Summary
The development of alveolar bone adjacent to the tooth root during tooth eruption is not well understood. This study tested the hypothesis that predominantly woven bone forms adjacent to tooth roots during tooth eruption, but that this immature structure transitions to lamellar bone when the tooth comes into function. Additionally, bone resorption was predicted to play a key role in transitioning immature bone to more mature, load-bearing tissue. Miniature pigs were compared at two occlusal stages, 13 weeks (n=3), corresponding with the mucosal penetration stage of M1 tooth eruption, and 23 weeks (n=3), corresponding with early occlusion of M1/M1. Bone samples for RNA extraction and qRT-PCR analysis were harvested from the diastema and adjacent to M1 roots on one side. Following euthanasia, bone samples for hematoxylin and eosin and TRAP staining were harvested from these regions on the other side. In contrast to expectations, both erupting and functioning molars had reticular fibrolamellar structure in alveolar bone adjacent to M1. However, the woven bone matrix in older pigs was thicker and had denser primary osteons. Gene expression data and osteoclast cell counts showed a tendency for more bone resorptive activity near the molars than at distant sites, but no differences between eruptive stages. Thus, although resorption does occur, it is not a primary mechanism in the transition in alveolar bone from eruption to function. Incremental growth of existing woven bone and filling in of primary osteons within the mineralized scaffold generated the fortification necessary to support an erupted and functioning tooth.
Keywords: alveolar bone, tooth eruption, resorption, qRT-PCR
Introduction
Alveolar bone is well known to develop as teeth erupt into function. However, the transitions in bone structure adjacent to the developing tooth root and mechanisms driving these changes are not well understood. Woven bone forms a crypt that surrounds a tooth during development and remodels as the tooth germ grows (Dixon et al., 1997, par Azeredo et al., 2000). With crown formation complete intraosseous tooth eruption begins, corresponding with the resorption of the coronal portion of the alveolar crypt and apposition occurring adjacent to the developing root (Wise et al., 2002, Pilipili et al., 1998, Marks and Cahill, 1986). Lamellar bone apposition and islets of chondroid tissue have been observed underlying the developing tooth root during intraosseous eruption of dog premolars, and woven bone and chondroid tissue filled the space unoccupied by the erupting tooth (Pilipili et al., 1998). Eruption continues as the crown penetrates the oral mucosa and moves toward the occlusal plane. The fully erupted and functional tooth relies on the anchorage of the periodontal ligament (PDL) within the alveolar bone proper (ABP) for immediate occlusal support. Away from the tooth root, cancellous bone fills the interior spaces of the alveolar crest and cortical plates support the outer surface of the dental arches. The architecture of tooth supporting alveolar bone in pigs is further described in a previous study (Yeh and Popowics, 2010) and differs from the structure of the non-supporting bone of the diastema in which the absence of teeth precludes the formation of ABP. The focus of this investigation is how alveolar bone develops adjacent to the tooth root during mucosal penetration and the first application of occlusal load, and thus achieves a structural configuration able to support an occluding tooth.
In particular, we tested the hypotheses that predominantly woven bone forms adjacent to the tooth root during the mucosal penetration stage of tooth eruption, but that this immature structure transitions to lamellar bone during early tooth function. The basis for this hypothesis is that the focused, consistently oriented load that is experienced only after the tooth is in functional occlusion, triggers adaptive remodeling (Yeh et al., 2010, Terespolsky et al., 2002, Saffar et al., 1997). During the mucosal penetration stage, the food bolus may apply low levels of load to the tooth crown through the partially penetrated mucosa, triggering woven bone apposition adjacent to the tooth roots. A similar response may also occur through the flexing of the mandible during chewing, whereby occlusion of adjacent erupted teeth may confer low levels of strain to the alveolar bone of erupting teeth. However, these loads are likely low and relatively unpredictable in orientation. In contrast, when a tooth reaches functional occlusion higher level loads will be transmitted through the crown to the alveolus, requiring the increased osseous support provided by lamellar bone. Frost hypothesized that cortical bone apposition is responsive to strain levels, and that above a threshold microstrain, apposition is activated in order to reduce peak strains (Frost, 1987). This “mechanostat” hypothesis has been supported for the loading of alveolar bone in rats, in that increasing occlusal loads through a bite block or hard diet resulted in increased cortical thickness (Mavropoulos et al., 2004).
In addition to the increased lamellar bone formation corresponding with the onset of tooth function, osteoclasts would have to play a key role in resorbing the woven bone accrued during tooth eruption. Woven bone is a poorly mineralized, highly cellular structure that is likely to be inadequate to withstand occlusal loads, and the early period of occlusion may result in alveolar microcracking. Indeed microcracks have been observed in pig alveolar bone during orthodontic tooth movement (Verna et al., 2004) and are known to stimulate remodeling activity (Burr, 1993, Taylor and Lee, 2003). Such targeted remodeling provides a mechanism for removing inadequate, injured bone structure and replacing it with denser lamellae. Additionally, alveolar bone turnover occurs at a higher rate than other skeletal regions (Huja et al., 2006), suggesting an important role for resorption in alveolar bone adaptation.
This study aims to define the eruptive transition in pig alveolar bone through molecular and structural assessment at the mucosal penetration stage of tooth eruption and at the onset of functional occlusion. Although pig mandibular bone has been studied previously (Powell et al., 1973), most work focused on the cortex and structural studies of large animal alveolar bone has been pursued in dogs (Deguchi et al., 2008, Marks and Cahill, 1986, Huja et al., 2006, Pilipili et al., 1998). Although several studies have addressed gene expression in porcine bone marrow mesenchymal stem cells in vitro (Bosch et al., 2006, Zeng et al., 2006, Zou et al., 2004), gene expression in pig alveolar bone remains undescribed in vivo. Here, gene expressions associated with the regulation of osteoclast formation, such as the receptor activator of NF-κB ligand (Rankl), osteoprotegerin (Opg), and colony stimulating factor (Csf-1) were measured in order to assess the role of osteoclast activity in bone adjacent to tooth roots during eruption. Additionally, genes associated with bone formation, such as Runx2 and Bsp were compared between eruptive stages. The bone adjacent to the lower first molar (M1) was compared with bone from the diastema region (which lacks teeth) in order to establish whether bone structure or gene expression differs in tooth supporting or non-tooth supporting regions.
Material and Methods
Histology
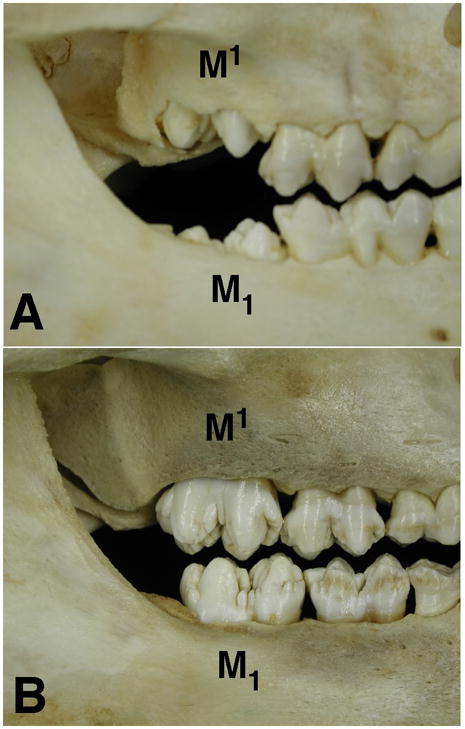
All procedures were humane and approved by the University of Washington Animal Care and Use Committee. Three Hanford miniature pigs were acquired at 13 and 23 weeks of age (n=6; Sinclair Research, St. Louis), corresponding with the mucosal penetration stage of M1 tooth eruption and early occlusion of M1/M1 (Figure 1), respectively. Pigs were euthanized by initial anesthetization with isoflurane and subsequent intracardiac injection of pentobarbital. Tissues for histological processing were collected postmortem, fixed in Bouin’s solution and decalcified in 8% EDTA or 10% formic acid solution. Decalcified tissues were dehydrated, paraffin embedded and sectioned sagittally at 7μm. Sections including the entire length of the distal root of M1 were stained with Hematoxylin and Eosin (H&E) and tartrate-resistant acid phosphatase (TRAP) according to the manufacturers’ instructions (Sigma kit 387A) and examined using light microscopy. In TRAP stained tissues, the bone posterior to the tooth root was divided into cervical (adjacent to the cementoenamel junction (CEJ)), middle and apical thirds. Two 1 × 1 mm regions were identified, one within the alveolar bone proper (ABP; adjacent to the periodontal ligament) and the other further distal in the cancellous bone (midway between the ABP and the lower second molar (M2) crypt). Osteoclasts were identified as darkly staining multi-nucleated cells and counted. Non-parametric statistics were used to compare osteoclast cell counts between regions and ages.
Figure 1.
Quantitative Real Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
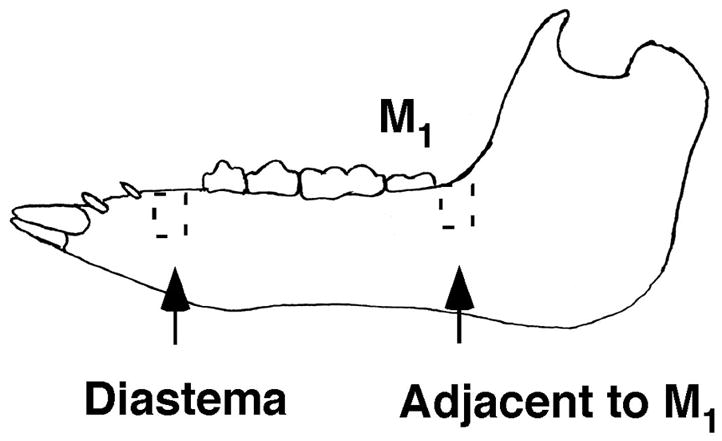
Alveolar bone samples from the mandibular diastema and distal M1 were collected from 3 pigs of each age group (Figure 2). In order to maximize RNA integrity, bone samples were collected from anesthetized animals antemortem with a reciprocal saw (Stryker) and flash frozen in liquid nitrogen. Each sample was ground to a powder using a mortar and pestle and liquid nitrogen. Cellular RNA was isolated from tissues using TRIZOL® Reagent (GIBCO/BRL) and utilized a MagNALyzer® homogenizer (Roche) in order to enhance the separation of protein from cells. An RNeasy Mini kit (Qiagen) was used to remove contaminating DNA from the extracted RNA samples. RNA yields and purity were quantified with a spectrophotometer, and only bone RNA samples showing 260/280 absorbance ratios that were higher than 1.90 were used. Selected samples were also tested to ensure RNA integrity. One RNA sample extracted from 13 week M1 distal bone (pig 4425) showed degradation and was omitted from further study.
Figure 2.
For PCR analysis, cDNA was synthesized using 1.0 μg total RNA with a cDNA synthesis kit (Transcriptor kit, Roche Diagnostic). 2.0 μL of the resulting cDNA product was used per 20 μL reaction in the Roche Lightcycler 480 system. qRT-PCR reactions were carried out with the DNA Master SYBR Green I kit (Roche). Primer sequences were designed from pig or cow partial coding sequences (Table 1) and are intron-spanning to amplify cDNA rather than genomic DNA. In the case of Runx2 and Opg, amplification products generated with cow primers were sequenced to generate partial coding sequences and primers for pig genes (GenBank accession # EU668154 and EF543195). A BLAST search of GenBank on the primer sequences was used to confirm specificity, and melting curve analysis of products post-PCR was checked for additional confirmation. Primers were used at a concentration of 0.5 μM. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) served as a housekeeping/reference gene for normalization. As described below, porcine osteoblast DNA was used for qRT-PCR calibration and template DNA was replaced with PCR-grade water as a negative control. The amplification profile used on the Lightcycler was: 95°C /60s; 58°C /60s; 72°C /60s and 50–55 cycles.
Table 1.
Primer sequences for Sus scrota specific genes.
| GenBank accession number | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | |
|---|---|---|---|
| Rankl | AY60682 | TGTGAGACTACTAAGCGG | GCAGGTTCCAGCATGA |
| Opg | EF543195 | GGGGCTCCTTCTAACT | CTCCGCAAGAAGTCCA |
| Csf-1 | AJ583506 | TCGTGCCAAATTGCCT | CGTCTCATAGAAAGTTCGGA |
| Bsp | L10363 | ACGAAGGCATGAATTGT | ACGGGTAGGTATCGTGA |
| Runx2 | EU668154 | CAAAGCCAGAGCGGAC | AATTTGGATTTAATAGCGTGC |
| Gapdh | AF017079 | GATCGTCAGCAATGCC | CCGGTAGAAGCAGGGA |
A porcine osteoblast cell culture was used to generate a calibrator DNA for qRT-PCR amplification. The role of the calibrator was to provide a stable ratio of target to reference genes and to normalize all samples within a qRT-PCR run. Pig bone marrow aspirates were obtained from the tibia and mononuclear cells were separated by centrifugation of aspirates through a solution of polysucrose and sodium diatrizoate (Histopaque; density, 1.077; Sigma) according to the protocol of Bosch (2006). Mononuclear cells pipetted from the opaque interface were washed twice in Dulbecco’s phosphate-buffered saline (D-PBS) and resuspended in Minimum Essential Medium (MEM) Alpha medium (Invitrogen) supplemented with 10% FBS. Cells were plated on plastic flasks at a density of approximately 500,000 cells/cm2 and the medium was changed every 24h, washing unattached cells away with the medium change. Adherent fibroblast-like, spindle shape cells grew for 10–14 days, with media replacement every 3rd day. Cells were passaged at 80–90% confluence by trypsinization (0.25% trypsin-EDTA solution) and reseeded at a density of 5000–6000 cells/cm2 in plastic flasks (Bosch et al., 2006). The cultured porcine mesenchymal cells (pMSCs) were induced to differentiate into osteogenic cells by exposure to media containing ascorbic acid and β-glycerophosphate. The cells’ osteoblastic identity was confirmed through staining of the mineralized extracellular matrix with alizarin red. Cells were harvested for RNA extraction and synthesized DNA was used as a calibrator in qRT-PCR experiments.
Each sample was amplified in triplicate for each gene and PCR products were identified with specific melting curves. Gene expression levels were standardized across reactions with corresponding coefficient curves and RelQuant software was used to measure different gene expression levels in different samples. The mean value for triplicate experiments was used to compare samples from different age groups and locations using non-parametric statistical analyses. A p value of less than 0.05 was considered significant.
Results
Bone Structure
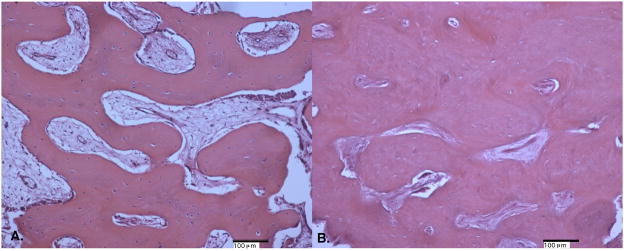
The bone distal to the erupting M1 in the 13 week pigs consisted mainly of reticular fibrolamellar bone adjacent to the tooth roots in all cervical through apical locations (Figure 3A). The mineralized woven bone matrix formed thin trabeculae interspersed with immature primary osteons with large diameter vascular marrow canals. The primary osteons were oriented obliquely throughout the matrix and the vascular canals anastomosed irregularly, agreeing with the category definition for reticular fibrolamellar bone (Francillon-Viellot et al., 1990). In some samples, the most cervical regions were reticular fibro lamellar bone adjacent to the root but laminar bone more distal from the root. Throughout the length of the tooth root, thin trabeculae projected into the periodontal ligament tissue. The diastema region appeared similar to the bone adjacent to the developing tooth root, including mainly fibrolamellar bone (Figure 4A).
Figure 3.
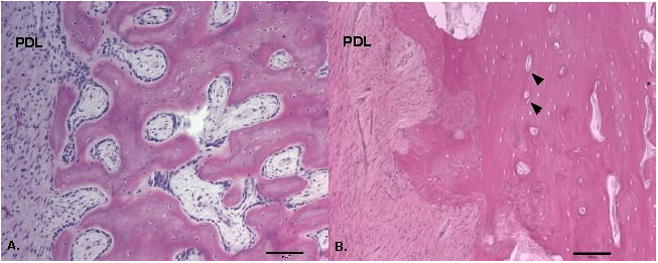
Figure 4.
In 23-week pigs, the bone distal to the erupted M1 was also mainly of reticular fibrolamellar structure, however the woven bone matrix was noticeably thicker than in the younger tissue and canals of the primary osteons were generally smaller (Figure 3B). The overall denser matrix gave the appearance of a less finely reticulated matrix than in the younger tissue. In some cervical locations the alveolar bone was especially dense and contained more longitudinally oriented vascular canals. The woven bone matrix formed a continuous surface or thick trabeculae that extended into the periodontal ligament. The fibrolamellar bone of the diastema showed a similar density to the bone distal to the erupted M1 with nearly all of the cellular space filled with primary osteons (Figure 4B).
Osteoclast Cell Numbers
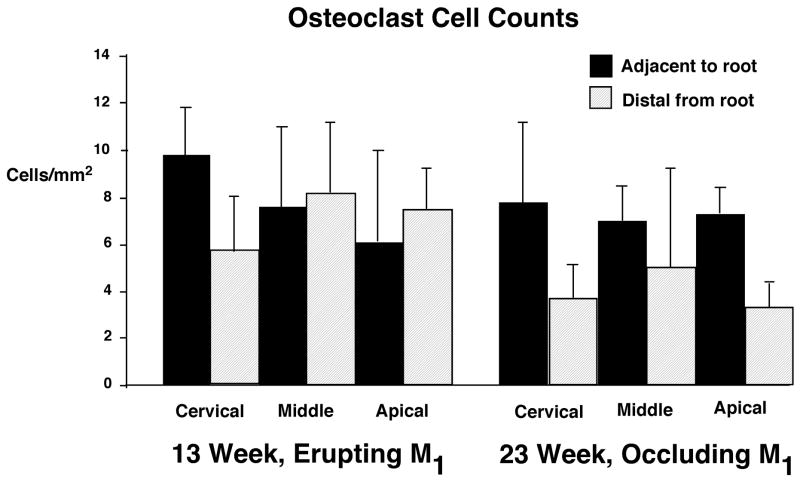
TRAP-stained osteoclasts were observed in all alveolar bone proper and cancellous bone regions adjacent to M1 and did not show marked differences among regions or between age groups (Figure 5). Within alveolar bone proper, osteoclast numbers ranged from 2–12/mm2 in 13 week specimens and 4–11/mm2 in 23 week specimens). Osteoclast numbers in the distal location were 0–10/mm2 at 13 weeks and 2–10/mm2 at 23 weeks. In comparing bone directly adjacent to the tooth root and bone more distal from the root, osteoclast numbers showed greater similarity in 13 week specimens. In 23 week specimens, osteoclast numbers in bone adjacent to the root tended to be higher than more distal from the root, however, this difference did not reach statistical significance.
Figure 5.
The tissue preservation of bone in the diastema region was insufficient to allow osteoclast cell counts; however the bone of the diastema appeared primarily formative. Although osteoclasts were visible in the diastema of both 13 and 23 week pigs, bone surfaces were predominantly smooth with only an occasional appearance of Howship’s lacunae.
qRT PCR
Gene expression values were similar between locations and age groups (Table 2). Negative controls produced no amplification of target gene DNAs, whereas the osteoblast DNA calibrator expressed target genes in all cases. When mean values were compared, Rankl expression was similar in the M1 alveolar bone in 13 week and 23 week pigs (0.52 vs. 0.50), and could not be distinguished from Rankl expression in the diastema (0.20 vs. 0.39, 13 week and 23 week pigs, respectively). For 4 of the 5 animals available for comparison (all but 5069), however, Rankl expression in the M1 bone was higher than in the diastema, resulting in overall higher mean expression in the M1 bone of pigs in each age group. Csf-1 expression was comparable between the M1 bone samples of 13 and 23 week pigs (means of 0.92 vs. 0.84), as well as in the diastema (means of 0.61 vs. 0.67; 13 and 23 weeks, respectively). As with Rankl expression, Csf-1 expression tended to be higher in each pig, with the exception of pig 5069, in the M1 distal bone versus the diastema. Opg expression was not reliably measured, as triplicate experiments for each sample were highly variable. Means and standard deviations for each sample are reported in Table 2; however, means for each age group were omitted from comparisons. In the case of Bsp and Runx2, gene expression values were very high, but were also highly variable among individual pigs of each age group, and thus, did not demonstrate differences. When individual animals were examined, these apposition-related genes were higher in M1 bone than the diastema in 13-week animals, but no consistent trend was seen in the 23-week animals.
Table 2.
Target gene expression relative to GAPDH from bone samples from the mandibular diastema and distal to the lower first molar.
| Pig Number | Age | Location | RANKL | CSF-1 | OPG | BSP | RUNX2 |
|---|---|---|---|---|---|---|---|
| 4425 | 13 wk | diastema | 0.13 ± 0.003 | 0.63 ± 0.04 | 24.68 ± 40.5 | 27,808 ± 156 | 4.19 ± 0.13 |
| 5060 | 13 wk | diastema | 0.25 ± 0.02 | 0.70 ± 0.03 | 188.32 ± 308 | 20,378 ± 3640 | 6.32 ± 0.31 |
| 5061 | 13 wk | diastema | 0.22 ± 0.03 | 0.49 ± 0.07 | 7.17 ± 8.7 | 20,538 ± 1612 | 3.61 ± 0.58 |
| Mean | 13 wk | diastema | 0.20 ± 0.06 | 0.61 ± 0.11 | 22,908 ± 4244 | 4.71 ± 1.42 | |
| 4501 | 23 wk | diastema | 0.07 ± 0.01 | 0.23 ± 0.10 | 18.99 ± 20 | 11,411 ± 1384 | 2.14 ± 0.07 |
| 5069 | 23 wk | diastema | 0.77 ± 0.04 | 1.11 ± 0.12 | 164.07 ± 245.7 | 211,673 ± 16,868 | 10.05 ± 0.37 |
| 5071 | 23 wk | diastema | 0.34 ± 0.02 | 0.66 ± 0.02 | 178.49 ± 222 | 66,639 ± 851 | 7.43 ± 0.33 |
| Mean | 23 wk | diastema | 0.39 ± 0.36 | 0.67 ± 0.44 | 96,574 ± 103,432 | 6.54 ± 4.03 | |
| 5060 | 13 wk | M1 distal | 0.61 ± 0.03 | 1.29 ± 0.16 | 6800 ± 11,611 | 44,838 ± 14,325 | 12.61 ± 2.04 |
| 5061 | 13 wk | M1 distal | 0.43 ± 0.03 | 0.54 ± 0.06 | 14,841 ± 20,981 | 44,796 ± 8683 | 6.18 ± 0.84 |
| Mean | 13 wk | M1 distal | 0.52 ± 0.13 | 0.92 ± 0.53 | 44, 817 ± 29 | 9.39 ± 4.55 | |
| 4501 | 23 wk | M1 distal | 0.22 ± 0.03 | 0.38 ± 0.04 | 177.25 ± 167.4 | 27,925 ± 3724 | 2.15 ± 0.17 |
| 5069 | 23 wk | M1 distal | 0.19 ± 0.03 | 0.66 ± 0.14 | 4.49 ± 5.7 | 3801 ± 334 | 4.20 ± 1.48 |
| 5071 | 23 wk | M1 distal | 1.10 ± 0.11 | 1.50 ± 0.25 | 66.20 ± 61.9 | 52,224 ± 4721 | 12.38 ± 1.33 |
| Mean | 23 wk | M1 distal | 0.50 ± 0.52 | 0.84 ± 0.58 | 27, 983 ± 24,211 | 6.24 ± 5.41 | |
Discussion
Contrary to expectations, the alveolar bone supporting fully erupted and functional first molars was not lamellar, but consisted of a dense fibrolamellar matrix that included primary osteons. Younger pigs also showed fibrolamellar structure surrounding the erupting first molars, however, it was less dense and more finely reticulated than the older bone. A previous study of the mandibular cortex in growing pigs demonstrated a plexiform structure that regionally varied in porosity (Powell et al., 1973), suggesting that this structure may characterize rapidly growing porcine bone. Although the alveolar bone proper is typically described as including lamellar tissue, it was not so in these animals. Nevertheless, the dense fibrolamellar structure observed adjacent to molars in juvenile pigs appears sufficient to support molars in occlusion. Lamellar structure may only occur in these locations with greater maturity or the larger occlusal forces that would correspond with increased body size.
Osteoclast activity was predicted to play a major role in the adaptation of alveolar bone to occlusal loading; however, osteoclast cell counts indicate at most a minor role for resorption in the osseous changes surrounding M1 tooth roots. Osteoclast numbers were similar adjacent to and distal from the root in 13 week tissues, suggesting a constitutive level of turnover in the alveolar bone during the mucosal penetration stage of M1 eruption. However, at 23 weeks, although osteoclast numbers were at levels similar to the 13 week bone, adjacent to the erupted molars, away from the root osteoclast numbers appeared to be dropping. This suggests the possibility of an age-related drop in constituitive resorption, while higher levels are maintained for bone anchoring PDL fibers in order to respond to occlusal loads transmitted through the PDL. This suggestion is consistent with previous observations in rat alveoli where resorption plays an active role in modeling of the alveolar bone proper (Saffar et al., 1997, Vignery and Baron, 1980).
The consistent expression of Rankl and Csf-1 in alveolar bone associated with erupting and functioning molars further indicates the presence of constitutive levels of resorption. The membrane of osteoblast/stromal cells expresses RANKL in response to bone resorbing signals, and all phases of osteoclastogenesis depend on the interaction between RANKL and the transmembrane signaling receptor, Rank, present on osteoclast progenitors (Yasuda et al., 1998, Yasuda et al., 1999). The cytokine CSF-1 is necessary for both proliferation and differentiation of osteoclast progenitors at remodeling sites (Katagiri and Takahashi, 2002, Van Wesenbeeck et al., 2002). The expression of Rankl and Csf-1 in porcine alveolar bone demonstrates the involvement of these genes in the promotion of osteoclast formation, but their similar expression levels indicate that peaks in resorption do not accompany the onset of occlusal activity. On the other hand, the Rankl gene expression levels distal to M1 showed a tendency to be higher than the diastema region, suggesting that bone surrounding tooth roots may have a higher turnover than toothless regions of the dental arch. Although osteoclast cell counts were unavailable in the poorly preserved diastema samples, bone surfaces in this region appear primarily formative, corresponding with lower Rankl expression in the diastema. Previous studies have shown that increases in Rankl expression correspond with increased remodeling (Fazzalari et al., 2001) and occur in fractured bone during repair processes (Kon et al., 2001). Studies of the bone overlying the tooth crown during the earlier intraosseous eruption demonstrate spikes in osteoclast cell numbers and expression of resorption associated genes Rankl and Csf-1 as an intraosseous eruption pathway is formed (Wise et al., 1999, Liu et al., 2005, Wise et al., 2005), but these features decline in basal regions surrounding the developing tooth root in mice at later eruptive stages (Heinrich et al., 2005). Application of excessive orthodontic forces on rat teeth can upregulate Rankl expression on the compression side of the alveolar bone (Ogasawara et al., 2004). Furthermore, a hyperocclusion mouse model has demonstrated increased osteoclasts and significant increases in Rankl expression in periodontal tissues (Walker et al., 2008). The tendency toward an increase in Rankl expression in bone distal to M1 tooth roots in the present study does not reach the magnitude previously observed during intraosseous eruption or in studies that manipulate the periodontal loads. Therefore, we conclude that although resorption is active in resorbing woven bone surrounding roots, a large-scale removal of inappropriately structured or microfractured alveolar tissue does not accompany the onset of occlusion.
Consistent expression of genes associated with osteogenesis, Runx2 and Bsp, suggests that incremental bone apposition generates the major structural differences in alveolar bone between 13 and 23 week pigs. Runx2 expression has been identified as a molecular switch stimulating mesenchymal cells to differentiate into osteoblasts and triggering expression of major bone matrix genes (Ducy, 2000, Zhang et al., 2009, Ducy et al., 1997, Komori, 2010), processes which in occur in alveolar bone cells as well as elsewhere (Perinpanayagam et al., 2006). Multiple signal transduction pathways regulate Runx2 expression, including those involved in mechanotransduction (Franceschi and Xiao, 2003). BSP is thought to nucleate hydroxyapatite at the bone mineralization front (Ogata, 2008) and is strongly expressed in osteocytes and osteoblasts within porcine alveolar bone (Chen et al., 1993). The present study did not detect differences in Runx2 or Bsp expression associated with age or eruptive status, indicating that bone growth or occlusal loading did not upregulate expression. Instead, high expression is probably associated with progressive apposition throughout the entire period of growth and eruption, thickening trabeculae and forming primary osteons within a pre-existing woven bone scaffold.
Although osteoblast gene expression has received much attention through study of in vitro cell cultures, little data are available on in vivo expression patterns, particularly in response to changes in functional conditions. The regulation of Rankl and Opg expression has been examined in bone marrow stromal cells (Rubin et al., 2002a, Gori et al., 2000), and cell cultures exposed to strain showed decreases in Rankl expression, whereas CSF expression was unaffected (Rubin et al., 2000, Rubin et al., 2002b). Different patterns of fluid flow can either downregulate (Kim et al., 2006) or upregulate (Liu et al., 2010) the Rankl/Opg expression ratio. In the periodontium, occlusal trauma, such as hyperocclusion may affect Rankl and Runx2 ratios, but in normally functioning animals expression levels remain steady (Claudino et al., 2010). In the present study, a trend toward higher Rankl expression was noted in the bone near molars relative to the diastema, but the gene expression patterns did not differ to the extent observed in the mouse hyperocclusion study. Overall, the present data indicate that transitions in bone structure are achieved in association with changes in tooth eruption and functional conditions despite similarities in gene expression.
Acknowledgments
Supported by NIH/NIDCR DE015815. Special thanks to the laboratories of Dr. Martha Somerman and Dr. Susan Herring for making these studies possible.
References
- Bosch P, Pratt SL, Stice SL. Isolation, characterization, gene modification, and nuclear reprogramming of porcine mesenchymal stem cells. Biol Reprod. 2006;74:46–57. doi: 10.1095/biolreprod.105.045138. [DOI] [PubMed] [Google Scholar]
- Burr DB. Remodeling and the repair of fatigue damage. Calcif Tissue Int. 1993;53:S75–S81. doi: 10.1007/BF01673407. [DOI] [PubMed] [Google Scholar]
- Chen J, Mcculloch CAG, Sodek J. Bone sialoprotein in developing porcine dental tissues: cellular expression and comparison of tissue localization with osteopontin and osteonectin. Archs Oral Biol. 1993;38:214–249. doi: 10.1016/0003-9969(93)90034-j. [DOI] [PubMed] [Google Scholar]
- Claudino M, Garlet TP, Cardoso CRB, Assis GFD, Taga R, Cunha FQ, Silva JOS, Garlet GP. Down-regulation of expression of osteoblast and osteocyte markers in periodontal tissues associated with the spontaneous alveolar bone loss of interleukin-10 knockout mice. Eur J Oral Sci. 2010;118:19–28. doi: 10.1111/j.1600-0722.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- Deguchi T, Takano-Yamamoto T, Yabuuchi T, Ando R, Roberts WE, Garetto LP. Histomorphometric evaluation of alveolar bone turnover between the maxilla and the mandible during experimental tooth movement in dogs. Am J Orthod Dentofac Orthop. 2008;133:889–97. doi: 10.1016/j.ajodo.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Dixon AD, Hoyte DAN, Ronning O. Fundamentals of Craniofacial Growth. CRC Press; New York: 1997. [Google Scholar]
- Ducy P. Cbfa1: A molecular switch in osteoblast biology. Dev Dyn. 2000;219:461–471. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1074>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Fazzalari NC, Kuliwaba JS, Atkins GJ, Forwood MR, Findlay DM. The ratio of messenger RNA levels of receptor activator of nuclear factor kB ligand to osteoprotegerin correlates with bone remodeling indices in normal human cancellous bone but not in osteoarthritis. J Bone Min Res. 2001;16:1015–1027. doi: 10.1359/jbmr.2001.16.6.1015. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003;88:446–454. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- Francillon-Viellot H, De Buffrénil V, Castanet J, Géraudie J, Meunier FJ, Sire JY, Zylberberg L, De Ricqlés A. Microstructure and mineralization of vertebrate skeletal tissues. In: Carter JG, editor. Skeletal Biomineralization: Patterns, Processes and Evolutionary trends. Vol. 1. Van Nostrand Reinhold; New York: 1990. [Google Scholar]
- Frost HM. Bone “Mass” and the “Mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL. The Expression of Osteoprotegerin and RANK Ligand and the Support of Osteoclast Formation by Stromal-Osteoblast Lineage Cells Is Developmentally Regulated. Endocrinology. 2000;141:4768–4776. doi: 10.1210/endo.141.12.7840. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Bsoul S, Barnes J, Woodruff K, Abboud S. CSF-1, RANKL and OPG regulate osteoclastogenesis during murine tooth eruption. Archs Oral Biol. 2005;50:897–908. doi: 10.1016/j.archoralbio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec Part A: Discoveries in Molecular, Cellular, and Evol Biol. 2006;288A:1243–1249. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Diseases. 2002;8:147–159. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Komori T. Regulation of bone development and extracellular matrix protein genes by Runx2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, Gerstenfeld LC, Einhorn TA. Expression of osteoprotegerin, receptor activator of NF-kB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Min Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao Y, Cheung WY, Gandhi R, Wang L, You L. Effects of cyclic hydraulic pressure on osteocytes. Bone. 2010;46:1449–56. doi: 10.1016/j.bone.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Yao S, Pan F, Wise GE. Chronology and regulation of gene expression of RANKL in the rat dental follicle. Eur J Oral Sci. 2005;113:404–409. doi: 10.1111/j.1600-0722.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- Marks SC, Cahill DR. Ultrastructure of alveolar bone during tooth eruption in the dog. Amer J Anat. 1986;177:427–428. doi: 10.1002/aja.1001770311. [DOI] [PubMed] [Google Scholar]
- Mavropoulos A, Kiliaridis S, Bresin A, Ammann P. Effect of different masticatory functional and mechanical demands on the structural adaptation of the mandibular alveolar bone in young growing rats. Bone. 2004;35:191. doi: 10.1016/j.bone.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Ogasawara T, Yoshimine Y, Kiyoshima T, Kobayashi I, Matsuo K, Akamine A, Sakai H. In situ expression of RANKL, RANK, osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J Periodontal Res. 2004;39:42–49. doi: 10.1111/j.1600-0765.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- Ogata Y. Bone sialoprotein and its transcriptional regulatory mechanism. J Periodont Res. 2008;43:127–135. doi: 10.1111/j.1600-0765.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- Par Azeredo RA, Watanabe IS, Beigno MIM, Lemos JLR, Liberti EA. The arrangement of the trabecular bone in the vestibular surface of the human fetus mandible. A scanning electron microscopy study. Morphologie. 2000;84:19–24. [PubMed] [Google Scholar]
- Perinpanayagam H, Martin T, Mithal V, Dahman M, Marzec N, Lampasso J, Dziak R. Alveolar bone osteoblast differentiation and Runx2/Cbfa1 expression. Archs Oral Biol. 2006;51:406–415. doi: 10.1016/j.archoralbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Pilipili CM, Goret-Nicaise M, Dhem A. Microradiographic aspects of the growing mandibular body during permanent premolar eruption in the dog. Eur J Oral Sci. 1998;106:429–436. doi: 10.1111/j.1600-0722.1998.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Powell K, Atkinson PJ, Woodhead C. Cortical bone structure of the pig mandible. Archs Oral Biol. 1973;18:171–180. doi: 10.1016/0003-9969(73)90136-2. [DOI] [PubMed] [Google Scholar]
- Rubin J, Ackert-Bicknell CL, Zhu L, Fan X, Murphy TC, Nanes MS, Marcus R, Holloway L, Beamer WG, Rosen CJ. IGF-I regulates osteoprotegerin (OPG) and receptor activator of nuclear factor-{kappa}B ligand in vitro and OPG in vivo. J Clin Endocrinol Metab. 2002a;87:4273–4279. doi: 10.1210/jc.2002-020656. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy T, Nanes MS, Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol. 2000;278:C1126–C1132. doi: 10.1152/ajpcell.2000.278.6.C1126. [DOI] [PubMed] [Google Scholar]
- Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor W. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Min Res. 2002b;17:1452–1460. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- Saffar JL, Lasfargues JJ, Cherruau M. Alveolar bone and the alveolar process: the socket that is never stable. Periodontology 2000. 1997;13:76–90. doi: 10.1111/j.1600-0757.1997.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Taylor D, Lee TC. Microdamage and mechanical behaviour: predicting failure and remodelling in compact bone. J Anat. 2003;203:203–211. doi: 10.1046/j.1469-7580.2003.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terespolsky MS, Brin I, Harari D, Steigman S. The effect of functional occlusal forces on orthodontic tooth movement and tissue recovery in rats. Amer J Orthodont Dentofacial Orthoped. 2002;121:620–628. doi: 10.1067/mod.2002.123342. [DOI] [PubMed] [Google Scholar]
- Van Wesenbeeck L, Odgren PR, Mackay CA, D’angelo M, Safadi FF, Popoff SN, Van Hul W, Marks SC., Jr The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: Evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. PNAS. 2002;99:14303–14308. doi: 10.1073/pnas.202332999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna C, Dalstra M, Lee TC, Cattaneo PM, Melsen B. Microcracks in the alveolar bone following orthodontic tooth movement: a morphological and morphometric study. Eur J Orthodont. 2004;26:459–467. doi: 10.1093/ejo/26.5.459. [DOI] [PubMed] [Google Scholar]
- Vignery A, Baron R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat Rec. 1980;196:191–200. doi: 10.1002/ar.1091960210. [DOI] [PubMed] [Google Scholar]
- Walker CG, Ito Y, Dangaria S, Luan X, Diekwisch TGH. RANKL, osteopontin, and osteoclast homeostasis in a hyperocclusion mouse model. Eur J Oral Sci. 2008;116:312–318. doi: 10.1111/j.1600-0722.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise GE, Frazier-Bowers S, D’souza RN. Cellular, molecular, and genetic determinants of tooth eruption. Crit Rev Oral Biol Med. 2002;13:323–334. doi: 10.1177/154411130201300403. [DOI] [PubMed] [Google Scholar]
- Wise GE, Huang H, Que BG. Gene expression of potential tooth eruption molecules in the dental follicle of the mouse. Eur J Oral Sci. 1999;107:482–486. doi: 10.1046/j.0909-8836.1999.eos107610.x. [DOI] [PubMed] [Google Scholar]
- Wise GE, Yao S, Odgren PR, Pan F. CSF-1 Regulation of Osteoclastogenesis for Tooth Eruption. J Dent Res. 2005;84:837–841. doi: 10.1177/154405910508400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N, Takahashi N, Suda T, Higashio K. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–113. doi: 10.1016/s8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Moringa T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. PNAS. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh K, Popowics T, Rafferty K, Herring S, Egbert M. The effects of tooth extraction on alveolar bone biomechanics in the miniature pig, Sus scrofa. Archs Oral Biol. 2010;55:663–9. doi: 10.1016/j.archoralbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh K-D, Popowics T. The impact of occlusal function on structural adaptation in alveolar bone of the growing pig, Sus Scrofa. Archs Oral Biol. 2010 doi: 10.1016/j.archoralbio.2010.08.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Rahrmann E, Hu Q, Lund T, Sandqist L, Felton M, O’brien TD, Zhang J, Verfaillie C. Multi-potent adult progenitor cells from swine bone marrow. Stem Cells. 2006;24:2355–66. doi: 10.1634/stemcells.2005-0551. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xiao Z, Luo J, He N, Mahlios J, Quarles LD. Dose-dependent effects of Runx2 on bone development. J Bone Min Res. 2009;24:1889–1904. doi: 10.1359/JBMR.090502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Li H, Chen L, Baatrup A, Bünger C, Lind M. Stimulation of porcine bone marrow stromal cells by hyaluronan, dexamethasone and rhBMP-2. Biomaterials. 2004;25:5375–5385. doi: 10.1016/j.biomaterials.2003.12.041. [DOI] [PubMed] [Google Scholar]