Abstract
Background & Aim
Previous studies have suggested that prior exposure to hepatitis B virus (HBV) infection may increase the risk of development of hepatocellular carcinoma (HCC) in patients with chronic hepatitis C. The aim of this study was to compare the prevalence of previous or occult HBV infection in a cohort of HBsAg-negative patients with histologically advanced chronic hepatitis C in the United States who did or did not develop HCC.
Methods
Stored sera from 91 patients with HCC and 182 matched controls who participated in the HALT-C Trial were tested for anti-HBc, anti-HBs and HBV DNA. Frozen liver samples from 28 HCC cases and 55 controls were tested for HBV DNA by real-time PCR.
Results
Anti-HBc (as a marker of previous HBV infection) was present in the serum of 41.8% HCC cases and 45.6% controls (P=0.54); anti-HBc alone was present in 16.5% of HCC cases and 24.7% of controls. HBV DNA was detected in the serum of only one control subject and no patient with HCC. HBV DNA (as a marker of occult HBV infection) was detected in the liver of 10.7% HCC cases and 23.6% controls (P=0.18).
Conclusion
Although almost half the patients in the HALT-C Trial had serological evidence of previous HBV infection there was no difference in prevalence of anti-HBc in serum or HBV DNA in liver between patients who did or did not develop HCC. In the United States, neither previous nor occult HBV infection is an important factor in HCC development among patients with advanced chronic hepatitis C.
Keywords: HBV DNA, hepatitis B core antibody, cirrhosis
Introduction
Hepatitis B virus (HBV) infection is marked by the presence of hepatitis B surface antigen (HBsAg) in serum. Clearance of HBsAg indicates recovery from infection; however, low levels of HBV DNA may persist within the liver and occasionally in the serum indicating that infection is not totally resolved in some patients. The presence of HBV DNA in HBsAg-negative persons has been referred to as “occult” HBV infection. It has been suggested that occult HBV infection may contribute to ongoing liver disease and the development of hepatocellular carcinoma (HCC) (1–3). There is evidence that previous HBV infection, marked only by the presence of antibodies to HBV, notably hepatitis B core antibody (anti-HBc) with or without hepatitis B surface antibody (anti-HBs), may also indicate persistent HBV infection. Previous HBV infection has also been linked with progressive liver disease, particularly as a co-factor among patients with another form of underlying liver disease such as chronic hepatitis C or alcoholic liver disease.
Prior studies have shown that the prevalence of occult HBV infection is higher in countries where HBV infection is prevalent, in patients with serological markers of previous HBV infection, and in patients who have risk factors for HBV infection such as those with human immunodeficiency virus (HIV) or hepatitis C virus (HCV) infection. What remains uncertain is the clinical significance of low level, persistent HBV infection, especially among patients with another cause of liver disease. Several studies, mostly from Europe and Japan, have found a higher rate of occult HBV infection in patients with chronic HCV infection who have HCC as compared to HCV-infected patients with no HCC (1–4). In some of these studies, the prevalence of occult HBV infection in HCV patients with HCC was as high as 60–70% (2–4). However, data on the prevalence of occult HBV infection in HCV patients and the contribution of occult HBV to HCC in the United States are limited.
The Hepatitis Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial prospectively followed patients with chronic HCV infection and advanced hepatic fibrosis for clinical outcomes including HCC. All the patients tested negative for HBsAg in the serum at enrollment. This large cohort provides an excellent opportunity to study the clinical significance of previous or occult HBV infection in patients with chronic HCV infection in the United States. The aims of this analysis were to compare the prevalence of (a) previous and (b) occult HBV infection in HALT-C patients who developed HCC and those who did not develop HCC. In addition we assessed the demographics, risk factors for HCV infection, laboratory and histological indicators of liver disease in HALT-C patients with and without (a) previous and (b) occult HBV infection.
Patients and Methods
The design of the HALT-C Trial has been described previously (5, 6). Briefly, patients with chronic hepatitis C had to meet the following criteria for enrollment: failure to achieve sustained virological response (SVR) after previous interferon treatment with or without ribavirin, the presence of advanced hepatic fibrosis on liver biopsy (Ishak fibrosis score ≥3), no history of hepatic decompensation or HCC, and the absence of defined exclusion criteria. Specifically, patients who were positive for HBsAg or HIV antibody were excluded from the trial.
All patients were required to have a liver biopsy before enrollment. For those in whom the entry biopsy was performed subsequent to consent into the HALT-C Trial, a portion of the biopsy was snap frozen and stored for future research after an adequate specimen was allocated for histologic assessment. The biopsies were initially stored at −70C at the clinical sites and then sent to a central repository (SeraCare Life Sciences, Gaithersburg, MD) on dry ice. Upon arrival at the central repository, the biopsies were stored in −70C freezers with back-up generators.
All patients had been previously treated for chronic hepatitis C with one or more courses of interferon, with the most recent course being a combination of peginterferon and ribavirin. Patients who remained viremic during treatment or experienced viral breakthrough or relapse after initial response were randomized to maintenance therapy (peginterferon alfa-2a 90 μg weekly) or to no further treatment for the next 3.5 years. Following completion of the 3.5 years of the randomized trial, all patients were invited to continue follow-up without treatment.
At entry, all patients were required to have an ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) with no evidence of hepatic mass lesions suspicious for HCC and to have an alpha fetoprotein (AFP) <200 ng/mL.
Patients were scheduled to be seen every 3 months during the 3.5 years of the randomized trial and every 6 months thereafter. At each visit, patients were evaluated clinically and blood tests were performed. Blood samples for research were collected on site and then centrifuged; sera were initially stored at −70C at the clinical sites and periodically sent on dry ice to SeraCare where they were stored in −70C freezers with back-up generators. Protocol-defined ultrasound examination was performed at intervals of 6 to 12 months (5–6). Patients with elevated or rising AFP and those with new lesions on ultrasound were further evaluated by CT or MRI.
Two definitions of HCC, one for presumed and one for definite, have been previously published (7). Definite HCC was defined by histology or a new mass on imaging with AFP levels increasing to ≥1,000 ng/mL. Presumed HCC was defined as a new mass on ultrasound in conjunction with two liver imaging studies showing a lesion with characteristics of HCC or evidence of progression on follow-up. All cases of HCC were reviewed by an Outcomes Review Panel comprised of panels of three clinical investigators.
To compare the prevalence of (a) previous and (b) occult HBV infection, a case-control study was performed. All HCC cases (definite or presumed) diagnosed at any time after enrollment into the HALT-C Trial were studied. Two controls were selected for each case of HCC, matched for fibrosis stratum on baseline biopsy (Ishak 3 or 4 vs. 5 or 6), treatment assignment (peginterferon vs. no treatment for randomized patients) and duration of follow-up. To ensure that control patients did not harbor early HCC, they were required to be followed for at least 12 months after the date of their matching with the HCC patient, and to not have HCC at any time during the HALT-C Trial.
Previous HBV infection was defined as the presence of anti-HBc with or without anti-HBs or HBV DNA in serum. Occult HBV infection was defined as the presence of HBV DNA in the liver.
HBV markers in serum
All patients tested negative for HBsAg in the clinical laboratory at the local HALT-C site prior to their enrollment into the HALT-C Trial. Stored serum samples were coded and sent to the clinical laboratory at University of California, Irvine where they were tested for anti-HBc (ETI-AB-COREK PLUS, DiaSorin Inc., Stillwater, MN) and anti-HBs (ADVIA Centaur anti-HBs, Siemens Healthcare Diagnostics, Inc., Tarrytown, NY) by enzyme immunoassays (anti-HBc) or chemiluminescent immunoassay (anti-HBs). Serum HBV DNA was tested by a real-time polymerase chain reaction (PCR) assay (COBAS TaqMan HBV Test, Roche Diagnostics, Indianapolis, IN) with a lower limit of quantification of 30 IU/mL and a lower limit of detection of 10 IU/mL.
HBV DNA in liver
Frozen liver samples from the HCC cases and selected controls, where available, were coded and tested for the presence of HBV DNA at the University of Michigan, Ann Arbor in the laboratory of one of the authors (A.S.F.L.). DNA was extracted from liver biopsies using the QIAamp DNA mini kit (QIAGEN, Valencia, CA) and HBV DNA was quantified in a real-time PCR assay, as described previously (8). Each sample was tested in duplicate with two sets of primers and probes (supplementary table 1), one spanning nucleotide (nt) positions 1167–1283 in the HBV polymerase gene and the other nt positions 333–476 in the HBV surface gene (that overlaps with the polymerase gene). To monitor for contamination during each step, sterile double-distilled water and liver specimens from uninfected persons (liver donors who were HBsAg negative and anti-HBc negative with undetectable HBV DNA in serum by PCR assay) were used as negative controls. Each assay also included explant liver from an HBsAg-positive patient who was previously demonstrated to have detectable hepatic HBV DNA as positive control. Quantification of β-actin was used to estimate the amount of genomic DNA and the number of hepatocytes in each liver sample and the amount of HBV DNA was expressed as IU/cell. The lower limit of detection of the assay was 5 IU/mL (8).
To verify reproducibility of the assay results, 6 of 54 samples with negative results in all 4 PCR reactions and 2 of 10 samples with positive results in all 4 PCR reactions were retested and all samples showed 100% concordance on retesting. All 22 samples with positive results in 1–3 PCR reactions were retested and only samples with positive results in at least 6 of 8 PCR reactions (i.e. positive results with both sets of primers) were considered to be positive.
Statistical analyses
Descriptive statistics were reported as number and percent or mean and standard deviation (SD). Baseline characteristics and HBV markers of HCC cases and matched controls were compared with conditional logistic regression for matched pairs. Controls were excluded if data were not available for their case. All analyses were performed at the data coordinating center (New England Research Institutes, Watertown, MA) with SAS statistical software (9.2, SAS Institute, Cary, NC). A 2-sided significance level of 5% was used for all analyses.
Results
Ninety-one HCC cases (definite and presumed) and 182 matched controls were included in this study. Among the HCC cases, three were diagnosed during the lead-in phase of HALT-C, one after achieving a sustained virologic response and the remaining 87 were non-responders. In the latter group, HCC was diagnosed a median of 4.2 (range 0.2–8.5) years after randomization. In comparing HCC cases and controls, those with HCC were older and more likely to have characteristics of more advanced liver disease, including lower platelet counts, lower serum albumin levels, higher bilirubin levels and more frequently had varices (Table 1). Risk factors for HCV infection, estimated duration of HCV infection, alcohol consumption and history of smoking were not different between HCC cases and controls.
Table 1.
Baseline Characteristic of HCC Cases and Controls
| HCC (n=91) | No HCC (n=182) | ||||
|---|---|---|---|---|---|
| Mean/% | SD | Mean/% | SD | P value | |
| DEMOGRAPHICS | |||||
| Age, years | 52.8 | 7.42 | 50.3 | 7.26 | 0.01 |
| Gender, male | 75.8% | 73.6% | 0.70 | ||
| Race | |||||
| White | 64.8% | 73.1% | 0.08 | ||
| Hispanic | 6.6% | 10.4% | |||
| Black | 23.1% | 15.9% | |||
| Other | 5.5% | 0.6% | |||
| VIRAL FACTORS | |||||
| HCV Genotype 1 | 91.2% | 96.1% | 0.09 | ||
| Log HCV RNA (IU/mL) | 6.37 | 0.51 | 6.47 | 0.52 | 0.16 |
| Duration of HCV infection, years | 30.7 | 8.79 | 27.7 | 8.19 | 0.01 |
| LABS | |||||
| Platelets ×1000/mm3 | 126.3 | 49.2 | 162.0 | 61.5 | <.0001 |
| Albumin, g/dL | 3.73 | 0.39 | 3.87 | 0.45 | 0.02 |
| AST, U/L | 112.0 | 63.3 | 91.3 | 68.0 | 0.02 |
| ALT, U/L | 128.2 | 90.6 | 110.0 | 88.9 | 0.13 |
| Alkaline phosphatase, U/L | 118.0 | 57.7 | 103.3 | 50.9 | 0.04 |
| Total bilirubin, mg/dL | 0.90 | 0.44 | 0.81 | 0.39 | 0.10 |
| INR | 1.09 | 0.12 | 1.04 | 0.11 | 0.002 |
| AFP, ng/ml | 27.5 | 31.6 | 14.4 | 24.5 | 0.002 |
| LIVER HISTOLOGY* | |||||
| Cirrhosis | 56.0% | 55.5% | 0.99 | ||
| Fibrosis | 44.0% | 44.5% | |||
| TREATMENT ASSIGNMENT | |||||
| Maintenance peginterferon | 42.5% | 46.7% | 0.99 | ||
| Control | 57.5% | 53.3% | |||
| SMOKING / ALCOHOL | |||||
| Ever smoked | 82.4% | 75.3% | 0.20 | ||
| Lifetime no. of drinks | 18,725 | 23,756 | 20,610 | 36,424 | 0.65 |
| PORTAL HYPERTENSION | |||||
| Esophageal varices on endoscopy | 43.5% | 28.0% | 0.02 | ||
| HEPATITIS C RISK FACTORS | |||||
| Transfusion of blood or blood products | 43.3% | 38.7% | 0.44 | ||
| Occupational exposure to human blood | 24.4% | 24.2% | 0.92 | ||
| Tattoos | 28.6% | 32.4% | 0.51 | ||
| Body piercing | 0.0% | 1.6% | 0.99 | ||
| Injection of recreational drugs | 44.0% | 44.5% | 0.93 | ||
| Snorted cocaine | 51.7% | 56.0% | 0.47 |
Controls were matched on fibrosis stage, treatment assignment, and duration of follow-up P value based on conditional logistic regression analysis
Frozen liver samples were available from 28 of 91 (30.8%) HCC cases and from 55 of 182 (30.2%) controls. Patients (HCC cases and controls) with liver samples were similar to those without liver samples regarding demographics, severity of liver disease, fibrosis stage, and treatment assignment. Among those with liver samples, the baseline characteristics of the cases and controls were similar except for lower serum albumin levels in the HCC cases.
HBV markers in serum
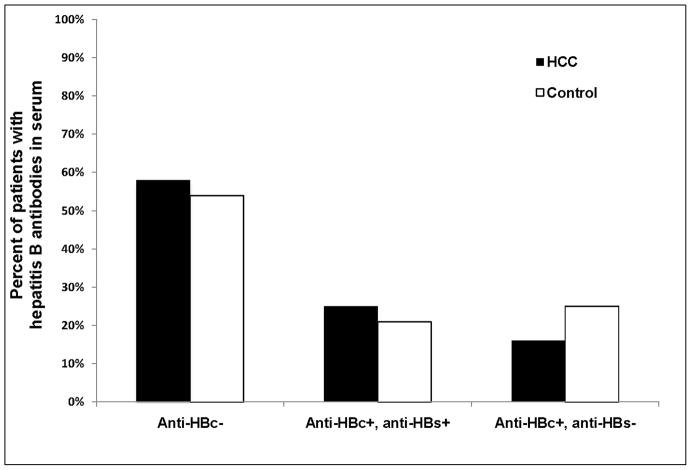
Almost half of the patients had evidence of previous HBV infection; thus 38 of 91 (41.8%) HCC cases and 83 of 182 (45.6%) controls had anti-HBc detectable in their serum (Table 2) (Figure 1). Of these, 15 (16.5%) HCC cases and 45 (24.7%) controls had isolated anti-HBc (without anti-HBs). Compared to patients who were seronegative for anti-HBc, the odds ratio and 95% confidence interval (OR, 95% CI) for HCC development was 0.85 (95% CI 0.51–1.43) for those who were anti-HBc positive (with or without anti-HBs) and 0.63 (95% CI 0.33–1.12) for those who were anti-HBc alone positive. The OR was similar when only patients with definite HCC were evaluated.
Table 2.
HBV Markers in Serum and Liver of HCC Cases and Controls
| HCC Cases | Controls | Odds Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|---|
| HBV antibodies in serum | (N=91) | (N=182) | |||
| Anti-HBc− | 53 (58%) | 99 (54%) | Ref | ||
| Anti-HBc+, anti-HBs+/− | 38 (42%) | 83 (46%) | 0.85 | 0.51–1.43 | 0.54 |
| Anti-HBc+, anti-HBs− | 15 (16%) | 45 (25%) | 0.63 | 0.33–1.12 | 0.12 |
| Anti-HBc+, anti-HBs+ | 23 (25%) | 38 (21%) | 1.13 | 0.60–2.138 | 0.27 |
| HBV DNA in liver | (N=28) | (N=55) | |||
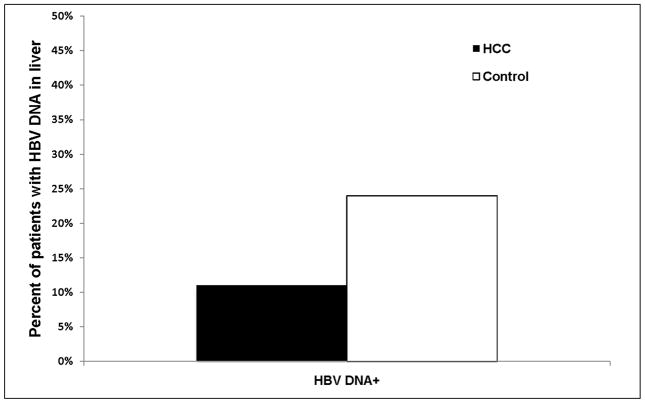
| HBV DNA− | 25 (89%) | 42 (76%) | Ref | ||
| HBV DNA+ | 3 (11%) | 13 (24%) | 0.42 | 0.12–1.52 | 0.18 |
Controls were matched 2:1 to cases for fibrosis strata on baseline biopsy, treatment assignment, and duration of follow-up. Matched odds ratios were determined by logistic regression analyses.
Figure 1.
Prevalence of anti-HBc and anti-HBs in the serum of HCC cases (n=91) and matched controls (n=182)
HBV DNA was detected in the serum of only one (0.4%) patient. This was a control subject with isolated anti-HBc. Serum HBV DNA level was very low (<30 IU/mL) but HBV DNA was detected in the liver
HBV DNA in liver
Three of 28 (10.7%) HCC cases and 13 of 55 (23.6%) controls had HBV DNA detectable in the liver (Figure 2). Compared to the patients with no detectable HBV DNA in the liver, those with HBV DNA in the liver had a matched odds ratio of 0.42 (95% CI 0.12–1.52, P = 0.18) for HCC development (Table 2). Median (range) HBV DNA concentrations in the cases and controls were 0.0047 ± 0.0056 and 0.0267 ± 0.0602 IU/cell, respectively. Two of the three (66.7%) HCC cases and eight of 13 (61.5%) controls with HBV DNA in the liver were anti-HBc positive in serum; of these, one HCC case and four controls had isolated anti-HBc.
Figure 2.
Prevalence of HBV DNA in the liver of HCC cases (n=28) and matched controls (n=55)
Characteristics of Patients with and without HBV Markers
Compared to patients who were anti-HBc negative, those who were anti-HBc positive were more likely to have a history of injection drug use and/or a history of snorting cocaine and less likely to have a history of blood transfusion; anti-HBc positive patients also were shown to have lower serum albumin levels (Table 3). Patients who were anti-HBc positive were also more likely to have a history of being tattooed and a history of body piercing and to be Black than patients who were anti-HBc negative, although these differences were not significant. Stage of hepatic fibrosis, presence of esophageal varices, and estimated duration of HCV infection were similar between those with and without anti-HBc.
Table 3.
Baseline Characteristics of Patients with and without HBV Markers in Serum
| Anti-HBc+ (N=121) | Anti-HBc− (N=152) | P-value* | |||||
|---|---|---|---|---|---|---|---|
| Anti-HBs− (N=60) | Anti-HBs+ (N=61) | ||||||
| Mean/% | SD | Mean/% | SD | Mean/% | SD | ||
| Demographics | |||||||
| Age, years | 50.4 | 5.59 | 51.8 | 7.38 | 51.1 | 8.01 | 0.96 |
| Gender, male | 83.3% | 73.8% | 71.1% | 0.16 | |||
| Race | 0.09 | ||||||
| White | 70.0% | 60.7% | 74.3% | ||||
| Hispanic | 5.0% | 9.8% | 10.5% | ||||
| Black | 23.3% | 24.6% | 13.8% | ||||
| Other | 1.7% | 4.9% | 1.3% | ||||
| Labs | |||||||
| Platelet, × 1000/mm3 | 150.4 | 53.5 | 141.4 | 47.1 | 153.4 | 66.7 | 0.30 |
| Albumin, g/dL | 3.77 | 0.44 | 3.74 | 0.39 | 3.88 | 0.44 | 0.02 |
| AST, U/L | 110.2 | 83.6 | 91.6 | 44.4 | 96.1 | 67.2 | 0.57 |
| ALT, U/L | 126.8 | 105.4 | 110.2 | 63.0 | 114.1 | 92.3 | 0.69 |
| Alkaline phosphatase, U/L | 112.6 | 65.4 | 111.1 | 54.4 | 105.3 | 48.0 | 0.32 |
| T bilirubin, mg/dL | 0.81 | 0.35 | 0.83 | 0.34 | 0.85 | 0.46 | 0.57 |
| INR | 1.07 | 0.11 | 1.06 | 0.11 | 1.05 | 0.13 | 0.17 |
| AFP, ng/mL | 19.0 | 31.9 | 23.4 | 36.4 | 16.8 | 21.1 | 0.20 |
| Cirrhosis | 60.0% | 49.2% | 56.6% | 0.74 | |||
| Maintenance peginterferon | 44.1% | 54.1% | 42.3% | 0.26 | |||
| Esophageal varices | 33.9% | 33.3% | 32.4% | 0.84 | |||
| Risk factors | |||||||
| Transfusion of blood or blood products | 33.9% | 32.8% | 45.7% | 0.04 | |||
| Occupational exposure | 23.3% | 26.2% | 23.8% | 0.86 | |||
| Tattoo | 33.3% | 41.0% | 26.3% | 0.054 | |||
| Body piercing | 0.0% | 4.9% | 0.0% | 0.09 | |||
| Injected recreational drugs | 63.3% | 52.5% | 33.5% | <.0001 | |||
| Snorted cocaine | 73.3% | 57.4% | 46.1% | 0.002 | |||
P-value for comparison of 121 anti-HBc+ vs. 152 anti-HBc- patients
Patients with and without HBV DNA in the liver were similar with regard to demographics, baseline laboratory values, fibrosis stage, presence of esophageal varices, risk factors for HCV infection, and estimated duration of HCV infection (data not shown).
Discussion
This nested case-control study of HBsAg-negative patients from the United States with advanced chronic hepatitis C showed that neither previous (presence of anti-HBc with or without anti-HBs in serum) nor occult (detectable HBV DNA in liver) HBV infection was associated with the development of HCC. In this study, HBV DNA was detected in the liver of 11% of patients with HCC and in 24% of matched controls without HCC (OR for HCC=0.42, 95% CI 0.12–1.52, P = 0.18). In studies from Japan and Italy, the reported frequency of HBV DNA detection in the liver of HBsAg-negative, anti-HCV-positive patients has ranged from 15% (9) to 49% (10) for patients without HCC and up to 73% among patients with HCC (11). Most (4, 12–14) but not all (9, 15–17) studies from Asia and Europe have found that patients with hepatitis C who had detectable HBV DNA in the liver or serum had an increased risk of HCC.
In the current study, 67% of HCC patients and 62% of matched controls with HBV DNA in the liver had anti-HBc in serum, an indication of previous HBV infection. Similarly, studies in Asia and Europe found that most but not all patients with occult HBV infection had markers of previous HBV infection in the serum (4, 10–13, 18, 19). HBV DNA was detected in the liver of 32 of 57 anti-HBc-negative patients with HCC in one study in Italy (4) and in 3 of 4 anti-HBc-negative patients with HCC in a study in Japan (11). Several mechanisms for occult HBV infection have been proposed. First, patients with chronic HBV infection who have undergone spontaneous loss of HBsAg often have persistent HBV DNA in the liver (20). These patients are often positive for anti-HBc only. Second, patients who have recovered from acute HBV infection may have detectable HBV DNA in the liver for many years after HBsAg to anti-HBs seroconversion (21, 22). These patients usually are anti-HBc positive and anti-HBs positive. Third, patients might be chronically infected with HBV variants with decreased HBsAg production or altered HBsAg epitopes leading to false negative results in standard enzyme immunoassays for HBsAg although this situation appears to be rare (23). These patients would be positive for anti-HBc. Fourth, patients co-infected with other hepatitis viruses such as HCV may have suppressed HBV replication as a result of viral interference (24). Finally, some patients may have primary occult HBV infection, i.e., low-dose infection and only a partial induction or a total lack of humoral immunity accounting for the absence not only of HBsAg but also anti-HBc and anti-HBs (25, 26). This phenomenon was first described in woodchucks (25) and confirmed in humans (26). In the latter study, anti-HBc-positive patients but not anti-HBc-negative patients with occult HBV infection showed a T-cell response typical of protective memory.
Nearly half of the patients in this study had anti-HBc in the serum indicating they had been infected with HBV in the past; however, the prevalence of anti-HBc was not different between the HCC cases and the controls: 42% vs. 46% (OR for HCC = 0.85, P = 0.54). Our results differ from the majority of studies from Japan and Italy, which found that HBsAg-negative patients with chronic hepatitis C who were anti-HBc positive are at increased risk for HCC (17, 27). Another difference between our findings and many other studies is the low prevalence of HBV DNA in serum. Only one of 273 patients tested had detectable serum HBV DNA at a very low level and this was observed in a control patient. By contrast, detection rates of low level DNA of up to 78% had been reported in Asian studies (9, 17, 19, 27). Although a higher percent of patients from Asia and southern Europe were anti-HBc positive, the difference in detection rate of HBV DNA in serum between our study and these studies cannot be explained by differences in prevalence of anti-HBc. The lower rate of HBV DNA detection in our patients who were anti-HBc positive may be related to the fact that most of the HALT-C patients likely acquired HBV infection as adults and cleared HBsAg after a transient infection while many of the anti-HBc-positive patients in Italy and Japan likely acquired HBV infection during childhood and did not clear HBsAg until after many years of chronic HBV infection. Several studies from Asia also reported very low rates of HBV DNA detection in serum, 2%–11%, despite a high prevalence of anti-HBc (17, 19).
Data on occult HBV infection and HCC in the United States are limited. Hsia et al. found HBV DNA in the liver of 17 of 31 (54.8%) HBsAg-negative North American patients with HCC; five of these patients were infected with hepatitis C and seven were anti-HBc positive (28). Kannangai et al. detected HBV DNA in the liver of three of 19 (16%) HBsAg-negative patients in the US; only five of the 19 were infected with hepatitis C (29). Shetty et al. found HBV DNA in the liver of 13 of 21 (62%) patients with HCV-related HCC and 9 of 23 (39%) patients with HCV-cirrhosis and no HCC (30). An erratum published by Shetty concluded that occult HBV was not associated with HCC (P = 0.36) (31). Our study differs from these three studies in that very small samples from liver biopsies rather than surgically resected tumors or explant livers were available for testing for HBV DNA. Moreover, the baseline biopsies from our patients were obtained 0.3 to 9.1 years (median 5.15) before the diagnosis of HCC was made while liver samples in the other studies were obtained at the time of HCC diagnosis.
Our study differed from studies in Asia and Europe in several additional respects. First, the prevalence of chronic HBV infection is low in the United States compared to Asia and southern Europe. Thus, the likelihood of detecting markers of previous or occult HBV infection in our patients would be expected to be lower than in patients from Asia or southern Europe. Nevertheless, we found HBV DNA in the liver in 19% and anti-HBc in the serum in 44% of our patients. The relatively high prevalence of occult HBV and previous HBV infection in a country with low endemicity is likely related to the shared risk factors for hepatitis B and hepatitis C. In this study, the controls were carefully matched to the HCC cases in having similar stage of fibrosis on liver biopsy as well as comparable duration of follow-up with no HCC. In prior studies, the frequency of HBV DNA detection increased with more advanced liver fibrosis (16, 18). Thus, studies which compared patients with HCC to those with less advanced fibrosis would be expected to show a more marked difference in HBV DNA or anti-HBc detection between the two groups. Indeed, some HBsAg-negative patients with HBV DNA in the liver or anti-HBc in the serum might have been chronically infected with HBV for decades before spontaneous loss of HBsAg, and the previous chronic HBV infection may have contributed to increased risk of HCC as well as increased risk of liver fibrosis. Several studies have shown that the risk of HCC persists if HBsAg clearance occurred after age 50 or after the development of cirrhosis (20, 32). HBV genotypes in our patients and those in Europe or Asia may also be different.
Our study had several potential limitations. First, the number of patients with HCC was relatively small. Nonetheless, this is the largest such study in the United States with 83 liver and 273 serum samples from patients with chronic HCV infection. Second, the liver tissue and serum samples had been stored for up to 9 years before being tested. However, all the samples had been stored at −70C and had not been subjected to freeze-thaw previously. While it is possible that DNA may have been degraded during long-term storage, serum antibodies should be robust, and there is no reason to expect more rapid DNA degradation in the samples from HCC patients than controls. Third, only 2 to 3 mm of liver tissue was generally available for HBV DNA detection. In many other studies, surgically resected HCC and/or surrounding non-cancerous liver tissue or explant liver were used for HBV DNA detection. It is possible that the HBV DNA detection rate may be higher if larger samples of liver tissue were available but the increase in yield would have to be substantial for us to show a statistically significant difference between patients with or without HCC. Fourth, PCR amplification of DNA from liver samples was performed from only two regions of the HBV genome in this study and both reactions must be positive for the sample to be considered as positive while some of the prior studies performed PCR reactions in three or four regions of the HBV genome and considered samples with positive results in two of three or two of four regions as positive. The likelihood that our method led to a gross under-detection of HBV DNA in the liver is low because other studies have shown that HBV DNA sequences are generally preserved and HBV DNA detection rate is similar with primers in different regions of the HBV genome (3, 4, 33). Fifth, although HALT-C Trial is a prospective study, we performed a case-control study and did not test stored serum and liver samples from all patients in the study. However, the nested case control study used here is an efficient design that allows reasonable inference for the entire HALT-C cohort. Sixth, frozen liver samples were available in only 31% of HCC cases but there was no difference between HCC cases with and without liver samples regarding demographics, severity of liver disease, fibrosis stage, treatment assignment, and risk factors for HCV infection. Finally, despite matching cases and controls for baseline fibrosis stage, the HCC cases were older and had laboratory values suggesting more advanced liver disease.
In summary, patients with HCC in the HALT-C cohort did not have a higher rate of detection of anti-HBc in serum or HBV DNA in liver compared to matched controls with no HCC. Our data suggest that neither previous nor occult HBV infection is an important factor in HCC development among patients with histologically-advanced chronic hepatitis C in the United States.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc. (now Genentech), through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Herbert L. Bonkovsky, MD, Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Jules L. Dienstag, MD, Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01), Gregory T. Everson, MD, Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John C. Hoefs, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) William M. Lee, MD, Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Richard K. Sterling, MD, MSc, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: Marc G. Ghany, MD, T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD, Elizabeth C. Wright, PhD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) Chihiro Morishima, MD, David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Teresa M. Curto, MSW, MPH
Inova Fairfax Hospital, Falls Church, VA: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HCC
hepatocellular carcinoma
- anti-HBc
hepatitis B core antibody
- anti-HBs
hepatitis B surface antibody
- HIV
human immunodeficiency virus
- HCV
hepatitis C virus
- HALT-C
Hepatitis Antiviral Long-term Treatment against hepatitis C
- AFP
alpha fetoprotein
Footnotes
This is publication #68 of the HALT-C Trial.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
Financial Disclosures
Financial relationships of the authors with Hoffmann-La Roche, Inc. (now Genentech), are as follows: A.S. Lok is a consultant and receives research support; A.M. Di Bisceglie is a consultant and receives research support; and T.R. Morgan receives research support. Authors with no financial relationships related to this project are: J.E. Everhart, H.-Y. Kim, and M. Hussain.
Contributor Information
Anna S. Lok, Email: ASLok@umich.edu.
James E. Everhart, Email: EverhartJ@extra.niddk.nih.gov.
Adrian M. Di Bisceglie, Email: dibiscam@slu.edu.
Hae-Young Kim, Email: hkim@neriscience.com.
Munira Hussain, Email: hussain@med.umich.edu.
Timothy R. Morgan, Email: timothy.morgan@va.gov.
References
- 1.Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49(4):652–7. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2(8):479–86. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 3.Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Brechot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? [see comments] Hepatology. 2001;34(1):194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- 4.Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126(1):102–10. doi: 10.1053/j.gastro.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359(23):2429–41. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25(5):472–92. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136(1):138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain M, Soldevila-Pico C, Emre S, Luketic V, Lok AS. Presence of intrahepatic (total and ccc) HBV DNA is not predictive of HBV recurrence after liver transplantation. Liver Transpl. 2007;13(8):1137–44. doi: 10.1002/lt.21179. [DOI] [PubMed] [Google Scholar]
- 9.Obika M, Shinji T, Fujioka S, Terada R, Ryuko H, Lwin AA, et al. Hepatitis B virus DNA in liver tissue and risk for hepatocarcinogenesis in patients with hepatitis C virus-related chronic liver disease. A prospective study. Intervirology. 2008;51(1):59–68. doi: 10.1159/000121363. [DOI] [PubMed] [Google Scholar]
- 10.Koike K, Shimotouno K, Okada S, Okamoto H, Hayashi N, Ueda K, et al. Survey of hepatitis B virus co-infection in hepatitis C virus-infected patients suffering from chronic hepatitis and hepatocellular carcinoma in Japan. Jpn J Cancer Res. 1999;90(11):1270–2. doi: 10.1111/j.1349-7006.1999.tb00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Momosaki S, Nakashima Y, Kojiro M, Tabor E. HBsAg-negative hepatitis B virus infections in hepatitis C virus-associated hepatocellular carcinoma. J Viral Hepat. 2005;12(3):325–9. doi: 10.1111/j.1365-2893.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, et al. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology. 2008;51(5):352–61. doi: 10.1159/000187720. [DOI] [PubMed] [Google Scholar]
- 13.Squadrito G, Pollicino T, Cacciola I, Caccamo G, Villari D, La Masa T, et al. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer. 2006;106(6):1326–30. doi: 10.1002/cncr.21702. [DOI] [PubMed] [Google Scholar]
- 14.Miura Y, Shibuya A, Adachi S, Takeuchi A, Tsuchihashi T, Nakazawa T, et al. Occult hepatitis B virus infection as a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C in whom viral eradication fails. Hepatol Res. 2008;38(6):546–56. doi: 10.1111/j.1872-034X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 15.Shintani Y, Yotsuyanagi H, Moriya K, Fujie H, Tsutsumi T, Takayama T, et al. The significance of hepatitis B virus DNA detected in hepatocellular carcinoma of patients with hepatitis C. Cancer. 2000;88(11):2478–86. [PubMed] [Google Scholar]
- 16.Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol. 2002;40(11):4068–71. doi: 10.1128/JCM.40.11.4068-4071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi S, Shibuya A, Miura Y, Takeuchi A, Nakazawa T, Saigenji K. Impact of occult hepatitis B virus infection and prior hepatitis B virus infection on development of hepatocellular carcinoma in patients with liver cirrhosis due to hepatitis C virus. Scand J Gastroenterol. 2008;43(7):849–56. doi: 10.1080/00365520801935459. [DOI] [PubMed] [Google Scholar]
- 18.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341(1):22–6. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Prevalence of low-level hepatitis B viremia in patients with HBV surface antigen-negative hepatocellular carcinoma with and without hepatitis C virus infection in Japan: analysis by COBAS TaqMan real-time PCR. Intervirology. 2007;50(4):241–4. doi: 10.1159/000101911. [DOI] [PubMed] [Google Scholar]
- 20.Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135(4):1192–9. doi: 10.1053/j.gastro.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2(10):1104–8. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 22.Michalak TI, Pardoe IU, Coffin CS, Churchill ND, Freake DS, Smith P, et al. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29(3):928–38. doi: 10.1002/hep.510290329. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Horikita M, Tsuda F, Itoh K, Akahane Y, Yotsumoto S, et al. Naturally occurring escape mutants of hepatitis B virus with various mutations in the S gene in carriers seropositive for antibody to hepatitis B surface antigen. J Virol. 1994;68(4):2671–6. doi: 10.1128/jvi.68.4.2671-2676.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih CM, Lo SJ, Miyamura T, Chen SY, Lee YH. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol. 1993;67(10):5823–32. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalak TI, Mulrooney PM, Coffin CS. Low doses of hepadnavirus induce infection of the lymphatic system that does not engage the liver. J Virol. 2004;78(4):1730–8. doi: 10.1128/JVI.78.4.1730-1738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zerbini A, Pilli M, Boni C, Fisicaro P, Penna A, Di Vincenzo P, et al. The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology. 2008;134(5):1470–81. doi: 10.1053/j.gastro.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K, Marusawa H, Osaki Y, Nakamura T, Kitajima N, Yamashita Y, et al. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med. 2007;146(9):649–56. doi: 10.7326/0003-4819-146-9-200705010-00008. [DOI] [PubMed] [Google Scholar]
- 28.Hsia CC, Scudamore CH, Di Bisceglie AM, Tabor E. Molecular and serological aspects of HBsAg-negative hepatitis B virus infections in North America. J Med Virol. 2003;70(1):20–6. doi: 10.1002/jmv.10353. [DOI] [PubMed] [Google Scholar]
- 29.Kannangai R, Molmenti E, Arrazola L, Klein A, Choti M, Thomas DL, et al. Occult hepatitis B viral DNA in liver carcinomas from a region with a low prevalence of chronic hepatitis B infection. J Viral Hepat. 2004;11(4):297–301. doi: 10.1111/j.1365-2893.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 30.Shetty K, Hussain M, Nei L, Reddy KR, Lok AS. Prevalence and significance of occult hepatitis B in a liver transplant population with chronic hepatitis C. Liver Transpl. 2008;14(4):534–40. doi: 10.1002/lt.21284. [DOI] [PubMed] [Google Scholar]
- 31.Shetty K, Hussain M, Nei L, Reddy KR, Lok AS. Erratum: Prevalence and significance of occult hepatitis B in a liver transplant population with chronic hepatitis C. Liver Transpl. 2011;17(1):97. doi: 10.1002/lt.21284. [DOI] [PubMed] [Google Scholar]
- 32.Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123(4):1084–9. doi: 10.1053/gast.2002.36026. [DOI] [PubMed] [Google Scholar]
- 33.Pollicino T, Raffa G, Costantino L, Lisa A, Campello C, Squadrito G, et al. Molecular and functional analysis of occult hepatitis B virus isolates from patients with hepatocellular carcinoma. Hepatology. 2007;45(2):277–85. doi: 10.1002/hep.21529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.