Abstract
Our previous studies demonstrated that selective overexpression of the Ron receptor tyrosine kinase in the murine mammary epithelium leads to mammary tumor formation. Biochemical analysis of mammary tumor lysates showed that Ron overexpression was associated with increases in β-catenin expression and tyrosine phosphorylation. β-catenin has also been shown to be regulated through tyrosine phosphorylation by the receptor tyrosine kinases Met, Fer, and Fyn. However, the molecular and physiological roles of β-catenin and β-catenin tyrosine phosphorylation downstream of Ron are not known. To investigate this association, we show that Ron and β-catenin are coordinately elevated in human breast cancers. Our data also demonstrate that activation of Ron, through ligand binding by hepatocyte growth factor-like protein (HGFL), induces the tyrosine phosphorylation of β-catenin, primarily on tyrosine residues Tyr 654 and Tyr 670. In addition, HGFL mediated Ron activation induces both β-catenin nuclear localization and transcriptional activity, with Tyr 654 and Tyr 670 residues of β-catenin being critical for these processes. We also demonstrate that a knockdown of Ron in breast cancer cell lines leads to a loss of HGFL-induced β-catenin-dependent transcriptional activation and cell growth which can rescued by activation of canonical Wnt/β-catenin signaling. Moreover, we show that HGFL-dependent Ron activation mediates upregulation of the β-catenin target genes cyclin D1 and c-myc, and that expression of these target genes in breast cancer cells is decreased following inhibition of Ron and/or β-catenin. Finally, we show that genetic ablation of β-catenin in Ron-expressing breast cancer cells decreases cellular proliferation in vitro, as well as mammary tumor growth and metastasis following orthotopic transplantation into the mammary fat pad. Together, our data suggest that β-catenin is a crucial downstream regulator of Ron receptor activation and is an important mediator of mammary tumorigenesis.
Keywords: beta-catenin, hepatocyte growth factor like protein, Ron receptor, breast cancer, receptor tyrosine kinase
Introduction
Behind skin cancer, breast cancer is the most frequently diagnosed cancer in women in the United States. In 2010, it is estimated that over 200,000 new cases of invasive breast cancer will be diagnosed and over 40,000 women and men will die from this disease (American Cancer Society Statistics, 2010). While death rates attributed to breast cancer have slightly diminished over the past two decades, further efforts to understand the molecular pathways governing breast cancer initiation and progression are needed. In numerous cancers, alterations in the expression and activation of cell surface receptor tyrosine kinases and in the activation of signaling molecules downstream of these receptors have been shown to play important roles in cancer development. A number of receptor tyrosine kinases, including the epidermal growth factor receptor (EGFR), Her 2, platelet derived growth factor, and the c-Met receptor, have been shown to be aberrantly expressed in human breast cancers (Coombes et al 1990, Graveel et al 2009, Shien et al 2005, Slamon et al 1989). Recently, several studies have also documented an increase in Ron receptor tyrosine kinase expression in human breast cancer (Lee et al 2005, Maggiora et al 1998, O’Toole et al 2006, Welm et al 2007).
The Ron receptor tyrosine kinase is overexpressed in about 50% of human breast cancers. In clinical studies with node-negative breast cancer specimens, Ron expression was shown to be an independent predictor of distant breast cancer metastases and breast cancer patients with high Ron expression had significantly worse 10-year disease-free survival (Lee et al 2005). In line with increased metastasis, activation of the Ron receptor signaling system, including Ron expression and expression of the Ron ligand, hepatocyte growth factor-like protein (HGFL), was shown to be a strong independent indicator of both metastasis and death in human breast cancer patients (Welm et al 2007). In murine breast cancer models, exogenous HGFL overexpression was able to promote breast tumor growth and broaden the spectrum of metastases (Welm et al 2007). Conversely, deletion of the Ron receptor in an oncogene-driven mouse model of breast tumorigenesis resulted in delayed tumor formation and decreased metastasis (Peace et al 2005).
To examine the significance of Ron receptor overexpression in breast cancers in vivo, our laboratory previously generated a transgenic mouse model wherein the Ron receptor tyrosine kinase was overexpressed selectively in the mammary epithelium (Zinser et al 2006). Ron overexpression was sufficient to induce mammary tumors in 100% of female mice and was associated with a high degree of breast cancer metastasis. We also showed that Ron overexpressing breast tumors exhibited elevated levels of tyrosine phosphorylated β-catenin. These studies suggested that Ron may be a causative factor in breast tumorigenesis and provided a model to dissect the molecular pathways downstream of Ron which are important for tumor growth and metastasis.
β-catenin is a pivotal transcription factor that plays diverse roles in adherens junctions and in cell signaling events including cellular proliferation and motility [reviewed in (Brembeck et al 2006)]. Increases in β-catenin cytosolic and nuclear accumulation have been associated with a variety of malignancies including colon and breast cancer and elevated levels of β-catenin have been shown to be inversely correlated with patient survival (Apte et al 2006, Gavert and Ben-Ze’ev 2007, Kizildag et al 2008, Lin et al 2000, Nakopoulou et al 2006, Ranganathan et al 2005, Takahashi-Yanaga and Kahn 2010). Nuclear and cytosolic localization of β-catenin is also associated with stem cell enrichment, vimentin expression, and with an epithelial to meschenymal transition (Micalizzi et al 2010, Takahashi-Yanaga and Kahn 2010). Interestingly, several reports have implicated β-catenin accumulation and activation downstream of receptor tyrosine kinase activation in multiple tumor types, although the in vivo significance of these findings is still unclear (Apte et al 2006, Castellone et al 2009, Danilkovitch-Miagkova et al 2001, Gujral et al 2008, Lee et al 2010, Zinser et al 2006).
In this report we sought to examine the biological significance of β-catenin as a downstream mediator of Ron receptor activation in breast cancer as well as the requirement β-catenin in breast tumorigenesis. Our data shows for the first time that Ron and β-catenin are coordinately overexpressed in human breast cancers and their combined high expression are associated with reduced survival and increased lymph node metastasis. In addition, we show that ligand induced Ron activation leads to β-catenin accumulation and tyrosine phosphorylation. Our data also demonstrate that ligand induced Ron activation leads to the nuclear localization and transcriptional activation of β-catenin and the expression of β-catenin dependent target genes. We also show that tyrosine residues 654 and 670 of β-catenin are important in mediating Ron-induced β-catenin transcriptional activation and cell growth. Moreover, we show that reduced breast cancer cell growth and β-catenin transcriptional activity as a result of Ron knockdown, can be rescued by activation of canonical Wnt/β-catenin signaling. Finally, in examining the independent contribution of β-catenin signaling in breast cancer cell growth, we show that loss of β-catenin expression reduces cell growth in vitro and completely abolishes tumorigenesis in vivo following orthotopic transplantation. These studies provide insights into the multiple modes of β-catenin regulation and function both downstream of the Ron receptor tyrosine kinase and as an important signaling molecule regulating breast tumor growth in vivo and in vitro.
Results
Coordinate expression of Ron and β-catenin in human breast cancer tissue and their expression in breast cancer cell lines
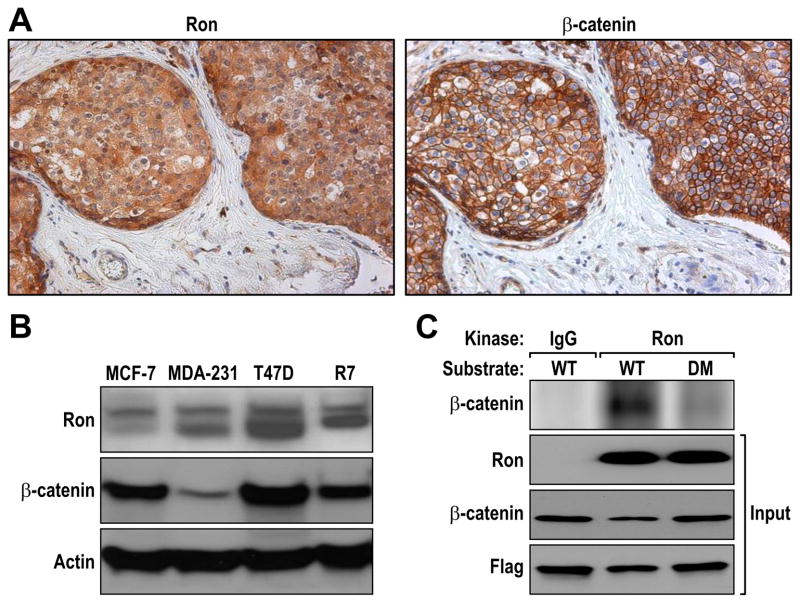
Given that our prior studies showed increased β-catenin accumulation in mouse mammary tumors following Ron overexpression in the mammary epithelium, we sought to examine the potential correlation of Ron and β-catenin overexpression in human breast cancers and cell lines. To accomplish this, we performed immunohistochemical staining on serial sections of human breast cancer tissue arrays for Ron and β-catenin expression. A representative breast tumor having both high Ron and β-catenin expression is depicted in Figure 1A. In our analysis, we found that 34 of the 76 breast cancer samples exhibited high levels of Ron expression (Table 1). In addition, we observed a statistically significant correlation between high Ron expression and high β-catenin expression with 23 of the 34 samples that expressed high levels of Ron also exhibiting increased β-catenin staining (Table 1). Tissues with high β-catenin expression exhibited a heterogeneous mixture of membranous, cytosolic and nuclear β-catenin accumulation (Figure 1A and data not shown). While the clinical follow up data on patients in this cohort was limited, our data demonstrate that patients with high Ron and high β-catenin expression (3/3, 100%) had significantly reduced survival within a 30 month follow-up period compared to patients with low Ron and β-catenin expression (5/14, 35.7%) (P<0.05). In addition, more lymph node metastasis was observed in patients with both high Ron and β-catenin levels (2/5, 40%) compared to patients with low levels of both proteins (0/14) (P<0.05). We further analyzed Ron and β-catenin expression in a panel of human breast cancer cell lines compared to the murine R7 breast cancer cell line which was established from a mammary tumor from mice which selectively overexpress Ron in the mammary epithelium (McClaine et al 2010, Zinser et al 2006). Figure 1B illustrates that that T47D human and R7 murine breast cancer cells expressed high levels of Ron, compared to MCF-7 and MDA-MB-231 breast cancer cell lines, with these cell lines also expressing high levels of β-catenin.
Figure 1. Ron and β-catenin expression and phosphorylation in human breast cancer.
A, Serial sections from human breast cancer tissue arrays were stained for Ron and β-catenin. Representative images are shown. B, Human breast cancer cell lines were screened for Ron and β-catenin expression by Western analysis. Actin was used as a loading control. C, In vitro kinase assay were performed using WT and DM β-catenin as the substrate. Flag-tagged plasmids expressing WT and DM β-catenin were transfected into HEK293 cells and the corresponding protein immunoprecipiated with an-anti-Flag antibody. Equal amounts of WT and DM β-catenin were subsequently utilized in an in vitro kinase assay with equal amounts of Ron immunoprecipitated from R7 breast cancer cell lysates or from an isotype control immunoprecipitation. A representative kinase assay is depicted demonstrating strong phosphorylation of WT β-catenin but not DM β-catenin in combination with Ron (Top). The total amount of input for Ron, β-catenin and Flag is shown as a control (Input). The data is representative of three independent experiments with similar results.
Table 1.
Ron and β-catenin expression in human breast cancer specimens.
| Total Samples | β-cat High | β-cat Low | |
|---|---|---|---|
| Ron High | 34/76 (45%) | 23/34 (68%) | 11/34 (32%) |
| Ron Low | 42/76 (55%) | 15/42 (36%) | 27/42 (64%) |
Two independent human breast cancer tissue arrays were stained for both Ron and β-catenin. The percent of Ron high and low expressing tissue samples is listed [Total Samples]. The number in parentheses shows the percentage of samples in the indicated group. There was a significant (p=0.0012) difference in the percentage of high β-catenin between the low and high Ron groups.
Ron and β-catenin associate and Ron kinase activity leads to tyrosine phosphorylation of β-catenin
Our prior studies on Ron overexpressing murine mammary tumors demonstrated an association between Ron and β-catenin by co-immunoprecipitation and with β-catenin in the immunoprecipitated tissue exhibiting tyrosine phosphorylation (Zinser et al 2006). To further data on this interaction and the role of select tyrosine residues in β-catenin which may be phosphorylated in association with Ron, in vitro kinase assays were performed. Given that previous studies have shown that β-catenin tyrosine phosphorylation at residues Tyr 654 and Tyr 670 is required for HGF-induced Met-mediated β-catenin nuclear translocation and ensuing transcriptional activation (Zeng et al 2006), we focused our studies on these tyrosine residues in β-catenin. For our kinase assays, we utilized plasmids containing either a Flag-tagged wild type β-catenin expression construct (WT) or a Flag-tagged expression construct containing a double mutant (DM) of β-catenin wherein Tyr residues 654 and 670 were replaced with phenylalanine (Zeng et al 2006). The WT and DM constructs were transfected into HEK-293 cells and β-catenin (WT or DM) was immunoprecipitated with an anti-Flag antibody. As depicted in Figure 1C, Ron, immunoprecipiated from ligand activated R7 mammary tumor cells, was able to induce the phosphorylation of WT β-catenin. A decrease in β-catenin phosphorylation was observed when the DM form of β-catenin was used as the substrate for Ron. Similar results were also observed when a purified kinase domain of Ron was utilized (data not shown), suggesting that Ron may directly phosphorylate β-catenin and may do this primarily on tyrosine residues 654 and 670 of β-catenin.
Tyrosine residues Tyr 654 and Tyr 670 are important in Ron-mediated β-catenin phosphorylation and nuclear localization
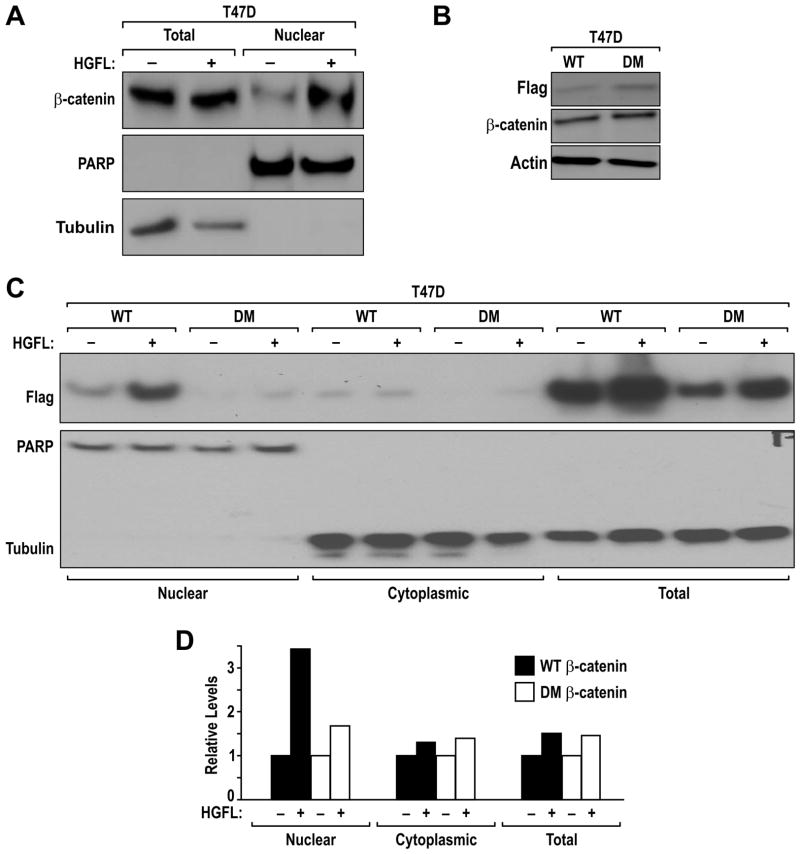
Given the similarities between the Ron and Met receptor tyrosine kinases, we sought to examine the role of HGFL-induced Ron activation on β-catenin nuclear localization and the importance of β-catenin Tyr 654 and Tyr 670 in this process. As depicted in Figure 2A, we show that HGFL treatment of T47D cells induces nuclear localization of β-catenin compared to vehicle treated cells. Similar results were obtained in the murine R7 breast cancer cells (data not shown). To test the importance of β-catenin tyrosine residues Tyr 654 and Tyr 670 in HGFL-induced β-catenin nuclear localization, WT and DM β-catenin expression constructs were transfected into T47D cells. Stable pools of T47D clones expressing similar levels of the Flag-tagged β-catenin were generated and labeled T47D-WT and T47D-DM (Figure 2B). To investigate the relevance of Tyr 654 and Tyr 670 phosphorylation of β-catenin on nuclear localization in response to Ron activation, the T47D-WT and T47D-DM cells were treated with HGFL or vehicle. Two hours after treatment, the cells were isolated into nuclear and cytoplasmic fractions. Each fraction, in addition to total cell extracts, was examined by Western analysis. As depicted in Figure 2C, HGFL treatment significantly increased the amount of WT β-catenin observed in the nuclear fraction compared to vehicle treated cells. In contrast, only a limited amount of the β-catenin DM was able to localize to the nucleus in the absence of HGFL stimulation compared to WT β-catenin and this level was not substantially increased by HGFL. Of note, the majority of exogenous WT and DM β-catenin protein was associated with the cellular membrane. Figure 2D is a representative quantification of the results from Figure 2C demonstrating that HGFL treatment increases the nuclear accumulation of WT β-catenin with minimal alterations in the nuclear amounts of DM β-catenin. Slight but consistent increases in β-catenin (either WT, DM or endogenous) levels were observed following HGFL treatment. Similar to the Met receptor, single mutations at Tyr 654 or Tyr 670 were not able to inhibit HGFL-induced nuclear localization of β-catenin suggesting that both tyrosine residues are required for this effect [data not shown and (Zeng et al 2006)].
Figure 2. Ron induces β-catenin nuclear localization and tyrosine phosphorylation.
A, Ron activation induces nuclear localization of endogenous β-catenin. T47D cells were treated with HGFL (+) or vehicle (−). After 2 hours of treatment, nuclear and whole cell extracts were generated and examined by Western analysis for β-catenin levels. Expression of PARP and Tubulin were utilized as loading controls for the purity of the nuclear and whole cell extracts, respectively. B, T47D overexpressing WT and DM β-catenin were lysed and immunoblotted for Flag and β-catenin expression. Actin was used as loading control. C, Ron induces nuclear localization of exogenous WT β-catenin but not the DM form of β-catenin. T47D cells stably expressing exogenous WT and DM Flag tagged β-catenin were treated with vehicle or HGFL for 2 hours and fractioned cell extracts were examined by Western analysis as in A with the inclusion of an antibody recognizing Flag tagged exogenous β-catenin. D, Quantification of the level of exogenous WT or DM β-catenin normalized to controls (PARP or Tubulin) in the nuclear, cytoplasmic or total cell lysate of vehicle or HGFL treated cells. Note, the T47D cells transfected with DM β-catenin showed attenuated levels of β-catenin nuclear localization in response to HGFL compared to controls. All results are representative of at least 4 independent experiments.
Ron activation promotes β-catenin mediated reporter activity and target gene expression
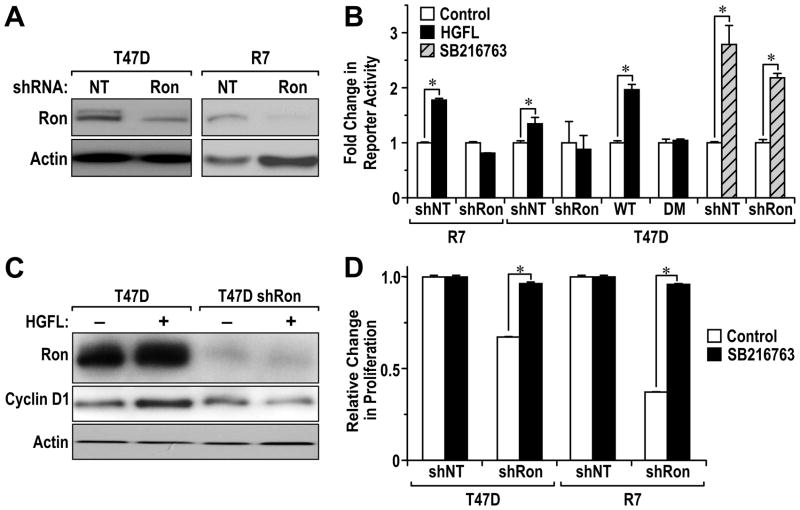
To investigate the role of HGFL induced Ron activation on β-catenin dependent reporter activity, we utilized T47D and R7 breast cancer cell lines along with cell lines containing variations in Ron and β-catenin expression. Specifically, we generated T47D and R7 cells with a stable knockdown of Ron utilizing lentiviral constructs containing shRNA against Ron or utilized a nontargeting (NT) control shRNA sequence. The knockdown of Ron in each cell type was efficient with Ron protein levels reduced by approximately 60 to 90% (Figure 3A). T47D cells stably expressing WT Flag-tagged and DM Flag-tagged β-catenin were also utilized with Figure 2B depicting similar levels of exogenous WT and DM protein (Flag) and overall β-catenin levels in these cells. Each cell line was transiently transfected with a β-catenin/TCF dependent reporter plasmid (TOPFLASH) and 48 hours later the cells were stimulated with HGFL or vehicle. Two hours after stimulation, the cells were collected and examined for luciferase activity. The reporter activity for each vehicle treated cell line was normalized to 1. T47D shNT and T47D shRon cells were similarly transfected with the β-catenin/TCF dependent reporter plasmid followed by treatment with a GSK-3β inhibitor (SB216763) for 16 hours to allow for β-catenin stabilization in cells with and without reduced Ron expression. As shown in Figure 3B, HGFL treatment of both T47D and R7 control cells leads to a significant induction of β-catenin-dependent reporter activity. Ron receptor expression is required for this induction as a knockdown of Ron in both cell types abrogates reporter activity. Treatment of T47D shNT and T47D shRon cells with SB216763 significantly increased β-catenin reporter activity in both cells compared to vehicle treated controls. This data suggests that while HGFL is not able to induce β-catenin activity in T47D Ron knockdown cells, canonical β-catenin activation, through GSK-3β inhibition, can be achieved. HGFL was also able to induce β-catenin-dependent reporter activity in T47D-WT cells to levels over that of endogenous β-catenin. In contrast, however, overexpression of the 654/670 double tyrosine mutant β-catenin in T47D-DM cells abolished HGFL-induced reporter activity.
Figure 3. Ron activation induces β-catenin-dependent activity.
A, Stable Ron knockdown in T47D and R7 breast cancer cells. T47D and R7 cells were transduced with Ron shRNA (shRon) or Non-target (NT) shRNA lentiviral plasmids and cells were lysed and analyzed for Ron expression by immunoblotting. Actin was used as loading control. B, Ron activation by HGFL induced β-catenin-dependent TOP-FLASH reporter activity. T47D shNT, T47D shRon, T47D-WT β-catenin, T47D-DM β-catenin, R7 shNT and R7 shRon cells were transiently transfected with a β-catenin reporter plasmid (TOP-FLASH) and a control reporter plasmid pRL-TK for normalization of transfection efficiency. Following transfection, the cells were treated with vehicle or HGFL (100ng/ml) for 2 hours or were treated with SB216763 or vehicle for 16 hours and a dual-luciferase assay was performed. Columns represent the average fold change of three independent experiments; bars represent Standard Error (SE). *, P < 0.05 compared to the corresponding vehicle treated control group. These results are representative of three independent experiments with similar results. C, HGFL-induced cyclin D1 expression in T47D cells is inhibited following a Ron knockdown. Control T47D shNT cells and T47D shRon cells were treated with or without HGFL for 2 hours. Cell lysates were generated and examined for Ron and cyclin D1 expression. Actin was used as loading control. D, SB216763 treatment rescues the proliferation defect in Ron knockdown cells. T47D shNT, T47D shRon, R7 shNT and R7 shRon cells were treated with vehicle or SB216763 (5 μM) for 72 hours. Cell number was measured utilizing a MTT colorimetric assay. For each experiment, the value of the vehicle treated shNT control group was set at 1 and relative values for the corresponding groups were determined. Columns represent the average fold change in proliferation over the control group from three independent experiments; bars represent Standard Error (SE). *, P < 0.05.
To examine the impact of HGFL on the production of well-established β-catenin target genes such as cyclin D1 and c-myc, T47D shNT and T47D shRon cells were treated in presence or absence of HGFL for 2 hours and total cell extracts were examined by Western analysis. As depicted in Figure 3C, HGFL stimulation increased expression of cyclin D1 in T47D cells but not in T47D cells with a Ron knockdown. Similar results were obtained with c-myc (data not shown). Knockdown of β-catenin in the T47D cells also abolished the ability of HGFL to induce cyclin D1 levels (data not shown) demonstrating the specificity of HGFL-induced Ron activation on β-catenin for the induction of cyclin D1 under these conditions. In addition, treatment of T47D and T47D-WT cells with HGFL promoted expression of both cyclin D1 and c-myc and this effect was blunted in the T47D-DM cells (Supplemental Figure S1).
To test for the ability of β-catenin to rescue the effects of Ron loss, we examined the growth of T47D and R7 cells with and without a Ron knockdown. As shown in Figure 3D (White bars), reduction of Ron levels leads to decreased growth in both cell types. However, in the presence of the GSK-3β inhibitor SB216763, which leads to increases in canonical β-catenin signaling, the growth of the Ron knockdown cells was similar to control cells. These data suggest that β-catenin activation can rescue the proliferation defect observed as a result of Ron depletion.
β-catenin deletion in MMTV-Ron cells decreases their proliferation in vitro
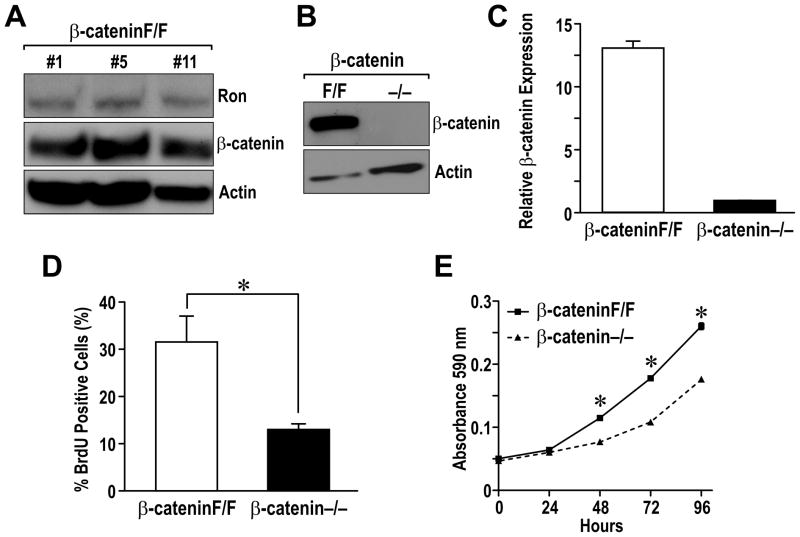
To examine the requirement of β-catenin downstream of Ron, mammary tumor cells lines were generated from MMTV-Ron mice that were bred to contain the β-catenin gene flanked by LoxP sites (Huelsken et al 2001). Three independent cell lines (labeled β-cateninF/F #1, #5 and #11) were generated as depicted in Figure 4A and express similar levels of Ron and β-catenin. To effectively eliminate β-catenin, the cell lines were transiently infected with an adenovirus dually expressing the Cre recombinase and GFP. Following infection, GFP-positive (β-catenin−/−) and negative cells (β-cateninF/F) were isolated by fluorescent activated cell sorting. As depicted in Figure 4B and 4C, β-catenin protein and RNA expression was efficiently deleted in GFP-positive cells. To examine the consequences of β-catenin loss in vitro, BrdU incorporation assays were performed. As quantified in Figure 4D, the β-catenin−/− cells exhibited a 50% reduction in BrdU-positive cells compared to β-cateninF/F. This decrease in proliferation was further supported by monitoring the growth of β-cateninF/F and β-catenin−/− cells over time (Figure 4E). These data indicated that deletion of β-catenin dramatically decreases proliferation of MMTV-Ron derived breast cancer cells in vitro.
Figure 4. β-catenin deletion in breast cancer cell lines leads to a decrease in cell proliferation in vitro.
A, Ron and β-catenin expression was analyzed by Western in three independent mammary tumor cell lines (β-cateninF/F) derived from MMTV-Ron expressing mice containing a Floxed β-catenin allele. Actin expression was used as a loading control. B, The β-cateninF/F cells were infected with an adenovirus expressing Cre recombinase and GFP. GFP-positive (β-catenin−/−) and negative (β-cateninF/F) cells were isolated by FACS sorting and analyzed for β-catenin expression Western analysis. C, Quantative real-time PCR was utilized to examine β-catenin mRNA expression in β-cateninF/F and β-catenin−/− cells. Relative mRNA levels are shown normalized to an internal control. D, Deletion of β-catenin leads to decreased proliferation. β-cateninF/F and β-catenin−/− cells were incubated with BrdU for 4 hours and immunocytostaining for BrdU incorporation was performed. The percent of cells straining positive for BrdU was quantitated from three independent high power fields and the data tabulated is a result from three independent experiments. *, P < 0.05. E, β-cateninF/F and β-catenin−/− cells were plated in triplicate in 24 well plates. Cell number was measured at 0, 24, 48, 72 and 96 hours by crystal violet staining assays. The graph is representative of the three independent experiments. *, P < 0.05 compared to the corresponding β-catenin−/− cells.
Exogenous expression of WT β-catenin but not DM β-catenin rescues the proliferation defect observed in the β-catenin−/− cells
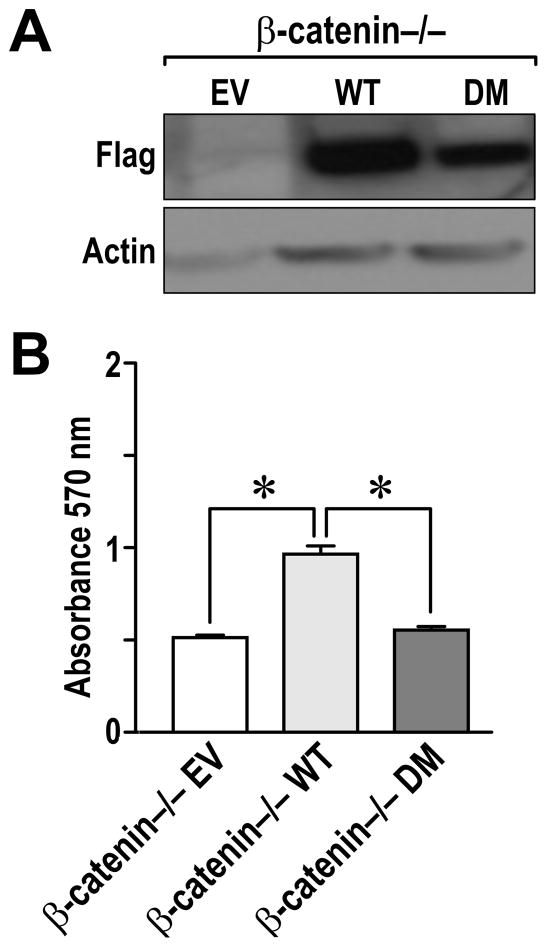
To examine the ability of WT or DM of β-catenin to rescue the proliferation defect in the β-catenin−/− cells, the β-catenin−/− cells were transiently transfected with WT β-catenin, DM β-catenin or with the empty vector (EV) as a negative control. Figure 5A demonstrates similar levels of β-catenin expression in the WT and DM transfected cells. As shown in Figure 5B, expression of exogenous WT β-catenin is able to rescue the defect in cell growth observed in the β-catenin−/− cells. In contrast, expression of DM β-catenin did not change the growth of the β-catenin−/− cells.
Figure 5. Transfection of WT β-catenin but not DM β-catenin partly rescues cell growth of β-catenin−/− cells.
A, β-catenin−/− cells were transiently transfected with Flag-tagged empty vector (EV), WT, or DM β-catenin. After 48 hours, cell lysates were generated and examined for Flag expression by Western analysis. Actin expression is provided as a loading control. B, β-catenin−/− cells were transfected with Flag-tagged empty vector (EV), WT, or DM β-catenin. After 48 hours, the cell number was quantiated utilizing an MTT assay. The experiment was performed three times in triplet and the data shown represents the mean of all experiments ± SE. *, P <0.05.
β-catenin deletion abolishes the growth of MMTV-Ron derived breast cancer cells in vivo
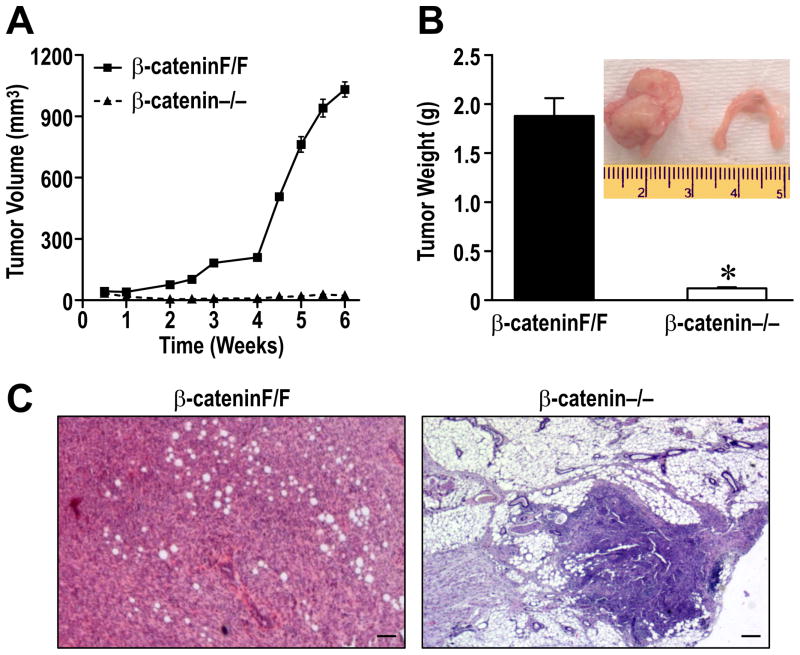
To examine the growth potential of β-cateninF/F and β-catenin−/− cells in vivo, equal number of cells were implanted orthopotically into the mammary fat pads of athymic nude mice. Tumor growth was measured biweekly by using digital calipers. As depicted in Figure 6A, the β-cateninF/F cells exhibited dramatic increases in tumor volume over the course of 6 weeks. In contrast, however, no growth was observed from the implanted β-catenin−/− cells (Figure 6A). At 6 weeks following orthotopic implantation, the mammary glands were harvested. Figure 6B demonstrates a significant increase in mammary gland weight from glands implanted with the β-cateninF/F cells compared to glands with β-catenin−/− cells. As an inset, a representative picture of each gland is provided. While these studies do not preclude the fact that β-catenin loss might simply hamper proliferation of any cancer cell line, independent of Ron signaling, they do show for the first time that β-catenin is essential for breast tumor growth in vivo in cells transformed by Ron overexpression. Following histological analysis, representative mammary tumor histology is depicted for the β-cateninF/F cells (Figure 6C). Interestingly, the β-catenin−/− cells were still observed in the mammary glands of mice after 6 weeks suggesting that cells are viable but exhibit an inability to grow in vivo (Figure 6C). In addition to primary tumor formation, extensive metastasis was observed in mice implanted with the β-cateninF/F cells. Metastases were observed in various organs including colon, pancreas, kidney, lung, and liver (Supplemental Figure S2 and Table 2). No metastasis was observed in mice transplanted with the β-catenin−/− cells.
Figure 6. Deletion of β-catenin prohibits mammary tumor formation and metastasis following implantation in the mammary fat pad of nude mice.
A, β-cateninF/F and β-catenin−/− cells were orthotopically transplanted into the mammary fat pads of nude mice. Mammary tumor growth was measured biweekly for up to 6 weeks and tumor volume was plotted over time. B, Mammary tumor weight of mice transplanted with β-catenin−/− and β-cateninF/F cells at 6 weeks. The inset depicts a representative picture of the mammary glands. *, P < 0.05 compared to the corresponding weight of the β-cateninF/F implanted glands. C, Hemotoxlyn and Eoisin staining of mammary tissue of glands implanted β-catenin F/F and β-catenin−/− cells. Bars=200 μm.
Table 2.
Metastatic dissemination of β-cateninF/F cells.
| Intestine | Kidney | Pancreas | Lung | Liver |
|---|---|---|---|---|
| 100% | 16.7% | 16.7% | 83.3% | 50% |
| (6/6) | (1/6) | (1/6) | (5/6) | (3/6) |
The organ site of metastatic dissemination and percentage of mice exhibiting site specific metastatic tumor cell dissemination were determined in mice at 6 weeks post-orthotopic implantation of β-cateninF/F cells into the mammary fat pad. The percentage of mice injected which exhibited metastasis to the specific organ site is indicated. The number in parentheses represents the number of mice with site specific metastasis/number of mice examined.
Discussion
Ron receptor overexpression in both human and murine breast cancers is associated with a highly aggressive tumor phenotype and with a high incidence of metastasis. Despite the importance of this receptor in breast cancer, downstream effectors of Ron signaling in breast cancer have not been well-characterized. Previous studies in our laboratory have shown that mammary-specific Ron overexpression in mice leads to breast tumor formation and metastasis in vivo (Zinser et al 2006). We also demonstrated that Ron overexpression in these breast tumors was associated with elevated levels of tyrosine phosphorylated β-catenin. In this study, we examined the significance of Ron and β-catenin in human breast cancer. Our study provides a number of novel findings. First, we show that Ron and β-catenin are coordinately elevated in human breast cancers and that overexpression of both is associated with reduced survival and increased lymph node metastasis as compared to patients with low Ron and β-catenin levels. Second, our data demonstrate that HGFL-induced Ron activation leads to β-catenin nuclear localization and increased β-catenin reporter activity, and that tyrosine residues 654 and 670 of β-catenin are important for these processes. Third, we demonstrate a loss of β-catenin transcriptional activity and cellular proliferation in breast cancer cells in response to HGFL following a Ron knockdown and that this loss can be compensated for by activation of canonical β-catenin signaling. Moreover, while mutation of tyrosine residues 654 and 670 of β-catenin leads to a loss of HGFL mediated β-catenin transcriptional activation, activation of canonical β-catenin signaling is unaffected. Finally, we also show that targeted deletion of β-catenin in Ron expressing breast cancer cells significantly diminishes mammary tumor growth that can be compensated for by exogenous expression of wild type β-catenin but not β-catenin mutated at Y654 and Y670. These studies not only document importance of β-catenin signaling downstream of Ron but point to the critical requirement of β-catenin expression in breast tumorigenesis.
In human breast cancer, our data demonstrate that Ron receptor overexpression significantly correlates with elevated β-catenin levels. In addition to Ron, there is strong evidence implicating β-catenin signaling in breast tumorigenesis. In humans, breast tumors frequently exhibit elevated levels of β-catenin with higher expression levels correlating with decreased patient survival (Lin et al 2000). In mice, overexpression of an activated form of β-catenin leads to the development mammary hyperplasia and adenocarcinomas (Tsukamoto et al 1988). These studies suggest that Ron receptor overexpression and activation may contribute to β-catenin signaling in breast cancer and suggests β-catenin expression and activity may serve as a biomarker for assessing the prognosis of Ron overexpressing breast cancers. Although our data set was limited in number and follow up characteristics, our studies did find that overexpression of both Ron and β-catenin are associated with reduced survival and increased lymph node metastasis within a 30 month follow-up period compared to patients with low Ron and β-catenin.
Interestingly, overexpression of other receptor tyrosine kinases including Met, EGFR and RET have been associated with increased free β-catenin pools. Moreover, tyrosine phosphorylation of β-catenin has been shown to promote β-catenin escape from the APC/Axin/GSK3β-mediated destruction and disruption of E-cadherin association and the disassembly of adherens junctions (Castellone et al 2009, Roura et al 1999). Utilizing in vitro kinase assays, our data demonstrate that Ron kinase activation leads to the tyrosine phosphorylation of β-catenin and that mutation of tyrosine residues 654 and 670 to phenylalanine significantly diminishes this effect. Our data also show that Ron receptor activation by HGFL leads to the tyrosine phosphorylation, nuclear localization and activation of β-catenin in breast cancer cell lines. Furthermore, we show that mutation of β-catenin at residues 654 and 670 from tyrosine to phenylalanine leads to a loss of HGFL-induced, Ron-dependent β-catenin nuclear localization, transcriptional activity and target gene expression. We further show that targeted deletion of β-catenin in Ron expressing breast cancer cells results in decreased cell proliferation in vitro which can be rescued by expressing wild type β-catenin but not the 654/670 mutant of β-catenin. Interestingly, while the β-catenin knockout cells grew approximately 50% less efficiently than β-catenin expressing cell in vitro, β-catenin expression was required for growth and metastasis of Ron expressing breast cancer cells in vivo. While these experiments demonstrate the importance of β-catenin in breast tumorigenesis in vivo, the data from this experiment does not specify that β-catenin deletion downstream of Ron is essential for this process but that disruption or deletion of β-catenin, as can occur through alteration of multiple signaling pathways (e.g. downstream of Wnt signaling, through various receptor tyrosine kinases, or through the disruption of cadherin functions), has an important impact in breast cancer growth and metastasis in vivo. The studies herein are consistent with reports whereby knockdown of β-catenin expression was able to reduce RET-mediated tumor growth and invasiveness of RET overexpressing NIH 3T3 cells in subcutaneous xenograft models in nude mice (Gujral et al 2008). In addition, mutation of β-catenin at both tyrosine residues 654 and 670 was able to block hepatocyte growth factor (HGF) induced β-catenin nuclear translocation, activation and proliferation downstream of Met receptor activation in hepatic cancer cell lines (Zeng et al 2006). Combined, these studies show that β-catenin is a critical factor downstream of a variety of receptor tyrosine kinases for promoting tumor cell proliferation. Further, our studies combined with previous reports on the Met receptor, demonstrating that WT β-catenin but not the 654/670 mutant of β-catenin can restore cellular proliferation, suggest a novel physiological role of receptor tyrosine kinases in inducing β-catenin-dependent proliferation. Our data also support the contention that β-catenin is an important downstream effector or Ron-mediated cell proliferation in vitro and tumor growth in vivo.
In addition to β-catenin accumulation, we have also shown that HGFL-induced Ron activation results in increased levels of both cyclin D1 and c-myc. The expression of cyclin D1 and c-myc in response to HGFL was dependent on both Ron and β-catenin expression as knockdown of either Ron or β-catenin in the breast cancer cells was able to inhibit cyclin D1 and c-myc induction. In addition, expression of the β-catenin 654/670 double mutant of β-catenin was also able to blunt the induction of these genes in response to HGFL stimulation. Both c-myc and cyclin D1 have been shown to be overexpressed in different human cancers including breast cancer. While amplification of the cyclin D1 gene has been observed in about 15% of breast cancers, overexpression of cyclin D1 at the mRNA and protein levels is observed in about 50% of human breast cancers and is mostly associated with ER-positive tumors (Taneja et al 2010). Alterations in c-myc are a common event in breast cancer, although c-myc levels alone have been difficult to correlate as a predictive or prognostic factor based on the complexity of c-myc expression and activity (Hynes and Stoelzle 2009). Interestingly both c-myc and cyclin D1 are converging effectors of both estrogen and growth factor signaling cascades in breast cancer with the potential to mediate resistance to endocrine directed therapy. It is interesting to speculate that Ron receptor overexpression might be an important marker of breast tumorigenesis and endocrine responsiveness prompting the need for a greater understanding of Ron and Ron downstream mediators in breast cancer.
In summary, our studies have identified a novel downstream pathway of Ron receptor activation that is important for breast cancer cell proliferation, tumor growth and spread. We show that HGFL stimulation of Ron induces tyrosine phosphorylation, nuclear localization and transcriptional activation of β-catenin. This association of Ron and β-catenin is observed in both human and murine breast cancers and supports the potential of anti-Ron therapy in breast cancer due to the impact of Ron receptor signaling on tumor-established pathways.
Materials/Subjects and Methods
Cell Lines and Reagents
T47D, MDA-MB-231, and MCF-7 human breast cancer cells were obtained from the American Type Culture Collection (Manassas, VA). The murine R7 breast cancer cell line was derived from a mammary tumor obtained from transgenic mice overexpessing Ron in the mammary epithelium (MMTV-Ron mice) (Zinser et al 2006). β-cateninF/F mammary tumor cell lines were generated from mammary tumors derived from MMTV-Ron that were crossed to into a homozygous β-catenin floxed (F/F) background (Huelsken et al 2001). Antibodies for Western analyses included anti-Ron β (C-20) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-catenin (BD Biosciences, San Jose, CA and Cell Signaling Technology, Danvers, MA), anti-mouse cyclin D1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-human cyclin D1 and c-myc (Thermo Scientific, Fremont, CA), anti-Flag antibody (Sigma Aldrich, St. Louis, MO), and anti-phosphotyrosine 4G10 (Millipore, Temecula, CA). Recombinant HGFL was purchased from R&D Systems (Minneapolis, MN). SB216763 (a GSK-3β inhibitor) was obtained from Sigma-Aldrich (St. Louis, MO).
Immunohistochemistry on Human Breast Cancer Specimens
Immunohistochemistry was performed on tissue microarrays (Cat. #CCB2 and CBA2, SuperBioChips Laboratories, New York, NY). Tissue staining and scoring were performed as previously described (Thobe et al 2010). Samples with no primary antibody or an IgG control antibody served as negative controls. High Ron expression was set at 190 or above and high β-catenin expression was set at 225 or above.
Plasmids
Wild-type (WT) β-catenin and Tyr 654/670 Phe (DM) β-catenin cloned into the p3XFLAG-CMV vector have been previously described (Zeng et al 2006). The human Ron shRNA (cat # RHS3979-9571732) and mouse Ron shRNA (cat # RMM3981-9590952) viral vector constructs were purchased from Open Biosystems (Huntsville, AL).
Immunoprecipitation and Immunoblotting
Cells were serum starved overnight and treated with HGFL (100 ng/ml) for the indicated times. Nuclear and cytoplasmic extracts were made as described previously (Dignam et al 1983). Immunoprecipitations and Western analyses were performed as previously described (Zinser et al 2006).
Kinase Assays
To generate the substrate, Flag tagged WT and DM β-catenin expressing plasmids were transfected into HEK-293 cells and the corresponding protein immunoprecipitated with an anti-Flag antibody (Sigma Aldrich, St. Louis, MO). Ron was immunoprecipitated from Ron R7 mammary tumor cells with a murine anti-MSP-R antibody (R and D Systems, Minneapolis, MN) from 1mg of total lysate. Equal amounts of WT and DM β-catenin were utilized as input for the kinase reactions along with equal amounts of the Ron immunoprecipitate or immunoprecipitate with an isotype control antibody. Kinase assays were performed essentially as previously described (Thobe et al 2010).
Transient Reporter Assays
The cells were transfected with the TCF reporter plasmid TOP-FLASH (Upstate Biotechnology, Lake Placid, NY) expressing firefly luciferase and pRLTX expressing Renilla luciferase (Clontech, Mountain View, CA) with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) for 48 hours. The cells were then treated with HGFL (100 ng/ml) for 2 hours, or with SB216763 for 16 hours, followed by a determination of luciferase activity according to the manufacturer’s instructions (Promega, Madison, WI).
Viral Infection
T47D and R7 cells were infected with human Ron shRNA and mouse Ron shRNA lentivirus, respectively. Cells were selected in media containing 0.5μg/ml and 5.0μg/ml of puromycin, respectively, and polyclonal stable cell lines were generated. For genetic deletion of β-catenin, the β-cateninF/F cells were infected with Adenovirus Cre-GFP. The high GFP-expressing cells were isolated the following day utilizing a BD FACSAria sorter.
Cell Growth and Proliferation Assay
To examine cell number over time, β-cateninF/F and β-catenin−/− cells were plated in triplicate on 24-well plates at a density of 5000 cells/well. Crystal violet staining was performed at 0, 24, 48, 72, and 96 hours after plating as previously described (Thobe et al 2010). For measurements of proliferation, 3×104 β-cateninF/F or β-catenin−/− cells were plated on plastic coverslips. The cells were incubated with BrdU (3ug/ml) for 4 hours, and BrdU staining was performed according to the manufacturer’s protocol (GE Healthcare, Piscataway, NJ). For rescue experiments, β-cateninF/F and β-catenin−/− cells were plated in 12-well plates at a density of 25×104 cells/well. Cells were transfected utilizing the Nucleofectar II kit (Amaxa Biosystems, Gaithersburg, MD) with WT- or DM-β-catenin. After 48 hours, MTT assays were performed.
Quantitative Real-Time PCR
To measure β-catenin transcript levels, quantitative real time-PCR was performed as previously described (Meyer et al 2009). For these experiments, the following primers were utilized: β-catenin 5′-TCCCTGAGACGCTAGATGAGG-3′ and 5′-CGTTTAGCAGTT TTGTCAGCTC-3′. Gene expression values were normalized to β-glucuronidase 5′-TTGAGAACTGGTATAAGACGCATCAG-3′ and 5′-TCTGGTACTCCTCACTGAACATGC-3′ as an internal control. Relative gene expression results are reported. Real-time analyses were repeated twice with similar results using samples from 3 individual isolations.
Tumor Growth in Nude Mice
Athymic female nude mice were purchased from the National Cancer Institute. 2×106 β-cateninF/F (n=6) and β-catenin−/− (n=7) cells in 50% Matrigel (BD Biosciences, San Jose, CA) were injected into the mammary fat pad of the nude mice. Tumor measurements were collected and expressed as described previously (Miller et al 2006, Peace et al 2001). All the animal experiments were done following a protocol approved by University of Cincinnati Institutional Animal Care and Use Committee.
Statistical Analysis
Statistical significance for all experimental analyses was determined by Student’s t-test or one-way ANOVA utilizing GraphPad Prism 4.0 software (GraphPad Software, Inc. La Jolla, CA). For the tissue microarray analyses, Pearson correlation analysis, χ2 tests and the Wilcoxon rank sum tests were all utilized (SAS® version 9.1.3, Cary, NC).
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Susanne I. Wells for providing adenoviral reagents and Allie K Varner for her technical support. This work was supported by Public Health Service Grant CA100002 (S.E.W) from the National Institutes of Health, by grant 1I01BX000803 (S.E.W.) from the Cincinnati Veteran’s Administration Medical Center, by a University of Cincinnati Cancer Center Grant (S.E.W.) and University of Cincinnati Research Council Grant (P.K.W.)
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Apte U, Zeng G, Muller P, Tan X, Micsenyi A, Cieply B, et al. Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology. 2006;44:992–1002. doi: 10.1002/hep.21317. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Castellone MD, De Falco V, Rao DM, Bellelli R, Muthu M, Basolo F, et al. The beta-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation. Cancer Res. 2009;69:1867–1876. doi: 10.1158/0008-5472.CAN-08-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes RC, Barrett-Lee P, Luqmani Y. Growth factor expression in breast tissue. J Steroid Biochem Mol Biol. 1990;37:833–836. doi: 10.1016/0960-0760(90)90428-n. [DOI] [PubMed] [Google Scholar]
- Danilkovitch-Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ. Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the beta-catenin pathway. Mol Cell Biol. 2001;21:5857–5868. doi: 10.1128/MCB.21.17.5857-5868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze’ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- Graveel CR, DeGroot JD, Su Y, Koeman J, Dykema K, Leung S, et al. Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc Natl Acad Sci U S A. 2009;106:12909–12914. doi: 10.1073/pnas.0810403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral TS, van Veelen W, Richardson DS, Myers SM, Meens JA, Acton DS, et al. A novel RET kinase-beta-catenin signaling pathway contributes to tumorigenesis in thyroid carcinoma. Cancer Res. 2008;68:1338–1346. doi: 10.1158/0008-5472.CAN-07-6052. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Stoelzle T. Key signalling nodes in mammary gland development and cancer: Myc. Breast Cancer Res. 2009;11:210. doi: 10.1186/bcr2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizildag S, Zengel B, Vardar E, Sakizli M. beta-catenin gene mutation in invasive ductal breast cancer. J Buon. 2008;13:533–536. [PubMed] [Google Scholar]
- Lee CH, Hung HW, Hung PH, Shieh YS. Epidermal growth factor receptor regulates beta-catenin location, stability, and transcriptional activity in oral cancer. Mol Cancer. 2010;9:64. doi: 10.1186/1476-4598-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Chen HH, Chow NH, Su WC, Lin PW, Guo HR. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin Cancer Res. 2005;11:2222–2228. doi: 10.1158/1078-0432.CCR-04-1761. [DOI] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M, et al. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–2933. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- McClaine RJ, Marshall AM, Wagh PK, Waltz SE. Ron receptor tyrosine kinase activation confers resistance to tamoxifen in breast cancer cell lines. Neoplasia. 2010;12:650–658. doi: 10.1593/neo.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SE, Zinser GM, Stuart WD, Pathrose P, Waltz SE. The Ron receptor tyrosine kinase negatively regulates mammary gland branching morphogenesis. Dev Biol. 2009;333:173–185. doi: 10.1016/j.ydbio.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-Mesenchymal Transition in Cancer: Parallels Between Normal Development and Tumor Progression. J Mammary Gland Biol Neoplasia. 2010 doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Miller M, Mehrotra S, Agarwal B, Mock BH, Zheng QH, et al. A physiologic imaging pilot study of breast cancer treated with AZD2171. Clin Cancer Res. 2006;12:281–288. doi: 10.1158/1078-0432.CCR-05-0219. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Mylona E, Papadaki I, Kavantzas N, Giannopoulou I, Markaki S, et al. Study of phospho-beta-catenin subcellular distribution in invasive breast carcinomas in relation to their phenotype and the clinical outcome. Mod Pathol. 2006;19:556–563. doi: 10.1038/modpathol.3800562. [DOI] [PubMed] [Google Scholar]
- O’Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66:9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- Peace BE, Hughes MJ, Degen SJ, Waltz SE. Point mutations and overexpression of Ron induce transformation, tumor formation, and metastasis. Oncogene. 2001;20:6142–6151. doi: 10.1038/sj.onc.1204836. [DOI] [PubMed] [Google Scholar]
- Peace BE, Toney-Earley K, Collins MH, Waltz SE. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 2005;65:1285–1293. doi: 10.1158/0008-5472.CAN-03-3580. [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Tan X, Monga SP. beta-Catenin and met deregulation in childhood Hepatoblastomas. Pediatr Dev Pathol. 2005;8:435–447. doi: 10.1007/s10024-005-0028-5. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Shien T, Tashiro T, Omatsu M, Masuda T, Furuta K, Sato N, et al. Frequent overexpression of epidermal growth factor receptor (EGFR) in mammary high grade ductal carcinomas with myoepithelial differentiation. J Clin Pathol. 2005;58:1299–1304. doi: 10.1136/jcp.2005.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Kahn M. Targeting Wnt Signaling: Can We Safely Eradicate Cancer Stem Cells? Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Fry EA, et al. Classical and Novel Prognostic Markers for Breast Cancer and their Clinical Significance. Clin Med Insights Oncol. 2010;4:15–34. doi: 10.4137/cmo.s4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobe MN, Gurusamy D, Pathrose P, Waltz SE. The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells. Oncogene. 2010;29:214–226. doi: 10.1038/onc.2009.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- Welm AL, Sneddon JB, Taylor C, Nuyten DS, van de Vijver MJ, Hasegawa BH, et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A. 2007;104:7570–7575. doi: 10.1073/pnas.0702095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Apte U, Micsenyi A, Bell A, Monga SP. Tyrosine residues 654 and 670 in beta-catenin are crucial in regulation of Met-beta-catenin interactions. Exp Cell Res. 2006;312:3620–3630. doi: 10.1016/j.yexcr.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser GM, Leonis MA, Toney K, Pathrose P, Thobe M, Kader SA, et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res. 2006;66:11967–11974. doi: 10.1158/0008-5472.CAN-06-2473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.