniation, bowel obstruction, malnutrition, and gallstone disease) [306].
Use of RYGB to Improve Transplant Candidacy
Use of RYGB in obese patients awaiting kidney transplants is being investigated. Laparoscopic SG is already being used before surgery in obese patients waiting for liver and lung transplants [307]. These procedures can be performed in uremic and cirrhotic patients. Takata and colleagues showed that they lost enough weight to be suitable transplant candidates [307]. Outcomes after transplantation have yet to be published, but the risk/benefit ratio may support adding WLS to treatment of high-risk transplant patients.
Pediatric/Adolescent Patients
WLS in pediatric and adolescent populations is very controversial. Of all the obese subgroups in the United States, the pediatric age group is the fastest growing, having tripled in the last 20 years for children aged 12 to 17 years [308]. A full 28% of boys and 31% of girls age 15 are over the 85th percentile for BMI [309]. Furthermore, statistics show that the overwhelming majority of obese teenagers will become obese adults [310].
The results of WLS have been promising, with 52% to 68% EWL 1 year after RYGB [198,311]. Two years after AGB, patients had a mean weight loss of 40% to 59%. Some advocate holding off on WLS until BMI exceeds 50 kg/m2, but this approach would limit WLS to the sickest patients with the highest risk for complications [120]. In addition, extremely obese patients lose a lower % excess body weight than do those whose BMI is less than 50 kg/m2 [120]. Evidence suggests that it is better to operate on extremely obese teenagers before they reach the highest class of obesity.
On the other hand, 20% to 30% of obese pediatric patients may lose their excess weight with diet, exercise, and behavior modification [312].
These numbers are higher than those seen for adults, but there is more recidivism in the adolescent population, and it is not clear at what age or level of maturity patients will understand that lifelong therapy, continued exercise, and compliance with nutrition supplementation is essential. To date no randomized, prospective studies have been done on optimal timing for WLS in the pediatric population. The literature is limited to retrospective studies with small sample sizes.
Recommended criteria for WLS in the pediatric population are summarized in Table 14. These are guidelines only. All adolescents considering WLS should be evaluated by a multidisciplinary treatment team on a case-by-case basis. The team should include those mentioned earlier as well as a pediatric obesity specialist [14]. Pediatric WLS should be performed within the supportive environment of clinical trials in medical centers with expertise in bariatric surgical techniques [308].
Table 14.
Adolescent/pediatric weight loss surgery criteria
| Recommended criteria for weight loss surgery [47] | |
|---|---|
| Indications | Failure of at least 6 months of organized attempts at weight management, as determined by the primary care physician or multidisciplinary team |
| Attainment or near-attainment of physiologic maturity (Tanner stage IV), in some cases, bone age is required to determine skeletal maturity | |
| BMI > 40 kg/m2 with co-morbidities or BMI > 50 kg/m2 | |
| Commitment to comprehensive medical and surgical evaluations before and after surgery | |
| Avoidance of pregnancy for at least 1 year post operatively | |
| Capable and willing to adhere to nutritional guidelines postoperatively | |
| Must be able to show decisional capacity and maturity in psychological evaluation and provide informed assent | |
| Supportive and committed family environment | |
| Contraindications | Medically correctable cause of obesity |
| Substance abuse problem within the previous year | |
| Medical, psychiatric, or cognitive disability that impairs ability for adherence | |
| Current pregnancy or breastfeeding, including planned pregnancy within the first year after surgery | |
| Inability of willingness of the patient or parents to understand the procedure of its medical consequences, including the need to maintain lifelong dietary guidelines and supplementation |
BMI, body mass index.
In general, complications from WLS in adolescents mirror those of adults. Their incidence, however, may be inflated due to few subjects in the small number of series to date. The complication rate for all morbidities is approximately 39% to 50% after RYGB [313,314]. After AGB, the overall complication rate is 10%, with no reports of death [315].
Bone Growth
There is no evidence of growth retardation in adolescents after WLS. In a 6-year follow-up of 34 WLS patients aged 11 to 19 years at the time of operation, Rand and MacGregor found that none of the patients were outside the average height range for their age group, and that their average height was the median of that age group [311]. Centers that perform WLS on pediatric patients typically require skeletal radiographs as part of their screening process to help determine physical maturity [47].
Recidivism
Weight regain has been reported to range from 10% to 15% of total weight [198,313], and may start within the first year after WLS [314].
Emotional, Physical, and Psychological Maturity
Adolescents have less control over their food intake and activities than adults [47]. As such, their weight gain may be the result of their family’s socioeconomic status, parental neglect, poor living conditions, or some other family issue [316]. It is therefore recommended that a psychosocial assessment of the entire family be done for WLS candidates [47].
One of the underappreciated aspects of obesity in the pediatric population is psychological morbidity. Often these patients deal with low-self esteem and social isolation [317], and are more likely to engage in high-risk behaviors, such as smoking and alcohol use [318]. Rand and MacGregor have the longest follow-up to date, a mean of 6 years. During this time, 94% of patients felt unattractive, but not 1 was unemployed (62% were working, 26% were students, and 12% were housewives) and 56% had a serious adult relationship [311]. Improved QOL may be another potential benefit for adolescents who undergo WLS.
Pregnancy after WLS
Obese female patients who undergo WLS have increased fertility, decreased risk of pregnancy-related complications, and fewer fetal complications. Gerrits and colleagues found that after BPD, women had more regular menstrual cycles and increased fertility [298]. After weight loss, there are fewer and less frequent pregnancy-related morbidities (eg, hypertension, gestational diabetes, preeclampsia, eclampsia, fetal miscarriage, fetal macrosomia, fetal malformation, and late fetal death) [319,320].
Studies show no increase in the incidence of fetal malnutrition, low birth weight, or inadequate maternal weight gain after RYGB [321,322]. Dixon and colleagues found similar results as well as a 28-kg weight loss in obese women who had an AGB. Outcomes were compared with their penultimate pregnancy before WLS and obese women who did not have WLS [323].
Most bariatric surgeons recommend avoiding pregnancy during the first 12 to 18 months after WLS. Since many oral contraceptives are not as effective after malabsorptive weight loss procedures, female patients of child-bearing age should be referred to obstetricians to assist with appropriate methods of birth control [298]. In the studies mentioned above, many patients became pregnant within the first 18 months after WLS [321,324], during the period of very rapid weight loss. Nonetheless, the rate of pregnancy-related complications was still better compared with their obese counterparts who did not have WLS.
In a 2004 series of 44 women and 80 pregnancies after AGB, Skull and colleagues found that 2 (4%) needed to have their bands removed due to acute gastric prolapse (neither band was placed using a pars flaccida technique) [325]. Both pregnancies went to term without complications. It can be argued that obese women who have had a difficult time conceiving or complicated pregnancies should consider WLS to not only increase their chance of becoming pregnant, but also to decrease their risk of pregnancy-related complications. No studies have identified morbidity, economic, or conception advantages for women who have WLS.
Although pregnancy after WLS can be safe and with many proven benefits, pregnancy after JIB, in particular, has been complicated by a high incidence of small-for-gestational-age infants [326]and nutritional abnormalities [327]. Fortunately, that procedure has been abandoned and should not be an issue for WLS candidates who are still of child-bearing age. Data on BPD show safe pregnancies, but most authors recommend increased supplementation of iron, calcium, and fat-soluble vitamins during pregnancy [319,328]. Oral contraceptives are also likely to fail in this population due to malabsorption [298]. To help prevent unwanted pregnancies in the first 18 months after BPD, a consultation with an obstetrician is recommended.
The nutritional status of women who get pregnant after WLS must be followed diligently. In addition to the expected nutrient abnormalities seen in all pregnant women, WLS patients require further monitoring of vitamin B12 and folate levels. These should be kept above 600 pg/mL and 15 ng/mL, respectively, to decrease homocysteine levels in the mother and prevent neural tube defects in the fetus [106]. Pregnant women require up to 71 g of protein per day and at least 1200 to 1500 mg of calcium per day.
Appropriate weight gain is the amount recommended for current BMI. Patients who have had AGB may need to have the fluid in their band removed during early pregnancy to ensure adequate nutrition for early fetal development. Band migration and erosion also may occur after repeated emesis [325]. Fluid in the band can be adjusted later if there is excessive weight gain [324]. A WLS treatment team should be part of prenatal care to help ensure proper nutrition and appropriate weight gain. Wittgrove and colleagues demonstrated that pregnant patients who maintained close follow-up with their bariatric team had more appropriate weight gain than those who were only seen in the first trimester [322]. Follow-up by the multidisciplinary weight loss team after delivery should also be part of the pregnancy plan.
The general surgeon may be asked to consult on pregnant patients who have undergone WLS. They should be aware of common problems that pertain to WLS. After AGB, for instance, hyperemesis my be secondary to gastric prolapse, esophageal/pouch dilation, or acute stomal obstruction. Treatment may be as simple as removing fluid from the band. After RYGB or BPD, the emesis may be due to internal herniation or stenosis. Understanding the WLS operation will help guide the diagnosis.
Risks versus Rewards of WLS
Health Benefits
Patients are likely to ask general surgeons and primary care providers questions about WLS. Although they can simply refer them to the nearest bariatric Center of Excellence, the community health care provider can also help his or her patients make informed decisions about WLS. Surgeons should be familiar with the evaluation and proper multidisciplinary process that WLS candidates should have during screening.
NIH criteria for WLS includes patients with BMI greater than 40 kg/m2 or greater than 35 kg/m2 with obesity-related comorbidities, including CVD, diabetes, and sleep apnea [46] (Table 6). Of those referred to WLS programs, 30% are turned away; 14% fail to meet NIH criteria, but nearly one half of the time (48%) denial stems from lack of insurance coverage [329]. Patients older than 65 years are at increased risk of surgical complications [84]. Therefore, most centers evaluate them on a case-by-case basis [329].
It may be important for referring physicians to temper expectations that obese patients have about weight loss after surgery. Although the results and benefits can be dramatic (far better than diet and exercise alone), only a minority of patients sustain weight loss greater than 20% of their total body weight after VBG and AGB [3]. The rate of those who do not sustain 20% total weight loss after RYGB is more than 25% [330]. Wee and colleagues report that fewer WLS candidates would risk death for permanent loss of “just” 20% of their total weight [331].
Still, the amount of weight lost, in most cases, is decidedly superior to any other form of weight management. Those who are deemed appropriate candidates after a multidisciplinary evaluation will most likely benefit greatly from WLS. It is important for referring physicians to emphasize reduction of comorbidities rather than total amount of weight lost when discussing the benefits of WLS. Rather than weight loss alone, the goals of WLS should be to restore health and improve QOL.
Resolution of Comorbidities
WLS improves or resolves most comorbid conditions of obesity. The largest studies on outcomes report that it is more effective than nonsurgical treatment for the improvement and control of most obesity-related comorbidities in patients with a BMI greater than 40 kg/m [2,16,49]. It resolves type 2 diabetes in up to 76.8% of patients [332] and controls it in up 86.6% of them [49]. Data indicate that BPD-DS has better resolution, with rates of approximately 90%, followed by RYGB, SG, and AGB [332].
Diabetes control after AGB is superior to that achieved by the best medical management [2]. WLS also eliminates hypertension in an average of 89% of patients; eliminates or controls hyperlipidemia in 88% of them; and significantly improves obstructive sleep apnea [49].
WLS can improve or control risk factors for CVD, reducing the risk of morbidity and mortality. Batsis and colleagues recently demonstrated that RYGB reduces risk of cardiovascular events and related mortality over a 10-year period [78]. Other conditions that improve or resolve after WLS include: cardiac dysfunction, GERD, pseudotumor cerebri, polycystic ovarian syndrome, stress urinary incontinence, degenerative joint disease, venous stasis disease, and nonalcoholic hepatitic steatosis [49].
Improved Outcome from Other Operations
WLS provides financial and physical relief from comorbidities of obesity as well as improved outcomes with other surgeries, including abdominoplasty and hernia repair [333,334]. The recurrence rate after repair of large or complex ventral hernias is 18% within 15 months in patients with a BMI greater than 35 kg/m2 [333]. To circumvent problems, more surgeons are using a staged approach that includes a weight loss procedure with a primary repair of the hernia or placement of bioabsorbable mesh. After significant weight loss, they perform a permanent mesh repair and may add a component release or Stoppa procedure. Using RYGB before definitive hernia repair in 27 patients, Newcomb and colleagues reported a 0% recurrence rate with an average of 20 months of follow-up [335].
Lower BMI also improves outcomes in total hip and knee arthroplasties. Failure is defined as continued pain or the inability to ambulate. Patients with a BMI greater than 40 kg/m2 have a failure rate of 35% compared with 2% for normal-weight individuals [336]. One report out of Sweden that examined 2106 male total hip arthroplasty patients found that a high BMI increased the risk of implant dislocation [337].
Decreased Cancer Risk
Obesity is a risk factor for the development of several malignancies, including the two most common cancers seen in men and women: colon and breast [338]. In addition to such significant risk factors as family history, increasing age, nulliparity, and the use of menopausal hormonal therapy, a BMI greater than 35 has been linked to increased risk of breast cancer in women [339]. Obesity has also been implicated as a risk factor for prostate [338] and endometrial cancer [340]. One possible mechanism for these associations is that hormonally active adipocytes secrete adipokines that induce a proinflammatory state that induces malignant degeneration [338].
Weight loss can reduce this proinflammatory state, possibly reducing the risk of developing some forms of cancer.
A BMI greater than 35 kg/m2 is associated with a 12-fold greater risk of lymph node metastases at time of resection for pancreatic cancer as well as decreased estimated disease-free and overall survival rates [341].
Obese patients are also twice as likely to die or have a recurrence of cancer after pancreatectomy [341]. Colectomy patients with a BMI greater than 35 kg/m2 are more likely to develop a PE, renal failure, surgical site infection, and wound dehiscence, but without increased risk of mortality [342]. Emphasis on improved weight management and a healthy lifestyle at an early age may improve the outcomes of oncology procedures when (or if) malignancy occurs.
Improved Quality of Life
WLS can improve QOL. Several studies have used different questionnaires to assess the impact of WLS on QOL and functional status. These include the 36-Item Short Form (SF-36) [343], the Gastrointestinal Quality of Life Index (GIQLI) [344], and the Moorehead-Ardelt Questionnaire, part of the Bariatric Analysis and Reporting Outcome System (BAROS) [345]. QOL has improved significantly within 3 months of RYGB [28,346]. Choban and colleagues found that after 18 months, QOL data matched that of normal-weight populations [347]. Improvements have lasted out to 13 years after RYGB [348]. Data on QOL after AGB are inconsistent. Some studies show improvements [349,350], but one found no change in the GI-QOL score after 2 years [351], and two reported a BAROS score of failure to improve in 4% to 50% of patients [352,353]. These results indicate that WLS patients with less than optimal % EWL and reductions in comorbidities still report good overall satisfaction due to dramatic improvements in QOL.
Increased Life Expectancy
WLS increases life expectancy [5,23,354]. Christo and colleagues found that 5-year risk of mortality is reduced from 6.17% to 0.68% after WLS [355], a reduction in relative risk of 89%. The Swedish Obese Subjects study followed 4047 WLS patients over 10 years. The 2010 who underwent WLS showed a 28% reduction in overall mortality compared with conventionally treated controls [4]. However, other data indicate a 58% higher rate of deaths from accidents and suicides in patients who have WLS compared with obese controls [5].
Best Practices in WLS
Surgical Accreditation and Centers of Excellence
The Lehman Center Expert Panel report on WLS has served as a key impetus to the development of accreditation standards and entities. Since the first Lehman Center report in 2005 [13], the ACS and the American Society for Metabolic and Bariatric Surgery (ASMBS) have both established accreditation programs grounded in evidence-based best practice care. The Centers for Medicare and Medicaid Services (CMMS) and most third party payers only pay for WLS performed at accredited sites.
The ACS Bariatric Surgery Center Network Accreditation Program has 2 levels of accreditation for inpatient facilities and outpatient surgical care sites [356–358]. Level 1 centers have resources devoted to WLS and provide complete care. They have high volume (>125 operations annually) and at least 2 credentialed and experienced weight loss surgeons who have individually performed a minimum of 100 weight loss operations in the prior 24 months. Centers with high volume, defined as more than 100 cases per year, have lower 30-day mortality than lower volume centers [83].
Level 1 centers must also have a program director and a program coordinator.
Level II centers must do at least 25 WLS procedures annually, with the recommendation to operate on lower risk patients (ie, BMI <50 kg/m2) [358].
The centers undergo review of their practice every 3 years and are expected to capture 100% of their WLS outcomes. Outcome measurements are monitored using the ACS National Surgical Quality Improvement Program (NSQIP).
The ASMBS Centers of Excellence program has similar criteria and accredits facilities as well as surgeons [359]. Organizations and surgeons initially apply for provisional status. That designation focuses on facility resources, the training and experience of the surgeons and surgical group, and whether criteria for provisional status are met. If so, after 2 years, hospitals can apply for full approval as an ASMBS Bariatric Surgery Center of Excellence [360].
Criteria for applicant institutions include performance of at least 125 bariatric surgical cases per year; for weight loss surgeons, the standard is a total of 125 or more cases in their lifetimes, with at least 50 in the preceding 12-month period. Other requirements include a designated physician medical director for WLS, a multidisciplinary team able to be summoned within 30 minutes, and equipment and instruments specifically designed for the care of WLS patients [360]. This program’s goal, like that of the ACS, is to help ensure that best practices are adhered to and that each institution’s outcome data are collected [359].
Other organizations are working to make outcomes from surgical procedures as transparent as possible. The Leapfrog Group, for example, uses surveys that focus on hospital adoption of evidence-based practices to improve medical care [361]. Bariatric surgery is one area that is being examined. It behooves general surgeons to know and adopt best practices for patient safety, and in the case of WLS, to follow the guidelines for excellence set forth by the ACS, the ASMBS, and the Lehman Center reports.
Lifelong Follow-Up
It is important to emphasize that WLS is a beginning and not a “last ditch effort” at weight loss management. The objective is to achieve a BMI that increases life expectancy and resolves or improves obesity- related conditions. Simply undergoing a procedure will not cure obesity or control its associated comorbidities. Changes in diet and lifestyle are imperative. Lifelong follow-up is mandated for WLS patients [362].
Those who have not seen their bariatric surgeon for several years should be referred to a multidisciplinary weight loss team, even if they are doing well. Sometimes such patients will have good weight control but, in fact, be malnourished. Maladies such as those reviewed earlier can occur several years, or even decades, after surgery. More likely, though, those who forego follow-up have regained weight. They may even be feeling worse or ashamed, believing that they “failed” even with surgery.
Reintroduction to a multidisciplinary weight loss program can help identify the factors leading to weight regain. These may be mechanical (eg, a fistula or pouch dilation), psychological (eg, stress eating), or behavioral (eg, poor food choices and lack of exercise). In each instance, there are ways to effect change and improve the outcome of the initial procedure. Although WLS enables obese patients to lose (and sustain) significant amounts of weight, the best outcomes still require patients to live a healthy lifestyle that includes a balanced diet and regular exercise.
Exercise and WLS
Although exercise is recommended for its general health benefits [363], more studies are needed to determine the optimal exercise regimen for the pre- and postoperative period of elective operations [364]. For WLS candidates, interventions to enhance preoperative weight loss via aerobic exercise and increased caloric expenditure may be misguided. Class III obese patients often suffer from locomotive handicaps that may preclude their ability to achieve aerobic activity levels of sufficient intensity and duration to facilitate weight loss [365]. The negative correlation between levels of physical activity and BMI indicates that obesity may be a barrier to physical activity [366]. Therefore, dietary changes might serve as the primary intervention for achieving desirable preoperative weight loss. However, an impaired capacity to perform physical activity is the strongest negative predictor of decreased EWL after WLS [367].
A potentially meaningful pre- and postsurgical exercise intervention to maximize weight loss after RYGB is one that addresses barriers to physical activity in patients with Class III obesity. Preoperative exercise protocols for these patients may be enhanced with activities that improve strength, fitness, and mobility. Patients should be encouraged to perform aerobic activity for its cardiovascular health benefits [368]. However, they may also benefit from such exercise routines as progressive resistance training (PRT), which targets large muscle groups for skeletal muscle biogenesis and hypertrophy [369].
PRT has been used successfully in several complicated patient cohorts to help ameliorate comorbid conditions associated with poor muscle quality, insulin resistance, and obesity [370]. The short-bout, isolated movements of resistance training may also serve as a more accessible form of exercise for patients with class III obesity. Increasing preoperative strength, mobility, and fitness can reduce the risk of perioperative complications, decrease postoperative recovery time, and result in superior long-term weight loss. To help them prepare for their operation, bariatric surgeons will often ask their patients to exercise 30 minutes daily before WLS.
After surgery, continued moderate aerobic activity can result in superior weight loss. One study found that an extra 150 minutes per week of a moderate aerobic activity, such as brisk walking, over 2.5 years produced a clinically significant increase in weight loss [371]. Another reported that using an extra 2500 kcals per week can sustain better weight loss [372]. The most appropriate exercise regimen to achieve optimal postoperative weight loss has yet to be determined. For patients who present several years after their WLS with complaints of weight regain, a full history should be conducted to determine their nutrition and exercise habits.
Nutrition and WLS
As was covered earlier, preoperative nutrition assessment is a critical component of caring for the WLS patient. Nutritional care extends far beyond assessing the macronutrient content of a diet. It includes such important factors as anthropometrics, weight, diet and medical history, psychosocial issues (eg, level of motivation and readiness to change), weight loss expectations, emotional connections with food, coping mechanisms, and dietary intake patterns.
Extensive preoperative diet education should include diet instruction that addresses pre- and postoperative diet stages, texture progression, and the importance of protein, hydration, and vitamin supplementation. It also needs to focus on key elements of behavior modification. Among others, these include the importance of taking personal responsibility for self- care and lifestyle choices, techniques for self-monitoring, keeping daily food journals, and setting realistic goals [373]. Patients who are not willing to make these changes, or who do not seem to understand these requirements, may not be ready to undergo surgery.
Preoperative Diet and Weight Loss
Weight loss before surgery has been used to reduce preoperative morbidity and reduce the risk of perioperative complications [374]. A preoperative weight loss of 5% to 10% is suggested, specifically in patients with a BMI greater than 50 kg/m2 or obesity-related comorbidities [25]. Patients should be encouraged to avoid last-minute binging before their surgery. Substituting meal replacements for regular meals 1 to 2 times per day is an effective strategy for preoperative weight loss. Portion-controlled meal replacements provide a release from complex dieting and produce successful, gradual weight loss of 1 to 2 lb per week [375].
An analysis of 6 studies on weight management using meal replacements compared a partial meal replacement (PMR) diet to a reduced calorie diet (RCD) with conventional food. Using 1 to 2 MRs per day with an additional regular food meal, the PMR group lost 7.8% of body weight compared with 3.7% in the RCD group [375]. Those following a PMR diet should choose meal replacements that have a balance of macronutrients, provide 180 to 200 calories and 15 to 20 g of protein, and are low in sugar (:::16 g of sugar per 200 calories).
Meal Planning
The suggested WLS preoperative diet is calorie reduced, with a macronutrient breakdown of 40% complex carbohydrate, 30% protein, and 30% fat [376]. To achieve this healthy balance, patients should be encouraged to follow the “New American Plate Model” from the American Institute of Cancer Research. One half of the plate should be filled with vegetables, ¼ with lean protein, and the remaining ¼ with complex carbohydrates. The foods that are highest in nutrients—fiber and water, which lengthen satiety— are usually the lowest in calories. Patients should be guided to add more fruits, vegetables, whole grains, and beans into meals (Table 15). Clinical experience suggests that weight loss before surgery makes procedures less technically challenging by reducing the size of the liver [377].
Table 15.
Suggested preoperative diet [375]
| Carbohydrate | Protein | Fats |
|---|---|---|
| ~40% of total caloric intake. The total should not be less than 130 g/day. A minimum of 20–35 g of fiber per day |
~30% of total caloric intake | ~30% of total caloric intake Choose mono- and polyunsaturated fats: olive oil, canola oil nuts/seeds fish, particularly those high in omega-3 fatty acids (eg, salmon, herring, trout, sardines, fresh tuna) 2 times/wk |
| Choose less of these foods | Choose more of these foods | |
|
|
|
Two weeks before surgery, patients should go on a meal plan consisting of 3 meal replacements per day plus 1 “real” food (Table 16).
TABLE 16.
Preoperative diet at 2 weeks before operation [375]
| 1200 calories: 2 week preoperative diet example (recommended for men) Meal |
|---|
| 1: Meal replacement |
| Meal 2: Meal replacement |
| Meal 3: Meal replacement |
| Meal 4: “Real” food (see below) |
| Meal 4: “Real” food options |
Proteins/meats (select 1 from this list per day)
|
Carbohydrate/starch servings (select 1 from this list per day)
|
Vegetable servings (select 1 from this list per day)—see “free” list for seasoning ideas
|
Fruit serving (select 1 from this list per day)
|
Dairy serving (select 1 from this list per day)
|
Fat servings (select 1 from this list per day)*Remember you may need to use your fat serving when cooking your meal*
|
Substitute for “real” food meal
|
Postoperative Diet
The objectives of postoperative nutrition are to [378]: maintain adequate hydration and nutrition status; support homeostasis of bodily functions; promote wound healing; preserve lean muscle mass; facilitate safe and sustained weight loss; and nurture a healthier lifestyle. These goals are achieved through a multiphase diet progression (Table 17).
TABLE 17.
Multiphase diet progression [379]
| Location and approximate schedule | Stage | General description | Length of stage (variable) |
|---|---|---|---|
| Starts after surgery with IV fluids continuing | 1 | Water (1 oz per hour) | One day or less |
| Completed in the hospital | 2 | Low sugar, decaffeinated, noncarbonated clear liquids (<3 oz at a time) | One day or less |
| Getting ready for discharge; introduced to stage 3; evaluated for tolerance | 3 | Low sugar, high-protein modified full liquids (4–8 oz per hour) | 3 weeks |
| Home; follow-up in clinic (3 weeks postoperatively) | 4 | Lean pureed/ground | 4–5 weeks |
| Home; follow-up in clinic (~8 weeks postoperatively) | 5 | Lean meat, fish, poultry, and protein bars, fresh fruit and vegetables, whole grains and legumes, healthy-fat and low-fat dairy | Lifetime |
Stages 1 and 2 are brief and completed in the hospital. Stage 3 provides high quality, low-fat, low-sugar, and low-lactose protein sources. During stage 3, the patient is dependent on liquid protein supplements or meal replacements as the primary source of nutrition. Either a protein supplement that provides all the indispensable amino acids or a combination of products must be used when protein supplements are the primary source of dietary protein intake. Stage 4 consists of pureed and ground foods. It gradually reintroduces the system to solid food. During stage 5, the diet for life, patients transition to reasonable portion sizes of regular healthy foods (Table 18).
TABLE 18.
Stage and fluid guidelines [379]
| Diet stage | Protein goal | Fluid recommendations | Recommended choices |
|---|---|---|---|
| Stage 3 | 60–80 g/day | 2–4 L/day (supplements included) | Liquid supplements: high quality protein, low-sugar, low-lactose, low-fat supplements with at least 15 g per 240 mL |
Food options: liquids/soft foods:
|
|||
Beverage options:
|
|||
| Stage 4 | 1.0–1.5 g/kg | 2–4 L per day | Liquid supplements: (same as stage 3) used to enhance protein intake not obtained from food choices below |
Food options: pureed and ground foods,1–2 oz servings:
|
|||
Avoid:
|
|||
| Beverage options: same as stage 3 | |||
| Stage 5 | 30% of total calories | 2–4 L per day | Food options: balanced and healthy foods: small portions of regular texture food over 3–4 meals per day
|
Avoid:
|
|||
|
Eating Strategies
After operation, patients must learn new eating strategies aimed at reducing uncomfortable side effects brought on by their new anatomical restriction [379] (Table 19).
Table 19.
Rules of gastric eating [379]
|
If there is vomiting or regurgitation:
|
Dehydration
The restrictive aspect of the procedure puts WLS patients at risk of dehydration. To avoid dehydration, it is critical to encourage patients to consume fluids slowly throughout the day and be aware of the signs and symptoms of dehydration (dry mouth, low urine output or dark concentrated urine, chronic headache, lightheadedness, dizziness, and fatigue). Fluid recommendations are described in Table 18.
Food Intolerances
Food intolerances, which frequently involve meat products, are prevalent after WLS, but usually diminish by the first postoperative year. New nutritional habits should be reinforced continuously to help minimize troubling gastrointestinal symptoms, and patients should be encouraged to eat alternative protein sources. The use of protein supplements is optional.
Patients who have had malabsorptive procedures are at particularly high risk of clinically important nutrient deficiencies. Initially, 1 to 2 tablets of a chewable multivitamin-mineral supplement are better tolerated than nonchewable preparations [380].
Protein Depletion and Supplementation
Hypoalbuminemia is rare after standard RYGB, but protein-deficient meals are common. This is generally noted at 3 to 6 months after WLS, and is largely attributed to intolerance of protein-rich foods. Seventeen percent of patients who experience persistent intolerance of such foods limit their intake of protein to less than 50% of recommended amounts. Even patients with completely resolved food intolerances often fail to meet daily recommended intake of protein. The nutritional status should be assessed regularly, and supplementation with protein modular sources pursued if protein consumption remains less than 60 g daily [380] (Table 20).
Table 20.
Postoperative calorie guidelines [380]
Postoperative calorie recommendations:
|
Weight maintenance calorie recommendations:
|
Cost-Benefit and Economics of WLS
Obesity-related illness costs the U.S. economy an estimated $100 billion a year, making it second only to smoking for overall annual medical expenditures [381]. Obese patients are 2 to 5 times more likely to miss work [382] and cost employers over $2200 more every year than their normal weight colleagues (in 2005 dollars) [383]. WLS is likely to reduce costs via reduced absenteeism and presenteeism, and increased productivity.
In a recent review on the economic impact of WLS, Cremieux and colleagues studied 3651 severely obese WLS patients and compared them to matched controls. Outcomes showed that the initial cost of bariatric surgery is approximately $17,000 to $26,000, and that for payers, all costs would be recouped within 2 years for laparoscopic surgery patients and within 4 years for open surgery patients [19]. The cost of diabetes alone is an estimated $10,634 annually [384].
WLS improves or resolves more than 30 obesity-related conditions, including type 2 diabetes, heart disease, sleep apnea, hypertension, and high cholesterol [18]. Data indicate that even the least amount of weight loss from WLS is superior to the best results from nonsurgical means [158]. Patients also have improved QOL and functional status even with relatively modest weight loss. As surgeons gain experience, techniques improve, and the use of multidisciplinary care teams becomes the norm, the risk will continue to decline and outcomes will continue to improve[27].
Despite the exponential increase in the number of weight loss surgeries performed between 1998 and 2004, the national inpatient death rate declined from 0.89% to 0.19% [385]. The incidence of respiratory failure after WLS fell from 7.7% to 4.5% between 1990 and 2000 [80]. Early mortality remains low even with more operations on patients older than the age of 50, and leak, hernia, infection, and pneumonia rates have all declined [27].
These improvements are happening in parallel with declining costs. Laparoscopy alone has reduced expense by approximately 12%, and AGB by 20% even with complications, readmissions, hospital stay, and the risks of an older patient population [27]. The decrease in cost is across-the- board, in patients with or without complications, and even in those who require readmission [27].
Conclusions
With nearly 250,000 weight loss operations performed each year, most health care providers will eventually be caring for patients who have undergone a band, bypass, or sleeve procedure. They should be familiar with the basics of the devices, anatomical changes, nutritional challenges, and early signs and symptoms of common complications. The emergency room doctor or cross-covering general surgeon may be the first to evaluate a patient with vomiting and abdominal pain years after gastric bypass. This monograph emphasizes complications of WLS and the importance of prompt and accurate diagnosis.
Over the last 5 years, the ACS and ASMBS/SRC have developed accreditation standards for hospitals and surgeons based on evidence based best practice standards. The Lehman Center reports have served as the impetus and framework for those criteria now used by the CMMS and third party payers to determine reimbursement policies.
Alarmed by extremely fast growth in the 1990s, the field of WLS has established measures to assure provider competence and protect patient safety. In turn, morbidity and mortality rates have continued to fall, even among the sickest patients. However, much still needs to be done to protect patient safety and ensure access to WLS and other life-saving treatments.
Stereotypes, biases, and prejudice work against obese people in every area of their lives, from the workplace to the doctor’s office. As a nation, we need to attack the prevention of obesity and the eradication of stigma with equal zeal. Obesity extracts a high price from those who suffer from it as well as society at large. It behooves America and other nations affected by the obesity crisis to invest in effective treatments and early intervention in schools, communities, and workplaces.
The fight against obesity can serve as a model for collaboration among industry, government, academia, and other stakeholders as they join forces to improve health care in the United States. In his May 2009 address to the American Medical Association, President Obama tasked physicians with making the latest research available for immediate consumption. This includes timely, comprehensive, evidence-based best practice standards like those developed and updated by the Lehman Center. The delivery of safe and effective WLS not only protects the best interests of patients, but also those of physicians, surgeons, insurers, and all who seek the speedy delivery of safe and effective health care throughout America and around the world.
Acknowledgments
The authors gratefully acknowledge Cine-Med for permission to use their illustrations and Sam Wollner, Rita Buckley, and Lisa Lim for their support and editorial assistance. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or United States Government.
References
- 1.Robinson MK. Surgical treatment of obesity—weighing the facts. N Engl J Med. 2009;361:520–1. doi: 10.1056/NEJMe0904837. [DOI] [PubMed] [Google Scholar]
- 2.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 3.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 5.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 6.Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varela JE, Asolati M, Huerta S, Anthony T. Outcomes of laparoscopic and open colectomy at academic centers. Am J Surg. 2008;196:403–6. doi: 10.1016/j.amjsurg.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S. Adverse events in coronary artery bypass graft (CABG) trials: a systematic review and analysis. Heart. 2003;89:767–72. doi: 10.1136/heart.89.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemp JA, Finlayson SR. Outcomes of laparoscopic and open colectomy: a national population-based comparison. Surg Innov. 2008;15:277–83. doi: 10.1177/1553350608327171. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Encinosa W. Statistical Brief #23. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Bariatric surgery utilization and outcomes in 1998 and 2004. [PubMed] [Google Scholar]
- 11.Jones SB, Jones DB. Obesity Surgery: Patient Safety and Best Practices. Wood-bury, CT: Cine-Med, Inc; 2009. [Google Scholar]
- 12.Dembner A. Push for Patient Safety honors writer. The Boston Globe. 2004 January 12; [Google Scholar]
- 13.Lehman Center Weight Loss Surgery Expert Panel. Commonwealth of Massachusetts. Betsy Lehman Center for Patient Safety and Medical Error Reduction. Expert Panel on Weight Loss Surgery: Executive Report. Obes Res. 2005;13:206–26. doi: 10.1038/oby.2005.30. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn GL, Hu FB, Hutter MM. Updated evidence—based recommendations for best practices in weight loss surgery. Obesity (Silver Spring) 2009;17:839–41. doi: 10.1038/oby.2008.572. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn GL, Hutter MM, Harvey AM, Apovian CM, Boulton HR, Cummings S, et al. Expert panel on weight loss surgery: executive report update. Obesity (Silver Spring) 2009;17:842–62. doi: 10.1038/oby.2008.578. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 17.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, et al. Safety and efficacy of bariatric surgery: longitudinal assessment of bariatric surgery. Surg Obes Relat Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fact Sheet. Metabolic & Bariatric Surgery. [Accessed August 8, 2009];American Society for Metabolic & Bariatric Surgery. Available at: http://www.asbs.org/Newsite07/media/asmbs_fs_surgery.pdf.
- 19.Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14:589–96. [PubMed] [Google Scholar]
- 20.Becker’s ASC Review. [Accessed April 2009];Update on Weight-Loss Surgery: 3 Current Trends. Available at: http://www.beckersasc.com/news-analysis-asc/business-financial-benchmarking/update-on-weight-loss-surgery-3-current-trends.html.
- 21.World Health Organization. [Accessed April 2009];Obesity and Overweight. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.htm.
- 22.Omalu BI, Luckasevic T, Shakir AM, Rozin L, Wecht CH, Kuller LH. Postbariatric surgery deaths, which fall under the jurisdiction of the coroner. Am J Forensic Med Pathol. 2004;25:237–42. doi: 10.1097/01.paf.0000136638.26060.78. [DOI] [PubMed] [Google Scholar]
- 23.Sowemimo OA, Yood SM, Courtney J, Moore J, Huang M, Ross R, et al. Natural history of morbid obesity without surgical intervention. Surg Obes Relat Dis. 2007;3:73–7. doi: 10.1016/j.soard.2006.10.017. discussion 77. [DOI] [PubMed] [Google Scholar]
- 24.McMahon MM, Sarr MG, Clark MM, Gall MM, Knoetgen J, 3rd, Service FJ, et al. Clinical management after bariatric surgery: value of a multidisciplinary approach. Mayo Clin Proc. 2006;81(10 Suppl):S34–45. doi: 10.1016/s0025-6196(11)61179-8. [DOI] [PubMed] [Google Scholar]
- 25.Saltzman E, Anderson W, Apovian CM, Boulton H, Chamberlain A, Cullum-Dugan D, et al. Criteria for patient selection and multidisciplinary evaluation and treatment of the weight loss surgery patient. Obes Res. 2005;13:234–43. doi: 10.1038/oby.2005.32. [DOI] [PubMed] [Google Scholar]
- 26.Buchwald H, Williams SE. Bariatric surgery training in the United States. Surg Obes Relat Dis. 2006;2:52–5. doi: 10.1016/j.soard.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Encinosa WE, Bernard DM, Du D, Steiner CA. Recent improvements in bariatric surgery outcomes. Med Care. 2009;47:531–5. doi: 10.1097/MLR.0b013e31819434c6. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–89. doi: 10.1097/00000658-200109000-00002. discussion 289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones KB, Jr, Afram JD, Benotti PN, Capella RF, Cooper CG, Flanagan L, et al. Open versus laparoscopic Roux-en-Y gastric bypass: a comparative study of over 25,000 open cases and the major laparoscopic bariatric reported series. Obes Surg. 2006;16:721–7. doi: 10.1381/096089206777346628. [DOI] [PubMed] [Google Scholar]
- 30.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–64. doi: 10.1381/0960892042387057. [DOI] [PubMed] [Google Scholar]
- 31.Griffen WO, Jr, Bivins BA, Bell RM. The decline and fall of the jejunoileal bypass. Surg Gynecol Obstet. 1983;157:301–8. [PubMed] [Google Scholar]
- 32.O’Brien PE, Dixon JB, Brown W, Schachter LM, Chapman L, Burn AJ, et al. The laparoscopic adjustable gastric band (Lap-Band): a prospective study of medium-term effects on weight, health and quality of life. Obes Surg. 2002;12:652–60. doi: 10.1381/096089202321019639. [DOI] [PubMed] [Google Scholar]
- 33.Jones DB, Schneider BE, Maithel SK. Atlas of Minimally Invasive Surgery. Woodbury, CT: Cine-Med, Inc; 2006. pp. 332–55. [Google Scholar]
- 34.Jones DB, Schneider BE, Maithel SK. Atlas of Minimally Invasive Surgery. Woodbury, CT: Cine-Med, Inc; 2006. pp. 298–331. [Google Scholar]
- 35.Curry TK, Carter PL, Porter CA, Watts DM. Resectional gastric bypass is a new alternative in morbid obesity. Am J Surg. 1998;175:367–70. doi: 10.1016/S0002-9610(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 36.Trelles N, Gagner M. Sleeve gastrectomy. Oper Tech Gen Surg. 2007:123–31. [Google Scholar]
- 37.Cottam D, Qureshi FG, Mattar SG, Gianetta E, Traverso E, Friedman D, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859–63. doi: 10.1007/s00464-005-0134-5. [DOI] [PubMed] [Google Scholar]
- 38.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–7. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 39.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253–71. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 40.Scopinaro N, Adami GF, Marinari GM, Gianetta E, Traverso E, Friedman D, et al. Biliopancreatic diversion. World J Surg. 1998;22:936–46. doi: 10.1007/s002689900497. [DOI] [PubMed] [Google Scholar]
- 41.Van Hee RH. Biliopancreatic diversion in the surgical treatment of morbid obesity. World J Surg. 2004;28:435–44. doi: 10.1007/s00268-004-7364-x. [DOI] [PubMed] [Google Scholar]
- 42.Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, Potvin M, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–54. doi: 10.1007/s002689900498. [DOI] [PubMed] [Google Scholar]
- 43.Lagacé M, Marceau P, Marceau S, Hould FS, Potvin M, Bourque RA, et al. Biliopancreatic diversion with a new type of gastrectomy: some previous conclusions revisited. Obes Surg. 1995;5:411–8. doi: 10.1381/096089295765557511. [DOI] [PubMed] [Google Scholar]
- 44.Parikh M, Pomp A. Laparoscopic duodenal switch. In: Rosenthal R, Jones DB, editors. Weight Loss Surgery: A Multidisciplinary Approach. Edgemont, PA: Matrix Medical Communications; 2008. pp. 297–310. [Google Scholar]
- 45.Almogy G, Crookes PF, Anthone GJ. Longitudinal gastrectomy as a treatment for the high-risk super-obese patient. Obes Surg. 2004;14:492–7. doi: 10.1381/096089204323013479. [DOI] [PubMed] [Google Scholar]
- 46.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–61. [PubMed] [Google Scholar]
- 47.Xanthakos SA, Daniels SR, Inge TH. Bariatric surgery in adolescents: an update. Adolesc Med Clin. 2006;17:589–612. doi: 10.1016/j.admecli.2006.06.001. abstract x. [DOI] [PubMed] [Google Scholar]
- 48.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142:621–32. doi: 10.1016/j.surg.2007.07.018. discussion 632–5. [DOI] [PubMed] [Google Scholar]
- 49.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–59. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 50.Ridley N Betsy Lehman Center for Patient Safety. Obesity surgery. Patient Safety and Best Practices. 2009:177–80. [Google Scholar]
- 51.Lancaster RT, Hutter MM. Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center, risk-adjusted ACS-NSQIP data. Surg Endosc. 2008;22:2554–63. doi: 10.1007/s00464-008-0074-y. [DOI] [PubMed] [Google Scholar]
- 52.Peters TG, Steinmetz SR, Cowan GS., Jr Splenic injury and repair during bariatric surgical procedures. South Med J. 1990;83:166–9. doi: 10.1097/00007611-199002000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Schneider BE, Villegas L, Blackburn GL, Mun EC, Critchlow JF, Jones DB. Laparoscopic gastric bypass surgery: outcomes. J Laparoendosc Adv Surg Tech A. 2003;13:247–55. doi: 10.1089/109264203322333575. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez R, Murr MM. Anastomotic leaks following gastric bypass surgery. In: Jones DB, Rosenthal R, editors. Weight Loss Surgery: A Multidisciplinary Approach. Edgemont, PA: Matrix Medical Communications; 2008. pp. 369–70. [Google Scholar]
- 55.Schwartz ML, Drew RL, Andersen JN. Induction of pneumoperitoneum in morbidly obese patients. Obes Surg. 2003;13:601–4. doi: 10.1381/096089203322190817. discussion 604. [DOI] [PubMed] [Google Scholar]
- 56.Madan AK, Taddeucci RJ, Harper JL, Tichansky DS. Initial trocar placement and abdominal insufflation in laparoscopic bariatric surgery. J Surg Res. 2008;148:210–3. doi: 10.1016/j.jss.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 57.Ahmad G, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2008;(2):CD006583. doi: 10.1002/14651858.CD006583.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Kothari SN, Fundell LJ, Lambert PJ, Mathiason MA. Use of transabdominal ultrasound to identify intraabdominal adhesions prior to laparoscopy: a prospective blinded study. Am J Surg. 2006;192:843–7. doi: 10.1016/j.amjsurg.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen NT, Rivers R, Wolfe BM. Factors associated with operative outcomes in laparoscopic gastric bypass. J Am Coll Surg. 2003;197:548–55. doi: 10.1016/S1072-7515(03)00648-3. discussion 555–7. [DOI] [PubMed] [Google Scholar]
- 60.Lee CW, Kelly JJ, Wassef WY. Complications of bariatric surgery. Curr Opin Gastroenterol. 2007;23:636–43. doi: 10.1097/MOG.0b013e3282f094b5. [DOI] [PubMed] [Google Scholar]
- 61.Miller K, Pump A, Hell E. Vertical banded gastroplasty versus adjustable gastric banding: prospective long-term follow-up study. Surg Obes Relat Dis. 2007;3:84–90. doi: 10.1016/j.soard.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Cariani S, Nottola D, Grani S, Vittimberga G, Lucchi A, Amenta E. Complications after gastroplasty and gastric bypass as a primary operation and as a reoperation. Obes Surg. 2001;11:487–90. doi: 10.1381/096089201321209396. [DOI] [PubMed] [Google Scholar]
- 63.Westling A, Ohrvall M, Gustavsson S. Roux-en-Y gastric bypass after previous unsuccessful gastric restrictive surgery. J Gastrointest Surg. 2002;6:206–11. doi: 10.1016/s1091-255x(01)00035-x. [DOI] [PubMed] [Google Scholar]
- 64.van Gemert WG, van Wersch MM, Greve JW, Soeters PB. Revisional surgery after failed vertical banded gastroplasty: restoration of vertical banded gastroplasty or conversion to gastric bypass. Obes Surg. 1998;8:21–8. doi: 10.1381/096089298765555006. [DOI] [PubMed] [Google Scholar]
- 65.Marshall JS, Srivastava A, Gupta SK, Rossi TR, DeBord JR. Roux-en-Y gastric bypass leak complications. Arch Surg. 2003;138:520–3. doi: 10.1001/archsurg.138.5.520. discussion 523–4. [DOI] [PubMed] [Google Scholar]
- 66.Mailapur R, Marema R, Buffington C. Oversewing the gastric staple-lines reduces the incidence of gastro-gastric fistulas with laparoscopic gastric bypass [abstract] Surg Endosc. 2004;18(S200) [Google Scholar]
- 67.Shikora SA, Kim JJ, Tarnoff ME. Reinforcing gastric staple-lines with bovine pericardial strips may decrease the likelihood of gastric leak after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:37–44. doi: 10.1381/096089203321136566. [DOI] [PubMed] [Google Scholar]
- 68.Thodiyil PA, Yenumula P, Rogula T, Gorecki P, Fahoum B, Gourash W, et al. Selective nonoperative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg. 2008;248:782–92. doi: 10.1097/SLA.0b013e31818584aa. [DOI] [PubMed] [Google Scholar]
- 69.Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138:957–61. doi: 10.1001/archsurg.138.9.957. [DOI] [PubMed] [Google Scholar]
- 70.Sapala JA, Wood MH, Schuhknecht MP, Sapala MA. Fatal pulmonary embolism after bariatric operations for morbid obesity: a 24-year retrospective analysis. Obes Surg. 2003;13:819–25. doi: 10.1381/096089203322618588. [DOI] [PubMed] [Google Scholar]
- 71.Gagner M, Milone L, Yung E, Broseus A, Gumbs AA. Causes of early mortality after laparoscopic adjustable gastric banding. J Am Coll Surg. 2008;206:664–9. doi: 10.1016/j.jamcollsurg.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 72.Melinek J, Livingston E, Cortina G, Fishbein MC. Autopsy findings following gastric bypass surgery for morbid obesity. Arch Pathol Lab Med. 2002;126:1091–5. doi: 10.5858/2002-126-1091-AFFGBS. [DOI] [PubMed] [Google Scholar]
- 73.De Pergola G, Pannacciulli N. Coagulation and fibrinolysis abnormalities in obesity. J Endocrinol Invest. 2002;25:899–905. doi: 10.1007/BF03344054. [DOI] [PubMed] [Google Scholar]
- 74.Steele K, Schweitzer MA, Hamad G, Rosenthal R, DeMaria E, Texeira J, et al. Prophylaxis of Venous Thromboembolism in Gastric Bypass Patients. In: Jones DB, Rosenthal R, editors. Weight Loss Surgery: A multidisciplinary approach. Edgemont, PA: Matrix Media Communications; 2008. pp. 113–7. [Google Scholar]
- 75.Sugerman HJ, Sugerman EL, Wolfe L, Kellum JM, Jr, Schweitzer MA, DeMaria EJ. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41–6. doi: 10.1097/00000658-200107000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Overby DW, Kohn GP, Cahan MA, Dixon RG, Stavas JM, Moll S, et al. Risk-group targeted inferior vena cava filter placement in gastric bypass patients. Obes Surg. 2009;19:451–5. doi: 10.1007/s11695-008-9794-2. [DOI] [PubMed] [Google Scholar]
- 77.Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007;246:1002–7. doi: 10.1097/SLA.0b013e31815c404e. discussion 1007–9. [DOI] [PubMed] [Google Scholar]
- 78.Batsis JA, Sarr MG, Collazo-Clavell ML, Thomas RJ, Romero-Corral A, Somers VK, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102:930–7. doi: 10.1016/j.amjcard.2008.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Escalante-Tattersfield T, Tucker O, Fajnwaks P, Szomstein S, Rosenthal RJ. Incidence of deep vein thrombosis in morbidly obese patients undergoing laparo-scopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4:126–30. doi: 10.1016/j.soard.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Trus TL, Pope GD, Finlayson SR. National trends in utilization and outcomes of bariatric surgery. Surg Endosc. 2005;19:616–20. doi: 10.1007/s00464-004-8827-8. [DOI] [PubMed] [Google Scholar]
- 81.Alamoudi OS. Long-term pulmonary complications after laparoscopic adjustable gastric banding. Obes Surg. 2006;16:1685–8. doi: 10.1381/096089206779319329. [DOI] [PubMed] [Google Scholar]
- 82.Poulose BK, Griffin MR, Zhu Y, Smalley W, Richards WO, Wright JK, et al. National analysis of adverse patient safety for events in bariatric surgery. Am Surg. 2005;71:406–13. doi: 10.1177/000313480507100508. [DOI] [PubMed] [Google Scholar]
- 83.Hollenbeak CS, Rogers AM, Barrus B, Wadiwala I, Cooney RN. Surgical volume impacts bariatric surgery mortality: a case for centers of excellence. Surgery. 2008;144:736–43. doi: 10.1016/j.surg.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Flum DR, Salem L, Elrod JA, Dellinger EP, Cheadle A, Chan L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294:1903–8. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 85.Al-Jiffry BO, Shaffer EA, Saccone GT, Downey P, Kow L, Toouli J. Changes in gallbladder motility and gallstone formation following laparoscopic gastric banding for morbid obesity. Can J Gastroenterol. 2003;17:169–74. doi: 10.1155/2003/392719. [DOI] [PubMed] [Google Scholar]
- 86.Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000–5. [PubMed] [Google Scholar]
- 87.Shiffman ML, Sugerman HJ, Kellum JM, Moore EW. Changes in gallbladder bile composition following gallstone formation and weight reduction. Gastroenterology. 1992;103:214–21. doi: 10.1016/0016-5085(92)91115-k. [DOI] [PubMed] [Google Scholar]
- 88.Sugerman HJ, Brewer WH, Shiffman ML, Brolin RE, Fobi MA, Linner JH, et al. A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg. 1995;169:91–6. doi: 10.1016/s0002-9610(99)80115-9. discussion 96–7. [DOI] [PubMed] [Google Scholar]
- 89.Miller K, Hell E, Lang B, Lengauer E. Gallstone formation prophylaxis after gastric restrictive procedures for weight loss: a randomized double-blind placebo-controlled trial. Ann Surg. 2003;238:697–702. doi: 10.1097/01.sla.0000094305.77843.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lalor PF, Tucker ON, Szomstein S, Rosenthal RJ. Complications after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2008;4:33–8. doi: 10.1016/j.soard.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 91.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, et al. Sleeve gastrectomy: a restrictive procedure? Obes Surg. 2007;17:57–62. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 92.Lee CM, Cirangle PT, Jossart GH. Vertical gastrectomy for morbid obesity in 216 patients: report of two-year results. Surg Endosc. 2007;21:1810–6. doi: 10.1007/s00464-007-9276-y. [DOI] [PubMed] [Google Scholar]
- 93.Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7:569–75. doi: 10.1097/00075197-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Giusti V, Suter M, Heraief E, Gaillard RC, Burckhardt P. Effects of laparoscopic gastric banding on body composition, metabolic profile and nutritional status of obese women: 12-months follow-up. Obes Surg. 2004;14:239–45. doi: 10.1381/096089204322857636. [DOI] [PubMed] [Google Scholar]
- 95.Chapman AE, Kiroff G, Game P, Foster B, O’Brien P, Ham J, et al. Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic literature review. Surgery. 2004;135:326–51. doi: 10.1016/S0039-6060(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 96.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–31. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 97.Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, Kenler HA, et al. Are vitamin B12 and folate deficiency clinically important after roux-en-Y gastric bypass? J Gastrointest Surg. 1998;2:436–42. doi: 10.1016/s1091-255x(98)80034-6. [DOI] [PubMed] [Google Scholar]
- 98.Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, Sarr MG. Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 1993;218:91–6. doi: 10.1097/00000658-199307000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003;67:979–86. [PubMed] [Google Scholar]
- 100.Brolin RE, Leung M. Survey of vitamin and mineral supplementation after gastric bypass and biliopancreatic diversion for morbid obesity. Obes Surg. 1999;9:150–4. doi: 10.1381/096089299765553395. [DOI] [PubMed] [Google Scholar]
- 101.Davies DJ, Baxter JM, Baxter JN. Nutritional deficiencies after bariatric surgery. Obes Surg. 2007;17:1150–8. doi: 10.1007/s11695-007-9208-x. [DOI] [PubMed] [Google Scholar]
- 102.Halverson JD. Micronutrient deficiencies after gastric bypass for morbid obesity. Am Surg. 1986;52:594–8. [PubMed] [Google Scholar]
- 103.Knudsen LB, Kallen B. Gastric bypass, pregnancy, and neural tube defects. Lancet. 1986;2:227. doi: 10.1016/s0140-6736(86)92531-6. [DOI] [PubMed] [Google Scholar]
- 104.Bellamy MF, McDowell IF, Ramsey MW, Brownlee M, Bones C, Newcombe RG, et al. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation. 1998;98:1848–52. doi: 10.1161/01.cir.98.18.1848. [DOI] [PubMed] [Google Scholar]
- 105.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 106.Dixon JB, Dixon ME, O’Brien PE. Elevated homocysteine levels with weight loss after Lap-Band surgery: higher folate and vitamin B12 levels required to maintain homocysteine level. Int J Obes Relat Metab Disord. 2001;25:219–27. doi: 10.1038/sj.ijo.0801474. [DOI] [PubMed] [Google Scholar]
- 107.Jacobson P, Lindroos A, Sjostrom C, et al. Long-term changes in homocysteine following weight loss in the SOS study [abstract] Int J Obes. 2000;24:S175. [Google Scholar]
- 108.Henning BF, Tepel M, Riezler R, Gillessen A, Doberauer C. Vitamin supplementation during weight reduction–favourable effect on homocysteine metabolism. Res Exp Med (Berl) 1998;198:37–42. doi: 10.1007/s004330050087. [DOI] [PubMed] [Google Scholar]
- 109.Borson-Chazot F, Harthe C, Teboul F, Labrousse F, Gaume C, Guadagnino L, et al. Occurrence of hyperhomocysteinemia 1 year after gastroplasty for severe obesity. J Clin Endocrinol Metab. 1999;84:541–5. doi: 10.1210/jcem.84.2.5476. [DOI] [PubMed] [Google Scholar]
- 110.Rhode BM, Shustik C, Christou NV, MacLean LD. Iron absorption and therapy after gastric bypass. Obes Surg. 1999;9:17–21. doi: 10.1381/096089299765553656. [DOI] [PubMed] [Google Scholar]
- 111.Kalfarentzos F, Kechagias I, Soulikia K, Loukidi A, Mead N. Weight loss following vertical banded gastroplasty: intermediate results of a prospective study. Obes Surg. 2001;11:265–70. doi: 10.1381/096089201321336566. [DOI] [PubMed] [Google Scholar]
- 112.Kushner RF, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104:1393–7. doi: 10.1016/j.jada.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 113.Loh Y, Watson WD, Verma A, Chang ST, Stocker DJ, Labutta RJ. Acute Wernicke’s encephalopathy following bariatric surgery: clinical course and MRI correlation. Obes Surg. 2004;14:129–32. doi: 10.1381/096089204772787437. [DOI] [PubMed] [Google Scholar]
- 114.Chaves LC, Faintuch J, Kahwage S, Alencar Fde A. A cluster of polyneuropathy and Wernicke-Korsakoff syndrome in a bariatric unit. Obes Surg. 2002;12:328–34. doi: 10.1381/096089202321088093. [DOI] [PubMed] [Google Scholar]
- 115.Hocking MP, Davis GL, Franzini DA, Woodward ER. Long-term consequences after jejunoileal bypass for morbid obesity. Dig Dis Sci. 1998;43:2493–9. doi: 10.1023/a:1026698602714. [DOI] [PubMed] [Google Scholar]
- 116.Sola E, Morillas C, Garzon S, Ferrer JM, Martin J, Hernandez-Mijares A. Rapid onset of Wernicke’s encephalopathy following gastric restrictive surgery. Obes Surg. 2003;13:661–2. doi: 10.1381/096089203322190934. [DOI] [PubMed] [Google Scholar]
- 117.Sasaki I, Fujii S, Ichihara N, Hatanaka Y. Vitamin B1 deficiency polyneuropathy presenting homolateral imitative synkinesia. No To Shinkei. 1999;51:638–40. [PubMed] [Google Scholar]
- 118.Cirignotta F, Manconi M, Mondini S, Buzzi G, Ambrosetto P. Wernicke-Korsakoff encephalopathy and polyneuropathy after gastroplasty for morbid obesity: report of a case. Arch Neurol. 2000;57:1356–9. doi: 10.1001/archneur.57.9.1356. [DOI] [PubMed] [Google Scholar]
- 119.Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14:182–9. doi: 10.1381/096089204322857537. [DOI] [PubMed] [Google Scholar]
- 120.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–6. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 121.Dolan K, Hatzifotis M, Newbury L, Lowe N, Fielding G. A clinical and nutritional comparison of biliopancreatic diversion with and without duodenal switch. Ann Surg. 2004;240:51–6. doi: 10.1097/01.sla.0000129280.68540.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brolin RE, LaMarca LB, Kenler HA, Cody RP. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–203. doi: 10.1016/s1091-255x(01)00022-1. discussion 204–5. [DOI] [PubMed] [Google Scholar]
- 123.Marinari GM, Murelli F, Camerini G, Papadia F, Carlini F, Stabilini C, et al. A 15-year evaluation of biliopancreatic diversion according to the Bariatric Analysis Reporting Outcome System (BAROS) Obes Surg. 2004;14:325–8. doi: 10.1381/096089204322917828. [DOI] [PubMed] [Google Scholar]
- 124.Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006;331:219–25. doi: 10.1097/00000441-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 125.Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg. 2005;15:145–54. doi: 10.1381/0960892053268264. [DOI] [PubMed] [Google Scholar]
- 126.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarent-zos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–8. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]
- 127.Rinaldi Schinkel E, Pettine SM, Adams E, Harris M. Impact of varying levels of protein intake on protein status indicators after gastric bypass in patients with multiple complications requiring nutritional support. Obes Surg. 2006;16:24–30. doi: 10.1381/096089206775222168. [DOI] [PubMed] [Google Scholar]
- 128.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–5. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 129.Bell NH. Bone loss and gastric bypass surgery for morbid obesity. J Clin Endocrinol Metab. 2004;89:1059–60. doi: 10.1210/jc.2003-032162. [DOI] [PubMed] [Google Scholar]
- 130.Pugnale N, Giusti V, Suter M, Zysset E, Héraïef E, Gaillard RC, et al. Bone metabolism and risk of secondary hyperparathyroidism 12 months after gastric banding in obese pre-menopausal women. Int J Obes Relat Metab Disord. 2003;27:110–6. doi: 10.1038/sj.ijo.0802177. [DOI] [PubMed] [Google Scholar]
- 131.Guney E, Kisakol G, Ozgen G, Yilmaz C, Yilmaz R, Kabalak T. Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg. 2003;13:383–8. doi: 10.1381/096089203765887705. [DOI] [PubMed] [Google Scholar]
- 132.Goldner WS, O’Dorisio TM, Dillon JS, Mason EE. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg. 2002;12:685–92. doi: 10.1381/096089202321019693. [DOI] [PubMed] [Google Scholar]
- 133.De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61. doi: 10.1097/00000441-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 134.Slater GH, Ren CJ, Siegel N, Williams T, Barr D, Wolfe B, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–54. doi: 10.1016/j.gassur.2003.09.020. discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 135.Kellum JM, DeMaria EJ, Sugerman HJ. The surgical treatment of morbid obesity. Curr Prob Surg. 1998;35:791–858. doi: 10.1016/s0011-3840(98)80009-9. [DOI] [PubMed] [Google Scholar]
- 136.Diniz Mde F, Diniz MT, Sanches SR, Salgado PP, Valadão MM, Araújo FC, et al. Elevated serum parathormone after Roux-en-Y gastric bypass. Obes Surg. 2004;14:1222–6. doi: 10.1381/0960892042386959. [DOI] [PubMed] [Google Scholar]
- 137.Collazo-Clavell ML, Jimenez A, Hodgson SF, Sarr MG. Osteomalacia after Roux-en-Y gastric bypass. Endocr Pract. 2004;10:195–8. doi: 10.4158/EP.10.3.195. [DOI] [PubMed] [Google Scholar]
- 138.Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–7. doi: 10.1038/oby.2004.7. [DOI] [PubMed] [Google Scholar]
- 139.Scopinaro N, Marinari GM, Pretolesi F, Papadia F, Murelli F, Marini P, et al. Energy and nitrogen absorption after biliopancreatic diversion. Obes Surg. 2000;10:436–41. doi: 10.1381/096089200321594309. [DOI] [PubMed] [Google Scholar]
- 140.Hatizifotis M, Dolan K, Newbury L, Fielding G. Symptomatic vitamin A deficiency following biliopancreatic diversion. Obes Surg. 2003;13:655–7. doi: 10.1381/096089203322190916. [DOI] [PubMed] [Google Scholar]
- 141.Lee WB, Hamilton SM, Harris JP, Schwab IR. Ocular complications of hypovitaminosis a after bariatric surgery. Ophthalmology. 2005;112:1031–4. doi: 10.1016/j.ophtha.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 142.Trostler N, Mann A, Zilberbush N, Charuzi II, Avinoach E. Nutrient intake following vertical banded gastroplasty or gastric bypass. Obes Surg. 1995;5:403–10. doi: 10.1381/096089295765557502. [DOI] [PubMed] [Google Scholar]
- 143.Abarbanel JM, Berginer VM, Osimani A, Solomon H, Charuzi I. Neurologic complications after gastric restriction surgery for morbid obesity. Neurology. 1987;37:196–200. doi: 10.1212/wnl.37.2.196. [DOI] [PubMed] [Google Scholar]
- 144.Juhasz-Pocsine K, Rudnicki SA, Archer RL, Harik SI. Neurologic complications of gastric bypass surgery for morbid obesity. Neurology. 2007;68:1843–50. doi: 10.1212/01.wnl.0000262768.40174.33. [DOI] [PubMed] [Google Scholar]
- 145.Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004;63:1462–70. doi: 10.1212/01.wnl.0000142038.43946.06. [DOI] [PubMed] [Google Scholar]
- 146.Herpertz S, Kielmann R, Wolf AM, Hebebrand J, Senf W. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review. Obes Res. 2004;12:1554–69. doi: 10.1038/oby.2004.195. [DOI] [PubMed] [Google Scholar]
- 147.Black DW, Goldstein RB, Mason EE. Prevalence of mental disorder in 88 morbidly obese bariatric clinic patients. Am J Psychiatry. 1992;149:227–34. doi: 10.1176/ajp.149.2.227. [DOI] [PubMed] [Google Scholar]
- 148.Sarwer DB, Wadden TA, Fabricatore AN. Psychosocial and behavioral aspects of bariatric surgery. Obes Res. 2005;13:639–48. doi: 10.1038/oby.2005.71. [DOI] [PubMed] [Google Scholar]
- 149.Kalarchian MA, Marcus MD, Wilson GT, Labouvie EW, Brolin RE, LaMarca LB. Binge eating among gastric bypass patients at long-term follow-up. Obes Surg. 2002;12:270–5. doi: 10.1381/096089202762552494. [DOI] [PubMed] [Google Scholar]
- 150.Norris L. Psychiatric issues in bariatric surgery. Psychiatr Clin North Am. 2007;30:717–38. doi: 10.1016/j.psc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 151.van Gemert WG, Severeijns RM, Greve JW, Groenman N, Soeters PB. Psychological functioning of morbidly obese patients after surgical treatment. Int J Obes Relat Metab Disord. 1998;22:393–8. doi: 10.1038/sj.ijo.0800599. [DOI] [PubMed] [Google Scholar]
- 152.Bauchowitz AU, Gonder-Frederick LA, Olbrisch ME, Azarbad L, Ryee MY, Woodson M, et al. Psychosocial evaluation of bariatric surgery candidates: a survey of present practices. Psychosom Med. 2005;67:825–32. doi: 10.1097/01.psy.0000174173.32271.01. [DOI] [PubMed] [Google Scholar]
- 153.Dixon JB, Schachter LM, O’Brien PE. Sleep disturbance and obesity: changes following surgically induced weight loss. Arch Intern Med. 2001;161:102–6. doi: 10.1001/archinte.161.1.102. [DOI] [PubMed] [Google Scholar]
- 154.Waters GS, Pories WJ, Swanson MS, Meelheim HD, Flickinger EG, May HJ. Long-term studies of mental health after the Greenville gastric bypass operation for morbid obesity. Am J Surg. 1991;161:154–7. doi: 10.1016/0002-9610(91)90377-p. discussion 157–8. [DOI] [PubMed] [Google Scholar]
- 155.Masheb RM, White MA, Toth CM, Burke-Martindale CH, Rothschild B, Grilo CM. The prognostic significance of depressive symptoms for predicting quality of life 12 months after gastric bypass. Compr Psychiatry. 2007;48:231–6. doi: 10.1016/j.comppsych.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 156.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 157.Mitchell JE, Lancaster KL, Burgard MA, Howell LM, Krahn DD, Crosby RD, et al. Long-term follow-up of patients’ status after gastric bypass. Obes Surg. 2001;11:464–8. doi: 10.1381/096089201321209341. [DOI] [PubMed] [Google Scholar]
- 158.Pories WJ, MacDonald KG, Jr, Morgan EJ, Sinha MK, Dohm GL, Swanson MS, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55(2 Suppl):582S–5S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 159.Omalu BI, Cho P, Shakir AM, Agumadu UH, Rozin L, Kuller LH, et al. Suicides following bariatric surgery for the treatment of obesity. Surg Obes Relat Dis. 2005;1:447–9. doi: 10.1016/j.soard.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 160.Spitzer RL, Yanovski S, Wadden T, Wing R, Marcus MD, Stunkard A, et al. Binge eating disorder: its further validation in a multisite study. Int J Eat Disord. 1993;13:137–53. [PubMed] [Google Scholar]
- 161.Saunders R. “Grazing”: a high-risk behavior. Obes Surg. 2004;14:98–102. doi: 10.1381/096089204772787374. [DOI] [PubMed] [Google Scholar]
- 162.Israel A, Sebbag G, Fraser D, Levy I. Nutritional behavior as a predictor of early success after vertical gastroplasty. Obes Surg. 2005;15:88–94. doi: 10.1381/0960892052993512. [DOI] [PubMed] [Google Scholar]
- 163.Ogden J, Clementi C, Aylwin S, Patel A. Exploring the impact of obesity surgery on patients’ health status: a quantitative and qualitative study. Obes Surg. 2005;15:266–72. doi: 10.1381/0960892053268291. [DOI] [PubMed] [Google Scholar]
- 164.DeMaria EJ, Sugerman HJ, Meador JG, Doty JM, Kellum JM, Wolfe L, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233:809–18. doi: 10.1097/00000658-200106000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Suter M, Calmes JM, Paroz A, Giusti V. A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg. 2006;16:829–35. doi: 10.1381/096089206777822359. [DOI] [PubMed] [Google Scholar]
- 166.Mittermair RP, Aigner F, Obermuller S. Results and complications after Swedish adjustable gastric banding in older patients. Obes Surg. 2008;18:1558–62. doi: 10.1007/s11695-008-9709-2. [DOI] [PubMed] [Google Scholar]
- 167.Shen R, Ren CJ. Removal of peri-gastric fat prevents acute obstruction after Lap-Band surgery. Obes Surg. 2004;14:224–9. doi: 10.1381/096089204322857609. [DOI] [PubMed] [Google Scholar]
- 168.Gulkarov I, Wetterau M, Ren CJ, Fielding GA. Hiatal hernia repair at the initial laparoscopic adjustable gastric band operation reduces the need for reoperation. Surg Endosc. 2008;22:1035–41. doi: 10.1007/s00464-007-9684-z. [DOI] [PubMed] [Google Scholar]
- 169.Spivak H, Favretti F. Avoiding postoperative complications with the LAP-BAND system. Am J Surg. 2002;184(6B):31S–7. doi: 10.1016/s0002-9610(02)01177-7. [DOI] [PubMed] [Google Scholar]
- 170.Watson M, Jones D. Lap-Band Companion Handbook. Woodbury, CT: Cine-Med, Inc; 2007. [Google Scholar]
- 171.Keidar A, Szold A, Carmon E, Blanc A, Abu-Abeid S. Band slippage after laparoscopic adjustable gastric banding: etiology and treatment. Surg Endosc. 2005;19:262–7. doi: 10.1007/s00464-003-8261-3. [DOI] [PubMed] [Google Scholar]
- 172.Chevallier JM, Zinzindohoué F, Douard R, Blanche JP, Berta JL, Altman JJ, et al. Complications after laparoscopic adjustable gastric banding for morbid obesity: experience with 1,000 patients over 7 years. Obes Surg. 2004;14:407–14. doi: 10.1381/096089204322917954. [DOI] [PubMed] [Google Scholar]
- 173.Abu-Abeid S, Keidar A, Gavert N, Blanc A, Szold A. The clinical spectrum of band erosion following laparoscopic adjustable silicone gastric banding for morbid obesity. Surg Endosc. 2003;17:861–3. doi: 10.1007/s00464-002-9195-x. [DOI] [PubMed] [Google Scholar]
- 174.Mittermair RP, Weiss HG, Nehoda H, Peer R, Donnemiller E, Moncayo R, et al. Band leakage after laparoscopic adjustable gastric banding. Obes Surg. 2003;13:913–7. doi: 10.1381/096089203322618768. [DOI] [PubMed] [Google Scholar]
- 175.Van Den Bossche B, Goethals I, Dierckx RA, Villeirs G, Pattyn P, Van de Wiele C. Leakage assessment in adjustable laparoscopic gastric banding: radiography versus (99m)Tc-pertechnetate scintigraphy. Eur J Nucl Med Mol Imaging. 2002;29:1128–31. doi: 10.1007/s00259-002-0825-2. [DOI] [PubMed] [Google Scholar]
- 176.Poole N, Al Atar A, Bidlake L, Fienness A, McCluskey S, Nussey S, et al. Pouch dilatation following laparoscopic adjustable gastric banding: psychobehavioral factors (can psychiatrists predict pouch dilatation?) Obes Surg. 2004;14:798–801. doi: 10.1381/0960892041590827. [DOI] [PubMed] [Google Scholar]
- 177.Gustavsson S, Westling A. Laparoscopic adjustable gastric banding: complications and side effects responsible for the poor long-term outcome. Semin Laparosc Surg. 2002;9:115–24. [PubMed] [Google Scholar]
- 178.Keidar A, Carmon E, Szold A, Abu-Abeid S. Port complications following laparoscopic adjustable gastric banding for morbid obesity. Obes Surg. 2005;15:361–5. doi: 10.1381/0960892053576604. [DOI] [PubMed] [Google Scholar]
- 179.Lattuada E, Zappa MA, Mozzi E, Antonini I, Boati P, Roviaro GC. Injection port and connecting tube complications after laparoscopic adjustable gastric banding. Obes Surg. 2008 Jun 10; doi: 10.1007/s11695-008-9561-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 180.Dargent J. Surgical treatment of morbid obesity by adjustable gastric band: the case for a conservative strategy in the case of failure—a 9-year series. Obes Surg. 2004;14:986–90. doi: 10.1381/0960892041719545. [DOI] [PubMed] [Google Scholar]
- 181.Clinical Issues Committee of American Society for Metabolic and Bariatric S. Sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2007;3:573–6. doi: 10.1016/j.soard.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 182.Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–9. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 183.Gumbs AA, Gagner M, Dakin G, Pomp A. Sleeve gastrectomy for morbid obesity. Obes Surg. 2007;17:962–9. doi: 10.1007/s11695-007-9151-x. [DOI] [PubMed] [Google Scholar]
- 184.Frezza EE. Laparoscopic vertical sleeve gastrectomy for morbid obesity. The future procedure of choice? Surg Today. 2007;37:275–81. doi: 10.1007/s00595-006-3407-2. [DOI] [PubMed] [Google Scholar]
- 185.Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450–6. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 186.Weiner RA, Weiner S, Pomhoff I, Jacobi C, Makarewicz W, Weigand G. Laparoscopic sleeve gastrectomy—influence of sleeve size and resected gastric volume. Obes Surg. 2007;17:1297–305. doi: 10.1007/s11695-007-9232-x. [DOI] [PubMed] [Google Scholar]
- 187.Baltasar A, Serra C, Perez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15:1124–8. doi: 10.1381/0960892055002248. [DOI] [PubMed] [Google Scholar]
- 188.Consten EC, Gagner M, Pomp A, Inabnet WB. Decreased bleeding after laparoscopic sleeve gastrectomy with or without duodenal switch for morbid obesity using a stapled buttressed absorbable polymer membrane. Obes Surg. 2004;14:1360–6. doi: 10.1381/0960892042583905. [DOI] [PubMed] [Google Scholar]
- 189.Moy J, Pomp A, Dakin G, Parikh M, Gagner M. Laparoscopic sleeve gastrectomy for morbid obesity. Am J Surg. 2008;196:e56–9. doi: 10.1016/j.amjsurg.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 190.Braghetto I, Korn O, Valladares H, Gutiérrez L, Csendes A, Debandi A, et al. Laparoscopic sleeve gastrectomy: surgical technique, indications and clinical results. Obes Surg. 2007;17:1442–50. doi: 10.1007/s11695-008-9421-2. [DOI] [PubMed] [Google Scholar]
- 191.Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen J, et al. Laparoscopic sleeve gastrectomy—volume and pressure assessment. Obes Surg. 2008;18:1083–8. doi: 10.1007/s11695-008-9576-x. [DOI] [PubMed] [Google Scholar]
- 192.Rubin M, Yehoshua RT, Stein M, Lederfein D, Fichman S, Bernstine H, et al. Laparoscopic sleeve gastrectomy with minimal morbidity. Early results in 120 morbidly obese patients. Obes Surg. 2008;18:1567–70. doi: 10.1007/s11695-008-9652-2. [DOI] [PubMed] [Google Scholar]
- 193.Dapri G, Cadiere GB, Himpens J. Laparoscopic seromyotomy for long stenosis after sleeve gastrectomy with or without duodenal switch. Obes Surg. 2009;19:495–9. doi: 10.1007/s11695-009-9803-0. [DOI] [PubMed] [Google Scholar]
- 194.Dapri G, Vaz C, Cadiere GB, Himpens J. A prospective randomized study comparingtwo different techniques for laparoscopic sleeve gastrectomy. Obes Surg. 2007;17:1435–41. doi: 10.1007/s11695-008-9420-3. [DOI] [PubMed] [Google Scholar]
- 195.Langer FB, Bohdjalian A, Felberbauer FX, Fleischmann E, Reza Hoda MA, Ludvik B, et al. Does gastric dilatation limit the success of sleeve gastrectomy as a sole operation for morbid obesity? Obes Surg. 2006;16:166–71. doi: 10.1381/096089206775565276. [DOI] [PubMed] [Google Scholar]
- 196.van Dielen FM, Soeters PB, de Brauw LM, Greve JW. Laparoscopic adjustable gastric banding versus open vertical banded gastroplasty: a prospective randomized trial. Obes Surg. 2005;15:1292–8. doi: 10.1381/096089205774512456. [DOI] [PubMed] [Google Scholar]
- 197.MacLean LD, Rhode BM, Sampalis J, Forse RA. Results of the surgical treatment of obesity. Am J Surg. 1993;165:155–60. doi: 10.1016/s0002-9610(05)80420-9. discussion 160–2. [DOI] [PubMed] [Google Scholar]
- 198.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–7. doi: 10.1016/S1091-255X(02)00125-7. discussion 107–8. [DOI] [PubMed] [Google Scholar]
- 199.Gonzalez RJ. Weight Loss Surgery: A Multidisciplinary Approach. Edgemont, PA: Matrix Medical Communications; 2008. [Google Scholar]
- 200.MacLean LD, Rhode BM, Forse RA. Late results of vertical banded gastroplasty for morbid and super obesity. Surgery. 1990;107:20–7. [PubMed] [Google Scholar]
- 201.Calmes JM, Giusti V, Suter M. Reoperative laparoscopic Roux-en-Y gastric bypass: an experience with 49 cases. Obes Surg. 2005;15:316–22. doi: 10.1381/0960892053576785. [DOI] [PubMed] [Google Scholar]
- 202.Moreno P, Alastrué A, Rull M, Formiguera X, Casas D, Boix J, et al. Band erosion in patients who have undergone vertical banded gastroplasty: incidence and technical solutions. Arch Surg. 1998;133:189–93. doi: 10.1001/archsurg.133.2.189. [DOI] [PubMed] [Google Scholar]
- 203.Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoileal bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308:995–9. doi: 10.1056/NEJM198304283081703. [DOI] [PubMed] [Google Scholar]
- 204.Requarth JA, Burchard KW, Colacchio TA, Stukel TA, Mott LA, Greenberg ER, et al. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg. 1995;130:318–25. doi: 10.1001/archsurg.1995.01430030088018. [DOI] [PubMed] [Google Scholar]
- 205.Haria DM, Sibonga JD, Taylor HC. Hypocalcemia, hypovitaminosis d osteopathy, osteopenia, and secondary hyperparathyroidism 32 years after jejunoileal bypass. Endocr Pract. 2005;11:335–40. doi: 10.4158/EP.11.5.335. [DOI] [PubMed] [Google Scholar]
- 206.Evans DJ, Berney DM, Pollock DJ. Symptomatic vitamin E deficiency diagnosed after histological recognition of myometrial lipofuscinosis. Lancet. 1995;346:545–6. doi: 10.1016/s0140-6736(95)91384-x. [DOI] [PubMed] [Google Scholar]
- 207.Solhaug JH, Grundt I. Metabolic changes after jejuno-ileal bypass for obesity. Scand J Gastroenterol. 1978;13:169–75. doi: 10.3109/00365527809181744. [DOI] [PubMed] [Google Scholar]
- 208.Rogers EL, Douglass W, Russell RM, Bushman L, Hubbard TB, Iber FL. Deficiency of fat soluble vitamins after jejunoileal bypass surgery for morbid obesity. Am J Clin Nutr. 1980;33:1208–14. doi: 10.1093/ajcn/33.6.1208. [DOI] [PubMed] [Google Scholar]
- 209.Dyckner T, Hallberg D, Hultman E, Wester PO. Magnesium deficiency following jejunoileal bypass operations for obesity. J Am Coll Nutr. 1982;1:239–46. doi: 10.1080/07315724.1982.10718992. [DOI] [PubMed] [Google Scholar]
- 210.Hey H, Lund B, Sorensen OH, Lund B. Delayed fracture healing following jejunoileal bypass surgery for obesity. Calcif Tissue Int. 1982;34:13–5. doi: 10.1007/BF02411201. [DOI] [PubMed] [Google Scholar]
- 211.Leff RD, Towles W, Aldo-Benson MA, Madura J, Biegel AA. A prospective analysis of the arthritis syndrome and immune function in jejunoileal bypass patients. J Rheumatol. 1983;10:612–8. [PubMed] [Google Scholar]
- 212.Ross CB, Scott HW, Pincus T. Jejunoileal bypass arthritis. Baillieres Clin Rheumatol. 1989;3:339–55. doi: 10.1016/s0950-3579(89)80025-1. [DOI] [PubMed] [Google Scholar]
- 213.Drenick EJ, Roslyn JJ. Cure of arthritis-dermatitis syndrome due to intestinal bypass by resection of nonfunctional segment of blind loop. Dig Dis Sci. 1990;35:656–60. doi: 10.1007/BF01540416. [DOI] [PubMed] [Google Scholar]
- 214.Ross CB, Shull HJ, Jr, Pincus T, Scott HW., Jr Bypass arthritis and the blind intestinal loop. South Med J. 1987;80:768–72. doi: 10.1097/00007611-198706000-00025. [DOI] [PubMed] [Google Scholar]
- 215.O’Leary JP. Hepatic complications of jejunoileal bypass. Semin Liver Dis. 1983;3:203–15. doi: 10.1055/s-2008-1040686. [DOI] [PubMed] [Google Scholar]
- 216.Piringer P, Buder R, Firlinger F, Kapral C, Luft C, Sega W, et al. Steatohepatitis and cirrhosis: first manifestation 23 years after jejunoileal bypass surgery. Wien Klin Wochenschr. 2007;119:733–8. doi: 10.1007/s00508-007-0850-z. [DOI] [PubMed] [Google Scholar]
- 217.Lowell JA, Shenoy S, Ghalib R, Caldwell C, White FV, Peters M, et al. Liver transplantation after jejunoileal bypass for morbid obesity. J Am Coll Surg. 1997;185:123–7. doi: 10.1016/s1072-7515(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 218.Das S, Joseph B, Dick AL. Renal failure owing to oxalate nephrosis after jejunoileal bypass. J Urol. 1979;121:506–9. doi: 10.1016/s0022-5347(17)56845-7. [DOI] [PubMed] [Google Scholar]
- 219.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–9. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 220.Mole DR, Tomson CR, Mortensen N, Winearls CG. Renal complications of jejuno-ileal bypass for obesity. QJM. 2001;94:69–77. doi: 10.1093/qjmed/94.2.69. [DOI] [PubMed] [Google Scholar]
- 221.Hassan I, Juncos LA, Milliner DS, Sarmiento JM, Sarr MG. Chronic renal failure secondary to oxalate nephropathy: a preventable complication after jejunoileal bypass. Mayo Clin Proc. 2001;76:758–60. doi: 10.4065/76.7.758. [DOI] [PubMed] [Google Scholar]
- 222.Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg. 2006;192:657–62. doi: 10.1016/j.amjsurg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 223.ASMBS. The Story of Surgery for Obesity. Brief History and Summary of Bariatric Surgery. [Accessed April 1, 2009];Chapter 3: gastric bypass. Available at: http://www.asbs.org/html/patients/bypass.html.
- 224.Carucci LR, Turner MA, Conklin RC, DeMaria EJ, Kellum JM, Sugerman HJ. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology. 2006;238:119–27. doi: 10.1148/radiol.2381041557. [DOI] [PubMed] [Google Scholar]
- 225.Pitt T, Brethauer S, Schauer P. Obesity Surgery: Patients Safety and Best Practices. CH 40. Woodbury, CT: Cine-Med, Inc; 2008. [Google Scholar]
- 226.Edwards MA, Jones DB, Ellsmere J, Grinbaum R, Schneider BE. Anastomotic leak following antecolic versus retrocolic laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2007;17:292–7. doi: 10.1007/s11695-007-9048-8. [DOI] [PubMed] [Google Scholar]
- 227.Hamilton EC, Sims TL, Hamilton TT, Mullican MA, Jones DB, Provost DA. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17:679–84. doi: 10.1007/s00464-002-8819-5. [DOI] [PubMed] [Google Scholar]
- 228.Mehran A, Szomstein S, Zundel N, Rosenthal R. Management of acute bleeding after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:842–7. doi: 10.1381/096089203322618623. [DOI] [PubMed] [Google Scholar]
- 229.Nguyen NT, Stevens CM, Wolfe BM. Incidence and outcome of anastomotic stricture after laparoscopic gastric bypass. J Gastrointest Surg. 2003;7:997–1003. doi: 10.1016/j.gassur.2003.09.016. discussion 1003. [DOI] [PubMed] [Google Scholar]
- 230.Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients—what have we learned? Obes Surg. 2000;10:509–13. doi: 10.1381/096089200321593706. [DOI] [PubMed] [Google Scholar]
- 231.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y—500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10:233–9. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- 232.Blachar A, Federle MP, Pealer KM, Ikramuddin S, Schauer PR. Gastrointestinal complications of laparoscopic Roux-en-Y gastric bypass surgery: clinical and imaging findings. Radiology. 2002;223:625–32. doi: 10.1148/radiol.2233011323. [DOI] [PubMed] [Google Scholar]
- 233.Fisher BL, Atkinson JD, Cottam D. Incidence of gastroenterostomy stenosis in laparoscopic Roux-en-Y gastric bypass using 21- or 25-mm circular stapler: a randomized prospective blinded study. Surg Obes Relat Dis. 2007;3:176–9. doi: 10.1016/j.soard.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 234.Carrodeguas L, Szomstein S, Zundel N, Lo Menzo E, Rosenthal R. Gastrojejunal anastomotic strictures following laparoscopic Roux-en-Y gastric bypass surgery: analysis of 1291 patients. Surg Obes Relat Dis. 2006;2:92–7. doi: 10.1016/j.soard.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 235.Dallal RM, Bailey LA. Ulcer disease after gastric bypass surgery. Surg Obes Relat Dis. 2006;2:455–9. doi: 10.1016/j.soard.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 236.DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235:640–5. doi: 10.1097/00000658-200205000-00005. discussion 645–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gumbs AA, Duffy AJ, Bell RL. Incidence and management of marginal ulceration after laparoscopic Roux-Y gastric bypass. Surg Obes Relat Dis. 2006;2:460–3. doi: 10.1016/j.soard.2006.04.233. [DOI] [PubMed] [Google Scholar]
- 238.Livingston EH. Complications of bariatric surgery. Surg Clin North Am. 2005;85:853–68. vii. doi: 10.1016/j.suc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 239.Ng EK, Chung SC, Sung JJ, Lam YH, Lee DW, Lau JY, et al. High prevalence of Helicobacter pylori infection in duodenal ulcer perforations not caused by non-steroidal anti-inflammatory drugs. Br J Surg. 1996;83:1779–81. doi: 10.1002/bjs.1800831237. [DOI] [PubMed] [Google Scholar]
- 240.Mason EE, Munns JR, Kealey GP, Wangler R, Clarke WR, Cheng HF, et al. Effect of gastric bypass on gastric secretion. Am J Surg. 1976;131:162–8. doi: 10.1016/0002-9610(76)90090-8. [DOI] [PubMed] [Google Scholar]
- 241.Flickinger EG, Sinar DR, Pories WJ, Sloss RR, Park HK, Gibson JH. The bypassed stomach. Am J Surg. 1985;149:151–6. doi: 10.1016/s0002-9610(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 242.Macgregor AM, Pickens NE, Thoburn EK. Perforated peptic ulcer following gastric bypass for obesity. Am Surg. 1999;65:222–5. [PubMed] [Google Scholar]
- 243.Zerey M, Sigmon LB, Kuwada TS, Heniford BT, Sing RF. Bleeding duodenal ulcer after roux-en-Y gastric bypass surgery. J Am Osteopath Assoc. 2008;108:25–7. [PubMed] [Google Scholar]
- 244.Ahmed AR, Husain S, Saad N, Patel NC, Waldman DL, O’Malley W. Accessing the common bile duct after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3:640–3. doi: 10.1016/j.soard.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 245.Malfertheiner P, Mégraud F, O’Morain C, Bell D, Bianchi Porro G, Deltenre M, et al. Current European concepts in the management of Helicobacter pylori infection—the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG) Eur J Gastroenterol Hepatol. 1997;9:1–2. doi: 10.1097/00042737-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 246.Capella RF, Iannace VA, Capella JF. Bowel obstruction after open and laparo-scopic gastric bypass surgery for morbid obesity. J Am Coll Surg. 2006;203:328–35. doi: 10.1016/j.jamcollsurg.2006.05.301. [DOI] [PubMed] [Google Scholar]
- 247.Hwang RF, Swartz DE, Felix EL. Causes of small bowel obstruction after laparoscopic gastric bypass. Surg Endosc. 2004;18:1631–5. doi: 10.1007/s00464-004-8804-2. [DOI] [PubMed] [Google Scholar]
- 248.Lauter DM. Treatment of nonadhesive bowel obstruction following gastric bypass. Am J Surg. 2005;189:532–5. doi: 10.1016/j.amjsurg.2005.01.024. discussion 535. [DOI] [PubMed] [Google Scholar]
- 249.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–29. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nguyen NT, Huerta S, Gelfand D, Stevens CM, Jim J. Bowel obstruction after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2004;14:190–6. doi: 10.1381/096089204322857546. [DOI] [PubMed] [Google Scholar]
- 251.Kasotakis G, Sudan R. Retrograde intussusception after Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2009;19:381–4. doi: 10.1007/s11695-008-9775-5. [DOI] [PubMed] [Google Scholar]
- 252.Goverman J, Greenwald M, Gellman L, Gadaleta D. Antiperistaltic (retrograde) intussusception after Roux-en-Y gastric bypass. Am Surg. 2004;70:67–70. [PubMed] [Google Scholar]
- 253.Efthimiou E, Court O, Christou N. Small bowel obstruction due to retrograde intussusception after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19:378–80. doi: 10.1007/s11695-008-9737-y. [DOI] [PubMed] [Google Scholar]
- 254.Edwards MA, Grinbaum R, Ellsmere J, Jones DB, Schneider BE. Intussusception after Roux-en-Y gastric bypass for morbid obesity: case report and literature review of rare complication. Surg Obes Relat Dis. 2006;2:483–9. doi: 10.1016/j.soard.2006.04.232. [DOI] [PubMed] [Google Scholar]
- 255.Zainabadi K, Ramanathan R. Intussusception after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17:1619–23. doi: 10.1007/s11695-007-9291-z. [DOI] [PubMed] [Google Scholar]
- 256.Simper SC, Erzinger JM, McKinlay RD, Smith SC. Retrograde (reverse) jejunal intussusception might not be such a rare problem: a single group’s experience of 23 cases. Surg Obes Relat Dis. 2008;4:77–83. doi: 10.1016/j.soard.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 257.Ukleja A. Dumping syndrome: pathophysiology and treatment. Nutr Clin Pract. 2005;20:517–25. doi: 10.1177/0115426505020005517. [DOI] [PubMed] [Google Scholar]
- 258.Mathews DH, Lawrence W, Jr, Poppell JW, Vanamee P, Randall HT. Change in effective circulating volume during experimental dumping syndrome. Surgery. 1960;48:185–94. [PubMed] [Google Scholar]
- 259.Deitel M. The change in the dumping syndrome concept. Obes Surg. 2008;18:1622–4. doi: 10.1007/s11695-008-9756-8. [DOI] [PubMed] [Google Scholar]
- 260.Scavini M, Pontiroli AE, Folli F. Asymptomatic hyperinsulinemic hypoglycemia after gastric banding. N Engl J Med. 2005;353:2822–3. doi: 10.1056/NEJMc052356. [DOI] [PubMed] [Google Scholar]
- 261.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–54. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 262.Clancy TE, Moore FD, Jr, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10:1116–9. doi: 10.1016/j.gassur.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 263.Alvarez GC, Faria EN, Beck M, Girardon DT, Machado AC. Laparoscopic spleen-preserving distal pancreatectomy as treatment for nesidioblastosis after gastric bypass surgery. Obes Surg. 2007;17:550–2. doi: 10.1007/s11695-007-9096-0. [DOI] [PubMed] [Google Scholar]
- 264.Goldenberg L. Weight Loss Surgery: A Multidisciplinary Approach. Edgemont, PA: Matrix; 2008. Hyperinsulinemic hypoglycemia following weight loss surgery; pp. 431–6. [Google Scholar]
- 265.Zagury L, Moreira RO, Guedes EP, Coutinho WF, Appolinario JC. Insulinoma misdiagnosed as dumping syndrome after bariatric surgery. Obes Surg. 2004;14:120–3. doi: 10.1381/096089204772787419. [DOI] [PubMed] [Google Scholar]
- 266.Z’Graggen K, Guweidhi A, Steffen R, Potoczna N, Biral R, Walther F, et al. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18:981–8. doi: 10.1007/s11695-008-9480-4. [DOI] [PubMed] [Google Scholar]
- 267.Lee MG, Jones DB. Staple-line buttressing material in gastric-bypass surgery. Expert Rev Med Devices. 2005;2:599–603. doi: 10.1586/17434440.2.5.599. [DOI] [PubMed] [Google Scholar]
- 268.Ellsmere JC, Thompson CC, Brugge WR, Chuttani R, J Desilets D, Rattner DW, et al. Endoscopic interventions for weight loss surgery. Obesity (Silver Spring) 2009;17:929–33. doi: 10.1038/oby.2008.588. [DOI] [PubMed] [Google Scholar]
- 269.Sekhar N, Torquati A, Youssef Y, Wright JK, Richards WO. A comparison of 399 open and 568 laparoscopic gastric bypasses performed during a 4-year period. Surg Endosc. 2007;21:665–8. doi: 10.1007/s00464-006-9151-2. [DOI] [PubMed] [Google Scholar]
- 270.Hutter MM, Randall S, Khuri SF, Henderson WG, Abbott WM, Warshaw AL. Laparoscopic versus open gastric bypass for morbid obesity: a multicenter, prospective, risk-adjusted analysis from the National Surgical Quality Improvement Program. Ann Surg. 2006;243:657–62. doi: 10.1097/01.sla.0000216784.05951.0b. discussion 662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248:10–5. doi: 10.1097/SLA.0b013e31816d953a. [DOI] [PubMed] [Google Scholar]
- 272.Kendrick ML, Dakin GF. Surgical approaches to obesity. Mayo Clin Proc. 2006;81(10 Suppl):S18–24. doi: 10.1016/s0025-6196(11)61177-4. [DOI] [PubMed] [Google Scholar]
- 273.Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, Simard S, et al. Twenty years of biliopancreatic diversion: what is the goal of the surgery? Obes Surg. 2004;14:160–4. doi: 10.1381/096089204322857492. [DOI] [PubMed] [Google Scholar]
- 274.Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg. 2005;15:408–16. doi: 10.1381/0960892053576695. [DOI] [PubMed] [Google Scholar]
- 275.Rabkin RA, Rabkin JM, Metcalf B, Lazo M, Rossi M, Lehmanbecker LB. Laparoscopic technique for performing duodenal switch with gastric reduction. Obes Surg. 2003;13:263–8. doi: 10.1381/096089203764467180. [DOI] [PubMed] [Google Scholar]
- 276.Anthone GJ, Lord RV, DeMeester TR, Crookes PF. The duodenal switch operation for the treatment of morbid obesity. Ann Surg. 2003;238:618–27. doi: 10.1097/01.sla.0000090941.61296.8f. discussion 627–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pomp A. The biliopancreatic diversion with duodenal switch. In: Jones DB, Jones SB, editors. Obesity Surgery, Patient Safety and Best Practices. Woodbury, CT: Cine-Med, Inc; 2008. pp. 285–9. [Google Scholar]
- 278.Herron DM. Biliopancreatic diversion with duodenal switch vs. gastric bypass for severe obesity. J Gastrointest Surg. 2004;8:406–7. doi: 10.1016/j.gassur.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 279.Strain GW, Gagner M, Inabnet WB, Dakin G, Pomp A. Comparison of effects of gastric bypass and biliopancreatic diversion with duodenal switch on weight loss and body composition 1–2 years after surgery. Surg Obes Relat Dis. 2007;3:31–6. doi: 10.1016/j.soard.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 280.Prachand VN, Davee RT, Alverdy JC. Duodenal switch provides superior weight loss in the super-obese (BMI >50 kg/m2) compared with gastric bypass. Ann Surg. 2006;244:611–9. doi: 10.1097/01.sla.0000239086.30518.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Michielson D, Van Hee R, Hendrickx L. Complications of biliopancreatic diversion surgery as proposed by Scopinaro in the treatment of morbid obesity. Obes Surg. 1996;6:416–20. doi: 10.1381/096089296765556485. [DOI] [PubMed] [Google Scholar]
- 282.Cossu ML, Noya G, Tonolo GC, Profili S, Meloni GB, Ruggiu M, et al. Duodenal switch without gastric resection: results and observations after 6 years. Obes Surg. 2004;14:1354–9. doi: 10.1381/0960892042583851. [DOI] [PubMed] [Google Scholar]
- 283.Headstrom PD, Surawicz CM. Chronic diarrhea. Clin Gastroenterol Hepatol. 2005;3:734–7. doi: 10.1016/s1542-3565(05)00298-3. [DOI] [PubMed] [Google Scholar]
- 284.Ocón Bretón J, Pérez Naranjo S, Gimeno Laborda S, Benito Ruesca P, García Hernández R. Effectiveness and complications of bariatric surgery in the treatment of morbid obesity. Nutr Hosp. 2005;20:409–14. [PubMed] [Google Scholar]
- 285.Parikh M, Pomp A. Weight Loss Surgery: Multidisciplinary Approach. Edgemont, PA: Matrix Medical Communications; 2008. pp. 297–310. [Google Scholar]
- 286.Weber M, Muller MK, Michel JM, Belal R, Horber F, Hauser R, et al. Laparoscopic Roux-en-Y gastric bypass, but not rebanding, should be proposed as rescue procedure for patients with failed laparoscopic gastric banding. Ann Surg. 2003;238:827–33. doi: 10.1097/01.sla.0000098623.53293.bb. discussion 833–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Dolan K, Fielding G. Bilio pancreatic diversion following failure of laparoscopic adjustable gastric banding. Surg Endosc. 2004;18:60–3. doi: 10.1007/s00464-003-8802-9. [DOI] [PubMed] [Google Scholar]
- 288.Bernante P, Foletto M, Busetto L, Pomerri F, Pesenti FF, Pelizzo MR, et al. Feasibility of laparoscopic sleeve gastrectomy as a revision procedure for prior laparoscopic gastric banding. Obes Surg. 2006;16:1327–30. doi: 10.1381/096089206778663797. [DOI] [PubMed] [Google Scholar]
- 289.Cohen R, Pinheiro JS, Correa JL, Schiavon C. Laparoscopic revisional bariatric surgery: myths and facts. Surg Endosc. 2005;19:822–5. doi: 10.1007/s00464-004-8826-9. [DOI] [PubMed] [Google Scholar]
- 290.Keshishian A, Zahriya K, Hartoonian T, Ayagian C. Duodenal switch is a safe operation for patients who have failed other bariatric operations. Obes Surg. 2004;14:1187–92. doi: 10.1381/0960892042387066. [DOI] [PubMed] [Google Scholar]
- 291.Schouten R, van Dielen FM, Greve JW. Re-operation after laparoscopic adjustable gastric banding leads to a further decrease in BMI and obesity-related co-morbidities: results in 33 patients. Obes Surg. 2006;16:821–8. doi: 10.1381/096089206777822386. [DOI] [PubMed] [Google Scholar]
- 292.Brolin RE, Cody RP. Weight loss outcome of revisional bariatric operations varies according to the primary procedure. Ann Surg. 2008;248:227–32. doi: 10.1097/SLA.0b013e3181820cdf. [DOI] [PubMed] [Google Scholar]
- 293.Genco A, Bruni T, Doldi SB, Forestieri P, Marino M, Busetto L, et al. BioEnterics intragastric balloon: the Italian experience with 2,515 patients. Obes Surg. 2005;15:1161–4. doi: 10.1381/0960892055002202. [DOI] [PubMed] [Google Scholar]
- 294.Allen J. Chapter 40: Intragastric balloon. In: Jones DB, Jones SB, editors. Obesity Surgery: Patient Safety and Best Practices. Woodbury, CT: Cine-Med, Inc; 2008. pp. 299–305. [Google Scholar]
- 295.Gottig S, Daskalakis M, Weiner S, Weiner RA. Analysis of safety and efficacy of intragastric balloon in extremely obese patients. Obes Surg. 2009;19:677–83. doi: 10.1007/s11695-009-9820-z. [DOI] [PubMed] [Google Scholar]
- 296.Herve J, Wahlen CH, Schaeken A, Dallemagne B, Dewandre JM, Markiewicz S, et al. What becomes of patients one year after the intragastric balloon has been removed? Obes Surg. 2005;15:864–70. doi: 10.1381/0960892054222894. [DOI] [PubMed] [Google Scholar]
- 297.Sallet JA, Marchesini JB, Paiva DS, Komoto K, Pizani CE, Ribeiro ML, et al. Brazilian multicenter study of the intragastric balloon. Obes Surg. 2004;14:991–8. doi: 10.1381/0960892041719671. [DOI] [PubMed] [Google Scholar]
- 298.Gerrits EG, Ceulemans R, van Hee R, Hendrickx L, Totte E. Contraceptive treatment after biliopancreatic diversion needs consensus. Obes Surg. 2003;13:378–82. doi: 10.1381/096089203765887697. [DOI] [PubMed] [Google Scholar]
- 299.Koutelidakis I, Dragoumis D, Papaziogas B, Patsas A, Katsougianopoulos A, Atmatzidis S, et al. Gastric perforation and death after the insertion of an intragastric balloon. Obes Surg. 2009;19:393–6. doi: 10.1007/s11695-008-9706-5. [DOI] [PubMed] [Google Scholar]
- 300.Ruiz D, Vranas K, Robinson DA, Salvatore L, Turner JW, Addasi T. Esophageal perforation after gastric balloon extraction. Obes Surg. 2009;19:257–60. doi: 10.1007/s11695-008-9605-9. [DOI] [PubMed] [Google Scholar]
- 301.Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51–8. doi: 10.1016/j.gie.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 302.Deitel M, Shikora SA. Introduction. Gastric pacing for obesity. Obes Surg. 2002;12(Suppl 1):2S. doi: 10.1007/BF03342138. [DOI] [PubMed] [Google Scholar]
- 303.Shikora SA. Weight loss surgery and outcomes: gastric pacing. In: Jones DB, Jones SB, editors. Obesity Surgery: Patient Safety and Best Practices. Woodbury, CT: Cine-Med, Inc; 2008. pp. 307–13. [Google Scholar]
- 304.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–9. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Depaula AL, Macedo AL, Mota BR, Schraibman V. Laparoscopic ileal interposition associated to a diverted sleeve gastrectomy is an effective operation for the treatment of type 2 diabetes mellitus patients with BMI 21–29. Surg Endosc. 2009;23:1313–20. doi: 10.1007/s00464-008-0156-x. [DOI] [PubMed] [Google Scholar]
- 307.Takata MC, Campos GM, Ciovica R, Rabl C, Rogers SJ, Cello JP, et al. Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surg Obes Relat Dis. 2008;4:159–64. doi: 10.1016/j.soard.2007.12.009. discussion 164–5. [DOI] [PubMed] [Google Scholar]
- 308.Speiser PW, Rudolf MC, Anhalt H, Camacho-Hubner C, Chiarelli F, Eliakim A, et al. Childhood obesity. J Clin Endocrinol Metab. 2005;90:1871–87. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- 309.Flodmark CE, Lissau I, Pietrobelli A. Child and adolescent obesity: why we need to fight! Acta Paediatr Suppl. 2005;94:4–7. doi: 10.1111/j.1651-2227.2005.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 310.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–8. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 311.Rand CS, MacGregor AM. Adolescents having obesitysurgery:a 6-year follow-up. South Med J. 1994;87:1208–13. doi: 10.1097/00007611-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 312.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 313.Strauss RS, Bradley LJ, Brolin RE. Gastric bypass surgery in adolescents with morbid obesity. J Pediatr. 2001;138:499–504. doi: 10.1067/mpd.2001.113043. [DOI] [PubMed] [Google Scholar]
- 314.Lawson ML, Kirk S, Mitchell T, Chen MK, Loux TJ, Daniels SR, et al. One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: a multi-center study from the Pediatric Bariatric Study Group. J Pediatr Surg. 2006;41:137–43. doi: 10.1016/j.jpedsurg.2005.10.017. discussion 137–43. [DOI] [PubMed] [Google Scholar]
- 315.Nadler EP, Youn HA, Ren CJ, Fielding GA. An update on 73 US obese pediatric patients treated with laparoscopic adjustable gastric banding: comorbidity resolution and compliance data. J Pediatr Surg. 2008;43:141–6. doi: 10.1016/j.jpedsurg.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 316.Zeller M, Daniels S. The obesity epidemic: family matters. J Pediatr. 2004;145:3–4. doi: 10.1016/j.jpeds.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 317.Grace DM. Patient selection for obesity surgery. Gastroenterol Clin North Am. 1987;16:399–413. [PubMed] [Google Scholar]
- 318.Strauss RS. Childhood obesity and self-esteem. Pediatrics. 2000;105:e15. doi: 10.1542/peds.105.1.e15. [DOI] [PubMed] [Google Scholar]
- 319.Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr. 1988;7:147–53. doi: 10.1080/07315724.1988.10720232. [DOI] [PubMed] [Google Scholar]
- 320.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 321.Lo Menzo E, Podkameni D, Wong-Swartz E, Szomstein S, Rosenthal RJ. Weight Loss Surgery: A Multidisciplinary Approach. Edgemont, PA: Matrix; 2008. Pregnancy after bariatric surgery; pp. 501–4. [Google Scholar]
- 322.Wittgrove AC, Jester L, Wittgrove P, Clark GW. Pregnancy following gastric bypass for morbid obesity. Obes Surg. 1998;8:461–4. doi: 10.1381/096089298765554368. discussion 465–6. [DOI] [PubMed] [Google Scholar]
- 323.Dixon JB, Dixon ME, O’Brien PE. Birth outcomes in obese women after laparoscopic adjustable gastric banding. Obstet Gynecol. 2005;106(5 Pt 1):965–72. doi: 10.1097/01.AOG.0000181821.82022.82. [DOI] [PubMed] [Google Scholar]
- 324.Dixon JB, Dixon ME, O’Brien PE. Pregnancy after Lap-Band surgery: management of the band to achieve healthy weight outcomes. Obes Surg. 2001;11:59–65. doi: 10.1381/096089201321454123. [DOI] [PubMed] [Google Scholar]
- 325.Skull AJ, Slater GH, Duncombe JE, Fielding GA. Laparoscopic adjustable banding in pregnancy: safety, patient tolerance and effect on obesity-related pregnancy outcomes. Obes Surg. 2004;14:230–5. doi: 10.1381/096089204322857618. [DOI] [PubMed] [Google Scholar]
- 326.Taylor JL, O’Leary JP. Pregnancy following jejunoileal bypass. Effects on fetal outcome. Obstet Gynecol. 1976;48:425–7. [PubMed] [Google Scholar]
- 327.Hey H, Niebuhr-Jorgensen U. Jejuno-ileal bypass surgery in obesity. Gynecological and obstetrical aspects. Acta Obstet Gynecol Scand. 1981;60:135–40. [PubMed] [Google Scholar]
- 328.Friedman D, Cuneo S, Valenzano M, Marinari GM, Adami GF, Gianetta E, et al. Pregnancies in an 18-year follow-up after biliopancreatic diversion. Obes Surg. 1995;5:308–13. doi: 10.1381/096089295765557692. [DOI] [PubMed] [Google Scholar]
- 329.Tsuda S, Barrios L, Schneider B, Jones DB. Factors affecting rejection of bariatric patients from an academic weight loss program. Surg Obes Relat Dis. 2009;5:199–202. doi: 10.1016/j.soard.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 330.Wee CC. Health utility assessment. In: Jones DB, Jones SB, editors. Obesity Surgery: Patient Safety and Best Practices. Woodbury, CT: Cine-Med, Inc; 2008. pp. 79–86. [Google Scholar]
- 331.Wee CC, Jones DB, Davis RB, Bourland AC, Hamel MB. Understanding patients’ value of weight loss and expectations for bariatric surgery. Obes Surg. 2006;16:496–500. doi: 10.1381/096089206776327260. [DOI] [PubMed] [Google Scholar]
- 332.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56. e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 333.Raftopoulos I, Courcoulas AP. Outcome of laparoscopic ventral hernia repair in morbidly obese patients with a body mass index exceeding 35 kg/m2. Surg Endosc. 2007;21:2293–7. doi: 10.1007/s00464-007-9406-6. [DOI] [PubMed] [Google Scholar]
- 334.Mulhall KJ, Ghomrawi HM, Mihalko W, Cui Q, Saleh KJ. Adverse effects of increased body mass index and weight on survivorship of total knee arthroplasty and subsequent outcomes of revision TKA. J Knee Surg. 2007;20:199–204. doi: 10.1055/s-0030-1248043. [DOI] [PubMed] [Google Scholar]
- 335.Newcomb WL, Polhill JL, Chen AY, Kuwada TS, Gersin KS, Getz SB, et al. Staged hernia repair preceded by gastric bypass for the treatment of morbidly obese patients with complex ventral hernias. Hernia. 2008;12:465–9. doi: 10.1007/s10029-008-0381-1. [DOI] [PubMed] [Google Scholar]
- 336.Amin AK, Clayton RA, Patton JT, Gaston M, Cook RE, Brenkel IJ. Total knee replacement in morbidly obese patients. Results of a prospective, matched study. J Bone Joint Surg Br. 2006;88:1321–6. doi: 10.1302/0301-620X.88B10.17697. [DOI] [PubMed] [Google Scholar]
- 337.Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79:141–7. doi: 10.1080/17453670710014897. [DOI] [PubMed] [Google Scholar]
- 338.Murthy NS, Mukherjee S, Ray G, Ray A. Dietary factors and cancer chemoprevention: an overview of obesity-related malignancies. J Postgrad Med. 2009;55:45–54. doi: 10.4103/0022-3859.43549. [DOI] [PubMed] [Google Scholar]
- 339.Lacey JV, Jr, Kreimer AR, Buys SS, Marcus PM, Chang SC, Leitzmann MF, et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer. 2009;9:84. doi: 10.1186/1471-2407-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hjartaker A, Langseth H, Weiderpass E. Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med Biol. 2008;630:72–93. doi: 10.1007/978-0-387-78818-0_6. [DOI] [PubMed] [Google Scholar]
- 341.Fleming JB, Gonzalez RJ, Petzel MQ, Lin E, Morris JS, Gomez H, et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg. 2009;144:216–21. doi: 10.1001/archsurg.2008.580. [DOI] [PubMed] [Google Scholar]
- 342.Merkow RP, Bilimoria KY, McCarter MD, Bentrem DJ. Effect of body mass index on short-term outcomes after colectomy for cancer. J Am Coll Surg. 2009;208:53–61. doi: 10.1016/j.jamcollsurg.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 343.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 344.Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–22. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 345.Oria HE, Moorehead MK. Updated Bariatric Analysis and Reporting Outcome System (BAROS) Surg Obes Relat Dis. 2009;5:60–6. doi: 10.1016/j.soard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 346.Dymek MP, le Grange D, Neven K, Alverdy J. Quality of life and psychosocial adjustment in patients after Roux-en-Y gastric bypass: a brief report. Obes Surg. 2001;11:32–9. doi: 10.1381/096089201321454088. [DOI] [PubMed] [Google Scholar]
- 347.Choban PS, Onyejekwe J, Burge JC, Flancbaum L. A health status assessment of the impact of weight loss following Roux-en-Y gastric bypass for clinically severe obesity. J Am Coll Surg. 1999;188:491–7. doi: 10.1016/s1072-7515(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 348.de Zwaan M, Lancaster KL, Mitchell JE, Howell LM, Monson N, Roerig JL, et al. Health-related quality of life in morbidly obese patients: effect of gastric bypass surgery. Obes Surg. 2002;12:773–80. doi: 10.1381/096089202320995547. [DOI] [PubMed] [Google Scholar]
- 349.Freys SM, Tigges H, Heimbucher J, Fuchs KH, Fein M, Thiede A. Quality of life following laparoscopic gastric banding in patients with morbid obesity. J Gastroi-ntest Surg. 2001;5:401–7. doi: 10.1016/s1091-255x(01)80069-x. [DOI] [PubMed] [Google Scholar]
- 350.Ahroni JH, Montgomery KF, Watkins BM. Laparoscopic adjustable gastric banding: weight loss, co-morbidities, medication usage and quality of life at one year. Obes Surg. 2005;15:641–7. doi: 10.1381/0960892053923716. [DOI] [PubMed] [Google Scholar]
- 351.Favretti F, Cadiere GB, Segato G, Busetto L, Loffredo A, Vertruyen M, et al. Bariatric analysis and reporting outcome system (BAROS) applied to laparoscopic gastric banding patients. Obes Surg. 1998;8:500–4. doi: 10.1381/096089298765554052. [DOI] [PubMed] [Google Scholar]
- 352.Victorzon M, Tolonen P. Bariatric Analysis and Reporting Outcome System (BAROS) following laparoscopic adjustable gastric banding in Finland. Obes Surg. 2001;11:740–3. doi: 10.1381/09608920160558696. [DOI] [PubMed] [Google Scholar]
- 353.Martikainen T, Pirinen E, Alhava E, Poikolainen E, Pääkkönen M, Uusitupa M, et al. Long-term results, late complications and quality of life in a series of adjustable gastric banding. Obes Surg. 2004;14:648–54. doi: 10.1381/096089204323093435. [DOI] [PubMed] [Google Scholar]
- 354.Favretti F, Segato G, Ashton D, Busetto L, De Luca M, Mazza M, et al. Laparoscopic adjustable gastric banding in 1,791 consecutive obese patients: 12-year results. Obes Surg. 2007;17:168–75. doi: 10.1007/s11695-007-9043-0. [DOI] [PubMed] [Google Scholar]
- 355.Christo NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–23. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Schirmer B, Jones DB. The American College of Surgeons Bariatric Surgery Center Network: establishing standards. Bull Am Coll Surg. 2007;92:21–7. [PubMed] [Google Scholar]
- 357.Schirmer B, Jones DB. The American College of Surgeons Bariatric Surgery Center Network: establishing standards. Bull Am Coll Surg. 2007;92:21–7. [PubMed] [Google Scholar]
- 358.Jones RS. ACS Bariatric Surgery Network. In: Jones DB, Jones SB, editors. Obesity Surgery: Patient Safety and Best Practices. Woodbury, CT: Cine-Med, Inc; 2008. pp. 191–4. [Google Scholar]
- 359.Champion JK, Pories WJ. Centers of Excellence for Bariatric Surgery. Surg Obes Relat Dis. 2005;1:148–51. doi: 10.1016/j.soard.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 360.Surgical Review Corporation. [Accessed August 11, 2009];Provisional Status. Available at: http://www.surgicalreview.org/pcoe/tertiary/tertiary_provisional.aspx.
- 361.Jha AK, Orav EJ, Ridgway AB, Zheng J, Epstein AM. Does the Leapfrog program help identify high-quality hospitals? Jt Comm J Qual Patient Saf. 2008;34:318–25. doi: 10.1016/s1553-7250(08)34040-9. [DOI] [PubMed] [Google Scholar]
- 362.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults–The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 363.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 364.Cho H, Tsuburaya A, Sakamoto J, Morita S, Oba K, Yoshikawa T, et al. A randomized phase II trial of preoperative exercise to reduce operative risk in gastric cancer patients with metabolic syndrome: adjuvant exercise for general elective surgery (AEGES) study group. Jpn J Clin Oncol. 2008;38:71–3. doi: 10.1093/jjco/hym134. [DOI] [PubMed] [Google Scholar]
- 365.Ferraro KF, Su YP, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. Am J Public Health. 2002;92:834–40. doi: 10.2105/ajph.92.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hemmingsson E, Ekelund U. Is the association between physical activity and body mass index obesity dependent? Int J Obes (Lond) 2007;31:663–8. doi: 10.1038/sj.ijo.0803458. [DOI] [PubMed] [Google Scholar]
- 367.Hatoum IJ, Stein HK, Merrifield BF, Kaplan LM. Capacity for physical activity predicts weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring) 2009;17:92–9. doi: 10.1038/oby.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 369.Feigenbaum MS, Pollock ML. Prescription of resistance training for health and disease. Med Sci Sports Exerc. 1999;31:38–45. doi: 10.1097/00005768-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 370.Willey KA, Singh MA. Battling insulin resistance in elderlyobese people with type 2 diabetes: bring on the heavy weights. Diabetes Care. 2003;26:1580–8. doi: 10.2337/diacare.26.5.1580. [DOI] [PubMed] [Google Scholar]
- 371.Evans RK, Bond DS, Wolfe LG, Meador JG, Herrick JE, Kellum JM, et al. Participation in 150 min/wk of moderate or higher intensity physical activity yields greater weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2007;3:526–30. doi: 10.1016/j.soard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 372.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–9. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 373.Aills L, Blankenship J, Buffington C, Furtado M, Parrott J Allied Health Sciences Section Ad Hoc Nutrition Committee. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S73–108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 374.van de Weijgert EJ, Ruseler CH, Elte JW. Long-term follow-up after gastric surgery for morbid obesity: preoperative weight loss improves the long-term control of morbid obesity after vertical banded gastroplasty. Obes Surg. 1999;9:426–32. doi: 10.1381/096089299765552693. [DOI] [PubMed] [Google Scholar]
- 375.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537–49. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 376.Campbell A. Tackling “diabesity” head-on. Joslin Diabetes Center’s new nutrition guideline. Diabetes Self Manag. 2005;22(40):42–4. [PubMed] [Google Scholar]
- 377.Hollingsworth KG, Abubacker MZ, Joubert I, Allison ME, Lomas DJ. Low-carbohydrate diet induced reduction of hepatic lipid content observed with a rapid non-invasive MRI technique. Br J Radiol. 2006;79:712–5. doi: 10.1259/bjr/23166141. [DOI] [PubMed] [Google Scholar]
- 378.Lysen LK. Quick Reference to Clinical Dietetics. 2. Sudbury, MA: Jones and Bartlett Publishers, Inc; 2006. [Google Scholar]
- 379.Kral JG. International Textbook of Obesity. New York: John Wiley and Sons; 2001. [Google Scholar]
- 380.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Spitz AF, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity. 2009;17:S1–70. v. doi: 10.1038/oby.2009.28. [DOI] [PubMed] [Google Scholar]
- 381.Powers KA, Rehrig ST, Jones DB. Financial impact of obesity and bariatric surgery. Med Clin North Am. 2007;91:321–38. ix. doi: 10.1016/j.mcna.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 382.Thompson D, Edelsberg J, Kinsey KL, Oster G. Estimated economic costs of obesity to U.S. business. Am J Health Promot. 1998;13:120–7. doi: 10.4278/0890-1171-13.2.120. [DOI] [PubMed] [Google Scholar]
- 383.Finkelstein E, Fiebelkorn C, Wang G. The costs of obesity among full-time employees. Am J Health Promot. 2005;20:45–51. doi: 10.4278/0890-1171-20.1.45. [DOI] [PubMed] [Google Scholar]
- 384.Association AD. Direct and Indirect Costs of Diabetes in the United States. [Accessed April 1, 2009]. [Google Scholar]
- 385.Kelly J, Tarnoff M, Shikora S, Thayer B, Jones DB, Forse RA, et al. Best practice recommendations for surgical care in weight loss surgery. Obes Res. 2005;13:227–33. doi: 10.1038/oby.2005.31. [DOI] [PubMed] [Google Scholar]
- 386.Scopinaro N, Marinari GM, Camerini G. Laparoscopic standard biliopancreatic diversion: technique and preliminary results. Obes Surg. 2002;12:241–4. doi: 10.1381/096089202762552692. [DOI] [PubMed] [Google Scholar]