Abstract
Life span researchers have long been interested in how and why fundamental aspects of human ontogeny differ between cohorts of people who have lived through different historical epochs. When examined at the same age, later born cohorts are often cognitively and physically fitter than earlier born cohorts. Less is known, however, about cohort differences in the rate of cognitive aging and if, at the very end of life, pervasive mortality-related processes overshadow and minimize cohort differences. We used data on 5 primary mental abilities from the Seattle Longitudinal Study (Schaie, 2005) to compare both age-related and mortality-related changes between earlier born cohorts (1886–1913) and later born cohorts (1914–1948). Our models covary for several individual and cohort differences in central indicators of life expectancy, education, health, and gender. Age-related growth models corroborate and extend earlier findings by documenting level differences at age 70 of up to 0.50 SD and less steep rates of cognitive aging on all abilities between 50 and 80 years of age favoring the later born cohort. In contrast, mortality-related models provide limited support for positive cohort differences. The later born cohort showed steeper mortality-related declines. We discuss possible reasons why often reported positive secular trends in age-related processes may not generalize to the vulnerable segment of the population that is close to death and suggest routes for further inquiry.
Keywords: cognitive aging, cohort, terminal decline, intelligence
Life span psychological and life course sociological research has long examined the extent to which historical processes and contextual factors shape fundamental aspects of individual development (Baltes, Cornelius, & Nesselroade, 1979; Bengtson, Biblarz, & Roberts, 2002; Bronfenbrenner, 1986; Elder, 1974; Riley, 1973; Rosow, 1978; Ryder, 1965; Schaie, 1965; Settersten, 2005). For example, evidence of mean-level differences in cognition and health suggests that, when examined at the same age range, later born cohorts are typically cognitively and physically fitter than earlier born cohorts (Alwin, 2008; Bäckman, Small, Wahlin, & Larsson, 2000; Crimmins, Hayward, & Saito, 1996; Flynn, 2007; Manton, Gu, & Lowrimore, 2008; Schaie, 2005). However, little is known about the extent to which rates of cognitive aging differ between cohorts. It is also an open question whether mortality-related processes at the very end of life differ between successive cohorts. Alternatively, terminal decline may be so pervasive that it overshadows and erases prior cohort differences. In the current study, we used cohort-sequential, multiwave data from the Seattle Longitudinal Study (SLS) to describe and examine how two broadly defined cohorts (born 1886–1913 vs. 1914–1948) differ in rates of cognitive aging and terminal cognitive decline. To separate the expected cognitive cohort differences from known individual and cohort differences in other domains, our models covary for central indicators of life expectancy, education, health, and gender.
Understanding the size, direction, and mechanisms underlying cohort differences in cognitive aging and terminal decline is important for several reasons. First, separating the effects of cohorts and historical time from those processes underlying aging and dying has long been acknowledged as a challenging but necessary condition for fully understanding the intricacies of development (Baltes et al., 1979; Schaie, 1965). Second, cohort differences provide important illustrations of key tenets of life span theory about the malleability and plasticity of ontogenetic development (Baltes, Lindenberger, & Staudinger, 2006). For example, the existence of positive cohort effects is generally taken to indicate that culture-based efforts result in an improvement of individual functioning over historical time. Cohort effects at advanced ages or in the context of mortality-related processes would indicate that sociocultural factors operate even among particularly vulnerable segments of the population (for a discussion, see Baltes & Smith, 2003), with potential implications for interventions. In addition, positive cohort differences in level of function or in rates at which that function changes over time bear profound implications for extending the phases of successful and productive aging, for example, by postponing the onset of cognitive impairments (Rowe & Kahn, 1997; Ryff & Singer, 1998). In addition to these conceptual perspectives, important methodological issues are addressed by the examination of cohort differences. For example, from a design perspective, the confounding of age effects and cohort effects constitutes a central threat to internal and external validity and/or a necessary element to consider in examining when and how development proceeds (e.g., generalization restricted to a given cultural epoch and cultural setting).
Cohort Differences in Cognitive Aging
Cohort differences in cognitive aging trajectories have been the focus of several recent longitudinal studies of older adults, but findings are mixed and do not allow for conclusive inferences. Finkel, Reynolds, McArdle, and Pedersen (2007) applied growth curve models to the Swedish Adoption/Twin Study on Aging (SATSA) data and compared the age-related changes occurring from age 62 to age 78 between two broadly defined birth cohorts. The results suggested that participants born during the first and the second quarters of the 20th century did not differ in rates of cognitive aging for a variety of abilities, including performances on spatial and verbal tests. Similarly, Zelinski and Kennison (2007) applied growth models to data from the Long Beach Longitudinal Study (LBLS) and reported that the rate of cognitive aging from age 55 to age 87 on measures of reasoning, spatial abilities, and vocabulary showed minimal if any differences between age-matched cohorts born 1893–1923 and those born 1908–1940.1 In contrast, Bowles, Grimm, and McArdle (2005), using growth models and data from the national General Social Survey (GSS), found cohort differences in rates of decline on vocabulary knowledge. Relative to those born later, cohorts born before 1940 showed steeper age-related declines on advanced vocabulary knowledge, although such differences were not evident at overlapping age ranges (ages 35–45 and 55–65; cf. Finkel et al., 2007). Finally, Schaie, Willis, and Pennak (2005) reported cohort differences in rates of cognitive aging among SLS participants that typically favored later born cohorts, particularly on reasoning and verbal meaning abilities. Taken together, these reports suggest that cohort differences in levels of cognitive functioning known from studies of children and young adults (see Flynn, 2007) extend to midlife and old age. However, it remains to be seen whether the gains in levels of cognitive functioning held by later born cohorts mitigate cognitive aging (i.e., the rates of age-related changes across adulthood and old age).
All of the above studies examined cohorts across overlapping age ranges in later adulthood and old age, but, unlike the reports from the SLS, reports from the SATSA, the LBLS, and the GSS defined birth cohorts very broadly and made use of contemporary techniques in the analysis of change (i.e., growth curve models). Our first objective in the present study therefore was an attempt to reconcile between-study differences in cognitive aging–cohort interactions by mimicking the procedures used in the other reports. To do so, we selected SLS participants from two broad and separable cohorts that had provided data over a 30-year overlapping age range, age 50 to age 80, and used growth curve methods to examine cohort differences in change over chronological age in Thurstone and Thurstone’s (1949) five primary mental abilities (PMA). These measures include both abilities recognized to evince normative declines relatively early in adulthood (e.g., inductive reasoning or spatial orientation) and abilities that begin to show normative declines only in later adulthood and old age (e.g., verbal meaning).
Cohort Differences in Terminal Cognitive Decline
Late-life changes in cognitive ability are often influenced by factors associated with impending death (e.g., terminal decline). Conceptual notions about the precipitous cognitive declines occurring at the very end of life (Kleemeier, 1962; Palmore & Cleveland, 1976; Riegel & Riegel, 1972; Siegler, 1975) have received strong empirical support over the last two decades (for overviews, see Bäckman & MacDonald, 2006; Small & Bäckman, 1999). Recently, several large-scale longitudinal studies have used time to death as a time metric for operationalizing and examining such mortality-related processes (Gerstorf et al., 2008, 2010; Sliwinski et al., 2006; Wilson, Beck, Bienias, & Bennett, 2007; Wilson, Beckett, Bienias, Evans, & Bennett, 2003).
Of pivotal interest is the extent to which cohort differences exist in mortality-related cognitive decline. The broader conceptual argument for such an examination is borrowed from the compression-of-morbidity perspective, according to which positive secular trends result in a delayed onset of chronic diseases and an increasingly short period of morbidity at the very end of life (Fries, 1980). Generally consistent with this notion, empirical evidence indicates that disability hazards are indeed lower for later born cohorts (Crimmins et al., 1996; Graham, Blakely, Davis, Sporle, & Pearce, 2004; Manton et al., 2008; Robine, Romieu, & Michel, 2003). However, such reports have almost exclusively relied upon comparing birth cohorts at a similar range of chronological ages. More direct tests of the compression-of-morbidity notion require moving from an age metric to a time-to-death metric. To the best of our knowledge, ours is the first study to compare birth cohorts at a similar range of years of time to death.2 A prediction of the compression-of-morbidity notion in the context of terminal cognitive decline is that later born cohorts are expected to experience shallower mortality-related declines than are earlier born cohorts. The factors contributing to such positive secular trends may be the same as those used to explain cohort differences in cognitive aging, including technological advances (e.g., access and organization of information; expanding use of informatics in daily life; Schaie & Charness, 2003), improvements in health care (e.g., treatment of cardiovascular disease), demographic trends (e.g., higher education, greater job complexity), or individual behaviors (e.g., engagement in preventive health behaviors).
It is an open empirical question to what extent positive secular trends reported for age-related processes generalize to mortality-related processes in cognitive functioning and change at the very end of life. The pervasive nature of mortality-related processes may bring about a sharp end to the possibilities afforded by positive secular trends. As a consequence, preexisting (cohort) differences may be minimized or erased at the end of life by mortality-related processes. Our second objective in this study was to address these questions and determine if cohort differences exist in terminal decline across the PMA.
The Current Study
Following in the footsteps of previous examinations of differences among 7-year birth cohorts in the SLS (for overview, see Schaie, 2005, 2008), we compare the cognitive aging and terminal decline trajectories of two broadly defined cohorts (born 1886–1913 vs. 1914–1948) of SLS participants. Defining cohorts with respect to the onset of World War I was in part based on methodological necessity (i.e., to minimize procedural differences with previous cohort studies) and data availability (e.g., need for sufficient sample size and number of deceased participants).
To ensure that the expected cohort differences do not simply reflect well-known differences in cognitive functioning and change related to distinct early-life or later life experiences of the two cohorts, our models include education, health, and gender as covariates. For example, major legislative changes in the first 20 years of the 20th century (e.g., compulsory school attendance, extended length of the school year) contributed to cohort differences in educational attainment. Such quantitative differences in schooling may underlie cohort differences in intellectual performance (e.g., by promoting cognitive reserve; Rönnlund, Nyberg, Bäckman, & Nilsson, 2005; Stern, Albert, Tang, & Tsai, 1999). We also covary for possible health differences in later life experiences. In particular, the earlier born cohort reached later adulthood and old age in the 1960s and 1970s, whereas our later born cohort largely represented the parents of the baby boomers, having reached old age in the 1980s and 1990s. Cardiovascular and major circulatory diseases, such as heart disease and stroke, have shown persistent declines over the past 30 years, particularly among men (see Aldwin, Spiro, & Park, 2006; Manton et al., 2008). Given that health-related indices are among the factors most consistently linked to adult cognitive changes (for review, see Anstey & Christensen, 2000), substantial advances in and broader access to nutrition, hygiene, health behaviors, and medical care among recent generations can conjointly be expected to have contributed to preserved cognitive functioning into older ages and until the end of life. Another pivotal health variable on which cohorts may differ is cancer. Annual incidence rates for all forms of cancer had reached an all-time high for people who had entered old age in the 1980s and 1990s (Altekruse et al., 2009). Finally, gender differences in cognitive functioning have long been documented, with men typically outperforming women on tasks assessing visuospatial and arithmetric processing, whereas women typically outperform men on tasks involving reasoning and verbal abilities (Maitland, Herlitz, Nyberg, Backman, & Nilsson, 2004; Meinz & Salthouse, 1998). Less consistent evidence has been gathered regarding gender differences in cognitive change in later adulthood and old age (Finkel, Reynolds, McArdle, Gatz, & Pederson, 2003), but some studies indeed have reported gender-differential change (Schaie, 1994; Zelinski & Stewart, 1998).
In our analysis, we asked two sets of questions. First, we used contemporary methods for the analysis of change and examined cohort differences in the rate of cognitive aging. Guided by earlier findings from the SLS (Schaie, 2005), we expected that the later born cohort would show shallower age-related cognitive declines than the earlier born cohort. Second, we adapted the analyses to explore whether and how cohorts differed in the rate of terminal decline. Guided by notions of terminal decline (Bäckman & MacDonald, 2006), we expected that the pervasive nature of mortality-related processes might diminish or even nullify previously existing positive cohort differences at the very end of life. All our models covaried for known individual and cohort differences, including life expectancy and indicators of education, health, and gender.
Method
Data were drawn from the SLS, an ongoing interdisciplinary longitudinal panel study that was launched in 1956. Detailed descriptions of variables and procedures can be found in Schaie (2005). Select information particularly relevant to the present study is presented below.
Participants and Procedure
The SLS is a cohort-sequential study that has collected data on close to 6,000 participants between the ages of 22 and 101 years. Participants were recruited randomly from gender and age/cohort groups within the membership of a large health maintenance organization (HMO) in the Seattle, Washington, area. The sampling frame was a community-dwelling population representing a wide range of occupational, educational, and economic backgrounds (Schaie, 2005). Data have been collected at 7-year intervals since 1956. With each wave, new participants were recruited over the original age range (22–70 years) plus an additional 7-year interval to match the ages reached by the original sample. All participants were able to complete the measurement battery.
Included in the subsample analyzed here were all participants from the SLS who (a) were born between 1886 and 1913 (earlier born cohort) or 1914 and 1948 (later born cohort); (b) provided one or more data points for one or more cognitive measures; (c) were between age 50 and age 80 (for the age-based change models) or were within 25 years of their death and had provided one or more data points within 10 years of their death (for the mortality-based change models). In total, the longitudinal data spanned 49 years with eight waves obtained at 7-year intervals (1956, 1963, 1970, 1977, 1984, 1991, 1998, and 2005).
Measures
To examine cognitive change in the SLS, we made use of those ability measures that have been assessed in every wave since study inception. These include a set of five subtests from the 1948 PMA 11-17 version of Thurstone’s Primary Mental Abilities Test (Thurstone & Thurstone, 1949).
Spatial Orientation is a test requiring the ability to visualize object rotation in two-dimensional space. Participants were asked to identify each of six options that represent a direct rotation (i.e., not mirror images) of a given stimulus figure. Inductive Reasoning constitutes a test of rule induction from an alphabetic series and involves logical problem solving and planning. Participants were asked to identify patterns in a letter series and to select from among six items the one that logically followed in the stimulus sequence. Word Fluency represents the ability to apply a lexical rule and retrieve words from long-term storage. Participants were asked to list as many words as possible beginning with the letter S in 5 minutes; the test is scored as the number of valid S words. Number ability was measured with a test assessing simple addition skills that asked participants to decide whether or not a given problem was solved correctly. Number scores were based on the frequency of correct responses minus the frequency of wrong responses. Verbal Meaning is a test of recognition vocabulary. Out of four alternatives, participants were asked to identify the correct synonym of a given word. All tests showed high test–retest reliabilities over 1 month (based on N = 705; r ≥ .78; see Schaie, 2005).
Covariates
Information about age at death was obtained from family members, Social Security death records, or the HMO. Gender was coded as 0 for men and 1 for women. Information about each participant’s educational attainment (schooling in number of years) was obtained from the self-administered personal data form. Records of (complete) medical histories are available for a subset of participants. Medical data were abstracted and coded according to the International Classification of Diseases (ICD; U.S. Public Health Service, 1968). Interrater reliabilities were high (Cronbach’s α > .90; for details, see Bosworth & Schaie, 1997; Hertzog, Schaie, & Gribbin, 1978). Since 1997, the HMO has provided computerized ICD disease codes. These codes were then used to construct a circulatory disease indicator and a cancer indicator, operationally defined as whether (1) or not (0) participants had at any point been diagnosed with either of these diseases. Diagnoses of the circulatory system included hypertension, hypotension, ischemic heart disease, (acute) myocardial infarction, cerebrovascular disease, or other forms of heart disease. Cancer diagnoses included malignant neoplasms of esophagus, stomach, liver and intrahepatic bile ducts, pancreas, female breast, prostate, trachea, bronchus, and lung.
Time-as-process metrics: Age and time to death
Age at each wave was calculated as the number of years since an individual’s birth (centered at 70 years). Time to death at each wave was calculated as the difference between the year of assessment and the year of an individual’s death. Both time metrics were coded with integer number of years. We note that age and mortality models are based on different subsamples that only partly overlap. Thus, the two change models were not directly compared with one another to determine which time variable provided a better representation of the data (see Ram, Gerstorf, Fauth, Zarit, & Malmberg, 2010).
Data Preparation
We scaled the raw scores on all cognitive tests to T scores (M = 50, SD = 10), using the data for all cross-sectional participants through 2005 as the base population (N ≈ 6,000). Table 1 provides descriptive statistics for the two cohorts on our covariates, separately for the two sets of models. It can be seen that our sample is larger for the cognitive aging models (N = 1,980) than for our terminal decline models (N = 891). Nevertheless, the general pattern of cohort differences is consistent in both sets of SLS subsamples, with the later born cohort having experienced more years of education and a lower risk of being diagnosed with circulatory diseases. No cohort differences were found in the frequency of cancer diagnoses.
Table 1.
Descriptive Statistics for the Measures Entered Into the Models of Cognitive Aging and Terminal Decline, Separately by Cohort
| Cognitive aging models |
Terminal decline models |
|||
|---|---|---|---|---|
| Variable | Earlier born | Later born | Earlier born | Later born |
| Years of birth | 1883–1913 | 1914–1948 | 1883–1913 | 1914–1948 |
| N | 1,242 | 738 | 603 | 288 |
| Education | 12.61a (3.47) | 14.20b (2.63) | 12.90a (3.57) | 14.34b (2.79) |
| % circulatory diseases | 61a | 48a | 59a | 47b |
| % cancer | 18a | 17a | 16a | 18a |
| % women | 53a | 57a | 51a | 40b |
| Age at study entry | 62.00a (10.37) | 46.52b (8.77) | ||
| Age at death | 83.75a (7.96) | 78.88b (10.58) | ||
Note. Age is in years. Parenthetical values are standard deviations. Subscripts that differ between columns for a given model are statistically significant at p < .05.
Also of note for the terminal decline model are cohort differences in the age at first assessment and the age at death. The design characteristics of our study meant that participants in the later born cohort were tested at and died at earlier ages relative to those in the earlier born cohorts. To examine if secular trends emerge in terminal decline that are independent of known cohort differences, we statistically controlled for these differences in our models (i.e., age at Time 1 [T1] and age at death were used as covariates), but note that the later born cohort likely was still somewhat favored regarding our research questions. In the age models, in contrast, our objective was to minimize procedural differences to other cohort reports (Bowles et al., 2005; Finkel et al., 2007; Zelinski & Kennison, 2007). We thus followed the procedures used in these reports and did not include mortality information in these age models. A considerable number of participants included in the analyses were still alive (earlier born cohort: 24%; later born cohort: 55%) and thus did not lend themselves to the examination of mortality-related questions.
Data Analysis
To examine cohort differences in cognitive aging and terminal decline, we examined age-related and mortality-related representations of cognitive change and how these representations of change differed by cohort membership, independent of differences in education, circulatory disease, cancer, gender, age at assessment, and age at death. To do so, we first fitted separate growth curve (i.e., multilevel) models for each of the five cognitive abilities over chronological age and effectively modeled interindividual differences in how each individual’s abilities changed from age 50 to age 80 years. We proceeded in an analogous fashion and fitted separate growth curve models for the five abilities over time to death, modeling how abilities changed in relation to impending mortality (i.e., up to 25 years prior to death). These models were specified as
| (1) |
where person i’s ability at time t, abilityti, is a function of an individual-specific intercept parameter (β0i), individual-specific linear and quadratic slope parameters (β1i and β2i) that capture the rate and acceleration of change per year over the selected time metric (age or time-to-death), and residual error (eti). Following standard multilevel/latent growth modeling procedures (Ram & Grimm, 2007; Singer & Willett, 2003), we modeled individual-specific intercepts, β0i, and slopes, β1i and β2i, (from the Level 1 model given in Equation 1) as
| (2) |
(i.e., Level 2 model), where γ00, γ10, and γ20 are sample means and u0i and u1i are individual deviations from those means that are assumed to be multivariate normally distributed, correlated with each other, and uncorrelated with the residual errors, eti.
To examine the extent to which the between-person variance in the trajectories (over age or time to death) was related to birth cohort, we expanded the growth model by adding the cohort membership variable as a predictor at the between-person level (Level 2). To control for the effects of other factors known to differ between individuals and cohorts, we covaried for interindividual differences in education, circulatory disease, cancer, and gender, as well as for the mortality-related change models, age at study entry, and age at death. Cohort and the covariates were effect coded/centered, so that the regression parameters indicated the average trajectory (across all individuals) and the extent of differences associated with a particular variable (rather than for a particular group). Positive parameters indicate differences favoring the later born cohort, individuals with higher levels of education, individuals with circulatory disease, those with cancer, and women. The expanded age-related change model took the form
| (3) |
In the mortality-related modes, we additionally took into account the effects of differences in age (at study entry, as a “cross-sectional” between-person difference) and age at death:
| (4) |
Individual deviations for the quadratic slope (i.e., quadratic random effects, u2i,) were tested but were not significant for six of the 10 models. For parsimony, main effects for cohort on the curvature of the average change trajectories were tested in absence of the random effects. Models were fit to the data with SAS (Proc Mixed; Littell, Miliken, Stoup, & Wolfinger, 1996). The time variable was centered at age 70 years in the age-related change models and at 3 years prior to death in the mortality-related change models. As a consequence, intercept means, intercept variances, intercept–slope covariances, and the effects of the covariates (including cohort) were interpreted to indicate effects at age 70 years and at 3 years prior to death, respectively. Interaction terms with the cohort variable were tested for all covariates, with only statistically significant terms retained in the final models. We note that the inclusion of age at study entry, age at death, education, circulatory disease, cancer, and gender into our models served to covary for possible confounds and were also attrition-informative variables. These factors thus helped to accommodate longitudinal selectivity under the assumption that incomplete data were missing at random (Little & Rubin, 1987; McArdle, 1994).
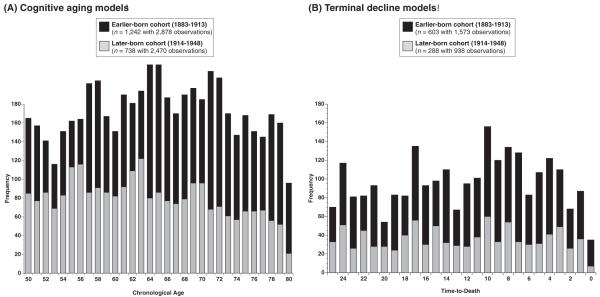
Figure 1 illustrates the frequencies of observations for the cognitive aging models (Panel A) and for the terminal decline models (Panel B), separately for both cohorts. For the cognitive aging models, 2,400+ observations were available per cohort, the large majority of which were longitudinal in nature (>85% for both cohorts). Our efforts to match the age range of the two cohorts were reasonably successful. A large number of observations were available to represent each of the three age decades under study for both the earlier born cohort (50s: n = 738; 60s: n = 1,007; 70s: n = 1,133) and the later born cohort (50s: n = 892; 60s: n = 897; 70s: n = 681). However, the average age of the total set of observations was slightly younger for the later born cohort (M = 63.60 years, SD = 8.32) than for the earlier born cohort (M = 66.14 years, SD = 8.42, respectively), F(1, 5346) = 122.70, p < .001. As shown in Panel B of Figure 1 for the terminal decline models, 900+ observations were available for each cohort, again with the large majority being longitudinal (>92% for both cohorts). The average time to death of all observations was only slightly greater for the later born cohort (M = 12.81 years, SD = 7.20) than for the earlier born cohort (M = 12.01 years, SD = 6.96), F(1, 2509) = 7.56, p < .01. The majority of participants provided data points in the last 5 years of life (earlier born, 56%; later born, 66%), indicating that analyses are based on data assessed in a time window during which terminal decline effects can be expected to occur. Overall, the data provide a reasonably large sample of observations on which to examine cohort differences in change.
Figure 1.
Frequency of observations in the Seattle Longitudinal Study in relation to chronological age (Panel A) and time-to-death (Panel B), separately for the two birth cohorts. For the age models, each cohort encompassed more than 700 participants who contributed more than 2,400 data points each over a 30-year observation period that was, on average, comparable across cohorts. For the time-to-death models, each cohort encompassed more than 280 participants who contributed more than 900 data points each over a 25-year observation period that was, on average, comparable across cohorts.
Results
As a preliminary check, we estimated the relative amount of between-person and within-person variance in each outcome. All five measures of cognitive ability exhibited sizable proportions of within-person variation, ranging between .21 for reasoning and .33 for spatial orientation in the sample used for the cognitive aging models and between .30 for word fluency and .44 for spatial orientation in the sample used for the terminal decline models. Using age-related and mortality-related growth models, we described and evaluated how this variation was structured across persons and over time.
Cohort Differences in Cognitive Aging
In a first set of analyses, growth curve models were used to examine whether the earlier born cohort and the later born cohort differed in age-related changes between ages 50 and 80 years. Results from these age models for each of the five abilities are given in Table 2. Consistent with previous work on age-related changes in cognitive abilities (Hofer & Alwin, 2008; Singer, Verhaeghen, Ghisletta, Lindenberger, & Baltes, 2003), all models indicate a prototypical trajectory that is characterized by linear decline with some acceleration. For example, the linear component of decline for reasoning amounted to about a quarter of a standard deviation per 10 years of age (e.g., γ10 = −0.297), which together with some concave curvature (γ20 = − 0.007) brought the average individual to an ability level at age 70 (γ00 = 43.722) that was more than half a standard deviation below the mean for all cross-sectional SLS participants (N ≈ 6,000; M = 50, SD = 10). We also found that the covariates included relate to levels and changes in cognitive functioning. For example, the negative coefficient for gender indicates that men, on average, outperformed women on measures of spatial orientation (γ04 = −2.745) and number (γ04 = −1.020), whereas the positive gender coefficient shows that women performed, on average, better than men on tasks assessing reasoning (γ04 = 2.035), fluency (γ04 = 3.602), and verbal meaning (γ04 = 2.257). Similarly, more educated persons performed better on all cognitive tests (e.g., for spatial orientation, γ01 = 0.310), and the presence of circulatory disease related to steeper cognitive aging trajectories on reasoning (γl2 = −0.045) and fluency (γl2 =−0.044).
Table 2.
Growth Curve Models of Cognitive Aging: The Role of Cohort and the Covariates
| Spatial orientation |
Inductive reasoning |
Word fluency |
Number |
Verbal meaning |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE |
| Fixed effects | ||||||||||
| Intercept,a γ00 | 47.056* | 0.358 | 43.722* | 0.334 | 46.114* | 0.449 | 50.689* | 0.447 | 45.715* | 0.372 |
| Linear slope, γ10 | 0.304* | 0.024 | −0.297* | 0.021 | −0.289* | 0.026 | −0.417* | 0.023 | −0.422* | 0.023 |
| Quadratic slope, γ20 | −0.005* | 0.001 | −0.007* | 0.001 | −0.004* | 0.001 | −0.010* | 0.001 | −0.011* | 0.001 |
| Education | 0.310* | 0.052 | 0.974* | 0.049 | 0.848* | 0.061 | 0.873* | 0.074 | 1.273* | 0.054 |
| Cancer | −0.725 | 0.440 | −0.607 | 0.418 | −0.089 | 0.519 | −0.648 | 0.545 | −0.679 | 0.460 |
| Circulatory disease | 0.864 | 1.274 | 0.297 | 0.318 | 0.294 | 0.398 | 0.120 | 0.419 | 0.270 | 0.352 |
| Gender | −2.745* | 0.330 | 2.035* | 0.311 | 3.602* | 0.483 | −1.020* | 0.412 | 2.257* | 0.345 |
| Cohort | 4.975 * | 0.361 | 5.818 * | 0.337 | 2.353 * | 0.609 | 0.457 | 0.443 | 5.408 * | 0.375 |
| Education × Linear slope | −0.002 | 0.003 | −0.018* | 0.003 | −0.008* | 0.003 | −0.007* | 0.003 | −0.004 | 0.003 |
| Cancer × Linear Slope | −0.034 | 0.024 | 0.004 | 0.021 | 0.003 | 0.027 | 0.021 | 0.024 | 0.006 | 0.023 |
| Circulatory Disease × Linear Slope | −0.010 | 0.019 | −0.045* | 0.016 | −0.044* | 0.021 | 0.012 | 0.018 | −0.012 | 0.018 |
| Gender × Linear Slope | 0.072* | 0.018 | −0.001 | 0.016 | −0.023 | 0.020 | 0.070* | 0.018 | 0.044* | 0.017 |
| Cohort × Linear Slope | 0.085 * | 0.028 | 0.138 * | 0.023 | 0.070 * | 0.030 | 0.060 * | 0.026 | 0.231 * | 0.027 |
| Cohort × Quadratic Slope | 0.003 | 0.002 | 0.004 * | 0.002 | − 0.002 | 0.002 | 0.004 * | 0.001 | 0.008 * | 0.002 |
| Gender × Cohort | −2.089* | 0.767 | ||||||||
| Education × Cohort | −0.553* | 0.147 | ||||||||
| Random effects | ||||||||||
| Variance intercept | 38.684* | 1.690 | 38.043* | 1.502 | 58.479* | 2.331 | 70.178* | 2.612 | 44.485* | 1.864 |
| Variance linear slope | 0.006 | 0.004 | 0.017* | 0.003 | 0.029* | 0.005 | 0.018* | 0.004 | 0.006 | 0.004 |
| Covariance intercept, slope | −0.067 | 0.062 | −0.054 | 0.050 | 0.277* | 0.081 | −0.394* | 0.075 | 0.291* | 0.067 |
| Residual variance | 21.953* | 0.632 | 12.521* | 0.366 | 20.598* | 0.594 | 16.505* | 0.476 | 19.451* | 0.572 |
| −2LL | 35,078 | 33,170 | 35,694 | 35,258 | 34,702 | |||||
Note. Scores standardized to a T metric (M = 50, SD = 10) based on cross-sectional data spanning all participants data at study entry. Unstandardized estimates and standard errors are presented. Boldface type highlights cohort differences in cognitive aging. Ns range from 1,979 (Reasoning) to 1,980 (Verbal meaning), with 5,338 to 5,348 observations. All covariates were effect coded/centered. Parameter estimates indicate the average trajectory and the extent of differences of a particular covariate. Positive parameters indicate differences favoring individuals with higher levels of education, those with cardiovascular disease and cancer, women, and the later born cohort. Cohort = earlier born (1886–1913; n = 1,242) versus later born (1914–1948; n = 738). −2LL = −2 log likelihood, a relative model fit statistic.
Intercept is centered at age 70 years.
p < .05.
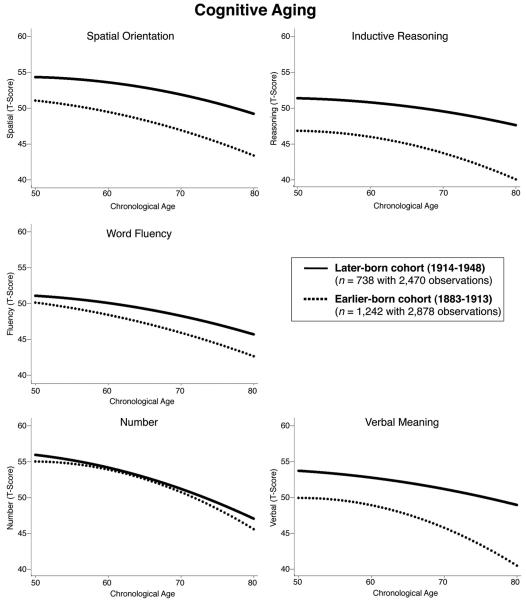
Most important for answering the questions posed in this study, birth cohort related positively to intercepts and linear age-related changes in four of the five abilities. With the exception of number ability, cohort differences in intercept ranged between 2.353 for fluency and 5.818 for reasoning, indicating that the later born cohort outperformed the earlier born cohort at age 70 by up to half a standard deviation. Cohorts also differed significantly in linear rates of cognitive aging. Effects ranged between 0.060 for number and 0.231 for verbal meaning, each being indicative of shallower cognitive aging trajectories for SLS participants born in 1914 or later. In addition, the concave curvature was somewhat less pronounced for the later born cohort on three of the five abilities tested (reasoning, 0.004; number, 0.004; verbal, 0.008). Figure 2 illustrates cohort differences in rates of cognitive aging. Overall, age-related growth models, covarying for education, circulatory disease, cancer, and gender, revealed substantial cohort differences in cognitive aging between age 50 and age 80 years. The later born cohort was favored both in level of cognitive functioning at age 70 years and in rate of cognitive decline.
Figure 2.
Illustrating cohort differences in cognitive aging from age 50 to age 80 on Thurstone’s five primary mental abilities of spatial orientation, inductive reasoning, word fluency, number, and verbal meaning, after residualizing for differences in education (years of schooling), health (circulatory disease and cancer), and gender. Except on number ability, later born cohorts (solid lines) outperformed earlier born cohorts (dashed lines) at age 70 by up to 0.50 SD and also showed shallower rates of cognitive decline on all abilities.
Cohort Differences in Terminal Cognitive Decline
In a second set of analyses, we examined whether cohort differences also exist in mortality-related cognitive changes. To do so, we applied growth models and compared, along with further covariates, the earlier born cohort and the later born cohort of now-deceased SLS participants who had provided one or more observations in the last 10 years of their lives. Data that spanned the 25 years prior to death were considered. Results are shown in Table 3.
Table 3.
Growth Curve Models of Terminal Decline: The Role of Cohort and the Covariates
| Spatial orientation |
Inductive reasoning |
Word fluency |
Number |
Verbal meaning |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE |
| Fixed effects | ||||||||||
| Intercept,a γ00 | 41.250* | 0.569 | 39.818* | 0.529 | 41.888* | 0.718 | 44.203* | 0.720 | 38.296* | 0.654 |
| Linear slope, γ10 | −0.631* | 0.065 | −0.298* | 0.056 | −0.489* | 0.065 | −0.744* | 0.066 | −0.853* | 0.065 |
| Quadratic slope, γ20 | −0.011* | 0.003 | −0.003* | 0.002 | −0.008* | 0.003 | −0.013* | 0.003 | −0.020* | 0.003 |
| Age at T1 | −0.182* | 0.028 | −0.230* | 0.025 | −0.056 | 0.036 | −0.088* | 0.035 | −0.162* | 0.032 |
| Age at death | −0.222* | 0.030 | −0.159* | 0.027 | −0.276* | 0.038 | −0.361* | 0.037 | −0.307* | 0.035 |
| Education | 0.207* | 0.087 | 0.458* | 0.080 | 0.661* | 0.112 | 0.579* | 0.113 | 0.950* | 0.106 |
| Cancer | −0.356 | 0.678 | −1.012 | 0.612 | 0.106 | 0.864 | 0.290 | 0.836 | −0.028 | 0.782 |
| Circulatory disease | −0.864 | 0.527 | −0.716 | 0.475 | −0.569 | 0.668 | 0.026 | 0.648 | −0.528 | 0.605 |
| Gender | −1.985* | 0.528 | 0.487 | 0.578 | 4.648* | 0.671 | −0.121 | 0.786 | 2.106* | 0.607 |
| Cohort | 0.928 | 0.733 | − 0.627 | 0.781 | 0.178 | 0.913 | − 4.654 * | 1.064 | 2.660 * | 0.834 |
| Age at T1 × Linear Slope | −0.002 | 0.002 | −0.002 | 0.002 | −0.003 | 0.002 | −0.005* | 0.002 | 0.001 | 0.002 |
| Age at Death × Linear Slope | −0.004 | 0.003 | −0.005* | 0.002 | −0.006* | 0.002 | −0.012* | 0.003 | −0.011* | 0.002 |
| Education × Linear Slope | 0.007 | 0.005 | −0.014* | 0.005 | 0.007 | 0.005 | −0.011 | 0.010 | −0.004 | 0.007 |
| Cancer × Linear Slope | 0.029 | 0.029 | −0.021 | 0.021 | −0.001 | 0.041 | 0.021 | 0.044 | −0.007 | 0.044 |
| Circulatory Disease × Linear Slope | −0.035 | 0.034 | −0.062* | 0.030 | −0.078* | 0.033 | 0.023 | 0.035 | −0.023 | 0.035 |
| Gender × Linear Slope | 0.077 | 0.033 | −0.071* | 0.038 | 0.056 | 0.040 | 0.048 | 0.045 | 0.032 | 0.035 |
| Cohort × Linear Slope | − 0.247 * | 0.098 | − 0.390 * | 0.083 | − 0.274 * | 0.099 | − 0.446 * | 0.098 | − 0.037 | 0.097 |
| Cohort × Quadratic Slope | − 0.009 * | 0.004 | − 0.014 * | 0.004 | − 0.009 * | 0.004 | − 0.010 * | 0.004 | − 0.008 a | 0.004 |
| Age at Death × Cohort × Linear Slope | −0.010* | 0.004 | ||||||||
| Women × Cohort | 3.168* | 0.992 | 4.327* | 1.359 | ||||||
| Women × Cohort × Linear Slope | 0.129* | 0.059 | 0.170* | 0.055 | 0.243* | 0.070 | ||||
| Education × Cohort | 0.305* | 0.149 | 0.759* | 0.144 | 0.402* | 0.199 | 0.519* | 0.227 | 0.859* | 0.210 |
| Education × Cohort × Linear Slope | 0.024* | 0.012 | 0.026* | 0.012 | ||||||
| Random effects | ||||||||||
| Variance intercept | 35.803* | 2.870 | 32.347* | 2.283 | 71.175* | 4.436 | 67.426* | 4.132 | 55.475* | 3.781 |
| Variance linear slope | 0.047* | 0.012 | 0.052* | 0.008 | 0.042* | 0.011 | 0.074* | 0.011 | 0.081* | 0.013 |
| Covariance intercept, slope | 0.572* | 0.153 | 0.424* | 0.114 | 0.872* | 0.174 | 0.381* | 0.172 | 1.360* | 0.190 |
| Residual variance | 19.800* | 0.916 | 12.418* | 0.569 | 19.380* | 0.880 | 17.029* | 0.747 | 17.947* | 0.852 |
| −2LL | 16,249 | 15,448 | 16,697 | 16,732 | 16,384 | |||||
Note. Scores standardized to a T metric (M = 50, SD = 10) based on all data available at Time 1 (T1). Unstandardized estimates and standard errors are presented. Boldface type highlights cohort differences in terminal decline. N = 891 individuals who provided between 2,496 (reasoning) and 2,511 observations (verbal meaning). Time to death centered at 3 years prior to death. All covariates were effect coded/centered. Parameter estimates indicate the average trajectory and the extent of differences of a particular covariate. Positive parameters indicate differences favoring individuals tested at an older age, individuals having died at an older age, those with higher levels of education, those with cardiovascular disease and cancer, women, and the later born cohort. Cohort = earlier born (1886–1913; n = 603) versus later born (1914–1948; n = 288). −2LL = −2 log likelihood, a relative model fit statistic.
Intercept is centered at 3 years prior to death.
p < .05.
The overall pattern of cognitive change is consistent with other reports of the precipitous proximate-to-death declines across various measures of cognitive abilities (Bäckman & MacDonald, 2006; Ghisletta, McArdle, & Lindenberger, 2006). Average levels of abilities at 3 years prior to death were up to one standard deviation below the mean of the cross-sectional (at study entry) sample (reasoning: γ00 = 39.818). Similarly, average rates of mortality-related linear decline ranged between γ10 = −0.298 for reasoning and γ10 = −0.853 for verbal meaning, and all measures showed accelerations in decline (i.e., quadratic change, e.g., γ20 = −0.020 for verbal meaning). Again, the covariates included in our models showed significant associations with aspects of the cognitive trajectories. For example, having died at an older age related to both lower levels of cognitive capacity at 3 years prior to death and steeper rates of terminal decline (e.g., for word fluency: γ02 = −0.276; γl2 = −0.006). Similarly, SLS participants diagnosed with circulatory diseases experienced steeper mortality-related decline on reasoning (γl4 = −0.062) and fluency (γl4 = −0.078).
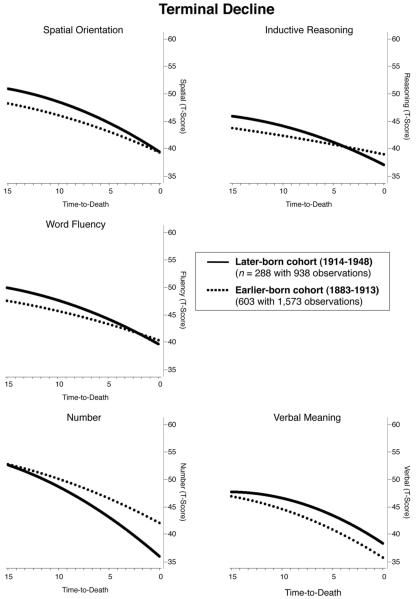
Most important for our research questions, mortality-related models indicated that positive cohort differences in terminal decline were present on verbal meaning only. The later born cohort outperformed, on average, the earlier born cohort at 3 years prior to death (γ07 = 2.660). In contrast, no significant cohort differences in ability levels were found for spatial orientation, inductive reasoning, and word fluency. Results even indicated evidence of negative cohort effects. For example, the later born cohort scored, on average, 0.465 SD lower on number ability at 3 years before death than did the earlier born cohort. In a similar vein, the later born cohort experienced steeper linear rates of terminal decline on four of the five abilities, with effect sizes ranging between 0.247 for spatial orientation and 0.446 for number ability. In addition, the later born cohort was characterized by greater convex curvature on all five abilities (e.g., reasoning: −0.014). We also note several interaction effects, conjointly indicating that advantages for women and those with higher education were somewhat more pronounced in the later born cohort. For example, the positive Education × Cohort interaction indicates that differences in cognitive performances between educational strata were more pronounced in the later born cohort than in the earlier born cohort. As shown in Figure 3, mortality-related growth models covarying for differences in age at T1, age at death, education, circulatory disease, cancer, and gender provided little evidence of positive secular trends. In contrast, the later born cohort was found to experience steeper mortality-related declines than the earlier born cohort.
Figure 3.
Illustrating cohort differences in terminal decline on Thurstone’s five primary mental abilities of spatial orientation, inductive reasoning, word fluency, number, and verbal meaning, after residualizing for differences in age at study entry, age at death, education (years of schooling), health (circulatory disease and cancer), and gender. Mortality-related models suggest no evidence for positive secular trends except on verbal meaning. In contrast, later born cohorts (solid lines) showed steeper mortality-related declines than earlier born cohorts (dashed lines) on four of the five abilities tested.
Discussion
Our goal in this study was to examine whether and how successive cohorts differ in the rates of age-related and mortality-related cognitive decline on the five PMAs. To do so, we compared two broad cohorts, those who had reached old age in the 1960s and 1970s (born 1886–1913) and those who had reached old age in the 1980s and 1990s (born 1914–1948). To disentangle cohort differences in cognitive change trajectories from known cohort differences in other domains, our models covaried for central indicators of life expectancy, education, health (circulatory diseases and cancer), and gender. For cognitive aging, we found a clear-cut pattern: Relative to those born earlier, individuals in the later born cohort showed, on average, considerably better cognitive functioning at age 70 as well as shallower rates of cognitive decline from age 50 to age 80. For terminal decline, in contrast, the growth models revealed very little evidence of positive cohort differences. In contrast, the later born cohort experienced steeper mortality-related declines. We discuss possible factors underlying these findings and conclude that positive secular trends reported for age-related processes do not generalize to mortality-related processes. We close by suggesting routes for further inquiry to substantiate and expand upon our results.
Cohort Differences in Cognitive Aging
The current study adds to previous research showing that historical transitions and events cumulatively shape individual development (Elder, 1974; Flynn, 2007; Helson & Moane, 1987; Schaie, 2008). Making use of the cohort-sequential design of the SLS, we corroborate and expand earlier reports of cohort differences in adult cognitive functioning and change in a number of ways. Whereas most previous studies have focused on questions revolving around cohort-related shifts in mean levels of (cognitive) performance, we were primarily interested in cohort-related shifts in developmental/aging trajectories. We extend earlier longitudinal studies through examination of changes in cognition spanning 30 years from age 50 to age 80, a period of life during which age-related declines can be expected, in individuals born between 1883 and 1948.
Our analyses indicate that initial cohort differences in cognitive performance are maintained throughout aging and are even exacerbated with advancing age by shallower age-related declines among individuals born later. Not surprisingly, historical cohort or generational improvements in mental capacity do not outweigh the negative effects of aging-related factors. Instead, cohort differences may be seen as a proxy for moderator variables that at best slow the rate of decline. In our age models, we attempted to minimize procedural differences with prior studies by using broad cohorts, including data only for the overlapping age range at which both cohorts were assessed, and applying growth curve modeling. We can only speculate as to why our study produced results divergent from those of other studies in the United States (Zelinski & Kennison, 2007) and Europe (Finkel et al., 2007). Over and above issues of design and measures, further study variations may originate in differences in years of birth (e.g., SLS cohorts were partly born earlier), socioeconomic strata (e.g., more blue-collar workers in the LBLS), or regional and country specifics regarding the implementation of public health measures such as improved hygiene (Condran & Crimmins-Gardner, 1978).
The only measure that did not reveal evidence for positive cohort effects was number ability. This finding is consistent with earlier reports from the SLS (Schaie, 1994, 2005) indicating that mathematical training approaches in elementary school among earlier born cohorts have reinforced numerical processing and arithmetic abilities such as simple rote addition and multiplication skills. Previous, more fine-grained analyses had revealed that number skills reached a peak with the 1924 birth cohort and showed negative cohort differences thereafter. Our later born cohort may thus include both those with increasing and those with decreasing number skills. Hence, our attempt to be consistent with prior studies and to consider two broad cohorts came at the costs of neglecting within-cohort heterogeneity, changes therein, or possible nonlinear cohort-related shifts (for discussion, see Dannefer, 2003).
Cohort Differences in Terminal Cognitive Decline
It is well established in the cognitive literature that older adults typically show steep declines in cognitive functioning in the years before death (see Bäckman & MacDonald, 2006). However, to the best of our knowledge, this is the first study to examine cohort differences in terminal decline. We posed the question whether previously observed cohort differences may extend into the last years of life or whether the magnitude of secular trends is altered with approaching death. The overall pattern that emerged in the terminal decline models relative to the cognitive aging models indicated very few positive secular trends. It was only on verbal meaning that later born participants outperformed earlier born participants. In contrast, in the last years of life no differences in average levels of cognitive functioning were apparent on three of the five PMAs, and evidence of negative secular trends emerged. In particular, the later born cohort performed lower on number ability and showed steeper mortality-related declines on all five abilities.
Our results are in line with the idea that mortality-related mechanisms and the progressive processes leading toward death (e.g., deteriorating health) are so pervasive that they override historical, cohort-related effects that were apparent earlier in life. Conceptually, this finding is consistent with life span tenets about the vulnerabilities and constraints that appear in very old age and at the end of life (Baltes & Smith, 2003). These notions suggest that despite an increasing need for cultural resources, there is an ever declining efficiency of those resources to overcome age-related decrements. More generally, our findings add to current debates in the field about the intricacies involved in alleviating rates of cognitive decline (Hertzog, Kramer, Wilson, & Lindenberger, 2009; Salthouse, 2006).
We note that two additional sets of factors may have contributed to diminished or even eliminated cohort differences proximate to death. First, survival at the end of life becomes increasingly more “manufactured” (Olshansky, Hayflick, & Carnes, 2002). Provided sufficient technological support, members of the later born cohort may have survived impairments or diseases that have resulted in death among members of earlier born cohorts. One way to interpret our results is that previously higher levels of functioning are not maintained during manufactured survival. In contrast, positive secular trends that may have existed at an earlier point in life may be offset or even reversed. It will be intriguing to examine the implications of an extended life in future analyses using data for cohorts who are entering old age now or in the near future (e.g., the baby boomers). Second, rapid increases in life expectancy imply that a greater number of relatively lower functioning segments of one’s birth cohort reach higher and higher ages (Singer et al., 2003). A less positive selection of later born generations could have led to lower average levels of functioning for members of this generation, thereby offsetting positive secular trends.
It must be noted that our study is primarily descriptive in nature and was not designed to tie cohort differences to particular causal mechanisms. For example, it remains an open question as to how and why the various abilities showed different associations with cohort over the same time-to-death (or age) period. Leaving issues of statistical power aside, such differential trends suggest either that the abilities are impacted by different factors or that the same (set of) factors may act differently on the different abilities. We had advanced the interpretation that mortality-related processes override previously existing cohort differences. If so, positive secular trends for verbal meaning may simply have persisted because verbal abilities typically show particularly strong positive secular effect (for a recent meta-analysis, see Uttl & Van Alstine, 2003) that may not get completely washed out by mortality effects. In a similar vein, verbal abilities are more likely to be “practiced” until the end of life as compared with, for example, spatial abilities. Given that verbal meaning is the PMA that is conceptually closest to the crystallized ability domain (Schaie et al., 2005), our results are also consistent with the notion that later born cohorts have greater cognitive reserve, which may mitigate nearness-to-death effects particularly for acculturated abilities (see also Stern et al., 1999). It would thus be informative to examine whether our results can be corroborated targeting other key abilities (e.g., psychomotor speed, memory, and executive functioning) as well as less knowledge-loaded measures, such as brain efficiency. These and other etiological questions warrant attention and further exploration in future research. For the time being, our initial findings suggest that positive secular trends often reported for cognitive aging do not extend to terminal cognitive decline.
Possible Factors Underlying Cohort Differences
To provide a meaningful interpretation of cohort differences in cognitive aging and terminal decline, we covaried for a number of factors that are known to differ between individuals and cohorts. Our results are thus net of the effects of well-established secular trends in educational systems (operationally defined as years of schooling) as well as disease prevalence (operationally defined as the presence of circulatory diseases and cancer). Our rationale was that historical increases in the quantity of educational attainment during the 20th century are a major factor underlying cognitive cohort differences (Alwin & McCammon, 2001; Hauser & Huang, 1997; Rönnlund et al., 2005). However, cohorts not only differ in such quantitative aspects of education but often also have experienced qualitatively different educational systems (e.g., shift from rote learning to more participatory strategies, such as discovery learning; see Emirbayer, 1992; Schaie, 2008) for which we unfortunately have not had any direct data. Similarly, we have included the presence of circulatory diseases and cancer (at any point during the study) as key health factors. Effects would probably have been stronger if we had directly modeled how declining health impacted cognitive decline. It would also be instructive to examine whether the severity of circulatory diseases or cancer has different implications for cognitive decline in the two cohorts. For the earlier born cohort, minor forms of disease may already implicate steep cognitive declines. For the later born cohort, in contrast, morbidity-related cognitive declines may not set in until diseases become severe, probably because of advances in health care.
Of course, education and health as examined in our study are just two domains in which cohorts might differ. Further factors that operate may include technological advances (e.g., computers) and associated demands on inductive logic and problem solving (Blair, Gamson, Thorne, & Baker, 2005), increasing complexity and cognitive challenges of work contexts (Schooler & Caplan, 2008), or changes in gender roles and increased labor force participation among women. Further investigation pinpointing these and other factors that potentially alter fundamental aspects of human ontogeny and quantifying their relative contribution to cohort differences is certainly warranted (Baltes et al., 1979). For example, one would expect, based on two-component theories of intelligence (Baltes et al., 2006; Cattell, 1971; Horn, 1982), that differences in formal education account for the lion’s share of cohort differences in acculturated crystallized measures (e.g., verbal meaning), whereas health variables account for much of the cohort differences in less acculturated fluid measures (e.g., inductive reasoning).
Limitations and Outlook
To put our findings in perspective, we discuss four issues surrounding the specificity and generalizability of our findings. The SLS consists of a comprehensive and well-defined sampling frame that has been extensively described (Schaie, 2005). Participants were community-dwelling individuals in reasonably good health who approximately represent the upper 75% of the socio-economic spectrum. At the same time, we acknowledge that participants were recruited through membership in one large HMO in the Pacific Northwest area of the United States and may thus not necessarily be equally representative for the cohorts under study. Although secular trends would have suggested longer life expectancies, participants in our later born cohort had died at younger ages than those in the earlier born cohort, suggesting that this subsample was probably less representative and included relatively more unhealthy participants. Rather than indexing general secular trends, the negative cohort differences observed over time to death may instead be reflective of differences in lifestyles, health behaviors, and cause of death. It is possible, for example, that individuals in the later born cohort were more likely to die from aggressive forms of cancer and to experience steeper terminal decline. The precautionary steps we took to adjust for this possibility were to include age at assessment, age at death, and the presence of circulatory diseases and cancer as covariates into our models. This approach, however, may have only partially captured selectivity-related differences between the cohorts. We also note that opposite implications would arise for cohort differences over age. If participants in the later born cohort for the age models were indeed unhealthy, the positive secular trends documented over chronological age were underestimating effect sizes in the target population. Comparative analyses from the SLS suggest that the amount of positive selection of the general SLS participant base is relatively minor (e.g., educational attainment: Hauser & Featherman, 1976; cf. Schaie et al., 2005). Despite these and other supporting findings, a full quantification of population selectivity in the SLS is warranted.
A second implication arising from the approach we chose to define our cohorts is that the earlier born cohort primarily died in the 1980s or before (median year of death = 1987), whereas the later born cohort primarily died in the 1990s or after (median year of death = 2001). As a consequence, cohort differences may also have existed in the treatment of terminal illnesses such as cancer (e.g., chemotherapy, beta blockers), possible cognitive side effects of such treatments, the length of terminal illness and the age at which cognitive pathologies and dementia set in, or place of death (home vs. hospital vs. nursing home). Unfortunately, these variables were not available in the context of our study. In future inquiries, it may thus be instrumental to define cohorts based on criteria other than year of birth. Utilizing historical events would make it possible to examine if, for example, the implementation of the health care reform in the United States resulted in alleviated terminal declines in the cognitive and health domains.
Third, several methodological factors may have limited our ability to thoroughly address questions about the operation of mortality-related processes and between-person differences therein. These limitations include that statistical power was certainly lower in the terminal decline models relative to the cognitive aging models, simply because these analyses were based on fewer participants and fewer longitudinal observations. Although we modeled quadratic trends, the ability to detect interindividual differences in those trends was limited by the fact that participants contributed no more than five data points to the age models and no more than four data points to the mortality models. Furthermore, the 7-year intervals between measurement occasions may be too long to fully capture any “accelerations” in how the mortality-related processes unfold. Likely, changes in the rates of change happen along a much smaller timescale than can be determined by our lengthy interval between occasions. Finally, the suitability of the quadratic change models for the study of terminal decline might also be viewed as a suboptimal approximation of the terminal decline phenomena—which imply discrete shifts in the rate of decline, a phase transition—rather than as “continuous” (and invariant in time) changes in the rate of change. If more closely spaced assessments are available, the compression of morbidity notion could be operationalized more directly via multiphase models of change that estimate the point of onset of terminal decline (Gerstorf et al., 2008). Following notions of cognitive reserve (Hall et al., 2009; Stern et al., 1999), later born cohorts may enter such phases of precipitous decline later than earlier born cohorts. However, once terminal decline has set in, the amount of decline may then be steeper among those born later (e.g., because they have more to lose). Such a scenario would even be consistent with the pattern found in our single-phase models. Future studies are thus needed to substantiate and elaborate on our initial findings.
Finally, many studies including our own report have targeted cohort differences in a single domain of functioning (see also Grühn, Rebucal, Diehl, Lumley, & Labouvie-Vief, 2008; Idler, 1993; Mroczek & Spiro, 2003; Twenge, 2000). An initial step toward a systemic-multivariate perspective would be to consider cohort differences in profiles across multiple domains spanning cognitive, health, personality, social, and well-being functions (see also Gerstorf, Smith, & Baltes, 2006; Magnusson, 1998). If the compression-of-morbidity scenario holds true (Fries, 1980), we would expect that later born cohorts have a higher chance than earlier born cohorts to be in a profile group of individuals who maintain key aspects of functionality across domains into the very last years of life. This of course is an empirical question, and our univariate results based on the cognitive domain suggest otherwise.
Synopsis
Making the simplifying assumption of ignoring period or time-of-measurement effects (for discussion, see Schaie & Herzog, 1983), our study has shed light on the direction, size, and nature of cohort differences in age-related and mortality-related change trajectories in various measures of cognitive functioning. For cognitive aging, our results suggest systematic and substantial positive trends in cohort levels and in cohort changes. The societal implications of such findings are tremendous. With regard to established age norms, for example, it has repeatedly been argued that positive cohort differences (e.g., 70-year-olds nowadays perform like 65-year-olds did 30 years ago) in abilities such as reasoning can be taken to suggest that people can much longer be productively employed in professions that require strong reasoning skills (Schaie, 2008; Zelinski & Kennison, 2007). For terminal cognitive decline, in contrast, our findings suggest a less optimistic outlook, with very little evidence of positive historical effects and several more consistent indications of negative secular trends. It appears that cohort improvements made in age-related mental capacity do not necessarily generalize to the end of life and thereby to one of the most vulnerable segments of our societies. More work is certainly needed to better understand the pathways through which social- and individual-level processes are interconnected. An inquiry into cohort differences in late-life functioning and change necessitates not only examining the distinctive and lasting effects of early-life experiences but also studying the implications of living conditions in the later phase of life. Such insights in turn will have profound societal and health care implications for accommodating the needs of a growing elderly population that differs from cohorts born earlier.
Acknowledgments
We are grateful for the support provided by the National Institute on Aging (Grants RC1-AG035645, NIA R21-AG032379, and NIA R21-AG033109). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
As Zelinski and Kennison (2007) noted, the overlap in the birth years of the two cohorts does not confound comparisons because the age matching held the 16-year cohort difference constant.
One approach to study questions about the effects resulting from processes of mortality selection was recently proposed by Alwin (2008). He statistically controlled for between-cohort differences in expected age at death and found that later born cohorts in the national Health and Retirement Study showed significantly higher levels of test performances on measures of memory relative to their earlier born counterparts.
References
- Aldwin CM, Spiro A, III, Park CL. Health, behaviors, and optimal aging: A lifespan developmental perspective. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 6th ed. Elsevier; San Diego, CA: 2006. pp. 85–104. [Google Scholar]
- National Cancer Institute Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Edwards BK, editors. SEER cancer statistics review, 1975–2007. 2009 Retrieved from the. web-site: http://seer.cancer.gov/csr/1975_2007.
- Alwin DF. History, cohort, and patterns of cognitive aging. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Sage; Thousand Oaks, CA: 2008. pp. 9–38. [Google Scholar]
- Alwin DF, McCammon RJ. Aging, cohorts, and verbal ability. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2001;56:P151–P161. doi: 10.1093/geronb/56.3.s151. [DOI] [PubMed] [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. doi:10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Bäckman L, MacDonald SWS. Death and cognition: Synthesis and outlook. European Psychologist. 2006;11:224–235. [Google Scholar]
- Bäckman L, Small BJ, Wahlin A, Larsson M. Cognitive functioning in very old age. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Erlbaum; Mahwah, NJ: 2000. pp. 499–558. [Google Scholar]
- Baltes PB, Cornelius SW, Nesselroade JR. Cohort effects in developmental psychology. In: Nesselroade JR, Baltes PB, editors. Longitudinal research in the study of behavior and development. Academic Press; New York, NY: 1979. pp. 61–87. [Google Scholar]
- Baltes PB, Lindenberger U, Staudinger UM. Life-span theory in developmental psychology. In: Lerner RM, editor. Handbook of child psychology: Vol. 1. Theoretical models of human development. 6th ed. Wiley; New York, NY: 2006. pp. 569–664. [Google Scholar]
- Baltes PB, Smith J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology. 2003;49:123–135. doi: 10.1159/000067946. doi:10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- Bengtson VL, Biblarz TJ, Roberts REL. How families still matter: A longitudinal study of youth in two generations. Cambridge University Press; Cambridge, England: 2002. [Google Scholar]
- Blair C, Gamson DA, Thorne S, Baker DP. Rising mean IQ: Cognitive demand of mathematics education for young children, population exposure to formal schooling, and the neurobiology of the prefrontal cortex. Intelligence. 2005;33:93–106. doi:10.1016/j.intell.2004.07.008. [Google Scholar]
- Bosworth HB, Schaie KW. The relationship of social environment, social networks, and health outcomes in the Seattle Longitudinal Study: Two analytic approaches. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1997;52:P197–P205. doi: 10.1093/geronb/52b.5.p197. [DOI] [PubMed] [Google Scholar]
- Bowles RP, Grimm KJ, McArdle JJ. A structural factor analysis of vocabulary knowledge and relations to age. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2005;60:P234–P241. doi: 10.1093/geronb/60.5.p234. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. Ecology of the family as a context for human development research perspectives. Developmental Psychology. 1986;22:723–742. doi:10.1037/0012-1649.22.6.723. [Google Scholar]
- Cattell RB. Abilities: Their structure, growth, and action. Houghton Mifflin; Boston, MA: 1971. [Google Scholar]
- Condran GA, Crimmins-Gardner E. Public health measure and mortality in U.S. cities in the late nineteenth century. Human Ecology. 1978;6:27–54. doi: 10.1007/BF00888565. doi:10.1007/BF00888565. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Hayward MD, Saito Y. Differentials in active life expectancy in the older population of the United States. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1996;51:P111–P120. doi: 10.1093/geronb/51b.3.s111. [DOI] [PubMed] [Google Scholar]
- Dannefer D. Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2003;58:P327–P337. doi: 10.1093/geronb/58.6.s327. [DOI] [PubMed] [Google Scholar]
- Elder GH., Jr. Children of the Great Depression: Social change in life experience. University of Chicago Press; Chicago, IL: 1974. [Google Scholar]
- Emirbayer M. Beyond structuralism and voluntarism: The politics and discourse of progressive school reform, 1890–1930. Theory and Society. 1992;21:621–664. doi:10.1007/BF00993493. [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Developmental Psychology. 2003;39:535–550. doi: 10.1037/0012-1649.39.3.535. doi:10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Cohort differences in trajectories of cognitive aging. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2007;62:P286–P294. doi: 10.1093/geronb/62.5.p286. [DOI] [PubMed] [Google Scholar]
- Flynn JR. What is intelligence? Cambridge University Press; Cambridge, England: 2007. [Google Scholar]
- Fries JF. Aging, natural death, and the compression of morbidity. New England Journal of Medicine. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. doi:10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Estabrook R, Schupp J, Wagner GG, Lindenberger U. Life satisfaction shows terminal decline in old age: Longitudinal evidence from the German Socio-Economic Panel Study (SOEP) Developmental Psychology. 2008;44:1148–1159. doi: 10.1037/0012-1649.44.4.1148. doi:10.1037/0012-1649.44.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Mayraz G, Hidajat M, Lindenberger U, Wagner GG, Schupp J. Late-life decline in well-being across adulthood in Germany, the United Kingdom, and the United States: Something is seriously wrong at the end of life. Psychology and Aging. 2010;25:477–485. doi: 10.1037/a0017543. doi:10.1037/a0017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Smith J, Baltes PB. A systemic–wholistic approach to differential aging: Longitudinal findings from the Berlin Aging Study. Psychology and Aging. 2006;21:645–663. doi: 10.1037/0882-7974.21.4.645. doi:10.1037/0882-7974.21.4.645. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, McArdle JJ, Lindenberger U. Longitudinal cognition–survival relations in old and very old age: 13-year data from the Berlin Aging Study. European Psychologist. 2006;11:204–223. doi:10.1027/1016-9040.11.3.204. [Google Scholar]
- Graham P, Blakely T, Davis P, Sporle A, Pearce N. Compression, expansion, or dynamic equilibrium? The evolution of health expectancy in New Zealand. Journal of Epidemiology and Community Health. 2004;58:659–666. doi: 10.1136/jech.2003.014910. doi:10.1136/jech.2003.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grühn D, Rebucal K, Diehl M, Lumley M, Labouvie-Vief G. Empathy across the adult lifespan: Longitudinal and experience-sampling findings. Emotion. 2008;8:753–765. doi: 10.1037/a0014123. doi:10.1037/a0014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive abilities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. doi:10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RM, Featherman DL. Equality of schooling: Trends and prospects. Sociology of Education. 1976;49:99–120. doi:10.2307/2112516. [Google Scholar]
- Hauser RM, Huang M-H. Verbal ability and socioeconomic success: A trend analysis. Social Science Research. 1997;26:331–376. doi:10.1006/ssre.1997.0604. [Google Scholar]
- Helson R, Moane G. Personality change in women from college to midlife. Journal of Personality and Social Psychology. 1987;53:176–186. doi: 10.1037//0022-3514.53.1.176. doi:10.1037/0022-3514.53.1.176. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Schaie KW, Gribbin K. Cardiovascular disease and changes in intellectual functioning from middle to old age. Journal of Gerontology. 1978;33:872–883. doi: 10.1093/geronj/33.6.872. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Sage; Thousand Oaks, CA: 2008. [Google Scholar]
- Horn JL. The theory of fluid and crystallized intelligence in relation to concepts of cognitive psychology and aging in adulthood. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. Plenum; New York, NY: 1982. pp. 237–278. [Google Scholar]
- Idler EL. Age differences in self-assessments of health: Age changes, cohort differences, or survivorship? Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1993;48:P289–P300. doi: 10.1093/geronj/48.6.s289. [DOI] [PubMed] [Google Scholar]
- Kleemeier RW. Intellectual changes in the senium. Proceedings of the American Statistical Association. 1962;1:290–295. [Google Scholar]
- Littell RC, Miliken GA, Stoup WW, Wolfinger RD. SAS system for mixed models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; New York, NY: 1987. [Google Scholar]
- Magnusson D. The logic and implication of a person approach. In: Cairns RB, Bergman LR, Kagan J, editors. Methods and models for studying the individual. Sage; Thousand Oaks, CA: 1998. pp. 33–63. [Google Scholar]
- Maitland SB, Herlitz A, Nyberg L, Backman L, Nilsson L-G. Selective sex differences in declarative memory. Memory & Cognition. 2004;32:1160–1169. doi: 10.3758/bf03196889. doi:10.3758/BF03196889. [DOI] [PubMed] [Google Scholar]
- Manton KG, Gu X, Lowrimore GR. Cohort changes in active life expectancy in the U.S. elderly population: Experience from the 1982–2004 National Long-Term Care Survey. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2008;63:P269–P281. doi: 10.1093/geronb/63.5.s269. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Structural factor analysis experiments with incomplete data. Multivariate Behavioral Research. 1994;29:409–454. doi: 10.1207/s15327906mbr2904_5. doi:10.1207/s15327906mbr2904_5. [DOI] [PubMed] [Google Scholar]
- Meinz EJ, Salthouse TA. Is age kinder to females than to males? Psychonomic Bulletin & Review. 1998;5:56–70. doi:10.3758/BF03209457. [Google Scholar]
- Mroczek DK, Spiro RA., III Modeling intraindividual change in personality traits: Findings from the Normative Aging Study. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2003;58:P153–P165. doi: 10.1093/geronb/58.3.p153. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Hayflick L, Carnes BA. Position statement on human aging. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2002;57:M292–M297. doi: 10.1093/gerona/57.8.b292. [DOI] [PubMed] [Google Scholar]
- Palmore E, Cleveland W. Aging, terminal decline, and terminal drop. Journal of Gerontology. 1976;31:76–81. doi: 10.1093/geronj/31.1.76. [DOI] [PubMed] [Google Scholar]
- Ram N, Gerstorf D, Fauth B, Zarit SH, Malmberg B. Aging, disablement, and dying: Three time metrics for charting late-life change. Research in Human Development. 2010;7:27–44. doi: 10.1080/15427600903578151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Grimm KJ. Using simple and complex growth models to articulate developmental change: Matching method to theory. International Journal of Behavioral Development. 2007;31:303–316. doi:10.1177/0165025407077751. [Google Scholar]
- Riegel KF, Riegel RM. Development, drop, and death. Developmental Psychology. 1972;6:306–319. doi:10.1037/h0032104. [Google Scholar]
- Riley MW. Aging and cohort succession: Interpretations and misinterpretations. Public Opinion Quarterly. 1973;37:35–49. doi:10.1086/268058. [Google Scholar]
- Robine JM, Romieu I, Michel JP. Trends in health expectancies. In: Robine J, Jagger C, Mathers CD, Crimmins EM, Suzman RM, editors. Determining health expectancies. Wiley; West Sussex, England: 2003. pp. 75–101. [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, Nilsson L-G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. doi:10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Rosow I. What is a cohort and why. Human Development. 1978;21:65–75. doi:10.1159/000271575. [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Ryder NB. The cohort as a concept in the study of social changes. American Sociological Review. 1965;30:843–861. doi:10.2307/2090964. [PubMed] [Google Scholar]
- Ryff CD, Singer B. The contours of positive human health. Psychological Inquiry. 1998;9:1–28. doi:10.1207/s15327965pli0901_1. [Google Scholar]
- Salthouse TA. Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. doi:10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Schaie KW. A general model for the study of developmental problems. Psychological Bulletin. 1965;64:92–107. doi: 10.1037/h0022371. doi:10.1037/h0022371. [DOI] [PubMed] [Google Scholar]
- Schaie KW. The course of adult intellectual development. American Psychologist. 1994;49:304–313. doi: 10.1037//0003-066x.49.4.304. doi:10.1037/0003-066X.49.4.304. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on intelligence: The Seattle Longitudinal Study. Oxford University Press; New York, NY: 2005. doi:10.1093/acprof:oso/9780195156737.001.0001. [Google Scholar]
- Schaie KW. Historical processes and patterns of cognitive aging. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Sage; Thousand Oaks, CA: 2008. pp. 368–383. [Google Scholar]
- Schaie KW, Charness N, editors. Impact of technology on the aging individual. Springer; New York, NY: 2003. [Google Scholar]
- Schaie KW, Herzog C. Fourteen-year cohort-sequential analyses of adult intellectual development. Developmental Psychology. 1983;19:531–543. doi:10.1037/0012-1649.19.4.531. [Google Scholar]
- Schaie KW, Willis SL, Pennak S. An historical framework for cohort differences in intelligence. Research in Human Development. 2005;2:43–67. doi: 10.1080/15427609.2005.9683344. doi:10.1207/s15427617rhd0201&2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler C, Caplan LJ. Those who have, get: Social structure, environmental complexity, intellectual functioning, and self-directed orientations in the elderly. In: Schaie KW, Abeles RP, editors. Social structures and aging individuals: Continuing challenges. Springer; New York, NY: 2008. pp. 131–153. [Google Scholar]
- Settersten RA., Jr. Toward a stronger partnership between life-course sociology and life-span psychology. Research in Human Development. 2005;2:25–41. doi:10.1207/s15427617rhd0201&2_2. [Google Scholar]
- Siegler IC. The terminal drop hypothesis: Fact or artifact? Experimental Aging Research. 1975;1:169–185. doi: 10.1080/03610737508257957. doi:10.1080/03610737508257957. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: Six-year longitudinal findings in the Berlin Aging Study (BASE) Psychology and Aging. 2003;18:318–331. doi: 10.1037/0882-7974.18.2.318. doi:10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Stawski RS, Hall RB, Katz M, Verghese J, Lipton RB. On the importance of distinguishing pre-terminal and terminal cognitive decline. European Psychologist. 2006;11:172–181. doi:10.1027/1016-9040.11.3.172. [Google Scholar]
- Small BJ, Bäckman L. Time to death and cognitive performance. Current Directions in Psychological Science. 1999;8:168–172. doi:10.1111/1467-8721.00040. [Google Scholar]
- Stern Y, Albert S, Tang M-X, Tsai WY. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone TG. Examiner manual for the SRA Primary Mental Abilities Test. Science Research Associates; Chicago, IL: 1949. [Google Scholar]
- Twenge JM. The age of anxiety? Birth cohort change in anxiety and neuroticism, 1952–1993. Journal of Personality and Social Psychology. 2000;79:1007–1021. doi: 10.1037//0022-3514.79.6.1007. doi:10.1037/0022-3514.79.6.1007. [DOI] [PubMed] [Google Scholar]
- U.S. Public Health Service . International classification of disease, eighth revision, adapted for use in the United States. U.S. Government Printing Office; Washington, DC: 1968. DHHS Publication No. PHS 80–1260. [Google Scholar]
- Uttl B, Van Alstine CL. Rising verbal intelligence scores: Implications for research and clinical practice. Psychology and Aging. 2003;18:616–621. doi: 10.1037/0882-7974.18.3.616. doi:10.1037/0882-7974.18.3.616. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: Accelerated loss of cognition in the last years of life. Psychosomatic Medicine. 2007;69:131–137. doi: 10.1097/PSY.0b013e31803130ae. doi:10.1097/PSY.0b013e31803130ae. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Kennison RF. Not your parents’ scores: Cohort reduces psychometric aging effects. Psychology and Aging. 2007;22:546–557. doi: 10.1037/0882-7974.22.3.546. doi:10.1037/0882-7974.22.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Stewart ST. Individual differences in 16-year memory changes. Psychology and Aging. 1998;13:622–630. doi: 10.1037//0882-7974.13.4.622. doi:10.1037/0882-7974.13.4.622. [DOI] [PubMed] [Google Scholar]