Abstract
Objectives
To evaluate morbidity, mortality, and associated risk factors in late preterm term infants (34 0/7-36 6/7 wk) requiring extra-corporeal membrane oxygenation (ECMO).
Study design
We reviewed a total of 21,218 neonatal ECMO runs in Extracorporeal Life Support Organization (ELSO) registry data from 1986 to 2006. Infants were divided into 3 groups: Late Preterm (34 0/7 to 36 6/7), Early Term (37 0/7 to 38 6/7), and Full Term (39 0/7 to 42 6/7).
Results
There were 14,528 neonatal ECMO runs which met inclusion criteria. Late preterm infants experienced the highest mortality on ECMO (late preterm 26.2%, early term 18%, full term 11.2%. p<0.001) and had longer ECMO runs; they also had higher rates of serious complications. GA was a highly significant predictor for mortality. Late preterm infants with a primary diagnosis of sepsis and PPHN had 3-fold higher risk of mortality on ECMO than those with meconium aspiration.
Conclusion
Late preterm infants treated with ECMO havehigher morbidity and mortality than term infants. This underscores the need for special consideration of this vulnerable population in the diagnosis and treatment of hypoxic respiratory failure.
Keywords: Late preterm, near term, early term, preterm birth, late preterm infant, infant mortality, hypoxic respiratory failure, extra corporeal membrane oxygenation (ECMO)
Infants born between 34 and 36 weeks gestation (239 to 259 days gestation) are at greater risk for morbidity and mortality compared with term newborns [1]. These infants, now referred to as late preterm infants, are contributing to increased rates of prematurity[2]. Late preterm births now account for 71.7% of all preterm births and nearly one third of neonatal intensive care unit (NICU) admissions in the US [2, 3]. This combination of large numbers and greater vulnerability results in a substantially higher etiologic fraction of disease burden and death in the early neonatal period [4, 5].
Several studies documented a higher risk of respiratory morbidity in late preterm infants. Beginning often as delayed respiratory transition and transient tachypnea, the course of respiratory distress in late preterm infants can be unpredictable. Etiology can be varied and is often overlapping with respiratory distress syndrome [6-10], transient tachypnea of the newborn (TTN) [11-15], and persistent pulmonary hypertension (PPHN) [16-18]. The clinical picture is further complicated by a higher incidence of co-morbidities such as apnea, hypoglycemia, hypothermia, poor feeding, sepsis/infections and hyperbilirubinemia[11, 13, 19-21]. In a retrospective study of 228,668 deliveries with 19,334 late preterm births, the incidence of PPHN was 0.38% in late preterm infants compared with 0.08% in term infants and the incidence of respiratory failure was 0.94% in late preterm infants compared with 0.11% in term infants. [22] Many of these infants are asymptomatic immediately after birth but subsequently demonstrate an escalating need for respiratory support with evidence of PPHN, hence the term malignant TTN to describe this sequence [23]. Given the relatively high birth weight, these infants are often not appropriately managed or monitored. They are treated with oxy-hoods for prolonged periods, placing them at risk for alveolar collapse due to oxygen absorption[5, 24, 25]. Refractory hypoxemia from RDS, PPHN, respiratory failure, meconium aspiration, pneumonia and sepsis are some of the indications to place infants on ECMO [26]. Our study evaluates the risk of morbidity and mortality in late preterm infants with severe hypoxic respiratory failure requiring ECMO compared with term infants and identifies risk factors that predispose them to adverse outcomes.
METHODS
We reviewed Extracorporeal Life Support Organization (ELSO) registry data from Jan 1986 to Dec 2006 in an electronic database without patient identifiers. ELSO is an international consortium of healthcare professionals and scientists that maintains a registry of ECMO runs in its participating centers. Nearly 170 centers worldwide contribute to this registry, with cumulative data on nearly 35,000 patients requiring ECMO. 24,000 of these are newborns. All ELSO member institutions are required to have IRB approval from their respective IRB for this data collection.
Infants with gestational age (GA) ≥ 34 weeks and < 43 weeks were included in our study and were divided into 3 Gestational age groups: Late Preterm (34 0/7 to 36 6/7), Early Term (37 0/7 to 38 6/7), and Full Term (39 0/7 to 42 6/7). Early term infants were treated as a separate group, given the significant increase in their numbers in recent years, and their predisposition to neonatal complications. Infants with congenital diaphragmatic hernias, and other major congenital disorders including cardiac defects, chromosomal and genetic abnormalities were excluded. Those with unavailable diagnosis or gestational age were also excluded, and only data from the first ECMO run was used for the analysis.
Patient demographic characteristics, perinatal and pre-ECMO variables, and complications on ECMO were summarized descriptively both overall and between GA groups. Categorical outcomes were summarized with percentages and compared between GA groups using the Chi square test of Independence. For continuous measurements, means and medians were calculated as appropriate and tested for equality between GA groups using one-way ANOVA and the Kruskal-Wallis Test.
We next assessed mortality on ECMO for each GA group as well as for each of the demographic and perinatal characteristics using univariate analysis. Crude odds ratios for mortality and their confidence intervals were calculated. To understand the differential survival on ECMO by GA group after adjusting for other covariates, we conducted a series of logistic regression analyses using mortality as our outcome. We used the pre-ECMO and demographic variables that were found significant in the earlier univariate analysis as covariates. Our starting model (Model 1) considered GA, adjusting for pH, Apgar 1 min, sex and year. Due to missing values, the predictors race (16% missing), SAO2 (28% missing), MAP (34% missing), and delivery method (accurate only after 1990) were excluded from this analysis. PCO2 was excluded due to high collinearity with pH, as was Apgar 5 min (collinear with Apgar 1). Our initial model included all covariates as possible confounders and effect modifiers of GA, except for those that had a potentially intermediate role in the casual pathway from GA to mortality (birth weight, primary diagnosis). A 10% shift in the odds-ratios (OR) for GA mortality was used to determine confounding, and interactions were considered significant at the 0.05 level. Our second model was a subset analysis that included two variables that had a high number of missing values in the registry but were important variables for control (race, delivery method). We then considered a model that did not include race and delivery method but incorporated the intermediate variables (birth weight, primary diagnosis), and again evaluated the association of GA with mortality. Our final model controlled for the post-ECMO outcomes of complications and time on ECMO to determine if a GA-mortality effect remained beyond these outcomes. The study was reviewed and approved by the ELSO Review Panel.
RESULTS
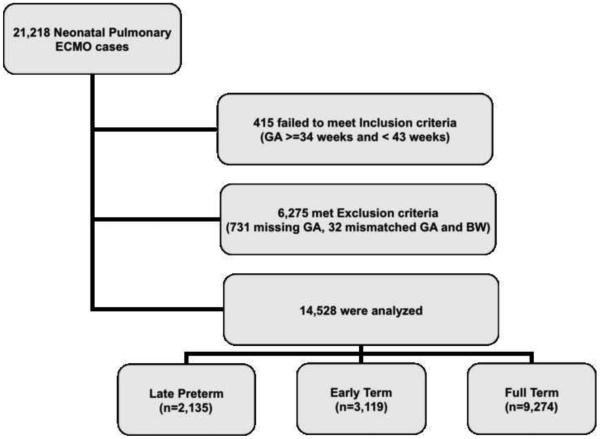
There were 21,218 neonatal pulmonary ECMO cases between 1986 and 2006, 415 failed to meet inclusion criteria, 6,275 met the exclusion criteria and a total of 14,528 infants were analyzed and were grouped as follows: late preterm: 2,135 infants, early term: 3,119 infants, and full term: 9,274 infants (Figure 1; available at www.jpeds.com). As would be expected, late preterm infants had a lower birth weight and gestational age (mean ±sd: birth weight, 2.8 (0.5) kg, and GA 35.4 (0.8) weeks. They were more likely to bemale, and non-Hispanic whites (Table I). Fifty six percent of the late preterm infants were delivered vaginally and 23% by elective C Section (delivery method data was available from 1990 onwards). The Apgar scores, pre-ECMO blood gas values and ventilator support type/magnitude in late preterm infants were statistically different from the term groups but the differences were not likely to beclinically significant.
Figure 1.
Study cohort derived from ECMO cases recorded in the ELSO database between 1986 and 2006. A total of 21,218 neonatal pulmonary ECMO cases were identified after ECMO runs initiated for cardiac conditions and post cardiac surgeries were excluded. Inclusion criteria was GA >= 34 weeks and <43 weeks. Exclusion criteria included infants with major congenital disorders including congenital diaphragmatic hernias, cardiac defects, chromosomal/genetic abnormalities, missing GA, mismatched GA and birth weight.
Table 1.
Demographic and Perinatal Characteristics
| Overall (n) |
Late Pre-Term % (n) |
Early Term % (n) |
Full Term % (n) |
|||
|---|---|---|---|---|---|---|
| (n = 14528) | 14.7 (n = 2135) | 21.5 (n = 3119) | 63.8% (n = 9274) | p1 | p2 | |
| Sex | < .0001 | < .0001 | ||||
| Female | 41.2 (5966/14472) | 33.7 (717/2118) | 35.4 (1098/3103) | 44.9 (4151/9241) | ||
| Male | 58.8 (8506/14472) | 66.3 (1411/2128) | 64.6 (2005/3103) | 55.1 (5090/9241) | ||
| Race | < .0001 | < .0001 | ||||
| White | 55.3 (6737/12180) | 71.9 (1194/1660) | 66.7 (1795/2692) | 47.9 (3748/7828) | ||
| Black | 24.4 (2974/12180) | 14.1 (234/1660) | 18.8 (505/2692) | 28.6 (2235/7828) | ||
| Hispanic | 12.4 (1510/12180) | 8.1 (135/1660) | 8.8 (237/2692) | 14.5 (1138/7828) | ||
| Asian | 3.6 (440/12180) | 2.8 (47/1660) | 2.3 (61/2692) | 4.2 (332/7828) | ||
| Other | 4.3 (519/12180) | 3.0 (50/1660) | 3.5 (94/2692) | 4.8 (375/7828) | ||
| Primary Diagnosis | < .0001 | < .0001 | ||||
| Sepsis | 16.4 (2375/14506) | 29.9 (638/2132) | 23.3 (725/3115) | 10.9 (1012/9259) | ||
| PPHN | 21.0 (3045/14506) | 21.7 (463/2132) | 28.3 (880/3115) | 18.4 (1702/9259) | ||
| RDS | 8.9 (1290/14506) | 28.2 (602/2132) | 13.6 (425/3115) | 2.8 (263/9259) | ||
| Respiratory Insufficiency | 2.6 (382/14506) | 3.9 (82/2132) | 4.1 (129/3115) | 1.9 (171/9259) | ||
| Meconium Aspiration | 44.9 (6514/14506) | 6.6 (140/2132) | 22.0 (686/3115) | 61.4 (5688/9259) | ||
| Other | 6.2 (900/14506) | 9.7 (207/2132) | 8.7 (270/3115) | 4.6 (423/9259) | ||
| Labor Status a | < .0001 | < .0001 | ||||
| Emergent C-section | 23.7 (2817/11884) | 20.9 (333/1595) | 24.7 (650/2627) | 23.9 (1834/7662) | ||
| Elective cesarean | 27.2 (3231/11884) | 23.2 (370/1595) | 22.7 (595/2627) | 29.6 (2266/7662) | ||
| Vaginal | 49.1 (5836/11884) | 55.9 (892/1595) | 52.6 (1382/2627) | 46.5 (3562/7662) | ||
| Ventilator Type | < .0001 | 0.0004 | ||||
| Conventional | 55.3 (5098/9219) | 52.3 (622/1189) | 52.1 (1069/2051) | 57.0 (3407/5979) | ||
| HFO | 35.9 (3311/9219) | 36.8 (438/1189) | 37.5 (768/2051) | 35.2 (2105/5979) | ||
| Other HFV | 8.8 (810/9219) | 10.9 (129/1189) | 10.4 (214/2051) | 7.8 (467/5979) | ||
| mean ± s.d. (n) | mean ± s.d. (n) | mean ± s.d. (n) | mean ± s.d. (n) | |||
| Birth Weight (kg) | 3.3 ± 0.6 (14250) | 2.8 ± 0.5 (2089) | 3.2 ± 0.5 (3062) | 3.5 ± 0.5 (9099) | < .0001 | < .0001 |
| Apgar, median (Q1,Q3): | ||||||
| 1 min | 6 (3, 8) (14167) | 7 (5, 8) (2087) | 7 (4, 8) (3057) | 5 (3, 7) (9023) | < .0001 | < .0001 |
| 5 min | 8 (6, 9) (14152) | 8 (7, 9) (2082) | 8 (7, 9) (3051) | 7 (6, 9) (9019) | < .0001 | < .0001 |
| Blood Gases | ||||||
| pH | 7.4 ± 0.2 (14211) | 7.3 ± 0.2 (2086) | 7.4 ± 0.2 (3053) | 7.4 ± 0.2 (9072) | < .0001 | < .0001 |
| PCo2 | 41.4 ± 20.0 (14162) | 43.4 ± 20.1 (2081) | 42.0 ± 20.1 (3035) | 40.7 ± 20.0 (9046) | < .0001 | < .0001 |
| Po2 | 44.2 ± 32.1 (14154) | 45.3 ± 34.5 (2083) | 44.9 ± 33.6 (3037) | 43.7 ± 30.9 (9034) | 0.05 | < .0001 |
| SaO2 | 73.0 ± 22.2 (10500) | 71.6 ± 22.8 (1442) | 72.8 ± 22.4 (2314) | 73.4 ± 21.9 (6744) | 0.02 | 0.006 |
| Ventilator Support | ||||||
| Rate | 52.8 ± 34.4 (12695) | 55.9 ± 35.8 (1844) | 50.6 ± 34.5 (2681) | 52.9 ± 34.0 (8170) | < .0001 | 0.0006 |
| FIO2 | 99.3 ± 4.8 (14114) | 99.3 ± 4.9 (2070) | 99.2 ± 5.4 (3007) | 99.3 ± 4.5 (9037) | 0.30 | 0.73 |
| PIP | 41.6 ± 10.7 (12583) | 42.6 ± 11.1 (1827) | 41.1 ± 10.8 (2660) | 41.5 ± 10.5 (8096) | < .0001 | < .0001 |
| PEEP | 4.7 ± 4.3 (11661) | 4.5 ± 3.9 (1766) | 4.8 ± 4.6 (2436) | 4.7 ± 4.2 (7459) | 0.05 | 0.03 |
| MAP | 19.5 ± 5.8 (9640) | 19.6 ± 5.9 (1290) | 19.4 ± 6.2 (2123) | 19.4 ± 5.6 (6217) | 0.46 | 0.23 |
Available for 1990 and later
p1 p-value for test of equality between late preterm, early term, full term
p2 p-value for test of equality between late preterm and full term only
Sepsis and respiratory distress syndrome (RDS) were the most common primary diagnoses among late preterm infants (29.9% and 28.2%, respectively); they were less likely to have a primary diagnosis of meconium aspiration syndrome than early term and full term infants (Table I). Early term and late preterm infants had the highest incidence of persistent pulmonary hypertension. Compared with term infants, late preterm infants had longer runs on ECMO and were more likely to die on ECMO or have ECMO support discontinued prior to lung recovery.
Univariate Analysis
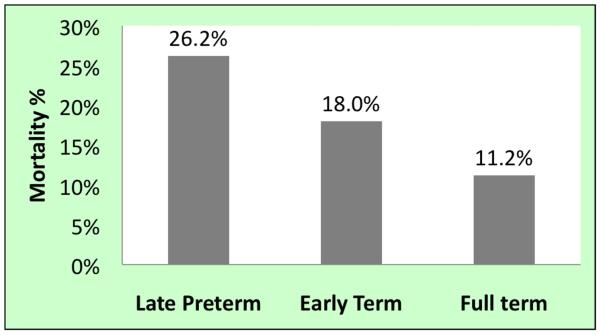
Univariate associations with ECMO mortality are presented in Table II. Late preterm infants had the highest mortality (26.2%), followed by early term (18%) and full term infants (11.2%) (Figure 2; available at www.jpeds.com). Compared with full term infants, late preterm infants had an unadjusted mortality OR of 2.81 (95% CI: [2.50, 3.16]) and early term infants had an OR of 1.73 (1.55, 1.94). Infants with lower birth weights fared worse on ECMO, with 26.4% mortality in the 2000 – 2499 g birth weight group compared with 11.4% among infants weighing between 3500 and 3999 g. (unadjusted. OR [95% CI] = 2.78 [2.32, 3.33]).
Table 2.
Mortality on ECMO (unadjusted)
| % Mortality | Total | Unadjusted. | OR (95% CI) | |
|---|---|---|---|---|
| Gestational Age | ||||
| Late pre-term | 26.2 | (560/2135) | 2.81 | (2.50, 3.16) |
| Early term | 18.0 | (560/3119) | 1.73 | (1.55, 1.94) |
| Full term | 11.2 | (804/7632) | ref. | ref. |
| Sex | ||||
| Female | 15.1 | (901/5966) | 1.03 | (0.94, 1.13) |
| Male | 14.7 | (1254/8506) | ref. | ref. |
| Race | ||||
| White | 17.2 | (1157/5580) | ref. | ref. |
| Black | 11.2 | (333/2974) | 0.61 | (0.53, 0.69) |
| Hispanic | 14.4 | (217/1510) | 0.81 | (0.69, 0.95) |
| Asian | 15.2 | (67/440) | 0.87 | (0.66, 1.13) |
| Other | 17.9 | (93/519) | 1.05 | (0.83, 1.33) |
| Birth Weight | ||||
| < 2000g | 24.9 | (84/337) | 2.58 | (1.97, 3.36) |
| 2000 – 2499g | 26.4 | (234/888) | 2.78 | (2.32, 3.33) |
| 2500 – 2999g | 20.5 | (609/2968) | 2.00 | (1.75, 2.29) |
| 3000 – 3499g | 13.0 | (607/4659) | 1.16 | (1.02, 1.33) |
| 3500 – 3999g | 11.4 | (426/3732) | ref. | ref. |
| 4000 – 4499g | 9.9 | (149/1501) | 0.86 | (0.70, 1.04) |
| 4500 – 4999g | 10.6 | (38/357) | 0.92 | (0.65, 1.31) |
| ≥ 5000g | 16.3 | (14/72) | 1.51 | (0.84, 2.70) |
| Primary Diagnosis | ||||
| Sepsis | 24.8 | (589/2375) | 5.10 | (4.44, 5.85) |
| PPHN | 20.1 | (613/3045) | 3.89 | (3.40, 4.46) |
| RDS | 14.0 | (180/1290) | 2.51 | (2.08, 3.02) |
| Respiratory Insufficiency | 24.6 | (94/382) | 5.04 | (3.91, 6.50) |
| Meconium Aspiration | 6.1 | (396/6514) | ref. | ref. |
| Other | 31.2 | (281/900) | 7.01 | (5.90, 8.35) |
| Labor Status 1 | ||||
| Emergent C-section | 13.1 | (370/2817) | 0.66 | (0.58, 0.75) |
| Elective cesarean | 11.5 | (370/3231) | 0.56 | (0.50, 0.64) |
| Vaginal | 18.7 | (1090/5836) | ref. | ref. |
| Ventilator Type | ||||
| Conventional | 14.6 | (743/4355) | ref. | ref. |
| HFO | 15.8 | (523/2788) | 1.10 | (0.97, 1.24) |
| Other HFV | 14.8 | (120/690) | 1.02 | (0.83, 1.26) |
Figure 2.
Mortality on ECMO in neonates with hypoxic respiratory failure by gestational age group.
A tabulation of complications on ECMO by GA (Table III; available at www.jpeds.com), reveals that late preterm infants were more likely to require hemofiltration/dialysis on ECMO (late preterm: 19.3%, early term: 15.5%, full term: 12.1%, p < 0.0001) and were more prone to renal complications overall. They were more likely to have intraventricular hemorrhage (late preterm: 12.3%, early term: 7.6%, full term: 3.6%, p<0.0001) and other neurological complications on ECMO. They also experienced increased mechanical, metabolic and infectious complications on ECMO.
Table 3.
online: Complications on ECMO (unadjusted)
| Late Preterm | Early Term | Full Term | |||||
|---|---|---|---|---|---|---|---|
| n = 2135 | n = 3119 | n = 9274 | |||||
| % | (n) | % | (n) | % | (n) | p | |
| Hemorrhagic | 17.3 | 369 | 19.7 | 614 | 19.5 | 1805 | 0.05 |
| Mechanical | 39.3 | 840 | 38.6 | 1205 | 35.5 | 3290 | 0.0002 |
| Metabolic | 24.6 | 526 | 21.6 | 675 | 15.4 | 1426 | < .0001 |
| Neurological | 33.5 | 715 | 22.0 | 687 | 17.7 | 1643 | < .0001 |
| IVH | 12.3 | 263 | 7.6 | 237 | 3.6 | 337 | < .0001 |
| Other | 24.0 | 512 | 16.1 | 503 | 15.0 | 1395 | < .0001 |
| Renal | 25.3 | 541 | 21.1 | 659 | 17.0 | 1573 | < .0001 |
| Hemofiltration/dialysis | 19.3 | 412 | 15.5 | 484 | 12.1 | 1125 | < .0001 |
| Infection | 6.9 | 148 | 6.6 | 207 | 5.5 | 511 | 0.009 |
| Culture proven infection | 6.5 | 139 | 6.0 | 187 | 5.1 | 476 | 0.02 |
| Major cardiovascular | 32.9 | 702 | 34.6 | 1079 | 31.9 | 2956 | 0.02 |
| PDA | 5.0 | 107 | 5.5 | 171 | 4.3 | 400 | 0.02 |
| Other | 30.7 | 655 | 32.1 | 1001 | 29.9 | 2777 | 0.07 |
| Pulmonary hemorrhage | 4.1 | 87 | 3.4 | 107 | 3.3 | 301 | 0.16 |
| Pneumothorax | 5.0 | 107 | 5.2 | 163 | 5.4 | 500 | 0.76 |
Multivariate Analysis
The results for our multivariate assessment of differential mortality risk by GA are summarized in Table IV. Our starting model (Model 1) considered GA, adjusting for pH, apgar 1 min, sexand year. All interactions with GA were found to be non-significant and were dropped, resulting in a highly significant final model (p < .0001). No factors were found to be confounders of GA, but the following variables were significantly associated with mortality: pH (p < .0001), Apgar 1 (p < .0001), sex(p = 0.006), and year (p = 0.005). Gestational age group was a highly significant predictor of mortality (p < .0001), and the adjusted mortality OR for late preterm (compared with full term) infants was 2.73 (95% CI: [2.40, 3.09]) and for early term infants it was 1.63 (1.45, 1.85).
Table 4.
Adjusted risk of mortality on ECMO, by gestational age
| Mortality OR | ||||||
|---|---|---|---|---|---|---|
| Late preterm vs. Full term |
Early term vs. Full term |
|||||
| total n | unadj.OR | (95% CI) | unadj.OR | (95% CI) | ||
| Unadjusted | 14,528 | 2.81 | (2.50, 3.16) | 1.73 | (1.55, 1.94) | |
| Model | model n | adj. OR | (95% CI) | adj. OR | (95% CI) | |
| 1. | Adjusted for pH, Apgar 1, pO2, gender, year | 13,754 | 2.73 | (2.40, 3.09) | 1.63 | (1.45, 1.85) |
|
| ||||||
| 2. | Model 1 with race, delivery status | 11,008 | ||||
| interaction-specific results: | ||||||
| White | 1.47 | (1.32, 1.64) | 0.99 | (0.89, 1.09) | ||
| Black | 3.60 | (2.53, 5.11) | 1.44 | (1.05, 1.99) | ||
| Hispanic | 2.27 | (1.43, 3.59) | 1.37 | (0.92, 2.05) | ||
| Asian | 2.59 | (1.22, 5.49) | 1.26 | (0.60, 2.67) | ||
| Other | 1.80 | (0.86, 3.75) | 1.15 | (0.63, 2.10) | ||
|
| ||||||
| 3. | Model 1 with weight and primary diagnosis | 13,736 | 1.31 | (1.12, 1.53) | 1.06 | (0.93, 1.20) |
|
| ||||||
| 4. | Model 3 with post-ECMO variables | 13,730 | 1.15 | (0.97, 1.36) | 1.04 | (0.89, 1.19) |
Our next analysis (Model 2) added the two important control variables of race and delivery method for which data were limited (as explained above). For this subset model (n = 11008/11995, 91.8% of data from 1990 onwards), there was a highly significant interaction of race with GA (p < 0.0001) that resulted in considerable heterogeneity of GA risk between races (Table IV). White infants had the least comparative risk between late preterm and full term (OR, [95% CI]: 1.47 [1.32, 1.64]), and had no difference between early term and full term (0.99, [0.89, 1.09]). In contrast, late preterm black infants had the most elevated risk (3.60, [2.53, 5.11]) and early term risk (1.44, [1.05, 1.99]). Indeed, this effect is partially due to markedly better survival of black full term infants (black vs. white full term infant mortality OR = 0.72, 95% CI: 0.61, 0.85). A similar effect was observed for Hispanic infants.
The third model included birth weight and primary diagnosis. Although these covariates are important predictors of infant mortality on ECMO, they are partially resultant from GA and thus may play an intermediate role in the casual pathway from GA to ECMO mortality (i.e.: their inclusion in a model would bias the observed GA effect towards the null hypothesis). Understanding this, the inclusion of these variables in the original logistic model allows us to see how much of the GA effect is affected after incorporating these two variables. As shown in Table IV, the mortality OR for late preterm vs. full term infants was substantially diminished to 1.31 (95% CI: [1.12, 1.53]) as was that for early term vs. full term (1.06, [0.93, 1.20]). Birth weight group was significantly associated with mortality (p < .0001), and the adjusted OR for infants weighing 2000 – 2499g compared with 3500-3999g was 1.97 (95% CI: [1.60, 2.44]). Primary diagnosis was also highly significant (p < .0001) and compared with a diagnosis of meconium aspiration, infants with sepsis had a mortality OR of 4.11 (95% CI: [3.48, 4.85]), those with PPHN had and OR of 3.61 ([3.10, 4.21]), those with RDS had and OR of 2.11 (95% CI: [1.69, 2.64]), and those with respiratory insufficiency had an OR of 3.88 (95% CI: [2.92, 5.15]) (Table V; available at www.jpeds.com).
Table 5.
Adjusted risk of mortality for Late Preterm vs. Full Term infants. (Online only)
| Mortality OR | ||||
|---|---|---|---|---|
| Model | model n | adj. OR | (95% CI) | |
| 1. | Adjusted for pH, Apgar 1, pO2, gender, year | 13,754 | 2.73 | (2.40, 3.09) |
| pH(0.1 unit decrease) | 1.25 | (1.22, 1.28) | ||
| Apgar 1 (1 unit increase) | 1.05 | (1.03, 1.07) | ||
| pO2(10 unit increase) | 1.00 | (0.98, 1.01) | ||
| Female gender | 1.15 | (1.04, 1.27) | ||
| 2. | Model 1 with race, delivery status | 11,008 | ||
| Delivery mode | ||||
| Elective c-section | 0.69 | (0.60, 0.80) | ||
| Emergent c-section | 0.66 | (0.58, 0.77) | ||
| Vaginal | ref. | |||
| 3. | Model 1 with weight and primary diagnosis | 13,736 | 1.31 | (1.12, 1.53) |
| Birth weight | ||||
| < 2000g | 2.04 | (1.49, 2.79) | ||
| 2000 – 2499g | 1.97 | (1.60, 2.44) | ||
| 2500 – 2999g | 1.58 | (1.36, 1.85) | ||
| 3000 – 3499g | 1.04 | (0.90, 1.21) | ||
| 3500 – 3999g | ref. | |||
| 4000 – 4499g | 0.89 | (0.72, 1.10) | ||
| 4500 – 4999g | 0.94 | (0.64, 1.38) | ||
| ≥ 5000g | 1.18 | (0.63, 2.20) | ||
| Primary diagnosis | ||||
| Sepsis | 4.11 | (3.48, 4.85) | ||
| PPHN | 3.61 | (3.10, 4.21) | ||
| RDS | 2.11 | (1.69, 2.64) | ||
| Respiratory Insufficiency | 3.88 | (2.92, 5.15) | ||
| Meconium Aspiration | ref. | |||
| Other | 5.49 | (4.51, 6.70) | ||
| 4. | Model 3 with post-ECMO variables | 13,730 | 1.15 | (0.97, 1.36) |
| Hours on ECMO (48 hour increase) | 1.09 | (1.06, 1.12) | ||
| Complications | ||||
| Hemorrhagic | 1.54 | (1.36, 1.76) | ||
| Mechanical | 0.84 | (0.74, 094) | ||
| Metabolic | 1.29 | (1.13, 1.47) | ||
| Neurological | 3.65 | (3.24, 4.10) | ||
| Renal | 2.47 | (2.19, 2.79) | ||
| Infection | 1.92 | (1.58, 2.32) | ||
| Major cardiovascular | 1.20 | (1.06, 1.35) | ||
| Pulmonary hemorrhage | 3.30 | (2.62, 4.17) | ||
| Pneumothorax | 1.72 | (1.40, 2.12) | ||
Finally, we extended the model with birth weight and primary diagnosis to also include the post-ECMO variables of time on ECMO and complications on ECMO, which are also intermediate factors. Once this information was incorporated, no independent GA effect remained for late preterm vs. full term infants (1.15, [0.97, 1.36]) or early term vs. full term (1.04, [0.89, 1.19]).
Discussion
Their large size and presumed maturity notwithstanding, late preterm infants are now recognized to be at considerable risk for morbidity and mortality. This knowledge, coupled with the recent rise in late preterm births, has intensified the debate about the risks and benefits of medically indicated late preterm births. In this study, we were able to analyze outcomes of a large cohort of late preterm infants with severe hypoxic respiratory failure who were treated with ECMO. Our finding, that late preterm infants had poorer outcomes on ECMO than their more mature counterparts, underscores their developmental immaturity and vulnerability. Further, the large number of late preterm infants who required ECMO is surprising and not in keeping with the overall impression of their “mild” initial disease.
Late preterm infants were more likely to be male and non-Hispanic white and this is consistent with several previous reports.[19, 27, 28] Davidoff et al demonstrated that non-Hispanic whites comprised the majority of the increase in late preterm births.[28] Although non-Hispanic whites had a larger representation in our late preterm cohort, they fared better than black infants and had lower odds of death when compared with black infants. However, full term black infants had better survival on ECMO as compared with non-Hispanic whites. These divergent findings require further study.
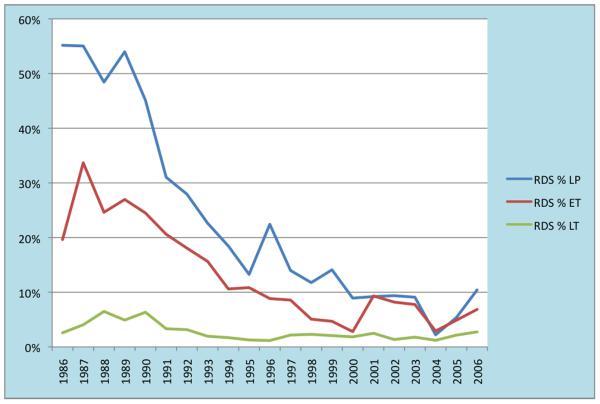
Nearly 23% of late preterm infants with severe hypoxic respiratory failure were born by elective cesarean section. This is higher than the 16.5% rate reported by Keszler et al in their 1985-91 data [18] and almost twice the estimated national rate of ~12% repeat cesarean sections of all live births in the United States [29]. Late preterm infants born by elective repeat cesarean section are over represented in the ECMO population. The lower mortality on ECMO in this group is somewhat surprising and cannot be explained, but probably reflects a bias towards more mature infants. Clark et al in their study of infants > 34 weeks also found that RDS was a leading cause of respiratory failure.[27] Sepsis, PPHN and RDS were the leading causes of respiratory failure among late preterm infants in our study, in contrast to other studies of ECMO patients where meconium aspiration syndrome was reported as the leading cause [30, 31]. RDS was the primary diagnosis in ~2.8% of the full term, 13.6% of the early term, and 28% in the late preterm ECMO population. Overall, data from the ELSO registry show that RDS has been declining as the primary diagnosis among the ECMO population (from ~55% in 1986 to 2% in 2004 among late preterm infants).This is likely due to the success of treatments such as surfactant, inhaled nitric oxide and high frequency ventilation. However, early term and late preterm infants in our study population were more likely to have a primary diagnosis of RDS than full term infants. Although the incidence of RDS seems to be stable among the full term infants, it seems to be rising again among the late preterm and early term infants, from 2% and 3% in 2004 to 10% and 7% in 2006 respectively; whether this trend will sustain remains to be seen (Figure 3; available at www.jpeds.com).
Figure 3.
Incidence of RDS as a primary diagnosis among neonates with severe hypoxic respiratory failure requiring ECMO. RDS among late preterm infants has declined from ~55% in 1986 to 2% in 2004. Though stable among the full term infants, it seems to be rising among the late preterm and early term infants, from 2% and 3% in 2004 to 10% and 7% in 2006 respectively.
The incidence of PPHN in our study population was significantly higher in late preterm and early term infants. The delayed clearance of lung fluid may be a significant contributor to progressive severe respiratory failure and PPHN[32]. This is consistent with our finding of the higher prevalence of elective c-section and PPHN in our late preterm infants. We have to mention that the primary diagnosis is assigned by the attending neonatologists at their respective institutions and often overlaps with other concurrent diagnoses.
Our study also validates recent reports of higher morbidity and mortality in early term infants (38-39 weeks).[29, 33] Late preterm and early term infants comprised 12.8% and 17.5% of all live births in the US respectively in 2006.[34] Their numbers were slightly higher in our ECMO population at 15% and 21% respectively. They had higher mechanical complications on ECMO, which could be explained partly by their longer ECMO runs, but they were also at higher risk for neurological (including IVH), metabolic and renal complications with increased risk of hemofilteration and dialysis. Tita et al in a recent study of cesarean births have shown a higher incidence of morbidity, including NICU stay or mechanical ventilation for infants born at 37 and 38 weeks [29]. We found that infants born only a few weeks prior to term gestation and even among those born early term had increased morbidity and mortality on ECMO. Given that in Tita's study nearly 36% of elective repeat cesarean sections were performed before 39 weeks, stricter guidelines and/or alternate strategies may be needed to prevent unintended/unnecessary elective repeat cesarean section births prior to 39 weeks.
Our logistic regression analysis attempted to determine the predictors of mortality by GA using three different models. When we included birth weight and primary diagnosis the odds of death decreased for late preterm infants compared with full term infants and the difference disappeared between early term and full term infants. However primary diagnosis and birth weight is a consequence of premature birth. For example RDS is more common among late preterm infants and therefore confounds the separate influences of GA and diagnosis at birth on mortality. As expected and consistent with previous studies, infants with lower birth weights had increased risk of death enetic abnormalities, missing GA, mismatched GA and birth weight.
Acknowledgments
Supported by the NIH (grant HL-2R01-063301 to L.J.).
Abbreviations
- ECMO
Extra corporeal membrane oxygenation
- ELSO
extra corporeal life support organization
- GA
gestational age
- OR
odds ratio
- TTN
transient tachypnea of the newborn
- PPHN
persistent pulmonary hypertension
- RDS
respiratory distress syndrome
- NICU
neonatal intensive care unit
- IVH
intra ventricular hemorrhage
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Bibliography
- 1.Raju TN. The problem of late-preterm (near-term) births: a workshop summary. Pediatric research. 2006;60:775–6. doi: 10.1203/01.pdr.0000246074.73342.1e. PHST- 2006/10/25 [aheadofprint] [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2005. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2007;56:1–103. [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH. Epidemiology of Neonatal Respiratory Failure in the United States . Projections from California and New York. Am J Respir Crit Care Med. 2001;164:1154–60. doi: 10.1164/ajrccm.164.7.2012126. [DOI] [PubMed] [Google Scholar]
- 4.Young PC, Glasgow TS, Li X, Guest-Warnick G, Stoddard G. Mortality of late-preterm (near-term) newborns in Utah. Pediatrics. 2007;119:e659–65. doi: 10.1542/peds.2006-2486. [DOI] [PubMed] [Google Scholar]
- 5.Tomashek KM, Shapiro-Mendoza CK, Davidoff MJ, Petrini JR. Differences in mortality between late-preterm and term singleton infants in the United States, 1995-2002. J Pediatr. 2007;151:450–6. doi: 10.1016/j.jpeds.2007.05.002. 6.e1. [DOI] [PubMed] [Google Scholar]
- 6.Roth-Kleiner M, Wagner BP, Bachmann D, Pfenninger J. Respiratory distress syndrome in near-term babies after caesarean section. Swiss Med Wkly. 2003;133:283–8. doi: 10.4414/smw.2003.10121. [DOI] [PubMed] [Google Scholar]
- 7.Hack M, Fanaroff AA, Klaus MH, Mendelawitz BD, Merkatz IR. Neonatal respiratory distress following elective delivery. A preventable disease? Am J Obstet Gynecol. 1976;126:43–7. doi: 10.1016/0002-9378(76)90462-2. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg RL, Nelson K. Iatrogenic respiratory distress syndrome. An analysis of obstetric events preceding delivery of infants who develop respiratory distress syndrome. Am J Obstet Gynecol. 1975;123:617–20. [PubMed] [Google Scholar]
- 9.Maisels MJ, Rees R, Marks K, Friedman Z. Elective delivery of the term fetus. An obstetrical hazard. Jama. 1977;238:2036–9. [PubMed] [Google Scholar]
- 10.Villar J, Carroli G, Zavaleta N, Donner A, Wojdyla D, Faundes A, et al. Maternal and neonatal individual risks and benefits associated with caesarean delivery: multicentre prospective study. Bmj. 2007;335:1025. doi: 10.1136/bmj.39363.706956.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 12.Engle WA, Tomashek KM, Wallman C. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390–401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 13.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372–6. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- 14.Rubaltelli FF, Bonafe L, Tangucci M, Spagnolo A, Dani C. Epidemiology of neonatal acute respiratory disorders. A multicenter study on incidence and fatality rates of neonatal acute respiratory disorders according to gestational age, maternal age, pregnancy complications and type of delivery. Italian Group of Neonatal Pneumology. Biol Neonate. 1998;74:7–15. doi: 10.1159/000014005. [DOI] [PubMed] [Google Scholar]
- 15.Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30:28–33. doi: 10.1053/j.semperi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Heritage CK, Cunningham MD. Association of elective repeat cesarean delivery and persistent pulmonary hypertension of the newborn. Am J Obstet Gynecol. 1985;152:627–9. doi: 10.1016/s0002-9378(85)80034-x. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Mitchell AA. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics. 2007;120:e272–82. doi: 10.1542/peds.2006-3037. [DOI] [PubMed] [Google Scholar]
- 18.Keszler M, Carbone MT, Cox C, Schumacher RE. Severe respiratory failure after elective repeat cesarean delivery: a potentially preventable condition leading to extracorporeal membrane oxygenation. Pediatrics. 1992;89:670–2. [PubMed] [Google Scholar]
- 19.Escobar GJ, Greene JD, Hulac P, Kincannon E, Bischoff K, Gardner MN, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125–31. doi: 10.1136/adc.2003.039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laptook A, Jackson GL. Cold stress and hypoglycemia in the late preterm (“near-term”) infant: impact on nursery of admission. Seminars in perinatology. 2006;30:24–7. doi: 10.1053/j.semperi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Bhutani VK, Johnson L. Kernicterus in late preterm infants cared for as term healthy infants. Seminars in perinatology. 2006;30:89–97. doi: 10.1053/j.semperi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 22.The Consortium on Safe L Respiratory Morbidity in Late Preterm Births. JAMA. 304:419–25. doi: 10.1001/jama.2010.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandrappa A, Jain L. Elective cesarean section: its impact on neonatal respiratory outcome. Clinics in perinatology. 2008;35:373–93. vii. doi: 10.1016/j.clp.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothen HU, Sporre B, Engberg G, Wegenius G, Hogman M, Hedenstierna G. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology. 1995;82:832–42. doi: 10.1097/00000542-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Prevention of atelectasis during general anaesthesia. Lancet. 1995;345:1387–91. doi: 10.1016/s0140-6736(95)92595-3. [DOI] [PubMed] [Google Scholar]
- 26.Brown KL, Sriram S, Ridout D, Cassidy J, Pandya H, Liddell M, et al. Extracorporeal membrane oxygenation and term neonatal respiratory failure deaths in the United Kingdom compared with the United States: 1999 to 2005. Pediatric Critical Care Medicine. 2010;11:60–5. doi: 10.1097/PCC.0b013e3181b0644e. [DOI] [PubMed] [Google Scholar]
- 27.Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251–7. doi: 10.1038/sj.jp.7211242. [DOI] [PubMed] [Google Scholar]
- 28.Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. UID - 19129525. The New England journal of medicine. 2009;360:111–20. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Rourke PP, Crone RK, Vacanti JP, Ware JH, Lillehei CW, Parad RB, et al. Extracorporeal Membrane Oxygenation and Conventional Medical Therapy in Neonates With Persistent Pulmonary Hypertension of the Newborn: A Prospective Randomized Study. Pediatrics. 1989;84:957–63. [PubMed] [Google Scholar]
- 31.Roy BJ, Rycus P, Conrad SA, Clark RH. The changing demographics of neonatal extracorporeal membrane oxygenation patients reported to the Extracorporeal Life Support Organization (ELSO) Registry. Pediatrics. 2000;106:1334–8. doi: 10.1542/peds.106.6.1334. [DOI] [PubMed] [Google Scholar]
- 32.Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Seminars in perinatology. 2006;30:34–43. doi: 10.1053/j.semperi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Cheng YW, Nicholson JM, Nakagawa S, Bruckner TA, Washington AE, Caughey AB. Perinatal outcomes in low-risk term pregnancies: do they differ by week of gestation? Am J Obstet Gynecol. 2008;199:e1–7. doi: 10.1016/j.ajog.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2006. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2009:1–102. [PubMed] [Google Scholar]