Abstract
Objective
Although a useful marker of heart failure in adults, the utility of brain natriuretic peptide concentration (BNP) for children after the Fontan procedure is not well studied.
Design
BNP was measured in 510 patients aged 6–18 years in the Pediatric Heart Network Fontan cross-sectional study at a median of 8.2 years after Fontan. Patients underwent echocardiography, exercise testing, magnetic resonance imaging (MRI) and functional health status questionnaires. Associations of BNP with baseline patient characteristics, medical history and cross-sectional assessment were examined with multivariable linear regression analyses.
Results
The distribution of BNP was highly skewed, median 13.0 pg/mL (inter-quartile range: 7.1, 25.9), and was normalized with logarithmic transformation (logBNP). Among medical history variables, logBNP was greater in females (p=0.02) and older patients (p<0.001). Presence of pre-Fontan systolic ventricular dysfunction, greater number of post-Fontan complications, and thrombosis after Fontan were independently associated with higher logBNP (R2=0.16). Age-adjusted logBNP was significantly related to Fontan connection type, (lower with extracardiac conduits, higher with atriopulmonary connection; p<0.001). Lower physical functioning health status (R2=0.05), lower chronotropic index during exercise (R2=0.17), indices of diastolic dysfunction measured by echocardiography (R2=0.15), and higher total ventricular mass on MRI (R2=0.33) were related to higher logBNP.
Conclusions
Despite a markedly abnormal circulation, BNP was variable but within a normal range in the majority of Fontan patients in this large outpatient cohort. Higher BNP was associated with several markers of suboptimal outcome, although associations were weak. The routine use of BNP as an outpatient surveillance tool in asymptomatic Fontan patients is not warranted.
Keywords: Fontan procedure, congenital heart defects, natriuretic peptides
INTRODUCTION
Brain natriuretic peptide is a naturally occurring hormone produced by cardiac ventricular and, to a lesser extent, atrial myocytes in physiologic situations associated with ventricular or atrial wall stress. Brain natriuretic peptide concentration (BNP) has been widely used as a diagnostic tool to differentiate cardiac from respiratory illnesses in adults.1, 2 Studies of BNP in pediatric patients with heart disease are limited and are frequently not adjusted for factors such as age and gender that confound measurements. In small studies involving children with heterogeneous congenital heart diagnoses, elevated BNP correlated with ventricular dysfunction.3 Patients with functionally univentricular hearts had higher BNP compared to those with a biventricular circulation.4 There is limited published information about BNP in Fontan patients and the potential diagnostic and prognostic value of BNP in this population is currently unknown.
The Pediatric Heart Network (PHN) recently completed a multi-institutional cross-sectional study of outpatient Fontan subjects aged 6 to 18 years.5, 6 The purpose of the present report is to describe the profile of BNP in children and adolescents after the Fontan procedure and its relationship to subject characteristics and medical history. Furthermore, we sought to identify laboratory factors that are associated with BNP using cross-sectional tests of functional health status, exercise performance, echocardiography and cardiac magnetic resonance imaging (MRI).
METHODS
Study Subjects
The seven centers comprising the PHN performed a multicenter, prospective, cross-sectional assessment of children who had undergone a Fontan procedure.6 The techniques used to collect medical history data and to perform standardized echocardiography, cardiac MRI, and exercise testing have been previously described. 5, 6 Potential study subjects were identified from medical record review, and deemed eligible if they were age 6 to 18 years at the time of enrollment; had undergone Fontan procedure at least 6 months before initial study testing; and agreed to have an echocardiogram and blood testing and to complete a parent report functional health status questionnaire within 3 months of enrollment at one of the study centers. Exclusion criteria included the presence of a non-cardiac medical or psychiatric disorder that would prevent successful completion or invalidate the results of study testing. Subjects (n=546) were evaluated at a mean age of nearly 12 years old, and at a mean interval of 8.6 years after Fontan completion.5 The protocol was approved by each center's institutional review board. Written informed consent and assent were obtained. The present analysis is limited to the 510 subjects with BNP measurements.
Measurements
Resting BNP in plasma was centrally measured with the Shinogi BNP-32 Human Assay (Mayo Clinical Trial Services, Rochester, MN).6 The samples were drawn from a venipuncture after patients had been in a sitting or supine position in a quiet room for 30 minutes. The lower detection limit was 4 pg/mL; this was used for 55 subjects whose values were denoted as below the detectable limit.
Among the 510 subjects with BNP measured, functional health status was assessed in 504 using the validated Parent Report Form of the Child Health Questionnaire (CHQ).7 In addition to scores for individual domains, summary scores were derived for Physical and Psychosocial Functioning. The Parent Report Form of the CHQ also required parents to identify from a list of associated health conditions those which were present in their child. A previous analysis reported associations between Physical Functioning Summary Score and patient and medical history characteristics.8 Exercise testing was performed in 390 of these subjects. Analysis of the 6 exercise variables was limited to the 158 subjects able to achieve maximal aerobic capacity (Peak Respiratory Exchange Ratio >1.1). Analysis of chronotropic index (maximal achieved heart rate- resting heart rate) ÷ (predicted maximal heart rate minus resting heart rate) was restricted to only the 135 non-paced subjects able to achieve maximal exercise. Among the other exercise variables, the number of missing values was small (<5 for each) and no imputation of missing values was performed. An echocardiogram was performed for 503 patients with measured BNP. Subjects were excluded from analysis of echocardiographic data if they had no available mass or volume data (n=114). Missing echocardiographic variable values were imputed in multivariable regression analyses using mean values from the subset with mass-volume data. An MRI was performed in 153 patients. Among the 7 MRI variables measured, very few values (<3 for each) were missing and imputation was not performed.
Data Analysis
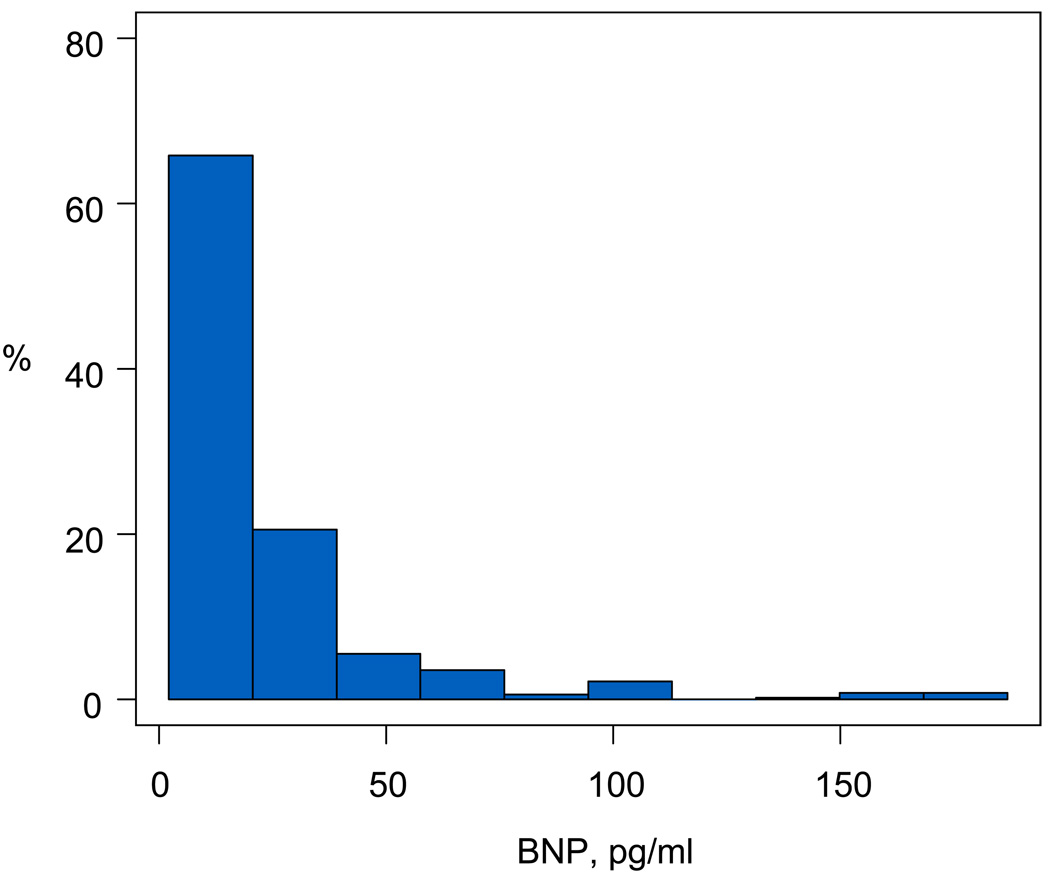
Data are described as frequencies, medians with minimum and maximum values or 25th and 75th percentile values, and means with standard deviations. The distribution of BNP was highly skewed towards lower values. (Figure 1). As a result, all analyses were performed after logarithmic transformation (logBNP, natural logarithm), which resulted in a more normal distribution. Variables explored for association with logBNP were initially analyzed within associated groups (e.g., echocardiography, exercise testing, MRI) in univariable models (Table 1) and then in multivariable models (Table 2). To determine the amount of variation in logBNP explained by each group of variables, multivariable linear regression analyses were performed with all variables from each group included in the model and the R2 adjusted for the number of variables are reported (Table 2). To determine which variables within each group were independently associated with logBNP, initial exploration was performed using bootstrap procedures to determine reliability for model inclusion, and then stepwise regression was performed with inclusion of gender and age at enrollment for adjustment in final models. Non-linearity was addressed by a variable transformation, as appropriate. Further adjustment for type of Fontan connection was performed by including this variable in final regression models for echocardiography, exercise testing and MRI variable groups. Given the large number of variables explored, only significant variables in multivariable regression analyses are reported (Table 3). All analyses were performed using SAS statistical software version 9 (SAS Institute, Inc, Cary, NC).
Figure 1.
Distribution of Brain natriuretic peptide (BNP) concentration excluding 4 patients with values exceeding 200 pg/ml.
TABLE 1.
Significant Variables in Univariable Testing for Association with Log Transformed BNP concentrations
| Variable | n | PE (SE) | p Value |
|---|---|---|---|
| Demographics | |||
| Age at enrollment | 510 | 0.05 (0.01) | <0.01 |
| Female gender | 510 | 0.23 (0.09) | <0.01 |
| Race | 0.02 | ||
| White | 406 | 0.23 (0.14) | |
| Black | 49 | 0.51 (0.19) | |
| Other | 54 | reference | |
| Body mass index Z score | 509 | 0.03 (0.01) | <0.01 |
| Years since Fontan | 510 | 0.04 (0.01) | <0.01 |
| Pre-Fontan Characteristics | |||
| History of stroke before Fontan | 504 | 0.55 (0.22) | 0.01 |
| Systolic ventricular dysfunction before Fontan | 468 | 0.46 (0.16) | <0.01 |
| No Cavopulmonary staging procedure performed | 510 | 0.33 (0.10) | <0.01 |
| Higher ventricular end-diastolic pressure | 486 | 0.03 (0.01) | 0.04 |
| Lower systemic oxygen saturation prior to Fontan | 494 | 0.03 (0.01) | <0.01 |
| Fontan Procedure Characteristics | |||
| Type of Fontan connection | <0.01 | ||
| Atriopulmonary | 69 | −0.37 (0.30) | |
| Intracardiac lateral tunnel | 301 | −0.76 (0.28) | |
| Extracardiac lateral tunnel | 63 | −1.08 (0.30) | |
| Extracardiac conduit | 66 | −1.22 (0.30) | |
| Other | 11 | reference | |
| Decreased systolic ventricular function at discharge | 506 | 0.60 (0.23) | <0.01 |
| Glycoside use at discharge | 505 | 0.18 (0.09) | 0.03 |
| Post-Fontan Follow-Up Characteristics | |||
| Total number of complications | 510 | 0.12 (0.03) | <0.01 |
| Post-Fontan thrombosis | 510 | 0.60 (0.16) | <0.01 |
| Post-Fontan arrhythmia | 509 | 0.28 (0.10) | <0.01 |
| Presence of other (non-cardiac) complications | 510 | 0.21 (0.10) | 0.03 |
| Number of other (non-cardiac) complications | 510 | 0.11 (0.04) | <0.01 |
| Number of post-Fontan cardiac surgeries | 510 | 0.10 (0.04) | 0.02 |
| Parent-Completed CHQ Scores | |||
| Poorer Physical Functioning Summary score | 483 | 0.01 (0.004) | <0.01 |
| Poorer Physical functioning domain | 503 | 0.01 (0.002) | <0.01 |
| Poorer Bodily pain domain | 498 | 0.01 (0.002) | 0.01 |
| Parent-Completed CHQ Health Conditions | |||
| Orthopedic problems | 498 | 0.50 (0.13) | <0.01 |
| Allergies / sinus problems | 496 | 0.32 (0.11) | <0.01 |
| Sleep disturbance | 498 | 0.36 (0.14) | 0.02 |
| Asthma | 491 | 0.27 (0.12) | 0.03 |
| Cardiopulmonary Exercise Testing | |||
| Lower Chronotropic index | 135 | 1.54 (0.61) | 0.01 |
| Echocardiography | |||
| Ventricular mass Z score | 382 | 0.11 (0.02) | <0.01 |
| Ventricular end-diastolic volume Z score | 389 | 0.11 (0.02) | <0.01 |
| Ventricular end-systolic volume Z score | 389 | 0.08 (0.02) | <0.01 |
| Ventricular stroke volume Z score | 389 | 0.09 (0.03) | <0.01 |
| Lower AW peak late diastolic velocity | 258 | 1.52 (0.42) | <0.01 |
| Duration of AW late diastolic inflow | 258 | 0.01 (0.002) | <0.01 |
| Ratio of early to late AW diastolic velocity | 258 | 0.31 (0.10) | <0.01 |
| Tei Index | 347 | 0.68 (0.29) | 0.02 |
| Magnetic Resonance Imaging | |||
| Total ventricular mass indexed to body surface area | 153 | 0.01 (0.003) | <0.01 |
| Ratio of total ventricular mass to end-diastolic volume | 153 | 0.80 (0.23) | <0.01 |
| Lower ventricular ejection fraction | 153 | 0.02 (0.01) | 0.04 |
PE, parameter estimate; SE, standard error; AW, atrioventricular valve; CHQ, Child Health Questionnaire; HLHS, hypoplastic left heart syndrome; PHN, Pediatric Heart Network; SV, single ventricle
TABLE 2.
The Proportion of Variance (Model R2) in Log Transformed Brain Natriuretic Peptide Concentrations* Explained in Multivariable Analysis by Groups of Variables
| Variable Group | n | Number of Variables |
R2 | Adjusted R2** |
|---|---|---|---|---|
| Demographics | 510 | 8 | 0.08 | 0.06 |
| Pre-Fontan characteristics | 510 | 20 | 0.05 | 0.02 |
| Fontan characteristics | 510 | 31 | 0.15 | 0.10 |
| Post-Fontan characteristics | 510 | 14 | 0.05 | 0.03 |
| Parent-completed CHQ summary scores | 483 | 2 | 0.02 | 0.01 |
| Parent-completed CHQ domain scores | 504 | 15 | 0.08 | 0.05 |
| Parent-completed CHQ health conditions | 504 | 15 | 0.10 | 0.08 |
| Cardiopulmonary exercise testing | 131 | 6 | 0.06 | 0.02 |
| Echocardiography† | 389 | 16 | 0.17 | 0.14 |
| Magnetic resonance imaging | 151 | 7 | 0.11 | 0.09 |
analyzed after logarithmic transformation (with the natural base) to normalize the distribution
adjusted for the number of variables in the model
CHQ, Child Health Questionnaire
Missing echocardiographic variable values were imputed from the subset with mass-volume data
TABLE 3.
Final Multivariable Models for Higher Log Transformed Brain Natriuretic Peptide Concentrations*
| PE (SE) | p Value | |
|---|---|---|
| Demographics, n=510, model R2=0.04, Adjusted R2=0.04** | ||
| Female gender | 0.21 (0.08) | 0.01 |
| Older age at enrollment | 0.05 (0.01) | <0.01 |
| Pre-Fontan Characteristics, n=466, model R2=0.06, Adjusted R2=0.06** | ||
| Presence of systolic ventricular dysfunction on pre-Fontan echocardiography | 0.52 (0.16) | <0.01 |
| Fontan Characteristics, n=510, model R2=0.10, Adjusted R2=0.09,** | ||
| Type of Fontan connection | <0.01 | |
| Atriopulmonary | −0.38 (0.30) | |
| Intracardiac lateral tunnel | −0.72 (0.28) | |
| Extracardiac lateral tunnel | −1.01 (0.31) | |
| Extracardiac conduit | −1.18 (0.30) | |
| Other | reference | |
| Post-Fontan Characteristics, n=510, model R2=0.08, Adjusted R2=0.08** | ||
| Greater total number of complications | 0.08 (0.03) | <0.01 |
| Post-Fontan thrombosis | 0.45 (0.17) | <0.01 |
| Combined Medical History Characteristics, n=468, model R2=0.16, Adjusted R2=0.15** | ||
| Presence of systolic ventricular dysfunction on pre-Fontan echocardiography | 0.48 (0.15) | <0.01 |
| Type of Fontan connection | <0.01 | |
| Atriopulmonary | −0.37 (0.32) | |
| Intracardiac lateral tunnel | −0.58 (0.30) | |
| Extracardiac lateral tunnel | −0.85 (0.33) | |
| Extracardiac conduit | −1.07 (0.32) | |
| Other | reference | |
| Greater number of post-Fontan complications during follow-up | 0.11 (0.03) | <0.01 |
| Post-Fontan thrombosis during follow-up | 0.41 (0.17) | 0.02 |
| Parent-Completed CHQ Summary Scores, n=483, model R2=0.05, Adjusted R2=0.04** | ||
| Lower Physical Functioning Summary score | 0.01 (0.004) | 0.02 |
| Parent-Completed CHQ Domain Scores, n=503, model R2=0.06, Adjusted R2=0.06** | ||
| Lower Physical Functioning Domain score | 0.007 (0.002) | <0.01 |
| Parent-Completed CHQ Health Conditions, n=494, model R2=0.07, Adjusted R2=0.06** | ||
| Orthopedic / bone / joint problems | 0.41 (0.13) | <0.01 |
| Allergy / sinus problems | 0.26 (0.11) | 0.02 |
| Cardiopulmonary Exercise Testing, n=135, model R2=0.17**, Adjusted R2=0.14* | ||
| Lower chronotropic index | 1.56 (0.60) | 0.01 |
| Echocardiography†, n=389, model R2=0.15, Adjusted R2=0.14*** | ||
| Lower AW peak late diastolic velocity (m/sec) | 1.40 (0.41) | <0.01 |
| Longer duration of AV late diastohc inflow (msec) | 0.01 (0.002) | <0.01 |
| Magnetic Resonance Imaging, n=153, model R2=0.33, Adjusted R2=0.31*** | ||
| Ventricular mass index > 95th percentile | 1.87 (0.28) | <0.01 |
after logarithmic transformation (with the natural base)
adjusted for age at enrollment and gender
adjusted for age at enrollment and gender, and type of Fontan connection
Missing echocardiographic variable values were imputed from the subset with mass-volume data
AVV, atrioventricular valve; CHQ, Child Health Questionnaire; PE, parameter estimate; SE, standard error
RESULTS
Study Subjects
BNP was measured in 510 of 546 subjects; 60% were male, 80% were white, 10% were black, and 10% were of another race. Subjects were a median age of 11.4 years (inter-quartile range [IQR]: 9.0 – 14.6 yrs) at the time of enrollment, and underwent their Fontan surgery at a median of 2.8 years (IQR: 2.1 – 4.0 yrs) of age. The 36 subjects without BNP measurements in the Fontan cross sectional study were significantly younger than the 510 with a measurement (10.8 ± 3.4 years versus 11.9 ± 3.4 years, p=0.05), likely due to reluctance to undergo venipuncture or technical difficulty. The mean BNP was 25 ± 48 pg/mL, with a median of 13 pg/mL (range 4 to 652 pg/mL; IQR 7 to 26 pg/mL). The distribution of BNP levels in the study sample is shown in Figure 1. Previous studies in patients with congenital heart disease have suggested that BNP > 40 pg/mL may be a marker of ventricular dysfunction 3 and may differentiate children with congestive heart failure from those with non-cardiac causes of respiratory distress.9 A value of 100pg/mL has been used as a marker for heart failure in adults. 2 Overall, 86% of subjects (n=438) had BNP < 40 pg/mL and 96% (n=489) had BNP <100pg/mL. Values below the detection level of 4pg/mL occurred in 11% of subjects (n=55).
Association of Patient Variables to BNP
Table 2 shows the magnitude of the relationship of variable groups with logBNP. For all variable groups, the proportion of variance in BNP explained by the variables (adjusted R2) was small, being highest for echocardiography at 0.14 or 14%, followed by MRI at 9%, and parent-completed CHQ associated health conditions at 8%.
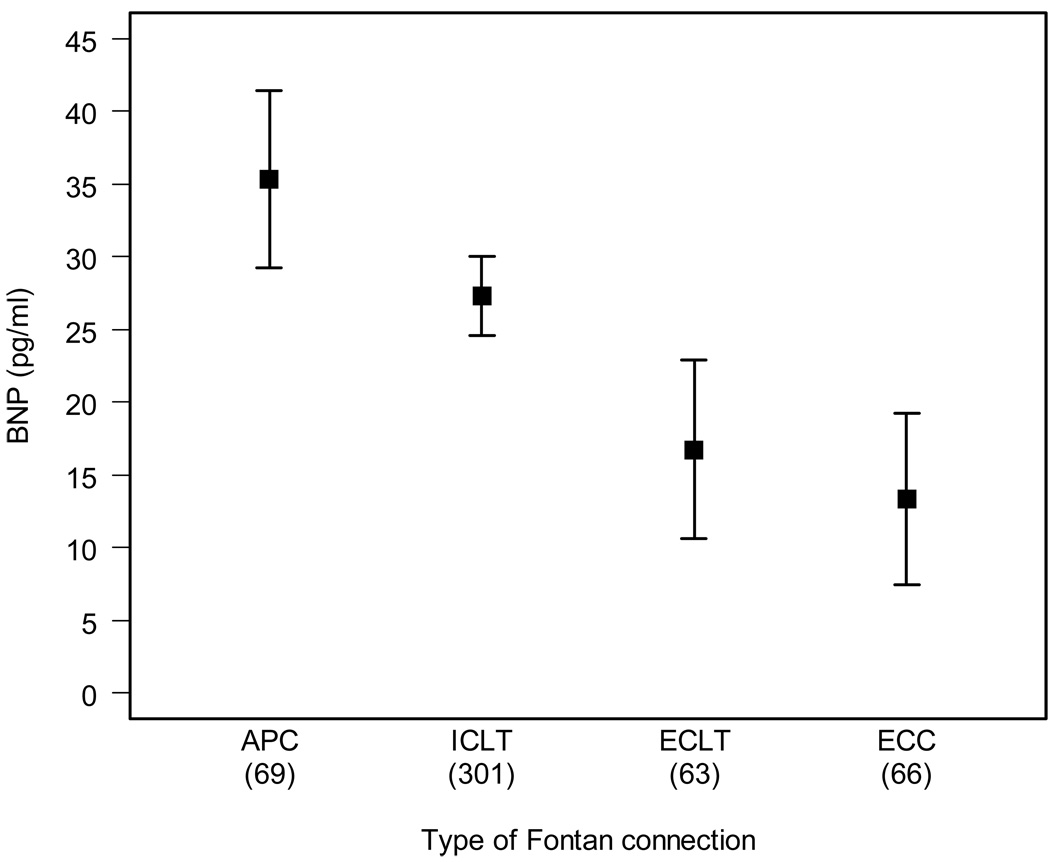
The results of the multivariable analyses for each variable group are shown in Table 3. Higher logBNP was significantly and independently related to female gender and older age at enrollment. Therefore, all other multivariable analyses were subsequently adjusted for gender and age. The only pre-Fontan procedure characteristic significantly associated with higher logBNP was the presence of systolic ventricular dysfunction by echocardiography. Type of Fontan connection was the only significant Fontan procedure characteristic variable independently associated with logBNP, (p< 0.01, Figure 2). Mean BNP (adjusted for age and gender) among subjects with atriopulmonary connection was 8pg/mL higher than for those with intracardiac lateral tunnel, which in turn was 11 units higher than BNP in those with an extracardiac lateral tunnel, and BNP in those with an extracardiac lateral tunnel were a mean of 4 pg/mL higher than those with an extracardiac conduit. Therefore, subsequent multivariable analyses of laboratory testing were adjusted for this additional variable of Fontan connection type. Post-Fontan characteristics independently associated with higher logBNP included a greater number of complications and the presence of an episode of post-Fontan thrombosis. For the parent-completed CHQ, higher logBNP was associated with lower Physical Functioning Summary and Physical Functioning domain scores, as well as the presence of associated bone/joint/orthopedic and allergy/sinus problems.
Figure 2.
Subgroup analysis of serum brain natriuretic peptide concentrations (BNP) by Fontan connection type. Mean ± SE of raw BNP adjusted for gender and age at enrollment
APC=atriopulmonary connection, ECC=extracardiac conduit, ECLT=extracardiac lateral tunnel, ICLT=intracardiac lateral tunnel.
Multivariable analyses of laboratory testing results were performed with adjustments for gender, age and type of Fontan connection (Table 3). From exercise testing, only lower chronotropic index was associated with higher logBNP. Among echocardiographic variables, indices of diastolic dysfunction were significantly related to higher logBNP. Among MRI variables, only greater total ventricular mass indexed to body surface area was associated with higher logBNP. To account for the non-linearity and improve model fit, a dichotomous variable separating observations in the 95th percentile of the indexed ventricular mass from the rest of the observations was used.
A number of variables were not associated with BNP. After adjustment for age at enrollment, gender and type of Fontan connection, the following were not independently associated with BNP: ventricular type (a dominant left, right or mixed ventricular dominance), age at Fontan completion, and post-Fontan history of arrhythmia. Protein losing enteropathy was documented in 19 subjects; median BNP was not different between those and the remaining 491 subjects, 13 vs, 10 pg/mL, p=0.5. The presence or magnitude of atrioventricular valve regurgitation assessed by echocardiogram was not associated with BNP. Ejection fraction (EF) assessed as a continuous variable by echocardiography and MRI was also not associated with BNP. However, when comparing subjects assessed by the echocardiography core lab to have a normal EF (z-score ≥−2, which corresponds to an EF ≥ 53%) with those with abnormal EF (z-score < −2, which corresponds to an EF < 53%), we found logBNP to be significantly lower in those with normal EF, p=0.01). When adjusted for age and gender, those with normal EF continued to have a lower logBNP (p=0.02)
DISCUSSION
This is the largest study to date examining BNP in Fontan patients. A strength of the study is its prospective design and centralized assay for BNP concentration. In addition, the analyses were adjusted for known confounders such as age and gender. We found that BNP varied considerably in this multicenter cohort of outpatient Fontan subjects, but was relatively low in the majority of patients, especially compared to values used to define congestive heart failure in adult patients. Higher BNP (or logBNP) was associated with older age, female gender and atriopulmonary type Fontan connection, as well as selected medical history variables. Higher BNP was weakly associated with diagnostic markers of suboptimal health status and ventricular performance.
Previous studies in patients with congenital heart disease have suggested that BNP > 40 pg/mL may be a marker of ventricular dysfunction 3 and may differentiate children with congestive heart failure from those with non-cardiac causes of respiratory distress.9, 10 Assigning specific cut-point values of BNP in pediatrics is uniquely problematic as normal values are not widely agreed upon and, similar to what we report, vary with both gender and age. 11, 12 In a group of 67 Fontan patents, BNP was reported as normal in 81%.13 The data presented here suggest that the uniform and unisex cut-points for normal values for BNP may be misleading and should not be used in this population.
BNP is elevated after initial palliative operations in single ventricle patients and falls in response to subsequent volume unloading surgeries such as the cavopulmonary anastomosis (Glenn) procedure.14 In a previous study involving single ventricle patients, plasma BNP was elevated when systemic ventricular dysfunction was present but not elevated compared to normal controls for patients with Glenn or Fontan physiology without ventricular dysfunction. 15 Among our subjects, ejection fraction, a marker of systolic function, was normal in 73% of subjects 5and those subjects had a lower logBNP when compared to those with an abnormal ejection fraction.
After controlling for age and gender, we found the type of Fontan connection was related to BNP. Similarly, BNP was higher in a small study of adult Fontan patients with atriopulmonary connections compared to those with total cavopulmonary connections. 16 Although the majority of BNP is secreted from ventricular myocytes, it is also secreted from atrial myocytes, and therefore may be expected to be higher in anatomic situations of increased atrial stretch such as with the atriopulmonary connection in comparison to extracardiac connections.
We found a higher BNP to be weakly associated with lower (poorer) Physical Functioning Summary Score and lower scores for the Physical Functioning domain. This is consistent with findings in a small group of children after Fontan where higher NT-pro BNP was associated with higher New York University Pediatric Heart Failure Index scores 17 and in an older Fontan population where lower BNP differentiated NYHA classification 2 from NYHA class 3 and 4. 16 The association of BNP to orthopedic/bone/joint problems and allergy/sinus problems has not been previously reported and is not intuitively explained, other than speculating that these findings may co-migrate with other indicators of poor cardiac status.
In the present study, a lower chronotropic index was independently associated with higher BNP after consideration of age, gender and Fontan connection type. A lower chronotropic index has been used as a marker for chronotropic incompetence, which has been shown to be a predictor of sudden cardiac death in patients with heart failure.18 Although not previously well studied in the Fontan population, chronotropic incompetence has been associated with higher BNP in adults with congenital heart disease decades after their surgical intervention. 19
The association of higher BNP with echocardiographic findings of diastolic dysfunction is consistent with a previous study involving 35 asymptomatic Fontan patients. 20 The association of BNP with increased ventricular mass noted in our patients undergoing MRI has not been previously reported in this population, but BNP has been associated with increased ventricular mass in adults with hypertrophic cardiomyopathy, 21 and in pediatric patients with increased left ventricular mass as a result of aortic stenosis.22
After adjustment for age, gender and Fontan connection type neither the presence nor magnitude of atrioventricular valve regurgitation was related to BNP. Again, this may reflect well compensated hemodynamics in this relatively healthy cross-sectional study of Fontan patients. Consistent with previous studies we found no difference in BNP related to the dominant ventricular morphology (right, left or mixed). 16 17
This study must be interpreted in light of some limitations. This was a cross-sectional study of pediatric Fontan outpatients, and as such, the most symptomatic Fontan patients may not have been included. This likely contributed to the rather narrow range of BNP values measured in our study and may have impeded our efforts to find strong associations. In addition, a cross-sectional study such as this cannot assess the predictive value of BNP for outcomes such as death or progressive heart failure. Not all patients were able to complete all tests, which might limit the ability to detect associations.
This study establishes a range of BNP concentrations in a large outpatient cohort of children after the Fontan procedure. Despite a markedly abnormal circulation, BNP is within the normal range for age in the majority of the subjects. Our data do not support the routine measurement of BNP in the outpatient follow up of relatively healthy patients after a Fontan procedure.
ACKNOWLEDGEMENTS
National Heart, Lung, and Blood Institute: Gail Pearson, Mario Stylianou, Judith Massicot-Fisher*, Marsha Mathis, Victoria Pemberton
Data Coordinating Center: New England Research Institutes, Lynn Sleeper, Steven Colan, Paul Mitchell*, Dianne Gallagher, Patti Nash, Gloria Klein, Minmin Lu
Network Chair: Lynn Mahony, University of Texas Southwestern Medical Center
Clinical Site Investigators: Children’s Hospital Boston, Jane Newburger (PI), Stephen Roth*, Roger Breitbart, Jonathan Rhodes, Jodi Elder, Ellen McGrath; Children’s Hospital of New York, Welton M. Gersony (PI), Daphne T. Hsu*, Seema Mital*, Beth Printz*, Ashwin Prakash*, Darlene Servedio*; Children’s Hospital of Philadelphia, Victoria Vetter (PI), Bernard J. Clark*, Mark Fogel, Steven Paridon, Jack Rychik, Margaret Harkins*, Jamie Koh; Duke University, Page A. W. Anderson (PI) - deceased, Rene Herlong*, Lynne Hurwitz, Jennifer S. Li, Ann Marie Nawrocki*; Medical University of South Carolina, J. Philip Saul (PI), Andrew M. Atz, Andrew D. Blaufox*, Girish Shirali, Jon Lucas*, Amy Blevins*; Primary Children’s Medical Center, Salt Lake City, Utah, LuAnn Minich (PI), Richard Williams, Linda Lambert, Michael Puchalski; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Timothy Bradley, Kevin Roman*, Jennifer Russell, Shi-Joon Yoo, Elizabeth Radojewski, Nancy Slater
Core Laboratories:
Cardiac MRI, Children’s Hospital Boston: Tal Geva (Director); Andrew J. Powell Echocardiography, Children’s Hospital Boston: Steven Colan (Director), Marcy Schwartz*, Renee Margossian
Protocol Review Committee: Michael Artman, Chair; Dana Connolly, Timothy Feltes, Julie Johnson, Jeffrey Krischer, G. Paul Matherne.
Data and Safety Monitoring Board: John Kugler, Chair; Kathryn Davis, David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Catherine L. Webb, Lawrence Wissow.
*no longer at the institution listed
FUNDING SOURCES
Supported by U01 grants from the NIH, National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288)
References
- 1.Maisel AS, Clopton P, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Steg G, Westheim A, Knudsen CW, Perez A, Kazanegra R, Bhalla V, Herrmann HC, Aumont MC, McCullough PA. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. Am Heart J. 2004;147:1078–1084. doi: 10.1016/j.ahj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;18:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 3.Law YM, Keller BB, Feingold BM, Boyle GJ. Usefulness of plasma B-type natriuretic peptide to identify ventricular dysfunction in pediatric and adult patients with congenital heart disease. Am J Cardiol. 2005;95:474–478. doi: 10.1016/j.amjcard.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Koch A, Zink S, Singer H. B-type natriuretic peptide in paediatric patients with congenital heart disease. Eur Heart J. 2006;27:861–866. doi: 10.1093/eurheartj/ehi773. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ., III Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleeper LA, Anderson P, Hsu DT, Mahony L, McCrindle BW, Roth SJ, Saul JP, Williams RV, Geva T, Colan SD, Clark BJ. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgraf JM. Measuring health-related quality of life in pediatric oncology patients: a brief commentary on the state of the art of measurement and application (discussion) Int J Cancer Suppl. 1999;12:147–150. doi: 10.1002/(sici)1097-0215(1999)83:12+<147::aid-ijc26>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.McCrindle BW, Williams RV, Mitchell PD, Hsu DT, Paridon SM, Atz AM, Li JS, Newburger JW. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113:1123–1129. doi: 10.1161/CIRCULATIONAHA.105.576660. [DOI] [PubMed] [Google Scholar]
- 9.Koulouri S, Acherman RJ, Wong PC, Chan LS, Lewis AB. Utility of B-type natriuretic peptide in differentiating congestive heart failure from lung disease in pediatric patients with respiratory distress. Pediatr Cardiol. 2004;25:341–346. doi: 10.1007/s00246-003-0578-0. [DOI] [PubMed] [Google Scholar]
- 10.Law YM, Hoyer AW, Reller MD, Silberbach M. Accuracy of plasma B-type natriuretic peptide to diagnose significant cardiovascular disease in children: the Better Not Pout Children! Study. J Am Coll Cardiol. 2009;54:1467–1475. doi: 10.1016/j.jacc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Saenger AK, Dalenberg DA, Bryant SC, Grebe SK, Jaffe AS. Pediatric brain natriuretic peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem. 2009;55:1869–1875. doi: 10.1373/clinchem.2009.123778. [DOI] [PubMed] [Google Scholar]
- 12.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch AM, Zink S, Singer H, Dittrich S. B-type natriuretic peptide levels in patients with functionally univentricular hearts after total cavopulmonary connection. Eur J Heart Fail. 2008;10:60–62. doi: 10.1016/j.ejheart.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Wahlander H, Westerlind A, Lindstedt G, Lundberg PA, Holmgren D. Increased levels of brain and atrial natriuretic peptides after the first palliative operation, but not after a bidirectional glenn anastomosis, in children with functionally univentricular hearts. Cardiol Young. 2003;13:268–274. [PubMed] [Google Scholar]
- 15.Law YM, Ettedgui J, Beerman L, Maisel A, Tofovic S. Comparison of plasma B-type natriuretic peptide levels in single ventricle patients with systemic ventricle heart failure versus isolated cavopulmonary failure. Am J Cardiol. 2006;98:520–524. doi: 10.1016/j.amjcard.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchi H, Takasugi H, Ohashi H, Yamada O, Watanabe K, Yagihara T, Echigo S. Abnormalities of neurohormonal and cardiac autonomic nervous activities relate poorly to functional status in fontan patients. Circulation. 2004;110:2601–2608. doi: 10.1161/01.CIR.0000145545.83564.51. [DOI] [PubMed] [Google Scholar]
- 17.Lechner E, Gitter R, Mair R, Pinter M, Schreier-Lechner E, Vondrys D, Tulzer G. Aminoterminal Brain Natriuretic Peptide Levels in Children and Adolescents After Fontan Operation Correlate with Congestive Heart Failure. Pediatr Cardiol. 2008:901–905. doi: 10.1007/s00246-008-9225-0. [DOI] [PubMed] [Google Scholar]
- 18.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 19.Norozi K, Wessel A, Alpers V, Arnhold JO, Binder L, Geyer S, Zoege M, Buchhorn R. Chronotropic incompetence in adolescents and adults with congenital heart disease after cardiac surgery. J Card Fail. 2007;13:263–268. doi: 10.1016/j.cardfail.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Man BL, Cheung YF. Plasma brain natriuretic peptide and systemic ventricular function in asymptomatic patients late after the Fontan procedure. Heart Vessels. 2007;22:398–403. doi: 10.1007/s00380-007-0993-x. [DOI] [PubMed] [Google Scholar]
- 21.Maron BJ, Tholakanahalli VN, Zenovich AG, Casey SA, Duprez D, Aeppli DM, Cohn JN. Usefulness of B-type natriuretic peptide assay in the assessment of symptomatic state in hypertrophic cardiomyopathy. Circulation. 2004;109:984–989. doi: 10.1161/01.CIR.0000117098.75727.D8. [DOI] [PubMed] [Google Scholar]
- 22.Cowley CG, Bradley JD, Shaddy RE. B-type natriuretic peptide levels in congenital heart disease. Pediatr Cardiol. 2004;25:336–340. doi: 10.1007/s00246-003-0461-z. [DOI] [PubMed] [Google Scholar]