Abstract
Background
Neuroticism is a personality trait reflecting the tendency to experience negative affect. It is a major risk for psychopathology, especially depression and anxiety disorders. Childhood maltreatment is another major risk factor for psychopathology and may influence personality. Maltreatment may interact with genotype to predict developmental outcomes. Variation in three polymorphisms of the CRHR1 gene has been found to moderate the association of childhood maltreatment with depression, and we hypothesized that it would also be linked to Neuroticism.
Methods
Variation in three CRHR1 SNPs (rs110402, rs242924, rs7209436) was assessed in 339 maltreated and 275 demographically similar nonmaltreated children, who participated in a day camp research program. Maltreated children were further categorized based on the number of types of maltreatment they had experienced and the most severe form of maltreatment experienced. Genotype and maltreatment status were used to predict the Big Five personality traits, as assessed by camp counselors following a week of interaction with children.
Results
CRHR1 genotype significantly moderated the association of maltreatment with Neuroticism but none of the other traits. Having two copies of the TAT haplotype of CRHR1 was associated with higher levels of Neuroticism among maltreated children relative to nonmaltreated children, with the exception of sexually abused children and children who had experienced 3 or 4 types of abuse. Effects sizes of these interactions ranged from η2 = .01 (p = .02) to η2 = .03 (p = .006).
Conclusions
Variation in CRHR1 moderates the association of maltreatment with Neuroticism. The effects of specific types of maltreatment on Neuroticism are differentially moderated by CRHR1 genotype, as are the effects of experiencing more or fewer types of maltreatment.
Keywords: Neuroticism, CRHR1, maltreatment, genetics, personality, HPA axis
Introduction
Maltreatment in childhood has dramatic developmental consequences, including a large increase in risk for psychopathology throughout life (Cicchetti & Valentino, 2006; Widom, DuMont, & Czaja, 2007). This risk is likely to be mediated by changes in the chronic patterns of emotional, behavioral, and cognitive functioning of the child—in other words, by changes in personality. Indeed, maltreated children display pervasive differences in personality, relative to nonmaltreated children (Cicchetti & Rogosch, 2007; Rogosch & Cicchetti, 2004). However, not every child responds to maltreatment in the same manner, and a crucial project is the identification of risk and protective factors for maladaptive sequelae of maltreatment (Cicchetti, 2010). Increasingly, attention is being focused on genetic variation as a moderator of the effects of maltreatment on risk for psychopathology (e.g., Caspi et al., 2002; Kim-Cohen et al., 2006). The present study examined moderation of the effect of maltreatment on personality by a key gene in the stress reactivity system.
Personality psychology is beginning to make significant advances in the identification of biological systems that underlie different personality traits (DeYoung & Gray, 2009; DeYoung et al., 2010). The most widely used and well validated taxonomy of personality in adulthood is the five-factor model or Big Five (John, Naumann, & Soto, 2008), and this taxonomy appears to provide an effective model of childhood personality as well, especially once it is linked to a theory of the psychological and biological functions that underlie each trait (Caspi & Shiner, 2006; DeYoung, 2010; Shiner & DeYoung, in press). The five dimensions of personality described by the Big Five are Extraversion, Neuroticism, Conscientiousness, Agreeableness, and Openness/Intellect. Maltreatment has been associated with differences in all of these dimensions except Extraversion (Rogosch & Cicchetti, 2004). The current study focused specifically on Neuroticism, which reflects the tendency to experience negative emotion. Neuroticism encompasses a variety of subtraits including anxiety, depression, irritability, self-consciousness, emotional lability, and emotional dysregulation, and it has been linked to the biological substrates of sensitivity to threat and punishment and of emotion regulation (Clark & Watson, 2008; DeYoung & Gray, 2009; DeYoung et al., 2010; Markon, Krueger, & Watson, 2005).
Neuroticism is a risk for most forms of psychopathology, with an especially strong relation to anxiety and depression (Fanous, Neale, Aggen, Kendler, 2007; Malouff, Thorsteinsson, & Schutte, 2005). Further, it accounts for much of the genetic risk for mood disorders (Kendler, Gatz, Gardner, & Pedersen, 2006). One recent study indicated that the economic costs of Neuroticism for society are even greater than the costs of common mental disorders and somatic disorders, in part because Neuroticism is associated not only with those disorders but also with personality disorders and somatoform disorders (Cuijpers et al., 2010). Another recent study suggests that Neuroticism is hard to distinguish statistically from the shared risk factor for mood and anxiety disorders that has been labeled “Internalizing” (Griffith et al., 2009). The biological systems involved in Neuroticism are thus of great importance in the etiology of internalizing disorders. Reactivity to stress is a major characteristic of Neuroticism, and the biological systems responsible for stress reactivity have been associated with Neuroticism, primarily through studies of the stress hormone cortisol (DeYoung & Gray, 2009). Genes involved in the systems that respond to stress are thus important candidates for studies of the genetic sources of Neuroticism, as well as for studies of genetic moderation of the effects of major stressors like maltreatment. The present study examined the association of Neuroticism with variation in a gene that plays an important role in the hypothalamic-pituitary-adrenal (HPA) axis, the major biological system for stress response.
Corticotropin-releasing hormone is the key activator of the HPA axis, binding to receptors that initiate the stress response, culminating with release of cortisol from the adrenal cortex. Recently, variation in the corticotropin-releasing hormone receptor 1 gene (CRHR1) has been linked to risk for depression, in the presence of childhood maltreatment (Bradley et al., 2008; Polanczyk et al., 2009). The tendency to experience depressed mood is one facet of Neuroticism (Costa & McCrae, 1992), and Neuroticism is a major risk for clinical diagnosis of depression (Griffith et al., 2009). Any change in risk for depression, caused by environmental stressors such as childhood maltreatment, is likely to be mediated by increased Neuroticism. The present study therefore undertook to examine the influence of CRHR1 variation on Neuroticism in interaction with maltreatment, in a large sample of maltreated children and a well matched nonmaltreated comparison group.
Two previous studies have reported moderation of the effects of maltreatment on depression by a three-allele haplotype of CRHR1, involving the SNPs rs7209436, rs110402, and rs242924. In both studies, the TAT haplotype was protective against depression for individuals who were severely maltreated (Bradley et al., 2008; Polanczyk et al., 2009). These findings led us to predict that the TAT haplotype might be associated with lower Neuroticism in severely maltreated children.
Additionally, in our sample we were able to address some of the limitations of the previous studies. Most importantly, the effect of CRHR1 in those studies was found in only three of the four samples examined. Of particular interest is the fact that the three samples producing the effect all used the same retrospective assessment of maltreatment, the Childhood Trauma Questionnaire (CTQ; Bernstein & Fink, 1998), whereas the one failure to replicate did not use the CTQ, but rather used a combination of prospective and retrospective assessments (Polanczyk et al., 2009). This failure to replicate is particularly notable because it occurred in a large, representative population sample, and thus is unlikely to be due to sampling variability or lack of power. This suggests that the gene by environment (GxE) interaction effect may be dependent in some way on the manner in which maltreatment is assessed.
Further, it is possible that the type of abuse constituting maltreatement is important in determining the effect of CRHR1 on depression. Previous studies have found that different types of maltreatment can have strikingly different outcomes, particularly in relation to functions of the HPA axis. Studies of cortisol levels in maltreated children have found different effects based on the presence or absence of physical or sexual abuse relative to other forms of maltreatment (Cicchetti & Rogosch, 2001, 2007; Cicchetti, Rogosch, Gunnar, & Toth, 2010). The present study therefore examined individuals according to whether their most severe form of maltreatment had been emotional maltreatment or neglect, physical abuse, or sexual abuse, using a sample that surmounted some of the limitations of previous CRHR1 studies in several ways. First, the sample was composed of maltreated children and closely matched non-maltreated children from the same demographic, socioeconomic, and geographic background. Second, children were assessed for maltreatment based on information obtained during childhood, rather than retrospectively as adults. Third, maltreatment was assessed from records of the Department of Human Services (DHS) using a rigorous coding scheme developed by Barnett, Manly, and Cicchetti (1993). Because of the different patterns of HPA axis dysfunction that have been seen in different subtypes of maltreatment, we hypothesized that subtype, in addition to general severity of maltreatment without regard to subtype, might be an important moderator of the effects of CRHR1 on Neuroticism.
To test this hypothesis, we performed three analyses, characterizing maltreatment in three different ways. First, all maltreated children were compared together against the nonmaltreated comparison group. Second, maltreatment was broken down according to whether children had experienced 1 or 2 forms of maltreatment (some maltreatment) or 3 or 4 forms of maltreatment (severe maltreatment). This procedure is directly analogous to the procedure used by Polanczyk et al. (2009) to determine the severity of maltreatment. Third, maltreatment was broken down according to the type of maltreatment received. Children were classified according to whether they had (a) experienced emotional maltreatment and/or neglect only; (b) experienced physical abuse (with or without emotional abuse or neglect); or (c) experienced sexual abuse (with or without emotional abuse, neglect, or physical abuse). Finally, because relatively few children had been sexually abused, and because sexual abuse was associated with a distinctive pattern of Neuroticism scores, we examined effects after excluding sexually abused children.
Materials and Methods
Participants
Participants were 624 children from an urban setting in upstate New York, who attended a day camp research program. Ten children were not assessed for personality, leaving 614 for the present analysis. Of this sample, 339 were maltreated. Children ranged in age from 8 to 13 years (mean = 11.3, SD = 1.0). The sample was ethnically and racially diverse, with 68% Black, 10% White, 18% Hispanic, and 4% other. The Add Health system for coding race and ethnicity was used (www.cpc.unc.edu/projects/addhealth/data/code/race; accessed Apr. 11, 2010), with the exception that “American Indian” was coded as “other” because only two participants were identified as such.
The day camp program was designed for comparison of developmental processes and functioning in maltreated and nonmaltreated, low-income, disadvantaged children (Cicchetti & Manly, 1990). A liaison from the DHS contacted families with a child meeting research criteria, provided information about the camp and associated research, and asked families for written permission to have their names released to project staff. (Due to confidentiality, the DHS liaison was not able to provide information regarding families who were not interested in participation.) Subsequently, parents of all participating children provided informed consent for their child’s participation, as well as consent for examination of any DHS records associated with the family; children provided assent. Children attended the camp free of charge and received small prizes for completing research measures; mothers received compensation ($25) for completing a research interview. The procedures in this investigation were approved by the Research Subjects Review Board of the University of Rochester.
Children in the maltreated group were recruited based on DHS records indicating they had experienced maltreatment. Those in the nonmaltreated group did not have records of maltreatment and were additionally screened through checks of the child abuse registry and interviews with their mothers utilizing the Maternal Maltreatment Classification Interview (Cicchetti, Toth, & Manly, 2003) to verify lack of DHS involvement and absence of maltreatment experiences. Families who received preventive services through DHS due to concerns over risk for maltreatment were excluded from the study to avoid inclusion of children with unidentified maltreatment in the comparison group.
Children attended a week-long day camp program and participated in research assessments. At the camp, children were assigned to groups of eight (4 maltreated, 4 nonmaltreated) same-age and same-sex peers. Each group was led by three trained camp counselors, who were unaware of the maltreatment status of children and the hypotheses of the study. Camp lasted 7 hrs/day for five days, providing 35 hours of interaction between children and counselors.
Maltreatment
Descriptions of maltreatment in DHS records were used to identify, for each child, the presence of sexual abuse, physical abuse, neglect, and/or emotional maltreatment. Trained raters coded DHS records using the operational criteria of the Maltreatment Classification System (Barnett et al., 1993), a well-validated approach for classifying maltreatment experiences. Among the maltreated children, 8.6% had experienced sexual abuse, 28.6% physical abuse, 78.5% neglect, and 52.2% emotional maltreatment; most children (59.1%) had experienced more than one type of maltreatment. Given the lower occurrence of sexual abuse and physical abuse, as well as differences in the extent to which different types of maltreatment violate societal norms (Manly, Cicchetti, & Barnett, 1994), a hierarchical ranking was used to designate a primary maltreatment subtype for each child. This hierarchy ranks four subtypes of abuse from most to least violative, in the following order: sexual abuse, physical abuse, neglect, emotional maltreatment. Children were assigned a primary subtype based on the most severe violation of societal norms they had experienced. Thus, children who experienced sexual abuse were classified as sexually abused regardless of whether they additionally experienced other forms of maltreatment; children who were physically abused without sexual abuse were classified as physically abused, and all remaining children were classified in a neglect/emotional maltreatment group. Of the maltreated children, 29 (8.6%; 20 girls, 9 boys) were classified as sexually abused, 82 (24.2%; 36 girls, 46 boys) were classified as physically abused, and 206 (60.8%; 105 girls, 101 boys) were classified in the neglect/emotional maltreatment group (without physical or sexual abuse). The majority of children in the sexual and physical abuse groups also had been neglected or emotionally maltreated. For 22 maltreated participants, the DHS information was not sufficiently complete to code the subtype of maltreatment; these children were excluded from analyses using maltreatment subgroups. Inter-rater agreement (kappa) for the presence of each subtype ranged from .72 to 1.0.
Personality
The Big Five personality traits were assessed using two instruments: the Big Five scales derived from the California Child Q-sort (CCQ; John, Caspi, Robins, Moffitt, & Stouthamer-Loeber, 1994) and a set of 46 trait descriptive adjectives (TDA) designed for assessment of the Big Five in children (Hagekull & Bohlin, 1998). The CCQ comprises 100 personality descriptive items that are sorted according to a fixed distribution into 9 categories, representing the degree to which each is characteristic of the child. The TDA comprises 46 items rated on a 5-point Likert scale. Each of these two instruments was completed by two adult camp counselors, trained in use of the instruments but unaware of research hypotheses and maltreatment status, after a week (35 hours) of extensive observation and interaction with participants. Interrater agreement was high, with the average intraclass correlation among pairs of raters ranging from .85 to .87 for the CCQ and from .74 to .89 for the TDA scales. Ratings for each item by each of the two raters were averaged before deriving scale scores for each instrument.
Big Five scores from the CCQ and TDA were standardized separately in order to combine scores across the two instruments. The standardized scores were then averaged, restandardized, and recentered by adding 1 (recentering was performed for clarity of graphical representation). Composite scores from these two inventories were very reliable, with Cronbach’s Alphas as follows: Extraversion: .95 (18 items), Agreeableness: .96 (25 items), Conscientiousness: .91 (18 items), Neuroticism: .90 (20 items), Openness/Intellect: .75 (10 items). (The lower Alpha for Openness/Intellect is attributable to its relatively fewer items.) Three items (one each from Agreeableness, Conscientiousness, and Openness/Intellect) were excluded from the calculation of trait scores because their correlations with the scale total (calculated without the item in question) were near zero and their inclusion reduced Cronbach’s Alpha. Scores calculated without these items correlated at .99 or higher with scores including them.
Items in the Neuroticism scale from the CCQ were “Is nervous and fearful”; “Worries about things for a long time”; “Freezes up when things are stressful, or else keeps doing the same thing over and over”; “Can bounce back or recover after a stressful or bad experience” (reversed); “Tends to go to pieces under stress; gets rattled when things are tough”; “Needs to have people tell him or her that he or she is doing well or ok; is not very sure of him- or herself”; “Tends to get sick when things go wrong or when there is a lot of stress. (For example, gets headaches, stomach aches, throws up.)”; “Gets nervous if he or she is not sure what’s going to happen or when it’s not clear what he or she supposed to do”; “Feels unworthy; has a low opinion of him- or herself”; and “His or her feelings get hurt easily if he or she is made fun of or criticized.” Items in the Neuroticism scale from the TDA were “nervous,” “tense,” “anxious,” “worries about things,” “fearful,” “relaxed” (reversed), “content” (reversed), “self-confident” (reversed), “oversensitive,” and “calm and stable” (reversed). Clearly, there is partial but not complete overlap between Neuroticism and symptoms of depression. As noted above, Neuroticism shows stronger overlap with the more general Internalizing construct than with depression specifically (Griffith et al., 2009).
Genotyping
Human genomic DNA was collected from all children using the Buccal Amp Kit (Epicentre, Cat. No. BQ0901SSC) and amplified using the Repli-g kit (Qiagen, Catalog No. 150043) per the kit instructions. DNA was whole-genome amplified to ensure the availability of data over the long term for this valuable sample. Amplified samples were then diluted to a working concentration and genotyped using assays for SNPs rs110402, rs242924, and rs7209436 purchased from Applied Biosystems, Inc. (ABI) as C 2544843 10, C2257689 10, and C 1570087 10, respectively. Individual allele determinations were made using TaqMan Genotyping Master Mix (Applied Biosystems, Catalog 4371357) with amplification in an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200.
If a genotype was unable to be determined after the first run, then it was repeated up to 4 times. If the null result persisted, then the whole-genome amplification reaction was repeated along with subsequent genotyping until a genotype could be confidently assigned to a participant. The resultant genotyping data were subjected to quadratic discriminant analysis using JMP statistical software from SAS. Samples with a predicted probability of 0.95 or less were repeated. The call rates for the 3 SNPs for CRHR1 were all 100% determined. There were no missing results.
Control samples were identified for these CRHR1 polymorphisms using DTCS chemistry on an ABI 3130xl. These results were confirmed using the TaqMan SNP genotyping reagents used in this study and run with each sample set as positive and negative controls for each allele.
None of the three SNPs deviated significantly from Hardy-Weinberg equilibrium (rs110402: χ2(1) = 0.10, p = .75; rs242924: χ2(1) = 0.00, p = .98; and rs7209436: χ2(1) = 0.15, p = .70). Haplotypes for the three SNPs were determined using Haplo Stats 1.4.0 (Sinnwell & Schaid, 2008). Because the three SNPs were very strongly correlated (all r > .94) Haplo Stats was able to estimate haplotypes for every participant with a posterior probability greater than .998, which allowed us to assign a score of 0, 1, or 2 copies of the TAT haplotype to every participant with a very high degree of certainty. The TAT haplotype accounted for 32% of all haplotypes in the sample, with its complement, CGG, accounting for an additional 65%. Number of TAT haplotypes was used in ANOVA to predict Neuroticism from haplotype score in interaction with maltreatment status.
Table 1 shows allele and haplotype frequencies for the sample, comparing maltreated and nonmaltreated children. The two groups did not differ by genotype (number of TAT haplotypes), χ2(2) = 1.05, p = . 59, which indicates absence of gene-environment correlation. In other words, CRHR1 genotype did not influence the likelihood that children would be maltreated.
Table 1.
Genotype and haplotype frequencies for three CRHR1 SNPs by maltreatment status.
| Maltreated (N = 339) |
Nonmaltreated (N = 275) |
|
|---|---|---|
| rs7209436 | ||
| CC | 157 (46%) | 110 (40%) |
| CT | 146 (45%) | 133 (48%) |
| TT | 36 (11%) | 32 (12%) |
| rs110402 | ||
| GG | 155 (45%) | 115 (42%) |
| AG | 150 (45%) | 127 (46%) |
| AA | 34 (10%) | 33 (12%) |
| rs242924 | ||
| GG | 160 (47%) | 117 (43%) |
| GT | 146 (43%) | 125 (45%) |
| TT | 33 (10%) | 33 (12%) |
| TAT haplotype | ||
| 0 copies | 164 (48%) | 120 (44%) |
| 1 copy | 142 (42%) | 124 (45%) |
| 2 copies | 33 (10%) | 31 (11%) |
Results
Table 2 shows genotypes according to maltreatment status, sex, and genotype. Sex was not significantly associated with number of TAT haplotypes, χ2(2) = 0.71, p = .70, or with most severe type of maltreatment χ2(3) = 5.44, p = .14, or with Neuroticism, F(1) = 0.01, p = .94. Race was not significantly associated with genotype, χ2(6) = 9.73, p = .14, or with Neuroticism, F(3) = 1.39, p = .24. However, race was significantly associated with most severe type of maltreatment, χ2(6) = 28.50, p = .001, which made it important to control for race in all analyses. Our third covariate was age, which was not associated with genotype, F(2) = 0.18, p = .98, but was marginally associated with Neuroticism, r = − .07, p = .09, and type of maltreatment, F(3) = 2.26, p = .08 (children who had experienced physical and/or sexual abuse were slightly older on average those who had experienced no maltreatment or only emotional maltreatment and/or neglect).
Table 2.
CRHR1 TAT haplotype frequencies by sex and race/ethnicity.
| Maltreated (N = 339) | Nonmaltreated (N = 275) | |||||
|---|---|---|---|---|---|---|
| TAT 0 | TAT 1 | TAT 2 | TAT 0 | TAT 1 | TAT 2 | |
| Sex | ||||||
| Female | 78 | 70 | 21 | 71 | 61 | 10 |
| Male | 86 | 72 | 12 | 49 | 63 | 21 |
| Race/Ethnicity | ||||||
| Black | 120 | 96 | 18 | 83 | 83 | 18 |
| White | 15 | 20 | 6 | 6 | 14 | 3 |
| Hispanic | 22 | 20 | 7 | 27 | 24 | 9 |
| Other | 7 | 6 | 2 | 4 | 3 | 1 |
Table 3 shows means and standard deviations for the Big Five in each group. Consistent with previous research (Rogosch & Cicchetti, 2004), maltreated children exhibited higher Neuroticism and lower Agreeableness, Conscientiousness, and Openness/Intellect than nonmaltreated children.
Table 3.
Means and standard deviations of the Big Five in maltreated and nonmaltreated children
| Maltreated (N = 339) |
Nonmaltreated (N = 275) |
||||||
|---|---|---|---|---|---|---|---|
| Maltreated (N = 339) |
Nonmaltreated (N = 275) |
||||||
| Mean | SD | Mean | SD | t(612) | p | d | |
| Neuroticism | 1.10 | 1.03 | 0.88 | 0.95 | 2.70 | .007 | 0.22 |
| Extraversion | 1.02 | 1.01 | 0.97 | 0.98 | 0.66 | .51 | 0.05 |
| Agreeableness | 0.83 | 1.02 | 1.21 | 0.94 | −4.72 | .000 | 0.39 |
| Conscientiousness | 0.82 | 1.02 | 1.22 | 0.94 | −4.95 | .000 | 0.39 |
| Openness/Intellect | 0.92 | 0.97 | 1.10 | 1.02 | −2.13 | .03 | 0.17 |
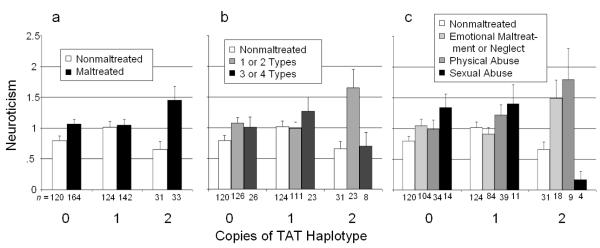
To assess the effects of GxE interactions on Neuroticism, we completed a series of three ANOVAs, controlling for age, sex, and race. (We also examined potential moderation by sex; sex did not significantly moderate any of the effects.) In the first GxE analysis (Table 4), maltreatment status was coded simply as maltreated versus nonmaltreated. The interaction of maltreatment with CRHR1 genotype was significant, and maltreated children with two copies of the TAT haplotype showed higher Neuroticism than all other groups (Figure 1a).
Table 4.
Analysis of Variance: Effects of CRHR1 TAT haplotypes and maltreatment on Neuroticism, using three different coding schemes for maltreatment
| Maltreated vs. Nonmaltreated |
Number of Types of Maltreatment |
Maltreatment Subtype | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | p | η 2 | F | df | p | η 2 | F | df | p | η 2 | |
| Age | 3.20 | 1 | .07 | .005 | 3.27 | 1 | .07 | .005 | 4.03 | 1 | .05 | .007 |
| Sex | 0.01 | 1 | .94 | .000 | 0.07 | 1 | .79 | .000 | 0.01 | 1 | .91 | .000 |
| Race | 1.14 | 3 | .33 | .005 | 1.49 | 3 | .22 | .007 | 1.40 | 3 | .24 | .007 |
| Maltreatment | 12.66 | 1 | .000 | .020 | 6.81 | 2 | .001 | .022 | 4.77 | 3 | .003 | .023 |
|
CHRH1 TAT haplotypes |
0.84 | 2 | .43 | .003 | 0.61 | 2 | .54 | .002 | 0.23 | 2 | .80 | .001 |
| Maltreatment × TAT haplotypes |
3.80 | 2 | .02 | .012 | 3.44 | 4 | .01 | .022 | 3.05 | 6 | .006 | .030 |
Figure 1.
Level of Neuroticism (with standard error of the mean) associated with maltreatment, depending on number of TAT haplotypes of the CRHR1 gene. (a) Maltreated children compared to nonmaltreated children. (b) Maltreatment categorized by number of types of maltreatment experienced. (c) Maltreatment categorized by most severe type of maltreatment experienced.
In the second analysis (Table 4), maltreatment status was coded according to number of types of maltreatment, as in previous research (Polanczyk et al., 2009). Three or 4 types of maltreatment were considered most severe, followed by 1 or 2 types of maltreatment, and finally no maltreatment. Here, too, the interaction of maltreatment with CRHR1 genotype was significant. The TAT haplotype was associated with greater Neuroticism in the presence of 1 or 2 types of maltreatment but not in the presence of 3 or 4 types (Figure 1b). Thus, the TAT haplotype appeared to be protective in severe maltreatment, as shown in previous research (Bradley et al., 2008; Polanczyk et al., 2009).
In the third analysis (Table 4), maltreatment status was coded as sexual abuse, physical abuse, neglect/emotional maltreatment, or no maltreatment. Once again the interaction of maltreatment with CRHR1 genotype was significant.1 As can be seen in Figure 1c, having two copies of the TAT haplotype was associated with different levels of Neuroticism depending on the maltreatment subtype. Children with two copies of the TAT haplotype who were physically abused showed the highest levels of Neuroticism, whereas children with two copies of the TAT haplotype who were sexually abused showed the lowest levels of Neuroticism, lower even than nonmaltreated children.
As a test of discriminant validity, we performed the same set of ANOVAs described above, using the other Big Five traits as outcome variables. None of them were significantly associated with CRHR1 variation, either as a main effect or in interaction with maltreatment. The CRHR1 GxE effect thus appears to be specific to Neuroticism.
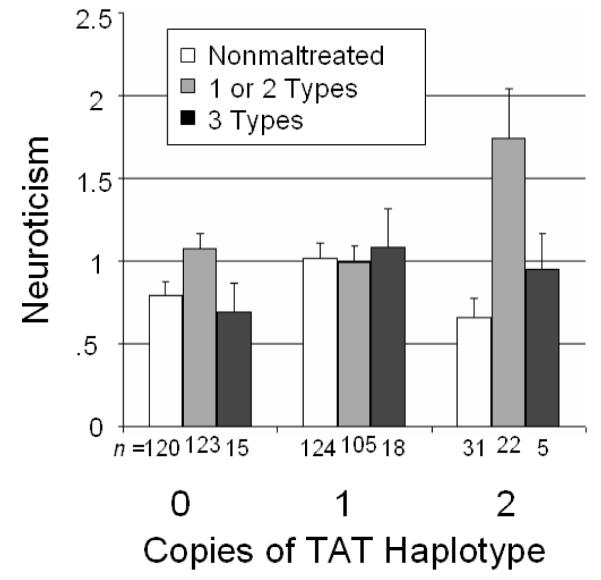
Because the number of sexually abused children in our sample was small, and because sexual abuse showed such a strikingly different pattern of interaction with CRHR1 than did other forms of maltreatment, we performed several post hoc analyses. First, we tested whether number of TAT haplotypes was significantly associated with Neuroticism just within the group of 29 sexually abused children (controlling for age, sex, and race), and found that it was, F(2) = 4.29, p = .027. Second we repeated the ANOVAs described above for number of subtypes and most severe subtype of maltreatment, after excluding all sexually abused children. The results of these analyses are presented in Table 5 and Figure 2 (the analysis of subtypes presented in Figure 1c is not represented in Figure 2 because means for other groups remained the same after exclusion of the sexually abused group). Interactions between CRHR1 and maltreatment remained significant in both.
Table 5.
Post hoc analysis of variance: Effects of CRHR1 TAT haplotypes and maltreatment on Neuroticism, excluding sexually abused children.
| Number of Types of Maltreatment |
Maltreatment Subtype | |||||||
|---|---|---|---|---|---|---|---|---|
| F | df | p | η 2 | F | df | p | η 2 | |
| Age | 3.51 | 1 | .06 | .006 | 4.31 | 1 | .04 | .007 |
| Sex | 0.21 | 1 | .65 | .000 | 0.08 | 1 | .78 | .000 |
| Race | 1.78 | 3 | .15 | .009 | 1.49 | 3 | .16 | .009 |
| Maltreatment | 7.54 | 2 | .001 | .026 | 6.88 | 2 | .001 | .023 |
|
CHRH1 TAT haplotypes |
1.24 | 2 | .29 | .004 | 2.53 | 2 | .08 | .009 |
| Maltreatment × TAT haplotypes |
3.36 | 4 | .01 | .023 | 3.04 | 4 | .02 | .021 |
Figure 2.
Level of Neuroticism (with standard error of the mean) associated with number of types of maltreatment experienced, depending on number of TAT haplotypes of the CRHR1 gene, excluding sexually abused participants.
Discussion
The association of childhood maltreatment with Neuroticism was found to be moderated by variation in the CRHR1 gene. Children with two copies of the TAT haplotype had different levels of Neuroticism depending on whether they had been maltreated or not and on the most severe type and the number of types of maltreatment they had experienced. This GxE interaction accounted for between 1 and 3 percent of the variance in Neuroticism. This is not a large effect, but it is fairly typical for size of effect of variation in single genes on strongly polygenic traits like Neuroticism or depression. Because CRHR1 produces the corticotropin-releasing hormone receptor, a key component of the HPA axis, this finding is consistent with the theory that the HPA axis is an important biological substrate of Neuroticism (DeYoung & Gray, 2009).
When maltreatment was categorized according to the number of types of maltreatment that had been experienced (as it was previously in two studies of CRHR1 and depression; Bradley et al., 2008; Polanczyk et al., 2009), having two copies of the TAT haplotype was associated with heightened levels of Neuroticism in children who had experienced 1 or 2 types of maltreatment, but not among those who had experienced 3 or 4 types of maltreatment. The fact that children who had experienced 3 or 4 types of maltreatment had levels of Neuroticism similar to those of the nonmaltreated group (Figure 1b) is reminiscent of the protective effect of the TAT haplotype on depression seen in previous research (Bradley et al., 2008; Polanczyk et al., 2009).
A rather different picture emerged, however, when maltreatment was categorized in terms of the most severe subtype of maltreatment experienced. Children with two copies of the TAT haplotype who had been emotionally maltreated, neglected, or physically abused had levels of Neuroticism considerably higher than the nonmaltreated comparison group, whereas those who had been sexually abused (often in addition to other forms of maltreatment) had levels of Neuroticism lower than the comparison group (Figure 1c). Although the sexually abused group was small, post hoc analysis showed that CRHR1 genotype significantly predicted Neuroticism in this group alone. Further, when the sexually abused group was excluded from analyses, the GxE interaction remained significant, indicating higher levels of Neuroticism for abused children with two copies of the TAT haplotype, but normal levels for those who had experience 3 types of abuse.
Our results suggest that having two copies of the TAT haplotype puts maltreated children at risk for higher levels of Neuroticism (and hence at greater risk for depression and other internalizing disorders), unless they have been sexually abused or have experienced 3 or 4 different types of maltreatment, in which case they may be protected from increased Neuroticism. The fact that both number of types and most severe type of maltreatment interacted with CRHR1 genotype to predict Neuroticism may be informative for studies of CRHR1 and depression. Such studies have been inconsistent in finding that the TAT haplotype is protective against depression in adults who experienced 3 or 4 types of abuse as children (Polanczyk et al., 2009). This inconsistency may reflect the possibility that most severe type of maltreatment, as well as number of types of maltreatment, is important in determining the effects of the TAT haplotype. It also may reflect the possibility that whether the TAT haplotype is a protective or risk factor depends on the exact nature of the maltreatment experienced.
The present findings raise a number of questions for future research. Why CRHR1 should be protective for individuals who experience 3 or 4 types of abuse but not 1 or 2 certainly warrants further study. Why sexual abuse should have unique effects, in interaction with genotype, is also an important question. Previous research has shown differences in HPA axis dysfunction among maltreated children depending on whether or not they have been sexually abused (Cicchetti & Rogosch, 2001, 2007; Cicchetti, Rogosch, Gunnar, & Toth, 2010). These differences may involve the corticotropin-releasing hormone receptor produced by CRHR1.
One limitation of the present study is the relatively small number of sexually abused children (four) with two copies of the TAT haplotype. The small size of this subgroup greatly limits ability to generalize from this finding to other sexually abused children and suggests that our results should be replicated before concluding that sexually abused children with this genotype show particularly low levels of Neuroticism. Notably, however, the standard error of the mean for this group was not large, indicating that all four children in this group were consistent in their level of Neuroticism. Regardless of conclusions regarding this small subgroup, our results indicate that increased Neuroticism associated with the TAT haplotype is not present in all maltreatment conditions.
Several issues must be considered when comparing our findings to research on depression in adults. Most obvious is that Neuroticism is not identical to depression, despite its relation to the general risk for depression and other internalizing disorders (Griffith et al., 2009). Future studies should directly examine the effects of CRHR1 on Neuroticism in adults and on depression in children. Additionally, HPA-axis function changes over time in chronically stressed individuals (Gunnar, & Vazquez, 2001; Miller, Chen, & Zhou, 2007), which could affect the influence of CRHR1 and render genetic effects in children different from effects in adults. Longitudinal effects should be considered in future research on the interaction of CRHR1 genotype with maltreatment. Finally, previous research has been in more racially homogeneous samples, whereas our sample was of diverse ethnic and racial background, which complicates genetic research. However, population stratification is unlikely to be responsible for our results, given that effects of CRHR1 on depression have previously been found in both White and African-American samples (Bradley et al., 2008; Polanczyk et al., 2009), that race was not associated with either CRHR1 genotype or Neuroticism, and that all of our analyses controlled for race.
Conclusion
Childhood maltreatment is a serious problem with extreme social and personal costs. Maltreatment typically leads to less resilient functioning and increased risk for many forms of psychopathology (Cicchetti & Rogosch, 2007; Cicchetti & Valentino, 2006; Widom, DuMont, & Czaja, 2007). However, the sequelae of maltreatment are manifest in different ways in different children, and the influence of genetic variation on response to maltreatment is a crucial topic for research. Genes involved in the HPA axis, the primary biological system for response to stress, are promising candidates for moderators of the effects of maltreatment on personality and psychopathology. The current study contributes to a growing body of evidence that variation in the CRHR1 gene moderates the effects of childhood maltreatment. It also provides evidence that the personality trait Neuroticism is associated with genetic variation related to the HPA axis.
Acknowledgments
This article was supported by grants awarded to Dante Cicchetti and Fred A. Rogosch from the National Institute on Drug Abuse (DA12903, DA17741) and the Spunk Fund, Inc. We would like to thank the children, families, counselors, and research staff at the Mt. Hope Family Center, Rochester, New York, who participated in this work.
Abbreviations
- CRHR1
corticotropin-releasing hormone receptor 1
- CTQ
Childhood Trauma Questionnaire
- CCQ
California Child Q-Sort
- TDA
trait descriptive adjectives
- DHS
Department of Human Services
Footnotes
The interaction effects for number of types and most severe type of maltreatment survived Bonferroni correction, and the interaction effect for the comparison of maltreated vs nonmaltreated was marginal when corrected (p = .07). However, a standard Bonferroni correction is strongly overly conservative in this case because the three tests were extremely non-independent, as all three simply reconfigure the maltreatment variable. Nonetheless, the p-values are small enough that two survive even the overly conservative correction.
The authors report no conflicts of interest.
Contributor Information
Colin G. DeYoung, University of Minnesota
Dante Cicchetti, Mt. Hope Family Center, University of Rochester and University of Minnesota
Fred A. Rogosch, Mt. Hope Family Center, University of Rochester
References
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Ablex; Norwood, NJ: 1993. pp. 7–73. [Google Scholar]
- Bernstein D, Fink L. Childhood Trauma Questionnaire Manual. The Psychological Corp.; San Antonio, TX: 1998. [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Shiner RL. Personality development. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology, Vol. 3. Social, emotional, and personality development. 6th ed Wiley; New York: 2006. pp. 300–365. [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective [Special Article] World Psychiatry. 2010;9:1–10. doi: 10.1002/j.2051-5545.2010.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of family research: Families at risk. Vol. 2. Erlbaum; Hillsdale, NJ: 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Development and Psychopathology. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, Manly JT. Maternal Maltreatment Interview. Rochester, NY: 2003. Unpublished manuscript. [Google Scholar]
- Cicchetti D, Valentino K. An ecological transactional perspective on child maltreatment: Failure of the average expectable environment and its influence upon child development. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2nd ed Vol. 3. Wiley; New York: 2006. pp. 129–201. [Google Scholar]
- Clark LA, Watson D. Temperament: An organizing paradigm for trait psychology. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. Guilford Press; New York: 2008. pp. 265–286. [Google Scholar]
- Costa PT, Jr., McCrae RR. Four ways five factors are basic. Personality and Individual Differences. 1992;13:653–665. [Google Scholar]
- Cuijpers P, Smit F, Penninx BWJH, de Graaf R, ten Have M, Beekman ATF. Economic costs of Neuroticism: A population-based study. Archives of General Psychiatry. 2010;67:1086–1093. doi: 10.1001/archgenpsychiatry.2010.130. [DOI] [PubMed] [Google Scholar]
- DeYoung CG. Toward a theory of the Big Five. Psychological Inquiry. 2010;21:26–33. [Google Scholar]
- DeYoung CG, Gray JR. Personality neuroscience: Explaining individual differences in affect, behavior, and cognition. In: Corr PJ, Matthews G, editors. The Cambridge handbook of personality psychology. Cambridge University Press; New York: 2009. pp. 323–346. [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience: Brain structure and the Big Five. Psychological Science. 2010;21 doi: 10.1177/0956797610370159. Epub ahead of print, 2010 Apr 30, doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Aggen SH, Kendler KS. A longitudinal study of personality and major depression in a population-based sample of male twins. Psychological Medicine. 2007;37:1163–1172. doi: 10.1017/S0033291707000244. [DOI] [PubMed] [Google Scholar]
- Griffith JW, Zinbarg RE, Craske MG, Mineka S, Rose RD, Waters AM, Sutton JM. Neuroticism as a common dimension in the internalizing disorders. Psychological Medicine. 2009 doi: 10.1017/S0033291709991449. e-Pub ahead of print. doi:10.1017/S0033291709991449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hagekull B, Bohlin G. Preschool temperament and environmental factors related to the Five-Factor Model of personality in middle childhood. Merrill-Palmer Quarterly. 1998;44:194–215. [Google Scholar]
- John OP, Caspi A, Robins RW, Moffitt TE, Stouthamer-Loeber M. The “little five”: Exploring the nomological network of the five-factor model of personality in adolescent boys. Child Devevelopment. 1994;65:160–178. [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big Five trait taxonomy: History: measurement, and conceptual issue. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. Guilford Press; New York: 2008. pp. 114–158. [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depres sion: A Swedish longitudinal, population-based twin study. Archives of General Psychiatry. 2006;63:1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: New evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Malouff JM, Thorsteinsson EB, Schutte NS. The relationship between the five factor model of personality and symptoms of clinical disorders: ameta-analysis. Journal of Psychopathology and Behavioral Assessment. 2005;27(2):101–114. [Google Scholar]
- Manly JT, Cicchetti D, Barnett D. The impact of subtype, frequency, chronicity, and severity of child maltreatment on social competence and behavior problems. Development and Psychopathology. 1994;6:121–143. [Google Scholar]
- Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: An integrative hierarchical approach. Journal of Personality and Social Psychology. 2005;88:139–157. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitarys–adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Archives of General Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D. Child maltreatment and emergent personality organization: Perspectives from the five-factor model. Journal of Abnormal Child Psychology. 2004;32:123–145. doi: 10.1023/b:jacp.0000019766.47625.40. [DOI] [PubMed] [Google Scholar]
- Shiner RL, DeYoung CG. The structure of temperament and personality traits: A developmental perspective. In: Zelazo PD, editor. The Oxford handbook of developmental psychology. Oxford University Press; New York: (in press) [Google Scholar]
- Sinnwell JP, Schaid DJ. Haplo Stats (version 1.4.0): Statistical methods for haplotypes when linkage phase is ambiguous. Mayo Clinic; Rochester, MN: 2008. [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of General Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]